Abstract
Processing and maturation of precursor RNA species is coupled to RNA Polymerase II transcription. Co-transcriptional RNA processing helps to ensure efficient and proper capping, splicing, and 3’ end processing of different RNA species to help ensure quality control of the transcriptome. Many improperly processed transcripts are not exported from the nucleus, are restricted to the site of transcription, and are in some cases degraded, which helps to limit any possibility of aberrant RNA causing harm to cellular health. These critical quality control pathways are regulated by the highly dynamic protein-protein interaction network at the site of transcription. Recent work has further revealed the extent to which the processes of transcription and RNA processing and quality control are integrated, and how critically their coupling relies upon the dynamic protein interactions that take place co-transcriptionally. This review focuses specifically on the intricate balance between 3’ end processing and RNA decay during transcription termination.
Keywords: RNAPII, RNA processing, co-transcriptional, quality control, capping, splicing, 3’ end processing, termination, transcription
Graphical Abstract
Dynamic coupling of RNAPII transcription complex and RNA processing machinery is the first-line of defense for nuclear RNA quality control.
Introduction
RNA polymerase II (RNAPII) is one of three major eukaryotic DNA-dependent RNA polymerases and is responsible for transcribing several RNA species, including messenger RNA (mRNA), noncoding RNA (ncRNA), and small nuclear/nucleolar RNAs (sn/snoRNA) (Tan-Wong, 2012; Wyers, 2005). Plants have two additional RNA Polymerases which were derived from RNAPII that have been reviewed in depth previously (Haag, 2011; Zhou, 2015). Transcription by RNAPII consists of three basic phases: initiation, RNAPII recruitment to the promoter, and synthesis of the first few RNA nucleotides; elongation, RNAPII moving further into the gene in the context of chromatin and extending the nascent RNA transcript; and termination, release of RNAPII and the fully processed nascent RNA transcript from the template DNA. Each of these steps requires a distinct set of proteins that aid and regulate RNAPII to ensure proper transcription and gene expression.
The majority of RNAPII transcripts need to be processed before serving their cellular purpose, and their class specific processing occurs in tandem with the transcription of nascent RNA. RNA processing machinery is recruited to RNAPII at the site of transcription and the success of processing is inherently linked to the progression of transcription and regulatory proteins the two processes share. The nature and extent of processing depends on the species of RNA, but the major processes are: 5’ end capping (Cho, 1997; McCracken, 1997a), splicing (Carrillo Oesterreich, 2010; Misteli, 1999), and 3’ end processing (Ahn, 2004; Kim, 2004a; Licatalosi, 2002). Under normal biological conditions, if RNA is not properly co-transcriptionally processed, it will be degraded often at or near the site of transcription. Although not often thought of in the context of quality control, proper processing ensures that the necessary transcripts are able to be exported from the nucleus and translated and is therefore essential to the maintenance of the transcriptome, and ultimately the proteome. Because it is so critical in determining the fate of nascent RNA, co-transcriptional processing mechanisms and their regulation will be highlighted in this review as primary steps in quality control. RNA transcripts that contain transcription errors or are improperly processed pose a threat to cell health. If these erroneous and/or unprocessed transcripts are not degraded, they can cause disease through multiple mechanisms such as non-functional protein expression, DNA damage through R-loop accumulation, and downregulation of functional protein expression through nuclear and/or cytoplasmic mRNA decay pathways (Bresson 2018). Degradation of erroneous transcripts in the nucleus is an important quality control strategy, and its intricate relationship with RNA processing will be prominently featured in this review.
The proteins that regulate co-transcriptional processing and degradation need to be recruited to the site of transcription at the appropriate time and perhaps withdraw immediately after their purpose is served to clear the way for the next wave of RNAPII interacting proteins. Proteins move on and off RNAPII during the transcription cycle, creating a highly dynamic interaction network at the site of transcription that governs the quality of the transcribed RNA (Kim, 2004a; Mayer, 2012; Mayer, 2010). This flexible precision is critical to ensure quality control of nascent transcripts in order to maintain proper gene expression. Altogether, this review will focus on the major protein groups that regulate quality control of RNA through balancing co-transcriptional RNA processing with degradation during eukaryotic RNAPII transcription termination. For more information on post-transcriptional RNA quality control refer to: Halbeisen, 2008; Inada, 2013; and/or Schaefke, 2018.
RECRUITMENT 101: UTILIZING THE C-TERMINAL DOMAIN AND PAUSING
Phosphorylation of the RNAPII CTD
RNAPII is distinguished from RNA polymerases I and III by the conserved C-terminal domain (CTD) of its largest subunit, Rpb1, which plays an integral role in the recruitment of proteins to RNAPII. The CTD consists of repeats of the peptide Y1S2P3T4S5P6S7, and is conserved from fungi to humans with variations in repeat number (Ahearn, 1987; Eick, 2013; Stiller, 2002; Yang, 2014). Five out of seven of the residues in this repeat can be phosphorylated, and the dynamic phosphorylation status of these residues throughout the transcription cycle is responsible for the specific recruitment of numerous regulatory proteins. Proteins with CTD-binding domains have binding affinity preferences for different phosphorylation patterns; as the pattern is modified throughout the transcription cycle, the CTD acts as a “landing pad” (Buratowski, 2003; Buratowski, 2009; Greenleaf, 1993) for the dynamic interactors at the site of transcription. Indeed, deletion of the RNAPII CTD has been shown to inhibit co-transcriptional processing of nascent RNA (Fong, 2001; McCracken, 1997b).
RNAPII exists in a hypo-phosphorylated state when it is recruited to the promoter by the preinitiation complex. However, very early in transcription CTD serine 5 (Ser5) levels rise and peak in early elongation. Ser5 phosphorylation is linked to recruiting both elongation factors and RNA processing proteins, such as the 5’ end capping complex (Ghosh, 2011; Komarnitsky, 2000) and the spliceosome (Nojima, 2018). Ser5 levels have also been shown to peak at actively spliced exons (Nojima, 2015) helping to recruit the spliceosome and regulate splicing (Harlen, 2016). As elongation proceeds, Ser5 phosphorylation levels fall, while serine 2 (Ser2) levels rise. This shift in the phosphorylation dynamic is integral in continued regulation of splicing, and recruitment of transcription termination factors and proteins involved in 3’ end processing and polyadenylation of the nascent RNA (Ahn, 2004; Davidson, 2014). Phosphorylation of both Tyr-1 and Thr-4 in the CTD repeats has also been shown to play a regulatory role in RNAPII transcription termination suggesting that this step, which is intimately coupled to mRNA 3’ end processing, is tightly regulated by the CTD phosphorylation state (Hsin, 2011; Mayer, 2012; Nemec, 2017; Schreieck, 2014). Finally, Ser-7 phosphorylation has been implicated in snRNA processing and recruitment of the Integrator complex (Egloff, 2007). Thus, the CTD has been shown to activate all three major RNA processing pathways, fitting in with the “recruitment model” (Bentley, 2014) of coupling RNA processing to transcription. Zaborowska, et al. reviews the “CTD code” in more depth (Zaborowska, 2016).
Pausing of RNAPII
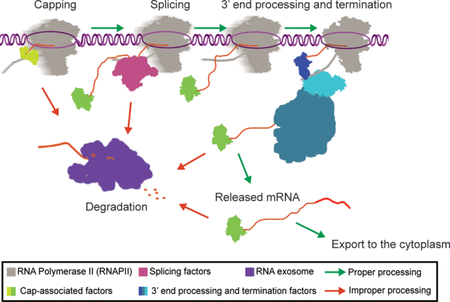
The “kinetic competition model” poses that as transcription occurs and the nascent RNA is extended, RNA-binding proteins are able to bind the transcript in a sequence specific (or non-specific) manner (Dujardin, 2013; Nilsen, 2010). The rate at which these binding sequences within the nascent RNA are synthesized during transcription elongation may allow competition between RNA-binding regulatory proteins that play important roles in RNA processing, RNAPII termination, and quality control checkpoints (Roberts, 1998). Since the rate of transcription has an impact on the kinetic recruitment of proteins to the site of transcription, an important consideration is RNAPII pausing. Pausing, or accumulation of RNAPII on DNA, can occur at any stage of transcription and provide a window of opportunity for recruitment of factors involved in the modulation of gene expression and co-transcriptional RNA processing (Henriques, 2013; Svejstrup, 2007). RNAPII pausing is associated with the three major co-transcriptional processing pathways, RNA-capping (Rasmussen, 1993), splicing (Alexander, 2010), and 3’ end processing, as well as RNA proofreading and degradation (Glover-Cutter, 2008; Kireeva, 2008; Nudler, 2012). This coupling of RNA processing to RNAPII activity may ensure that the nascent RNA is protected from degradation and efficiently matures into a functional mRNA (Fig. 1).
Figure 1: RNA processing and degradation.
Processing and degradation factors are recruited to the site of transcription. Shown are two polymerases moving in opposite directions demonstrating each of the major RNA processing pathways (as described in the key).
In metazoans, RNAPII pausing proximal to the promoter is a regulatory step in transcription for the majority of protein coding genes (Muse, 2007; Nechaev, 2010) and is thought to be used as a rate-limiting elongation checkpoint that can hold back RNAPII to give a “window of opportunity” for the recruitment of factors needed for transcription elongation and/or co-transcriptional RNA processing (Adelman, 2012; Henriques, 2013; Valen, 2011). Pausing may provide a timing opportunity and an interaction surface to facilitate capping, as interactions have been reported between the capping machinery and the pause-regulatory factor/positive transcription elongation factor, DSIF (Spt4/5) (Adelman, 2012; Mandal, 2004; Moore, 2006). Positive elongation factor b (P-TEFb) has been shown to phosphorylate DSIF and NELF to trigger pause release. The regulation of promoter proximal pause release may ensure that RNAPII does not proceed into productive elongation before it is appropriately modified for binding by the RNA processing factors and may provide a binding platform later in transcription for complexes carrying out 3’ end processing (Buratowski, 2009). Of note, recent findings have suggested that RNAPII release from pausing at highly abundant promoter proximal pause sites may occur through premature transcription termination up to 99% of the time (Erickson, 2018; Steurer, 2018). These findings suggest that termination is the predominant form of RNAPII removal from promoter proximal pause sites rather than release into productive elongation.
It is possible that the promoter proximal pause site could serve as a key location for mRNA 5’ capping quality control. This possibility is supported in elegant work showing that inhibition of the Cdk9 kinase in Schizosaccharomyces pombe leads to significant decreases in the phosphorylation of the elongation factor Spt5, which has been implicated in both promoter proximal pausing and the recruitment of capping enzyme components (Booth, 2018; Pei, 2003; Viladevall, 2009). The biological mechanisms that could underlie a large degree of RNAPII turnover at promoter proximal pause sites have not been explored, but this topic remains controversial since other groups have reported measurement of stable RNAPII pausing as a potential mechanism to poise the transcription machinery for rapid induction. Regardless, rapid RNAPII removal from promoter proximal pause sites could involve unique termination mechanisms such as potential termination coupled RNA degradation by the exosome, which to date has not been explored in metazoan cells (Lemay, 2014; Fox, 2015). RNAPII has also been shown to pause at a variety of splice sites throughout the genome and also once it reaches the 3’ end of genes and various polyadenylation sites (Kwak, 2013; Mayer, 2015; Nojima, 2015). Pausing is an important intermediate step leading to termination in many mammalian genes, providing the opportunity for the termination machinery to be recruited to chromatin (Andrulis, 2002; Gusarov, 1999). Additionally, it has been shown that RNAPII pausing sites change positions when alternative polyadenylation sites are used in cells (Fusby, 2016). Details regarding the role of pausing during each processing step are provided in their respective sections. Pausing and its effects on transcription have recently been reviewed in (Adelman, 2012; Chen, 2018; Mayer, 2017).
THE RNA EXOSOME: THE CLEAN-UP CREW
Proper maturation and processing of the mRNA lends protective features to the transcripts that keep them from being degraded. The major quality control mechanism for aberrant RNA is degradation. However, degradation is not solely restricted to incorrectly made or processed RNAs, as it also occurs during routine processing in the case of many ncRNAs. Arguably, the most significant role for degradation in RNA quality control is its role in the removal of RNAs produced from pervasive transcription. There are both nuclear and cytoplasmic RNA degradation systems, but this review will only focus on the nuclear mechanisms due to their connection to transcription and co-transcriptional RNA processing.
Processing transcripts while they are still attached to chromatin provides additional checkpoints for the removal of unprocessed and potentially deleterious transcripts and avoids wasteful transcription. The RNA exosome is a multi-subunit 3’−5’ exonuclease complex that has been shown to have a large number of regulatory roles in RNA biology including, 3’ end processing and the degradation of ncRNAs and unstable transcripts (Allmang, 1999; Chlebowski, 2013; Mitchell, 1997). The exosome is responsible for degrading mRNA transcripts that have been improperly processed (Bitton, 2015; Bousquet-Antonelli, 2000; Gudipati, 2012; Schneider, 2012; Szczepinska, 2015), including: lncRNAs (Pefanis, 2015; Wlotzka, 2011), cryptic unstable transcripts (CUTs) (Davis, 2006; Szczepinska, 2015; Wyers, 2005) and their human counterparts promoter upstream transcripts (PROMPTs) (Preker, 2008; Preker, 2011), and heterochromatin-forming repetitive elements such as rRNA and centromeres (Buhler, 2007; Houseley, 2007; Vasiljeva, 2008b). Defective transcripts that are destined for exosomal degradation often accumulate and result in the retention of RNAPII at the site of transcription (de Almeida, 2010; Eberle, 2010; Hilleren, 2001). Exosome-dependent degradation at the site of transcription could have an array of consequences such as increases in the local concentrations of nucleotides, which could facilitate RNAPII transcription at nearby genes.
The exosome has two catalytic subunits responsible for degradation of RNA, Dis3 and Rrp6, with Rrp6 able to function independently of the exosome core particle (Chlebowski, 2013). However, the exosome requires all subunits plus cofactors for optimal activity and appropriate substrate selection. Four such cofactors are the Trf4/5-Air1/2-Mtr4 polyadenylation (TRAMP) complex, the yeast Nrd1-Nab3-Sen1 (NNS) complex, and the human nuclear exosome targeting (NEXT) and poly-A tail exosome targeting (PAXT) complexes. The exosome and its cofactors were recently reviewed in Zinder, 2017. The TRAMP complex interacts with the exosome to both increase the hydrolytic activity of Rrp6 (Callahan, 2010) and to add short polyA tails to RNA substrates in order to make them more accessible for degradation (Schmidt, 2013). TRAMP function is generally coupled to that of the NNS complex (Arigo, 2006b; Schulz 2013; Thiebaut, 2006), which is further discussed later in this review. The exosome has an established role in post-transcriptional quality control (Lemieux, 2011; Schneider, 2012; Wang, 2008), but there is also evidence showing that Rrp6 and the exosome are recruited to transcribed genes (Andrulis, 2002; Hessle, 2012; Hieronymus, 2004; Lim, 2013), and that the exosome interacts with elongating RNAPII in metazoans (Andrulis, 2002).
Transcriptome analysis of quality control mutants revealed an important role for nucleases in removing aberrant mRNA species (Davis, 2006; Gudipati, 2012; Schneider, 2012; Wyers, 2005). The accumulation of RNA as a result of exosome mutations can have a variety of negative effects on the transcriptome. Aberrant RNA accumulation can negatively impact the cell in multiple ways that include: competing with properly processed RNA for RNA binding proteins (Coy, 2013), activating antiviral defense mechanisms that trigger autoimmunity (Eckard, 2014), or producing DNA-RNA hybrids (R-loops) that induce double-stranded DNA breaks and chromatin instability (Wahba, 2013). Mutations in the exosome have been linked to a variety of human pathologies, particularly to spinal motor neuron disorders such as pontocerebellar hypoplasia type 1B (Boczonadi, 2014; Wan, 2012). It has also been seen that Rrp6 mediates the transcriptional silencing of HIV-1 promoter and that the loss of Rrp6 function leads to a loss in inhibition of the HIV-1 promoter (Wagschal, 2012). Additionally, a mouse model with a mutation in the exosome presents with B lymphocyte dysfunction (Pefanis, 2014). It has been postulated that the phenotype is due to the fact that the exosome is required for class switch recombination and somatic hypermutation, both of which are necessary for antibody diversity. The multitude of disease phenotypes produced from defects in the exosome highlights the importance of proper quality control of nascent RNA transcripts. The following sections will highlight the different RNA processing reactions and their interconnectedness with degradation for proper RNA quality control. There are many recent reviews that cover RNA degradation and decay in more detail, and a selection is listed here: Bresson, 2018; Karousis, 2016; Palumbo, 2015; Schmid, 2018; Schmidt, 2013.
IN BRIEF: 5’ CAPPING AND SPLICING
Co-transcriptional capping and the initiation-elongation transition
Shortly after the 5’ end of the nascent transcript emerges from the RNA exit channel, a 7-methyl guanosine cap is added (Perales, 2009; Rasmussen, 1993). There are three steps to the capping process: conversion of the tri-phosphate group at the 5’ end of the nascent transcript to a diphosphate group; attachment of a GMP molecule; and methylation of the N7 atom of the guanosine base to produce a mature cap (Furuichi, 2000; Shuman, 1995). In yeast, two proteins (Cet1 and Ceg1) form a heterodimer capping complex, while mammals have one bifunctional enzyme for the first two steps (Itoh, 1987; Yue, 1997). In the nucleus, the 5’ cap protects against 5’−3’ exonucleolysis (Wilusz, 2001) and plays roles in processes such as pre-mRNA splicing (Izaurralde, 1994), 3’ end formation (Flaherty, 1997), and RNA export (Izaurralde, 1995). A combination of cryo-electron microscopy (Cryo-EM) and crosslinking mass spectrometry (XL-MS) determined the open and closed states of the capping enzyme (CE) in yeast and illuminated the position CE takes at the end of the RNAPII exit tunnel with its active sites facing the nascent RNA (Martinez-Rucobo, 2015). Their model provides a structural basis for understanding how capping continuously protects the 5’ end of the RNA from exonucleases. For more information on the function of the cap and cap-binding proteins refer to (Cougot, 2004; Topisirovic, 2011).
