Summary
Excretion is an essential process of an organism’s removal of the waste products of metabolism to maintain a constant chemical composition of the body fluids despite changes in the external environment. Excretion is performed by the kidneys in vertebrates and by Malpighian tubules (MTs) in Drosophila. Kidney serves as an excellent model organ to investigate the cellular and molecular mechanisms underlying organogenesis. Mammals and Drosophila share common principles of renal development. Tissue homeostasis, which is accomplished through self-renewal or differentiation of stem cells, is critical for the maintenance of adult tissues throughout the lifetime of an animal. Growing evidence suggests that stem cell self-renewal and differentiation is controlled by both intrinsic and extrinsic factors. Deregulation of stem cell behavior results in cancer formation, tissue degeneration, and premature aging. The mammalian kidney has a low rate of cellular turnover but has a great capacity for tissue regeneration following an ischemic injury. However, there is an ongoing controversy about the source of regenerating cells in the adult kidney that repopulate injured renal tissues. Recently, we identified multipotent stem cells in the MTs of adult Drosophila and found that these stem cells are able to proliferate and differentiate in several types of cells in MTs. Furthermore, we demonstrated that an autocrine Janus kinase–signal transducers and activators of transcription (JAK-STAT) signaling regulates stem cell self-renewal or differentiation of renal stem cells. The Drosophila MTs provide an excellent in vivo system for studying the renal stem cells at cellular and molecular levels. Understanding the molecular mechanisms governing stem cell self-renewal or differentiation in vivo not only is crucial to using stem cells for future regenerative medicine and gene therapy, but it also will increase our understanding of the mechanisms underlying cancer formation, aging, and degenerative diseases. Identifying and understanding the cellular processes underlying the development and repair of the mammalian kidney may enable more effective, targeted therapies for acute and chronic kidney diseases in humans.
Keywords: kidney, Malpighian tubules, kidney development, multipotent stem cells, renal and nephric stem cells, renal cancer, Drosophila
Introduction
Organisms need to perform processes that are vital for life. One of the life processes is excretion. Excretion is an essential process in the life of an organism, involving the removal of the waste products of metabolism to maintain a constant chemical composition of the fluids even when the external environment changes. While unicellular organisms discharge their waste products directly through the cell surface, multicellular organisms utilize more complex and specialized excretory organs. These organs vary in different animals but show a striking degree of similarity in form and function. For instance, excretion is performed by a single excretory cell in Caenorhabditis elegans; by Malpighian tubules (MTs) in insects; nephridia in annelids; rectal glands in sharks; and kidneys in all other vertebrates. In mammals, the kidney is an important excretory and homeostatic organ, embedded in a connective tissue matrix of the body. It performs several essential biological functions to maintain the homeostatic balance of the bodily fluids by excreting and reabsorbing water and inorganic electrolytes; it also regulates blood pressure, glucose metabolism, erythropoiesis, synthesis of vitamin D, and hormone secretion (Vainio and Lin, 2002, Dressler, 2006; Tomas and Kumar, 2008).
The MTs of Drosophila serve as the kidney which, together with hindgut, acts as an excretory and osmoregulatory organ system. The MTs of the fly are free-floating within the haemocoelic body cavity (Dow et al., 1998). MTs perform several functions, including excreting metabolic waste, excess water, and organic metabolites (certain synthetic dyes); selectively reabsorbing the fluid in the proximal tubules of MTs and hindgut; and acting as an autonomous immune system (Maddrell et al., 1974; Denholm et al., 2003; Wang et al., 2004; Dow and Davies, 2006; Jung et al., 2005; Ruiz-Sanchez and O’Donnell, 2007; Dow, 2007a; Dow, 2007b; Evans et al., 2008; Day et al., 2008). Mammalian and fly kidney requires the proper integration and coordination in the development of specialized cell types within a well-defined architectural framework that functions in an integrated manner to maintain homeostasis of body fluids, electrolytes, and nutrients (Jung et al., 2005).
Common elements of renal development in mammals and Drosophila
The development of an organism requires proper integration and coordination of cell behavior and cell–cell communication. Reciprocal inductive interactions among cells result in cellular differentiation and formation of tissues and organs. The kidney serves as an excellent model organ to investigate the cellular and molecular mechanisms underlying organogenesis. It is an important organ system in which to analyze most of the basic developmental biology events, including epithelial-mesenchymal interaction, branching morphogenesis, cell polarization, and pattern formation and differentiation (Vainio and Lin, 2002; Jung et al., 2005; Dressler, 2006). The mammalian kidney develops from the intermediate mesoderm and proceeds through three successive stages of development, each marked by the development of a more advanced pair of organs: the pronephros, the mesonephros, and the metanephros. The adult metanephros develops by cycles of ureteric bud (UB) branching and nephron formation. The cycles begin and are maintained by reciprocal inductive interactions and by feedback between epithelial UB tips and the surrounding metanephric mesenchyme (MM). In the process of these interactions, branching of the UB is dependent on the MM, and the differentiation of the nephrons is integrated with each new collecting tubule (Saxen, 1987, Vainio and Lin, 2002, Dressler, 2006). The epithelial UB emanates from the Wolffian duct (WD), grows toward, and invades the adjacent MM, and then undergoes multiple interactions of a branching program (mesenchymal-to-epithelial transition [MET]) that leads to the development of an extensive urinary collecting system (Dressler, 2006; Rosenblum, 2008). MM cells then condense around the tips of the growing and branching ureter and aggregate at the tip of the UB to form an epithelial tubule that proceeds through several morphological stages and ultimately gives rise to the nephron, an excretory unit of the kidney (Fig. 1, Saxen, 1987; Vainio and Lin, 2002; Wang et al., 2004; Jung et al., 2005; Dressler, 2006; Rosenblum, 2008). The Bowman’s capsule, glomeruli, proximal and distal tubules arise from MM and the collecting ducts and ureter arise form the UB (Fig. 1). The continuous MET generates ~12,000 nephrons in the adult mouse kidney and ~500,000 to 1,000,000 nephrons in the adult human kidney. Molecular genetic analyses of the mammalian system have revealed a complex network of more than 400 genes that control kidney organogenesis. These include the transcription factors, signaling pathways, growth factors molecules, and transmembrane protein and extracellular matrix molecules (Dressler, 2006; Park et al., 2007; Yokoo et al., 2008 Uhlenhaut and Treier, 2008). Elucidating the cells and the molecular basis of signaling events involved during development will increase our understanding of the molecular basis of hereditary and sporadic renal developmental defects, and lead to useful new diagnostic and therapeutic tools that can be used to treat kidney diseases and manipulate kidney function in adults.
Fig.1.
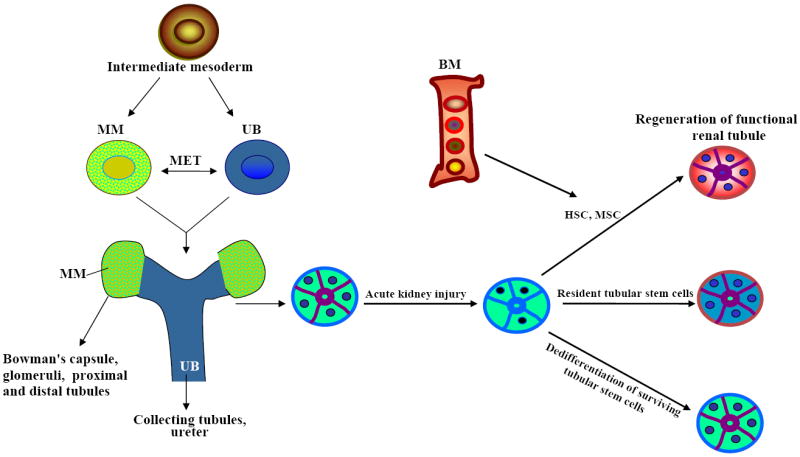
Proposed model of the mammalian kidney development and the origin of tubular epithelial regenerating cells after acute renal injury.
The Drosophila MTs provide an excellent model organ for studying cell fate specification, organization of epithelial sheets, and physiology of renal systems, and serve as an excellent model for studying human kidney diseases (Dow and Davies, 2006; Chintapalli et al., 2007). MTs develop from two sources: the ectodermal epithelial bud (hindgut primordium) and the mesenchymal mesoderm. The MTs consist of monolayer epithelial cells with a distinct apical-basal polarity. The development of Drosophila MTs, which is largely completed during embryogenesis, includes an allocation of tubule primordia and tube budding, followed by growth and elongation via cell rearrangements, and, eventually, cell differentiation (Ainsworth et al., 2000; Delholm et al., 2003; Jung et al., 2005; Dow, 2007a; Dow, 2007b). In the early stages of MT development, interaction between the midgut and hindgut anlagen redefines the expression of the two transcription factors, zinc-finger transcription factor, Krüppel (Kr) and homeodomain-containing protein, Cut in primordial cells (Gaul and Weigel, 1990, Liu and Jack, 1992). Each gene is expressed in the tubule primordia independently of the other and they act together to specify tubule cell fate (see Hatton-Ellis et al. 2007). Following tubule evagination, extension of the cylindrical buds into crescent-shaped tubes requires ribbon (rib), which encodes a BTB/POZ-type nuclear protein, faint sausage (fas), which encodes an extracellular, immunoglobulin-like protein, and Drosophila ret (Halm and Bishop, 2001). The tubule cell shape changes, and the rearrangement of the cycloskeleton leads to tube elongation, which is regulated by the walrus, zipper, hibris, rib, rolling pebbles, myoblast city, and rac genes (Liu et al., 1999). Signaling from the tip cell is needed for the proliferation of the distal cells of the tubule. The four MTs are derived from two cell populations: ectodermal epithelial buds and the surrounding mesenchymal mesoderm (Sozen et al., 1997). The tubules derived from the initial ectodermal epithelium buds consist of principal cells (PC). Mesenchymal cells from the visceral mesoderm migrate and polarize along the epithelial tubules, where they undergo a mesenchymal-to-epithelial transition (MET) and incorporate into tubules as stellate cells (SC) that require hibris, an ortholog of the vertebrate NEPHRIN (Denholm et al., 2003). Several genes and signaling pathways are known to play a crucial role in the early and later developmental events underlying the formation of functional MTs (Sozen et al., 1997; Wan et al., 2000; Ainsworth et al., 2000; Sudarsan et al., 2002; Denholm et al., 2003; Dow and Davies, 2006; Hatton-Ellis et al., 2007; Evans et al., 2008).
