Abstract
Interacting with manipulable objects (tools) requires the integration of diverse computations supported by anatomically remote regions. Previous functional neuroimaging research has demonstrated the left supramarginal (SMG) exhibits functional connectivity to both ventral and dorsal pathways, supporting the integration of ventrally-mediated tool properties and conceptual knowledge with dorsally-computed volumetric and structural representations of tools. This architecture affords us the opportunity to test whether interactions between the left SMG, ventral visual pathway, and dorsal visual pathway are differentially modulated when participants plan and generate tool-directed gestures emphasizing functional manipulation (tool use gesturing) or structure-based grasping (tool transport gesturing). We found that functional connectivity between the left SMG, ventral temporal cortex (bilateral fusiform gyri), and dorsal visual pathway (left superior parietal lobule/posterior intraparietal sulcus) was maximal for tool transport planning and gesturing, whereas functional connectivity between the left SMG, left ventral anterior temporal lobe, and left frontal operculum was maximal for tool use planning and gesturing. These results demonstrate that functional connectivity to the left SMG is differentially modulated by tool use and tool transport gesturing, suggesting that distinct tool features computed by the two object processing pathways are integrated in the parietal lobe in the service of tool-directed action.
Keywords: functional magnetic resonance imaging, tool processing, left supramarginal gyrus, dorsal stream, ventral stream, functional connectivity, manipulable object representations, tool use, tool transport
Introduction
Tool-directed grasping and tool use ability forms a core aspect of human cognitive function that is utilized on a moment-to-moment basis. Consider for example the difference between grasping a pair of scissors to pass to a friend and grasping a pair of scissors to cut a piece of paper: In the former, one can grasp the scissors in a number of ways depending on biomechanical comfort and given environmental constraints such as the location of the object with respect to the body, limbs, and hands; in contrast, in order to use scissors to cut paper one must arrange the hand and fingers in a manner that presupposes the retrieval of conceptual/functional knowledge for skilled object manipulation. Understanding how functional interactions among object representations in the ventral and dorsal pathways support tool use has been the focus of recent neurocognitive proposals of action representation in the brain (e.g., see Buxbaum, 2017; Cloutman, 2013; Freud, Plaut, & Behrmann, 2016; Mahon & Caramazza, 2011; Milner, 2017; Orban & Caruana, 2014; van Polanen & Davare, 2015). In this report we use functional connectivity based functional magnetic resonance imaging (fMRI) in a group of neurotypical volunteers to assess whether gesturing to move or transport a tool relies on qualitatively different functional interactions than when gesturing to functionally manipulate or use a tool.
Functional neuroimaging work focusing on tool processing has demonstrated that the retrieval of object manipulation knowledge, or physically generating tool use actions, elicits increased blood oxygen level-dependent (BOLD) signal in the left supramarginal gyrus (SMG; Boronat et al., 2005; Brandi, Wohlschlager, Sorg, & Hermsdorfer, 2014; Buchwald, Przybylski, & Kroliczak, 2018; Buxbaum, Kyle, Tang, & Detre, 2006; Canessa et al., 2008; Q.Chen, Garcea, Jacobs, & Mahon, 2018; Q.Chen, Garcea, & Mahon, 2016; Gallivan, McLean, Valyear, & Culham, 2013; Hermsdorfer, Terlinden, Muhlau, Goldenberg, & Wohlschlager, 2007; Johnson-Frey, Newman-Norlund, & Grafton, 2005; Kellenbach, Brett, & Patterson, 2003; Rumiati et al., 2004), whereas more superior regions in bilateral anterior and posterior intraparietal sulcus (IPS), and superior parietal-occipital cortex respond maximally when reaching to touch or grasp objects (for review, see Gallivan & Culham, 2015; Konen, Mruczek, Montoya, & Kastner, 2013).
A separate functional neuroimaging literature has focused on the representation of tools in the ventral visual pathway, and has demonstrated that viewing images of tools, relative to common baseline categories (e.g., animals, conspecifics), elicits increased BOLD contrast in bilateral medial fusiform gyri (Chao, Haxby, & Martin, 1999; Devlin, Rushworth, & Matthews, 2005; Garcea & Mahon, 2014; Mahon et al., 2007; Noppeney, Price, Penny, & Friston, 2006), left posterior middle temporal gyrus (Beauchamp, Lee, Haxby, & Martin, 2002; Chao et al., 1999; Kristensen, Garcea, Mahon, & Almeida, 2016; Mahon, Anzellotti, Schwarzbach, Zampini, & Caramazza, 2009; for review, see Lewis, 2006; A.Martin, 2007), and in the left middle occipital gyrus (‘dorsal occipital cortex’, e.g., see Garcea, Chen, Vargas, Narayan, & Mahon, 2018; Garcea, Kristensen, Almeida, & Mahon, 2016; Garcea & Mahon, 2014; for review see Lingnau & Downing, 2015). Other studies have shown that viewing images of typical functional grasps, relative to atypical functional grasps, engages lateral temporooccipital cortex in the vicinity of the left posterior middle temporal gyrus and lateral occipital cortex (e.g., see Valyear & Culham, 2010; see also Buxbaum et al., 2006).
A third parallel literature has focused on the representation of manipulable objects outside of parietal and temporooccipital regions. For example, Anzellotti and colleagues (2011) demonstrated that categorizing images of tools (relative to categorizing images of animals) led to differential BOLD contrast in the left ventral anterior temporal lobe (for neuropsychological evidence, see Brambati et al., 2006). Several repetitive transcranial magnetic stimulation (rTMS) studies have found that stimulation to the left anterior temporal lobe selectively slowed down conceptual/function judgments of tools but had no effect when participants made judgments about tool manipulation (Ishibashi, Lambon Ralph, Saito, & Pobric, 2011; see also Andres, Pelgrims, & Olivier, 2013; Pelgrims, Olivier, & Andres, 2011; Pobric, Jefferies, & Ralph, 2010), indicating that function knowledge of tools is represented in part in the left ventral anterior temporal lobe. Other studies reported the involvement of the left ventral premotor cortex (Chao & Martin, 2000; Grafton, Fadiga, Arbib, & Rizzolatti, 1997; Kroliczak & Frey, 2009), the left inferior frontal gyrus bordering pars opercularis (e.g., see Johnson-Frey et al., 2003), and the left insula (Brandi et al., 2014) when participants viewed tool images or were instructed to process the appropriate manner with which to grasp or manipulate objects. Neuropsychological evidence consistent with those fMRI findings suggests that lesions to the left inferior frontal gyrus and adjacent structures in the insula impair the ability to select the appropriate manner with which to grasp or use an object (e.g., see Watson & Buxbaum, 2015; see also Goldenberg, Hermsdorfer, Glindemann, Rorden, & Karnath, 2007; M.Martin et al., 2016).
Collectively, these studies suggest that the processing of manipulable objects engages a broad network of regions involved in conceptual processing of tools and their associated actions, which must be integrated with information extracted online about object structure and volume for the programming of arm and hand movements; furthermore, that information must interface with mechanisms involved in the selection of task-appropriate actions for subsequent object use. A recent neurocognitive model (the ‘Two Action Systems Plus Account’; 2AS+) advanced by Buxbaum, Binkofski and colleagues (Binkofski & Buxbaum, 2013; Buxbaum, 2017) articulates how tool-related information may be integrated in the service of interacting with and manipulating tools. Based in part on evidence from the macaque brain (Rizzolatti & Matelli, 2003), it has been argued that the dorsal visual pathway can be subdivided into two anatomically and computationally independent (but highly interactive) pathways for action processing. The dorso-dorsal pathway transforms current visual input into a sensory-motor representation in the service of reaching and grasping, supported in part by bilateral IPS, superior parietal-occipital cortex, and dorsal premotor cortex. In contrast, the ventro-dorsal pathway supports the transformation of visual information from environmental input and from stored representations of action appearance into a sensory-motor representation for functional manipulation and tool use, supported in part by the left SMG, left ventral premotor cortex, and left inferior frontal gyrus.
While both pathways function independently, it is through the integration of conceptual representations of tools and their associated actions supported in part by the ventral stream with online sensory-motor information extracted in part by the dorso-dorsal stream that ‘manipulation knowledge’ is aggregated in the left SMG, a portion of the ventro-dorsal stream, and transiently buffered. A biasing signal from the left inferior frontal gyrus enables selection of task-appropriate actions and subsequent relaying of the selected action to the motor system (see also Cisek & Kalaska, 2010; Schubotz, Wurm, Wittmann, & von Cramon, 2014).