Nuclear decay systems take advantage of co-transcriptional RNA processing to assess the quality of nascent RNA and degrade any non-optimal transcripts (Schmid, 2018). Mutations that lead to improper capping, whether directly or indirectly, result in mRNA decay and can cause premature termination of transcription (Jimeno-Gonzalez, 2010). These degradative quality control mechanisms are known to involve the 5’−3’ exonuclease Xrn2 (also known as Rat1), the decapping endonuclease Rai1 (also known as Dxo), and the Rai1 homolog Dxo1 that has both decapping and 5’−3’ exoRNAse activity (Brannan, 2012; Chang, 2012; Jiao, 2010; Xiang, 2009). Rai1 is already known to bind and stimulate the activity of Xrn2 for RNAPII transcription termination (Kim, 2004b; Xue, 2000). Rai1 has been shown to convert the 5’-triphosphate into monophosphates that can target these transcripts Xrn2 for degradation (Jiao, 2010; Xiang, 2009). The mammalian Rai1 homolog, DOXO, has pyrophosphatase, decapping, and exoribonuclease activity, and has been shown to have the ability to prepare both uncapped and unmethylated-capped RNAs for degradation (Jiao, 2013; Xiang, 2009). Co-deletion of yeast Rai1 and Dxo1 leads to accumulation of incompletely capped RNAs; depletion of the human homolog DXO leads to accumulation of aberrantly capped, unspliced, and inefficiently 3’ end cleaved RNAs (Chang, 2012; Jiao, 2013). So, it is thought that Rai1 and Dxo1 play critical roles in terminating RNAPII and degrading improperly capped nascent transcripts (Chang, 2012; Xiang, 2009). However, it is unknown how this process precisely intersects with the control of promoter proximal pause release and/or premature RNAPII termination. The process of decapping is also a major regulatory step for mRNA degradation, but occurs in the cytoplasm, and so it is not extensively reviewed here, but is addressed in the following publications: (Coller, 2004; Franks, 2008; Grudzien-Nogalska, 2017; Song, 2010; Zhai, 2014).
Co-transcriptional splicing
The splicing of introns out of mRNA occurs at many genes in eukaryotes coordinately with RNAPII transcription elongation (Carrillo Oesterreich, 2016). Alternative splice variants provide the opportunity for expanded diversity within the proteome without extensive genomic expansion. In order to ensure proper splicing, this processing event is often directly coupled to transcription. Electron microscopy studies in both Drosophila melanogaster and Chironomus tentans demonstrated that splicing does occur co-transcriptionally (Bauren, 1994; Beyer, 1988). Sequencing experiments in human (Ameur, 2011; Tilgner, 2012; Windhager, 2012), mouse (Khodor, 2012), yeast (Carrillo Oesterreich, 2010), and fly (Khodor, 2011) model systems have also shown that an extensive amount of splicing occurs co-transcriptionally. In their 2018 paper, Burke et al. developed a sequencing method to globally profile spliceosome bound pre-mRNA, intermediates, and spliced mRNA at single nucleotide resolution through combining biochemical purification of endogenous spliceosomes sequencing (Burke, 2018). This new method provides a tool for quantifiable studies of previously hard to identify RNA species and allowed measurement of differential splicing between three yeast species. Additionally, this method provides the ability to investigate splicing regulation through intron retention, which was previously not possible due to the transient nature of these RNA species and mutations that had to be made for stabilization. Further advances in our understanding of splicing have occurred through recently solved structures characterizing the spliceosome in different forms (Agafonov, 2016; Bertram, 2017a; Bertram, 2017b, Finci, 2018; Galej, 2016; Nguyen, 2015; Nguyen, 2016b; Ohi, 2007; Pomeranz Krummel, 2009; Wan, 2016; Yan, 2015). More in-depth reviews of structural studies of the spliceosome can be found here: (Fica, 2017; Nguyen, 2016a). Although not all splicing occurs co-transcriptionally, it is likely that most spliceosomes assemble on the nascent transcript (Pandya-Jones, 2009) with support from co-immunoprecipitation studies using antibodies specific for active spliceosomes that showed 80% of active spliceosomes to be bound to chromatin in HeLa cells (Girard, 2012). Herzel et al. recently reviewed co-transcriptional spliceosome assembly and function (Herzel, 2017).
Intron-containing mRNA is particularly prone to nuclear degradation (Kilchert, 2015). Mutations that delay splicing exacerbate this effect, and so unspliced or improperly spliced pre-mRNAs have been shown to be rapidly degraded (Danin-Kreiselman, 2003; Gudipati, 2012; Lemieux, 2011). However, the precise mechanisms that target intron containing mRNA for degradation and the factors involved in this nuclear quality control pathway are still unknown (Bresson, 2018). Failure to splice pre-mRNA may trigger any of the following responses in addition to others: transcription downregulation (Damgaard, 2008); failure of mRNA export (Luo, 1999; Reed, 2002); pre-mRNA degradation from the 5’ and 3’ ends by either Xrn2 and the nuclear exosome (Bousquet-Antonelli, 2000; Kufel, 2004) or via endonucleolysis by Swt1 (Skruzny, 2009); and post-transcriptional anchoring of the unspliced pre-mRNA at the NPC via the Mlp proteins (Galy, 2004). It has also been shown that defects in snRNP assembly lead to degradation by two distinct quality control mechanisms, either through the actions of the TRAMP complex and the RNA exosome or via decapping and 5’ to 3’ decay by Xrn1 (Shukla, 2014). Additionally, depletion of either core splicing or transcription termination components in Drosophila have been shown to increase the production of circular RNAs which occur through backsplicing reactions (Liang, 2017). Considering that many splicing reactions have been shown to occur efficiently during transcription elongation, backsplicing should be a rare event that occurs only when splicing rates are slowed to an extent allowing production of a downstream splice site donor before removal of an upstream intron with an intact splice acceptor (Wilusz, 2018). While backsplicing could be a normal scenario for some poorly spliced transcripts, the creation of circular RNAs may also serve as a fail-safe and/or quality control mechanism for a larger number of mRNAs when splicing and/or termination pathways are disrupted. This review does not delve in depth into the mechanisms of splicing however the following reviews on this topic are recommended: (Kaida, 2016; Sperling, 2017; Wilusz, 2018; Woodward, 2017).
THE END OF THE LINE: 3’ END PROCESSING AND DEGRADATION
The mechanisms of termination of RNAPII transcription are still not fully understood in eukaryotes although there is a wide array of knowledge on the factors that are required for termination to occur (Fong, 2015; Zhang, 2015). Termination of transcription occurs when RNAPII stops nucleotide addition to the nascent RNA and both the RNA transcript and RNAPII are released from the DNA template. Termination is highly dynamic and can occur at multiple sites within a single gene and is also coupled to mRNP export (Gilbert, 2004; Johnson, 2009; Luo, 2006). In order to be dynamic and flexible, termination is an extremely complex process with pathways that depend on numerous regulatory proteins (>100 proteins involved at protein coding genes in humans) (Shi, 2009). These multiple pathways for termination involve many proteins required for both for RNAPII termination and for RNA processing (Arndt, 2015; Porrua, 2015; Shi, 2009), as termination is inherently tied to co-transcriptional mRNA 3’ end processing. Many termination factors interact with RNA processing and degradation enzymes and will be discussed below. The decision as to which termination pathway is utilized has a large influence on the future of an RNA: stabilization and protection versus degradation.
Cleavage and polyadenylation of mRNAs
The major 3’ end processing pathway used in the context of mRNAs, but also utilized for some ncRNAs, is cleavage followed by polyadenylation of the resulting 3’-OH by polyA polymerase (Fig. 2) (Kuehner, 2011; Mischo, 2013; Xiang, 2014). In metazoans, the polyA polymerase interacts with a protein complex known as the cleavage and polyadenylation specificity factor (CPSF) which contains subunits responsible for recognition of the AAUAAA hexanucleotide. The metazoan cleavage complex is also known to contain: cleavage stimulation factor (CstF), cleavage factor I (CFI), and cleavage factor II (CFII). Similarly, in yeast the cleavage and polyadenylation factor (CPF) is made up of various sub-assemblies of protein complexes and interacts with a cleavage factor known as cleavage factor Ia (CFIa). Recent cryo-EM, XL-MS, and non-covalent nanoelectrospray ionization mass spectrometry (nanoESI-MS) studies of yeast CPF revealed a high degree of modularity within CPF, which has also been suggested for mammalian CPSF, by extensive biochemical analysis (Casanal, 2017; Hernández, 2007). Three modules were identified for S. cerevisiae CPF: nuclease, phosphatase, and polyA polymerase. The polyA polymerase module is very similar to the minimal mammalian components of CPSF needed for polyadenylation, suggesting high conservation between the yeast and metazoan machinery (Schonemann, 2014). Several components of the full cleavage and polyadenylation factor-cleavage factor (CPF-CF) complex both bind to RNAPII and recognize termination and processing signals in the 3’ UTR of the nascent RNA (Baejen, 2014; Pearson, 2014; Xiang, 2014). RNAs polyadenylated by CPF-CF are rapidly exported to the cytoplasm if they pass quality control steps (Mouaikel, 2013; Zenklusen, 2008). Mutants in the CPF-CF pathway in yeast can lead to accumulation of polyA RNA in the nucleus as a consequence of defective coupling of transcription termination and mRNA export (Amberg, 1992; Hammell, 2002). Furthermore, it was recently shown in human cells that cleavage via the CPSF processing endonuclease CPSF73 is required for efficient termination of protein-coding genes, as loss of CPSF73 activity lead to termination defects and increased readthrough transcription (Eaton, 2018). The increased readthrough transcription in mutants in the CPF complex can also lead to increased production of exonuclease-resistant circular RNAs, discussed in more detail below (Liang, 2017).
Figure 2: Cleavage and polyadenylation.
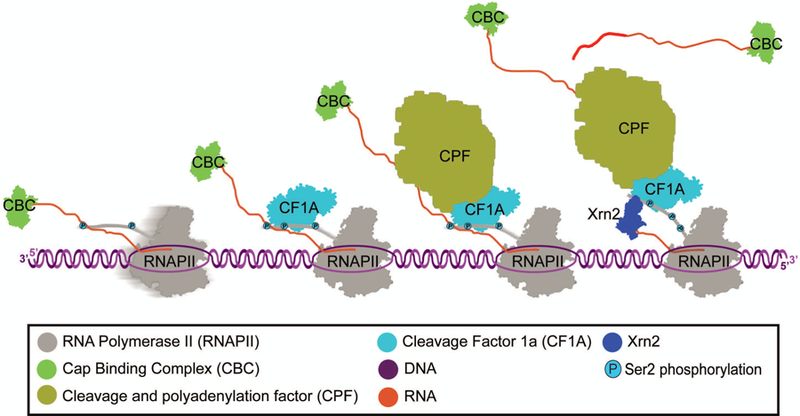
Cap binding complex (CBC) binds 5’ guanosine cap. Cleavage factor 1A (CF1A) is recruited to Ser2 phosphorylated CTD of RNAPII. Cleavage and polyadenylation factor (CPF) is recruited and cleaves RNA after polyadenylation signal. Poly(A) polymerase polyadenylates 3’ end of RNA following cleavage. Xrn2 degrades 5’ end of uncapped RNA and removes RNAPII from the DNA template.
Mutations in 3’ end processing factors often cause decreases in transcript levels (Baejen, 2017; Hilleren, 2001; Libri, 2002; Milligan, 2005; Torchet, 2002). One reason for this decrease could be interference with polyA tail addition, exposing the mRNA to 3’-exonucleocytic attack. This concept is supported by the fact that co-deletion of exosome components with 3’ end processing mutants leads to restoration of stable, yet likely incorrectly processed, mRNAs (Burkard, 2000; Libri, 2002; Milligan, 2005; Pefanis, 2015; Tan-Wong, 2012). The outcome of pre-mRNA 3’ end formation is determined by relative efficiencies of polyadenylation versus RNA decay. It has been shown that polyA polymerase interacts with Rrp6 (Burkard, 2000) and the exosome might directly influence the activity of the polyA machinery (Milligan, 2005; Saguez, 2008). RNAPII pauses downstream of the polyA site to allow time for co-transcriptional cleavage and polyadenylation (Glover-Cutter, 2008; Gromak, 2006). It is possible that this pause favors backtracking, leading to a potential ‘reverse torpedo’ mechanism to favor coupled termination and RNA degradation via the exosome (Fox, 2015; Lemay, 2014; Proudfoot, 2016). This model could be coupled with a post-transcriptional RNA cleavage and polyadenylation event to restrict exosome degradation (Bresson, 2018).
Two models for RNAPII release from the DNA template
Following cleavage and polyadenylation of the mRNA, RNAPII transcribes downstream ~150 nucleotides before being released from the DNA template (Creamer, 2011). The exact mechanisms responsible for RNAPII release during termination are still under investigation. It is proposed that pausing at the end of a transcription unit couples 3’ end processing to termination (Kuehner, 2011; Mayer, 2015; Nojima, 2015; Richard, 2009). Cleavage of the mRNA is important for RNAPII release from the template, as cleavage defective mutants tend to be impaired for termination, resulting in transcription read-through phenotypes (Sadowski, 2003). There are two models proposed for release of RNAPII from the DNA: an allosteric model and a torpedo model. In the allosteric model, binding of the termination complex results in a conformational change in the elongation complex as elongation factors are lost, leading to a decrease in processivity (reviewed in (Richard, 2009)). Support for this is shown by the fact that RNAPII loses elongation factors before being released (Ahn, 2004; Baejen, 2017; Kim, 2004a; Mayer, 2010). Also, in vitro studies have indicated that transcript cleavage, a key requirement for the torpedo model, is not required for RNAPII transcription termination (Zhang, 2015). In the torpedo model, cleavage of the 3’ end of the mRNA by CPF-CF provides an entry point for the Rat1/Xrn2 5’−3’ exonuclease to degrade the nascent RNA up to RNAPII and displace the elongation complex (Brannan, 2012; Kim, 2004b; Pearson, 2013; West, 2004).
Support for the torpedo model has been shown in yeast and humans through the occurrence of termination read-through transcription when Rat1 activity is defective, and through the use of in vitro termination assays with yeast Rat1 (Park, 2015). However, Rat1 and its interacting partner Rai1 may be facilitated in vivo through the CTD binding activity of Rtt103, a CTD-interaction domain (CID) protein that has been shown to recruit Rat1/Rai1 to 3’ end of genes to facilitate the dismantling of the elongation complex (Dengl, 2009; Kim, 2004b; Lunde, 2010; Luo, 2006). The human homologs of Rtt103, RPRD1a and 1b (also known as Kub5/Hera), have been implicated in a wide array of functions including interaction with Xrn2 and the serine 5 CTD phosphatase RPAP2 that has also been implicated in loss of RNAPII occupancy on protein coding genes in yeast (Hunter, 2016; Morales, 2014; Ni, 2014). A unified version of both the torpedo and allosteric models has also been proposed where a complex containing both Xrn2/Rat1 and CPF-CF assembles at polyA sites mediating cleavage, nascent RNA degradation, and termination through an allosteric change in the elongation complex (Baejen, 2017; Lunde, 2010; Luo, 2006). Depletion of the Rat1/Xrn2 exonuclease or the CPF subunits Cpsf73 or Symplekin in Drosophila cells can cause circular RNA production through the backsplicing of read-through RNA transcripts with retained introns from upstream genes (Liang, 2017). It is unclear, however, if the backsplicing reactions are coupled with fail-safe mechanisms that might facilitate RNAPII termination under conditions in which termination factors are limited. Perhaps RNAPII pausing associated with RNA backsplicing events (as reported for canonical splicing events) may provide an opportunity for alternative termination mechanisms to occur such as those discussed in the next section involving the helicase Senataxin/Sen1.
Other termination mechanisms
A number of other termination mechanisms have been described that employ additional factors for RNAPII termination coordinated with RNA processing and/or RNA decay, often in cases of premature or non-polyA dependent transcription termination. These pathways may also recruit a number of proteins and/or protein complexes that are involved in the polyA dependent termination pathway described above; however, we will focus on the unique players involved in these pathways in the following section.
