Abstract
Bacteria use a variety of mechanisms to translocate proteins from the cytoplasm where they are synthesized to the cell surface, extracellular environment, or directly into other cells, where they perform their ultimate functions. Type V Secretion Systems (T5SS) use β-barrel transporter domains to export passenger domains across the outer membranes of Gram-negative bacteria. Distinct among T5SS are Type Vb or Two Partner Secretion (TPS) systems in which the transporter and passenger are separate proteins, necessitating a mechanism for passenger-translocator recognition in the periplasm and providing the potential for reuse of the translocator. This review describes current knowledge of the TPS translocation mechanism, using Bordetella filamentous hemagglutinin (FHA) and its transporter FhaC as a model. We present the hypothesis that the TPS pathway may be a general mechanism for contact-dependent delivery of toxins to target cells.
INTRODUCTION
Bacteria use surface molecules to interact with inanimate objects during biofilm development, other bacteria during sociomicrobiological community activities, and host organisms during mutualistic, commensal, and parasitic symbioses. Among the mechanisms for delivering proteins to the surface of Gram-negative bacteria are Type V Secretion Systems (T5SS) (1–3). T5SS comprise a passenger domain and an associated β-barrel transporter domain that, once integrated into the outer membrane via the Bam assembly complex, is sufficient for export of the passenger from the periplasm to the cell surface. Based on domain architecture, T5SS are categorized into five classes, with Type Vb or Two-Partner Secretion (TPS) pathway systems being distinct because the passenger domain (referred to generically as a TpsA protein) is synthesized independently from the transporter domain (the TpsB protein). This arrangement requires a mechanism for passenger-transporter recognition in the periplasm, and may allow reuse of the transporter for export of multiple copies of the same, or closely related, passenger proteins.
TpsB proteins are members of the Omp85 superfamily that includes the β-barrel outer membrane insertases BamA in bacteria, Tob55/Sam50 in mitochondria, and Toc75 in chloroplasts (4, 5). The C-terminal ~350 amino acids (aa) of Omp85 family proteins form a β-barrel pore composed of 16 β-strands linked by loops and turns (Fig. 1A). At or near the N-termini are periplasmically-located polypeptide transport-associated (POTRA) domains (two in TpsB proteins), each about 80 aa in length with a βααββ structure, that mediate recognition of translocation substrates (6). TpsA proteins are large, often longer than 3,000 aa. They contain highly conserved N-terminal ~250 aa ‘TPS domains’ that fold into right-handed β-helices, with each turn of the helix formed from three β-strands in a triangular arrangement (7–10) (Fig. 1B). The remaining portions of TpsA proteins are not highly conserved, but are predicted to contain substantial β-helical regions, usually within the N-terminal half of the protein, as well as regions of undetermined structure and distinct globular domains located near the C-terminus.
Figure 1. Structures of FhaC and the TPS domain of FhaB.
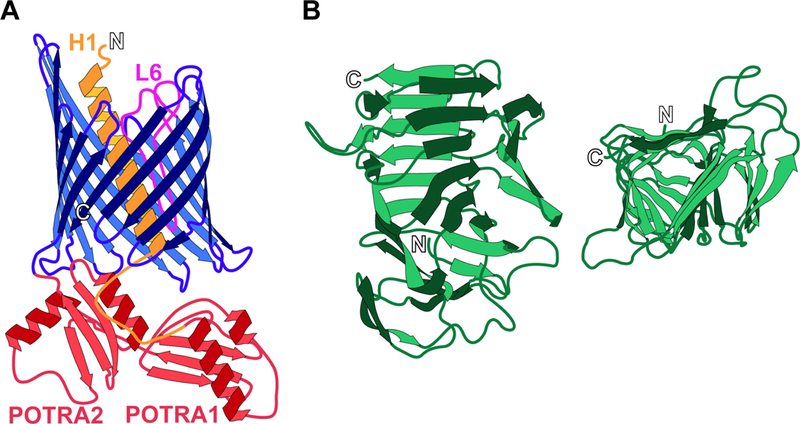
(A) Helix 1 (H1, orange) and loop 6 (L6, fuchsia) are located within the pore of the 16-stranded β barrel (blue) of FhaC when the transporter is in the “closed” state. The POTRA domains (POTRA1 and POTRA2, red) remain periplasmic for selective recognition of the FhaB TPS domain. (B) The TPS domain of FhaB adopts a triangular β-helical structure, shown from the side of the helix (left) and top-down in a C-terminus to N-terminus direction (right). Termini are indicated by outlined letter.
TPS systems are widespread among Gram-negative bacteria, and TpsA proteins perform a broad range of activities that includes adherence, cytolysis, iron acquisition, and interbacterial signaling and intoxication (11). The best characterized TPS system is filamentous hemagglutinin (FHA) and its transporter FhaC of Bordetella pertussis, the causal agent of human whooping cough, and Bordetella bronchiseptica, which infects the respiratory tracts of a broad range of mammals (12). FHA is a key Bordetella virulence factor. It is essential for establishing infection in the lower respiratory tract by mediating adherence to respiratory epithelia, and it plays an important role in suppressing the initial inflammatory response to infection, promoting bacterial persistence (13–15). In this chapter, we discuss what is known about FHA secretion as a prototypical TPS pathway protein. Although differences surely exist, many aspects of FHA secretion are likely to be conserved among TPS family members.
