Abstract
Introduction:
Monkeypox is a re-emerging viral zoonosis that occurs naturally in heavily forested regions of West and Central Africa. Inter-human transmission of monkeypox virus, although limited, drives outbreaks, particularly in household and health-care settings. But the available evidence suggests that without repeated zoonotic introductions, human infections would eventually cease to occur. Therefore, interrupting virus transmission from animals to humans is key to combating this disease.
Areas covered:
Herein we review laboratory and field studies examining the susceptibility of various animal taxa to monkeypox virus infection, and note the competence of various species to serve as reservoirs or transmission hosts. In addition, we discuss early socio-ecologic theories of monkeypox virus transmission in rural settings and review current modes of ecologic investigation – including ecologic niche modeling, and ecologic sampling – in light of their potential to identify specific animal species and features of the environment that are associated with heightened risk for human disease.
Expert opinion:
The role of disease ecology and scientific research in ongoing disease prevention efforts should be reinforced, particularly for wildlife-associated zoonoses such as monkeypox. Such efforts alongside those aimed at nurturing ‘One Health’ collaborations may ultimately hold the greatest promise for reducing human infections with this pathogen.
Keywords: Monkeypox, One Health, zoonosis, orthopoxvirus
1. Introduction
Monkeypox (MPX) is a viral zoonosis that was largely hidden throughout the era of smallpox, gaining recognition as a human disease only during the final stages of the smallpox eradication efforts. Its concealment could be attributed to two factors: one the difficulty to discern between MPX and discrete ordinary smallpox due to similar clinical presentations, and two, the lack of routine laboratory confirmation of smallpox cases in geographic areas endemic for the disease. The virus that causes MPX, monkeypox virus (MPXV), was initially discovered in 1958 as the agent of infection responsible for outbreaks of pustular rash illness among two serial cohorts of cynomolgus monkeys that had been shipped from Singapore to Denmark [1]. The illness, non-fatal, affected 20–30% of the animals in each shipment (von Magus, 1959). The isolation and subsequent characterization of the causative agent, using discriminatory techniques common for the era, revealed the entity to be unique, but closely related to viruses in the vaccinia-variola subgroup of poxviruses [2]. Over the course of the next 10 years, multiple outbreaks were seen among captive non-human primates (NHP) in Europe, and in the United States [3,4].
Evidence of human infection eventually emerged from specimens collected in 1970, during the early stages of the intensified program of smallpox eradication efforts in Africa [5]. The discovery emerged during the course of a routine investigation of a suspected case of smallpox detected in a remote health zone in the Democratic Republic of the Congo (DRC, then Zaire) that had been free of cases for two years. At the center of the investigation was a 9-month-old child who was severely ill and hospitalized with suspected smallpox. Diagnostic specimens collected from the child were ultimately tested at the World Health Organization (WHO) smallpox reference center in Moscow and the infection was determined to have been caused by MPXV, the same virus that had afflicted the primate facilities in Europe and the United States [5]. This discovery nevertheless presented several pressing concerns. Did the existence of this virus suggest that NHPs could also serve as reservoirs to sustain variola virus? Was MPX caused by a hybrid virus harboring elements of variola virus and an animal Orthopoxvirus? Could this zoonotic virus simply replace variola as a pathogenic threat to humans? [2].
Over the course of the next two decades, dedicated research efforts on the part of a cadre of international scientists led to several pivotal findings that largely allayed these fears. First, using historic reports and laboratory studies, scientists reconfirmed the low susceptibility and transmission potential of NHPs for variola virus [3]. In addition, they determined that the protective benefits of smallpox vaccination largely extended to monkeypox, suggesting that well-vaccinated populations would, for the most part, be protected from disease [6]. Adding further support, detailed epidemiologic examination of the interhuman transmission potential of the virus demonstrated a nearly 10-fold lower efficiency of transmission among household members than smallpox [7]. Indeed, stochastic mathematical models designed to explore worst-case scenarios of possible monkeypox epidemics unambiguously pointed to the requirement for continued zoonotic introductions to feed ongoing outbreaks. Given what was known about the epidemiologic parameters of the disease at that time – inter-human contact rates, virus transmissibility, vaccine effectiveness – monkeypox was assumed unable to engender sustained epidemics in human populations in the absence of a proximate source of virus in animal populations. (Figure 1)
Figure 1.
Number of human monkeypox cases resulting from the inter-human transmission, Democratic Republic of the Congo, 1980–1984 (Summarized from Fine et al. 1988 [70],) (blue bars). The total number of infections (orange bars) encompasses instances of co-primary cases (i.e. there were 98 episodes of primary zoonotic introduction, involving 114 cases, for which there was no subsequent inter-human transmission). During this time there was only 1 episode that extended to four generations of interhuman transmission, which resulted in five human MPXV infections.
One aspect of monkeypox virus that has received considerable attention, but which remains unanswered to the current day is how the virus is maintained in nature. This knowledge gap limits our ability to accurately predict how changes (e.g. rainfall, climate change, manmade habitat disturbance, etc.) will impact virus prevalence in nature, and, by extension, risks for human acquisition of monkeypox. Naturally-occurring monkeypox currently remains confined to the humid forest regions of West and Central Africa, and, until recently, consistent, sustained reporting of cases has been largely confined to DRC [8].
Disease reporting in DRC (and elsewhere) was most notable in the years between 1970 and 1985 [4,9]. During the subsequent decade, reports diminished in DRC and ceased entirely from other areas that had experienced sporadic cases in the past. Yet, since 2001 – approximately 20 years since the cessation of routine smallpox vaccinations – disease incidence in DRC has been on the rise [10,11], and cases have been identified and confirmed in the Republic of the Congo, Central African Republic, Cameroon, South Sudan, Sierra Leone, Liberia and Nigeria [8]. Of special relevance was a large outbreak of human and animal MPX the mid-western United States, which awakened authorities to the threats accompanying the importation of potentially infected animals (rodents) from Africa (in this case Ghana) [12]. Notable as well is the 2017–18 epidemic of monkeypox that occurred in Nigeria, which prompted the initiation of a robust surveillance initiative. This surveillance effort has led to the unmasking of what is thought to be prior, unrecognized low-level endemic disease transmission in that country ‘(pers. comm)’. (Figure 2)
Figure 2.
(a) Documented (laboratory confirmed) cases of monkeypox from 1970- April 2018, in West Africa (Sierra Leone, Liberia, Cote D’Ivoire, Nigeria) (green); *Central Africa excluding DRC (Republic of the Congo, Cameroon, Gabon, Central African Republic)(blue); Other (United States, South Sudan)(yellow). (b) Monkeypox cases notified to the Ministry of Health, DRC 1970–2015. Reported cases are inclusive of reported and laboratory-confirmed cases (DRC MOH, pers comm).