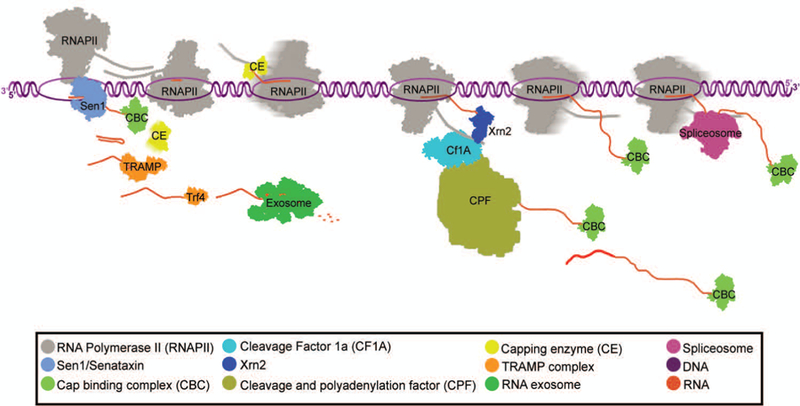
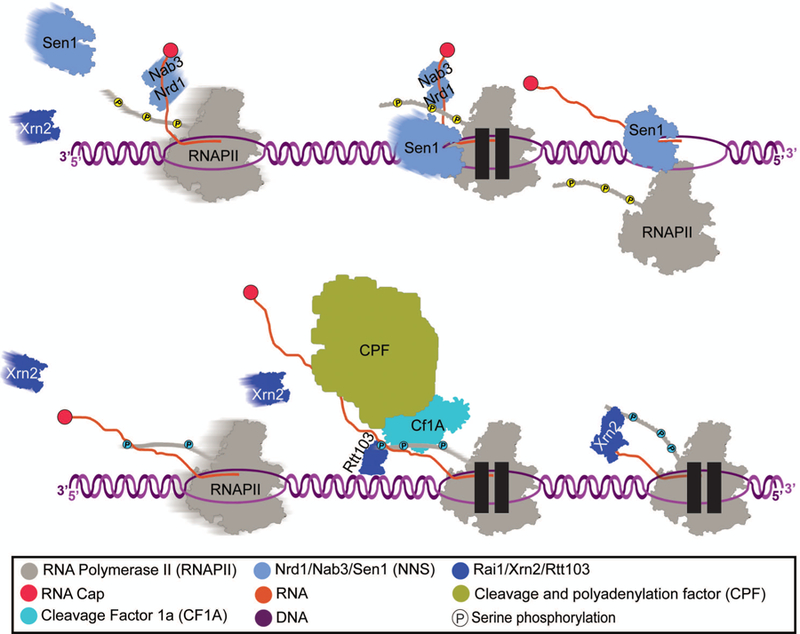
A well-characterized alternate termination pathway exists in budding yeast, the Nrd1-Nab3-Sen1 (NNS)-dependent pathway (Fig. 3). This pathway is responsible for termination at genes encoding snRNAs and snoRNAs (Steinmetz, 2001) and various types of pervasive transcripts such as yeast CUTs (Arigo, 2006b; Schulz, 2013; Thiebaut, 2006; Wyers, 2005). Nrd1-Nab3 binding sites have been shown to be enriched in regions upstream of promoters of CUTs and antisense transcription units (Cakiroglu, 2016; Carroll, 2004; Schulz, 2013; Wlotzka, 2011). NNS is generally considered to terminate shorter transcripts (typically less than 1000 nucleotides) compared to the traditional polyA-dependent pathway (Creamer, 2011; Gudipati, 2008; Schulz, 2013; Vasiljeva, 2008b). However, Nrd1-Nab3 dependent termination has also been shown to provide a fail-safe for transcripts that read past a polyA site, restricting mRNAs where 3’ end formation has failed (Rondon, 2009; Vasiljeva, 2006). Degradation intermediates originating from unspliced RNA species have also been UV-crosslinked to Nrd1, Nab3, or Trf4 (Wlotzka, 2011). It is not understood how Nrd1 is specifically recruited to aberrant RNAs in order to mediate their degradation by the exosome, but there is some evidence that Nrd1 is generally recruited to all RNAPII transcripts perhaps through its protein-protein interaction with the RNAPII CTD (Mayer, 2012; Schulz, 2013). Recently, Bresson et al.demonstrated that NNS and the TRAMP complex were targeted to transcripts that were being downregulated in response to glucose starvation (Bresson, 2017). This suggests that NNS could be a mechanism for selective degradation of RNAs in order to change transcriptional programming under different cellular and environmental signaling pathways.
Figure 3: Nrd1-Nab3-Sen1 Termination.
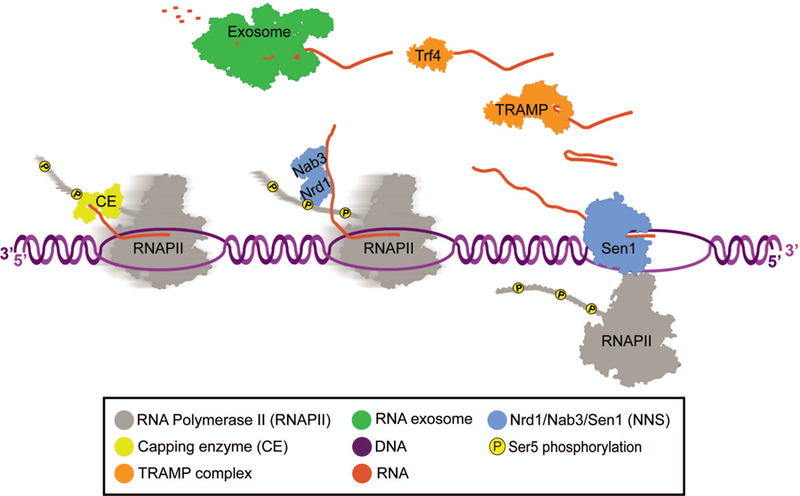
Nrd1 is recruited to the site of transcription by Ser5 phosphorylated CTD. Nab3 and Nrd1 form a heterodimer and bind to RNA via their RNA recognition motif domains. Nab3 and Nrd1 are thought to be able to recruit Sen1, which then catches RNAPII and unwinds the DNA/RNA hybrid (also known as R-loop). TRAMP unwinds RNA and its subunit Trf4 polyadenylates the 3’ end of RNA for processing and/or degradation. The exosome complex then degrades the RNA 3’−5’.
With the major exception of sn/snoRNA transcripts, many of the RNAs terminated by the NNS pathway are rapidly degraded after transcription by the RNA exosome and can only be fully detected when the exosome is perturbed. Increased recruitment of Nrd1 during transcription, even if it does not induce termination, can destabilize a transcript (Honorine, 2011; Vasiljeva, 2008b). Timely termination of CUTs is important for preventing transcription interference with the coding transcriptome. Nrd1 and Nab3 recognize specific sequence motifs on the RNA that are crucial to their specificity (Carroll, 2007; Creamer, 2011; Porrua, 2012; Wlotzka, 2011) and are often clustered with AU-rich sequences, contributing to termination efficiency (Porrua, 2012). Nrd1 also interacts with phosphorylated Ser5 on the RNAPII CTD (Heo, 2013; Kubicek, 2012; Tudek, 2014; Vasiljeva, 2008a), which is the predominant phospho-form during early elongation (Kim, 2011; Komarnitsky, 2000; Mayer, 2010; Tietjen, 2010). Nrd1 and Nab3 may act to ensure efficient and specific Sen1 recruitment since it is present at relatively low levels and appears to recognize RNA indiscriminately (Creamer, 2011; Ghaemmaghami, 2003; Porrua, 2013). Cleavage of the primary transcript has not been demonstrated for this pathway, although the CF protein Pcf11 has been implicated in NNS (Grzechnik, 2015). Human Pcf11 is also involved in snRNA gene termination (O’Reilly, 2014). The CTD phosphatases RPAP2/Rtr1 and Ssu72 have both also been implicated in sn/snoRNA termination control in metazoans while Ssu72 plays a major, although mechanistically uncharacterized, role in NNS termination in yeast (Dichtl, 2002; Egloff, 2012; Loya, 2012; O’Reilly, 2014). Upon release of the RNA transcript from RNAPII as a result of termination aided by Sen1, the exosome can facilitate either the processing or degradation of the transcript (Vasiljeva, 2006). In budding yeast, the exosome is particularly connected to the NNS-termination pathway, due to the type of unstable transcripts typically produced. Rrp6 and Dis3 trim the 3’ end of snRNA and snoRNA precursors to convert them into mature species (Allmang, 1999; Gudipati, 2012; van Hoof, 2000) and completely degrade CUTs (Arigo, 2006b; Gudipati, 2012; Thiebaut, 2006; Wyers, 2005). Nrd1 also recruits TRAMP through direct recognition of a CTD mimic in Trf4 of the TRAMP complex (Tudek, 2014). Efficient degradation and processing require the TRAMP complex which both catalyzes polyadenylation and facilitates degradation by the exosome.
Although a pathway directly homologous to the yeast NNS pathway has not been identified in higher eukaryotes, PROMPTs are produced through early RNAPII termination and degraded by the exosome (Preker, 2008; Preker, 2011). Furthermore, the human NEXT complex has been shown to play a role in RNA surveillance, cryptic transcript degradation, and the termination and 3’ end processing of snRNAs (Hrossova, 2015; Lubas, 2011). NEXT contains hMTR4, the zinc-finger protein ZCCHC8, the RNA-binding factor RBM7. Unlike NNS, which is highly sequence specific, RBM7 seems only to prefer U-rich regions and tends to be promiscuous (Hrossova, 2015; Lubas, 2015). NEXT mainly targets unprocessed transcripts and recent publications have provided evidence for a poly-A tail exosome targeting (PAXT) connection (Meola, 2016). Both NEXT and PAXT have been shown to have physical linkages to the cap-binding complex and its associated factors (Andersen, 2013; Lubas, 2015; Meola 2016), suggesting mechanisms for recruiting the exosome to capped transcripts. TRAMP, NEXT, and PAXT all contain hMTR4, suggesting a possible mechanism in which the exosome can target different transcripts through substitution of adaptors containing hMTR4 (Meola, 2016). The conservation of TRAMP and the role of the NEXT complex in recruiting the exosome to short transcripts suggests comparable mechanisms for early RNAPII termination and coupled RNA decay exist throughout eukaryotes.
Speed of transcription affects the genomic position at which RNAPII termination occurs and accordingly, pausing of RNAPII may provide the opportunity for Sen1 to locate RNAPII and aid in the termination of transcription via the Nrd1-Nab3-Sen1 (NNS) pathway (Hazelbaker, 2013; Jamonnak, 2011; Schaughency, 2014). Sen1 has also been shown to trigger forward translocation of stalled RNAPII complexes (Han, 2017). Sen1 also functions independently of NNS in yeast since: 1) Sen1 has been shown to bind to a number of polyA dependent termination sites (Creamer, 2011; Jamonnak, 2011), 2) Sen1 is sufficient to displace the elongation complex DNA in vitro through interaction with the nascent RNA which requires its ATP-dependent helicase activity (Han, 2017; Leonaite, 2017; Porrua, 2013), and 3) Sen1 inactivation (using an anchor away approach) leads to RNAPII accumulation at the 3’ end of both coding and noncoding genes (Schaughency, 2014). Human and yeast Senataxin/Sen1 have been shown to trigger RNAPII termination to resolve R-loops (Mischo, 2011; Skourti-Stathaki, 2014; Skourti-Stathaki, 2011), which occur in numerous cellular contexts (see Santos-Pereira, 2015 for review). R-loops can cause various types of genome instability and have recently been found to be required for efficient double strand break repair (Ohle, 2016). R-loops can also be formed by circular RNAs that are retained in the nucleus (Conn, 2017). However, it remains to be seen if the circular RNAs that are retained in the nucleus can facilitate RNAPII termination through existing R-loop resolving pathways. Interestingly, an R-loop resolution pathway has been characterized in human cells line in which the RNA-binding protein SMN binds to arginine 1810 symmetric dimethylation on the RNAPII CTD for stable Senataxin recruitment. Recruitment of Senataxin through SMN leads to both R-loop resolution and recruitment of Xrn2 to trigger premature RNAPII termination (Zhao, 2016). It is possible that circular RNAs could work in a similar way to form R-loops at particular genomic regions thereby recruiting R-loop binding proteins and Senataxin to specific sites to trigger RNAPII termination. R-loop resolution pathways may have both basal and fail-safe roles in the regulation and protection of the genome (Chédin, 2016; Santos-Pereira, 2015). Recent work has also shown that yeast Sen1 levels are tightly controlled by the anaphase-promoting complex likely for overall genome maintenance and control of RNAPII termination (Mischo, 2018). In addition, it was shown that overexpression of Sen1 leads to cellular fitness defects. It remains to be determined if Xrn2 is required for Sen1/Senataxin regulated RNAPII termination and vice versa, although previous studies have indicated that both Senataxin and Xrn2 may be required for torpedo function at a model polyA dependent terminator (Kawauchi, 2008; Mischo, 2011; Rondon, 2009; Skourti-Stathaki, 2011), as is suggested for other R-loop resolution pathways (Zhao, 2016).
In metazoans, the multi-subunit Integrator complex is responsible for 3’ end formation and processing of snRNAs (Baillat, 2005). Integrator has been shown to be recruited to a Ser2-P / Ser7-P CTD phosphoform of RNAPII with Ser5-P being inhibitory for recruitment (Egloff, 2010). As such, the recruitment of Integrator may have a dependence on the activity of the two conserved Ser5-P CTD phosphatases RPAP2 (known as Rtr1 in yeast) and Ssu72 which have both been shown to regulate Ser5-P levels in both yeast and mammalian cells (Egloff, 2012; Hunter, 2016; Mosley, 2009; Ni, 2014). In fact, knockdown studies for either phosphatase resulted in decreased snRNA processing efficiency, indicating that native levels of both RPAP2 and Ssu72 are required for proper recruitment/regulation of Integrator (Egloff, 2012; O’Reilly, 2014; Wani, 2014). Incorrect processing by Integrator could lead to exosome-dependent degradation of the nascent snRNA similar to defects caused by improper snRNP formation in SMN deficient cells (Shukla, 2014). The Integrator complex has also been implicated in the termination of numerous other noncoding RNAs including promoter proximal transcripts in a mechanism that terminates DSIF (Spt4/Spt5)- associated RNAPII elongation complexes and enhancer RNAs (Lai, 2015; Skaar, 2015). Considering that polyA-dependent termination factors are temporally recruited after dissociation of the core elongation machinery, which includes DSIF, it is possible that Integrator could also carry out premature RNAPII termination of pervasive transcripts in metazoans similar to the NNS termination pathway in yeast (Mayer, 2012; Mayer, 2010). It has been shown that Senataxin and Xrn2 are not required for RNAPII termination at U1 and U2 snRNA genes (O’Reilly, 2014). However it has not been determined if RNAPII termination of pervasive transcripts in mammalian cells requires Senataxin and/or Xrn2; but both proteins have been implicated in kinetic competition models of termination that could be facilitated by RNAPII pausing as a consequence of engagement of RNA processing machinery such as Integrator (Fong, 2015; Hazelbaker, 2013). The kinetic competition models provide a strong mechanistic foundation for the intimate coupling of RNA processing and RNAPII termination.
The mechanisms of transcription termination including some additional examples of unique pathways are reviewed in more detail in the following publications: (Kuehner, 2011; Porrua, 2016; Proudfoot, 2016).
Conclusions
Recent work has begun to reveal how intimately coupled the processes of transcription and RNA processing are, particularly in the regulation of RNA quality control pathways. The clearest example of this important connection is the requirement for both 5’ and 3’ exonucleases for proper control of RNAPII termination and RNA transport. The rate of RNAPII transcription and phosphorylation of the RNAPII CTD are both critical for the recruitment of the RNA processing machinery and this coupling of processes in return likely ensures proper quality control of the transcribed RNAs to maintain proper gene expression and cellular health. As discussed in this review, capping, splicing, and 3’ end processing are inherently tied to the site of transcription, while the basal transcription machinery coordinately plays a significant role in managing checkpoints of RNA quality control and degradation in the nucleus. Future work is needed to define the proteins and mechanisms involved in the coupled transcription – RNA quality checkpoints which remain poorly understood throughout eukaryotes.
Cutting-edge structural biology and sequencing method development have greatly contributed to a gain in the understanding of the molecular mechanisms that underpin how these processes work both individually and cooperatively. Cryo-EM in particular has helped to make a number of recent advances in mechanistic knowledge of the transient, and subsequently hard to study, RNA processing pathways. However, there are still many open questions when it comes to understanding the mechanisms of these processes, and full structures of the mRNA 3’ end processing machinery have not been reported. The precise interplay between RNA processing machine assembly/disassembly and RNAPII transcription elongation is still poorly understood. As discussed, a number of recent observations of RNAPII pausing at splice sites and termination sites suggests an intimate crosstalk between RNAPII progression and RNA processing that likely provides coordinated regulation of alternative splicing and polyadenylation.
Figure 4: Kinetic competition between RNAPII and Sen1/Xrn2.
The rate of transcription varies, due to factors that influence RNAPII kinetics and passage through chromatin, and these variations in rate can affect the termination window. In general, it is thought that slower elongation rates promote earlier termination, while faster elongation rates lead to termination spreading further downstream. The faster RNAPII moves along a gene, the harder it will be for the termination machinery to catch up, and how long it takes for RNAPII to be caught will help determine what termination pathway is used. Two termination-associated proteins thought to be tasked with catching RNAPII are Xrn2 (also known as Rat1) and Sen1 (yeast homolog of Senataxin). Pausing of RNAPII would promote termination via either Xrn2 or Sen1 by providing the ability for them to catch up to the elongation complex (Mischo, 2013).
PolyA Binding Protein Nuclear 1: A common resource for RNA processing and decay.
A good example of the interconnectedness of processing and degradation for quality control is polyA binding protein nuclear 1 (PABPN1). PABPN1 interacts with polyA polymerase to aid in the addition of the polyA tail to transcripts (Kerwitz 2003). One way in which PAPBN1 functions in quality control of RNAs is through regulation of alternative cleavage and polyadenylation (APA) (Jenal, 2012). Similar to alternative splicing, APA can provide a way in which multiple transcripts can be produced from a single gene, with as many as 50% of human genes derived in this manner (Tian, 2005). Moreover, usage of alternative polyadenylation sites can lead to differing lengths in 3’ UTRs, which can have effects on mRNA stability, localization, and translation efficiency by changing targets for RNA binding proteins and miRNAs (Andreassi, 2009; Fabian, 2010). APA has been described in more detail in the following reviews (Elkon, 2013; Di Giammartino, 2011; Lutz, 2011; Tian, 2013). PABPN1 and polyA polymerases have also been shown to have a role in RNA decay via the exosome (Beaulieu, 2012; Bresson, 2013; Bresson, 2015; Lee, 2009). This functional connection between PABPN1 and the exosome has been further supported by the discovery of a protein that physically links PABN1 to the human NEXT complex (Meola, 2016). The fact that this PABN1 plays important roles in both 3’ end processing and targeting transcripts to the exosome highlights how quality control is managed through the balance between processing and degradation.