Mammalian kidneys and Drosophila MTs show distinct similarities in development and function. The development of both systems involves the interaction of two distinct cell populations, one of which undergoes MET to acquire key functional units. Even some of the pathways and molecules are conserved in both systems during development (Ainsworth et al., 2000; Denholm, et al., 2003; Jung et al., 2005). The knowledge gained from the development and physiological function of Drosophila MTs will provide a unique insight into the role of the cellular pathways required in mammalian renal development and eventually lead to a better understanding of complex renal diseases.
Stem cells in regeneration and repair of mammalian kidney
In recent years, stem cells have emerged as one of the fundamental backbones of tissue biology. Stem cells are defined functionally as unspecialized cells that have an unlimited capacity to self-renew through mitotic cell division, as well as the ability to differentiate into a diverse range of specialized cell types that support normal development and tissue homeostasis. Both the self-renewal and differentiation processes must be tightly regulated by two types of proliferative behavior, asymmetric and symmetric division, to ensure the survival of an organism (Yamanaka, 2007; Morrison and Spradling, 2008; Yamashita and Fuller, 2008). These unique properties of stem cells will provide a powerful tool for future regenerative medicine and gene therapy. Growing evidence suggests that stem cell self-renewal and differentiation is controlled by both intrinsic and extrinsic factors, and that misregulation of stem cell behavior results in cancer formation, tissue degeneration, and premature aging.
In mammals, two kinds of stem cells, embryonic and adult, have been identified, and each has distinct functions and characteristics. Embryonic stem cells are considered as true stem cells, as they generate mature progeny of all cell types; in contrast, the adult stem cells are thought to have limited proliferation potential and can only differentiate into the mature tissue in which they reside. However, the accumulative evidence suggests that adult stem cells may have greater plasticity than previously recognized. Despite their extensive proliferative capacities, stem cells may be quiescent in vivo until injury or tissue degradation stimulates the regenerative signal. The adult stem cells may repair the damaged tissues by differentiating into appropriate cell phenotypes, by providing cytokines and other factors to enhance recovery of endogenous cells, or by undergoing cell fusion (Morris and Spradling, 2008). Adult stem cells play an important role in cell turnover and regeneration. However, several obstacles exist in the use of adult stem cells as disease therapy. First, the ability to identify most adult stem cells is hampered by the lack of stem cell markers. Second, in vitro systems for manipulating adult stem cell populations are often not well defined. Finally, our understanding of how adult stem cells are regulated within their niche is in its immature stage. Multipotent adult stem cells have been reported in many organs, such as bone marrow, skin, intestine, prostate, pancreas, lung, and testes, with unrestricted potential to form different cell types after tissue injury (Blanpain et al., 2007; Barker et al., 2008).
Adult stem cells play an important role in cell turnover and regeneration. The kidney, on the other hand, has a low rate of cellular turnover but has a great potential for tissue regeneration following an ischemic or toxic injury. Ischemic injury to the kidney causes acute renal failure, loss of tubular polarity, necrosis, and cell death, followed by tubular regeneration and recovery of renal function (Anglani et al., 2008; Gupta and Rosenberg, 2008; Yokoo et al., 2008; Vaidya et al., 2008). In humans, two broad categories of kidney disease have been characterized: acute and chronic. Acute renal failure is a leading cause of morbidity and mortality. Developmental studies reveal that the kidney has more than 26 terminally differentiated cells types, suggesting that the differentiation potential would be helpful in renal regeneration after injury. Many animal models provide evidence of regenerating completely degenerated renal tissues after injury (Elger et al., 2003; Haller et al., 2005), which can help us understand the cellular and molecular mechanisms of ischemic injury and tubular regeneration (Imai et al., 2007, Gupta and Rosenberg, 2008, Yokoo et al., 2008, Vaidya et al., 2008). There is an ongoing debate about the source of proliferating cells (stem cells) in the adult kidney that repopulate injured nephrons or regenerate the lost renal tissues. Whether the adult stem cells that repopulate the renal tubule following injury come from within the renal tubule or from extra-renal cells remain controversial. Kidney regeneration and repair occur through three possible sources of stem cells: dedifferentiation of surviving tubule cells, bone marrow–derived stem cells, or resident kidney stem cells (Fig. 1; Oliver et al., 2004; Cantley, 2005; Duffield et al., 2005; Gupta et al., 2006; Imai et al., 2007; Imberti et al. 2007; Bussolati et al., 2008; Gupta and Rosenberg, 2008; Yokoo et al., 2008; Vaidya et al., 2008). Stem cell–based renal regeneration would be critical in reducing the incidence and severity of acute renal failure and treatment of several other kidney diseases and cancer.
Several studies in preclinical models of acute and chronic kidney injury have demonstrated that cells from bone marrow (bone marrow–derived stem cells, mesenchymal stem cells) may migrate to the kidney and participate in the generation of new epithelial cells following injury (for details, see reviews, Gupta and Rosenberg, 2008; Sagrinati et al., 2008). Mesenchymal stem cells have also been found to produce several growth factors such as VEGF, HGF, IGF-1, BMP-7, and TGF-α, suggesting that, after renal injury, a paracrine effect of renal vasculature may provide regeneration and repair. Further studies have examined the role of bone marrow–derived stem cells in renal regeneration, with different results (Gupta and Rosenberg, 2008; Sagrinati et al., 2008). Moreover, several recent studies have shown that tubular cell replacement with bone marrow–derived stem cells occurs less frequently than previously recognized, suggesting that these cells may not represent a major tool in cell therapy after tubular injury and that regenerative cells originate from intra-renal cells (Lin et al., 2005). A recent study even suggests that treatment of renal failure with bone marrow stem cells can be offset by a partial maldifferentiation of bone marrow stem cells into adipocytes, resulting in glomerular sclerosis (Kunter et al., 2007). Thus, kidney-specific stem cells may be better for tissue replacement because of their inherent organ-specific identity, which can reduce the risk of maldifferentiation. Several other studies suggest that renal tubular injury may be repaired by the less damaged cells, which can migrate, proliferate, and ultimately repopulate into normal renal tubules by the dedifferentiation (Lin et al., 2005; Humpherys et al., 2008).
Accumulative evidence suggests that the repair of kidney injuries is predominately regulated by different endogenous renal stem cells (Table 1). However, the location and behavior of renal stem cells remain controversial. Adult kidney stem cells have been isolated using four different selection strategies that have been used to successfully isolate stem cells from other organs (Gupta and Rosenberg, 2008). First, because stem cells are slow-cycling cells and they can retain the 5-bromo-2-deoxyuridine (BrdU) for a long period of time. By BrdU labeling it has been shown that label-retaining cells function as a source of regenerating cells in adult kidney (Maeshima et al., 2003). These cells retain for long period of time at the interstitial cells of renal papilla, and are multipotent in vitro (Oliver et al., 2004). However, a recent study used the genetic fate mapping technique in mice to exclude the presence of stem cells in the interstitial renal cells, and the results indicate that surviving tubule epithelial cells are the predominant mechanism of adult mammalian kidney repair after ischemic injury (Humphreys et al., 2008). Second, isolating side-population (SP) cells because these cells extrude Hoechst dye through the activity of multidrug resistance proteins of ATP-binding cassette transporter superfamily (Gupta and Rosenberg, 2008). SP cells (Hoechst low cells) isolated from many different organs are multipotent stem cells (Challen and Littlle, 2006) Stem cells are also found in the side population (SP) cells of the adult kidney with multilineage differential potential (Iwatani et al., 2004; Challen et al., 2006). Third, identify and isolate kidney stem cells using specific cell surface markers that have been used to identify stem cells in other organs including kidney. Gupta et al. (2006) identified multipotent renal stem cells in the proximal tubules of the rat kidney and showed that these cells express several markers, including CD90, Oct4, and Pax2. Recently, multipotent renal stem cells have been identified in the adult human kidney in a subset of parietal epithelial cells located in Bowman’s capsule (Bussolati et al., 2005; Sagrinati et al., 2006). These progenitor cells have the ability to self-renew and differentiate into several cell types in the kidney, and are characterized by the expression of stem cell markers, such as CD24, CD133, SDF-1, CXCR4, and CXCR7 (Sagrinati et al., 2006; Mazzinghi et al., 2008). These findings in the adult human kidney suggest that CD24, CD133, SDF-1, CXCR4, and CXCR7 play an essential role in the therapeutic homing of human renal progenitor cells in acute renal failure, with important implications for the development of stem cell–based therapies for renal injury (Mazzinghi et al., 2008).
Table 1.
