Figure 3.
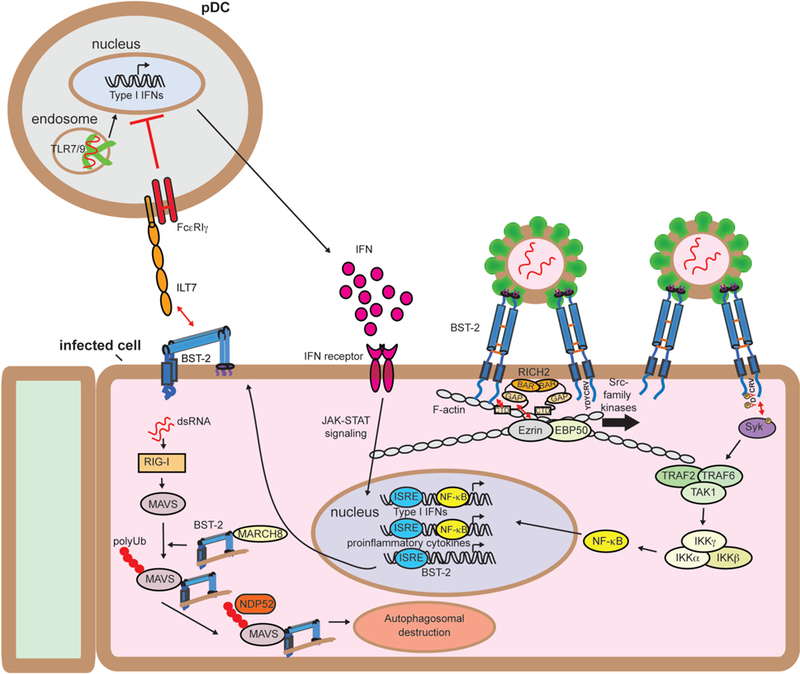
Immune signaling functions of BST-2. IFN produced in response to viral infection stimulates a cellular antiviral state by inducing the expression of hundreds of genes, including BST-2. IFN-induced BST-2 inhibits budding of newly-forming virions, creating BST-2 clusters at the plasma membrane. Clustered BST-2 is linked to the cortical actin cytoskeleton via RICH2, a BAR domain containing protein. This leads to phosphorylation of tyrosines in the YDYCRV motif in the cytoplasmic tail by Src-family kinases followed by the recruitment and activation of Syk. Syk activation recruits TRAF2, TRAF6, and TAK1 into a complex that leads to the activation of NF-kB and the induction of proinflammatory cytokines.[68] The upregulated BST-2 expressed on the surface of infected cells engages the plasmacytoid dendritic cell (pDC) receptor ILT7, resulting in inhibitory signaling that reduces the expression of IFNs by pDCs.[72, 73] BST-2 also negatively regulates RIG-I-like receptor innate immune signaling. RIG-I recognizes uncapped viral dsRNA and induces MAVS, resulting in IFN-I expression and subsequently BST-2 expression. BST-2 then recruits the ubiquitin E3-ligase MARCH8 to catalyze addition K27-linked polyUb chains to MAVS at K7. This chain is then recognized by NDP52, which recruits BST-2/MAVS to autophagosomes for destruction, thereby dampening IFN-I production.[74]