In the face of mounting case counts in Africa, and the possibility for additional translocation events, an obvious question emerges. Why now? There are a multitude of possible explanations, none of which necessarily exclude the others, each of which calls for a unique response in addition to the general One Health remedies that are often prescribed to combat zoonotic diseases [13]. The varied potential contributions of different factors–such as, waning vaccine-derived immunity [10,14,15], improved surveillance [16,17], ecologic shifts [18,19], and human interactions with wildlife [20,21]–to MPX re-emergence will be discussed below in light of what is currently known, and what could be ascertained, with additional scientific inquiry.
2. Subject matter literature review
2.1. Early insights into zoonotic sources of monkeypox
The discovery and early association of MPXV with illness in NHPs (in particularly animals in the genus Macaca) led to the supposition that the virus might be a primate pathogen of East or South Asian origin [22]. The subsequent identification of several human infections in remote African villages, however, upended the idea of an Asian origin of the virus. Nevertheless, the high proportion of early human cases reporting exposure to primates served initially to reinforce the notion that these animals were largely responsible for transmitting the infection to humans. Serostudies performed from 1970 to 1975 among sizable cohorts of wild and wild-caught NHPs demonstrated an absence of anti-Orthopoxvirus antibody among animals from Asia and areas of Africa from which human MPX has not been observed (n = 1614) [22]. Conversely, Orthopoxvirus seroreactivity was detected from among a portion of NHPs from West and Central Africa, including purportedly MPXV-specific antibody from 2 Cercopitecus aethiops collected in Cote d’Ivoire (n = 207) [23].
Curiously, however, despite vigorous attempts at virus culture, no isolates were recovered during these investigations. Furthermore, the epidemiology of human infections failed to provide strong indications that NHPs were the primary vehicle of virus transmission to humans. Contact with these animals was common among individuals living in affected areas, and the frequency of infections among children generally too young to hunt or prepare the meat of monkeys suggested an alternative source of infection, apart from or in addition to NHPs.
In 1979, a large-scale survey of animals (representing at least 43 species) in DRC revealed further serologic evidence of Orthopoxvirus exposure among NHPs, but also generated evidence implicating at least one species of terrestrial rodent, and more prominently squirrels, with the latter exhibiting presumed MPXV-specific serologic reactivity [24]. This observation was consistent with findings that ~12% of persons presumed to have been infected by contact with animals had recent contact with squirrels [25]. Furthermore, none of the domestic animals tested – 120 sheep and goats, 67 cats – exhibited serologic evidence of Orthopoxvirus infection [26].
Beginning in 1984, the focus of ecologic studies intensified, collections being now restricted to those areas in DRC having active human cases [27]. The results of this shift in emphasis were two-fold: ecologic investigations began increasingly to, first, encompass elements of societal structure and human behavior (common age- and gender-associated activities, food sources, etc.), and second, to explore the significance of landscape features (agricultural plots, remote forest hunting camps) in the immediate and more remote distance from village communities. This led to the hypothesis that the disturbed ‘agricultural’ zones around habitations – areas rich in squirrels of the genus Funisciurus and Heliosciurus and certain terrestrial rodents – are likely areas where human contact might lead to virus transmission [26,28]. Indeed, collections were subsequently enriched for these species, and for NHPs more distant from habitations, leading to a second advancement in our understanding of potential sources of virus maintenance and zoonotic transmission to humans, the isolation of MPXV from a captured, symptomatic squirrel (Funiscirurs anerythrus) in 1985 [24].
Although the socio-ecologic hypothesis for MPXV maintenance and transmission met with initial approval within the scientific community, there has been very little additional evidence generated over the years to support its veracity. The presumptive animal source of primary human infections continues to be cryptic [16,29] and the virus has remained elusive in its natural source. The only other instance of virus isolation from a wild animal was in 2012 from a juvenile sooty mangaby (Cercocebus atys) from Cote d’Ivoire [30]. The idea that NHPs may experience incidental infections similar to those of humans and may themselves be an incidental source of infection to humans, is now generally accepted. Aside from that, the contemporary picture of monkeypox is one of a wildlife zoonosis with a complex ecology and epizootology, potentially involving a network of maintenance hosts, plus additional susceptible species capable of transmitting the virus to humans [31].
2.2. Laboratory studies and field surveys of species susceptibility
Fundamental insights into the natural history of sylvatic zoonoses such as monkeypox often emerge from the combined efforts of laboratory-based studies of host susceptibility and/or tolerance, and field surveys evaluating infection prevalence and patterns of disease transmission. Such efforts are ongoing for MPXV.
The range of taxa capable of supporting infection with MPXV – either artificially in a laboratory setting, or naturally among animals in confinement – is extraordinarily broad, though several common peridomestic rodents are excepted. Adult white rabbits, and white rats (genus Rattus), have been observed to be refractory to infection with MPXV, regardless of the route of exposure (mucosal, intravenous, parenteral); newborns, on the other hand, are highly susceptible (reviewed in [32]). Nearly all sub-species of the common house mouse, Mus musculus, are resistant to challenge with MPXV when adult animals with functional immune systems are used [33]. One exception to this is the castaneous (CAST) subspecies of the house mouse, Mus musculus castaneus. In particular, a wild-derived inbred strain of CAST (CAST/EiJ; wild parental strain from Thailand) mice is highly susceptible to orthopoxvirus infection by intranasal and systemic routes [34]. The basis for susceptibility of this inbred line is thought to be due to intrinsic low levels of IFN-γ and TNF-α responses and overall fewer NK cells and CD4+ and CD8+ T cells as compared to a resistant classical inbred mouse strain [35,36]. These observations have been important as they allow scientists to infer immunologic characteristics associated with MPXV susceptibility, and because they underscore the impact of genetically based host restriction on the potential of monkeypox for geographic spread [37].
Further insights into the range of taxa that can support MPXV infection – some of which may be involved in transmitting the infection to humans – have emerged from outbreaks among captive animals on display or kept as pets. Two New World giant anteaters (Myrmecophaga tridactyla) formed the index of infection during an outbreak of MPX at the Rotterdam zoo in 1964, during which individuals from seven different species of NHPs became ill and in some cases died [38]. New World anteaters are not believed to play a role in the natural history of MPXV, but their role in igniting the outbreak at the zoo is undisputed, lending support to the notion that transmission hosts need not be natural, maintenance hosts of the virus. Significantly, this event highlights the ongoing circumstance that captive animals seem particularly vulnerable to the epizootic spread of MPXV, whether due to crowding, species mixing, or physiologic stress, a fact underscored by two recent outbreaks at primate sanctuaries in Cameroon [8].