Isolation and localization of the multipotent stem cells in mammalian and Drosophila kidney
| Species | Isolation method | Markers | Localization | Potential | References |
|---|---|---|---|---|---|
| Rat | Label retaining/marker | BrdU, HIF-1α | Papilla | Multipotent | Oliver et al. 2004 |
| Rat | Label retaining | BrdU | Proximal tubule | Unipotent | Maeshima et al. 2003 |
| Rat | Side population | Sca-1, C-kit, CD45 | Proximal tubule | None | Iwatani et al. 2004 |
| Mouse | Side population | Sca-1, Musculin/MyoR | Interstitium | Multipotent | Hishikawa et al. 2005 |
| Mouse | Side population | Sca-1, CD24, endoglin/CD105, c-kit, ki-67 | Proximal Tubule | Multipotent | Challan et al. 2006 |
| Mouse | Molecular marker | Sca-1 | Interstitium of papilla | Multipotent | Dekel et al. 2006 |
| Human | Molecular marker | CD133, Pax-2, CD44 | Interstitium | Pluripotent | Bussolati et al. 2005 |
| Human | Molecular marker | CD133, Oct-4, CD24, BMl1 | Bowman’s capsule | Multipotent | Sagrinati et al. 2006 |
| Mouse | Molecular marker | CD29, CD44, CD73, CD90 | Glomeruli | Multipotent | da Silva Meirelles et al. 2006 |
| Rat | Culture | Pax-2, Sca-1, musashi-1, wnt-1, wnt4 | S-3 segment of proximal tubule | Multipotent | Kitamura et al. 2005 |
| Rat | Culture | Oct-4, Rex-1, pax-2, CD44 | Proximal tubule | Multipotent | Gupta et al. 2006 |
| Rat/Human | Label retaining, molecular marker | BrdU, cyclin D1, Ki-67 | Proximal tubule | None | Vogetseder et al. 2008 |
| Mouse | Lineage labeling | BrdU, Six-2, ki-67 | Tubular epithelial cells | Multipotent | Humphreys et al. 2008 |
| Mouse | Subcapsular implant, molecular marker | Sca-1, Prominin-1, aquaporin-2 | Papilla cortex, outer medulla | Multipotent | Curtis et al. 2008 |
| Mouse/Human | Molecular marker | (SDF-1), CXCR4, CXCR7 | Bowman’s capsule | Multipotent | Mazzinghi et al. 2008 |
| Drosophila | Lineage labeling, molecular marker | BrdU, PH3, armadillo, Unpaired, STAT92E, escargot, kruppel, TSH, Cut | Lower tubules and ureters | Multipotent | Singh et al. 2007 |
The characterization of stem cells in other adult organs, together with evidence from animal and human models for renal tubule repair or regeneration after injury, strongly suggests the presence of adult renal stem cells. However, the precise source and location of renal stem cells in the adult kidney remain unclear. Subsequently, the stem cells markers in the adult kidney are also lacking or less well characterized, making it difficult to isolate, to define a renal stem cell niche, or to follow cell lineage progression in the normal or injured kidney (Gupta and Rosenberg, 2008). A summary of the isolation and localization of kidney stem cells, including candidate markers, in different species is presented in Table 1. However, precise identification and characterization of adult kidney stem cells and signaling pathways regulating their self-renewal or differentiation are poorly understood. Additionally, clonal analysis might be essential for comparing the different mechanisms underlying renal repairs. Understanding the signaling pathways regulating the proliferation and differentiation of adult kidney stem cells will undoubtedly provide the development of novel therapeutic strategies for the treatment of acute kidney injury (Humphreys et al., 2008).
Stem cells in the renal tubules of adult Drosophila
The development of Drosophila MTs is completed during embryogenesis. The MTs remain intact during metamorphosis but undergo some structural changes. Tissue homeostasis is critical for the maintenance of adult tissues. Most, if not all, adult epithelia of mammals and MTs have a well-defined organizational structure. Epithelial tissue can be classified into two broad categories based on turnover time: rapidly self-renewing and persistent. Most of the mammalian adult epithelial tissues, such as intestine, skin, cornea, and mammary gland, undergo rapid self-renewal and replace damaged or dead cells throughout the life of the animal. Studies on epithelial tissue turnover suggest that cell self-renewal varies among different types of epithelial tissues. Drosophila is a highly attractive model system for the study of several important, complex biological processes, including epithelial tissue turnover.
Historically, the adult organs of Drosophila have been viewed as strictly postmitotic. Detailed studies exist on the reproductive system of adult stem cells in Drosophila (see Hou and Singh, 2008). However, the existence of multipotent cells in adult Drosophila epithelial tissues did not become clear until recently (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Ohlstein and Spradling, 2007; Singh et al., 2007; Takashima et al., 2008). Recent studies have shown that Drosophila midgut contains many multipotent stem cells that lie close to the extracellular matrix, and Notch signaling regulates self-renewal or differentiation of these stem cells (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Ohlstein and Spradling, 2007). Additionally, there is an age-related increase in the number and activity of midgut stem cells and progenitor cells, regulated by PDGF/VEGF (Choi et al., 2008). Another study has identified adult stem cells in hindgut of Drosophila that are regulated by Wnt and Hh signaling (Takashima et al., 2008). During metamorphosis, the larval gut is degenerated, the gut is entirely remodeled, and the adult gut is formed. However, the Malpighian tubules are not remodeled during metamorphosis and remain almost unmodified in the adult. How cell turnover is managed in the adult MT cells and what factor(s) regulates MT cell self-renewal were unknown until we identified the cells with small nuclei in the region of the lower tubules and ureters of the MTs in adult Drosophila. These stem cells function as multipotent stem cells, capable of differentiating into all cell types in the tubule and regulated by an autocrine JAK-STAT signaling (Fig. 2A-H), Singh et al., 2007; Singh and Hou, 2008). The details of the identication and characterization of stem cells’ behavior in the renal tubules of the adult Drosophila are described below.
Fig. 2.
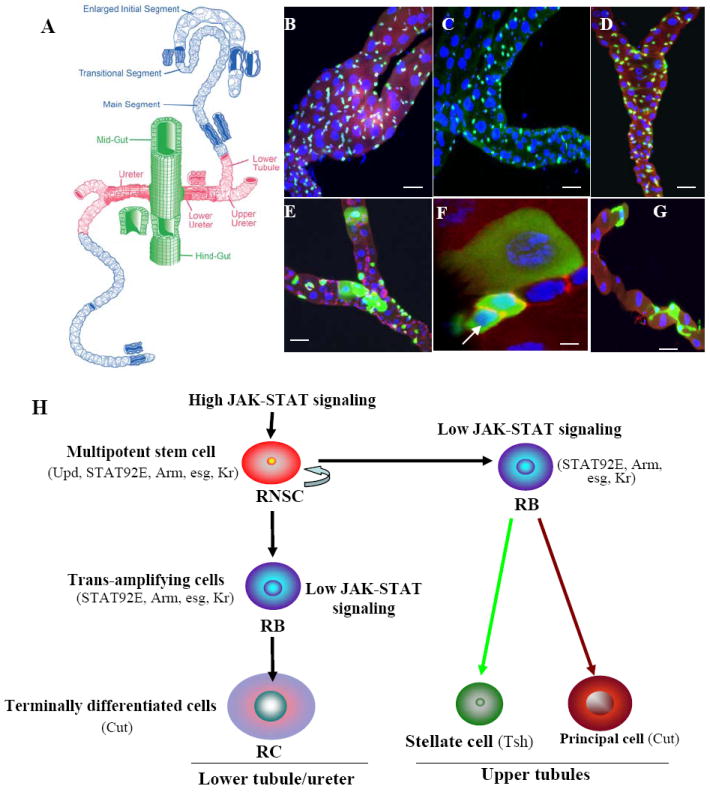
The Drosophila MTs and renal stem cell lineage in Drosophila. (A) Drawing of the Drosophila MTs (adapted from Wessing and Eichelberg, 1978). The adult MTs consist of two pairs of epithelial tubes: a longer, anterior pair runs through the hemolymph on both sides of the midgut, and a shorter, posterior pair runs along the hindgut. The pairs converge at a common ureter at the midgut–hindgut junction. Each tubule is divided into four compartments: initial, transitional, main, and proximal (lower tubules and ureter). (B-D) Expression pattern of unique molecular markers in the region of lower tubules and ureters of adult Drosophila MTs. (B). kr-Gal4/UAS-GFP is specifically expressed in the small nuclear cells (anti-GFP, green; anti-Arm, red; DAPI, blue). (C) upd-Gal4/UAS-GFP expressed in the region of lower tubules and ureters (anti-GFP, green; DAPI, blue). (D) Stat92E reporter-GFP is expressed only in small nuclear cells in the region of the lower tubules and ureters (anti-Arm, red; anti-GFP, green; DAPI, blue). (E-G) MTs with GFP-marked wild-type MARCM clones. (E) 6 days after clone induction, GFP marks cluster cells with small, intermediate, and large nuclei in the region of the lower tubules and ureters, and highlighted in F (arrow, RNSC), (anti-Arm, red; anti-GFP, green; DAPI, blue). (G) 10 days after clone induction in the upper tubule, the GFP labels both stellate and principal cells. (H) Schematic summary of renal and nephric stem cells’ lineage in Drosophila MTs (modified after Singh and Hou, 2008). Markers express in RNSCs, and their differentiated cells are shown in parentheses. Scale bars in (B-E) represent 10 μm, in (F-G), 5 μm.
