Abstract
Importance:
Major Depressive Disorder (MDD) might involve dopamine (DA) reductions. The dopamine transporter (DAT) regulates DA clearance and neurotransmission, and is sensitive to DA levels, with preclinical studies (including those involving inescapable stressors) showing that DAT density decreases when DA signaling is reduced. Despite compelling preclinical data, evidence of reduced DAT in MDD is inconclusive.
Objective:
Using a highly selective DAT positron emission tomography (PET) tracer ([11C] altropane), we probed DAT availability in unmedicated individuals with MDD. Levels of DAT expression were also evaluated in post-mortem tissues from donors with MDD who died by suicide.
Design:
Cross-sectional PET study enrolling consecutive unmedicated individuals with MDD and demographically matched healthy controls between January 2012 and March 2014. Post-mortem analyses of brain tissues obtained from the Douglas-Bell Canada Brain Bank.
Setting:
McLean Hospital and Douglas-Bell Canada Brain Bank.
Participants:
For the PET component, 25 unmedicated individuals with current MDD and 23 healthy controls recruited from McLean Hospital were included (all provided usable data). For the post-mortem component, 15 depressed individuals and 14 healthy controls were considered.
Intervention:
PET scan.
Main Outcome:
Striatal and midbrain DAT binding potential. For the post-mortem component, tyrosine hydroxylase and DAT levels were evaluated using Western Blots.
Results:
Relative to 23 controls (13 females, mean age: 26.49), 25 individuals with MDD (19 females, mean age: 26.52) showed significantly lower in vivo DAT availability in the bilateral putamen and ventral tegmental area (effect sizes: −0.62 to −0.71), and both effects were exacerbated with increasing numbers of depressive episodes. Unlike healthy controls, the MDD group failed to show age-related reduction in striatal DAT availability, with young MDD individuals being indistinguishable from older healthy controls. Moreover, DAT availability in the ventral tegmental area was lowest in MDD individuals reporting feeling trapped in stressful circumstances. Lower DAT (and tyrosine hydroxylase) in the putamen of MDD relative to healthy controls was replicated in post-mortem analyses (effect sizes: −0.92 to −1.15).
Conclusions and Relevance:
MDD, particularly with recurring episodes, is characterized by decreased striatal DAT expression, which might reflect a compensatory down-regulation due to low DA signaling within mesolimbic pathways.
Introduction
Despite decades of research, the molecular underpinnings of major depressive disorder (MDD) remain incompletely understood. Among various targets, dopamine (DA) has received considerable attention, particularly owing to its role in motivation, which is affected in MDD1. Growing data point to blunted DA transmission in MDD. The most direct evidence stems from dopamine depletion studies, which have described rapid increases in depressive symptoms after catecholamine depletion in remitted MDD2–4. Additionally, functional magnetic resonance imaging (fMRI) studies have shown that MDD is associated with reduced reward-related activation within DA-rich regions, including the ventral (nucleus accumbens, NAc) and dorsal (caudate, putamen) striatum5,6. Notably, such blunting could be normalized by a pharmacological challenge hypothesized to transiently increase DA signaling7, suggesting that blunted striatal responses might be linked to hypodopaminergic mechanisms. Third, animal models relevant to depression reliably induce anhedonic phenotypes and mesolimbic DA abnormalities1,8.
Neurobiologically, these preclinical models trigger long-lasting downregulation of mesolimbic DA pathways8 and reduced levels of the dopamine transporter (DAT)9. Reduced DAT–interpreted as reflecting compensatory DAT downregulation owing to blunted DA transmission–has been reported in the NAc, caudate, or putamen of animals exposed to chronic stressors9–11. Reduced striatal DAT has been also described in rats bred for increased vulnerability to depression12.
The DAT, which is localized on the membrane of presynaptic terminals, plays a central role in regulating the intensity and duration of dopaminergic transmission in the synaptic cleft by re-uptaking DA into presynaptic cells, in both the striatum and midbrain (e.g., ventral tegmental area (VTA)). In addition to regulating clearance of extracellular striatal DA, the DAT modulates the signal-to-noise ratio of DA neurotransmission and affects presynaptic DA levels13. Critically, DAT is regulated by extracellular DA levels. Specifically, DA synthesis depletion reduces striatal DAT density and function14,15, highlighting compensatory DAT downregulation to adjust to reduced DA concentration. Similarly, DAT downregulation was seen in surviving midbrain DA neurons after loss of striatal DA terminals 16. Collectively, these findings highlight plastic changes in DAT levels depending on striatal DA availability.
Despite these preclinical data, evidence from human studies is inconclusive. In vivo evidence from positron emission tomography (PET) or single photon emission computed tomography (SPECT) studies in MDD has been inconsistent. A recent meta-analysis revealed no significant effects17. However, 11 of the included 12 studies used SPECT, which has poorer spatial resolution relative to PET. Moreover, some of the tracers used (e.g., [123I]β-CIT) have incomplete specificity for DAT versus serotonin transporter, which complicates interpretations. This meta-analysis revealed a high degree of heterogeneity across studies, and it is unclear whether clinical heterogeneity contributed to inconsistencies. Similarly, few human postmortem studies have investigated DAT in MDD18, and none has assessed striatal DAT levels in MDD.