INITIAL STEPS IN THE SECRETION PROCESS
FHA is first synthesized as a ~370 kDa preproprotein (3,591 aa in B. pertussis Tohama I and 3,710 aa in B. bronchiseptica RB50) called FhaB. Its 71 aa signal sequence, which is removed during Sec-mediated translocation across the cytoplasmic membrane, contains a 25 aa N-terminal extended signal peptide region (ESPR) (16). ESPRs are highly conserved, present on a large number of T5SS proteins (17, 18), and have been implicated in regulating co-translational and post-translational Sec-mediated translocation (19–23). For FhaB, the ESPR appears to slow Sec-mediated post-translational translocation across the cytoplasmic membrane (24), although how it contributes to the overall secretion mechanism remains unknown.
Once in the periplasm, FhaB must find FhaC. Early studies showed that polypeptides corresponding to the N-terminal ~300 aa of FhaB are efficiently secreted into culture supernatants of B. pertussis in an FhaC-dependent manner and can be secreted by E. coli when co-produced with FhaC (7, 25, 26). These results indicated that the TPS domain contains all of the information necessary for recognition and translocation (of at least the TPS domain) by FhaC, and led to further use of such models to investigate initial steps in the TPS pathway. Using E. coli liposomes containing FhaC, Fan et al. showed that FhaC is, reciprocally, the only outer membrane protein required for translocation of the N-terminal 370 aa of FhaB and provided evidence that folding of the FhaB N-terminus occurs after translocation (27). Using a variety of protein-protein interaction approaches, Jacob-Dubuisson and colleagues showed that the POTRA domains of FhaC bind unfolded, but not folded, β-strands within the FhaB TPS domain, possibly via β augmentation (28, 29). Moreover, mutational analyses revealed that aa at the tip of loop six of FhaC, which is located within the channel in the crystal structure (Fig. 1A), are critical for secretion, perhaps by recognizing the presence of a secretion substrate (30). These studies identified regions within FhaC and the FhaB TPS domain that are important for recognition and secretion, but could not address the dynamics of TpsA translocation. To investigate the movement of FhaB across the outer membrane, the Jacob-Dubuisson group designed an approach to trap and characterize secretion intermediates (31). This study was the first to show translocation of a (truncated) substrate through the channel of an Omp85-family β-barrel (31). It revealed interactions between specific aa within FhaB and the POTRA domains, the interior of the channel, and the four-stranded B5-B8 extracellular β-sheet of FhaC. The data allowed Baud et al. to propose a model (Fig. 2) in which FhaC alternates between ‘open’ (helix 1 (H1) at the N-terminus of FhaC in the periplasm) and ‘closed’ (H1 in the channel) conformations in the absence of its substrate. Binding of extended amphipathic segments within the TPS domain of FhaB to the POTRA domains stabilizes the open conformation and allows the N-terminus of FhaB to thread through the channel. Specific anti-parallel β-strands near the N-terminus of the TPS domain then bind to the B5-B8 extracellular β-sheets of FhaC, while adjacent β-strands are translocated through the pore and begin folding into a β-helix on the cell surface, the stability of which prevents backsliding through the channel. Continued translocation of FhaB β-strands then extends the β-helix into the extracellular milieu.
Figure 2. Initial steps in secretion of FhaB.
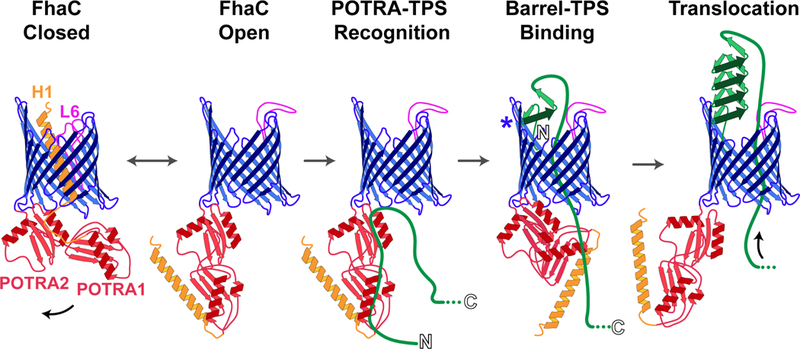
FhaC alternates between a closed state, in which H1 (orange) and L6 (fuchsia) plug the channel, and an open state, in which H1 and L6 localize to the periplasm and the extracellular space, respectively. The POTRA domains of FhaC (red) bind the unfolded FhaB TPS domain (green line), stabilizing FhaC in the open state. The N-terminus of FhaB then binds the interior of the FhaC barrel at β-strands B5-B8 (blue asterisk) and forms into a β-helix as the protein is translocated, preventing backsliding through the channel.