A larger natural experiment of this nature was inadvertently performed in 2003 in the United States when MPXV was introduced to several mid-western states through a consignment of African rodents (origin Ghana) destined for the pet trade. This outbreak led to 47 confirmed and probable human infections as well as documented infections among nine other animals species, most kept as ‘pocket pets’ [39] (Table 1). The susceptibility of several native rodent species to MPXV infection raised immediate concern regarding the possibility of sylvatic spread, but localized surveillance efforts performed at the site of animal carcass disposal failed to yield evidence of the virus in rodent populations.
Table 1.
Animal taxa susceptible to infection with monkeypox virus.
| Order | Family | Species | Common name | Method of determination | Comment | Citation |
|---|---|---|---|---|---|---|
| Didelphimorphia | Didelphidae | Monodelphis domestica | Gray short-tailed opossum | Outbreak among pocket pets | na | [39] |
| Didelphis marsupialis | Southern opossum | Outbreak among pocket pets | na | [39] | ||
| Eulipotyphla | Erinaceidae | Atelerix spp. | African hedge hog | Outbreak among pocket pets | na | [39] |
| Lagomorpha | Leporidae | Oryctolagus cuniculus | White rabbit | Laboratory infection studies | Adult animals generally not susceptible; outcomes vary by route of inoculation and genetic background of animal | [40] |
| Macroscelidea | Macroscelididae | Petrodromus tetradactylus | Four toed sengis (elephant shrews) | Field investigations | OPX antibody positive; no antigen or virus DNA detected | [29,31] |
| Pilosa | Myrmecophagidae | Myrmecophaga tridactyla | New World giant anteater | Outbreak, zoological park | Morbidity observed | [3] |
| Rodentia | Chinchillidae | Chinchilla lanigera | Chinchilla | Outbreak among pocket pets | OPX antibody positive; no antigen or virus DNA detected | [39] |
| Cricetidae | Sigmodon hispidus | Cotton rat | Laboratory infection studies | Unpublished laboratory infection studies (Shelukhina 1980) | [41] | |
| Dipodidae | Jaculus spp. | Jerboa | Outbreak among pocket pets | [39] | ||
| Gliridae | Graphiurus spp. | African dormouse | Outbreak among pocket pets; laboratory infection studies | In vivo imaging studies performed post-infection | [39,52] | |
| Muridae | Mus musculus | House mouse, laboratory mouse | Laboratory infection studies | Wild-derived castaneus strains were shown to be susceptible in laboratory studies; adult immune competent mice are generally resistant | [33,42] | |
| Mastomy natalensis | Multimammate mouse | Laboratory infection studies | Original studies unpublished | [43] | ||
| Oenomys hypoxanthus | Rufus-nosed rat | Field investigations | OPX antibody positive; no antigen or virus DNA detected | [31] | ||
| Nesomyidae | Cricetomys spp. | Giant pouched rat | Outbreak among pocket pets; laboratory infection studies | In vivo imaging studies performed post-infection | [39,53] | |
| Sciuridae | Cynomys ludovicanus | Black-tailed priarie dog | Outbreak among pocket pets; laboratory infection studies | In vivo imaging studies performed post-infection | [39,44] | |
| Funiscirus spp. | Rope squirrel | Outbreak among pocket pets; laboratory infection studies; virus isolate from wild-collected animal | F. anerythrus, F. pyrrphpus, F. congicus, F. lemniscatus demonstrated susceptibility even with low-dose inoculum* | [24,39,45] | ||
| Heliosciurus gambianus | Sun squirrel | Laboratory infection studies | Unpublished laboratory infection studies; animals recovered after mild illness with low-dose inoculum** | [45] | ||
| Protexerus strangeri | Forest giant squirrel | Laboratory infection studies | Unpublished laboratory infection studies | [45] | ||
| Marmota monax | groundhog | outbreak among pocket pets | na | [39] | ||
| Marmota bobak | Ground squirrel | laboratory infection studies | na | [46] | ||
| Spermophilus tridecemlineatus | 13-Lined ground squirrel | Laboratory infection studies | na | [47] | ||
| Sciurus vulgaris | Red squirrel | Laboratory infection studies | na | [45] | ||
| Xerus sp. | Unstriped ground squirrels | field investigations | MPXV DNA detected | [48] | ||
| Primates | Callitrichidae | Callithrix Jacchus | Marmoset | Laboratory infection studies | na | [49] |
| Ceropithicediae | Cercopithecus spp. | Guenons | Field investigations | OPX antibody positive; no antigen or virus DNA detected | [50] | |
| Colobus spp. | Colobuses | Field investigations | OPX antibody positive; no antigen or virus DNA detected | [50] | ||
| Cercocebus atys | Sooty mangabey | Field investigations | Viral isolate collected from wild animal found dead | [30] | ||
| Hominidae | Gorilla sp. | Gorilla | Outbreak, zoological park | Morbidity observed | [3] | |
| Pan troglodytes | Chimpanzee | Outbreak at sanctuaries in Cameroon | Viral isolates obtained | [8] | ||
| Pongo sp. | Orangutan | Outbreak, zoological park | Morbidity & mortality observed | [3] |
‘na’, no additional relevant information.
≤102 plaque forming units (pfu);
104 pfu.
Three genera of African rodent, Graphiurus, Cricetomys and Funisciurus (African dormice, giant pouched rat, rope squirrel, respectively) were implicated as vehicles of virus introduction during the initial importation event [39,51]. Studies performed subsequently to assess the competence of each species to serve as natural reservoirs of the virus demonstrated that, in general, though none showed ‘tolerance’ (i.e. virus amplification and shedding in the absence of evident disease), each was capable of being infected and of shedding viable virus for extended periods of time through varied routes [52–56]. All three species can be found in forest margins and in the disturbed agricultural (peridomestic) zone near human habitations in rural West and Central Africa [27,28]. Members of the Graphiurus and Funisciurus genera are largely arboreal, whereas Cricetomys are terrestrial (though they have a reputation as good climbers) [57]. Additionally, Cricetomys and Funisciurus can serve as protein sources for people living in rural communities suggesting enhanced opportunities for potential animal-human contact for these taxa [31,58,59].
While reservoir studies in the laboratory and in the field have, to date, not led to the definitive identification of a single MPXV maintenance host, several likely, candidates (outlined above) have been identified based on evidence generated from outbreak investigations, ecologic analysis, anthropologic information and laboratory studies of reservoir competence. Additional modes of investigation aimed at identifying elements of the physical environment that are associated with risk for the disease may reveal a set of ecologic requirements that are consistent (or inconsistent) with a range of presumptive MPXV hosts.