A prediction derived in part from the 2AS+ model, which forms a principal motivation for this study, is that functional connectivity to the left SMG should be modulated by action tasks emphasizing different tool-directed computations. To date, the available evidence suggests only that action versus non-action tasks differentially modulate the tool use network. For example, Garcea et al. (2018) asked a group of participants to pantomime the use of objects or to perform an n-back style picture matching task, and measured the degree to which functional connectivity was differentially driven by task. The items and stimulus presentation parameters were identical across tasks, thus any changes in connectivity were driven by the computations engaged by the task over and above the items used. Garcea and colleagues found that functional connectivity between the left SMG and ventral visual pathway (left medial fusiform gyrus) exhibited strong functional connectivity during the picture matching experiment, whereas functional connectivity between the left SMG, left middle temporal gyrus, left dorsal and ventral premotor cortex was maximal during the tool pantomiming experiment (see also Q.Chen, Garcea, Almeida, & Mahon, 2017; Hutchison & Gallivan, 2018; Kleineberg et al., 2018).
Here we use a similar experimental approach to extend prior observations and to contrast functional connectivity modulated when gesturing tool use actions against functional connectivity modulated when gesturing tool transport actions. We used a sparse event-related design to quantify functional connectivity as a function of planning and gesturing tool use actions separately from tool transport actions, and compared changes in functional connectivity over and above modulations of functional connectivity observed during baseline fixation events. Importantly, the same stimuli and stimulus presentation parameters were maintained across tool use and transport conditions, thus any observed changes in functional connectivity would be driven by computations engaged by the task over and above the stimuli used. Although tool use gestures are more heterogenous and diverse in the range and complexity of movement relative to tool transport gestures, we included tool transport planning and gesturing to serve as an important contrast to demonstrate that changes in functional connectivity for tool use would not be driven by any engagement of the hand and fingers to produce an action. In addition, we sought positive evidence that tool transport planning and gesturing may differentially modulate functional connectivity relative to tool use planning and gesturing.
We sought to test four predictions. First, we predicted that generating tool use actions, relative to tool transport actions, should elicit differential BOLD contrast in the left SMG, left middle temporal gyrus, and left inferior frontal gyrus. Given low-level motor differences between the gesturing of tool use actions relative to tool transport actions, we focused our BOLD contrast analyses on the planning phase. Second, we predicted that planning and generating tool use gestures should emphasize conceptual processing of tools and their associated actions, which should be reflected in increased functional connectivity between the left SMG, left middle temporal gyrus, and left ventral anterior temporal lobe. Third, we predicted that planning and gesturing tool use emphasizes the selection of task-appropriate actions from competing alternatives, which should elicit increased functional connectivity between the left SMG and left inferior frontal gyrus. Finally, we predicted that planning and generating tool transport gestures, by hypothesis, bypasses semantic processing and emphasizes sensory-motor processing of the stimulus, which should be reflected in increased functional connectivity between the left SMG and the dorso-dorsal stream (left posterior IPS/superior parietal cortex).
As a secondary aim, we assessed whether interactions between the left SMG and ventral temporal cortex (bilateral medial fusiform gyri) were differentially modulated by action type. Adjacent voxels in the left and right collateral sulci have been shown to respond to surface texture of objects more than to object form or orientation (e.g., see Cant & Goodale, 2007). What remains poorly understood is whether representations of material properties (e.g., surface texture) are differentially relevant when planning and generating tool use or tool transport gestures; thus we assessed how and whether interactions between the left SMG and bilateral ventral temporal cortex were differentially driven by tool use versus tool transport gesturing.
Methods
Participants
Thirty-four volunteers (16 female; mean age, 22 y; SD, 2.6 y) from the University of Pennsylvania participated in the study in exchange for payment. All participants were native English speakers, had normal or corrected-to normal vision, had no history of neurological illness, and were right hand dominant (established with the Edinburgh Handedness Questionnaire; Oldfield, 1971). All participants gave written informed consent in accordance with the Institutional Review Board at the University of Pennsylvania and the Albert Einstein Healthcare Network. One participant’s data were removed due to excessive head movement (> 2 SD of movement in translation and rotation in XYZ dimensions); all subsequent analyses were performed over the remaining 33 participants.
General Experimental Procedure
Stimulus presentation was controlled with E-Prime Professional Software 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA). All participants viewed the stimuli binocularly through a mirror attached to the head coil adjusted to allow for foveal viewing of a back-projected monitor (temporal resolution = 60 Hz). Each participant took part in one scanning session which began with a high resolution T1 anatomical scan; 22 of the 33 participants then took part in 4 runs of an object recognition experiment not germane to the focus of this study (using different stimuli), followed by 2 runs of the experiment proper. A second cohort of 12 individuals participated in a T1 anatomical scan, followed by 8 runs of the experiment proper. We collected 8 runs in those individuals to study the neural mechanisms supporting pantomiming of tool use not germane to the focus of this study; we include only the first two runs of data from these 12 additional participants to ensure that all participants contributed equal amounts of data to subsequent BOLD contrast and functional connectivity analyses.
Tool Viewing, Planning, and Pantomiming fMRI Experiment
Design.
There were 12 cells in the design of the experiment: Task type (three levels; view, plan, pantomime), Action type (two levels; tool use, tool transport), and Conflict type (two levels; high conflict items, low conflict items; see Watson & Buxbaum, 2015). (We collapse across conflict type in the focused analyses reported here, resulting in a design with 6 cells.) Eight photographs of manipulable objects (scissors, cork screw, key, screwdriver, axe, bottle opener, butcher knife, and fork) were presented in a sparse event-related design; there was one exemplar of each item, and each item was presented once per run (for original items, see Watson & Buxbaum, 20151; see Figure 1 for a schematic of the trial structure). The assignment of items to action type was counterbalanced within- and across-runs in an ABAB format. At the item level, half of the tools assigned to tool use events on odd runs were assigned to tool transport events on even runs (and vice versa for the other half of tools), such that after two runs every item was presented in every cell of the design. Tool use and tool transport trials were presented randomly within a run. Pictorial and word stimuli were centrally presented to allow for foveal viewing and to minimize eye movements.
Figure 1.
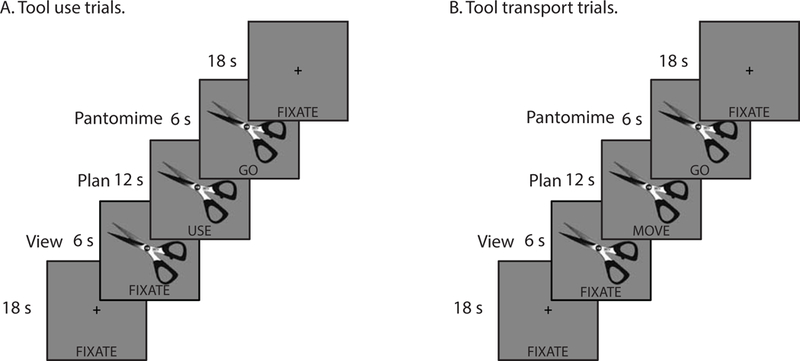
Schematic of trial structure in the tool viewing, planning, and pantomiming fMRI experiment.
Procedure.
During each 24-second trial, a picture of a tool was presented centrally for the duration of the event; the first 6 seconds of each trial began with the image and with the word “FIXATE” presented below it. During these ‘viewing’ events participants were instructed to pay attention to the presented image. After six seconds the “FIXATE” cue was replaced with the word “MOVE” or “USE”, which remained for 12 seconds. The word served as a cue for participants to begin planning and imagining interacting with the object in order to move it (i.e., interacting with the object to pick it up and displace it by several inches or to pass it to a friend; hereafter, tool transport) or use it (i.e., interacting with the object to functionally grasp it and use it). After 12 seconds the planning cue was replaced with the word “GO” for 6 seconds, during which time the participants generated a tool-directed gesture while the word remained on the screen.
Trial events were interspersed by 18-second rest periods in which a fixation cross was presented centrally with the word “FIXATE” positioned in the same physical location as the cues during the experimental trials. During these fixation events participants were instructed to relax and to wait for the next trial. We used the 18-second interstimulus period to temporally separate the principal events in the experiment for subsequent functional connectivity analyses, and to separately quantify functional connectivity in the absence of the experimental stimuli (i.e., during a baseline fixation period). As described below, this approach permits us to measure the modulation of functional connectivity maximally driven by tool use planning and pantomiming in relation to tool transport planning and pantomiming, over and above functional connectivity observed during this baseline period (i.e., a two-way functional connectivity interaction; for a similar experimental design and analysis pipeline, see Garcea et al., 2018; for a recent review on this technique, see Gonzalez-Castillo & Bandettini, 2017).