MECHANISTIC INSIGHT DERIVED FROM TOPOLOGICAL ANALYSIS OF FhaB/FHA
Experiments using the TPS domain provided substantial insight into the TPS translocation mechanism. Indeed, the ratcheted diffusion model proposed by Baud et al. helps explain how proteins can be moved across the hydrophobic outer membrane in the absence of an obvious chemical energy source (there is no ATP in the periplasm) - a major outstanding question for all T5SS. However, translocation of full-length TpsA proteins, the C-terminal halves of which are not predicted to fold into β-helices, is likely to be more complicated. Moreover, for FhaB (and possibly other TpsA proteins), the C- terminal ~1,200 aa (called the ‘prodomain’) are removed at some point during the secretion process to form the ~250 kDa FHA protein, which is both cell-associated and released into the extracellular milieu (32, 33). The prodomain does not appear to function as an independent protein as it is degraded rapidly and not detectable as a separate polypeptide in whole cell lysates or culture supernatants (26, 34). It was therefore proposed to function as some sort of chaperone, perhaps keeping the N- terminus of FhaB in an unfolded state within the periplasm (26), with FHA being the ‘mature’ molecule that is functional during infection. X-ray crystallography, electron microscopy, and molecular modeling analyses indicate that the N-terminal ~2,000 aa of FHA form a rigid β-helical rod, while the C-terminal ~500 aa (the mature C-terminal domain, or MCD) form a globular domain that appears to be bi-lobed in the released form of the protein (35–37).
In search of a protease responsible for cleavage of FhaB to create FHA, Coutte et al. identified SphB1, a subtilisin-like serine protease and classical autotransporter (Type Va Secretion System) protein (38). Deletion of sphBI in B. pertussis results in the production of a slightly longer FHA molecule (called FHA*) that is released much less efficiently into culture supernatants than FHA is (38). Coutte et al. proposed a model in which FhaB emerges from FhaC on the cell surface in an N-to-C terminal direction until the C-terminus of the MCD is exposed and cleaved by SphB1, resulting in formation and release of FHA into the extracellular milieu (Fig. 3A). Although attractive, this model did not account for the fact that a large amount of FHA is retained on the cell surface, and could not explain the biophysics of moving the globular ~500 aa MCD through FhaC. While seeking to understand the role of released FHA during infection, our lab found that, as in B. pertussis, ΔsphBI mutants of B. bronchiseptica produce a slightly larger FHA protein (FHA’) that is poorly released from the cell surface (34). Moreover and unexpectedly, fractionation, limited proteolysis, immunoblotting, and fluorescence microscopy approaches showed that FHA is anchored to the cell by its N-terminus, with the MCD located distally from the cell surface (34). Consistent with this orientation, antibodies against the MCD, but not antibodies recognizing the N-terminus, block FHA-dependent adherence to epithelial cells and macrophages (34). Additional studies showed that the prodomain remains in the periplasm due to a region called the prodomain N-terminus (PNT), and cysteine accessibility experiments showed that the prodomain is required for the MCD to achieve its normal conformation (39). These data, together with those of Baud et al., support a model in which FhaB is translocated through FhaC as a ‘hairpin’, with its N-terminus anchored to the B5-B8 extracellular β-sheets of FhaC and its β-helical shaft elongating by the addition of β-strands to the distal end (Fig. 3B). The stability of the β-helix, which ultimately extends about 40 nm, allows the globular MCD to be ‘pulled’ to the surface without backsliding until movement is stopped by the PNT reaching the periplasmic side of FhaC, through which it cannot pass. The MCD then samples limited conformations before folding into its final form, followed at some point by cleavage by SphB1, degradation of the prodomain, and release of FHA into the extracellular milieu (39).
Figure 3. Two models for FhaB secretion: distal N-terminus versus hairpin.