2.3. Mapping zoonotic risks through space and time: ecologic niche modeling approaches
To date, several studies have begun to probe associations between the ecologic or physical features of the environment and MPX risk. During a 2013 outbreak of monkeypox in the Bokungu health zone in DRC satellite imagery was used to compare the immediate environment around case homes as compared to unaffected homes [60]. For this a 50-m resolution multispectral Landsat composite image (obtained from the Central Africa Regional Program for the Environment, CARPE) was classified into five land cover categories: water (rivers), forest (tree canopy), disturbed (cleared forest, agricultural land), developed (human habitation, roads), and flooded (seasonal flooding). The analysis revealed no differences in the immediate vicinity between case households and other households in the community, but when considering land type out to 500 m, case households were significantly more likely to abut disturbed areas than noncase households [60].
More recently, a multi-year survey of small terrestrial animals sampled from a region of high MPX endemicity in DRC revealed a relative paucity of orthopoxvirus-seropositive animals from edaphic (seasonally flooded) forest areas, despite the presence of many of the same species in edaphic vs nearby dense forest areas [31]. This observation shed light on how landscape features can influence enzootic maintenance or spread of MPXV. More such studies are needed.
In a similar vein, ecologic niche models have been employed in attempts to better define the geographic extent of areas that are ecologically suitable for monkeypox [18,19,61,62]. Such models are based on, developing an understanding of ecologic factors at the location of human cases (using remote sensing data in conjunction with human MPX occurrence data), and then identifying the extent of other areas that fulfill the same conditions. Comparison of the projected area of suitability of MPXV with that of various presumptive hosts is one means of assessing the likelihood of each as a reservoir [63]. The lack of comprehensive, geographically coded collection information for many species of interest, however, is a current hindrance to this approach.
Ecologic niche models have been used at different scales to determine environmental associations for MPXV transmission and identify suitable areas. At the continental scale, models found that monkeypox transmission is mainly associated to the rainforest in Sub-Saharan Africa [61,64]. At the regional scale, models built using historic data and projected into contemporary environmental data in the Congo basin showed changes in the distribution of suitable conditions with potential expansion of at-risk areas [18]; in recent years, several human cases have been reported from these predicted areas [8]. At the local scale, models confirmed a higher risk of monkeypox in highly forested areas [65].
3. One Health and intersectoral responses during monkeypox outbreaks
Results from laboratory studies, field surveys and ‘natural experiments’ suggest that a variety of animals can be infected with OPXVs and may be competent to transmit the virus to humans. Protein supplementation from wild animal sources is common and nutritionally important in many parts of Africa where monkeypox occurs. In rural DRC, for example, people living in forested areas routinely encounter the carcasses of rodents, primates and other animals [58]. These are often collected for consumption or sold. This suggests that MPX educational campaigns should prioritize reducing human contact with suspect animals, focusing on those that commonly serve as sources of protein, e.g. primates and larger rodents [31]. In those instances where the cause of the animal’s death is not apparent or might have been due to an infection, messages that discourage the consumption or sale of found carcasses may enhance disease prevention efforts for MPX and other zoonoses that affect wildlife.
But, in the absence of other secure sources of protein, the impact of such messaging efforts may ultimately be limited. This highlights an important role for the agricultural sector in zoonotic disease prevention efforts, including for diseases such as MPX that are derived from wildlife. Food security remains a pressing concern in many rural communities in Africa. By reducing reliance on wildlife as a food source, human contact with potentially infected animals could be decreased thereby diminishing risks for many zoonotic diseases. Active promotion of local husbandry efforts to raise food animals is one way this could be accomplished. It must be noted, however, that changing people’s behaviors and food preferences have often proven to be challenging [66]. Consumption of ‘bush meat’ among urbanized populations, for example, is often driven by cultural preference rather than need. The use of animal products in traditional medicine may also be a contributing factor, increasing the extent of individuals at potential risk for infection. Such practices can lead to atypical outbreaks of MPX, such as those observed in more urban settings [67].
Multi-sectoral disease prevention strategies have gained visibility and traction through various global health initiatives, such as the Global Health Security Agenda [68], often falling under the egis of ‘One Health’ [69]. Conceptually, the goal of One Health is to improve overall health outcomes by recognizing that human and animal health are interdependent and integrally tied to ecosystems. In practice, a key objective of One Health has often been to improve both high-level and on-the-ground coordination and communication between human and veterinary public health services [70,71]. The idea that this latter approach will yield major benefits for wildlife-associated zoonoses can seem counterintuitive, as it is difficult to envision a role for veterinary public health services with diseases such as MPX, which are largely cryptic in nature and do not generate discernable epizootics with large die-offs of animals. In such instances, veterinary services may be of less direct relevance than ecologic science.
A more likely role for veterinary public health services emerges from the particular situation of MPX outbreaks involving animals in captivity. Captive animals can serve as sentinels to alert health authorities of the circulation of MPXV in the immediate captive environment or in the environment from which the animals originated. Recent examples of this include outbreaks among captive, roaming chimpanzees at primate sanctuaries in Cameroon (2014, 2016) [8] and the geographically widespread outbreak of MPX that affected ‘pocket pets’ (and their humans) in the Midwestern United States in 2003 ([12]). In both instances, veterinary personnel and animal caretakers were integral to outbreak response efforts and were themselves ultimately often at risk for infection [72]. The outbreak in the United States precipitated institution by public health authorities of a complete ban on importation of African rodents, either alive or dead (https://www.cdc.gov/importation/bringing-an-animal-into-the-united-states/african-rodents.html).
For MPX outbreaks of undetermined origin, live animal markets, zoos, commercial holding facilities and household pets should be considered as potential sources of infection, thus necessitating the institution of coordinated, inter-sectoral investigation approaches.