The race between RNAPII and termination factors.
The DNA:RNA helicase Sen1 and the 5’−3’ exonuclease Xrn2 are required for various termination pathways. In models of kinetic competition between RNAPII and Sen1/Xrn2, it has been proposed that slower-moving RNAPII will be able to be caught earlier in transcription, while fast-moving RNAPII won’t be caught until later in the gene. Pausing of RNAPII could provide an increased probability for termination via either Xrn2 or Sen1 (Fig. 4) (Mischo, 2013). Use of RNAPII trigger loop mutants (Fast mutant: rpb1-E1103G, Slow mutant: rpb1-N488D) has demonstrated that slow elongation leads to earlier termination, while fast elongation lead to later termination (Malagon, 2006). Growth and termination defects in some Xrn2 mutant cells can be overcome by RNAPII slow mutants (Fong, 2015; Jimeno-Gonzalez, 2010). Similarly, Sen1 mutants display read-through termination defects which can be suppressed by the introduction of slow RNAPII (Hazelbaker, 2013). In both cases, the slow RNAPII mutants provide a larger window of opportunity for proper termination by the mutant termination factors. In complementary work, it has recently been shown that Sen1 protein levels are modulated by the cell cycle (Mischo, 2018). Overexpression of Sen1 leads to a decrease in ncRNA production and an increase in efficiency of mRNA termination, with changes observed in both the occupancy of RNAPII and the position of termination (Mischo, 2018). The toxicity of Sen1 overexpression is likely caused by excess termination. This model is supported by the fact that mutations in other termination factors suppress this phenotype (Mischo, 2018).
Acknowledgments
We sincerely thank the members of the Mosley labs for critical discussions and comments.
Funding Information
ALM acknowledges support from NIH grant R01 GM099714 and NSF grant MCB 1515748. SAP is supported by T32 HL007910.
Contributor Information
Sarah A. Peck, Email: sapeck@iupui.edu, Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, IN 46220.
Katlyn D. Hughes, Email: katdhugh@iu.edu, Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, IN 46220.
Jose F. Victorino, Email: jvictori@iu.edu, Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, IN 46220.
Amber L. Mosley, Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, IN 46220.
References
- Adelman K, & Lis JT (2012). Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat Rev Genet, 13(10), 720–731. doi: 10.1038/nrg3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman K, Marr MT, Werner J, Saunders A, Ni Z, Andrulis ED, & Lis JT (2005). Efficient release from promoter-proximal stall sites requires transcript cleavage factor TFIIS. Mol Cell, 17(1), 103–112. doi: 10.1016/j.molcel.2004.11.028 [DOI] [PubMed] [Google Scholar]
- Agafonov DE, Kastner B, Dybkov O, Hofele RV, Liu WT, Urlaub H, Luhrmann R, & Stark H (2016). Molecular architecture of the human U4/U6.U5 tri-snRNP. Science, 351(6280), 1416–1420. doi: 10.1126/science.aad2085 [DOI] [PubMed] [Google Scholar]
- Ahearn JM Jr, Bartolomei MS, West ML, Cisek LJ, & Corden JL. (1987). Cloning and sequence analysis of the mouse genomic locus encoding the largest subunit of RNA polymerase II. J Biol Chem, (262)22, 10695–10705. [PubMed] [Google Scholar]
- Ahn SH, Kim M, & Buratowski S (2004). Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3’ end processing. Mol Cell, 13(1), 67–76. [DOI] [PubMed] [Google Scholar]
- Alexander RD, Innocente SA, Barrass JD, & Beggs JD (2010). Splicing-dependent RNA polymerase pausing in yeast. Mol Cell, 40(4), 582–593. doi: 10.1016/j.molcel.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allmang C, Kufel J, Chanfreau G, Mitchell P, Petfalski E, & Tollervey D (1999). Functions of the exosome in rRNA, snoRNA and snRNA synthesis. EMBO J, 18(19), 5399–5410. doi: 10.1093/emboj/18.19.5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg DC, Goldstein AL, & Cole CN (1992). Isolation and characterization of RAT1: an essential gene of Saccharomyces cerevisiae required for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev, 6(7), 1173–1189. [DOI] [PubMed] [Google Scholar]
- Ameur A, Zaghlool A, Halvardson J, Wetterbom A, Gyllensten U, Cavelier L, & Feuk L (2011). Total RNA sequencing reveals nascent transcription and widespread co-transcriptional splicing in the human brain. Nat Struct Mol Biol, 18(12), 1435–1440. doi: 10.1038/nsmb.2143 [DOI] [PubMed] [Google Scholar]
- Andersen PR, Domanski M, Kristiansen MS, Storvall H, Ntini E, Verheggen C, Schein A, Bunkenborg J, Poser I, Hallais M, Sandberg R, Hyman A, LaCava J, Rout MP, Andersen JS, Bertrand E, & Jensen TH (2013). The human cap-binding complex is functionally connected to the nuclear RNA exosome. Nat Struct Mol Biol, 20(12), 1367–1376. doi: 10.1038/nsmb.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassi C, & Riccio A (2009). To localize or not to localize: mRNA fate is in 3′UTR ends. Trends in Cell Biology, 19(9), 465–474. doi: 10.1016/j.tcb.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Andrulis ED, Werner J, Nazarian A, Erdjument-Bromage H, Tempst P, & Lis JT (2002). The RNA processing exosome is linked to elongating RNA polymerase II in Drosophila. Nature, 420(6917), 837–841. doi: 10.1038/nature01181 [DOI] [PubMed] [Google Scholar]
- Arigo JT, Carroll KL, Ames JM, & Corden JL (2006a). Regulation of yeast NRD1 expression by premature transcription termination. Mol Cell, 21(5), 641–651. doi: 10.1016/j.molcel.2006.02.005 [DOI] [PubMed] [Google Scholar]
- Arigo JT, Eyler DE, Carroll KL, & Corden JL (2006b). Termination of cryptic unstable transcripts is directed by yeast RNA-binding proteins Nrd1 and Nab3. Mol Cell, 23(6), 841–851. doi: 10.1016/j.molcel.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Arndt KM, & Reines D (2015). Termination of Transcription of Short Noncoding RNAs by RNA Polymerase II. Annu Rev Biochem, 84, 381–404. doi: 10.1146/annurev-biochem-060614-034457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baejen C, Andreani J, Torkler P, Battaglia S, Schwalb B, Lidschreiber M, Maier KC, Boltendahl A, Rus P, Esslinger S, Soding J, & Cramer P (2017). Genome-wide Analysis of RNA Polymerase II Termination at Protein-Coding Genes. Mol Cell, 66(1), 38–49 e36. doi: 10.1016/j.molcel.2017.02.009 [DOI] [PubMed] [Google Scholar]
- Baejen C, Torkler P, Gressel S, Essig K, Soding J, & Cramer P (2014). Transcriptome maps of mRNP biogenesis factors define pre-mRNA recognition. Mol Cell, 55(5), 745–757. doi: 10.1016/j.molcel.2014.08.005 [DOI] [PubMed] [Google Scholar]
- Baillat D, Hakimi MA, Naar AM, Shilatifard A, Cooch N, & Shiekhattar R (2005). Integrator, a multiprotein mediator of small nuclear RNA processing, associates with the C-terminal repeat of RNA polymerase II. Cell, 123(2), 265–276. doi: 10.1016/j.cell.2005.08.019 [DOI] [PubMed] [Google Scholar]
- Bauren G, & Wieslander L (1994). Splicing of Balbiani ring 1 gene pre-mRNA occurs simultaneously with transcription. Cell, 76(1), 183–192. [DOI] [PubMed] [Google Scholar]
- Beaulieu YB, Kleinman CL, Landry-Voyer AM, Majewski J, & Bachand F (2012). Polyadenylation-dependent control of long noncoding RNA expression by the poly(A)-binding protein nuclear 1. PLoS Genet, 8(11), e1003078. doi: 10.1371/journal.pgen.1003078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley DL (2014). Coupling mRNA processing with transcription in time and space. Nat Rev Genet, 15(3), 163–175. doi: 10.1038/nrg3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, & Bourne PE (2000). The Protein Data Bank. Nucleic Acids Research 28:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram K, Agafonov DE, Dybkov O, Haselbach D, Leelaram MN, Will CL, Urlaub H, Kastner B, Luhrmann R, & Stark H (2017a). Cryo-EM Structure of a Pre-catalytic Human Spliceosome Primed for Activation. Cell, 170(4), 701–713 e711. doi: 10.1016/j.cell.2017.07.011 [DOI] [PubMed] [Google Scholar]
- Bertram K, Agafonov DE, Liu WT, Dybkov O, Will CL, Hartmuth K, Urlaub H, Kastner B, Stark H, & Luhrmann R (2017b). Cryo-EM structure of a human spliceosome activated for step 2 of splicing. Nature, 542(7641), 318–323. doi: 10.1038/nature21079 [DOI] [PubMed] [Google Scholar]
- Beyer AL, & Osheim YN (1988). Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev, 2(6), 754–765. [DOI] [PubMed] [Google Scholar]
- Bitton DA, Atkinson SR, Rallis C, Smith GC, Ellis DA, Chen YY, Malecki M, Codlin S, Lemay JF, Cotobal C, Bachand F, Marguerat S, Mata J, & Bahler J (2015). Widespread exon skipping triggers degradation by nuclear RNA surveillance in fission yeast. Genome Res, 25(6), 884–896. doi: 10.1101/gr.185371.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boczonadi V, Muller JS, Pyle A, Munkley J, Dor T, Quartararo J, Ferrero I, Karcagi V, Giunta M, Polvikoski T, Birchall D, Princzinger A, Cinnamon Y, Lutzkendorf S, Piko H, Reza M, Florez L, Santibanez-Koref M, Griffin H, Schuelke M, Elpeleg O, Kalaydjieva L, Lochmuller H, Elliott DJ, Chinnery PF, Edvardson S, & Horvath R (2014). EXOSC8 mutations alter mRNA metabolism and cause hypomyelination with spinal muscular atrophy and cerebellar hypoplasia. Nat Commun, 5, 4287. doi: 10.1038/ncomms5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth GT, Parua PK, Sansó M, Fisher RP, Lis JT (2018). Cdk9 regulates a promoter-proximal checkpoint to modulate RNA polymerase II elongation rate in fission yeast. Nat Commun, 9, 543. doi: 10.1038/s41467-018-03006-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet-Antonelli C, Presutti C, & Tollervey D (2000). Identification of a regulated pathway for nuclear pre-mRNA turnover. Cell, 102(6), 765–775. doi:Doi 10.1016/S0092-8674(00)00065-9 [DOI] [PubMed] [Google Scholar]
- Brannan K, Kim H, Erickson B, Glover-Cutter K, Kim S, Fong N, Kiemele L, Hansen K, Davis R, Lykke-Andersen J, & Bentley DL (2012). mRNA decapping factors and the exonuclease Xrn2 function in widespread premature termination of RNA polymerase II transcription. Mol Cell, 46(3), 311–324. doi: 10.1016/j.molcel.2012.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresson SM, & Conrad NK (2013). The human nuclear poly(a)-binding protein promotes RNA hyperadenylation and decay. PLoS Genet, 9(10), e1003893. doi: 10.1371/journal.pgen.1003893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresson S, Tuck A, Staneva D, & Tollervey D (2017). Nuclear RNA Decay Pathways Aid Rapid Remodeling of Gene Expression in Yeast. Mol Cell, 65(5), 787–800 e785. doi: 10.1016/j.molcel.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresson S, & Tollervey D (2018). Surveillance-ready transcription: nuclear RNA decay as a default fate. Open Biol, 8(3). doi: 10.1098/rsob.170270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler M, Haas W, Gygi SP, & Moazed D (2007). RNAi-dependent and -independent RNA turnover mechanisms contribute to heterochromatic gene silencing. Cell, 129(4), 707–721. doi: 10.1016/j.cell.2007.03.038 [DOI] [PubMed] [Google Scholar]
- Buratowski S (2003). The CTD code. Nat Struct Biol, 10(9), 679–680. doi: 10.1038/nsb0903-679 [DOI] [PubMed] [Google Scholar]
- Buratowski S (2009). Progression through the RNA polymerase II CTD cycle. Mol Cell, 36(4), 541–546. doi: 10.1016/j.molcel.2009.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard KTD, & Butler JS (2000). A nuclear 3 ‘−5 ‘ exonuclease involved in mRNA degradation interacts with poly(A) polymerase and the hnRNA protein Npl3p. Molecular and Cellular Biology, 20(2), 604–616. doi:Doi 10.1128/Mcb.20.2.604-616.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JE, Longhurst AD, Merkurjev D, Sales-Lee J, Rao B, Moresco JJ, Yates JR 3rd, Li JJ, & Madhani HD (2018). Spliceosome Profiling Visualizes Operations of a Dynamic RNP at Nucleotide Resolution. Cell, 173(4), 1014–1030 e1017. doi: 10.1016/j.cell.2018.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakiroglu SA, Zaugg JB, & Luscombe NM (2016). Backmasking in the yeast genome: encoding overlapping information for protein-coding and RNA degradation. Nucleic Acids Res, 44(17), 8065–8072. doi: 10.1093/nar/gkw683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan KP, & Butler JS (2010). TRAMP complex enhances RNA degradation by the nuclear exosome component Rrp6. J Biol Chem, 285(6), 3540–3547. doi: 10.1074/jbc.M109.058396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelli T, Challal D, Briand JB, Boulay J, Porrua O, Colin J, & Libri D (2018). High-resolution transcription maps reveal the widespread impact of roadblock termination in yeast. EMBO J, 37(4). doi: 10.15252/embj.201797490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo Oesterreich F, Preibisch S, & Neugebauer KM (2010). Global analysis of nascent RNA reveals transcriptional pausing in terminal exons. Mol Cell, 40(4), 571–581. doi: 10.1016/j.molcel.2010.11.004 [DOI] [PubMed] [Google Scholar]
- Carrillo Oesterreich F, Herzel L, Straube K, Hujer K, Howard J, & Neugebauer KM (2016). Splicing of nascent RNA coincides with intron exit from RNA polymerase II. Cell, 165, 372–381. doi: 10.1016/j.cell.2016.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KL, Ghirlando R, Ames JM, & Corden JL (2007). Interaction of yeast RNA-binding proteins Nrd1 and Nab3 with RNA polymerase II terminator elements. RNA, 13(3), 361–373. doi: 10.1261/rna.338407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KL, Pradhan DA, Granek JA, Clarke ND, & Corden JL (2004). Identification of cis elements directing termination of yeast nonpolyadenylated snoRNA transcripts. Mol Cell Biol, 24(14), 6241–6252. doi: 10.1128/MCB.24.14.6241-6252.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanal A, Kumar A, Hill CH, Easter AD, Emsley P, Degliesposti G, Gordiyenko Y, Santhanam B, Wolf J, Wiederhold K, Dornan DL, Skehel M, Robinson CV, & Passmore LA (2017). Architecture of eukaryotic mRNA 3 ‘-end processing machinery. Science, 358(6366), 1056–1059. doi: 10.1126/science.aao6535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JH, Jiao XF, Chiba K, Oh C, Martin CE, Kiledjian M, & Tong L (2012). Dxo1 is a new type of eukaryotic enzyme with both decapping and 5 ‘−3 ‘ exoribonuclease activity. Nature Structural & Molecular Biology, 19(10), 1011–U1062. doi: 10.1038/nsmb.2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chédin F (2016) Nascent Connections: R-Loops and Chromatin Patterning. Trends Genet 32(12), 828–838. doi: 10.1016/j.tig.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FX, Smith ER, & Shilatifard A (2018). Born to run: control of transcription elongation by RNA polymerase II. Nat Rev Mol Cell Biol, 19(7), 464–478. doi: 10.1038/s41580-018-0010-5 [DOI] [PubMed] [Google Scholar]
- Cheung AC, & Cramer P (2011). Structural basis of RNA polymerase II backtracking, arrest and reactivation. Nature, 471(7337), 249–253. doi: 10.1038/nature09785 [DOI] [PubMed] [Google Scholar]
- Chlebowski A, Lubas M, Jensen TH, & Dziembowski A (2013). RNA decay machines: the exosome. Biochim Biophys Acta, 1829(6–7), 552–560. doi: 10.1016/j.bbagrm.2013.01.006 [DOI] [PubMed] [Google Scholar]
- Cho EJ, Takagi T, Moore CR, & Buratowski S (1997). mRNA capping enzyme is recruited to the transcription complex by phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev, 11(24), 3319–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin J, Candelli T, Porrua O, Boulay J, Zhu C, Lacroute F, Steinmetz LM, & Libri D (2014). Roadblock termination by reb1p restricts cryptic and readthrough transcription. Mol Cell, 56(5), 667–680. doi: 10.1016/j.molcel.2014.10.026 [DOI] [PubMed] [Google Scholar]
- Coller J, & Parker R (2004). Eukaryotic mRNA decapping. Annu Rev Biochem, 73(1), 861–890. doi: 10.1146/annurev.biochem.73.011303.074032 [DOI] [PubMed] [Google Scholar]
- Conn VM, Hugouvieux V, Nayak A, Conos SA, Capovilla G, Cildir G, Jourdain A, Tergaonkar V, Schmid M, Zubieta C, & Conn SJ (2017). A circRNA from SEPALLATA3 regulates splicing of its cognate mRNA through R-loop formation. Nat Plants, 3, 17053. doi: 10.1038/nplants.2017.53 [DOI] [PubMed] [Google Scholar]
- Cougot N, van Dijk E, Babajko S, & Seraphin B (2004). ‘Cap-tabolism’. Trends Biochem Sci, 29(8), 436–444. doi: 10.1016/j.tibs.2004.06.008 [DOI] [PubMed] [Google Scholar]