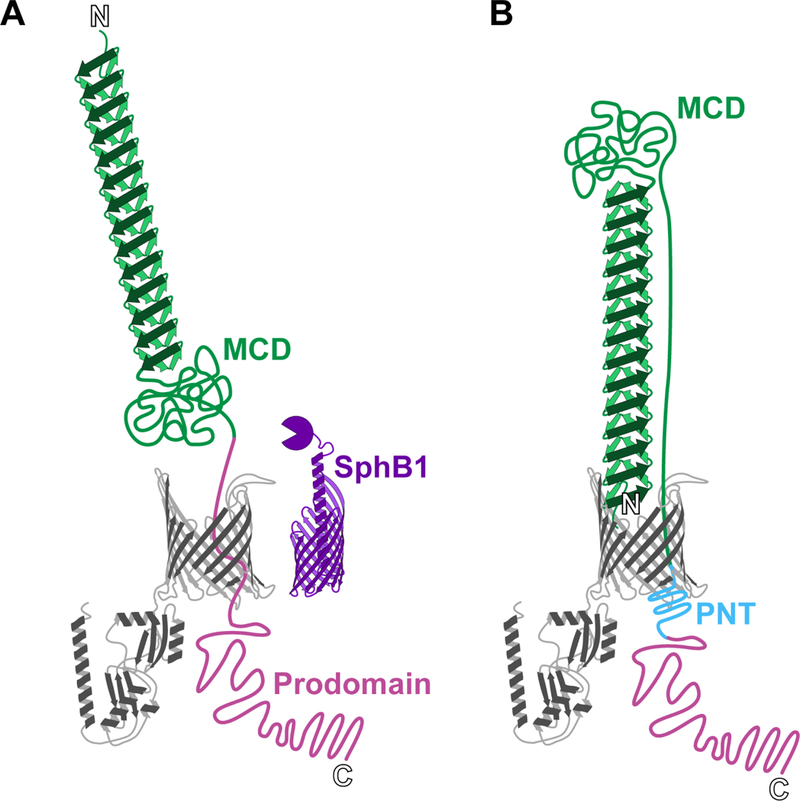
In the model proposed by Coutte et al. (A), the N-terminus of FhaB is pushed away from the membrane as more of the polypeptide translocates through FhaC. The protease SphB1 cleaves between the mature C-terminal domain (MCD) and the periplasmic prodomain, causing release of FHA. In the alternative “hairpin” model proposed by Mazar and Cotter (B), the N-terminus of FhaB remains bound to FhaC during secretion, and the MCD is located at the distal end of the β-helix. A portion of the MCD spans the helix length, as it is tethered to the periplasmic prodomain. The prodomain N-terminus (PNT) prevents translocation of the prodomain through FhaC.
ROLE OF THE FhaB PRODOMAIN
Although the prodomain remains in the periplasm, it appears not to be required for keeping the N-terminus of FhaB in a secretion-competent state because secretion and release of FHA occurs in strains producing truncated FhaB proteins that lack the prodomain (34). However, given its large size, it seems unlikely that prodomain function is limited to tethering the C-terminus of the MCD to the membrane, which might require only the ~145 aa PNT region. FhaB contains two distinct domains at its C-terminus: the extreme C-terminus (ECT), composed of the C-terminal ~100 aa that are invariant among all predicted FhaB proteins, and the highly conserved proline rich region (PRR), which is immediately N-terminal to the ECT. Deletion of the sequence encoding the ECT results in increased conversion of FhaB to FHA, while deletion of the sequence encoding the PRR results in no discernible phenotype in bacteria grown in culture in the laboratory (13). However, both ΔECT and ΔPRR mutants are cleared much faster than wild-type bacteria from the lungs of intranasally-inoculated mice, despite being indistinguishable from wild-type bacteria in their ability to adhere to respiratory epithelium (13). Because the prodomain does not appear to be an independently functional polypeptide, these data suggest that full-length FhaB is not simply an intermediate molecule in the maturation pathway of FHA, but plays an import role in the ability of the bacteria to resist inflammation-mediated clearance in the lower respiratory tract (13).
The fact that deletion of sphBI results in altered (rather than lack of) cleavage of FhaB (34, 38), together with the observation that the increased degradation of the prodomain that occurs in ΔECT mutants also occurs in ΔECTΔsphBI double mutants (13), suggests the existence of a periplasmic protease that degrades the prodomain in a manner that is somehow negatively regulated by the ECT. B. bronchiseptica gene BB0300, annotated as ctpA, is predicted to encode a member of the Prc/Tsp/CtpA family of periplasmic proteases that target C-termini. Deletion of ctpA abrogates increased conversion of FhaB to FHA in the ΔECT strain, as well as formation of FHA’ in a ΔsphBI mutant (Nash & Cotter, in revision). Moreover, although a version of FHA that is even longer than FHA’ is produced in a ΔctpAΔsphBI double mutant (indicating the existence of yet another protease that can cleave the prodomain in the periplasm), no form of FHA is detected in culture supernatants, indicating that cleavage by either CtpA or SphBI must occur for FHA (or FHA’) to be released. By inducing synthesis of FhaB synchronously, we found that cleavage of FhaB occurs in a step-wise manner, with CtpA-dependent degradation of the prodomain occurring first, followed by SphB1-mediated cleavage to form FHA (Nash & Cotter, in revision). We also found that the central portion of the prodomain (the region between the PNT and the PRR) is required for ECT-dependent control of CtpA-mediated degradation of the prodomain. Together, these data support a model (Fig. 4) in which full-length FhaB resides in the outer membrane with the MCD located distally from the cell surface and the prodomain in the periplasm with the ECT preventing CtpA-mediated degradation. In response to a signal that is propagated from the MCD to the prodomain, an unknown protease (P3 in Fig. 4) removes the ECT, exposing a C-terminus that is susceptible to degradation by CtpA, which occurs in a processive manner, ultimately creating FHA’ (or FHA* in B. pertussis), the C-terminus of which is located in the middle of the PNT. Removal of the C-terminal half of the PNT weakens its translocation-blocking activity, allowing the C-terminus of the MCD to move through FhaC to the surface, where it is cleaved by SphB1. The small peptide that is created (extending from the SphB1 cleavage site to the end of FHA’ or FHA*) is then free to slide back into the periplasm, the N-terminus of FHA disassociates from the B5-B8 β-sheets of FhaC, and FHA is released from the cell surface.