Our goal was to address these limitations by using a highly selective DAT tracer ([11C]altropane) in unmedicated individuals with MDD and demographically matched healthy controls. Compared to other DAT tracers, altropane offers several advantages, including a rapid and specific kinetics in DA-rich striatal regions and high selectivity for DAT19,20. Moreover, clinical heterogeneity was addressed by including only unmedicated individuals with MDD (mostly, non-smokers), who were characterized using clinical scales capturing constructs conceptually related to DA, including anhedonia and perception of entrapment. The latter was selected due to a convergence of preclinical and clinical findings. Preclinically, depression-like responses21,22 and DAT downregulation1,8 have been observed in stressful circumstances where escape is blocked. These data have been complemented by human findings that chronic stressors characterized by the perception of being trapped in inescapable situations are particularly depressogenic23,24 and prospectively predicts depression onset25 and re-occurrence26. Finally, DAT expression was evaluated in post-mortem tissues from donors with MDD for independent corroboration. We hypothesized that, relative to healthy controls, MDD would be characterized by lower striatal DAT density, reflecting a compensatory downregulation due to chronically reduced DA signaling.
Materials and Methods
PET study
Participants
Participants included 23 healthy controls and 25 unmedicated individuals with MDD (Table 1). Because DAT expression declines with age27, the age range was restricted to 18–45. Participants provided written informed consent to a protocol approved by the Partners Healthcare IRB. Eligibility was established using the Structured Clinical Interview for the DSM-IV (SCID28) (see eMethods). At screening, participants were administered several clinical scales, including the 17-item Hamilton Depression Inventory29.
Table 1.
Sociodemographic and clinical data in control (n = 25) and MDD (n = 23) subjects
| Control subjects (n = 23) | MDD subjects (n = 25) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Statistics | P value | |
| Agea | 26.49 | 7.26 | 26.52 | 5.92 | t=−0.02 | >0.98 |
| Gender ratio (Female/Male) | 13/10 | N/A | 19/6 | N/A | χ2 = 2.05 | >0.15 |
| Luteal (N, %)b | 5 (41.7%) | 6 (31.6%) | χ2 = 0.33 | >0.55 | ||
| Follicular (N, %) | 7 (58.3%) | 13 (68.4%) | ||||
| Education (years) | 15.67 | 1.93 | 16.40 | 2.42 | t=−1.14 | >0.25 |
| Ethnicity (N, % Caucasian) | 14 (60.9%) | N/A | 18 (72.0%) | N/A | χ2 = 0.67 | >0.40 |
| Marital status (N, % never married) | 17 (73.9%) | N/A | 23 (92.0%) | N/A | χ2 = 2.82 | >0.09 |
| Average Caffeine Consumption (mg/day) | 116.93 | 95.86 | 126.78 | 93.62 | t=−0.36 | >0.72 |
| Caffeine consumed 24 hours before the PET session (mg/day) | 77.55 | 120.84 | 103.60 | 101.73 | t=−0.80 | >0.42 |
| Current smokers (N, %)c | 2 (9.5%) | 3 (12.5%) | χ2 = 1.00 | >0.75 | ||
| Incomed: N, % <$50,000 | 13 (56.5%) | N/A | 16 (69.6%) | N/A | χ2 = 0.93 | >0.25 |
| N, % $50,000–100,000 | 8 (34.8%) | N/A | 6 (26.1%) | N/A | ||
| N, % >$100,000 | 2 (8.7%) | N/A | 1 (4.3%) | N/A | ||
| Age of MDD onset | N/A | N/A | 16.46 | 4.71 | N/A | N/A |
| Lifetime MDEe: 1 MDE | N/A | N/A | 3 | N/A | N/A | N/A |
| 2–3 MDEs | N/A | N/A | 8 | N/A | ||
| ≥5 MDEs | N/A | N/A | 13 | N/A | ||
| HRSD | 0.55 | 1.01 | 17.91 | 3.79 | t=−20.79 | <0.0001 |
| BDI-IIa | 0.48 | 1.31 | 25.80 | 8.65 | t=−8.90 | <0.0001 |
| SHAPSa | 20.74 | 5.54 | 33.20 | 4.11 | t=−13.89 | <0.0001 |
| External Entrapment Scalea | 1.44 | 2.15 | 19.64 | 8.53 | t=−9.94 | <0.0001 |
Notes: HRSD: Hamilton Rating Scale for Depression29; BDI-II: Beck Depression Inventory-II30; SHAPS: Snaith Hamilton Pleasure Scale31; External Entrapment Scale22
Variable assessed at the PET session;
Missing for 1 HC.
Missing for 2 HC and 1 MDD subject.
Missing for 2 MDD subjects.
Missing for 1 MDD subject.
Procedure
Approximately 10 mCi of [11C] altropane was injected intravenously (MDD: 9.00±0.47, controls: 9.19±0.34, p>0.13; range: 8.27–10.22 mCi) and serial PET images acquired. After the scan, participants filled out various questionnaires, including the Beck Depression Inventory-II (BDI-II30) and the Snaith Hamilton Pleasure Scale (SHPS31) to assess levels of depressive symptoms and anhedonia, respectively, as well as the External Entrapment Scale22 (see eMethods).
Apparatus & Data analyses
See eMethods.
Statistics
A multivariate analysis of covariance (MANCOVA) with Hemisphere (left, right), Striatal Region (caudate, putamen, NAc; see eFigure 1) and Group (MDD, healthy controls) as factors and age as covariate was run on binding potential (BPND). For the VTA, BPND values were averaged across the hemispheres, and entered in an ANCOVA with Group as factor (covariate: age). Analyses were also repeated excluding age as a covariate and using partial volume corrected (PVC) data32,33.
For regions showing group differences in BPND, correlation analyses were performed with three clinical scales (BDI, SHAPS, Entrapment Scale) and number of lifetime MDEs. For number of MDEs, because the distribution was skewed the right, we re-binned data by categorizing MDD subjects reporting one single MDE, between 2 and 4 MDE, and 5 or more MDE (see 34). For these analyses, healthy controls were included (MDE=0), and Spearman’s Rank correlations were performed. For the clinical scales, Pearson correlations were run in the MDD group only. All correlation analyses were conducted using age-residualized BPND values.