- Coy S, Volanakis A, Shah S, & Vasiljeva L (2013). The Sm complex is required for the processing of non-coding RNAs by the exosome. PLoS One, 8(6), e65606. doi: 10.1371/journal.pone.0065606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer TJ, Darby MM, Jamonnak N, Schaughency P, Hao H, Wheelan SJ, & Corden JL (2011). Transcriptome-wide binding sites for components of the Saccharomyces cerevisiae non-poly(A) termination pathway: Nrd1, Nab3, and Sen1. PLoS Genet, 7(10), e1002329. doi: 10.1371/journal.pgen.1002329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damgaard CK, Kahns S, Lykke-Andersen S, Nielsen AL, Jensen TH, & Kjems J (2008). A 5’ splice site enhances the recruitment of basal transcription initiation factors in vivo. Mol Cell, 29(2), 271–278. doi: 10.1016/j.molcel.2007.11.035 [DOI] [PubMed] [Google Scholar]
- Danin-Kreiselman M, Lee CY, & Chanfreau G (2003). RNAse III-mediated degradation of unspliced pre-mRNAs and lariat introns. Mol Cell, 11(5), 1279–1289. [DOI] [PubMed] [Google Scholar]
- Danko CG, Hah N, Luo X, Martins AL, Core L, Lis JT, Siepel A, & Kraus WL (2013). Signaling pathways differentially affect RNA polymerase II initiation, pausing, and elongation rate in cells. Mol Cell, 50(2), 212–222. doi: 10.1016/j.molcel.2013.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L, Kerr A, & West S (2012). Co-transcriptional degradation of aberrant pre-mRNA by Xrn2. EMBO J, 31(11), 2566–2578. doi: 10.1038/emboj.2012.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L, Muniz L, & West S (2014). 3’ end formation of pre-mRNA and phosphorylation of Ser2 on the RNA polymerase II CTD are reciprocally coupled in human cells. Genes Dev, 28(4), 342–356. doi: 10.1101/gad.231274.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CA, & Ares M Jr. (2006). Accumulation of unstable promoter-associated transcripts upon loss of the nuclear exosome subunit Rrp6p in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A, 103(9), 3262–3267. doi: 10.1073/pnas.0507783103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SF, Garcia-Sacristan A, Custodio N, & Carmo-Fonseca M (2010). A link between nuclear RNA surveillance, the human exosome and RNA polymerase II transcriptional termination. Nucleic Acids Res, 38(22), 8015–8026. doi: 10.1093/nar/gkq703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengl S, & Cramer P (2009). Torpedo nuclease Rat1 is insufficient to terminate RNA polymerase II in vitro. J Biol Chem, 284(32), 21270–21279. doi: 10.1074/jbc.M109.013847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichtl B, Blank D, Ohnacker M, Friedlein A, Roeder D, Langen H, & Keller W (2002). A role for SSU72 in balancing RNA polymerase II transcription elongation and termination. Mol Cell, 10(5), 1139–1150. [DOI] [PubMed] [Google Scholar]
- Dujardin G, Lafaille C, Petrillo E, Buggiano V, Gomez Acuña LI, Fiszbein A, Godoy Herz MA, Nieto Moreno N, Muñoz MJ, Alló M, Schor IE, & Kornblihtt AR (2013). Transcriptional elongation and alternative splicing. Biochim Biophys Acta, 1829(1), 134–140. doi: 10.1016/j.bbagrm.2012.08.005 [DOI] [PubMed] [Google Scholar]
- Eaton JD, Davidson L, Bauer DLV, Natsume T, Kanemaki MT, West S (2018). Xrn2 accelerates termination by RNA polymerase II, which is underpinned by CPSF73 activity. Genes Dev, 32(2), 127–139. doi: 10.1101/gad.308528.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle AB, Hessle V, Helbig R, Dantoft W, Gimber N, & Visa N (2010). Splice-site mutations cause Rrp6-mediated nuclear retention of the unspliced RNAs and transcriptional down-regulation of the splicing-defective genes. PLoS One, 5(7), e11540. doi: 10.1371/journal.pone.0011540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckard SC, Rice GI, Fabre A, Badens C, Gray EE, Hartley JL, Crow YJ, & Stetson DB (2014). The SKIV2L RNA exosome limits activation of the RIG-I-like receptors. Nat Immunol, 15(9), 839–845. doi: 10.1038/ni.2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff S, O’Reilly D, Chapman RD, Taylor A, Tanzhaus K, Pitts L, Eick D, & Murphy S (2007). Serine-7 of the RNA polymerase II CTD is specifically required for snRNA gene expression. Science, 318(5857), 1777–1779. doi: 10.1126/science.1145989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff S, Szczepaniak SA, Dienstbier M, Taylor A, Knight S, & Murphy S (2010). The integrator complex recognizes a new double mark on the RNA polymerase II carboxyl-terminal domain. J Biol Chem, 285(27), 20564–20569. doi: 10.1074/jbc.M110.132530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff S, Zaborowska J, Laitem C, Kiss T, & Murphy S (2012). Ser7 phosphorylation of the CTD recruits the RPAP2 Ser5 phosphatase to snRNA genes. Mol Cell, 45(1), 111–122. doi: 10.1016/j.molcel.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick D, & Geyer M (2013). The RNA polymerase II carboxy-terminal domain (CTD) code. Chem Rev, 113(11), 8456–8490. doi: 10.1021/cr400071f [DOI] [PubMed] [Google Scholar]
- Elkon R, Ugalde AP, & Agami R (2013). Alternative cleavage and polyadenylation: extent, regulation and function. Nat Rev Genet, 14(7), 496–506. doi: 10.1038/nrg3482 [DOI] [PubMed] [Google Scholar]
- Erickson B, Sheridan RM, Cortazar M, & Bentley DL (2018) Dynamic turnover of paused Pol II complexes at human promoters. Genes Dev, 32(17–18), 1215–1225. doi: 10.1101/gad.316810.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian MR, Sonenberg N, & Filipowicz W (2010). Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem, 79, 351–379. doi: 10.1146/annurev-biochem-060308-103103 [DOI] [PubMed] [Google Scholar]
- PDB ID: 4U4C. Falk S, Weir JR, Hentschel J, Reichelt P, Bonneau F, & Conti E (2014). The molecular architecture of the TRAMP complex reveals the organization and interplay of its two catalytic activities. Mol Cell, 55(6), 856–867. doi: 10.1016/j.molcel.2014.07.020 [DOI] [PubMed] [Google Scholar]
- Fica SM, & Nagai K (2017). Cryo-electron microscopy snapshots of the spliceosome: structural insights into a dynamic ribonucleoprotein machine. Nat Struct Mol Biol, 24(10), 791–799. doi: 10.1038/nsmb.3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finci LI, Zhang X, Huang X, Zhou Q, Tsai J, Teng T, Agrawal A, Chan B, Irwin S, Karr C, Cook A, Zhu P, Reynolds D, Smith PG, Fekkes P, Buonamici S, & Larsen NA (2018). The cryo-EM structure of the SF3b spliceosome complex bound to a splicing modulator reveals a pre-mRNA substrate competitive mechanism of action. Genes Dev, 32(3–4), 309–320. doi: 10.1101/gad.311043.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty SM, Fortes P, Izaurralde E, Mattaj IW, & Gilmartin GM (1997). Participation of the nuclear cap binding complex in pre-mRNA 3’ processing. Proceedings of the National Academy of Sciences of the United States of America, 94(22), 11893–11898. doi:DOI 10.1073/pnas.94.22.11893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong N, & Bentley DL (2001). Capping, splicing, and 3’ processing are independently stimulated by RNA polymerase II: different functions for different segments of the CTD. Genes Dev, 15(14):1783–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong N, Brannan K, Erickson B, Kim H, Cortazar MA, Sheridan RM, Nquyen T, Karp S, & Bentley DL (2015). Effects of Transcription Elongation Rate and Xrn2 Exonuclease Activity on RNA Polymerase II Termination Suggest Widespread Kinetic Competition. Mol Cell, 60(2), 256–267. doi: 10.1016/j.molcel.2015.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MJ, Gao H, Smith-Kinnaman WR, Liu Y, & Mosley AL (2015). The exosome component Rrp6 is required for RNA polymerase II termination at specific targets of the Nrd1-Nab3 pathway. PLoS Genet, 11(2), e1004999. doi: 10.1371/journal.pgen.1004999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks TM, & Lykke-Andersen J (2008). The control of mRNA decapping and P-body formation. Mol Cell, 32(5), 605–615. doi: 10.1016/j.molcel.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs G, Voichek Y, Benjamin S, Gilad S, Amit I, & Oren M (2014). 4sUDRB-seq: measuring genomewide transcriptional elongation rates and initiation frequencies within cells. Genome Biol, 15(5), R69. doi: 10.1186/gb-2014-15-5-r69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y, & Shatkin AJ (2000). Viral and cellular mRNA capping: past and prospects. Adv Virus Res, 55(0065–3527 (Print)), 135–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusby B, Kim S, Erickson B, Kim H, Peterson ML, & Bentley DL (2016). Coordination of RNA Polymerase II Pausing and 3’ End Processing Factor Recruitment with Alternative Polyadenylation. Mol Cell Biol, 36(2), 295–303. doi: 10.1128/MCB.00898-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galej WP, Wilkinson ME, Fica SM, Oubridge C, Newman AJ, & Nagai K (2016). Cryo-EM structure of the spliceosome immediately after branching. Nature, 537(7619), 197–201. doi: 10.1038/nature19316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy V, Gadal O, Fromont-Racine M, Romano A, Jacquier A, & Nehrbass U (2004). Nuclear retention of unspliced mRNAs in yeast is mediated by perinuclear Mlp1. Cell, 116(1), 63–73. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami S, Huh W, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, & Weissman JS (2003). Global analysis of protein expression in yeast. Nature, 425(6959), 737–741. doi: 10.1038/nature02046 [DOI] [PubMed] [Google Scholar]
- Ghosh A, Shuman S, & Lima CD (2011). Structural insights to how mammalian capping enzyme reads the CTD code. Mol Cell, 43(2), 299–310. doi: 10.1016/j.molcel.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giammartino DC, Nishida K, & Manley JL (2011). Mechanisms and consequences of alternative polyadenylation. Mol Cell, 43(6), 853–866. doi: 10.1016/j.molcel.2011.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W, & Guthrie C (2004). The Glc7p nuclear phosphatase promotes mRNA export by facilitating association of Mex67p with mRNA. Mol Cell, 13(2), 201–212. [DOI] [PubMed] [Google Scholar]
- Girard C, Will CL, Peng J, Makarov EM, Kastner B, Lemm I, Urlaub H, Hartmuth K, & Luhrmann R (2012). Post-transcriptional spliceosomes are retained in nuclear speckles until splicing completion. Nat Commun, 3, 994. doi: 10.1038/ncomms1998 [DOI] [PubMed] [Google Scholar]
- Glover-Cutter K, Kim S, Espinosa J, & Bentley DL (2008). RNA polymerase II pauses and associates with pre-mRNA processing factors at both ends of genes. Nat Struct Mol Biol, 15(1), 71–78. doi: 10.1038/nsmb1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf AL (1993). Positive patches and negative noodles: linking RNA processing to transcription? Trends Biochem Sci, 18(4), 117–119. [DOI] [PubMed] [Google Scholar]
- Gromak N, West S, & Proudfoot NJ (2006). Pause sites promote transcriptional termination of mammalian RNA polymerase II. Mol Cell Biol, 26(10), 3986–3996. doi: 10.1128/MCB.26.10.3986-3996.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudzien-Nogalska E, & Kiledjian M (2017). New insights into decapping enzymes and selective mRNA decay. Wiley Interdiscip Rev RNA, 8(1). doi: 10.1002/wrna.1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzechnik P, Gdula MR, & Proudfoot NJ (2015). Pcf11 orchestrates transcription termination pathways in yeast. Genes Dev, 29(8), 849–861. doi: 10.1101/gad.251470.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PDB ID: 3KYH. Gu M, Rajashankar KR, & Lima CD (2010). Structure of the Saccharomyces cerevisiae Cet1-Ceg1 mRNA capping apparatus. Structure, 18(2), 216–227. doi: 10.1016/j.str.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudipati RK, Villa T, Boulay J, & Libri D (2008). Phosphorylation of the RNA polymerase II C-terminal domain dictates transcription termination choice. Nat Struct Mol Biol, 15(8), 786–794. doi: 10.1038/nsmb.1460 [DOI] [PubMed] [Google Scholar]
- Gudipati RK, Xu Z, Lebreton A, Seraphin B, Steinmetz LM, Jacquier A, & Libri D (2012). Extensive degradation of RNA precursors by the exosome in wild-type cells. Mol Cell, 48(3), 409–421. doi: 10.1016/j.molcel.2012.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusarov I, & Nudler E (1999). The mechanism of intrinsic transcription termination. Mol Cell, 3(4), 495–504. [DOI] [PubMed] [Google Scholar]
- Haag JR, & Pikaard CS (2011). Multisubunit RNA polymerases IV and V: purveyors of non-coding RNA for plant gene silencing. Nat Rev Mol Cell Biol, 12(8), 483–492. doi: 10.1038/nrm3152 [DOI] [PubMed] [Google Scholar]
- Halbeisen RE, Galgano A, Scherrer T, & Gerber AP (2008). Post-transcriptional gene regulation: from genome-wide studies to principles. Cell Mol Life Sci, 65(5), 798–813. doi: 10.1007/s00018-007-7447-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammell CM, Gross S, Zenklusen D, Heath CV, Stutz F, Moore C, & Cole CN (2002). Coupling of termination, 3’ processing, and mRNA export. Mol Cell Biol, 22(18), 6441–6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Libri D, & Porrua O (2017). Biochemical characterization of the helicase Sen1 provides new insights into the mechanisms of non-coding transcription termination. Nucleic Acids Res, 45(3), 1355–1370. doi: 10.1093/nar/gkw1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlen KM, Trotta KL, Smith EE, Mosaheb MM, Fuchs SM, & Churchman LS (2016). Comprehensive RNA Polymerase II Interactomes Reveal Distinct and Varied Roles for Each Phospho-CTD Residue. Cell Rep, 15(10), 2147–2158. doi: 10.1016/j.celrep.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbaker Dane Z., Marquardt S, Wlotzka W, & Buratowski S (2013). Kinetic Competition between RNA Polymerase II and Sen1-Dependent Transcription Termination. Molecular Cell, 49(1), 55–66. doi: 10.1016/j.molcel.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques T, Gilchrist DA, Nechaev S, Bern M, Muse GW, Burkholder A, Fargo DC, & Adelman K (2013). Stable pausing by RNA polymerase II provides an opportunity to target and integrate regulatory signals. Mol Cell, 52(4), 517–528. doi: 10.1016/j.molcel.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo DH, Yoo I, Kong J, Lidschreiber M, Mayer A, Choi BY, Hahn Y, Cramer P, Buratowski S, & Kim M (2013). The RNA polymerase II C-terminal domain-interacting domain of yeast Nrd1 contributes to the choice of termination pathway and couples to RNA processing by the nuclear exosome. J Biol Chem, 288(51), 36676–36690. doi: 10.1074/jbc.M113.508267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández H, & Robinson CV (2007). Determining the stoichiometry and interactions of macromolecular assemblies from mass spectrometry. Nature Protocols, 2(3), 715–726. doi: 10.1038/nprot.2007.73 [DOI] [PubMed] [Google Scholar]
- Herzel L, Ottoz DSM, Alpert T, & Neugebauer KM (2017). Splicing and transcription touch base: co-transcriptional spliceosome assembly and function. Nat Rev Mol Cell Biol, 18(10), 637–650. doi: 10.1038/nrm.2017.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessle V, von Euler A, Gonzalez de Valdivia E, & Visa N (2012). Rrp6 is recruited to transcribed genes and accompanies the spliced mRNA to the nuclear pore. RNA, 18(8), 1466–1474. doi: 10.1261/rna.032045.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieronymus H, Yu MC, & Silver PA (2004). Genome-wide mRNA surveillance is coupled to mRNA export. Genes Dev, 18(21), 2652–2662. doi: 10.1101/gad.1241204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleren P, & Parker R (2001). Defects in the mRNA export factors Rat7p, Gle1p, Mex67p, and Rat8p cause hyperadenylation during 3 ‘-end formation of nascent transcripts. Rna-a Publication of the Rna Society, 7(5), 753–764. doi:Doi 10.1017/S1355838201010147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honorine R, Mosrin-Huaman C, Hervouet-Coste N, Libri D, & Rahmouni AR (2011). Nuclear mRNA quality control in yeast is mediated by Nrd1 co-transcriptional recruitment, as revealed by the targeting of Rho-induced aberrant transcripts. Nucleic Acids Res, 39(7), 2809–2820. doi: 10.1093/nar/gkq1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseley J, Kotovic K, El Hage A, & Tollervey D (2007). Trf4 targets ncRNAs from telomeric and rDNA spacer regions and functions in rDNA copy number control. EMBO J, 26(24), 4996–5006. doi: 10.1038/sj.emboj.7601921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrossova D, Sikorsky T, Potesil D, Bartosovic M, Pasulka J, Zdrahal Z, Stefl R, & Vanacova S (2015). RBM7 subunit of the NEXT complex binds U-rich sequences and targets 3’-end extended forms of snRNAs. Nucleic Acids Res, 43(8), 4236–4248. doi: 10.1093/nar/gkv240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin JP, Sheth A, & Manley JL (2011). RNAP II CTD phosphorylated on threonine-4 is required for histone mRNA 3’ end processing. Science, 334(6056), 683–686. doi: 10.1126/science.1206034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter GO, Fox MJ, Smith-Kinnaman WR, Gogol M, Fleharty B, & Mosley AL (2016). Phosphatase Rtr1 Regulates Global Levels of Serine 5 RNA Polymerase II C-Terminal Domain Phosphorylation and Cotranscriptional Histone Methylation. Mol Cell Biol, 36(17), 2236–2245. doi: 10.1128/MCB.00870-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T (2013). Quality control systems for aberrant mRNAs induced by aberrant translation elongation and termination. Biochim Biophys Acta, 1829(6–7), 634–642. doi: 10.1016/j.bbagrm.2013.02.004 [DOI] [PubMed] [Google Scholar]