Figure 4. Model for stepwise processing and release of FhaB.
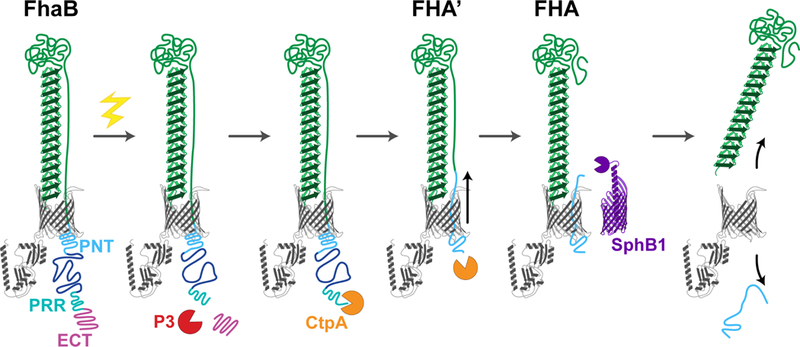
Upon receipt of an unknown maturation signal (yellow bolt), an as yet unidentified protease (P3) removes the extreme C-terminus (ECT) and exposes a substrate for the protease CtpA. CtpA processively degrades the prodomain through a portion of the PNT, forming FHA’ and shifting the polypeptide to expose the cleavage site of SphBI. FHA is formed from SphB1-dependent cleavage of FHA’, and it is retained at the membrane until the remaining portion of the prodomain exits FhaC (gray barrel).
A HYPOTHESIS: TPS AS A MECHANISM FOR REGULATED, SELECTIVE TOXIN DELIVERY
Questions raised by the model described above include the nature of the signal that is sensed by the MCD and the role of controlled degradation of the prodomain in FhaB/FHA function. The fact that ΔECT and ΔPRR mutants of B. bronchiseptica are indistinguishable from wild-type bacteria in their ability to adhere to epithelial cells in vitro and in vivo, but are unable to persist in the lower respiratory tract, suggests that full-length FhaB is required specifically for resisting clearance by phagocytic cells (13). Adenylate cyclase toxin (ACT) is a calmodulin-activated adenylyl cyclase that is produced by all classical bordetellae (B. pertussis, B. bronchiseptica, and B.parapertussis) that also contributes to defense against inflammation-mediated clearance (40, 41). ACT inhibits phagocytosis and oxidative burst in neutrophils (42), blocks complement-dependent phagocytosis in macrophages (43), and suppresses activation and chemotaxis in T lymphocytes (44). Although exported by a dedicated Type 1 Secretion System, ACT binds tightly to the MCD of FhaB/FHA on the cell surface (45), and there is evidence that it is newly secreted ACT, not that which has been released into culture supernatants, that is most efficient at intoxicating host cells (46). Moreover, both FHA and ACT have been shown to bind receptors, such as CD11b/CD18 and Very Late Antigen (VLA)-5 or Leukocyte Response Integrin-Integrin Associated Protein (LRI-IAP), that are present on neutrophils, dendritic cells, and macrophages (47–50). We hypothesize that ACT forms a complex with FhaB on the cell surface and that binding of this complex to receptors on phagocytes causes a conformational change in FhaB that is propagated across the membrane resulting in cleavage and degradation of the prodomain by the unknown protease and CtpA, followed by delivery of ACT to, and intoxication of, the host cell, hence promoting persistence of the bacteria in the face of a robust inflammatory response. In this way, FhaB may serve as an efficient ACT delivery system that prevents indiscriminant intoxication of unintended host cells, such as epithelial cells, and wasteful dispersal of ACT into the extracellular milieu. Once FHA is released from the cell surface, FhaC can be loaded with a new FhaB protein for another round of toxin delivery. The possibility that many, if not all, TPS systems function in this manner is supported by a recent report by Ruhe at el. (51) who proposed a somewhat similar mechanism for a TPS system that mediates interbacterial competition. In that system, the toxin that is delivered is the C-terminus of the TpsA protein and the target cells are non-sibling bacteria, but the concept that receptor binding by a region in the middle of the TpsA protein triggers a conformational change and toxin delivery is the same. Future experiments will be required to determine the generality of this strategy, as well as the mechanistic variations among TPS systems.
AN OUTSTANDING QUESTION: DOES FHA, EITHER SURFACE-ASSOCIATED OR RELEASED, PLAY A ROLE IN VIRULENCE?