Postmortem Study
Frozen tissue blocks containing the striatum from 15 depressed individuals and 14 psychiatrically healthy controls were obtained from the Douglas-Bell Canada Brain Bank (see eMethods).
Results
Imaging Studies
Groups were well matched with respect to sociodemographic variables, menstrual cycle, and caffeine consumption (Table 1). At screening, the MDD group had a mean 17-item HRSD score of 17.91 (range: 12–28, SD=3.79), indicating, on average, moderate depression; at the PET session, the MDD group reported, a mean BDI-II score of 25.80, also indicating moderate depression. Four MDD participants had a current comorbidity of social phobia (secondary to MDD) and one MDD participant had a current comorbidity of dysthymic disorders. Forty of 45 participants (88.9%) were non-smokers (smoking status missing for 2 healthy controls and 1 MDD participant).
Group differences in striatal and midbrain DAT BPND
A Group (HC, MDD) x Region (caudate, putamen, NAc) x Hemisphere MANCOVA (covariate: Age) yielded a significant main effect of Region (Wilks’ Lambda (2,44)=34.36, p<10−8), due to significantly higher BPND in the putamen than caudate, which in turn had significantly higher BPND than the NAc (all pairwise Bonferroni-corrected simple effects: ps< 0.00003). The effect of Age (covariate) was also significant (F(1,45)= 7.83, p<0.009), due to decreasing striatal BPND with increasing age. Critically, the Group x Region interaction (Wilks’ Lambda (2,44)=4.15, p<0.023) was significant (Figure 1a). Bonferroni-corrected simple effects revealed that, relative to controls, the MDD group had significantly lower BPND in the bilateral putamen (p<0.029; Cohen’s d=−0.66), whereas groups did not differ in the caudate (d=−0.30) and NAc (d=−0.23) (ps>0.28). When excluding age as a covariate, the Group x Region interaction (F(2,45)=4.20, p<0.021) and reduced bilateral putamen BPND in MDD (p<0.031; Cohen’s d=−0.62) remained, indicating that age was unrelated to the Group x Region. Groups also differed in putamen BPND when using PVC data (t(46)=2.04, p<0.047, d=−0.59).
Figure 1: DAT availability in major depressive disorder.
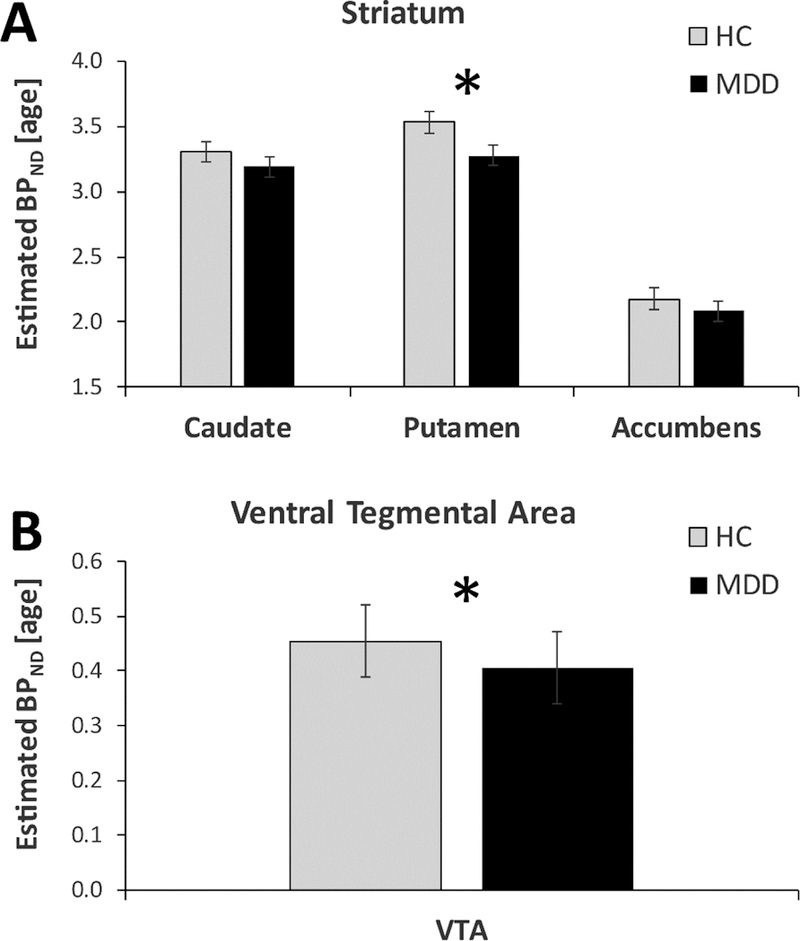
Age-residualized DAT availability (as assessed by binding potential (BPND)) in the healthy control (light gray; n=23) and MDD (black, n=25) groups for (A) striatal regions, (B) ventral tegmental area (VTA).
For the VTA, analyses including (F(1,48)= 6.04, p<0.018, d=-0.71) or excluding (t(46)=2.45, p<0.018; d=-0.71) age as a covariate revealed a main effect of Group, due to overall lower BPND in MDD than controls (Figure 1b). This group difference was not seen, however, when using PVC data (p>0.24). For both the putamen and VTA, all significant effects were confirmed when analyses were re-run only in non-smokers (eResults).
Relations to number of lifetime MDE
Among the MDD subjects, 3 reported one single MDE, 8 had experienced between 2 and 4 MDE, and 13 had experienced 5 or more MDE. Spearman’s Rank correlations among all participants (including HC) indicated that, for both the putamen (Rho=−0.36, p<0.013; Figure 2) and the VTA (Rho=-0.40, p<0.005), age-residualized BPND was negatively correlated with numbers of lifetime MDEs. Because number of MDEs and age could be strongly related, hierarchical regressions entering age in the first step were performed (eResults). For both the putamen and VTA, number of MDEs continued to predict raw (non-age-residualized) BPND (putamen: ΔR2=0.111, p<0.016; VTA: ΔR2=0.125, p<0.014).