- PDB ID: 52CY. Irani S, Yogesha SD, Mayfield J, Zhang M, Zhang Y, Matthews WL, Nie G, Prescott NA, & Zhang YJ (2016). Structure of Saccharomyces cerevisiae Rtr1 reveals an active site for an atypical phosphatase. Sci Signal, 9(417), ra24. doi: 10.1126/scisignal.aad4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Yamada H, Kaziro Y, & Mizumoto K (1987). Messenger-Rna Guanylyltransferase from Saccharomyces-Cerevisiae - Large-Scale Purification, Subunit Functions, and Subcellular-Localization. Journal of Biological Chemistry, 262(5), 1989–1995. [PubMed] [Google Scholar]
- Izaurralde E, Lewis J, Gamberi C, Jarmolowski A, McGuigan C, & Mattaj IW (1995). A cap-binding protein complex mediating U snRNA export. Nature, 376(6542), 709–712. doi: 10.1038/376709a0 [DOI] [PubMed] [Google Scholar]
- Izaurralde E, Lewis J, McGuigan C, Jankowska M, Darzynkiewicz E, & Mattaj IW (1994). A nuclear cap binding protein complex involved in pre-mRNA splicing. Cell, 78(4), 657–668. [DOI] [PubMed] [Google Scholar]
- Jamonnak N, Creamer TJ, Darby MM, Schaughency P, Wheelan SJ, & Corden JL (2011). Yeast Nrd1, Nab3, and Sen1 transcriptome-wide binding maps suggest multiple roles in post-transcriptional RNA processing. RNA, 17(11), 2011–2025. doi: 10.1261/rna.2840711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenal M, Elkon R, Loayza-Puch F, van Haaften G, Kühn U, Menzies FM, Oude Vrielink JAF, Bos AJ, Drost J, Rooijers K, Rubinstein DC, & Agami R (2012). The Poly(A)-Binding Protein Nuclear 1 Suppresses Alternative Cleavage and Polyadenylation Sites. Cell, 149(3), 538–553. doi: 10.1016/j.cell.2012.03.022 [DOI] [PubMed] [Google Scholar]
- Jiao X, Chang JH, Kilic T, Tong L, & Kiledjian M (2013). A mammalian pre-mRNA 5’ end capping quality control mechanism and an unexpected link of capping to pre-mRNA processing. Mol Cell, 50(1), 104–115. doi: 10.1016/j.molcel.2013.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Xiang S, Oh C, Martin CE, Tong L, & Kiledjian M (2010). Identification of a quality-control mechanism for mRNA 5’-end capping. Nature, 467(7315), 608–611. doi: 10.1038/nature09338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno-Gonzalez S, Haaning LL, Malagon F, & Jensen TH (2010). The yeast 5’−3’ exonuclease Rat1p functions during transcription elongation by RNA polymerase II. Mol Cell, 37(4), 580–587. doi: 10.1016/j.molcel.2010.01.019 [DOI] [PubMed] [Google Scholar]
- Johnson SA, Cubberley G, & Bentley DL (2009). Cotranscriptional Recruitment of the mRNA Export Factor Yra1 by Direct Interaction with the 3′ End Processing Factor Pcf11. Molecular Cell, 33(2), 215–226. doi: 10.1016/j.molcel.2008.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida D (2016). The reciprocal regulation between splicing and 3’-end processing. Wiley Interdiscip Rev RNA, 7(4), 499–511. doi: 10.1002/wrna.1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karousis ED, Nasif S, & Muhlemann O (2016). Nonsense-mediated mRNA decay: novel mechanistic insights and biological impact. Wiley Interdiscip Rev RNA, 7(5), 661–682. doi: 10.1002/wrna.1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi J, Mischo H, Braglia P, Rondon A, & Proudfoot NJ (2008). Budding yeast RNA polymerases I and II employ parallel mechanisms of transcriptional termination. Genes Dev, 22(8), 1082–1092. doi: 10.1101/gad.463408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwitz Y, Kuhn U, Lilie H, Knoth A, Scheuermann T, Friedrich H, Schwarz E, & Wahle E (2003). Stimulation of poly(A) polymerase through a direct interaction with the nuclear poly(A) binding protein allosterically regulated by RNA. EMBO J, 22(14), 3705–3714. doi: 10.1093/emboj/cdg347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodor YL, Menet JS, Tolan M, & Rosbash M (2012). Cotranscriptional splicing efficiency differs dramatically between Drosophila and mouse. RNA, 18(12), 2174–2186. doi: 10.1261/rna.034090.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodor YL, Rodriguez J, Abruzzi KC, Tang CH, Marr MT 2nd, & Rosbash M (2011). Nascent-seq indicates widespread cotranscriptional pre-mRNA splicing in Drosophila. Genes Dev, 25(23), 2502–2512. doi: 10.1101/gad.178962.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilchert C, Wittmann S, Passoni M, Shah S, Granneman S, & Vasiljeva L (2015). Regulation of mRNA Levels by Decay-Promoting Introns that Recruit the Exosome Specificity Factor Mmi1. Cell Rep, 13(11), 2504–2515. doi: 10.1016/j.celrep.2015.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HD, Choe J, & Seo YS (1999). The sen1(+) gene of Schizosaccharomyces pombe, a homologue of budding yeast SEN1, encodes an RNA and DNA helicase. Biochemistry, 38(44), 14697–14710. [DOI] [PubMed] [Google Scholar]
- Kim M, Ahn SH, Krogan NJ, Greenblatt JF, & Buratowski S (2004a). Transitions in RNA polymerase II elongation complexes at the 3’ ends of genes. EMBO J, 23(2), 354–364. doi: 10.1038/sj.emboj.7600053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Krogan NJ, Vasiljeva L, Rando OJ, Nedea E, Greenblatt JF, & Buratowski S (2004b). The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature, 432(7016), 517–522. doi: 10.1038/nature03041 [DOI] [PubMed] [Google Scholar]
- Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, Harper JW, & Gygi SP (2011). Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell, 44(2), 325–340. doi: 10.1016/j.molcel.2011.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kireeva ML, Nedialkov YA, Cremona GH, Purtov YA, Lubkowska L, Malagon F, Burton ZF, Strathern JN, & Kashlev M (2008). Transient reversal of RNA polymerase II active site closing controls fidelity of transcription elongation. Mol Cell, 30(5), 557–566. doi: 10.1016/j.molcel.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarnitsky P, Cho EJ, & Buratowski S (2000). Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev, 14(19), 2452–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek K, Cerna H, Holub P, Pasulka J, Hrossova D, Loehr F, Hofr C, Vanacova S, & Stefl R (2012). Serine phosphorylation and proline isomerization in RNAP II CTD control recruitment of Nrd1. Genes Dev, 26(17), 1891–1896. doi: 10.1101/gad.192781.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner JN, Pearson EL, & Moore C (2011). Unravelling the means to an end: RNA polymerase II transcription termination. Nat Rev Mol Cell Biol, 12(5), 283–294. doi: 10.1038/nrm3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufel J, Bousquet-Antonelli C, Beggs JD, & Tollervey D (2004). Nuclear pre-mRNA decapping and 5’ degradation in yeast require the Lsm2–8p complex. Mol Cell Biol, 24(21), 9646–9657. doi: 10.1128/MCB.24.21.9646-9657.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak H, Fuda NJ, Core LJ, Lis JT (2013). Precise maps of RNA polymerase reveal how promoters direct initiation and pausing. Science (339), 950–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Gardini A, Zhang A, & Shiekhattar R (2015). Integrator mediates the biogenesis of enhancer RNAs. Nature, 525(7569), 399–403. doi: 10.1038/nature14906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson DR, Zenklusen D, Wu B, Chao JA, & Singer RH (2011). Real-time observation of transcription initiation and elongation on an endogenous yeast gene. Science, 332(6028), 475–478. doi: 10.1126/science.1202142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, & Glaunsinger BA (2009). Aberrant herpesvirus-induced polyadenylation correlates with cellular messenger RNA destruction. PLoS Biol, 7(5), e1000107. doi: 10.1371/journal.pbio.1000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemay JF, Larochelle M, Marguerat S, Atkinson S, Bahler J, & Bachand F (2014). The RNA exosome promotes transcription termination of backtracked RNA polymerase II. Nat Struct Mol Biol, 21(10), 919–926. doi: 10.1038/nsmb.2893 [DOI] [PubMed] [Google Scholar]
- Lemieux C, Marguerat S, Lafontaine J, Barbezier N, Bahler J, & Bachand F (2011). A Pre-mRNA degradation pathway that selectively targets intron-containing genes requires the nuclear poly(A)-binding protein. Mol Cell, 44(1), 108–119. doi: 10.1016/j.molcel.2011.06.035 [DOI] [PubMed] [Google Scholar]
- Lennon JC, Wind M, Saunders L, Hock MB, & Reines D (1998). Mutations in RNA polymerase II and elongation factor SII severely reduce mRNA levels in Saccharomyces cerevisiae. Molecular and Cellular Biology, 18(10), 5771–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PDB ID: 5MZN. Leonaite B, Han Z, Basquin J, Bonneau F, Libri D, Porrua O, & Conti E (2017). Sen1 has unique structural features grafted on the architecture of the Upf1-like helicase family. EMBO J, 36(11), 1590–1604. doi: 10.15252/embj.201696174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Tatomer DC, Luo Z, Wu H, Yang L, Chen LL, Cherry S, & Wilusz JE (2017). The Output of Protein-Coding Genes Shifts to Circular RNAs When the Pre-mRNA Processing Machinery Is Limiting. Mol Cell, 68(5), 940–954 e943. doi: 10.1016/j.molcel.2017.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libri D, Dower K, Boulay J, Thomsen R, Rosbash M, & Jensen TH (2002). Interactions between mRNA Export Commitment, 3’-End Quality Control, and Nuclear Degradation. Molecular and Cellular Biology, 22(23), 8254–8266. doi: 10.1128/mcb.22.23.8254-8266.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licatalosi DD, Geiger G, Minet M, Schroeder S, Cilli K, McNeil JB, & Bentley DL (2002). Functional interaction of yeast pre-mRNA 3’ end processing factors with RNA polymerase II. Mol Cell, 9(5), 1101–1111. [DOI] [PubMed] [Google Scholar]
- Lim SJ, Boyle PJ, Chinen M, Dale RK, & Lei EP (2013). Genome-wide localization of exosome components to active promoters and chromatin insulators in Drosophila. Nucleic Acids Res, 41(5), 2963–2980. doi: 10.1093/nar/gkt037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loya TJ, O’Rourke TW, & Reines D (2012). A genetic screen for terminator function in yeast identifies a role for a new functional domain in termination factor Nab3. Nucleic Acids Res, 40(15), 7476–7491. doi: 10.1093/nar/gks377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubas M, Christensen MS, Kristiansen MS, Domanski M, Falkenby LG, Lykke-Andersen S, Anderson JS, Dziembowski A, & Jensen TH (2011). Interaction profiling identifies the human nuclear exosome targeting complex. Mol Cell, 43(4), 624–637. doi: 10.1016/j.molcel.2011.06.028 [DOI] [PubMed] [Google Scholar]
- Lubas M, Andersen PR, Schein A, Dziembowski A, Kudla G, & Jensen TH (2015). The human nuclear exosome targeting complex is loaded onto newly synthesized RNA to direct early ribonucleolysis. Cell Rep, 10(2), 178–192. doi: 10.1016/j.celrep.2014.12.026 [DOI] [PubMed] [Google Scholar]
- Luna R, Jimeno S, Marin M, Huertas P, Garcia-Rubio M, & Aguilera A (2005). Interdependence between transcription and mRNP processing and export, and its impact on genetic stability. Mol Cell, 18(6), 711–722. doi: 10.1016/j.molcel.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Lunde BM, Reichow SL, Kim M, Suh H, Leeper TC, Yang F, Mutschler H, Buratowski S, Meinhart A, & Varani G (2010). Cooperative interaction of transcription termination factors with the RNA polymerase II C-terminal domain. Nat Struct Mol Biol, 17(10), 1195–1201. doi: 10.1038/nsmb.1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo MJ, & Reed R (1999). Splicing is required for rapid and efficient mRNA export in metazoans. Proceedings of the National Academy of Sciences of the United States of America, 96(26), 14937–14942. doi:DOI 10.1073/pnas.96.26.14937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Johnson AW, & Bentley DL (2006). The role of Rat1 in coupling mRNA 3’-end processing to transcription termination: implications for a unified allosteric-torpedo model. Genes Dev, 20(8), 954–965. doi: 10.1101/gad.1409106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz CS, & Moreira A (2011). Alternative mRNA polyadenylation in eukaryotes: an effective regulator of gene expression. Wiley Interdisciplinary Reviews: RNA, 2(1), 22–31. doi: 10.1002/wrna.47 [DOI] [PubMed] [Google Scholar]
- Malagon F, Kireeva ML, Shafer BK, Lubkowska L, Kashlev M, & Strathern JN (2006). Mutations in the Saccharomyces cerevisiae RPB1 gene conferring hypersensitivity to 6-azauracil. Genetics, 172(4), 2201–2209. doi: 10.1534/genetics.105.052415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal SS, Chu C, Wada T, Handa H, Shatkin AJ, & Reinberg D (2004). Functional interactions of RNA-capping enzyme with factors that positively and negatively regulate promoter escape by RNA polymerase II. Proceedings of the National Academy of Sciences of the United States of America, 101(20), 7572–7577. doi: 10.1073/pnas.0401493101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Rucobo Fuensanta W., Kohler R, van de Waterbeemd M, Heck Albert J. R., Hemann M, Herzog F, Stark H, & Cramer P (2015). Molecular Basis of Transcription-Coupled Pre-mRNA Capping. Molecular Cell, 58(6), 1079–1089. doi: 10.1016/j.molcel.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Mayer A, di Iulio J, Maleri S, Eser U, Vierstra J, Reynolds A, Sandstrom R, Stamatoyannopoulos JA, & Churchman LS (2015). Native elongating transcript sequencing reveals human transcriptional activity at nucleotide resolution. Cell, 161(3), 541–554. doi: 10.1016/j.cell.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Heidemann M, Lidschreiber M, Schreieck A, Sun M, Hintermair C, Kremmer E, Eick D, & Cramer P (2012). CTD tyrosine phosphorylation impairs termination factor recruitment to RNA polymerase II. Science, 336(6089), 1723–1725. doi: 10.1126/science.1219651 [DOI] [PubMed] [Google Scholar]
- Mayer A, Landry HM, & Churchman LS (2017). Pause & go: from the discovery of RNA polymerase pausing to its functional implications. Curr Opin Cell Biol, 46, 72–80. doi: 10.1016/j.ceb.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Lidschreiber M, Siebert M, Leike K, Soding J, & Cramer P (2010). Uniform transitions of the general RNA polymerase II transcription complex. Nat Struct Mol Biol, 17(10), 1272–1278. doi: 10.1038/nsmb.1903 [DOI] [PubMed] [Google Scholar]
- PDB ID: 1H2T. Mazza C, Segref A, Mattaj IW, & Cusack S (2002). Large-scale induced fit recognition of an m(7)GpppG cap analogue by the human nuclear cap-binding complex. EMBO J, 21(20), 5548–5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken S, Fong N, Rosonina E, Yankulov K, Brothers G, Siderovski D, Hessel A, Foster S, Shuman S, & Bentley DL (1997a). 5’-Capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II. Genes Dev, 11(24), 3306–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken S, Fong N, Yankulov K, Ballantyne S, Pan G, Greenblatt J, Patterson SD, Wickens M, & Bentley DL (1997b). The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature, 385(6614), 357–361. doi: 10.1038/385357a0 [DOI] [PubMed] [Google Scholar]
- Meola N, Domanski M, Karadoulama E, Chen Y, Gentil C, Pultz D, Vitting-Seerup K, Lykke-Andersen S, Andersen JS, Sandelin A, Jensen TH (2016). Identification of a Nuclear Exosome Decay Pathway for Processed Transcripts. Mol Cell, 64(3), 520–533. doi: 10.1016/j.molcel.2016.09.025 [DOI] [PubMed] [Google Scholar]
- Milligan L, Torchet C, Allmang C, Shipman T, & Tollervey D (2005). A nuclear surveillance pathway for mRNAs with defective polyadenylation. Mol Cell Biol, 25(22), 9996–10004. doi: 10.1128/MCB.25.22.9996-10004.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischo HE, Chun Y, Harlen KM, Smalec BM, Dhir S, Churchman LS, & Buratowski S (2018). Cell-Cycle Modulation of Transcription Termination Factor Sen1. Mol Cell, 70(2), 312–326 e317. doi: 10.1016/j.molcel.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischo HE, Gomez-Gonzalez B, Grzechnik P, Rondon AG, Wei W, Steinmetz L, Aguilera A, & Proudfoot NJ (2011). Yeast Sen1 helicase protects the genome from transcription-associated instability. Mol Cell, 41(1), 21–32. doi: 10.1016/j.molcel.2010.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischo HE, & Proudfoot NJ (2013). Disengaging polymerase: Terminating RNA polymerase II transcription in budding yeast. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms, 1829(1), 174–185. doi: 10.1016/j.bbagrm.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli T, & Spector DL (1999). RNA polymerase II targets pre-mRNA splicing factors to transcription sites in vivo. Mol Cell, 3(6), 697–705. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Petfalski E, Shevchenko A, Mann M, & Tollervey D (1997). The exosome: a conserved eukaryotic RNA processing complex containing multiple 3’-->5’ exoribonucleases. Cell, 91(4), 457–466. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Schwartzfarb EM, Silver PA, & Yu MC (2006). Differential recruitment of the splicing machinery during transcription predicts genome-wide patterns of mRNA splicing. Mol Cell, 24(6), 903–915. doi: 10.1016/j.molcel.2006.12.006 [DOI] [PubMed] [Google Scholar]