Prior to the scan, careful training and instruction was given to participants to pantomime tool use and tool transport actions. The training procedure was identical to previous training instructions we have administered to participants (e.g., see Q.Chen et al., 2017; Q.Chen et al., 2018; Q.Chen et al., 2016; Erdogan, Chen, Garcea, Mahon, & Jacobs, 2016; Garcea et al., 2018) in which participants are trained to generate a gesture as if the tool stimulus was ‘in-hand’. Importantly, because participants are in a physically constrained environment in the bore of the magnet, they were instructed to generate gestures at a slow rate (~ 1 gesture per second) for the duration of the event; because there was no visual feedback during scanning (i.e., the participants’ right hand was out of sight during the scan), the experimenter ensured that the participants memorized the tool transport and tool use actions prior to the scan by asking them to generate each action from memory. During each run the experimenter visually inspected the participant’s gesturing action in real time to verify that the participants were generating actions when cued by the experiment. Participants were given feedback between runs to ensure tool use and tool transport actions were gestured correctly and with minimal inadvertent head movement.
MR Acquisition Parameters
MRI Parameters
Whole brain BOLD imaging was conducted on a Siemens 3-Tesla PRISMA scanner with a 64-channel head coil located at the Hospital of the University of Pennsylvania. High-resolution structural T1 contrast images were acquired using a magnetization-prepared rapid gradient echo (MP-RAGE) pulse sequence at the start of each participant’s scanning session (TR = 1850 ms, TE = 3.91 ms, flip angle = 8 degrees, FOV = 256 mm, matrix = 256 × 192, 160 left-to-right slices, voxel size = 1×0.94×0.94 mm). An echo-planar imaging pulse sequence was used for T2* contrast (TR = 3000 ms, TE = 30 ms, flip angle = 90 degrees, FOV = 192 × 192 mm, matrix = 64 × 64, 48 inferior-to-superior slices, voxel size = 3×3×3 mm). Due to an error in image acquisition, two participants’ data were acquired using a multi-echo sequence (TR = 3000 ms, TE = 14 ms, flip angle = 90 degrees, FOV = 216 × 216 mm, matrix = 68 × 68, 43 inferior-to-superior slices, voxel size = 3.17×3.17×3.5 mm). This acquisition procedure generated 3 dicoms per volume; the second dicom in each volume corresponds to the BOLD component of the MR signal (Kundu et al., 2013; Kundu, Inati, Evans, Luh, & Bandettini, 2012), and thus we included only those dicoms in subsequent analyses. Importantly, the results reported below do not change qualitatively when removing the two participants’ data, therefore we include the two participants in all analyses. The first 6 volumes of each run were discarded to allow for signal equilibration (4 volumes during image acquisition and 2 at preprocessing).
Preprocessing of fMRI data
fMRI data were analyzed with the BrainVoyager software package (Version 2.8.2) and in-house scripts drawing on the BVQX toolbox written in MATLAB (http://support.brainvoyager.com/available-tools/52-matlab-tools-bvxqtools/232-getting-started.html). Preprocessing of the functional data included, in the following order, slice scan time correction (sinc interpolation), 3D motion correction with respect to the first volume of the first functional run, and linear trend removal in the temporal domain (cutoff: 2 cycles within the run). Functional data were registered (after contrast inversion of the first volume) to high-resolution deskulled anatomy on a participant-by-participant basis in native space. For each participant, echo-planar and anatomical volumes were transformed into standardized space (Talairach & Tournoux, 1988). All functional data were smoothed at 6 mm FWHM (2 voxels). The general linear model (GLM) was used to fit beta estimates to the experimental events of interest. Experimental events were convolved with a standard 2-gamma hemodynamic response function. The first derivatives of 3D motion correction from each run were added to all models as regressors of no interest to attract variance attributable to head movement.
Whole-brain BOLD contrast of tool use and tool transport viewing and planning
Upon completing the pre-processing steps described above, a group-level GLM was created with the 33 participants’ data (i.e., random effects GLM). We began by computing the simple effect of Task (two levels; viewing, planning) separately for tool use and tool transport trials. Given previous literature demonstrating increased BOLD contrast in frontoparietal action circuits when viewing images of manipulable objects (e.g., see Chao & Martin, 2000; Mahon et al., 2007), our goal was to isolate regions that exhibited increased BOLD contrast for action planning over and above tool viewing, and to determine where the effect was differentially modulated by tool use relative to tool transport. Thus we carried out the BOLD contrast analysis to replicate previous findings (Brandi et al., 2014) and we sought to extend those findings by demonstrating that the effect was present in the planning phase of a tool use action. In subsequent analyses we collapse across planning and pantomiming when computing whole-brain functional connectivity; we return to this issue below.
To measure the differential effect of tool use planning relative to tool transport planning, and to demonstrate the specificity of the planning effect over and above tool viewing, we computed a voxelwise directional interaction analysis using the contrast ‘[Planning Tool Use – Viewing Tool Use] – [Planning Tool Transport – Viewing Tool Transport]’. Thus, any differential changes in the BOLD signal would be driven by planning tool use actions relative to planning tool transport actions, over and above the tool viewing condition which preceded the planning phase. The contrast was computed on a participant-by-participant basis, and a whole-brain one-sample t-test against 0 was computed to determine at the group-level which voxels exhibited significant differential BOLD contrast for tool use planning (positive t-values) and tool transport planning (negative t-values). This analysis identified five regions-of-interest (ROIs) that were entered in a subsequent functional connectivity analysis: i) the left SMG, ii) the left middle temporal gyrus, iii) the left frontal cortex (including the inferior and middle frontal gyri), iv) the left anterior insula, and v) the left posterior insula extending laterally to include the left ventral premotor cortex (see Figure 2Ai; see also Figure 3).
Figure 2.
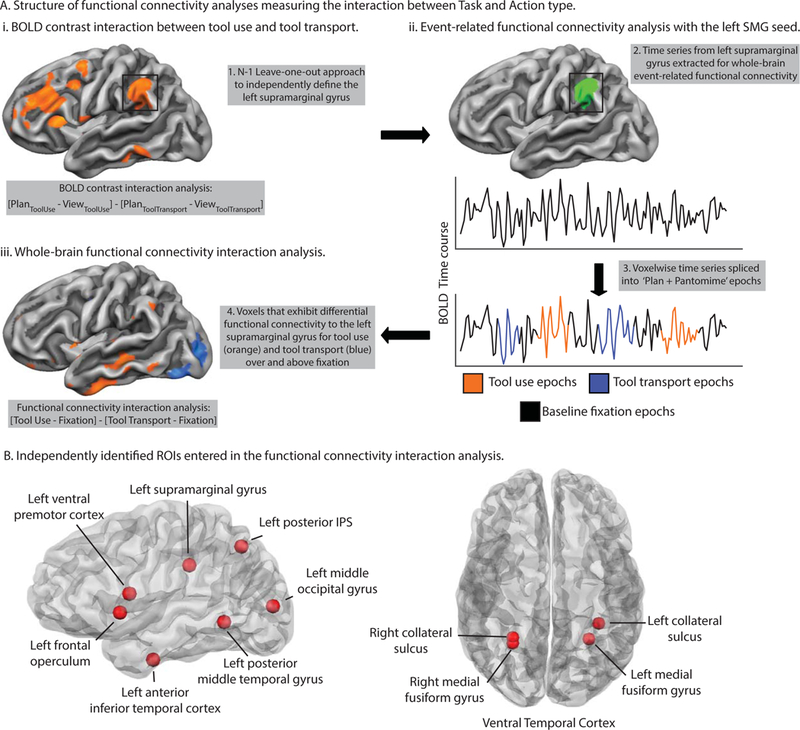
Task-based functional connectivity processing pipeline and independent literature-defined ROIs entered in the two-way functional connectivity interaction analysis.
Figure 3.