Figure 2: DAT availability as a function of number of major depressive episodes.
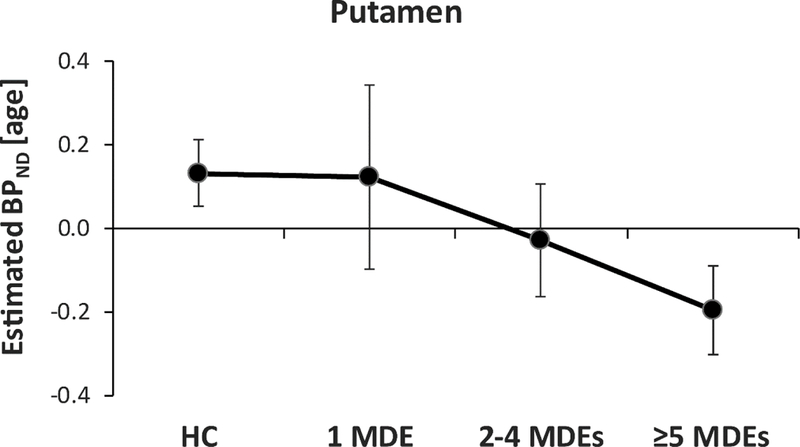
Age-residualized DAT availability (as assessed by binding potential (BPND)) in the mean (averaged across left and right) putamen as a function of lifetime number of major depressive episodes (MDEs).
Relations to Clinical Symptoms (MDD group; n=25)
Owing to the group findings reported above, correlations were performed between (1) mean age-residualized BPND in the putamen or VTA and (2) scores on three clinical scales (SHAPS, BDI, Entrapment scale), leading to a total of 6 tests (Bonferroni correction: p<0.05/6=0.0083). Contrary to hypotheses, no correlations emerged for SHAPS scores (rs<0.38, p>0.062). Mean age-residualized VTA BPND was negatively associated with scores on the External Entrapment Scale (r=−0.43, p<0.032), indicating that increasing perception of entrapment was associated with lower VTA BPND (Figure 3). Highlighting the specificity of this finding, External Entrapment Scale scores predicted VTA BPND even when entering BDI and SHAPS scores in the first step of a hierarchical regression (ΔR2=0.193, ΔF(1,21)=6.31, p<0.020).
Figure 3: DAT availability as a function of perceived entrapment.
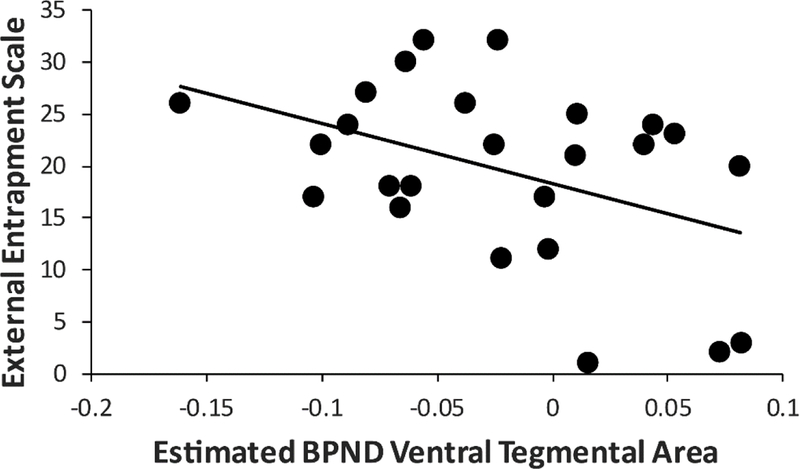
Scatterplot between external entrapment scale score and mean (averaged across left and right) ventral tegmental area (VTA) DAT availability within the MDD group.
Effects of age on striatal and midbrain DAT BPND
See eResults.
Human Postmortem Studies
In light of the PET findings, Western blots on putamen were used to measure DAT expression (the VTA was unavailable). Owing to the hypothesized role of the NAc in MDD, Western blots analyses were also performed on the NAc (see eResults). Depressed and control groups did not differ in age, sex ratio, post-mortem time interval, or pH values (all ps>0.26; see eTable 1).
TH and DAT are decreased in the putamen of individuals with MDD
In the MDD group, TH expression was significantly lower in the putamen (p=0.0071; d=-1.11) (Figure 4, eTable 2). Expression of the mature form of DAT (80 kDa)35,36 were significantly decreased in MDD (p=0.0045, d=−1.20). In addition, DAT 50 kDa (p=0.019; d=−0.99) and 60 kDa (p=0.013, d=-0.99), thought to represent intermediate glycosylated forms, were also significantly decreased. In contrast, the putative DAT precursor (40 kDa) did not differ. For both TH and DAT, none of the covariates significantly affected group effects. Only the TH and 80 kDa group differences survived a Bonferroni correction (p<0.05/5=0.010).
Figure 4: DAT and TH expression in post-mortem analyses.