Because of its first-identified role, and demonstrated importance, as an adhesin, the efficient release of FHA into culture supernatants (and assumed release of FHA in vivo) has been enigmatic. Indeed, a desire to determine if FHA release is important during infection is what initially motivated our laboratory to investigate the FhaB/FHA secretion mechanism. Although ΔsphBI mutants are severely defective in mouse models ((52) and our unpublished data), their in vivo phenotype(s) cannot be wholly attributed to lack of FHA release because SphB1 cleaves multiple surface proteins in addition to FhaB (our unpublished data). Discovery that FhaB/FHA secretion, processing, and release is much more complicated than initially imagined, also reveals the challenge of creating a mutant in which FhaB and surface-associated FHA remain functional while FHA release is prevented, which would be required to query the function of FHA release in vivo. Moreover, although the fact that ΔECT and ΔPRR mutants are defective specifically for resisting inflammation-mediated clearance supports a role for FhaB during infection, the fact that these mutants do not differ from wild-type bacteria in their ability to adhere to respiratory epithelium in vitro and in vivo or to suppress the initial inflammatory response does not rule out the possibility that it is FhaB, and not FHA, that is important for these functions. Hence, whether ‘mature’ FHA plays any role during the Bordetella mammalian host interaction is now an open question. Continued investigation of the mechanism of FhaB/FHA secretion and processing, with accompanying infection experiments, will hopefully answer the outstanding questions regarding this important virulence factor, as well as provide a roadmap for investigating other members of the large TPS family.
REFERENCES
- 1.Leo JC, Grin I, Linke D. 2012. Type V secretion: mechanism(s) of autotransport through the bacterial outer membrane. Phil Trans R Soc B 367:1088–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa TRD, Felisberto-Rodrigues C, Meir A, Prevost MS, Redzej A, Trokter M, Waksman G. 2015. Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nature Rev Microbiol 13:343–359. [DOI] [PubMed] [Google Scholar]
- 3.Fan E, Chauhan N, Udatha DBRKG, Leo JC, Linke D. 2016. Type V Secretion Systems in Bacteria. Microbiol Spectr 4:305–335. [DOI] [PubMed] [Google Scholar]
- 4.Ulrich T, Rapaport D. 2015. Biogenesis of beta-barrel proteins in evolutionary context. International Journal of Medical Microbiology 305:259–264. [DOI] [PubMed] [Google Scholar]
- 5.Gentle I, Gabriel K, Beech P, Waller R, Lithgow T. 2004. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. The Journal of Cell Biology 164:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmerman RF, Dave AM, Bruce BD. 2014. Structure and Function of POTRA Domains of Omp85/TPS Superfamily. International Review of Cell and Molecular Biology 308:1−34. [DOI] [PubMed] [Google Scholar]
- 7.Clantin B, Hodak H, Willery E, Locht C, Jacob-Dubuisson F, Villeret V. 2004. The crystal structure of filamentous hemagglutinin secretion domain and its implications for the two-partner secretion pathway. Proc Natl Acad Sci USA 101:6194–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo HJ, Yokoyama T, Walkiewicz K, Biological YKJO, 2007. The structure of the Haemophilus influenzae HMW1 pro-piece reveals a structural domain essential for bacterial two-partner secretion. ASBMB. [DOI] [PubMed] [Google Scholar]
- 9.Weaver TM, Hocking JM, Bailey LJ, Wawrzyn GT, Howard DR, Sikkink LA, Ramirez-Alvarado M, Thompson JR. 2009. Structural and functional studies of truncated hemolysin a from Proteus mirabilis. J Biol Chem 284:jbc.M109.014431–22309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baelen S, Dewitte F, Clantin B, Villeret V. 2013. Structure of the secretion domain of HxuA from Haemophilus influenzae. Acta Crystallographica Section F 69:1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guérin J, Bigot S, Schneider R, Buchanan SK, Jacob-Dubuisson F. 2017. Two-Partner Secretion: Combining Efficiency and Simplicity in the Secretion of Large Proteins for Bacteria-Host and Bacteria-Bacteria Interactions. Front Cell Infect Microbiol 7:1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melvin JA, Scheller EV, Miller JF, Cotter PA. 2014. Bordetella pertussis pathogenesis: current and future challenges. Nature Publishing Group 12:274–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melvin JA, Scheller EV, Noël CR, Cotter PA. 2015. New Insight into Filamentous Hemagglutinin Secretion Reveals a Role for Full-Length FhaB in Bordetella Virulence. MBio 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inatsuka CS, Julio SM, Cotter PA. 2005. Bordetella filamentous hemagglutinin plays a critical role in immunomodulation, suggesting a mechanism for host specificity. Proc Natl Acad Sci USA 102:18578–18583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonso S, Pethe K, Mielcarek N, Raze D, Locht C. 2001. Role of ADP-ribosyltransferase activity of pertussis toxin in toxin-adhesin redundancy with filamentous hemagglutinin during Bordetella pertussis infection. Infect Immun 69:6038–6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob-Dubuisson F, Buisine C, Mielcarek N, Clément E, Menozzi FD, Locht C. 1996. Amino-terminal maturation of the Bordetella pertussis filamentous haemagglutinin. Mol Microbiol 19:65–78. [DOI] [PubMed] [Google Scholar]