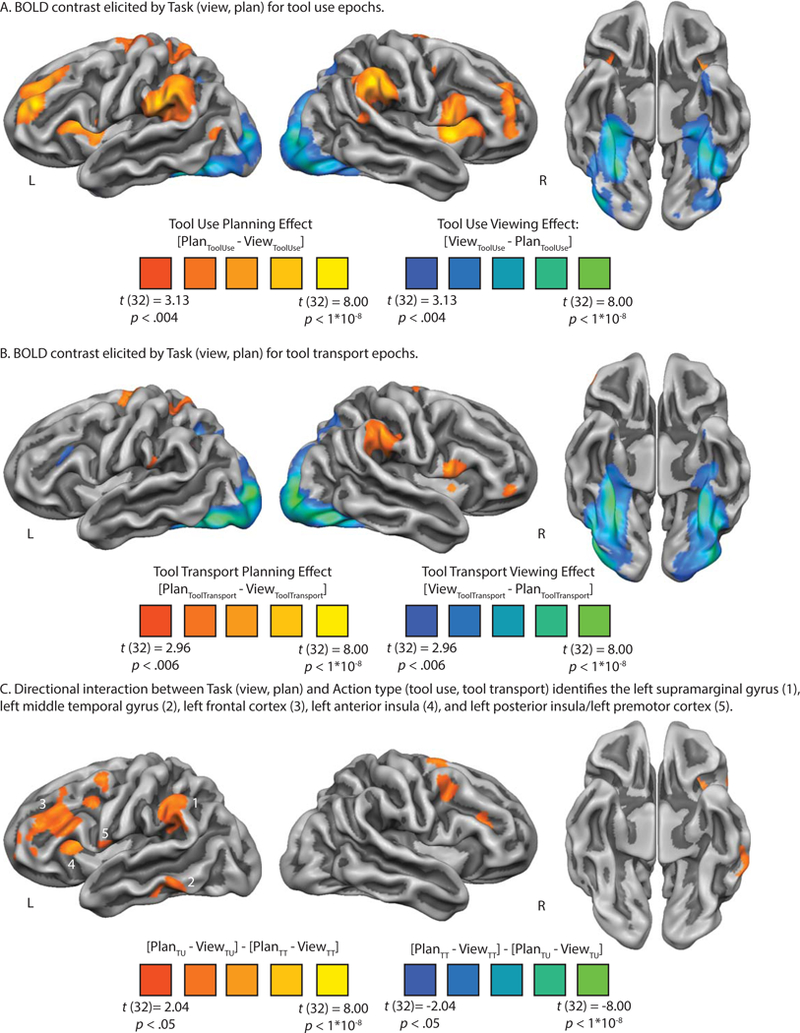
BOLD contrast interaction analysis identifies a swath of left hemisphere regions involved in tool use planning.
Ensuring Independence of ROI Selection from ROI Testing
We took deliberate care to ensure that our results were statistically robust and independent. We selected ROIs for subsequent functional connectivity analyses using three approaches: i) Using a leave-one-out approach to define the Nth participant’s ROIs with N - 1 participants’ data, iterating this procedure across participants to define each ROI independently (e.g., see Kriegeskorte, Simmons, Bellgowan, & Baker, 2009); ii) Using literature-defined ROIs from previously published fMRI research on tool representation and action (e.g., see Anzellotti, Mahon, Schwarzbach, & Caramazza, 2011; Cant & Goodale, 2007; Q.Chen et al., 2016; Gallivan et al., 2013; Garcea et al., 2018); and iii) Using the Neurosynth database (http://neurosynth.org).
The left SMG, left middle temporal gyrus, left frontal cortex (inferior and middle frontal gyri), left anterior insula, and left posterior insula/ventral premotor cortex exhibited differential BOLD contrast for tool use planning from the interaction analysis, and we used a leave-one-out approach to define those regions on a participant-by-participant basis.
In a complementary approach using literature-defined regions, we selected 4 ROIs previously published by Gallivan et al. (2013), including the left posterior intraparietal sulcus, left posterior middle temporal gyrus, left SMG, and left ventral premotor cortex. These regions were shown to encode information about reaching-to-touch or reaching-to-grasp actions. Next, we selected the right and left medial fusiform gyri previously published by Chen, Garcea, and Mahon (2016), and the left dorsal occipital cortex (hereafter, left middle occipital gyrus) published by Garcea et al. (2018); both bilateral medial fusiform gyri and the middle occipital gyrus were shown to exhibit differential BOLD contrast for tool viewing relative to animal viewing. We used the right and left collateral sulci (published by Cant and Goodale (2007)), two regions that responded more strongly to surface texture than to orientation or object form, and we used a tool-preferring region in the left ventral anterior temporal lobe published in Anzellotti et al. (2011). Finally, we used the Neurosynth fMRI database to define the left inferior frontal gyrus/frontal operculum using the search term ‘frontal operculum’. Using the Harvard-Oxford cortical structural atlas in FSL we verified that the peak region was situated in left pars opercularis (Brodmann Area 44), and we converted the peak MNI coordinate to Talairach space for subsequent analyses.
As outlined in the introduction, we selected these 11 regions because they represent a literature that has focused on the processing of tools and their associated actions from the perspective of object processing in the ventral visual pathway, sensory-motor processing in the dorsal visual pathway, and selection mechanisms supported by prefrontal and premotor regions. For all regions entered into the functional connectivity analysis, we created a spherical ROI 10 mm in diameter centered on each participant-specific, literature-defined, and Neurosynth-defined peak coordinate (see Table 1 for peak Talairach coordinates). Thus all ROIs were on equal footing with respect to the number of voxels in each sphere.
Table 1.
Peak Talairach coordinates from the BOLD contrast-defined and literature-defined regions-of-interest entered in the functional connectivity interaction analysis.
| Source of Peak Voxel Coordinates | Peak Talairach Coordinates |
||
|---|---|---|---|
| X | Y | Z | |
| Gallivan et al (2013) | |||
| Left Posterior Intraparietal Sulcus | −22 | −68 | 45 |
| Left Posterior Middle Temporal Gyrus | −53 | −57 | −3 |
| Left Supramarginal Gyrus | −56 | −35 | 33 |
| Left Ventral Premotor Cortex | −52 | 3 | 15 |
| Chen et al (2016) | |||
| Left Medial Fusiform Gyrus | −27 | −53 | −13 |
| Right Medial Fusiform Gyrus | 27 | −52 | −14 |
| Cant & Goodale (2007) | |||
| Left Collateral Sulcus | −37 | −42 | −15 |
| Right Collateral Sulcus | 27 | −56 | −13 |
| Garcea et al (2018) | |||
| Left Dorsal Occipital Cortex | −31 | −88 | 7 |
| Anzellotti et al (2011) | |||
| Left Anterior Temporal Lobe | −52 | −12 | −27 |
| Neurosynth-defined Region | |||
| Left Inferior Frontal Gyrus/Frontal Operculum | −49 | 9 | 3 |
| BOLD Contrast-defined Regions | |||
| Left Supramarginal Gyrus | −54 | −37 | 37 |
| Left Frontal Cortex | −51 | 23 | 25 |
| Left Middle Temporal Gyrus | −63 | −40 | −8 |
| Left Anterior Insula | −30 | 17 | 13 |
| Left Posterior Insula | −39 | −4 | 1 |
The modulation of functional connectivity by the interaction of task and action type
The principal focus of the analysis was to measure the degree to which functional connectivity was modulated by the interaction of Task (two levels; plan + pantomime, fixation) and Action Type (two levels; tool use, tool transport). We modeled functional connectivity for the planning and pantomiming phase as one condition for three reasons: i) The planning phase of our task was too short in duration (12 seconds, 4 volumes) to compute functional connectivity in isolation (i.e., there is an increased risk of committing Type I error when computing functional connectivity over a shorter duration); ii) Because pantomiming epochs followed planning epochs closely in time, we were unable to separate functional connectivity driven by planning processes from functional connectivity driven by pantomiming processes; iii) The hand posture required to generate a tool transport gesture was more homogeneous across actions than tool use actions. For these reasons we modeled both events together into a ‘plan + pantomime’ epoch, and we used an identical analytic approach as was carried out by Garcea and colleagues (2018) to measure where functional connectivity was differentially modulated for tool use epochs relative to tool transport epochs, over and above functional connectivity measured during fixation epochs (see Figure 2A). Prior to computing functional connectivity, the change in head position (translation and rotation in XYZ dimensions) across volumes was regressed out of the time series data (after the preprocessing steps described above); all functional connectivity analyses were conducted over the residuals of that regression model. We did not regress the global mean time course, as work has suggested that this procedure is at best unnecessary, and at worst may introduce spurious correlation patterns to the data (e.g., see Gotts, Jo, et al., 2013; Gotts, Saad, et al., 2013; Saad et al., 2013).
To measure the differential modulation of functional connectivity, we extracted the BOLD time series data that corresponded to trial events for tool use and tool transport epochs, and separately, for the fixation event portion of the BOLD time series that immediately followed each trial (see Figure 2Aii). We extracted 6 volumes (18 seconds) of time series data aligned to the onset of each plan type (4 volumes) and the subsequent pantomime event (2 volumes). There were 8 trials presented in each run; thus, we derived eight 6-volume-long time series segments for each run and correlated the time series data extracted from a given ROI for those volumes with the time series data for those volumes in every other voxel of the brain. Functional connectivity was computed with the NeuroElf toolbox (https://neuroelf.net) in MATLAB using the ‘vtc_CrossCorrelate’ function. The functional connectivity analysis resulted in 8 whole-brain functional connectivity maps per run. We then Fisher-transformed each functional connectivity map and averaged together the 8 whole-brain maps for tool use planning and pantomiming (4 maps from run 1; 4 maps from run 2) and the 8 whole-brain maps for tool transport planning and pantomiming. In a manner identical to the analysis of tool use and tool transport events described above, we computed whole-brain functional connectivity during the post-stimulus fixation events and averaged together the fixation baseline maps separately for events that followed tool use epochs and tool transport epochs. See Figure 2A for a schematic of the two-way functional connectivity analysis.