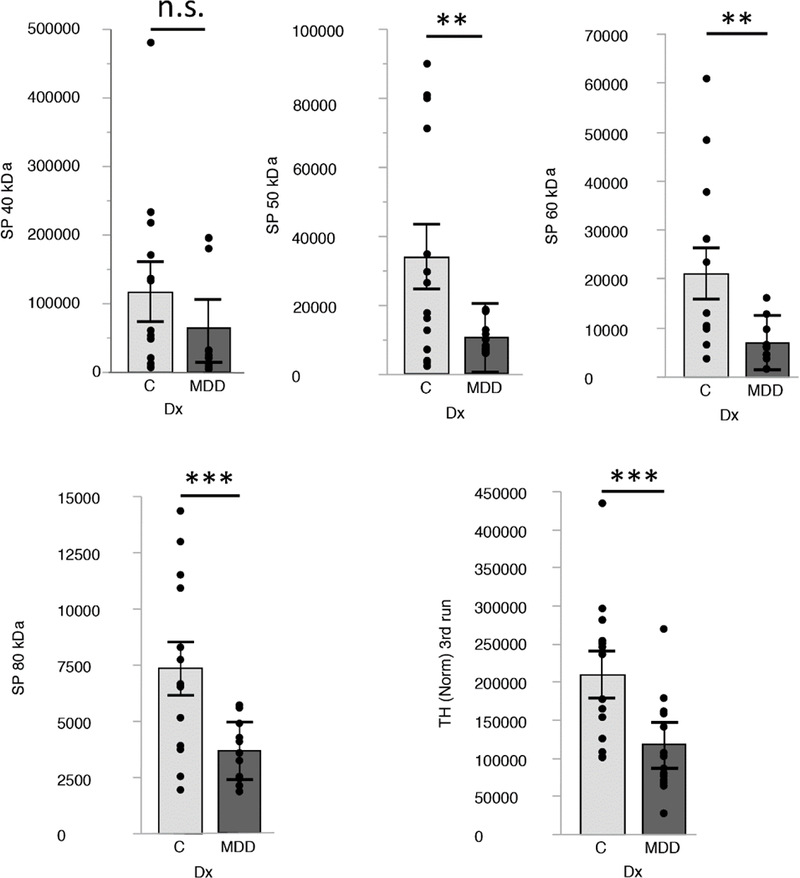
DAT and TH expression levels are decreased in the putamen of people with MDD. Box blots show expression levels (mean and SE) for DAT (40, 50, 60/65 and 80 kDa) and TH (60 kDa). Highly significant changes (p= 0.01 to 0.001) are marked with ** and significant changes to (0.05 to 0.01) are marked with *. Not significant (≥ 0.05; ns). Protein levels are expressed as normalized signal in fluorescence arbitrary units (A.U.; LI-COR Odyssey CLx scanner).
Discussion
Dopamine dysfunction has been implicated in MDD, but in vivo evidence has been elusive1,17. Abundant evidence indicates that DAT plays a pivotal role in synaptic DA regulation, and reflects the integrity and function of the DA system13. Due to evidence that stress-related animal models of depression and pharmacological manipulations depleting DA lead to reduced DAT–interpreted as reflecting a compensatory downregulation to regulate DA levels–we hypothesized that unmedicated individuals with MDD would show blunted striatal and midbrain DAT availability. We further hypothesized that such dysfunction would be greatest in MDD individuals reporting elevated anhedonic symptoms and perceived entrapment (a construct related to helplessness; eMethods). Several important findings emerged.
First, relative to controls, the MDD group had significantly lower DAT availability in the bilateral putamen and VTA. Notably, putamen and VTA availability were inversely related to lifetime number of MDE. Although this latter finding is novel, prospective studies are needed to disentangle whether these findings represent a cumulative effect or potential premorbid marker of increased recurrence risk. Second, unlike controls, the MDD group failed to show age-related declines in DAT availability. Third, contrary to our hypotheses, striatal and midbrain DAT availability was not moderated by anhedonic symptoms. In the VTA, however, a negative correlation between external entrapment scores and VTA DAT availability emerged (eResults). Thus, MDD individuals reporting of being trapped in putatively inescapable circumstances showed the lowest VTA DAT availability. These findings are intriguing, particularly in light of prior reports that external entrapment scores prospectively predicted depression37 and MDD re-occurrence26 12–16 months later. Finally, important corroboration of reduced putamen DAT in MDD emerged in post-mortem analyses.
The current findings of lower striatal DAT availability in MDD agree with a prior PET study38, but findings have been inconsistent17. However, 11 of the 12 studies included in a prior meta-analysis17 as well as 14 of 15 studies included in a recent literature review39 used SPECT and suboptimal DAT tracers. Notably, the only PET study38 reported reduced DAT availability in the putamen in a small MDD group (N=9). In addition to showing a 28-fold selectivity for DAT over the serotonin transporter (vs. 1:1 for [123I]β-CIT and 3:1 for TRODAT; 19,20), altropane accumulates within 30 min almost exclusively to DA-rich striatal regions40, and the putamen:cerebellum ratio is 120:1, highlighting exceptional degree of binding selectivity40. We speculate that the use of this highly selective PET tracer allowed us to more reliably probe DAT function in unmedicated MDD. Our findings fit prior reports of negative correlations between dorsal striatal DAT availability and depressive symptoms in patients with MDD41 and Parkinson’s Disease42. Moreover, recovery from MDD has been associated with an increase in midbrain DAT availability43 and 6-week treatment with escitalopram increased striatal DAT availability by 20%41.
Important strengths of the current study are that all individuals with MDD were unmedicated, had minimal degrees of comorbidity, and the vast majority were non-smokers (88.9%). Focus on an unmedicated sample is particularly important due to evidence that even brief treatment with SSRIs led to a 10–20% increase in striatal DAT availability44.
A novel finding is that, in MDD, perception of being trapped in inescapable circumstances was associated with lower bilateral VTA availability. This link is intriguing, particularly in light of abundant evidence indicating that (1) exposure to stressful situations where escape is blocked suppresses approach behavior and downregulates mesolimbic DA pathways1,8; and (2) stressful life events characterized by entrapment are particularly depressogenic31–34. However, because this correlation finding did not survive correction for multiple comparisons, replication is warranted.