Identification and localization of renal stem cells in MTs
The adult MTs consist of two pairs of epithelial tubes: a longer, anterior pair that runs through the hemolymph on both side of the midgut, and a shorter, posterior pair that runs along the hindgut. These pairs converge in the ureter at the midgut–hindgut junction (Fig. 2A). Initially, only three regions (initial, transitional, and main segments) and two cell types (principal, or type I, cells, and stellate, or type II, cells) were described in the MTs. However, genetic techniques for enhancer trapping, molecular marking, and clonal analysis revealed six regions and six cell types in the tubules (Sozen et al., 1997; Dow and Davies, 2006; Hatton-Ellis et al., 2007). These regions are highly specified, and the cells have a precise positional identity due to interaction of the combinations of transcription factors (Wang et al., 2004). Each tubule is divided into four compartments (Fig. 2A): initial, transitional, main (secretion), and proximal (lower tubules and ureter-readsorption). The initial, intermediate, and main segments of each tubule consist of two cell types comprising more than 150 cells. Type I cells in the MTs express Cut, and represent the majority (~80 %) of cell types found in the initial, transitional, and main segments, and the region of lower tubules and ureters; Type I cells transport cations and organic solutes. Type II cells which express teashirt (Tsh), conduct water and chloride ions, and are found in the initial, transitional, and main segments, but not in the region of lower tubules and ureters (Wessing and Eichelberg, 1978; Sozen et al., 1997). In addition, the proximal compartment (including the lower tubules and ureters) contains “tiny” cell types, homologs of myoendocrine cells in the ants Formica that collect the urine in the ureter and secrete neurohormones in the hemolymph to regulate ion transport (Sozen et al., 1997). However, because a detailed analysis of the different types of cells in the proximal tubules had not been performed, we reanalyzed the different cell types in the MTs using known molecular markers that had been shown to express in MTs, such as antibody to Cut and TshlacZ enhancer trap line (Sozen et al., 1997). As reported previously, we found that principal cells, express Cut, have large nuclei, and are distributed throughout the tubules, whereas the Tsh positive cells are SC, have small nuclei, and are restricted to the upper tubules that include the initial, transitional, and main segments. Further, using phallodin and Dapi staining in the tubule, we found that three types of cells, each with a different-sized nucleus, occupy distinct positions in the region of lower tubules and ureters. The first type has a small nucleus and lies primarily close to the tubular walls and is similar to the previously reported “tiny” cells in the lower tubules and ureters; the second type has a large, oval nucleus and is distant from the tubular walls. The third type of cell has an intermediate-size nucleus and may be a transition-type cell. We also found that cells of the MTs are different from those of the midgut because of the difference in the expression of the markers in both systems (Cut and Kr express only in the MT; Su(H) and Prospero express only in the midgut).
We also asked whether cells in the MTs are proliferating and mitotically active throughout adult life. The epithelial cells can be labeled by continuous incorporation of the nucleotide analog Brdu for a long period of time; during the chase period, the cells dilute their label through cell division. The more slowly dividing cells, however, can retain the labeling for a longer period, and cell division of these labeled, slowly-dividing cells can be observed in postmitotic tissues. We found that when BrdU (a proliferating marker) is incorporated, it labels all three cell types in the lower tubules and ureters. However, no Brdu-labeled cells have been seen in the upper tubules. Since many cells undergo endoreplication in both larvae and adult tissues, the Brdu can label both endoreplicating and dividing cells. To distinguish endoreplicating cells from dividing cells in the MTs, we stained the tissue for phosphorylated-histone-H3 (H3P), which plays an important role in gene expression, chromatin remodeling, chromosome condensation, and cell division. H3P may initiate at different phases of the cell division in different organisms, but metaphase chromosomes are always found to be heavily phosphorylated. H3P is used as a mitotic marker in a wide range of organisms. In contrast to Brdu labeling, which expresses in all three cell types in the lower tubules and ureters, we detected H3P staining in cells with small nuclei, suggesting that the cells with small nuclei divide and the cells with intermediate and large nuclei undergo endoreplication. Additionally, we characterized several cell-specific molecular markers, such as two transcription factors, escargot (esg), and kr and a memberance protein (Fig 2B), Armadillo (Arm; the β-catenin homolog), that express in small-nuclear-sized cells in the lower tubules and ureters. Further, we found that both esg and kr sometimes label a pair of H3P-positive dividing cells. Since the function of the esg gene is to maintain cells as diploid in Drosophila imaginal cells, the esg-postive cells in the MTs are most likely diploid cells.
Cells with small nuclei in the proximal tubules of MTs are multipotent stem cells
To determine whether stem cells maintain cell turnover in the MTs, and if so, whether stem cells are able to recruit different types of cells to the tubule, we used a positively marked mosaic lineage (PMML) labeling technique (Kirilly et al., 2005) to label and trace cells that undergo mitotic divisions. Genetic lineage labeling in Drosophila involves using heat shock to induce a mitotic genetic recombination event that results in permanent expression of an easily visualized reporter transgenes [(such as green fluorescent protein (GFP) or lacZ (beta galactosidase)] in a proliferating cell as well as in all progeny subsequently descending from that cell. Because the recombination event is infrequent, only single cells in a small area are labeled, and upon proliferation of these cells over time, the labeled cells can make a small cluster of their descendants. We heat-shocked the flies and observed the fate of the GFP-marked clones in adult MTs from two to ten days after clone induction. Two days after clone induction, GFP labeled primarily a few cells with small nuclei in the basal region of the ureter and lower tubules, and we observed a few transient clones with large nuclear cells and limited proliferation potential. Four to six days after clone induction, clones were restricted mainly to the region of the lower tubules and ureter, with individual, cells with small nuclei and clusters of cells with small, intermediate, and large nuclei. We found that most basal diploid cells with small nuclei function as stem cells, and we term these the renal and nephric stem cells (RNSCs). The RNSCs contacted their immature diploid daughters, which we term the renalblasts (RBs), and these RBs underwent two fates: In the lower tubules and ureters, the RBs began to expand in size and DNA content, become intermediate and large nuclear cells in ~ 5 days, and express Cut. We term the intermediate and large nuclear cells early and late renalcytes (RCs) respectively (Fig. E-F, H). Ten days following clone induction, the RBs moved toward the distal upper tubules and finally differentiated into Cut-positive cells as PCs and Tsh-positive cells as SCs in the transitional and initial segments (Fig. 2G-H). Using different molecular markers and clonal analysis, we also found that, out of ~500 cells in one pair of anterior MTs, >90 cells are RNSCs. This analysis suggests that cells with small nuclei in the regions of the lower tubules and ureters function as multipotent stem cells in the renal tubules because they are able to proliferate and differentiate into several types of cells (Fig. 2E-H).
Regulation of stem cell behavior in MTs
Understanding what regulates stem cell behavior will allow to us to predict that behavior during development and homeostasis, and after a disease. Additionally, determining how signaling pathways or molecules regulate stem cell self-renewal and differentiation is a critical step toward manipulating adult stem cells for therapeutic purposes. The various stem cell niches found in organisms from insects to mammals appear to have common signal transduction pathways, including the JAK-STAT, canonical Wingless (Wnt), Hedgehog (Hh), and Notch pathways (Li and Xie, 2005; Ohlstein and Spradling, 2007 Hou and Singh, 2008; Morrison and Spradling, 2008, Takashima et al., 2008, Nusse, 2008). The existence of a stem cell niche has been proposed for several adult stem cell systems (Martinez-Agosto, et al. 2007; Jones and Wagers, 2008). The precise spatial organization of the stem cells and the surrounding supporting cells plays an important role in the niche’s ability to provide the proliferative signal and to avoid factors that enhance differentiation. Maintaining the balance between proliferation-inhibitory and proliferation-stimulating signals is the key to maintaining tissue homeostasis. Adhesion between stem cells and support cells plays an important role in keeping stem cells within the niche so that they may receive the self-renewal signal. Additionally, the niche may provide the polarity signal, which orients stem cells within the niche (Yamashita and Fuller, 2008). Stem cells niches are well characterized in the adult testis and ovary of Drosophila, where a fixed niche regulates stem cell self-renewal (Kiger et al. 2001, Lin, 2002, Brawley and Matunis, 2004, Li and Xie, 2005; Decotto and Spradling, 2005, Wang et al., 2006; Nystul and Spradling 2007; Hou and Singh, 2008, Voog et al. 2008). However, in the MTs, the renal stem cells do not adhere to a particular cell type and are scattered over the region of proximal tubules. If there is no fixed niche for renal stem cells in MTs, what factor(s) regulate their self-renewal and differentiation?. To determine what signaling or molecules regulate renal stem cells behavior in MTs, we examined the expression pattern of the JAK-STAT pathway components because the signaling regulates stem cell self-renewal in several other stem cell systems. The Drosophila JAK-STAT pathway components are illustrated in Fig. 3. These components are: JAK, encoded by the hopscotch (hop) gene; STAT, encoded by the stat92E (signal-transducer and activator of: transcription protein at 92E) gene; a ligand, encoded by the unpaired (upd) gene; a receptor, encoded by the domeless (dome)/Master of Marrelle (mom) gene; and three negative regulators, Socs36E, ken, and barbie (Luo and Dearolf, 2001; Hou et al., 2002; Agaisse and Perrimon, 2004; Singh et al., 2005; Hombría and Sotillos, 2006, Arbouzova and Zeidler, 2006; Gregory et al. 2008). A. The activated Stat92E enters the nucleus to activate its target genes’ transcription (Fig. 3). We observed that most of the components of JAK-STAT signaling in MTs express in cells with small nuclei at the lower tubules and ureter region (Fig. 2C-D). They are also sometimes positive for the H3P, suggesting that these cells are mitotically active and are expressed in renal stem cell or their immediate daughter RBs.