This procedure resulted in four whole-brain maps per participant, which were entered in a two-way interaction analysis to determine where functional connectivity was differentially modulated by tool use planning and pantomiming in relation to tool transport planning and pantomiming, over and above modulation in functional connectivity observed during fixation events ‘[Tool Use plan + pantomime - Fixation tool use] – [Tool transport plan + pantomime - Fixation tool transport]’. We then computed a one-sample t-test against 0 in each voxel to determine the extent to which the directional two-way functional connectivity interaction was differentially modulated by tool use planning and acting (positive t-values) and tool transport planning and acting (negative t-values) across participants (see Figure 2Aiii).
We took two approaches to interpreting functional connectivity. First, we used the left SMG region derived from the BOLD contrast interaction analysis as a seed, and we used the remaining regions (i.e., left middle temporal gyrus, left frontal cortex, left anterior insula, left posterior insula/ventral premotor) as mask regions from which to extract functional connectivity for interpretation. In a parallel analysis, we use 11 independently defined regions from the tool and action literature reviewed above to serve as seed and mask regions, and we tested for functional connectivity modulations differentially driven by tool use actions relative to tool transport actions (see Figure 2B).
Results
BOLD contrast analysis of planning tool use and tool transport actions
There were four factors in our design that were directly contrasted in the BOLD analysis: Task type (two levels; view, plan) and Action type (two levels; tool use, tool transport). We began by testing the directional interaction between task type separately for tool use ([Plan Tool Use – View Tool Use]) and tool transport ([Plan Tool Transport – View Tool Transport]) actions. We then directly contrasted tool use planning against tool transport planning ([Plan Tool Use – View Tool Use] – [Plan Tool Transport – View Tool Transport]) to determine the anatomical loci differentially engaged by planning tool use or tool transport actions.
Simple Effect:
Tool Use Planning. The whole-brain contrast of tool use planning against tool viewing is plotted in Figure 3A. All voxels plotted survive FDR correction (q < .05). Relative to tool viewing, tool use planning elicited increased BOLD contrast across a swath of cortical and subcortical regions, including bilateral supramarginal gyri, the left superior parietal lobule, bilateral ventral premotor cortex extending medially into the insula and adjacent subcortical voxels in the vicinity of the basal ganglia, bilateral middle frontal gyri extending superiorly into the superior frontal gyri, and bilateral dorsal premotor cortex ending medially into the supplementary motor area. A contiguous cluster of voxels not pictured on the cortical rendering was identified in the left superior pre- and postcentral gyrus. In contrast, tool viewing during the use epochs elicited increased BOLD contrast in bilateral ventral temporal cortex, including bilateral inferior temporal gyri and medial fusiform gyri, extending anteriorly into the anterior ventral temporal lobe. Superior parietal-occipital cortices also exhibited increased BOLD contrast for tool viewing (see Table 2A for peak voxel coordinates and t-values associated with the tool use contrast map).
Table 2.
Peak Talairach coordinates, maximum t-value, cluster size, and anatomical region for the BOLD contrast analysis reported in Figure 3.
| Region Name | Peak X, Y, Z Talairach Coordinates |
Maximum t-value |
Anatomical Voxels (1mm3) |
||
|---|---|---|---|---|---|
| A. Tool use planning > tool viewing | |||||
| Left middle frontal gyrus | −30 | 44 | 25 | t(32) = 6.91, p < .001 | 8633 |
| Right supramarginal gyrus | 51 | −37 | −37 | t(32) = 6.75, p < .001 | 11391 |
| Right putamen | 24 | −1 | 13 | t(32) = 6.60, p < .001 | 15146 |
| Left supramarginal gyrus | −57 | −31 | 25 | t(32) = 6.26, p < .001 | 36439 |
| Right middle frontal gyrus | 33 | 44 | 34 | t(32) = 6.26, p < .001 | 8222 |
| Left putamen | −24 | −7 | 13 | t(32) = 6.12, p < .001 | 12759 |
| Left cerebellum | −33 | −37 | −35 | t(32) = 4.84, p < .001 | 2815 |
| Left precentral gyrus | −52 | 2 | 43 | t(32) = 4.57, p < .001 | 877 |
| Right cerebellum | 39 | −37 | −38 | t(32) = 4.22, p < .001 | 475 |
| Left posterior middle temporal gyrus | −45 | −64 | 7 | t(32) = 4.02, p < .001 | 452 |
| Right prefrontal cortex | 24 | 59 | 3 | t(32) = 3.64, p < .001 | 326 |
| Right medial fusiform gyrus | 27 | −58 | −11 | t(32) = −7.93, p < .001 | 44555 |
| Left medial fusiform gyrus | −33 | −46 | −14 | t(32) = −7.53, p < .001 | 26835 |
| Right cingulate cortex | −9 | −25 | 28 | t(32) = −4.67, p < .001 | 1289 |
| Left anterior temporal lobe | −27 | 8 | −29 | t(32) = −4.58, p < .001 | 1375 |
| B. Tool transport planning > tool viewing | |||||
| Left putamen | −27 | −7 | 1 | t(32) = 6.18, p < .001 | 1926 |
| Left somatosensory cortex | −21 | −19 | 64 | t(32) = 5.53, p < .001 | 7779 |
| Right supramarginal gyrus | 60 | −34 | 37 | t(32) = 4.76, p < .001 | 4875 |
| Right inferior frontal gyrus | 51 | 8 | 13 | t(32) = 4.67, p < .001 | 3538 |
| Right prefrontal cortex | 45 | 41 | 1 | t(32) = 4.55, p < .001 | 834 |
| Right putamen | 24 | −7 | 7 | t(32) = 4.52, p < .001 | 614 |
| Left anterior cingulate cortex | −12 | 44 | 16 | t(32) = 4.16, p < .001 | 516 |
| Left supramarginal gyrus | −54 | −28 | 22 | t(32) = 3.95, p < .001 | 529 |
| Right middle occipital gyrus | 30 | −82 | 2 | t(32) = −10.58, p < .001 | 49959 |
| Left middle occipital gyrus | −27 | −88 | 4 | t(32) = −9.23, p < .001 | 43292 |
| Right thalamus | 18 | −28 | −3 | t(32) = −4.55, p < .001 | 494 |
| Left middle/inferior frontal gyrus | −39 | 20 | 25 | t(32) = −3.86, p < .001 | 798 |
Simple Effect:
Tool Transport Planning. The whole-brain contrast of tool transport planning against tool viewing is plotted in Figure 3B. All voxels plotted survive FDR correction (q < .05). We observed qualitatively similar results as was observed for tool use planning, albeit quantitatively weaker for tool transport planning. Relative to tool viewing, tool transport planning elicited increased BOLD contrast in the right SMG, in the left superior parietal lobule, in the left dorsal premotor cortex extending medially into the left supplementary motor area, in the medial aspect of the left and right inferior frontal gyrus adjacent to the insula, and in bilateral putamen. Relative to tool transport planning, tool viewing in the transport epochs elicited a pattern of BOLD contrast virtually identical to those depicted in the contrast map in Figure 3A. Specifically, we observed robust increase in BOLD contrast in bilateral inferior temporal gyri and medial fusiform gyri, which extended anteriorly into the anterior and ventral temporal lobe; in addition, we observed increased BOLD contrast in bilateral superior parietal-occipital cortices (see Figure 3B and Table 2B for peak voxel coordinates and t-values associated with the tool transport contrast map).
Interaction between Task and Action Type.
Next, we directly contrasted tool use planning against tool transport planning using a whole-brain directional two-way interaction analysis. Specifically, we computed at the voxel level the degree to which BOLD contrast was differentially modulated by tool use planning relative to tool transport planning, over and above changes observed for viewing. We found five left hemisphere cortical clusters that exhibited a preference for tool use planning: i) the left SMG, ii) the left middle temporal gyrus, iii) the left frontal cortex (including the inferior and middle frontal gyrus), iv) the left anterior insula, and v) the left posterior insula extending laterally to the left ventral premotor cortex (see Figure 3C). Consistent with Brandi and colleagues (2014), we find no left hemisphere cortical voxels that exhibit a differential BOLD response for tool transport relative to tool use (but see Supplemental Figure 1). In a supplemental analysis, we modeled the gesturing phase in isolation from the planning phase when contrasting tool use against tool transport, and we observed results for tool use gesturing that were qualitatively similar to those displayed in Figure 3C (see Supplemental Figure 1).
It’s important to note that the left middle temporal gyrus peak here (peak: −63 −40 −8) is anterior to the oft-reported left posterior middle temporal gyrus locus identified in previous tool use fMRI studies in neurotypical adults (Q.Chen et al., 2016; Gallivan et al., 2013; Garcea & Mahon, 2014) and in neuropsychological populations (Campanella, D’Agostini, Skrap, & Shallice, 2010; Kalenine & Buxbaum, 2016). To address this anatomical discrepancy we include a literature-defined left posterior middle temporal gyrus in our functional connectivity analyses (see below).