Although PET imaging cannot pinpoint the source of DAT downregulation, increased inflammation and oxidative stress might be candidate mechanisms. Based on preclinical findings16,46, it is possible that increased inflammation and/or oxidative stress contributes to reducing DA signaling, which could eventually result in compensatory DAT downregulation. Consistent with this, mounting evidence points to increased inflammation in MDD47, and inflammation acutely decreases striatal DAT expression levels48. Moreover, and replicating prior findings27, DAT availability was negatively correlated with age among the control–but not MDD–group. Notably, MDD participants below the median age (21.72±1.48 years) were indistinguishable from healthy controls (32.09±6.70 years) and MDD individuals (30.94±4.86 years) older than the median age. Importantly, relatively younger and older MDD individuals did not differ in their number of lifetime MDEs (eFigure 2) and age was not correlated with depressive (HRSD and BDI) or anhedonic (SHAPS) symptoms, indicating that these variables did not confound findings. Larger samples should evaluate whether the current findings reflect accelerated aging in MDD49. Because oxidative stress has been strongly implicated in aging50 and DA uptake has been found to be inhibited by oxidative stress51,52, it is possible that the current striatal and midbrain DAT downregulation might be due to increased oxidative stress in MDD. Two additional, not reciprocally exclusive, potential mechanisms are suggested by postmortem results. First, a disruption of DAT expression may be due to altered posttranslational modifications leading to a decrease of its active, mature form35,36. Our postmortem results show that levels of the immature DAT form were not altered in MDD donors, while increasingly glycosylated forms up to the active 80 kDa form were decreased. These findings suggest that a disruption of glycosylation mechanisms may lead to decreased DAT activity. Notably, reduction of mature (glycosylated) forms of DAT has been described in surviving DA neurons after loss of striatal DA terminals due to an oxidative injury in models of Parkinson’s disease16. Second, concurrent TH and DAT decreases in MDD fit the possibility that DA terminals within the putamen may be reduced in MDD. If so, the result would be an overall decrease of DA tone. This possibility may be at odds with results showing normal levels of the immature forms of DAT, suggesting that dopaminergic terminals are still present in depression, but express an immature, inactive form of DAT. However, it is plausible that both mechanisms may be involved and contribute to our findings. This possibility will be tested in future studies.
Despite strengths, including the inclusion of unmedicated participants, the use of a highly selective PET tracer, groups well matched for variables that could affect DA (e.g., age, menstrual phase, smoking status, caffeine use), and replication of the main finding in post-mortem analyses, limitations should be emphasized. First, the MDD imaging sample was relatively young and moderately depressed; thus, it is unclear whether the current findings will replicate in different samples. This limitation is, at least partially, mitigated by parallel findings in postmortem studies, comprised of older donors with severe MDD, likely to have led to suicide. Although results from postmortem studies cannot exclude that suicide, rather than MDD, is associated with DAT and TH decreases, convergence between imaging and postmortem results suggest otherwise. In addition, in the PET sample, suicidal ideation did not affect results (eResults). Second, information on pharmacological treatment for subjects included in postmortem studies was limited to toxicology data. It is possible that long-term treatment may impact DAT and TH expression. Again, strong similarities between postmortem and imaging findings, the latter including unmedicated subjects, mitigates this concern. Third, our cross-sectional design prevented us to test whether DAT downregulation is a potential cause or consequence of recurrence risk. Fourth, group differences in the VTA were not confirmed when using PVC data, indicating that caution should be used when interpreting VTA findings. Finally, it is unclear why BPND differences emerged in the dorsal, but not, ventral striatum, although it is important to emphasize that, in humans, DAT expression is highest in the dorsal striatum and weakest in the NAc53. These limitations notwithstanding, the current findings provide convergent evidence from in vivo molecular imaging and post-mortem assays that MDD is characterized by DAT downregulation–likely reflecting a compensatory downregulation due to blunted DA signaling within reward-related pathways–particularly with increasing number of prior MDE.
Supplementary Material
Key Points.
Question:
Are unmedicated individuals with major depressive disorder characterized by lower dopamine transporter within the brain reward system relative to psychiatrically healthy control subjects?
Findings:
In both analyses of positron emission tomography and post-mortem data, MDD was linked to lower dopamine transporter in the dorsal striatum. In the imaging data, this dysfunction was exacerbated by more episodes of depression.
Meaning:
Decreased dopamine transporter availability might represent a compensatory down-regulation due to low dopaminergic signaling within mesolimbic pathways.
Acknowledgments
This project was supported by R01 MH068376 and R37 MH068376 from the National Institute of Mental Health (Dr. Pizzagalli). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Drs. Pizzagalli. Berretta and Pilobello (McLean Hospital) and Drs. Wooten and Normandin (MGH) conducted and are responsible for the data analysis. Dr. Pizzagalli had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Trial Registration: NCT01701141
Over the past 3 years, DAP has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Compass, Posit Science, and Takeda Pharmaceuticals for activities unrelated to the current review. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors.
Footnotes
Conflict of Interest
All other authors report no biomedical financial interests.