Fig. 3.
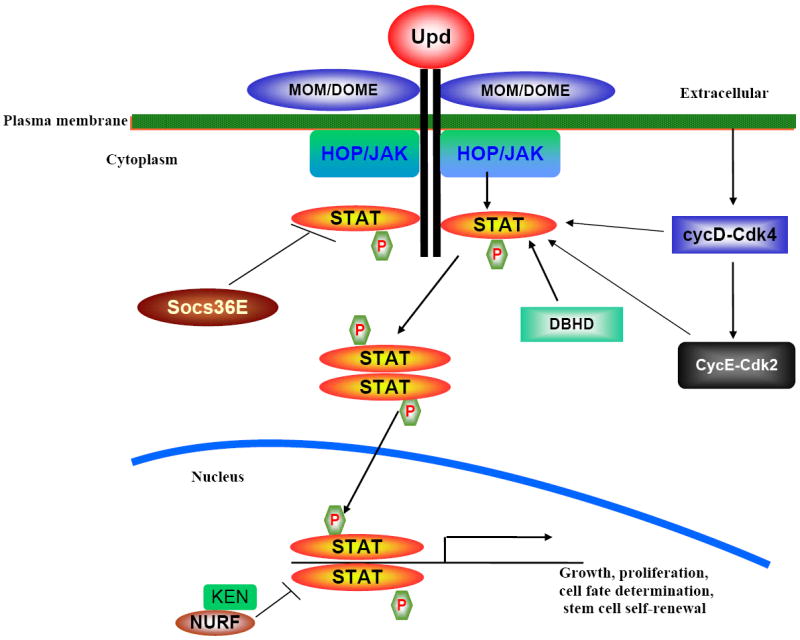
Schematic diagram of the JAK-STAT pathway. The components of JAK-STAT are: JAK, encoded by the hopscotch (hop) gene; STAT, encoded by the stat92E (signal-transducer and activator of: transcription protein at 92E) gene; a ligand, encoded by the unpaired (upd) gene; a receptor, encoded by the domeless (dome)/Master of Marrelle (mom) gene; and three negative regulators, Socs36E, ken, and barbie. The activated STAT92E enters the nucleus to activate its target genes’ transcription. Several other molecules interact and regulate the STAT activity such as cyclin D (cycD-Cdk4), cyclinE (CycE-Cdk2), and Drosophila homolog of BHD (DBHD).
We also examined the role of JAK-STAT signaling in renal stem cells by using the PMML technique. We found that when we overexpressed the JAK-STAT ligand Upd, the size of the MT increased. We also saw a dramatic increase in the expression of STAT92E, as well as in the number of proliferating cells, mitotically active cells, and renal stem cells in overexpressed Upd flies, compared with wild-type MTs. These observations suggest that overexpression of Upd makes renal stem cells more active and accelerate cell division of the RNSCs. When we reduced the JAK-STAT signaling by using the PMML technique to overexpress socs36E, the renal stem cells differentiated prematurely. Furthermore, we determined whether JAK-STAT signaling directly required in renal stem cells. Using the mosaic analysis with repressible cell marker (MARCM) technique (Lee and Luo, 2001), we analyzed the STAT92E homozygous clones in the MTs and found that the absence of JAK-STAT signaling promotes the differentiation of RNSCs as well as the loss of stem cell population. Further, using Apoptag staining we found that the loss of RNSCs is not due to cell death. Next, we observed the expression pattern of the JAK-STAT ligand Upd and its receptor Dome in the MTs and found that both ligand and its receptor, along with STAT92E protein, express in RNSCs. Using these findings, we proposed that self-renewal of RNSCs is controlled by autocrine JAK-STAT signaling and do not need a fixed niche. This is similar to intestinal stem cells (ISCs) in adult Drosophila midgut, where ISCs do not rely on any fixed anatomic niche because ISCs themselves play an active role in supplying signals, such as Delta ligand, to their daughters to control their proliferation and differentiation (Ohlstein and Spradling, 2006, 2007). However, the RNSCs reside primarily in the lower tubules and ureters, even in the Upd overexpressed flies, suggesting that, in addition to JAK-STAT signaling, other signaling or molecules restrict the RNSCs to the lower tubules and ureters. Furthermore, in observing the activity of JAK-STAT signaling in MTs by using STAT92E reporter GFP, which is expressed in cells with small nuclei between the ureter and the lower part of the main segment, we noticed that some cells with small nuclei express strong GFP, and others express weak GFP. These observations suggest that high JAK-STAT signaling regulates RNSC self-renewal, whereas weak JAK-STAT signaling prepares RBs for differentiation into an RC in the lower tubules and ureters, and into a PC or SC in the upper tubules (Fig. 2H). These findings on the MTs of adult Drosophila suggest that resident stem cells may be involved in to repair/ regeneration of the renal tissues after acute/ischemic injury in the mammalian kidney, and that a fixed niche may not be needed to maintain the self-renewal of renal stem cells (Singh et al., 2007, Affolter and Barde, 2007; Singh and Hou, 2008).
Stem cells and kidney cancer
The normal development of the mammalian kidney begins with a reciprocal inductive interaction between the UB and MM that leads to the evolution of the collecting duct system and nephrons, respectively. This process requires a proper sequence and balance of proliferation, cell–cell interactions, apoptosis, and differentiation. Any misregulation in these processes may lead to kidney disease, including acute or chronic renal failure, polycystic kidney disease or glomerulonephritis or kidney cancer. Globally, more than 500 million individuals have some form of chronic kidney disease (CKD). Most of these individuals depend on dialysis and rarely receive transplants because of the limited availability of the organ.
Cancer is a leading cause of death worldwide. Cancer begins when a group of cells display uncontrolled growth, invasion and sometimes metastasis. The malignant tumors develop through the accumulation of genetic changes in proliferating cells, such as the activation of oncogenes, dysfunction of stability genes, or inactivation of tumor-suppressor genes. Kidney cancer is a combination of different types of cancer, each with a different histology, requiring different clinical courses and responding to different forms of therapy, and each associated with the alteration of a different gene. Renal cell carcinoma (RCC) is the most common type of kidney cancer, accounting for more than 90% of malignant kidney tumors. RCC originates primarily in proximal renal tubules and, rarely, in collecting ducts (Fig. 4). Like most cancers, RCC is difficult to treat once it has metastasized. The five human genes associated with predisposition to RCC are von Hippel-Lindau (VHL; clear cell RCC); met proto-oncogene (c-MET; papillary RCC); fumarate hydratase (FH; papillary RCC); Birt-Hogg-Dubé (BHD/FLCN-chromophore, oncocytomas, clear cell); and hyperparathyroidism 2 (HRPT2; papillary RCC) (Fig. 4). RCC could develop following chronic renal regeneration and repair in individuals with polycystic kidney disease or in renal allograft. A detailed investigation of kidney neoplasms suggests that some RCCs, such as Wilms’ tumor (WT) and hereditary papillary renal carcinoma, are caused by mutations in the genes involved in normal nephrogenesis. WT is a pediatric kidney cancer that arises from multipotent embryonic renal precursors of the metanepric blastema that fail to differentiate. Some of the neoplasms are caused by mutations in genes expressed during normal development; for example, RCC is associated with the tuberous sclerosis complex (TSC) gene, and clear cell renal carcinoma with the VHL gene. The majority of RCCs develop in abnormalities of renal epithelial cells, and only a mutation in TSC causes abnormalities in both mesenchymal cells and epithelial cells (Fig. 4; Henske, 2005; Pfaffenroth and Linehan, 2008)
Fig. 4.
Schematic summary of genes regulating RCC in mammalian kidney. Kidney tissues are made up of many types of cells. These cells grow and divide in a controlled way to produce more cells as they are needed to keep the kidney healthy. When normal cells are damaged beyond repair, they are eliminated by apoptosis. When alteration in the genetic material of a cell happens, cells avoid apoptosis and continue to multiply in an unregulated manner accumulating mutations that affect normal cell growth and division and results in tumor formation. Model shows that the renal tumors caused by different genes mutation. Renal progenitor cells are regulated by TSCs (TSC1 and TSC2) that are able to differentiate into either mesenchymal or epithelial cells. Mutations in TSC1 or TSC2 cause either mesenchymal (oncocytomas and cyst) or epithelial (angiomyolipomas) tumors. However, most of the common tumors are epithelial RCC type. RCC originates primarily in the proximal renal tubules and, rarely, in collecting ducts. These tumors are clear cell carcinoma; papillary; chromophobe; oncocytomas. Some of the tumors are of epithelial origin and are seen, only seen in children (Wilms’ tumor); some of the tumors are also caused by the combination of both epithelial and stroma cells (transitional cell carcinoma).
Studies of the genetic conditions associated with RCC, such as VHL, BHD, and MET, as well as genetic analyses of the tumors have provided considerable insight into the pathogenesis of these lesions. Although RCC is resistant to chemotherapy, kinase inhibitors and interleukin-2 are used to treat advanced RCC. However, because of the side effects, these therapies are not effective. Cancer is considered a stem cell disease because of both its propagation by a minority of cells with stem-cell-like properties (termed cancer stem cells, or CSCs) and its possible derivation from normal-tissue stem cells (Sneddon and Werb, 2008). Furthermore, overlapping sets of molecules and pathways regulate both stem cell migration and cancer metastasis. Normally, most adult stem cells reside in a quiescent state. However, the effect of repeated injury (as in chronic kidney injury, for example) would, over time, increase the pool size of stem cells in an active state of renewal and increase cancer incidence. The presence of more stem cells in a tissue may enhance the possibility of a stem cell being trapped in the activated state by an oncogenic event (Beachy et al., 2004).