4. Conclusion
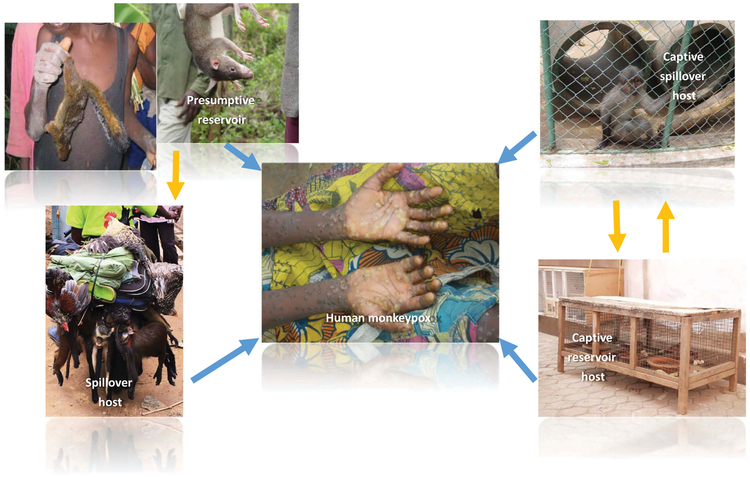
Epidemiologic observations – bolstered by theoretical models of disease transmission – point to the fact that inter-human transmission of MPXV cannot be sustained indefinitely [73,74]. The virus is maintained in wildlife populations and occasionally spills over to affect humans. There are various potential sources of spillover to humans including, reservoir hosts; incidentally infected, wild hosts; and replication permissive, captive animals. (Figure 3) A critical next step in preventing the further emergence and spread of this disease will be to gain a better understanding of the principal sources and modes of MPXV transmission at the human-animal-ecosystem interface. Inter-sectoral research initiatives (ecologic, epidemiologic) undertaken before, during, and after MPX occurrence events will be key to generating new insights and hypotheses and will foster the development of new tools for monitoring and forecasting MPX disease risks.
Figure 3.
Potential sources of zoonotic transmission of MPXV to humans. Yellow arrows denote potential MPXV virus transmission routes between (non-human) animals. Blue arrows denote potential routes of virus transmission to humans.
5. Expert opinion
The improvements made to disease detection capacity in the wake of the 2014 Ebola Epidemic is one factor that has undoubtedly contributed to the recent upsurge in the identification of MPX cases in West and Central Africa [8]. Further improvements in response capacity – especially in the areas of infection prevention and control (IPC) and medical countermeasure utilization – should result in fewer instances of inter-human transmission. What remains then is the quandary of how to approach prevention of primary zoonotic infections of humans. Where should resources be devoted? to ecologic studies aimed at understanding the natural transmission cycles of MPXV? To ecologic modeling studies to identify and predict geographic areas prone to disease risk? Or to further developing One Health frameworks for coordinated surveillance and response?
Each has some favorable aspects. One Health emphasizes a role for veterinary medicine and public health in control of zoonotic diseases, and further promotes the importance of a unity of health across animal, human and environmental sectors [71]. But in natural settings, MPXV may not be a significant animal health issue. Pathogens are a part of healthy ecosystems, and indeed, sick, MPXV-infected animals are rarely found during the course of active scientific investigation. On the other hand, there is some suggestion that the utilization of wild animals in traditional medicine and as food sources may be associated with enhanced risk for MPX [29]. This is certainly the case for certain other serious zoonotic infections [75,76]. Decreasing human reliance on wild animals as food sources through agricultural extension efforts, may, therefore, have positive benefits overall with regard to reducing zoonotic infections associated with wild animals. Fostering social movements to dissuade consumption of ‘bush meat’ among urban populations could yield similar benefits. Such efforts could also engender improved conservation and wildlife management outcomes, i.e. better environmental health.
Ultimately, however, the problem of how to prevent MPX in humans boils down to the need to understand where virus exists in nature and how it most commonly enters human communities. With this information in hand, useful disease prevention recommendations and effective interventions could be put into place. Reaching this endpoint will, however, require the combined application of ecologic, epidemiologic and behavioral science. In the interim, outbreak control measures organized around a nexus of stakeholders – from the public health sector, agricultural and animal health sectors, and environmental health and conservation sectors – may offer the best path forward. Ensuring that the latter two groups are not only represented in outbreak response planning and actions, but are also adequately provisioned with resources, will expand opportunities to identify and reduce primary sources of MPXV in the immediate environment, and will build critical capacity to combat other zoonotic infections.
Specific MPX knowledge gaps and weaknesses in public health practice that merit timely attention and allocation of resources are detailed below.
5.1. What are key weaknesses hindering current efforts to halt the resurgence of MPX?
Underdeveloped capacity for human disease MPX surveillance, detection, and diagnosis in areas with endemic disease and in areas at risk for the sporadic occurrence or importation events.
Time lags in achieving laboratory confirmation of MPXV infection, which often precludes the ability to perform a timely investigation of events at the start of an outbreak.
Lack of systematic, longitudinal surveys for evidence of infection in relevant animal populations in monkeypox endemic areas, which limits our ability to identify those animal species that maintain the virus in nature and inform prevention strategies.
Lack of robust animal infection data from endemic areas to inform predictive risk modeling at a community level.
Lack of the understanding of natural history of the virus and common sources of human infection.
Lack of comprehensive strategy for vaccine utilization.
5.2. What research approaches would be useful and what are the potential outcomes?
Research efforts encompassing predictive risk modeling across different landscape types and scales (including fine-scale, village level analyses) could help to pinpoint potential sources of environmental risk both proximal and more distal to human habitations.
Population genetic studies of MPXV could complement fine-scale understanding of virus transmission patterns in different ecologies.
Longitudinal studies of suspected reservoirs in disease-endemic areas would inform hypotheses regarding the reservoir status of various species and to generate knowledge about the ecology of the reservoir itself.
Ecologic risk mapping studies could be performed to merge nascent theories of MPXV disease ecology with the known biology of presumptive hosts in order to more accurately pinpoint potential sources of risk.
Theoretical mathematical modeling studies could be performed to demonstrate whether MPXV transmission can be sustained in nature by a single host or whether it requires multiple reservoir species.
Surveys performed among human groups at-risk for primary MPXV introduction would lead to a better understanding of the specific interactions with wildlife that lead to risk for infection.
5.3. How can current impediments to coordinated, multisectoral engagement be minimized?
Guidelines should be established within the human public health, agriculture and environmental sectors to facilitate work in a coordinated fashion to ensure the best and most efficient use of resources (e.g. labs, expertise in diagnostics, fieldwork, biosafety, etc.).
Veterinary and environmental health sectors are chronically under-resourced for zoonotic disease investigations. Outbreak investigations conducted within the framework of an Emergency Operations Center typically emphasize and provide resource support for traditional human public health activities to the detriment of other vital stakeholder activities. For MPX, the investigation of live animal markets, wildlife, and wildlife products should be considered priorities, especially when the cause of the outbreak is undetermined. Resources made available to allow the work to be accomplished within the appropriate sector Table 2.
Active engagement with environmental partners in the academic community should be explored to support rapid response and longer-term monitoring of virus in environment. Such engagements offer mutual benefit including the training of the next generation of scientists and public health professionals.