- Morales JC, Richard P, Rommel A, Fattah FJ, Motea EA, Patidar PL, Xiao L, Leskov K, Wu SY, Hittelman WN, Chiang CM, Manley JL, & Boothman DA (2014). Kub5-Hera, the human Rtt103 homolog, plays dual functional roles in transcription termination and DNA repair. Nucleic Acids Res, 42(8), 4996–5006. doi: 10.1093/nar/gku160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley AL, Pattenden SG, Carey M, Venkatesh S, Gilmore JM, Florens L, Workman JL, & Washburn MP (2009). Rtr1 is a CTD phosphatase that regulates RNA polymerase II during the transition from serine 5 to serine 2 phosphorylation. Mol Cell, 34(2), 168–178. doi: 10.1016/j.molcel.2009.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouaikel J, Causse SZ, Rougemaille M, Daubenton-Carafa Y, Blugeon C, Lemoine S, Devaux F, Darzacq X, & Libri D (2013). High-frequency promoter firing links THO complex function to heavy chromatin formation. Cell Rep, 5(4), 1082–1094. doi: 10.1016/j.celrep.2013.10.013 [DOI] [PubMed] [Google Scholar]
- Muse GW, Gilchrist DA, Nechaev S, Shah R, Parker JS, Grissom SF, Zeitlinger J, & Adelman K (2007). RNA polymerase is poised for activation across the genome. Nature Genetics, 39(12), 1507–1511. doi: 10.1038/ng.2007.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag A, & Steitz JA (2012). Tri-snRNP-associated proteins interact with subunits of the TRAMP and nuclear exosome complexes, linking RNA decay and pre-mRNA splicing. RNA Biol, 9(3), 334–342. doi: 10.4161/rna.19431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechaev S, Fargo DC, dos Santos G, Liu L, Gao Y, & Adelman K (2010). Global analysis of short RNAs reveals widespread promoter-proximal stalling and arrest of Pol II in Drosophila. Science, 327(5963), 335–338. doi: 10.1126/science.1181421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemec CM, Yang F, Gilmore JM, Hintermair C, Ho YH, Tseng SC, Heidemann M, Zhang Y, Florens L, Gasch AP, Eick D, Washburn MP, Varani G, & Ansari AZ (2017). Different phosphoisoforms of RNA polymerase II engage the Rtt103 termination factor in a structurally analogous manner. Proc Natl Acad Sci U S A, 114(20), E3944–E3953. doi: 10.1073/pnas.1700128114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Galej WP, Bai XC, Savva CG, Newman AJ, Scheres SH, & Nagai K (2015). The architecture of the spliceosomal U4/U6.U5 tri-snRNP. Nature, 523(7558), 47–52. doi: 10.1038/nature14548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Galej WP, Fica SM, Lin PC, Newman AJ, & Nagai K (2016a). CryoEM structures of two spliceosomal complexes: starter and dessert at the spliceosome feast. Curr Opin Struct Biol, 36, 48–57. doi: 10.1016/j.sbi.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen THD, Galej WP, Bai XC, Oubridge C, Newman AJ, Scheres SHW, & Nagai K (2016b). Cryo-EM structure of the yeast U4/U6.U5 tri-snRNP at 3.7 A resolution. Nature, 530(7590), 298–302. doi: 10.1038/nature16940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Xu C, Guo X, Hunter GO, Kuznetsova OV, Tempel W, Marcon E, Zhong G, Guo H, Huo WH, Li J, Young P, Olsen JB, Wan C, Loppnau P, El Bakkouri M, Senisterra GA, He H, Huang H, Sidhu SS, Emili A, Murphy S, Mosley AL, Arrowsmith CH, Min J, & Greenblatt JF (2014). RPRD1A and RPRD1B are human RNA polymerase II C-terminal domain scaffolds for Ser5 dephosphorylation. Nat Struct Mol Biol, 21(8), 686–695. doi: 10.1038/nsmb.2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen TW, & Graveley BR (2010). Expansion of the eukaryotic proteome by alternative splicing. Nature, 463(7280), 457–463. doi: 10.1038/nature08909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima T, Gomes T, Grosso ARF, Kimura H, Dye MJ, Dhir S, Carmo-Fonseca M, & Proudfoot NJ (2015). Mammalian NET-Seq Reveals Genome-wide Nascent Transcription Coupled to RNA Processing. Cell, 161(3), 526–540. doi: 10.1016/j.cell.2015.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima T, Rebelo K, Gomes T, Grosso AR, Proudfoot NJ, & Carmo-Fonseca M (2018). RNA Polymerase II Phosphorylated on CTD Serine 5 Interacts with the Spliceosome during Co-transcriptional Splicing. Mol Cell, 72(2), 369–379 e364. doi: 10.1016/j.molcel.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudler E (2012). RNA polymerase backtracking in gene regulation and genome instability. Cell, 149(7), 1438–1445. doi: 10.1016/j.cell.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly D, Kuznetsova OV, Laitem C, Zaborowska J, Dienstbier M, & Murphy S (2014). Human snRNA genes use polyadenylation factors to promote efficient transcription termination. Nucleic Acids Res, 42(1), 264–275. doi: 10.1093/nar/gkt892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi MD, Ren L, Wall JS, Gould KL, & Walz T (2007). Structural characterization of the fission yeast U5.U2/U6 spliceosome complex. Proc Natl Acad Sci U S A, 104(9), 3195–3200. doi: 10.1073/pnas.0611591104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohle C, Tesorero R, Schermann G, Dobrev N, Sinning I, & Fischer T (2016). Transient RNA-DNA Hybrids Are Required for Efficient Double-Strand Break Repair. Cell, 167(4), 1001–1013 e1007. doi: 10.1016/j.cell.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Palumbo MC, Farina L, & Paci P (2015). Kinetics effects and modeling of mRNA turnover. Wiley Interdiscip Rev RNA, 6(3), 327–336. doi: 10.1002/wrna.1277 [DOI] [PubMed] [Google Scholar]
- Pandya-Jones A, & Black DL (2009). Co-transcriptional splicing of constitutive and alternative exons. RNA, 15(10), 1896–1908. doi: 10.1261/rna.1714509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kang M, & Kim M (2015). Unraveling the mechanistic features of RNA polymerase II termination by the 5’−3’ exoribonuclease Rat1. Nucleic Acids Res, 43(5), 2625–2637. doi: 10.1093/nar/gkv133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson E, & Moore C (2014). The evolutionarily conserved Pol II flap loop contributes to proper transcription termination on short yeast genes. Cell Rep, 9(3), 821–828. doi: 10.1016/j.celrep.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson EL, & Moore CL (2013). Dismantling promoter-driven RNA polymerase II transcription complexes in vitro by the termination factor Rat1. J Biol Chem, 288(27), 19750–19759. doi: 10.1074/jbc.M112.434985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Schwer B & Shuman S (2003) Interactions between fission yeast Cdk9, its cyclin partner Pch1, and mRNA capping enzyme Pct1 suggest an elongation checkpoint for mRNA quality control. J. Biol. Chem 278, 7180–7188. doi: 10.1074/jbc.M211713200 [DOI] [PubMed] [Google Scholar]
- Pefanis E, Wang J, Rothschild G, Lim J, Chao J, Rabadan R, Economides AN, & Basu U (2014). Noncoding RNA transcription targets AID to divergently transcribed loci in B cells. Nature, 514(7522), 389–393. doi: 10.1038/nature13580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pefanis E, Wang J, Rothschild G, Lim J, Kazadi D, Sun J, Federation A, Chao J, Elliott O, Liu ZP, Economides AN, Bradner JE, Rabadan R, & Basu U (2015). RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity. Cell, 161(4), 774–789. doi: 10.1016/j.cell.2015.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales R, & Bentley D (2009). “Cotranscriptionality”: the transcription elongation complex as a nexus for nuclear transactions. Mol Cell, 36(2), 178–191. doi: 10.1016/j.molcel.2009.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz Krummel DA, Oubridge C, Leung AK, Li J, & Nagai K (2009). Crystal structure of human spliceosomal U1 snRNP at 5.5 A resolution. Nature, 458(7237), 475–480. doi: 10.1038/nature07851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrua O, Boudvillain M, & Libri D (2016). Transcription Termination: Variations on Common Themes. Trends Genet, 32(8), 508–522. doi: 10.1016/j.tig.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Porrua O, Hobor F, Boulay J, Kubicek K, D’Aubenton-Carafa Y, Gudipati RK, Stefl R, & Libri D (2012). In vivo SELEX reveals novel sequence and structural determinants of Nrd1-Nab3-Sen1-dependent transcription termination. EMBO J, 31(19), 3935–3948. doi: 10.1038/emboj.2012.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrua O, & Libri D (2013). A bacterial-like mechanism for transcription termination by the Sen1p helicase in budding yeast. Nat Struct Mol Biol, 20(7), 884–891. doi: 10.1038/nsmb.2592 [DOI] [PubMed] [Google Scholar]
- Porrua O, & Libri D (2015). Transcription termination and the control of the transcriptome: why, where and how to stop. Nat Rev Mol Cell Biol, 16(3), 190–202. doi: 10.1038/nrm3943 [DOI] [PubMed] [Google Scholar]
- Preker P, Nielsen J, Kammler S, Lykke-Andersen S, Christensen MS, Mapendano CK, Schierup MH, & Jensen TH (2008). RNA exosome depletion reveals transcription upstream of active human promoters. Science, 322(5909), 1851–1854. doi: 10.1126/science.1164096 [DOI] [PubMed] [Google Scholar]
- Preker P, Almvig K, Christensen MS, Valen E, Mapendano CK, Sandelin A, & Jensen TH (2011). PROMoter uPstream Transcripts share characteristics with mRNAs and are produced upstream of all three major types of mammalian promoters. Nucleic Acids Res, 39(16), 7179–7193. doi: 10.1093/nar/gkr370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot NJ (2016). Transcriptional termination in mammals: Stopping the RNA polymerase II juggernaut. Science, 352(6291), aad9926. doi: 10.1126/science.aad9926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen EB, & Lis JT (1993). In vivo transcriptional pausing and cap formation on three Drosophila heat shock genes. Proc Natl Acad Sci U S A, 90(17), 7923–7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PDB ID: 5LQW. Rauhut R, Fabrizio P, Dybkov O, Hartmuth K, Pena V, Chari A, Kumar V, Lee CT, Urlaub H, Kastner B, Stark H, & Luhrmann R (2016). Molecular architecture of the Saccharomyces cerevisiae activated spliceosome. Science, 353(6306), 1399–1405. doi: 10.1126/science.aag1906 [DOI] [PubMed] [Google Scholar]
- Reed R, & Hurt E (2002). A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell, 108(4), 523–531. [DOI] [PubMed] [Google Scholar]
- Reines D, Conaway RC, & Conaway JW (1999). Mechanism and regulation of transcriptional elongation by RNA polymerase II. Curr Opin Cell Biol, 11(3), 342–346. doi: 10.1016/S0955-0674(99)80047-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reines D, Ghanouni P, Gu W, Mote J Jr., & Powell W (1993). Transcription elongation by RNA polymerase II: mechanism of SII activation. Cell Mol Biol Res, 39(4), 331–338. [PubMed] [Google Scholar]
- Richard P, & Manley JL (2009). Transcription termination by nuclear RNA polymerases. Genes Dev, 23(11), 1247–1269. doi: 10.1101/gad.1792809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GC, Gooding C, Mak HY, Proudfoot NJ, & Smith CW (1998). Co-transcriptional commitment to alternative splice site selection. Nucleic Acids Res, 26(24), 5568–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Molina Juan B., Tseng Sandra C., Simonett Shane P., Taunton J, & Ansari Aseem Z. (2016). Engineered Covalent Inactivation of TFIIH-Kinase Reveals an Elongation Checkpoint and Results in Widespread mRNA Stabilization. Molecular Cell, 63(3), 433–444. doi: 10.1016/j.molcel.2016.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondon AG, Mischo HE, Kawauchi J, & Proudfoot NJ (2009). Fail-safe transcriptional termination for protein-coding genes in S. cerevisiae. Mol Cell, 36(1), 88–98. doi: 10.1016/j.molcel.2009.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K, Gabunilas J, Gillespie A, Ngo D, & Chanfreau GF (2016). Common genomic elements promote transcriptional and DNA replication roadblocks. Genome Res, 26(10), 1363–1375. doi: 10.1101/gr.204776.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski M, Dichtl B, Hubner W, & Keller W (2003). Independent functions of yeast Pcf11p in pre-mRNA 3 ‘ end processing and in transcription termination. Embo Journal, 22(9), 2167–2177. doi:DOI 10.1093/emboj/cdg200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saguez C, Schmid M, Olesen JR, Ghazy MA, Qu X, Poulsen MB, Nasser T, Moore C, & Jensen TH (2008). Nuclear mRNA surveillance in THO/sub2 mutants is triggered by inefficient polyadenylation. Mol Cell, 31(1), 91–103. doi: 10.1016/j.molcel.2008.04.030 [DOI] [PubMed] [Google Scholar]
- Santos-Pereira JM, & Aguilera A (2015). R loops: new modulators of genome dynamics and function. Nat Rev Genet, 16(10), 583–597. doi: 10.1038/nrg3961 [DOI] [PubMed] [Google Scholar]
- Schaefke B, Sun W, Li YS, Fang L, & Chen W (2018). The evolution of posttranscriptional regulation. Wiley Interdiscip Rev RNA, e1485. doi: 10.1002/wrna.1485 [DOI] [PubMed] [Google Scholar]
- Schaughency P, Merran J, & Corden JL (2014). Genome-wide mapping of yeast RNA polymerase II termination. PLoS Genet, 10(10), e1004632. doi: 10.1371/journal.pgen.1004632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, & Jensen TH (2018). Controlling nuclear RNA levels. Nat Rev Genet doi: 10.1038/s41576-018-0013-2 [DOI] [PubMed] [Google Scholar]
- Schmidt K, & Butler JS (2013). Nuclear RNA surveillance: role of TRAMP in controlling exosome specificity. Wiley Interdiscip Rev RNA, 4(2), 217–231. doi: 10.1002/wrna.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Kudla G, Wlotzka W, Tuck A, & Tollervey D (2012). Transcriptome-wide analysis of exosome targets. Mol Cell, 48(3), 422–433. doi: 10.1016/j.molcel.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonemann L, Kuhn U, Martin G, Schafer P, Gruber AR, Keller W, Zavolan M, & Wahle E (2014). Reconstitution of CPSF active in polyadenylation: recognition of the polyadenylation signal by WDR33. Genes Dev, 28(21), 2381–2393. doi: 10.1101/gad.250985.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreieck A, Easter AD, Etzold S, Wiederhold K, Lidschreiber M, Cramer P, & Passmore LA (2014). RNA polymerase II termination involves C-terminal-domain tyrosine dephosphorylation by CPF subunit Glc7. Nat Struct Mol Biol, 21(2), 175–179. doi: 10.1038/nsmb.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PDB ID: 6FSZ. Schuller JM, Falk S, Fromm L, Hurt E, & Conti E (2018). Structure of the nuclear exosome captured on a maturing preribosome. Science, 360(6385), 219–222. doi: 10.1126/science.aar5428 [DOI] [PubMed] [Google Scholar]
- Schulz D, Schwalb B, Kiesel A, Baejen C, Torkler P, Gagneur J, Soeding J, & Cramer P (2013). Transcriptome surveillance by selective termination of noncoding RNA synthesis. Cell, 155(5), 1075–1087. doi: 10.1016/j.cell.2013.10.024 [DOI] [PubMed] [Google Scholar]
- Shi Y, Di Giammartino DC, Taylor D, Sarkeshik A, Rice WJ, Yates JR 3rd, Frank J, & Manley JL (2009). Molecular architecture of the human pre-mRNA 3’ processing complex. Mol Cell, 33(3), 365–376. doi: 10.1016/j.molcel.2008.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, & Parker R (2014). Quality control of assembly-defective U1 snRNAs by decapping and 5’-to-3’ exonucleolytic digestion. Proc Natl Acad Sci U S A, 111(32), E3277–3286. doi: 10.1073/pnas.1412614111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S (1995). Capping enzyme in eukaryotic mRNA synthesis. Prog Nucleic Acid Res Mol Biol, 50(0079–6603 (Print)), 101–129. [DOI] [PubMed] [Google Scholar]