Abnormal functioning of signaling pathways is believed to contribute to the pathogenesis of many malignancies and is particularly relevant to renal cancers. To understand the cancer stem cells in tumors, we need to know first what signaling pathways and pathway interactions regulate cancer formation. It has been shown that TSC complex (hamartin/tuberin [TSC1/TSC2]) regulates early renal progenitor cells because individuals with TSC mutations have an increased incidence of RCC. TSC mutations activate the mammalian target of rapamycin (mTOR) by inhibiting the Rheb (ras homolog enriched in brain) and biochemically resemble VHL phenotypes. In addition, mTOR has been shown to regulate hypoxia inducible factor (HIF) activity, and much evidence implicates mTOR as a valid target for treatment of renal cell carcinoma. Epidermal growth factor receptor (EGFR) activates pI3K and downstream targets including AKT (protein kinase B) and mTOR, resulting in increased HIF-1 expression leading to tumor metastasis. The c-MET proto-oncogene, expressed in both stem and cancer cells, is a key regulator of invasive growth in normal conditions because it binds with HGF to induce receptor dimerization and phosphorylation, and interacts with several other intracellular factors, including the ras oncogene–mitogen-activated protein kinase (RAS-MAPK) and protein kinase B (AKT) pathways. VHL, a tumor-suppressor gene, plays a role in the regulation of tumor angiogenesis by targeting the hypoxia-inducible growth factor-1 (HIF-1) for ubiquitin-mediated degradation. HIF-1 induces the dedifferentiation of cancer cells, maintains stem cell identity, and increases the metastatic potential (Kondo et al., 2003). VHL interacts with HIF-1, resulting in the loss of VHL and overproduction of the HIF-1, which contributes to the development RCC. These findings suggest that HIF-1 is downstream of VHL and are able to treat RCC caused by VHL. Further, genetic inactivation of VHL prevents HIF-1 down-regulation, leading to the expression of the c-MET proto-oncogene, an important regulator of invasion and metastasis. VHL and glycogen synthase kinase 3 (GSK3) function together in a ciliary-maintenance signaling network, disruption of which enhances the vulnerability of cells to lose their cilia, thereby promoting cyst formation. Recently it has been reported that PTEN (phosphatase and tensin homolog) tumor-suppressor protein cooperates with VHL to regulate kidney tumorigenesis (Frew et al., 2008). STRA13 is a cancer-associated protein regulated by VHL and HIF-1 pathway and is overexpressed in many common malignancies. VHL deficiency or HIF-1 activation results in the repression of endogenous STAT1, which possesses tumor-suppressor properties and is mediated by STRA13 (Ivanov et al., 2007). BHD is another dominantly inherited hamartoma syndrome (Schmidt et al., 2005) that shares several features with TSC; mutation in both genes causes renal carcinoma, which suggests that the BHD and TSC proteins may function within a common pathway. However, clinical phenotypes and the risk of malignancy are higher in BHD than in TSC. Recently, we demonstrated that amplifying the JAK-STAT signaling by overexpressing its ligand Upd stimulates the RNSCs to proliferate and differentiate into RCs, which results in tumorous overgrowth in the MT (Singh et al., 2007). Previously, we have reported that BHD interacts with JAK-STAT signaling and regulates germline stem cell in the Drosophila testis and functions downstream of JAK-STAT signaling pathways (Singh et al., 2006). Therefore, the Drosophila RNSC system may also be a valuable in vivo system in which to study cancer stem cell regulation in renal tubules. The genetic interactions of different oncogenes and tumor-suppressor genes regulating renal carcinoma and other kidney cancers are summarized in Fig. 5. A key goal in clinical oncology is the development of medical therapies specific to pathways that are misregulated in cancer. Understanding the biological pathways involving VHL, MET, FH, BHD, and HRPT2 will provide new therapeutic approaches for kidney cancer.
Fig. 5.
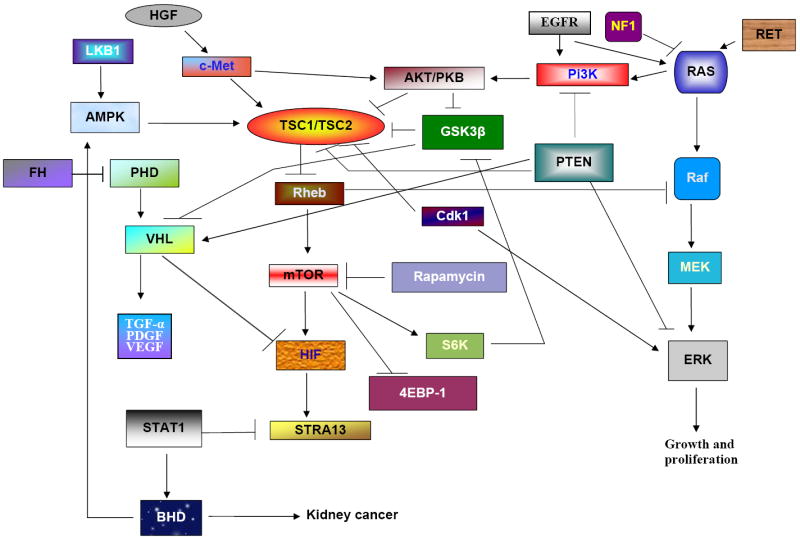
Proposed model of molecular pathways that regulate kidney tumors in mammals.
The incidence of end-stage renal disease in patients with chronic kidney disease is predicted to rise in the near future, and likely will not be counterbalanced by currently available renal replacement therapies such as hemodialysis and hemofiltration (Braam et al., 2007). These therapies repair or replace only the filtration function for small solutes and do not replace the lost transport, metabolic, and endocrine functions of the kidney. In addition to conventional methods of renal therapy, four innovative ways have been suggested to restore the normal function of the kidney cells after chronic disease or ischemic injury. First, damaged kidneys can be restored by stem cell technology (cell-targeted therapy) and knowledge of developmental programming; second, using therapeutic cloning, a kidney may be grown in vitro and transplanted into the recipient; third, other organs may be used to replace various renal functions; and finally, the artificial kidney may be used, which has the potential to be supplemented with human cells (Challen et al., 2006; Braam et al., 2007). These innovative therapies to replace the functions of the kidney, particularly stem cell therapy, may provide invaluable treatments for renal failure. Further, understanding the response to tissue injury, as well as the signals that regulate the activation of tissue stem cells, will provide potential strategies for the use of stem cells in cancer prevention and therapy.
Conclusions and future prospects
Regardless of several differences, the general organization and physiology of mammalian kidneys and Drosophila MTs show clear similarities in development and function. Both use common strategies of development, and even some of the pathways and molecules are conserved in both systems. Adult stem cells have been well defined to contribute to tissue regeneration after injury in various organ systems. The recruitment of stem cells to the injured tissue therefore appears to be the prerequisite for repair, and understanding the mechanisms that regulate their migration is crucial for the success of any clinical strategy involving stem cells. Despite the abundant promise of progress, there remains the essential challenge in understanding the role of stem cells in the kidney. Therefore, identifying and characterizing this role is critical to renal research, which must move forward before approaches to therapy and repair, can be fully realized.
Using genetic-labeling techniques and molecular markers, we have identified many multipotent stem cells in the MTs of the adult Drosophila. These renal stem cells have the ability to generate all cell types of the adult. Further, we found that autocrine JAK-STAT signaling regulates self-renewal or differentiation of renal stem cells. However, several important questions remain regarding the biological properties of multipotent stem cells in Drosophila renal tubules, such as how the self-renewal or differentiation is balanced in the MTs to avoid tissue overgrowth; whether their self-renewal is unlimited; what are the molecular mechanisms controlling asymmetric stem cell division: whether cell polarity, centrosomal asymmetry, and cell cycle regulators, determines asymmetric division of RNSCs, as in other stem cells system, and finally, how the tumor suppressor genes regulate stem cell proliferation and self-renewal in MTs. Answering these questions is crucial and will provide a better understanding of the mechanisms behind self-renewal, proliferation and differentiation of stem cells in general.