Additional studies should be performed to address inter- and intra-specific MPXV transmission parameters among captive animals.
Table 2.
Investigation modalities for zoonotic sources of monkeypox virus.
| Possible MPXV source location |
Potential source | Investigation | Probable Investigation Stakeholders |
|---|---|---|---|
| Wildlife habitat, forest | Live animals, animal carcasses, multiple species | Longitudinal or sentinel surveillance; intensive sampling (trapping) during presumptive epizootic | Ecologic scientists (University and/or Government based); Governmental sectors with oversight of Forests, the Environment, Conservation; Veterinary and/or human public health laboratories |
| Market | Skins, meat, non-meat wildlife products (amulet, traditional medicines, quills, ointments) | During outbreak–tissue samples, swabs, fragment of products | Food inspection authorities; Governmental sectors with oversight of Agriculture; Veterinary and/or human public health laboratories |
| Live animal market/zoos and animal sanctuaries | Live animals, animal carcasses, ill animals, in captive settings for commence or display | During outbreak–tissue samples, swabs, necropsy specimens | Governmental sectors with oversight of Forests, the Environment, Conservation; Governmental sectors with oversight of Agriculture, and Human Public Health; Veterinary and/or human public health laboratories |
| Peridomestic environment | Live animals, animal carcasses, multiple species | During outbreak – intensive sampling (trapping) around location of affected human habitations | Ecologic scientists (University and/or Government based); Governmental sectors with oversight of Human Public Health and Sanitation; Veterinary and/or human public health laboratories |
5.4. What is the risk of failing to make progress?
In the absence of a firm understanding of the principal species responsible for transmission of MPXV to humans, opportunities for ‘bush meat’-associated, urban outbreaks of MPX may increase in certain parts of West and Central Africa where the transportation infrastructure and economic purchasing power of urban dwellers has improved. Conversely, the social strife and armed conflict in other MPX-prone areas of Africa could also lead to more outbreaks, owing to an increased reliance on marginal protein sources among displaced people.
As healthcare-associated transmission of MPXV is increasingly observed, the risk of transmission to especially vulnerable populations – pregnant women, HIV-infected persons, immune-compromised persons – is magnified.
Though MPX outbreaks in recent years have increased in frequency and size, the vast majority of human infections have occurred in areas of known endemicity. However, given the diversity of taxa that are capable of supporting MPXV replication, it is conceivable that the virus could spillover into a broadly-ranging, permissive animal species. One with the potential to, at least temporarily, greatly expand the geographic range of monkeypox and, concomitantly, the magnitude of the human population at risk.
Enhanced research and multisector engagement to identify important sources of zoonotic transmission can reduce primary human infections through behavioral avoidance. Coordinated investigations and research in the human, animal and environmental sectors will generate knowledge about the natural history of the disease (i.e. reservoir(s), maintenance of virus in nature, identification of at-risk populations, etc.) as well as socioeconomic and behavioral factors associated with primary transmission into humans that, together, will inform interventions to reduce risk of human disease. Introduction and use of vaccines to prevent human-to-human transmission can further reduce the threat of this infection in MPXV enzootic countries and virtually eliminate the threat of its spread beyond.
Article highlights.
Human monkeypox is a re-emerging zoonotic disease threat. In recent years, cases of human monkeypox have been reported in areas/countries from which the disease was last reported decades ago (e.g. Liberia, Sierra Leone, Nigeria, Cameroon, Cote d’Ivoire), which may represent the re-emergence of monkeypox in the region.
Monkeypox is a zoonotic disease known since the 1970s, yet many aspects of the natural history and zoonotic transmission of monkeypox virus remain unknown. However, recent studies in a wide variety of fields have contributed to the identification of potential reservoir species of the virus; environmental conditions that could determine the geographic distribution of the disease; factors associated with the zoonotic transmission of the virus; and local elements of disease transmission into and within affected human populations.
Approaches to the control of monkeypox should focus on intersectoral collaboration, but not necessarily under a traditional One Health rubric. Future studies and research should focus on integrative approaches that include coordinated efforts from the human, animal and environmental sectors to understand the various aspects of this disease system and propose effective interventions to protect public health. The One Health concept proffers a framework within which human, animal, and environmental health are interrelated; however, the implementation of coordinated efforts between sectors can prove challenging without common goals, understanding and guidelines to create synergy among all stakeholders.
Acknowledgments
Funding
This paper was not funded.
Footnotes
Declaration of interest
All authors receive salary support from the US Federal Government. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.von Magnus P, Andersen E, Petersen K, et al. A pox-like disease in Cynomolgus monkeys. Acta Pathol Microbiol Scand. 1959;46:156–176. [Google Scholar]
- 2.Cho CT, Wenner HA. Monkeypox virus. Bacteriol Rev. 1973;37:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arita I, Henderson DA. Smallpox and monkeypox in non-human primates. Bull World Health Organ. 1968;39:277–283.• Paper summarizes findings from the earliest investigations of monkeypox virus outbreaks in captive animals and describes sporadic, naturally-occurring outbreaks of smallpox among wild primates.
- 4.Breman JG. Monkeypox: an emerging infection for humans? In: Scheld WM, Craig WA, Hughes JM, editors. Emerging infections 4. Washington (DC): ASM Press; 2000. p. 45–67. [Google Scholar]
- 5.Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu territory, democratic republic of the Congo. Bull World Health Organ. 1972;46:593–597. [PMC free article] [PubMed] [Google Scholar]
- 6.Jezek Z, Marennikova SS, Mutumbo M, et al. Human monkeypox: a study of 2,510 contacts of 214 patients. J Infect Dis. 1986;154:551–555. [DOI] [PubMed] [Google Scholar]
- 7.Jezek Z, Gromyko AI, Szczeniowski MV. Human monkeypox. J Hyg Epidemiol Microbiol Immunol. 1983;27:13–28. [PubMed] [Google Scholar]
- 8.Durski KN, McCollum AM, Nakazawa Y, et al. Emergence of monkeypox – west and Central Africa, 1970–2017. MMWR. 2018;67:306–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breman JG, Henderson DA. Poxvirus dilemmas–monkeypox, smallpox, and biologic terrorism. N Engl J Med. 1998;339:556–559. [DOI] [PubMed] [Google Scholar]
- 10.Rimoin AW, Mulembakani PM, Johnston SC, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the democratic republic of Congo. Proc Natl Acad Sci USA. 2010;107:16262–16267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoff NA, Doshi RH, Colwell B, et al. Evolution of a disease surveillance system: an increase in reporting of human monkeypox disease in the democratic republic of the Congo, 2001–2013. Int J Trop Dis Health. 2017;25:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350.• Paper describes a large outbreak of monkeypox in the United States ensuing from the introduction of monkeypox virus into a ‘pocket pet’ retail facility.