- 17.Henderson IR, Navarro-Garcia F, Nataro JP. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol 6:370–378. [DOI] [PubMed] [Google Scholar]
- 18.Desvaux M, Cooper LM, Filenko NA, Scott-Tucker A, Turner SM, Cole JA, Henderson IR. 2006. The unusual extended signal peptide region of the type V secretion system is phylogenetically restricted. FEMS Microbiol Lett 264:22–30. [DOI] [PubMed] [Google Scholar]
- 19.Sijbrandi R, Urbanus ML, Hagen-Jongman ten CM, Bernstein HD, Oudega B, Otto BR, Luirink J. 2003. Signal recognition particle (SRP)-mediated targeting and Sec-dependent translocation of an extracellular Escherichia coli protein. J Biol Chem 278:4654–4659. [DOI] [PubMed] [Google Scholar]
- 20.Peterson JH, Woolhead CA, Bernstein HD. 2003. Basic amino acids in a distinct subset of signal peptides promote interaction with the signal recognition particle. J Biol Chem 278:46155–46162. [DOI] [PubMed] [Google Scholar]
- 21.Desvaux M, Scott-Tucker A, Turner SM, Cooper LM, Huber D, Nataro JP, Henderson IR. 2007. A conserved extended signal peptide region directs posttranslational protein translocation via a novel mechanism. Microbiology (Reading, Engl) 153:59–70. [DOI] [PubMed] [Google Scholar]
- 22.Szabady RL, Peterson JH, Skillman KM, Bernstein HD. 2005. An unusual signal peptide facilitates late steps in the biogenesis of a bacterial autotransporter. Proceedings of the National Academy of Sciences 102:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson JH, Szabady RL, Bernstein HD. 2006. An unusual signal peptide extension inhibits the binding of bacterial presecretory proteins to the signal recognition particle, trigger factor, and the SecYEG complex. J Biol Chem 281:9038–9048. [DOI] [PubMed] [Google Scholar]
- 24.Chevalier N, Moser M, Koch H-G, Schimz K-L, Willery E, Locht C, Jacob-Dubuisson F, Müller M. 2004. Membrane targeting of a bacterial virulence factor harbouring an extended signal peptide. J Mol Microbiol Biotechnol 8:7–18. [DOI] [PubMed] [Google Scholar]
- 25.Jacob-Dubuisson F, Buisine C, Willery E, Renauld-Mongénie G, Locht C. 1997. Lack of functional complementation between Bordetella pertussis filamentous hemagglutinin and Proteus mirabilis HpmA hemolysin secretion machineries. J Bacteriol 179:775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renauld-Mongénie G, Cornette J, Mielcarek N, Menozzi FD, Locht C. 1996. Distinct roles of the N-terminal and C-terminal precursor domains in the biogenesis of the Bordetella pertussis filamentous hemagglutinin. J Bacteriol 178:1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan E, Fiedler S, Jacob-Dubuisson F, Müller M. 2012. Two-partner secretion of gram-negative bacteria: a single β-barrel protein enables transport across the outer membrane. J Biol Chem 287:2591–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodak H, Clantin B, Willery E, Villeret V, Locht C, Jacob-Dubuisson F. 2006. Secretion signal of the filamentous haemagglutinin, a model two-partner secretion substrate. Mol Microbiol 61:368–382. [DOI] [PubMed] [Google Scholar]
- 29.Delattre A-S, Saint N, Clantin B, Willery E, Lippens G, Locht C, Villeret V, Jacob-Dubuisson F. 2011. Substrate recognition by the POTRA domains of TpsB transporter FhaC. Molecular Microbiology 81:99–112. [DOI] [PubMed] [Google Scholar]
- 30.Clantin B, Delattre AS, Rucktooa P, Saint N, Meli AC, Locht C, Jacob-Dubuisson F, Villeret V. 2007. Structure of the Membrane Protein FhaC: A Member of the Omp85-TpsB Transporter Superfamily. Science 317:957–961. [DOI] [PubMed] [Google Scholar]
- 31.Baud C, Guérin J, Petit E, Lesne E, Dupré E, Locht C, Jacob-Dubuisson F. 2014. Translocation path of a substrate protein through its Omp85 transporter. Nat Commun 5:5271. [DOI] [PubMed] [Google Scholar]
- 32.Aricó B, Nuti S, Scarlato V, Rappuoli R. 1993. Adhesion of Bordetella pertussis to eukaryotic cells requires a time-dependent export and maturation of filamentous hemagglutinin. Proceedings of the National Academy of Sciences 90:9204–9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domenighini M, Relman D, Capiau C, Falkow S, Prugnola A, Scarlato V, Rappuoli R. 1990. Genetic characterization of Bordetella pertussis filamentous haemagglutinin: a protein processed from an unusually large precursor. Mol Microbiol 4:787–800. [DOI] [PubMed] [Google Scholar]