Studies to determine the sources of kidney regeneration after injury and the existence of stem cells and their location have produced conflicting results in the mammalian system, and several experiments are needed to identify the sources and types of cells involved in this process. Furthmore, strategies must be developed to overcome the severity of tubular injury and find a better way to improve the capability of tubular regeneration after acute renal failure. Identification of multipotent stem cells in adult Drosophila MTs suggests that resident stem cells may be responsible for repair and regeneration of completely damaged tissues in the mammalian kidney (Humphreys et al. 2008). Understanding the function of multipotent kidney stem cell in adults, combined with powerful, state-of-the-art forward- and reverse-genetic approaches, genomics, and proteomics tools, and studies of the Drosophila renal stem cell system, will lead to a better understanding of kidney development. Understanding this process has important clinical implications for early detection, prevention, and treatment of several kidney diseases, including cancer.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
List of abbreviations
- Akt
protein kinase B
- Arm
armadillo
- BHD
Birt-Hogg-Dubé
- Brdu
5-bromo-2-deoxyuridine
- c-MET
met-proto-oncogene
- CSC
cancer stem cell
- CKD
chronic kidney disease
- Dome
domeless
- EGFR
epidermal growth factor receptor
- esg
escargot
- fas
faint sausage
- FH
fumerate hydratase
- GFP
green fluorescent protein
- GSK3
glycogen synthase kinase 3
- H3P
phospho-Histone H3
- Hh
hedgehog
- HGF
hepatocyte growth factor
- HIF-1
hypoxia inducible factor-1
- hop
hopscotch
- HRPT-2
hyperparathyoridism-2
- IGF-1
insulin-like Growth Factor-1
- JAK
Janus kinases
- Kr
Krüppel
- lacZ
beta galactosidase
- MAPK
mitogen-activated protein kinases
- MARCM
mosaic analysis with a repressible cell marker
- MET
mesenchymal-to-epithelial transitions
- MM
metanephric mesenchyme
- mom
master of Marrelle
- mTOR
mammalian target of rapamycin
- MTs
Malpighian tubules
- BMP-7
bone Morphogenetic Protein-7
- PC
principal cells
- PMML
positively marked mosaic lineage
- PDGF
Platelet-derived growth factor
- PTEN
phosphatase and tensin homolog
- RAS
ras oncogene
- RBs
renalblasts
- RC
renalcyte
- RCC
renal cell carcinoma
- Rheb
ras homolog enriched in brain
- rib
ribbon
- RNSCs
renal and nephric stem cells
- SC
stellate cells
- SP
side population
- STAT
signal Transducers and Activators of Transcription
- STAT92E
signal-transducer and activator of transcription protein at 92E
- Su(H)
suppressor of hairless
- TGF-α
transforming growth factor-α
- TSC
tuberous sclerosis complex
- TSC1
hamartin
- TSC2
tuberin
- Tsh
teashirt
- UB
ureteric bud
- Upd
unpaired
- VEGF
vascular endothelial growth factor
- VHL
von Hippel-Lindau
- WD
Wolffian duct
- Wnt
wingless
- WT
Wilms’ tumor
References
- Affolter M, Barde Y. Self-renewal in the fly kidney. Dev Cell. 2007;13:321–322. doi: 10.1016/j.devcel.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Agaisse H, Perrimon N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol Rev. 2004;198:72–82. doi: 10.1111/j.0105-2896.2004.0133.x. [DOI] [PubMed] [Google Scholar]
- Ainsworth C, Wan S, Skaer H. Coordinating cell fate and morphogenesis in Drosophila renal tubules. Philos Trans R Soc Lond B Biol Sci. 2000;355:931–937. doi: 10.1098/rstb.2000.0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglani F, Ceol M, Mezzabotta F, Torregrossa R, Tiralongo E, Tosetto E, Del Prete D, D’Angelo A. The renal stem cell system in kidney repair and regeneration. Front Biosci. 2008;13:6395–6405. doi: 10.2741/3161. [DOI] [PubMed] [Google Scholar]
- Arbouzova NI, Zeidler MP. JAK/STAT signaling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:1605–1616. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam B, Verhaar M, Blankestijn P, Boer WH, Joles JA. Technology insight: innovative options for end-stage renal disease—from kidney refurbishment to artificial kidney. Nat Clin Pract Nephrol. 2007;3:564–572. doi: 10.1038/ncpneph0600. [DOI] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Tetta C, Camussi G. Contribution of stem cells to kidney repair. Am J Nephrol. 2008;28:813–822. doi: 10.1159/000137681. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley LG. Adult stem cells in the repair of the injured renal tubule. Nat Clin Pract Nephrol. 2005;1:22–32. doi: 10.1038/ncpneph0021. [DOI] [PubMed] [Google Scholar]
- Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- Challen GA, Bertoncello I, Deane JA, Ricardo SD, Little MH. Kidney side population reveals multilineage potential and renal functional capacity but also cellular heterogeneity. J Am Soc Nephrol. 2006;17:1896–1912. doi: 10.1681/ASN.2005111228. [DOI] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JA. Using fly atlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis LM, Chen S, Chen B, Agarwal A, Klug CA, Sanders PW. Contribution of intrarenal cells to cellular repair after acute kidney injury: subcapsular implantation technique. Am J Physiol Renal Physiol. 2008;295:F310–F314. doi: 10.1152/ajprenal.90205.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Day JP, Wan S, Allan AK, Kean L, Davies SA, Gray JV, Dow JA. Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa. J Cell Sci. 2008;121:2612–2619. doi: 10.1242/jcs.033084. [DOI] [PubMed] [Google Scholar]
- Dekel B, Zangi L, Shezen E, Reich-Zeliger S, Eventov-Friedman S, Katchman H, Jacob-Hirsch J, Amariglio N, Rechavi G, Margalit R, Reisner Y. Isolation and characterization of nontubular sca-1+lin- multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol. 2006;17:3300–3314. doi: 10.1681/ASN.2005020195. [DOI] [PubMed] [Google Scholar]
- Denholm B, Sudarsan V, Pasalodos-Sanchez S, Artero R, Lawrence P, Maddrell S, Baylies M, Skaer H. Dual origin of the renal tubules in Drosophila: mesodermal cells integrate and polarize to establish secretory function. Curr Biol. 2003;13:1052–1057. doi: 10.1016/s0960-9822(03)00375-0. [DOI] [PubMed] [Google Scholar]
- Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell. 2005;9:501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Dow JA. Integrative physiology, functional genomics and the phenotype gap: a guide for comparative physiologists. J Exp Biol. 2007a;210:1632–1640. doi: 10.1242/jeb.002691. [DOI] [PubMed] [Google Scholar]
- Dow JA. Model organisms and molecular genetics for endocrinology. Gen Comp Endocrinol. 2007b;153:3–12. doi: 10.1016/j.ygcen.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Dow JA, Davies SA. The Malpighian tubule: rapid insights from post-genomic biology. J Insect Physiol. 2006;52:365–738. doi: 10.1016/j.jinsphys.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Dow JA, Davies SA, Sözen MA. Fluid Secretion by the Drosophila Malpighian Tubule. Amer Zool. 1998;38:450–460. [Google Scholar]
- Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elger M, Hentschel H, Litteral J, Wellner M, Kirsch T, Luft FC, Haller H. Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J Am Soc Nephrol. 2003;14:1506–1518. doi: 10.1097/01.asn.0000067645.49562.09. [DOI] [PubMed] [Google Scholar]
- Evans JM, Day JP, Cabrero P, Dow JA, Davies SA. A new role for a classical gene: white transports cyclic GMP. J Exp Biol. 2008;211:890–899. doi: 10.1242/jeb.014837. [DOI] [PubMed] [Google Scholar]
- Frew IJ, Thoma CR, Georgiev S, Minola A, Hitz M, Montani M, Moch H, Krek W. pVHL and PTEN tumour suppressor proteins cooperatively suppress kidney cyst formation. EMBO J. 2008;27:1747–1757. doi: 10.1038/emboj.2008.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Rosenberg ME. Do stem cells exist in the adult kidney? Am J Nephrol. 2008;28:607–613. doi: 10.1159/000117311. [DOI] [PubMed] [Google Scholar]
- Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y, Luttun A, Rosenberg ME. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17:3028–3040. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- Gaul U, Weigel D. Regulation of Kruppel expression in the anlage of the Malpighian tubules in the Drosophila embryo. Mech Dev. 1990;33:57–67. doi: 10.1016/0925-4773(90)90135-9. [DOI] [PubMed] [Google Scholar]
- Gregory L, Came PJ, Brown S. Stem cell regulation by JAK/STAT signaling in Drosophila. Semin Cell Dev Biol. 2008;19:407–413. doi: 10.1016/j.semcdb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Haller H, Groot KD, Bahlmann F, Elger M, Fliser D. Stem cells and progenitor cells in renal disease. Kidney Int. 2005;68:1932–1936. doi: 10.1111/j.1523-1755.2005.00622.x. [DOI] [PubMed] [Google Scholar]
- Hahn M, Bishop J. Expression pattern of Drosophila ret suggests a common ancestral origin between the metamorphosis precursors in insect endoderm and the vertebrate enteric neurons. Proc Natl Acad Sci USA. 2001;98:1053–1058. doi: 10.1073/pnas.98.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton-Ellis E, Ainsworth C, Sushama Y, Wan S, VijayRaghavan K, Skaer H. Genetic regulation of patterned tubular branching in Drosophila. Proc Natl Acad Sci U S A. 2007;104:169–174. doi: 10.1073/pnas.0606933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henske EP. Tuberous sclerosis and the kidney: from mesenchyme to epithelium, and beyond. Pediatr Nephrol. 2005;20:854–847. doi: 10.1007/s00467-004-1795-3. [DOI] [PubMed] [Google Scholar]
- Hishikawa K, Marumo T, Miura S, Nakanishi A, Matsuzaki Y, Shibata K, Ichiyanagi T, Kohike H, Komori T, Takahashi I, et al. Musculin/MyoR is expressed in kidney side population cells and can regulate their function. J Cell Biol. 2005;169:921–928. doi: 10.1083/jcb.200412167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombría JC, Sotillos S. JAK/STAT signalling: STAT cannot play with Ken and Barbie. Curr Biol. 2006;16:R98–100. doi: 10.1016/j.cub.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Hou SX, Singh SR. Methods Mol Biol. Vol. 450. Humana Press; 2008. Germline stem cells. [DOI] [PubMed] [Google Scholar]