Modulation of functional connectivity by the interaction of task and action type.
Interaction analysis with BOLD contrast-defined regions.
Next, we sought to determine whether the left SMG exhibits increased functional connectivity for tool use planning and pantomiming relative to tool transport planning and pantomiming, over and above fixation baseline functional connectivity, to the regions identified in the BOLD contrast analysis. We observed increased functional connectivity to the left middle temporal gyrus for tool use epochs (relative to fixation baseline; t(32) = 5.36, p < .001) and for tool transport epochs (relative to fixation baseline; (t(32) = 4.28, p < .001); this increase in functional connectivity for tool use epochs was not significantly stronger than tool transport epochs (t(32) = 1.30, p = .20; see Supplemental Figure 2)2. A similar pattern was present when measuring functional connectivity to the left frontal cortex ROI: we found increased functional connectivity for tool use epochs (t(32) = 3.62, p < .001) and tool transport epochs (t(32) = 3.51, p < .001) relative to baseline, however the interaction was not significant (t < 1). Lastly, there was no differential functional connectivity to the left anterior insula or to the left posterior insula ROIs (all t-values < 1; see Supplemental Figure 2C).
Interaction analysis with independently-defined regions.
Next, we used the same analytic approach with our independent literature-defined and Neurosynth-defined ROIs to determine the extent to which functional connectivity among the 11 regions was differentially driven by tool use and tool transport epochs. The analysis proceeded in two steps. In the first step, we used each ROI as a seed region to compute whole-brain functional connectivity, and we used the 10 remaining regions as masks to extract functional connectivity values; this resulted in an 11-by-11 two-way interaction matrix. In the second step, we interpreted the significance of the data if the observed functional connectivity values satisfied three constraints: i) Functional connectivity must be significant bi-directionally (e.g., when using region A as a seed and region B as a mask, and vice versa), ii) at an alpha level of at least p < .01, and iii) the effect size for the two-way interaction must be moderate in magnitude (Hedges’ G of 0.40 or higher). After imposing those constraints we found 7 significant ROI-to-ROI effects: For tool use epochs, we observed a significant two-way interaction between the left SMG and i) the left ventral anterior temporal lobe, and ii) the left frontal operculum/inferior frontal gyrus (see Figure 4A and 4B); for tool transport epochs, we observed a significant two-way interaction between the left SMG and iii) right collateral sulcus, iv) the left medial fusiform gyrus, v) the right medial fusiform gyrus, vi) the left middle occipital gyrus, and vii) between the left middle occipital gyrus and left posterior middle temporal gyrus (see Figure 4B). Functional connectivity that was bi-directionally robust but did not survive a p value cutoff of .01 is marked with a ‘/’; all other non-significant functional connectivity is marked with a ‘X’ (see Figure 4A; see also Table 3 for t-values and effect sizes associated with the two-way analysis; see Supplemental Table 1 for the average group-level functional connectivity values and p-values).
Figure 4.
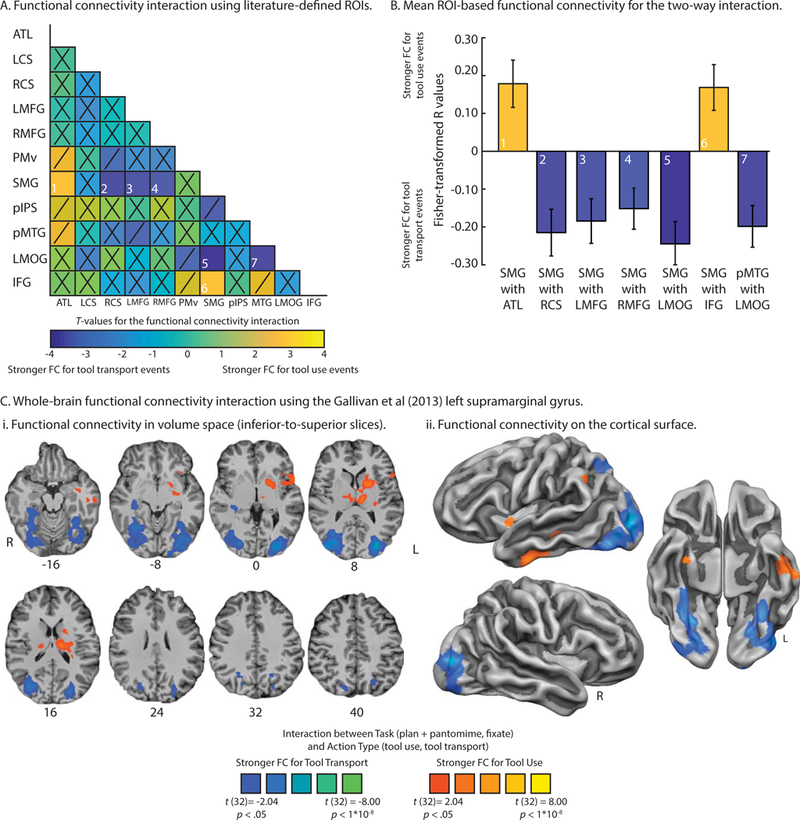
Modulation of functional connectivity for the interaction between Task and Action type.
Table 3.
t-values associated with the two-way interaction between Task (plan + pantomime, fixate) and Action type (tool use, tool transport). t-values are reported if they satisfy the joint constraint of reaching significance at p < .01 across data folds with an effect size (Hedge’s G) that is moderate in magnitude (minimum value of 0.40); the magnitude of the effect size is given in parentheses. Functional connectivity values that do not meet those constraints but that are significant across data folds (p < .05) are listed in italics. Columns correspond to the seed regions and rows correspond to mask regions from which functional connectivity was extracted. Abbreviations. ATL, left anterior temporal lobe; LCS, left collateral sulcus; RCS, right collateral sulcus; LMFG, left medial fusiform gyrus; RMFG, right medial fusiform gyrus; PMv, left ventral premotor cortex; SMG, left supramarginal gyrus; pIPS, left posterior intraparietal sulcus; pMTG, left posterior middle temporal gyrus; LMOG, left middle occipital gyrus; IFG, left frontal operculum/left inferior frontal gyrus.
| ATL | LCS | RCS | LMFG | RMFG | PMv | SMG | pIPS | pMTG | LMOG | IFG | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ATL | - | - | - | - | - | 2.78 |
3.13 (0.58) |
- | 2.61 | - | - |
| LCS | - | - | - | - | - | - | - | - | - | - | - |
| RCS | - | - | - | - | - | −2.22 |
−3.50 (0.54) |
- | - | - | - |
| LMFG | - | - | - | - | - | - |
−2.86 (0.47) |
- | −2.14 | - | - |
| RMFG | - | - | - | - | - | - |
−2.95 (0.46) |
- | - | - | - |
| PMv | 2.46 | - | −2.00 | - | - | - | - | - | - | - | - |
| SMG |
2.86 (0.53) |
- |
−3.48 (0.57) |
−3.12 (0.52) |
−2.77 (0.41) |
- | - | −2.20 | - |
−3.40 (0.60) |
2.71 (0.63) |
| pIPS | - | - | - | - | - | - | −2.62 | - | - | - | - |
| pMTG | 2.44 | - | - | −2.29 | - | - | - | - | - |
−3.08 (0.67) |
2.74 |
| LMOG | - | - | - | - | - | - |
−4.13 (0.75) |
- |
−3.60 (0.76) |
- | - |
| IFG | - | - | - | - | - | - |
2.78 (0.67) |
- | 2.35 | - | - |
Lastly, to confirm the results of the functional connectivity analysis using the BOLD contrast-defined left SMG, we projected the whole-brain two-way interaction map using the Gallivan et al (2013) left SMG seed in volume space (Figure 4Ci) and on the cortical surface (Figure 4Cii). The resulting map confirms the ROI-based functional connectivity analysis: Tool use epochs elicited increased functional connectivity to the left anterior ventral temporal cortex (Z = −16), and to the left frontal operculum extending into the left posterior insula to include aspects of the basal ganglia (Z = 0, 8; for cluster-level analysis see Table 4). In contrast, tool transport epochs elicited increased functional connectivity to a bilateral swath of cortex in ventral temporal cortex, including bilateral medial fusiform gyri extending into the collateral sulci (Z = −16), bilateral middle occipital gyri (Z = 0, 8), and posterior intraparietal sulcus extending into the superior parietal lobe (Z = 24, 32, 40; see Table 4; see also Supplemental Figure 2A).
Table 4.