- 34.Mazar J, Cotter PA. 2006. Topology and maturation of filamentous haemagglutinin suggest a new model for two-partner secretion. Mol Microbiol 62:641–654. [DOI] [PubMed] [Google Scholar]
- 35.Kajava AV, Cheng N, Cleaver R, Kessel M, Simon MN, Willery E, Jacob-Dubuisson F, Locht C, Steven AC. 2001. Beta-helix model for the filamentous haemagglutinin adhesin of Bordetella pertussis and related bacterial secretory proteins. Mol Microbiol 42:279–292. [DOI] [PubMed] [Google Scholar]
- 36.Makhov AM, Hannah JH, Brennan MJ, Trus BL, Kocsis E, Conway JF, Wingfield PT, Simon MN, Steven AC. 1994. Filamentous hemagglutinin of Bordetella pertussis. A bacterial adhesin formed as a 50-nm monomeric rigid rod based on a 19-residue repeat motif rich in beta strands and turns. J Mol Biol 241:110–124. [DOI] [PubMed] [Google Scholar]
- 37.Kajava AV, Steven AC. 2006. The turn of the screw: variations of the abundant beta-solenoid motif in passenger domains of Type V secretory proteins. J Struct Biol 155:306–315. [DOI] [PubMed] [Google Scholar]
- 38.Coutte L, Antoine R, Drobecq H, Locht C, Jacob-Dubuisson F. 2001. Subtilisin-like autotransporter serves as maturation protease in a bacterial secretion pathway. EMBO J 20:5040–5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noël CR, Mazar J, Melvin JA, Sexton JA, Cotter PA. 2012. The prodomain of the Bordetella two-partner secretion pathway protein FhaB remains intracellular yet affects the conformation of the mature C-terminal domain. Mol Microbiol 86:988–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fedele G, Schiavoni I, Adkins I, Klimova N, Sebo P. 2017. Invasion of Dendritic Cells, Macrophages and Neutrophils by the Bordetella Adenylate Cyclase Toxin: A Subversive Move to Fool Host Immunity. Toxins (Basel) 9:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novak J, Cerny O, Osickova A, Linhartova I, Masin J, Bumba L, Sebo P, Osicka R. 2017. Structure-Function Relationships Underlying the Capacity of Bordetella Adenylate Cyclase Toxin to Disarm Host Phagocytes. Toxins (Basel) 9:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Confer DL, Eaton JW. 1982. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science 217:948–950. [DOI] [PubMed] [Google Scholar]
- 43.Kamanova J, Kofronova O, Masin J, Genth H, Vojtova J, Linhartova I, Benada O, Just I, Sebo P. 2008. Adenylate cyclase toxin subverts phagocyte function by RhoA inhibition and unproductive ruffling. J Immunol 181:5587–5597. [DOI] [PubMed] [Google Scholar]
- 44.Paccani SR, Dal Molin F, Benagiano M, Ladant D, D’Elios MM, Montecucco C,Baldari CT. 2008. Suppression of T-lymphocyte activation and chemotaxis by the adenylate cyclase toxin of Bordetella pertussis. Infect Immun 76:2822–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffman C, Eby J, Gray M, Heath Damron F, Melvin J, Cotter P, Hewlett E. 2016. Bordetella adenylate cyclase toxin interacts with filamentous haemagglutinin to inhibit biofilm formation in vitro. Mol Microbiol 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray MC, Donato GM, Jones FR, Kim T, Hewlett EL. 2004. Newly secreted adenylate cyclase toxin is responsible for intoxication of target cells by Bordetella pertussis. Mol Microbiol 53:1709–1719. [DOI] [PubMed] [Google Scholar]
- 47.Guermonprez P, Khelef N, Blouin E, Rieu P, Ricciardi-Castagnoli P, Guiso N, Ladant D, Leclerc C. 2001. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18). J Exp Med 193:1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Strijp JA, Russell DG, Tuomanen E, Brown EJ, Wright SD. 1993. Ligand specificity of purified complement receptor type three (CD11 b/CD18, alpha m beta 2, Mac-1). Indirect effects of an Arg-Gly-Asp (RGD) sequence. J Immunol 151:3324–3336. [PubMed] [Google Scholar]
- 49.Ishibashi Y, Yoshimura K, Nishikawa A, Claus S, Laudanna C, Relman DA. 2002. Role of phosphatidylinositol 3-kinase in the binding of Bordetella pertussis to human monocytes. Cell Microbiol 4:825–833. [DOI] [PubMed] [Google Scholar]
- 50.Ishibashi Y 2002. Bordetella pertussis infection of human respiratory epithelial cells up-regulates intercellular adhesion molecule-1 expression: role of filamentous hemagglutinin and pertussis toxin. Microbial Pathogenesis 33:115–125. [DOI] [PubMed] [Google Scholar]
- 51.Ruhe ZC, Subramanian P, Song K, Nguyen JY, Stevens TA, Low DA, Jensen GJ, Hayes CS. 2018. Programmed Secretion Arrest and Receptor- Triggered Toxin Export during Antibacterial Contact- Dependent Growth Inhibition. Cell 175:921–933.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coutte L, Alonso S, Reveneau N, Willery E, Quatannens B, Locht C, Jacob-Dubuisson F. 2003. Role of adhesin release for mucosal colonization by a bacterial pathogen. J Exp Med 197:735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]