- Sigurdsson S, Dirac-Svejstrup AB, & Svejstrup JQ (2010). Evidence that transcript cleavage is essential for RNA polymerase II transcription and cell viability. Mol Cell, 38(2), 202–210. doi: 10.1016/j.molcel.2010.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar JR, Ferris AL, Wu X, Saraf A, Khanna KK, Florens L, Washburn MP, Hughes SH, & Pagano M (2015). The Integrator complex controls the termination of transcription at diverse classes of gene targets. Cell Res, 25(3), 288–305. doi: 10.1038/cr.2015.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skourti-Stathaki K, Kamieniarz-Gdula K, & Proudfoot NJ (2014). R-loops induce repressive chromatin marks over mammalian gene terminators. Nature, 516(7531), 436–439. doi: 10.1038/nature13787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skourti-Stathaki K, Proudfoot NJ, & Gromak N (2011). Human senataxin resolves RNA/DNA hybrids formed at transcriptional pause sites to promote Xrn2-dependent termination. Mol Cell, 42(6), 794–805. doi: 10.1016/j.molcel.2011.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skruzny M, Schneider C, Racz A, Weng J, Tollervey D, & Hurt E (2009). An endoribonuclease functionally linked to perinuclear mRNP quality control associates with the nuclear pore complexes. PLoS Biol, 7(1), e8. doi: 10.1371/journal.pbio.1000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somesh BP, Reid J, Liu WF, Sogaard TM, Erdjument-Bromage H, Tempst P, & Svejstrup JQ (2005). Multiple mechanisms confining RNA polymerase II ubiquitylation to polymerases undergoing transcriptional arrest. Cell, 121(6), 913–923. doi: 10.1016/j.cell.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Song MG, Li Y, & Kiledjian M (2010). Multiple mRNA decapping enzymes in mammalian cells. Mol Cell, 40(3), 423–432. doi: 10.1016/j.molcel.2010.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R (2017). The nuts and bolts of the endogenous spliceosome. Wiley Interdiscip Rev RNA, 8(1). doi: 10.1002/wrna.1377 [DOI] [PubMed] [Google Scholar]
- Steinmetz EJ, Conrad NK, Brow DA, & Corden JL (2001). RNA-binding protein Nrd1 directs poly(A)-independent 3’-end formation of RNA polymerase II transcripts. Nature, 413(6853), 327–331. doi: 10.1038/35095090 [DOI] [PubMed] [Google Scholar]
- Steinmetz EJ, Ng SB, Cloute JP, & Brow DA (2006). cis- and trans-Acting determinants of transcription termination by yeast RNA polymerase II. Mol Cell Biol, 26(7), 2688–2696. doi: 10.1128/MCB.26.7.2688-2696.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steurer B, Janssens RC,Geverts B,Geijer ME, Wienholz F,Theil AF, Chang J, Dealy S, Pothof J, van Cappellen WA, Houtsmuller AB, & Marteijn JA (2018). Live-cell analysis of endogenous GFP-RPB1 uncovers rapid turnover of initiating and promoter-paused RNA Polymerase II. Proc Natl Acad Sc, 115(19), E4368–E4376. doi: 10.1073/pnas.1717920115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller JW, & Hall BD (2002). Evolution of the RNA polymerase II C-terminal domain. Proc Natl Acad Sci U S A, 99(9), 6091–6096. doi: 10.1073/pnas.082646199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svejstrup JQ (2007). Contending with transcriptional arrest during RNAPII transcript elongation. Trends Biochem Sci, 32(4), 165–171. doi: 10.1016/j.tibs.2007.02.005 [DOI] [PubMed] [Google Scholar]
- PDB ID: 3HOU. Sydow JF, Brueckner F, Cheung AC, Damsma GE, Dengl S, Lehmann E, Vassylyev D, & Cramer P (2009). Structural basis of transcription: mismatch-specific fidelity mechanisms and paused RNA polymerase II with frayed RNA. Mol Cell, 34(6), 710–721. doi: 10.1016/j.molcel.2009.06.002 [DOI] [PubMed] [Google Scholar]
- Szczepinska T, Kalisiak K, Tomecki R, Labno A, Borowski LS, Kulinski TM, Adamska D, Kosinska J, & Dziembowski A (2015). DIS3 shapes the RNA polymerase II transcriptome in humans by degrading a variety of unwanted transcripts. Genome Res, 25(11), 1622–1633. doi: 10.1101/gr.189597.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan-Wong SM, Zaugg JB, Camblong J, Xu Z, Zhang DW, Mischo HE, Ansari AZ, Luscombe NM, Steinmetz LM, & Proudfoot NJ (2012). Gene loops enhance transcriptional directionality. Science, 338(6107), 671–675. doi: 10.1126/science.1224350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut M, Kisseleva-Romanova E, Rougemaille M, Boulay J, & Libri D (2006). Transcription termination and nuclear degradation of cryptic unstable transcripts: a role for the nrd1-nab3 pathway in genome surveillance. Mol Cell, 23(6), 853–864. doi: 10.1016/j.molcel.2006.07.029 [DOI] [PubMed] [Google Scholar]
- Tian B, Hu J, Zhang H, & Lutz CS (2005). A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic Acids Res, 33(1), 201–212. doi: 10.1093/nar/gki158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, & Manley JL (2013). Alternative cleavage and polyadenylation: the long and short of it. Trends Biochem Sci, 38(6), 312–320. doi: 10.1016/j.tibs.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietjen JR, Zhang DW, Rodriguez-Molina JB, White BE, Akhtar MS, Heidemann M, Li X, Chapman RD, Shokat K, Keles S, Eick D, & Ansari AZ (2010). Chemical-genomic dissection of the CTD code. Nat Struct Mol Biol, 17(9), 1154–1161. doi: 10.1038/nsmb.1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilgner H, Knowles DG, Johnson R, Davis CA, Chakrabortty S, Djebali S, Curado J, Snyder M, Gingeras TR, & Guigo R (2012). Deep sequencing of subcellular RNA fractions shows splicing to be predominantly co-transcriptional in the human genome but inefficient for lncRNAs. Genome Res, 22(9), 1616–1625. doi: 10.1101/gr.134445.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topisirovic I, Svitkin YV, Sonenberg N, & Shatkin AJ (2011). Cap and cap-binding proteins in the control of gene expression. Wiley Interdiscip Rev RNA, 2(2), 277–298. doi: 10.1002/wrna.52 [DOI] [PubMed] [Google Scholar]
- Torchet C, Bousquet-Antonelli C, Milligan L, Thompson E, Kufel J, & Tollervey D (2002). Processing of 3’-extended read-through transcripts by the exosome can generate functional mRNAs. Mol Cell, 9(6), 1285–1296. [DOI] [PubMed] [Google Scholar]
- Tous C, Rondon AG, Garcia-Rubio M, Gonzalez-Aguilera C, Luna R, & Aguilera A (2011). A novel assay identifies transcript elongation roles for the Nup84 complex and RNA processing factors. EMBO J, 30(10), 1953–1964. doi: 10.1038/emboj.2011.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudek A, Porrua O, Kabzinski T, Lidschreiber M, Kubicek K, Fortova A, Lacroute F, Vanacova S, Cramer P, Stefl R, & Libri D (2014). Molecular Basis for Coordinating Transcription Termination with Noncoding RNA Degradation. Molecular Cell, 55(3), 467–481. doi: 10.1016/j.molcel.2014.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turowski TW, & Tollervey D (2015). Cotranscriptional events in eukaryotic ribosome synthesis. Wiley Interdiscip Rev RNA, 6(1), 129–139. doi: 10.1002/wrna.1263 [DOI] [PubMed] [Google Scholar]
- Uptain SM, Kane CM, & Chamberlin MJ (1997). Basic mechanisms of transcript elongation and its regulation. Annu Rev Biochem, 66, 117–172. doi: 10.1146/annurev.biochem.66.1.117 [DOI] [PubMed] [Google Scholar]
- Valen E, Preker P, Andersen PR, Zhao X, Chen Y, Ender C, Dueck A, Meister G, Sandelin A, & Jensen TH (2011). Biogenic mechanisms and utilization of small RNAs derived from human protein-coding genes. Nat Struct Mol Biol, 18(9), 1075–1082. doi: 10.1038/nsmb.2091 [DOI] [PubMed] [Google Scholar]
- van Hoof A, Lennertz P, & Parker R (2000). Yeast exosome mutants accumulate 3’-extended polyadenylated forms of U4 small nuclear RNA and small nucleolar RNAs. Mol Cell Biol, 20(2), 441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiljeva L, & Buratowski S (2006). Nrd1 interacts with the nuclear exosome for 3’ processing of RNA polymerase II transcripts. Mol Cell, 21(2), 239–248. doi: 10.1016/j.molcel.2005.11.028 [DOI] [PubMed] [Google Scholar]
- Vasiljeva L, Kim M, Mutschler H, Buratowski S, & Meinhart A (2008a). The Nrd1-Nab3-Sen1 termination complex interacts with the Ser5-phosphorylated RNA polymerase II C-terminal domain. Nat Struct Mol Biol, 15(8), 795–804. doi: 10.1038/nsmb.1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiljeva L, Kim M, Terzi N, Soares LM, & Buratowski S (2008b). Transcription termination and RNA degradation contribute to silencing of RNA polymerase II transcription within heterochromatin. Mol Cell, 29(3), 313–323. doi: 10.1016/j.molcel.2008.01.011 [DOI] [PubMed] [Google Scholar]
- Veloso A, Kirkconnell KS, Magnuson B, Biewen B, Paulsen MT, Wilson TE, & Ljungman M (2014). Rate of elongation by RNA polymerase II is associated with specific gene features and epigenetic modifications. Genome Res, 24(6), 896–905. doi: 10.1101/gr.171405.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viladevall L, St Amour CV, Rosebrock A, Schneider S, Zhang C, Allen JJ, … Fisher RP (2009). TFIIH and P-TEFb coordinate transcription with capping enzyme recruitment at specific genes in fission yeast. Mol Cell, 33(6), 738–751. doi: 10.1016/j.molcel.2009.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagschal A, Rousset E, Basavarajaiah P, Contreras X, Harwig A, Laurent-Chabalier S, Nakamura M, Chen X, Zhang K, Meziane O, Boyer F, Parrinello H, Berkhout B, Terzian C, Benkirane M, & Kiernan R (2012). Microprocessor, Setx, Xrn2, and Rrp6 Co-operate to Induce Premature Termination of Transcription by RNAPII. Cell, 150(6), 1147–1157. doi: 10.1016/j.cell.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba L, Gore SK, & Koshland D (2013). The homologous recombination machinery modulates the formation of RNA-DNA hybrids and associated chromosome instability. Elife, 2, e00505. doi: 10.7554/eLife.00505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Yourshaw M, Mamsa H, Rudnik-Schoneborn S, Menezes MP, Hong JE, Leong DW, Senderek J, Salman MS, Chitayat D, Seeman P, von Moers A, Geaul-Neumann L, Kornberg AJ, Castro-Gago M, Sobrido MJ, Sanefuji M, Shieh PB, Salamon N, Kim RC, Vinters HV, Chen Z, Zerres K, Ryan MM, Nelson SF, & Jen JC (2012). Mutations in the RNA exosome component gene EXOSC3 cause pontocerebellar hypoplasia and spinal motor neuron degeneration. Nat Genet, 44(6), 704–708. doi: 10.1038/ng.2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Yan C, Bai R, Wang L, Huang M, Wong CC, & Shi Y (2016). The 3.8 A structure of the U4/U6.U5 tri-snRNP: Insights into spliceosome assembly and catalysis. Science, 351(6272), 466–475. doi: 10.1126/science.aad6466 [DOI] [PubMed] [Google Scholar]
- Wang SW, Stevenson AL, Kearsey SE, Watt S, & Bahler J (2008). Global role for polyadenylation-assisted nuclear RNA degradation in posttranscriptional gene silencing. Mol Cell Biol, 28(2), 656–665. doi: 10.1128/MCB.01531-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani S, Yuda M, Fujiwara Y, Yamamoto M, Harada F, Ohkuma Y, & Hirose Y (2014). Vertebrate Ssu72 regulates and coordinates 3’-end formation of RNAs transcribed by RNA polymerase II. PLoS One, 9(8), e106040. doi: 10.1371/journal.pone.0106040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S, Gromak N, & Proudfoot NJ (2004). Human 5 ‘-> 3 ‘ exonuclease Xrn2 promotes transcription termination at co-transcriptional cleavage sites. Nature, 432(7016), 522–525. doi: 10.1038/nature03035 [DOI] [PubMed] [Google Scholar]
- Wilusz CJ, Wormington M, & Peltz SW (2001). The cap-to-tail guide to mRNA turnover. Nat Rev Mol Cell Biol, 2(4), 237–246. doi: 10.1038/35067025 [DOI] [PubMed] [Google Scholar]
- Wilusz JE (2018). A 360° view of circular RNAs: From biogenesis to functions. WIREs RNA 9, e1478 10.1002/wrna.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wind M, & Reines D (2000). Transcription elongation factor SII. Bioessays, 22(4), 327–336. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wind-Rotolo M, & Reines D (2001). Analysis of gene induction and arrest site transcription in yeast with mutations in the transcription elongation machinery. J Biol Chem, 276(15), 11531–11538. doi: 10.1074/jbc.M011322200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windhager L, Bonfert T, Burger K, Ruzsics Z, Krebs S, Kaufmann S, Malterer G, L’Hernault A, Schilhabel M, Schreiber S, Rosenstiel P, Zimmer R, Eick D, Friedel CC, & Dolken L (2012). Ultrashort and progressive 4sU-tagging reveals key characteristics of RNA processing at nucleotide resolution. Genome Res, 22(10), 2031–2042. doi: 10.1101/gr.131847.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlotzka W, Kudla G, Granneman S, & Tollervey D (2011). The nuclear RNA polymerase II surveillance system targets polymerase III transcripts. EMBO J, 30(9), 1790–1803. doi: 10.1038/emboj.2011.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LA, Mabin JW, Gangras P, & Singh G (2017). The exon junction complex: a lifelong guardian of mRNA fate. Wiley Interdiscip Rev RNA, 8(3). doi: 10.1002/wrna.1411 [DOI] [PubMed] [Google Scholar]
- Wyers F, Rougemaille M, Badis G, Rousselle JC, Dufour ME, Boulay J, Regnault B, Devaux F, Namane A, Seraphin B, Libri D, & Jacquier A (2005). Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell, 121(5), 725–737. doi: 10.1016/j.cell.2005.04.030 [DOI] [PubMed] [Google Scholar]
- PDB ID: 3O2Q. Xiang K, Nagaike T, Xiang S, Kilic T, Beh MM, Manley JL, & Tong L (2010). Crystal structure of the human symplekin-Ssu72-CTD phosphopeptide complex. Nature, 467(7316), 729–733. doi: 10.1038/nature09391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang K, Tong L, & Manley JL (2014). Delineating the structural blueprint of the pre-mRNA 3’-end processing machinery. Mol Cell Biol, 34(11), 1894–1910. doi: 10.1128/MCB.00084-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PDB ID: 3FQD. Xiang S, Cooper-Morgan A, Jiao XF, Kiledjian M, Manley JL, & Tong L (2009). Structure and function of the 5 ‘-> 3 ‘ exoribonuclease Rat1 and its activating partner Rai1. Nature, 458(7239), 784–U130. doi: 10.1038/nature07731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Bai XX, Lee I, Kallstrom G, Ho J, Brown J, Stevens A, & Johnson AW (2000). Saccharomyces cerevisiae RAI1 (YGL246c) is homologous to human DOM3Z and encodes a protein that binds the nuclear exoribonuclease Rat1p. Molecular and Cellular Biology, 20(11), 4006–4015. doi:Doi 10.1128/Mcb.20.11.4006-4015.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CY, Hang J, Wan RX, Huang M, Wong CCL, & Shi YG (2015). Structure of a yeast spliceosome at 3.6-angstrom resolution. Science, 349(6253), 1182–1191. doi: 10.1126/science.aac7629 [DOI] [PubMed] [Google Scholar]
- Yang C, Hager PW, & Stiller JW (2014). The identification of putative RNA polymerase II C-terminal domain associated proteins in red and green algae. Transcription, 5(5), e970944. doi: 10.4161/21541264.2014.970944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Maldonado E, Pillutla R, Cho H, Reinberg D, & Shatkin AJ (1997). Mammalian capping enzyme complements mutant Saccharomyces cerevisiae lacking mRNA guanylyltransferase and selectively binds the elongating form of RNA polymerase II. Proc Natl Acad Sci U S A, 94(24), 12898–12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborowska J, Egloff S, & Murphy S (2016). The pol II CTD: new twists in the tail. Nat Struct Mol Biol, 23(9), 771–777. doi: 10.1038/nsmb.3285 [DOI] [PubMed] [Google Scholar]
- Zenklusen D, Larson DR, & Singer RH (2008). Single-RNA counting reveals alternative modes of gene expression in yeast. Nat Struct Mol Biol, 15(12), 1263–1271. doi: 10.1038/nsmb.1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai LT, & Xiang S (2014). mRNA quality control at the 5’ end. J Zhejiang Univ Sci B, 15(5), 438–443. doi: 10.1631/jzus.B1400070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Rigo F, & Martinson HG (2015). Poly(A) Signal-Dependent Transcription Termination Occurs through a Conformational Change Mechanism that Does Not Require Cleavage at the Poly(A) Site. Mol Cell, 59(3), 437–448. doi: 10.1016/j.molcel.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Zhang K, Fischer T, Porter RL, Dhakshnamoorthy J, Zofall M, Zhou M, Veenstra T, & Grewal SI (2011). Clr4/Suv39 and RNA quality control factors cooperate to trigger RNAi and suppress antisense RNA. Science, 331(6024), 1624–1627. doi: 10.1126/science.1198712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Fu J, & Gilmour DS (2005). CTD-dependent dismantling of the RNA polymerase II elongation complex by the pre-mRNA 3’-end processing factor, Pcf11. Genes Dev, 19(13), 1572–1580. doi: 10.1101/gad.1296305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DY, Gish G, Braunschweig U, Li Y, Ni Z, Schmitges FW, … Greenblatt JF (2016). SMN and symmetric arginine dimethylation of RNA polymerase II C-terminal domain control termination. Nature, 529(7584), 48–53. doi: 10.1038/nature16469 [DOI] [PubMed] [Google Scholar]
- Zhou M, & Law JA (2015). RNA Pol IV and V in gene silencing: Rebel polymerases evolving away from Pol II’s rules. Curr Opin Plant Biol, 27, 154–164. doi: 10.1016/j.pbi.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder JC, & Lima CD (2017). Targeting RNA for processing or destruction by the eukaryotic RNA exosome and its cofactors. Genes Dev, 31(2), 88–100. doi: 10.1101/gad.294769.116 [DOI] [PMC free article] [PubMed] [Google Scholar]