Peak Talairach coordinates, peak t-value, p-value, and cluster size for the two-way functional connectivity interaction reported in Figure 4C.
| Region Name | Peak X, Y, Z Talairach Coordinates |
Peak t-value |
Anatomical Voxels (1mm3) |
||
|---|---|---|---|---|---|
| Tool Use > Tool Transport | |||||
| Left thalamus | −12 | −16 | 10 | t(32) = 4.09, p < .001 | 4500 |
| Left putamen | −18 | 5 | 7 | t(32) = 4.13, p < .001 | 6214 |
| Left ventral inferior temporal gyrus | −45 | −13 | −23 | t(32) = 3.97, p < .001 | 4302 |
| Tool Transport > Tool Use | |||||
| Right middle occipital gyrus | 39 | −82 | 7 | t(32) = 5.23, p < .001 | 27981 |
| Left middle occipital gyrus | −30 | −79 | 4 | t(32) = 6.00, p < .001 | 23625 |
General Discussion
The 2AS+ model predicts that functional connectivity to the left SMG should be modulated by action tasks emphasizing diverse tool-directed computations. To assess that prediction, we conducted a task-based functional connectivity study in which neurotypical adult participants planned and pantomimed tool use and tool transport actions. We first demonstrated a greater engagement of the left SMG, left middle temporal gyrus, left frontal cortex, left anterior insula, and left posterior insula extending laterally into ventral premotor cortex for tool use planning relative to tool transport planning. Next, we modeled increases in functional connectivity separately for planning and pantomiming epochs for tool use versus transport, and contrasted the modulation in functional connectivity between those conditions over and above baseline levels of functional connectivity quantified during fixation events. We found strong increases in functional connectivity for tool use between the left SMG and left middle temporal gyrus, however the effect was only marginally stronger relative to tool transport. In a final series of analyses we computed functional connectivity among 11 literature-defined regions implicated in object processing, sensory-motor processing, and action selection. For tool use epochs there was increased functional connectivity between the left SMG and ventral anterior temporal lobe, as well as between the left SMG and inferior frontal gyrus/frontal operculum; in contrast, for tool transport epochs there was increased functional connectivity between the left SMG and right collateral sulcus, left and right medial fusiform gyri, left middle occipital gyrus, and between the left posterior middle temporal gyrus and left middle occipital gyrus.
Our results demonstrate that functional interactions with the left SMG are differentially modulated by tool-directed pantomime actions emphasizing semantic or sensory-motor processing demands. Producing a tool use action, by hypothesis, presupposes the integration of conceptual knowledge of tools with knowledge of the appropriate posture of the fingers, hand, and joints required for skilled tool manipulation. In contrast, tool transport pantomiming bypasses semantic processing, and emphasizes the extraction of three-dimensional volumetric properties of a tool in the environment relative to the hand and body in order to displace or transport a tool several inches. In the sections below, we evaluate our findings in relation to prominent neurocognitive models of action production and tool use.
Our BOLD contrast results are consistent with previous studies demonstrating that the left SMG processes tool-associated manipulation knowledge (Boronat et al., 2005; Canessa et al., 2008; Johnson-Frey et al., 2005; Kellenbach et al., 2003; for discussion, see Orban & Caruana, 2014). For example, Brandi and colleagues (2014) found that generating tool use actions, relative to tool transport actions, elicited increased BOLD signal in the left SMG, lateral occipital complex (in the vicinity of the left posterior middle temporal gyrus) and ventral premotor cortex (among other areas). Moreover, we extend their findings by controlling for tool viewing; this is an important control, as prior research indicates that the left SMG, middle temporal gyrus, and ventral premotor cortex exhibit increased BOLD contrast for tool stimuli in passive viewing paradigms (Chao & Martin, 2000; Garcea et al., 2016; Garcea & Mahon, 2014; Grafton et al., 1997; Mahon et al., 2007; Noppeney et al., 2006).
One discrepancy between our results and previous findings relates to the difference between the left middle temporal gyrus identified in the BOLD contrast analysis and the literature-defined left posterior middle temporal gyrus. For example, although the peak of our BOLD contrast-defined left SMG was in good agreement with the Gallivan et al. (2013) peak (4.9 mm in Euclidean distance), our left middle temporal gyrus was 20.4 mm in Euclidean distance from the Gallivan et al. (2013) left posterior middle temporal gyrus peak. Nevertheless, there was strong functional connectivity for tool use epochs between our BOLD contrast-defined left SMG and left middle temporal gyrus, replicating prior work demonstrating these regions exhibit resting state connectivity (Simmons & Martin, 2012) and task-based functional connectivity driven by tool use (Garcea et al., 2018; Hutchison & Gallivan, 2018; Kleineberg et al., 2018). Future studies using participant-specific ROIs derived from independent localizer tasks will be able to resolve subtle differences in peak voxels used in functional connectivity analyses.
In our BOLD contrast analysis and ROI-based functional connectivity analysis the left inferior frontal gyrus (IFG) was identified as a region involved in tool use planning and pantomiming. Previously, we have provided evidence that the left IFG may provide a top-down biasing signal that resolves competition between candidate tool use actions, supported by functional connectivity with the left SMG (Buxbaum, 2017; see also Cisek & Kalaska, 2010). Other proposals argue that subregions within left IFG participate in distinct components of action, supported by separate white matter fiber tracts connected to the left inferior parietal lobule. For example, Hamzei et al. (2016) used fMRI to parcellate the left IFG into subregions that responded maximally when observing actions, imitating actions, or when grasping a tool without visual feedback. The investigators found that Brodmann area 44 dorsal (BA44d) responded maximally to action observation and imitation, whereas Brodmann Area 44 ventral (BA44v; approximately 4.4 mm in Euclidean distance from our Neurosynth-defined IFG/frontal operculum ROI) responded maximally when grasping a tool without visual feedback. Follow-up diffusion tractography analyses identified white matter connectivity between BA44d and the left inferior parietal lobule via the superior longitudinal fasciculus (SLF; dorsal route), whereas ventral Area 44 (BA44v) and the left inferior parietal lobule were connected via the extreme capsule (ventral route). Consistent with these findings, Vry et al. (2015) identified the extreme capsule as connecting regions involved in pantomiming tool use, including pars triangularis of the left IFG, the left middle temporal gyrus, and the left inferior parietal regions (SMG, IPS); in contrast, frontal and parietal regions involved in imitation of object use and imitation of meaningless gestures were connected by the dorsal route, principally via the SLF. On the other hand, our own prior research has identified the SLF as critical to error-free pantomime production in patients with stroke (e.g., see Watson & Buxbaum, 2015). Thus, an area of continued interest for future work is the extent to which subregions of left prefrontal cortex integrate distinct aspects of action supported via the ventro-dorsal stream, and the ventral (extreme capsule) and dorsal (SLF) fiber tracts reported by Vry et al. (2015) and Hamzei et al. (2016).
We also found strong functional connectivity between the left SMG and left ventral anterior temporal lobe for tool use actions (see Figure 4). Prior TMS studies have found that repetitive stimulation to the left ventral anterior temporal lobe selectively slowed down function judgments of tools, but had no effect on response time when participants made manipulation judgments of tools (Andres et al., 2013; Ishibashi et al., 2011; for discussion, see Garcea & Mahon, in press). Comparable findings in the fMRI literature have been reported by Chen and colleagues (Q.Chen et al., 2018; Q.Chen et al., 2016). For example, when participants were asked to generated tool use gestures to the visual presentation of objects, Chen and colleagues (2016) found that mesial and anterior temporal cortex encoded gestures that shared the same function or purpose of use (see also Canessa et al., 2008), whereas the left SMG encoded gestures that were similar in hand posture and tool use kinematics (see also Gallivan et al., 2013). As noted in the introduction, tool use planning and gesturing emphasizes the integration of semantic processing (including the processing of function knowledge) with skilled tool use action processes, which may be supported via the ventral white matter route proposed by Vry et al. (2015).
In contrast, planning and pantomiming tool transport actions elicited increased functional connectivity between the left SMG and bilateral posterior parietal cortices (see Figure 4C). A core function of the dorso-dorsal stream is to transform current visual input into a sensory-motor representation in the service of reaching and grasping, supported in part by bilateral IPS, superior parietal lobule, and dorsal premotor cortex. Although our analyses combined the planning and pantomiming phase when computing functional connectivity, in a supplemental analysis we measured functional connectivity modulated by the pantomiming phase of each trial, and found robust increases in functional connectivity driven by tool transport pantomiming between the left SMG and bilateral superior parietal lobule, bilateral dorsal premotor cortex, and bilateral somatosensory cortex (see Supplemental Figure 3). Thus, generating a tool transport pantomime engages a bilateral network of regions consistent with the neuroanatomic substrates of the dorso-dorsal stream.