References
- 1.Pizzagalli DADA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu. Rev. Clin. Psychol 2014;10:393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasler G, Fromm S, Carlson PJ, Luckenbaugh DA, Waldeck T, Geraci M, Roiser JP, Neumeister A, Meyers N, Charney DS, Drevets WC. Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Arch. Gen. Psychiatry 2008;65(5):521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasler G, Luckenbaugh DA, Snow J, Meyers N, Waldeck T, Geraci M, Roiser J, Knutson B, Charney DS, Drevets WC. Reward Processing After Catecholamine Depletion in Unmedicated, Remitted Subjects with Major Depressive Disorder. Biol. Psychiatry 2009;66(3):201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homan P, Neumeister A, Nugent AC, Charney DS, Drevets WC, Hasler G. Serotonin versus catecholamine deficiency: behavioral and neural effects of experimental depletion in remitted depression. Transl. Psychiatry 2015;5(3):e532–e532. doi: 10.1038/tp.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am. J. Psychiatry 2009;166(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry 2009;166(6):702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Admon R, Kaiser RH, Dillon DG, Beltzer M, Goer F, Olson DP, Vitaliano G, Pizzagalli DA. Dopaminergic enhancement of striatal response to reward in Major Depression. Am. J. Psychiatry 2017;174(4):378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabib S, Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci. Biobehav. Rev 2012;36(1):79–89. [DOI] [PubMed] [Google Scholar]
- 9.Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, Sakai RR, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience 2004;124(2):449–457. [DOI] [PubMed] [Google Scholar]
- 10.Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci 2004;19(7):1863–1874. [DOI] [PubMed] [Google Scholar]
- 11.Lucas LR, Wang CJ, McCall TJ, McEwen BS. Effects of immobilization stress on neurochemical markers in the motivational system of the male rat. Brain Res 2007;1155:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiao X, Pare WP, Tejani-Butt S, Paré WP, Tejani-Butt S. Strain differences in the distribution of dopamine transporter sites in rat brain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003;27(6):913–919. [DOI] [PubMed] [Google Scholar]
- 13.Sulzer D, Cragg SJ, Rice ME. Striatal dopamine neurotransmission: Regulation of release and uptake. Basal Ganglia 2016;6(3):123–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon I, Weizman R, Rehavi M. Modulatory effect of agents active in the presynaptic dopaminergic system on the striatal dopamine transporter. Eur. J. Pharmacol 1996;298(1):27–30. [DOI] [PubMed] [Google Scholar]
- 15.Han S, Rowell PP, Carr LA. D2 autoreceptors are not involved in the down-regulation of the striatal dopamine transporter caused by alpha-methyl-p-tyrosine. Res. Commun. Mol. Pathol. Pharmacol 1999;104(3):331–8. [PubMed] [Google Scholar]
- 16.Afonso-Oramas D, Cruz-Muros I, Barroso-Chinea P, de la Rosa DÁ, Castro-Hernández J, Salas-Hernández J, Giráldez T, González-Hernández T. The dopamine transporter is differentially regulated after dopaminergic lesion. Neurobiol. Dis 2010;40(3):518–530. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, He Y, Tang J, Zong X, Hu M, Chen X. Molecular imaging of striatal dopamine transporters in major depression—A meta-analysis. J. Affect. Disord 2015;174:137–143. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald ML, Kassir SA, Underwood MD, Bakalian MJ, Mann JJ, Arango V. Dysregulation of striatal dopamine receptor binding in suicide. Neuropsychopharmacology 2017;42(4):974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischman AJ, Bonab AA, Babich JW, Livni E, Alpert NM, Meltzer PC, Madras BK. [(11)C, (127)I] Altropane: a highly selective ligand for PET imaging of dopamine transporter sites. Synapse 2001;39(4):332–42. [DOI] [PubMed] [Google Scholar]
- 20.Madras BK, Meltzer PC, Liang AY, Elmaleh DR, Babich J, Fischman AJ. Altropane, a SPECT or PET imaging probe for dopamine neurons: I. Dopamine transporter binding in primate brain. Synapse 1998;29(2):93–104. [DOI] [PubMed] [Google Scholar]
- 21.Dixon AK, Fisch HU, Huber C, Walser A. Ethological studies in animals and man, their use in psychiatry. Pharmacopsychiatry 1989;22 Suppl 1(S 1):44–50. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert P, Allan S. The role of defeat and entrapment (arrested flight) in depression: an exploration of an evolutionary view. Psychol Med 1998;28(3):585–598. [DOI] [PubMed] [Google Scholar]
- 23.Brown GW, Harris TO, Hepworth C. Loss, humiliation and entrapment among women developing depression: a patient and non-patient comparison. Psychol Med 1995;25(1):7–21. [DOI] [PubMed] [Google Scholar]
- 24.Keller MC, Neale MC, Kendler KS. Association of different adverse life events with distinct patterns of depressive symptoms. Am. J. Psychiatry 2007;164(10):1521–9. [DOI] [PubMed] [Google Scholar]
- 25.Broadhead JC, Abas MA. Life events, difficulties and depression among women in an urban setting in Zimbabwe. Psychol Med 1998;28(1):29–38. [DOI] [PubMed] [Google Scholar]
- 26.Sturman ED, Mongrain M. Entrapment and perceived Status in graduate students experiencing a recurrence of Major Depression. Can. J. Behav. Sci 2008;40(3):505–19. [Google Scholar]
- 27.Spencer TJ, Biederman J, Madras BK, Dougherty DD, Bonab AA, Livni E, Meltzer PC, Martin J, Rauch S, Fischman AJ. Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol. Psychiatry 2007;62(9):1059–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, William JB. Structure Clinical Interview for DSM-IV-TR Axis I Disorders-Non-patient Edition (SCID-I/NP, 11/2002 revision) New York, NY Biometric Res. Dep. New York State Psychiatr. Inst; 2002. [Google Scholar]
- 29.Hamilton M A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960;23(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck AT, steer RA, Brown GK. Beck Depression Inventory Manual; 1996.