- Hou SX, Zheng Z, Chen X, Perrimon N. The JAK/STAT pathway in model organisms: emerging roles in cell movement. Dev Cell. 2002;3:765–778. doi: 10.1016/s1534-5807(02)00376-3. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Imai N, Hishikawa K, Marumo T, Hirahashi J, Inowa T, Matsuzaki Y, Okano H, Kitamura T, Salant D, Fujita T. Inhibition of histone deacetylase activates side population cells in kidney and partially reverses chronic renal injury. Stem Cells. 2007;25:2469–2475. doi: 10.1634/stemcells.2007-0049. [DOI] [PubMed] [Google Scholar]
- Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, Abbate M, Zoja C, Remuzzi G. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- Ivanov SV, Salnikow K, Ivanova AV, Bai L, Lerman MI. Hypoxic repression of STAT1 and its downstream genes by a pVHL/HIF-1 target DEC1/STRA13. Oncogene. 2007;26:802–812. doi: 10.1038/sj.onc.1209842. [DOI] [PubMed] [Google Scholar]
- Iwatani H, Ito T, Imai E, Matsuzaki Y, Suzuki A, Yamato M, Okabe M, Hori M. Hematopoietic and nonhematopoietic potentials of Hoechst(low)/side population cells isolated from adult rat kidney. Kidney Int. 2004;65:1604–1614. doi: 10.1111/j.1523-1755.2004.00561.x. [DOI] [PubMed] [Google Scholar]
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- Jung A, Denholm B, Skaer H, Affolter M. Renal tubule development in Drosophila: a closer look at the cellular level. J Am Soc Nephrol. 2005;16:322–328. doi: 10.1681/ASN.2004090729. [DOI] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kirilly D, Spana EP, Perrimon N, Padgett RW, Xie T. BMP signaling is required for controlling somatic stem cell self-renewal in the Drosophila ovary. Dev Cell. 2005;9:651–662. doi: 10.1016/j.devcel.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Yamasaki Y, Kinomura M, Sugaya T, Sugiyama H, Maeshima Y, Makino H. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J. 2005;19:1789–1797. doi: 10.1096/fj.05-3942com. [DOI] [PubMed] [Google Scholar]
- Kondo K, Kim WY, Lechpammer M, Kaelin WG., Jr Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1:E83. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunter U, Rong S, Boor P, Eitner F, Müller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, Milovanceva-Popovska M, Kerjaschki D, Floege J. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol. 2007;18:1754–1764. doi: 10.1681/ASN.2007010044. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002;3:931–940. doi: 10.1038/nrg952. [DOI] [PubMed] [Google Scholar]
- Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest. 2005;115:1756–1764. doi: 10.1172/JCI23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Jack J. Regulatory interactions and role in cell type specification of the Malpighian tubules by the cut, Kruppel, and caudal genes of Drosophila. Dev Biol. 1992;150:133–143. doi: 10.1016/0012-1606(92)90013-7. [DOI] [PubMed] [Google Scholar]
- Liu X, Kiss I, Lengyel JA. Identification of genes controlling Malpighian tubule and other epithelial morphogenesis in Drosophila melanogaster. Genetics. 1999;151:685–695. doi: 10.1093/genetics/151.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Dearolf CR. The JAK/STAT pathway and Drosophila development. Bioessays. 2001;23:1138–1147. doi: 10.1002/bies.10016. [DOI] [PubMed] [Google Scholar]
- Maddrell SH, Gardiner BO, Pilcher DE, Reynolds SE. Active transport by insect Malpighian tubules of acidic dyes and of acylamides. J Exp Biol. 1974;61:357–377. doi: 10.1242/jeb.61.2.357. [DOI] [PubMed] [Google Scholar]
- Maeshima A, Yamashita S, Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol. 2003;14:3138–3146. doi: 10.1097/01.asn.0000098685.43700.28. [DOI] [PubMed] [Google Scholar]
- Martinez-Agosto JA, Mikkola HK, Hartenstein V, Banerjee U. The hematopoietic stem cell and its niche: a comparative view. Genes Dev. 2007;21:3044–3060. doi: 10.1101/gad.1602607. [DOI] [PubMed] [Google Scholar]
- Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, Parente E, Mancina R, Netti GS, Becherucci F, et al. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205:479–490. doi: 10.1084/jem.20071903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystul T, Spradling A. An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell. 2007;1:277–285. doi: 10.1016/j.stem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential Notch signaling. Science. 2007;1315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Valerius MT, McMahon AP. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134:2533–2539. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- Pfaffenroth EC, Linehan WM. Genetic basis for kidney cancer: opportunity for disease-specific approaches to therapy. Expert Opin Biol Ther. 2008;8:779–790. doi: 10.1517/14712598.8.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum ND. Developmental biology of the human kidney. Semin Fetal Neonatal Med. 2008;13:125–132. doi: 10.1016/j.siny.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Ruiz-Sanchez E, O’Donnell MJ. Effects of chronic exposure to dietary salicylate on elimination and renal excretion of salicylate by Drosophila melanogaster larvae. J Exp Biol. 2007;210:2464–2471. doi: 10.1242/jeb.003152. [DOI] [PubMed] [Google Scholar]
- Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- Sagrinati C, Ronconi E, Lazzeri E, Lasagni L, Romagnani P. Stem-cell approaches for kidney repair: choosing the right cells. Trends Mol Med. 2008;14:277–285. doi: 10.1016/j.molmed.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Saxen L. Organogenesis of the kidney. New York: Cambridge University Press; 1987. [Google Scholar]
- Schmidt LS, Nickerson ML, Warren MB, Glenn GM, Toro JR, Merino MJ, Turner ML, Choyke PL, Sharma N, Peterson J, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dubé syndrome. Am J Hum Genet. 2005;76:1023–1033. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SR, Hou XS. Lessons learned about adult kidney stem cells from the Malpighian tubules of Drosophila. J Am Soc Nephrol. 2008;19:660–666. doi: 10.1681/ASN.2007121307. [DOI] [PubMed] [Google Scholar]
- Singh SR, Chen X, Hou SX. JAK/STAT signaling regulates tissue outgrowth and male germline stem cell fate in Drosophila. Cell Res. 2005;15:1–5. doi: 10.1038/sj.cr.7290255. [DOI] [PubMed] [Google Scholar]
- Singh SR, Liu W, Hou XS. The adult Drosophila Malpighian tubules are maintained by multipotent stem cell. Cell Stem Cell. 2007;1:191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SR, Zhen W, Zheng ZY, Wang H, Oh SW, Liu W, Zbar B, Schmidt LS, Hou SX. The Drosophila homologue of the human tumor suppressor gene BHD regulates male germline stem cell maintenance and functions downstream of Dpp. Oncogene. 2006;25:5933–5941. doi: 10.1038/sj.onc.1209593. [DOI] [PubMed] [Google Scholar]
- Sneddon JB, Werb Z. Location, location, location: the cancer stem cell niche. Cell Stem Cell. 2007;1:607–611. doi: 10.1016/j.stem.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sözen MA, Armstrong JD, Yang MY, Kaiser K, Dow JAT. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc Natl Acad Sci U S A. 1997;94:5207–5212. doi: 10.1073/pnas.94.10.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarsan V, Pasalodos-Sanchez S, Wan S, Gampel A, Skaer H. A genetic hierarchy establishes mitogenic signalling and mitotic competence in the renal tubules of Drosophila. Development. 2002;129:935–944. doi: 10.1242/dev.129.4.935. [DOI] [PubMed] [Google Scholar]
- Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature. 2008;454:651–655. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- Thomas L, Kumar R. Control of renal solute excretion by enteric signals and mediators. J Am Soc Nephrol. 2008;19:207–212. doi: 10.1681/ASN.2007101122. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut NH, Treier M. Transcriptional regulators in kidney disease: gatekeepers of renal homeostasis. Trends Genet. 2008;24:361–371. doi: 10.1016/j.tig.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S, Lin Y. Coordinating early kidney development: lessons from gene targeting. Nat Rev Genet. 2002;3:533–543. doi: 10.1038/nrg842. [DOI] [PubMed] [Google Scholar]
- Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B, Le Hir M. The proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol. 2008;294:C22–C28. doi: 10.1152/ajpcell.00227.2007. [DOI] [PubMed] [Google Scholar]
- Voog J, D’Alterio C, Jones DL. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature. 2008;454:1132–1136. doi: 10.1038/nature07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Cato AM, Skaer H. Multiple signalling pathways establish cell fate and cell number in Drosophila Malpighian tubules. Dev Biol. 2000;217:153–165. doi: 10.1006/dbio.1999.9499. [DOI] [PubMed] [Google Scholar]
- Wang H, Singh SR, Zheng ZY, Oh SW, Chen X, Edwards K, Hou SX. A Rap-GEF/Rap GTPase signaling controls stem cell maintenance through regulating adherens junction positioning and cell adhesion in Drosophila testis. Dev Cell. 2006;10:117–126. doi: 10.1016/j.devcel.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Wang J, Kean L, Yang J, Allan AK, Davies SA, Herzyk P, Dow JA. Function-informed transcriptome analysis of Drosophila renal tubule. Genome Biol. 2004;5:R69. doi: 10.1186/gb-2004-5-9-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessing A, Eichelberg D. Malpighian tubules, rectal papillae and excretion. In: Ashburner A, Wright TRF, editors. The Genetics and Biology of Drosophila. 2c. London: Academic Press; 1978. pp. 1–42. [Google Scholar]
- Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Fuller MT. Asymmetric centrosome behavior and the mechanisms of stem cell division. J Cell Biol. 2008;180:261–266. doi: 10.1083/jcb.200707083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoo T, Fukui A, Matsumoto K, Okabe M. Stem cells and kidney organogenesis. Front Biosci. 2008;13:2814–2832. doi: 10.2741/2888. [DOI] [PubMed] [Google Scholar]