- 13.Bidaisee S, Macpherson CN. Zoonoses and one health: a review of the literature. J ParasitolRes 2014;2014:874345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karem KL, Reynolds M, Olson V, et al. Monkeypox outbreak diagnostics and implications for vaccine protective effect. Nat Med. 2006;12:495–496. [DOI] [PubMed] [Google Scholar]
- 15.Karem KL, Reynolds M, Hughes C, et al. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. ClinVaccine Immunol. 2007;14:1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doshi RH, Guagliardo SAJ, Dzabatou-Babeaux A, et al. Strengthening of surveillance during monkeypox outbreak, republic of the Congo, 2017. Emerg Infect Dis. 2018;24:1158–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds MG, Emerson GL, Pukuta E, et al. Detection of human monkeypox in the republic of the Congo following intensive community education. Am J Trop Med Hyg. 2013;88:982–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakazawa Y, Lash RR, Carroll DS, et al. Mapping monkeypox transmission risk through time and space in the Congo Basin. PLoS One. 2013;8:e74816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomassen HA, Fuller T, Asefi-Najafabady S, et al. Pathogen-host associations and predicted range shifts of human monkeypox in response to climate change in central Africa. PLoS One. 2013;8:e66071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalthan E, Dondo-Fongbia JP, Yambele S, et al. [Twelve cases of monkeypox virus outbreak in Bangassou District (Central African Republic) in December 2015]. Bull Soc Pathol Exot. 2016;109:358–363. [DOI] [PubMed] [Google Scholar]
- 21.Quiner CA, Moses C, Monroe BP, et al. Presumptive risk factors for monkeypox in rural communities in the democratic republic of the Congo. PLoS One. 2017;12:e0168664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arita I, Henderson DA. Monkeypox and whitepox viruses in west and Central Africa. Bull World Health Organ. 1976;53:347–353. [PMC free article] [PubMed] [Google Scholar]
- 23.Gispen R, Brand-Saathof BB, Hekker AC. Monkeypox-specific antibodies in human and simian sera from the Ivory Coast and Nigeria. Bull World Health Organ. 1976;53:355–360. [PMC free article] [PubMed] [Google Scholar]
- 24.Khodakevich L, Jezek Z, Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet. 1986;1:98–99.• Details the first instance of monkeypox virus isolation from a wild animal.
- 25.Arita I, Jezek Z, Khodakevich L, et al. Human monkeypox: a newly emerged orthopoxvirus zoonosis in the tropical rain forests of Africa. Am J Trop Med Hyg. 1985;34:781–789. [DOI] [PubMed] [Google Scholar]
- 26.Khodakevich L, Szczeniowski M, Nambu MD, et al. Monkeypox virus in relation to the ecological features surrounding human settlements in Bumba zone, Zaire. Trop Geogr Med. 1987;39:56–63. [PubMed] [Google Scholar]
- 27.Khodakevich L, Jezek Z, Messinger D. Monkeypox virus: ecology and public health significance. Bull World Health Organ. 1988;66:747–752. [PMC free article] [PubMed] [Google Scholar]
- 28.Khodakevich L, Szczeniowski M, Manbu MD, et al. The role of squirrels in sustaining monkeypox virus transmission. Trop Geogr Med. 1987;39:115–122. [PubMed] [Google Scholar]
- 29.Hutin YJ, Williams RJ, Malfait P, et al. Outbreak of human monkeypox, democratic republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7:434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radonic A, Metzger S, Dabrowski PW, et al. Fatal monkeypox in wild-living sooty mangabey, Cote d’Ivoire, 2012. Emerg Infect Dis. 2014;20:1009–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doty JB, Malekani JM, Kalemba LN, et al. Assessing monkeypox virus prevalence in small mammals at the human-animal interface in the democratic republic of the Congo. Viruses. 2017;9:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future. Virol 2013;8:129–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Americo JL, Moss B, Earl PL. Identification of wild-derived inbred mouse strains highly susceptible to monkeypox virus infection for use as small animal models. J Virol. 2010;84:8172–8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Americo JL, Sood CL, Cotter CA, et al. Susceptibility of the wild-derived inbred CAST/Ei mouse to infection by orthopoxviruses analyzed by live bioluminescence imaging. Virology. 2014;449:120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Earl PL, Americo JL, Moss B. Lethal monkeypox virus infection of CAST/EiJ mice is associated with a deficient gamma interferon response. J Virol. 2012;86:9105–9112.• Addresses the biological basis for natural susceptibility and resistance to monkeypoxvirus infection among genetic lineages of mice.