- 31.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry 1995;167(1):99–103. [DOI] [PubMed] [Google Scholar]
- 32.Greve DN, Salat DH, Bowen SL, Izquierdo-Garcia D, Schultz AP, Catana C, Becker JA, Svarer C, Knudsen GM, Sperling RA, Johnson KA. Different partial volume correction methods lead to different conclusions: An (18)F-FDG-PET study of aging. Neuroimage 2016;132:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greve DN, Svarer C, Fisher PM, Feng L, Hansen AE, Baare W, Rosen B, Fischl B, Knudsen GM. Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. Neuroimage 2014;92:225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MT, Chakravarty MM, Dutra SJ, Polli FE, Iosifescu DV, Fava M, Gabrieli JD, Pizzagalli DA. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol. Psychiatry 2015;77(3):285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol. Sci 1993;14(2):43–9. [DOI] [PubMed] [Google Scholar]
- 36.Afonso-Oramas D, Cruz-Muros I, Alvarez de la Rosa D, Abreu P, Giráldez T, Castro-Hernández J, Salas-Hernández J, Lanciego JL, Rodríguez M, González-Hernández T. Dopamine transporter glycosylation correlates with the vulnerability of midbrain dopaminergic cells in Parkinson’s disease. Neurobiol. Dis 2009;36(3):494–508. [DOI] [PubMed] [Google Scholar]
- 37.Griffiths AW, Wood AM, Maltby J, Taylor PJ, Tai S. The prospective role of defeat and entrapment in depression and anxiety: A 12-month longitudinal study. Psychiatry Res 2014;216(1):52–59. [DOI] [PubMed] [Google Scholar]
- 38.Meyer JH, Kruger S, Wilson AA, Christensen BK, Goulding VS, Schaffer A, Minifie C, Houle S, Hussey D, Kennedy SH. Lower dopamine transporter binding potential in striatum during depression. Neuroreport 2001;12(18):4121–4125. [DOI] [PubMed] [Google Scholar]
- 39.Nikolaus S, Mamlins E, Hautzel H, Müller H-W. Acute anxiety disorder, major depressive disorder, bipolar disorder and schizophrenia are related to different patterns of nigrostriatal and mesolimbic dopamine dysfunction. Rev. Neurosci 2018;0(0). doi: 10.1515/revneuro-2018-0037. [DOI] [PubMed] [Google Scholar]
- 40.Madras BK, Gracz LM, Meltzer PC, Liang AY, Elmaleh DR, Kaufman MJ, Fischman AJ. Altropane, a SPECT or PET imaging probe for dopamine neurons: II. distribution to dopamine-rich regions of primate brain. Synapse 1998;29(2):105–115. [DOI] [PubMed] [Google Scholar]
- 41.Rominger A, Cumming P, Brendel M, Xiong G, Zach C, Karch S, Tatsch K, Bartenstein P, la Fougère C, Koch W, Pogarell O. Altered serotonin and dopamine transporter availabilities in brain of depressed patients upon treatment with escitalopram: A [123I]β-CIT SPECT study. Eur. Neuropsychopharmacol 2015;25(6):873–881. [DOI] [PubMed] [Google Scholar]
- 42.Hesse S, Meyer PM, Strecker K, Barthel H, Wegner F, Oehlwein C, Isaias IU, Schwarz J, Sabri O. Monoamine transporter availability in Parkinson’s disease patients with or without depression. Eur. J. Nucl. Med. Mol. Imaging 2009;36(3):428–435. [DOI] [PubMed] [Google Scholar]
- 43.Laasonen-Balk T, Viinamäki H, Kuikka JT, Husso-Saastamoinen M, Lehtonen J, Tiihonen J. 123I-beta-CIT binding and recovery from depression. A six-month follow-up study. Eur. Arch. Psychiatry Clin. Neurosci 2004;254(3):152–5. [DOI] [PubMed] [Google Scholar]
- 44.Merens W, Booij L, Van Der Does AJ. Residual cognitive impairments in remitted depressed patients. Depress. Anxiety 2008;25(6):E27–36. [DOI] [PubMed] [Google Scholar]
- 45.Siddaway AP, Taylor PJ, Wood AM, Schulz J. A meta-analysis of perceptions of defeat and entrapment in depression, anxiety problems, posttraumatic stress disorder, and suicidality. J. Affect. Disord 2015;184:149–159. [DOI] [PubMed] [Google Scholar]
- 46.Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, Freeman AA, Rye DB, Goodman MM, Howell LL, Miller AH. Chronic interferon-α decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology 2013;38(11):2179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Current Topics in Behavioral Neurosciences 2012;14:135–151. [DOI] [PubMed] [Google Scholar]
- 48.Lai Y-T, Tsai Y-PN, Cherng CG, Ke J-J, Ho M-C, Tsai C-W, Yu L. Lipopolysaccharide mitagates methamphetamine-induced striatal dopamine depletion via modulating local TNF-α and dopamine transporter expression. J. Neural Transm 2009;116(4):405–415. [DOI] [PubMed] [Google Scholar]
- 49.Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress. Anxiety 2010;27(4):327–338. [DOI] [PubMed] [Google Scholar]
- 50.Barja G Free radicals and aging. Trends Neurosci 2004;27(10):595–600. [DOI] [PubMed] [Google Scholar]
- 51.Berman SB, Zigmond MJ, Hastings TG. Modification of dopamine transporter function: effect of reactive oxygen species and dopamine. J. Neurochem 1996;67(2):593–600. [DOI] [PubMed] [Google Scholar]
- 52.Fleckenstein AE, Metzger RR, Beyeler ML, Gibb JW, Hanson GR. Oxygen radicals diminish dopamine transporter function in rat striatum. Eur. J. Pharmacol 1997;334(1):111–4. [DOI] [PubMed] [Google Scholar]
- 53.González-Hernández T, Barroso-Chinea P, de la Cruz Muros I, del Mar Pérez-Delgado M, Rodríguez M. Expression of dopamine and vesicular monoamine transporters and differential vulnerability of mesostriatal dopaminergic neurons. J. Comp. Neurol 2004;479(2):198–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