- 36.Earl PL, Americo JL, Moss B. Insufficient innate immunity contributes to the susceptibility of the castaneous mouse to orthopoxvirus infection. J Virol. 2017;91:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynolds MG, Carroll DS, Karem KL. Factors affecting the likelihood of monkeypox’s emergence and spread in the post-smallpox era. Curr.Opin.Virol 2012;2:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gispen R, Verlinde JD, Zwart P. Histopathological and virological studies on monkeypox. Arch Gesamte Virusforsch. 1967;21:205–216. [DOI] [PubMed] [Google Scholar]
- 39.Hutson CL, Lee KN, Abel J, et al. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am J Trop Med Hyg. 2007;76:757–768. [PubMed] [Google Scholar]
- 40.Marennikova SS, Seluhina EM. Susceptibility of some rodent species to monkeypox virus, and course of the infection. Bull World Health Organ. 1976;53:13–20. [PMC free article] [PubMed] [Google Scholar]
- 41.Shelukhina EM, Shenkman LS, Rozina EE, et al. Possible mechanism of orthopoxvirus preservation in nature. Vopr Virusol. 1979;Jul-Aug:368–372. [PubMed] [Google Scholar]
- 42.Earl PL, Americo JL, Moss B. Genetic studies of the susceptibility of classical and wild-derived inbred mouse strains to monkeypox virus. Virology. 2015;481:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jezek Z, Fenner F. Human monkeypox. Monog Virol. 1988;17:1–140. [Google Scholar]
- 44.Falendysz EA, Londono-Navas AM, Meteyer CU, et al. Evaluation of monkeypox virus infection of black-tailed prairie dogs (Cynomys ludovicianus) using in vivo bioluminescent imaging. J Wildl Dis. 2014;50:524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marennikova SS, Shelukhina EM, Zhukova OA. Experimental infection of squirrels Sciurus vulgaris by monkey pox virus. Acta Virol. 1989;33:399. [PubMed] [Google Scholar]
- 46.Sergeev AA, Kabanov AS, Bulychev LE, et al. Using the ground squirrel (marmota bobak) as an animal model to assess monkeypox drug efficacy. Transbound Emerg Dis. 2017;64:226–236. [DOI] [PubMed] [Google Scholar]
- 47.Sbrana E, Xiao SY, Newman PC, et al. Comparative pathology of north american and central african strains of monkeypox virus in a ground squirrel model of the disease. Am J Trop Med Hyg. 2007;76:155–164. [PubMed] [Google Scholar]
- 48.Reynolds MG, Carroll DS, Olson VA, et al. A silent enzootic of an orthopoxvirus in Ghana, West Africa: evidence for multi-species involvement in the absence of widespread human disease. Am J Trop Med Hyg. 2010;82:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mucker EM, Chapman J, Huzella LM, et al. Susceptibility of marmosets (Callithrix jacchus) to monkeypox virus: a low dose prospective model for monkeypox and smallpox disease. PLoS One. 2015;10: e0131742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breman JG, Nakano JH, Coffi E, et al. Human poxvirus disease after smallpox eradication. Am J Trop Med Hyg. 1977;26:273–281. [DOI] [PubMed] [Google Scholar]
- 51.US Centers for Disease Control and Prevention. Multistate outbreak of monkeypox-Illinois, Indiana, and Wisconsin, 2003. Mmwr. 2003;52:537–540. [PubMed] [Google Scholar]
- 52.Earl PL, Americo JL, Cotter CA, et al. Comparative live bioluminescence imaging of monkeypox virus dissemination in a wild-derived inbred mouse (Mus musculus castaneus) and outbred African dormouse (Graphiurus kelleni). Virology. 2015;475:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Falendysz EA, Lopera JG, Lorenzsonn F, et al. Further assessment of monkeypox virus infection in gambian pouched rats (cricetomys gambianus) using in vivo bioluminescent imaging. PLoS Negl Trop Dis. 2015;9:e0004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falendysz EA, Lopera JG, Doty JB, et al. Characterization of monkeypox virus infection in African rope squirrels (Funisciurus sp.). PLoS Negl Trop Dis. 2017;11:e0005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hutson CL, Nakazawa YJ, Self J, et al. Laboratory investigations of African pouched rats (cricetomys gambianus) as a potential reservoir host species for monkeypox virus. PLoS Negl Trop Dis. 2015;9:e0004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schultz DA, Sagartz JE, Huso DL, et al. Experimental infection of an African dormouse (Graphiurus kelleni) with monkeypox virus. Virology. 2009;383:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kastenmayer RJ, Moak HB, Jeffress EJ, et al. Management and care of African dormice (Graphiurus kelleni). J Am Assoc Lab Anim Sci. 2010;49:173–176. [PMC free article] [PubMed] [Google Scholar]
- 58.Monroe BP, Doty JB, Moses C, et al. Collection and utilization of animal carcasses associated with zoonotic disease in Tshuapa district, the democratic republic of the Congo, 2012. J Wildl Dis. 2015;51:734–738. [DOI] [PubMed] [Google Scholar]
- 59.Rimoin AW, Alfonso VH, Hoff NA, et al. Human exposure to wild animals in the Sankuru province of the democratic republic of the Congo. Ecohealth. 2017;14:552–563. [DOI] [PubMed] [Google Scholar]
- 60.Nolen LD, Osadebe L, Katomba J, et al. Introduction of monkeypox into a community and household: risk factors and zoonotic reservoirs in the democratic republic of the Congo. Am J Trop Med Hyg. 2015;93:410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levine RS, Peterson AT, Yorita KL, et al. Ecological niche and geographic distribution of human monkeypox in Africa. PLoS One. 2007;2:e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakazawa Y, Emerson GL, Carroll DS, et al. Phylogenetic and ecologic perspectives of a monkeypox outbreak, southern Sudan, 2005. Emerg Infect Dis. 2013;19:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monroe BP, Nakazawa YJ, Reynolds MG, et al. Estimating the geographic distribution of human Tanapox and potential reservoirs using ecological niche modeling. Int J Health Geogr. 2014;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ellis CK, Carroll DS, Lash RR, et al. Ecology and geography of human monkeypox case occurrences across Africa. J Wildl Dis. 2012;48:335–347. [DOI] [PubMed] [Google Scholar]
- 65.Fuller T, Thomassen HA, Mulembakani PM, et al. Using remote sensing to map the risk of human monkeypox virus in the Congo basin. Ecohealth. 2011;8:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dounias E, Ichikawa M. Seasonal bushmeat hunger in the Congo basin. Ecohealth. 2017;14:575–590. [DOI] [PubMed] [Google Scholar]
- 67.Yinka-Ogunleye A, Aruna O, Ogoina D, et al. Reemergence of human monkeypox in Nigeria, 2017. Emerg Infect Dis. 2018;24:1149–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gronvall G, Boddie C, Knutsson R, et al. One health security: an important component of the global health security agenda. Biosecur Bioterror. 2014;12:221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cleaveland S, Sharp J, Abela-Ridder B, et al. One Health contributions towards more effective and equitable approaches to health in low- and middle-income countries. Philos Trans R Soc Lond B BiolSci. 2017;372:20160168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corning S World Organisation for Animal Health: strengthening veterinary services for effective One Health collaboration. Rev Sci Tech. 2014;33:639–650. [DOI] [PubMed] [Google Scholar]
- 71.Nuttall I, Miyagishima K, Roth C, et al. The United Nations and One Health: the International Health Regulations (2005) and global health security. Rev Sci Tech. 2014;33:659–668. [DOI] [PubMed] [Google Scholar]
- 72.Croft DR, Sotir MJ, Williams CJ, et al. Occupational risks during a monkeypox outbreak, Wisconsin, 2003. Emerg Infect Dis. 2007;13:1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pe F, Jezek Z, Grab B, et al. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17:643–650.• Early mathematical modeling study that led to the conclusion that monkeypox could not be sustained in human populations in the absence of repeated zoonotic introductions.
- 74.Jezek Z, Grab B, Dixon H. Stochastic model for interhuman spread of monkeypox. Am J Epidemiol. 1987;126:1082–1092. [DOI] [PubMed] [Google Scholar]
- 75.Judson SD, Fischer R, Judson A, et al. Ecological contexts of index cases and spillover events of different ebolaviruses. PLoS Pathog. 2016;12:e1005780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leendertz FH, Ellerbrok H, Boesch C, et al. Anthrax kills wild chimpanzees in a tropical rainforest. Nature. 2004;430:451–452. [DOI] [PubMed] [Google Scholar]