In a secondary aim, we measured functional connectivity between the left SMG and ventral temporal cortex (bilateral medial fusiform gyri and bilateral collateral sulci). Whereas previous work has reported functional connectivity between the left SMG and left medial fusiform gyrus during tool use tasks (e.g., see Garcea et al., 2018; Garcea & Mahon, 2014), we found bilateral medial fusiform gyri exhibited differentially greater functional connectivity to the left SMG for tool transport planning and pantomiming, an action type emphasizing dorso-dorsal stream computations. To our knowledge this is the first study to contrast functional connectivity modulated by tool transport actions against tool use actions; thus it remains an open question whether the computations supported in part by medial ventral temporal cortex (e.g., processing of surface texture) are differentially relevant for tool transport actions relative to tool use actions. One prominent view proposed by Gallivan and Culham (2015) suggests that the ventral visual pathway may receive the outputs of visuomotor processes in frontal-parietal circuits prior to the onset of action (i.e., during the planning phase) to serve as a prediction mechanism of to-be performed actions. Consistent with this possibility, a recent lesion-activity mapping fMRI study in pre-operative neurosurgery patients found that lesions involving the left SMG and adjacent voxels in the left anterior IPS were associated with reduced BOLD contrast for tools in the left medial fusiform gyrus, indicating that lesions to parietal action areas, including the left SMG, disrupt processing in the ventral visual pathway (Garcea et al., in press). Although speculative, in the tool transport condition the left SMG may query ventral stream representations of surface texture, as participants were instructed to imagine grasping the tool to move or transport it; in contrast, tool use epochs emphasize action kinematics and relative positioning of joint angles for accurate tool use, which may not emphasize to the same extent the processing of an object’s material properties, including surface texture. It will also be important to consider if visual properties of tools facilitate tool use (e.g., that a keyboard has buttons that can be pressed) akin to structural affordances of tools that facilitate tool-directed grasping (e.g., that an object has a principal axis that “affords” grasping), and whether tool use affordances are neurocognitively distinct from structural and volumetric affordances of tools. Future functional connectivity research emphasizing diverse object properties when cueing an action (e.g., the coarseness of an object; that an object is slippery; that an object is heavy; e.g., see Cavina-Pratesi et al., 2018; Gallivan, Cant, Goodale, & Flanagan, 2014) can address the extent to which functional interactions between parietal action areas and ventral stream representations of objects are differentially modulated by the transport or use of manipulable objects.
A limitation of our study derives from the fact that tool transport gestures are more uniform and homogeneous than tool use gestures in terms of the structure of the hand and fingers for grasping, and in movement trajectory. While this limitation is not unique to our study (e.g., see Brandi et al., 2014), we designed our experiment such that participants explicitly planned and engaged in thinking about producing the action prior to physically generating a pantomime, permitting us to measure differences in BOLD contrast between tool use and tool transport actions independent of motoric differences between action types, and motoric similarity among tool transport actions. Furthermore, because every item was used in a tool use and tool transport epoch we know that the effects cannot reduce to low-level differences between the items, and instead must be driven by high-level processes that were differentially engaged by the task.
We also found that planning tool transport gestures engaged the right SMG to a greater extent than the left SMG (see Figure 3B). This may be due in part to the nature of the tool transport task (i.e., imagining picking an object up and passing it to a friend, or displacing it several inches), which may emphasize spatial imagery processes and encoding of the extrinsic position of the tool relative to the environment. Thus, it will be of interest for future research to consider how different tool-directed actions (e.g., pantomiming compared to imitation of use and transport actions) differentially engage right hemisphere regions.
Conclusion
A number of fMRI studies have demonstrated there is a high degree of cross-talk between the dorsal and ventral visual pathways as a function of grasping or generating tool-directed gestures (e.g., see Budisavljevic, Dell’Acqua, & Castiello, 2018; Garcea et al., 2018; Hutchison & Gallivan, 2018) or when viewing objects or making judgments about object manipulation (e.g., J.Chen, Snow, Culham, & Goodale, 2018; Q.Chen et al., 2017; Freud, Rosenthal, Ganel, & Avidan, 2015; Kleineberg et al., 2018; Sim, Helbig, Graf, & Kiefer, 2015; for discussion, see Orban & Caruana, 2014; van Polanen & Davare, 2015). Our results offer a novel interpretation of the role that the left SMG plays in tool-directed actions: Tool manipulation knowledge is not ‘represented’ in the left SMG; rather the left SMG sits at the nexus of the dorso-dorsal and ventro-dorsal visual pathways, and serves as an intermediary or “hub” region aggregating i) representations of object properties and conceptual knowledge in the ventral stream with ii) online sensory-motor information processed in the dorsal stream, iii) which is informed by top-down biasing signals from prefrontal cortex to resolve competition between candidate tool use actions. Future task-based functional connectivity studies using diverse tool-directed actions to bias processing to the ventro-dorsal or dorso-dorsal stream will permit a direct evaluation of these hypotheses and of the predictions of the 2AS+ model.
Supplementary Material
Acknowledgements.
We would like to thank Christine Watson and Harrison Stoll for their assistance in data collection. This research was supported by NIH grant R01 NS099061 to L.J. Buxbaum. Preparation of this manuscript was supported by a Moss Rehabilitation Research Institute/University of Pennsylvania postdoctoral training fellowship (NIH 5T32HD071844-05).
Footnotes
The 8 tool items are part of a larger battery of 40 objects used to test pantomime ability in limb apraxic patients; items were independently equated for number of affordances, name agreement, and familiarity; 6 of the 8 items were equated for average number of moveable parts. For details see Watson & Buxbaum, 2015.
When using a more restrictive statistical threshold to define the left middle temporal gyrus peak, we replicate the significant increase in functional connectivity for tool use (relative to fixation baseline; t(32) = 7.04, p < .001) and tool transport epochs (relative to fixation baseline; (t(32) = 3.75, p < .001), and we found that the increase in functional connectivity for tool use epochs was differentially stronger than tool transport epochs (t(32) = 2.28, p < .05; i.e., a significant two-way interaction).
References
- Andres M, Pelgrims B, & Olivier E (2013). Distinct contribution of the parietal and temporal cortex to hand configuration and contextual judgements about tools. Cortex, 49(8), 2097–2105. doi: 10.1016/j.cortex.2012.11.013 [DOI] [PubMed] [Google Scholar]
- Anzellotti S, Mahon BZ, Schwarzbach J, & Caramazza A (2011). Differential activity for animals and manipulable objects in the anterior temporal lobes. J Cogn Neurosci, 23(8), 2059–2067. doi: 10.1162/jocn.2010.21567 [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, & Martin A (2002). Parallel visual motion processing streams for manipulable objects and human movements. Neuron, 34(1), 149–159. [DOI] [PubMed] [Google Scholar]
- Binkofski F, & Buxbaum LJ (2013). Two action systems in the human brain. Brain Lang, 127(2), 222–229. doi: 10.1016/j.bandl.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronat CB, Buxbaum LJ, Coslett HB, Tang K, Saffran EM, Kimberg DY, & Detre JA (2005). Distinctions between manipulation and function knowledge of objects: evidence from functional magnetic resonance imaging. Brain Res Cogn Brain Res, 23(2–3), 361–373. doi: 10.1016/j.cogbrainres.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Brambati SM, Myers D, Wilson A, Rankin KP, Allison SC, Rosen HJ, . . . Gorno-Tempini ML (2006). The anatomy of category-specific object naming in neurodegenerative diseases. J Cogn Neurosci, 18(10), 1644–1653. doi: 10.1162/jocn.2006.18.10.1644 [DOI] [PubMed] [Google Scholar]
- Brandi ML, Wohlschlager A, Sorg C, & Hermsdorfer J (2014). The neural correlates of planning and executing actual tool use. J Neurosci, 34(39), 13183–13194. doi: 10.1523/JNEUROSCI.0597-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald M, Przybylski L, & Kroliczak G (2018). Decoding Brain States for Planning Functional Grasps of Tools: A Functional Magnetic Resonance Imaging Multivoxel Pattern Analysis Study. J Int Neuropsychol Soc, 24(10), 1013–1025. doi: 10.1017/S1355617718000590 [DOI] [PubMed] [Google Scholar]
- Budisavljevic S, Dell’Acqua F, & Castiello U (2018). Cross-talk connections underlying dorsal and ventral stream integration during hand actions. Cortex, 103, 224–239. doi: 10.1016/j.cortex.2018.02.016 [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ (2017). Learning, remembering, and predicting how to use tools: Distributed neurocognitive mechanisms: Comment on Osiurak and Badets (2016). Psychol Rev, 124(3), 346–360. doi: 10.1037/rev0000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Tang K, & Detre JA (2006). Neural substrates of knowledge of hand postures for object grasping and functional object use: evidence from fMRI. Brain Res, 1117(1), 175–185. doi: 10.1016/j.brainres.2006.08.010 [DOI] [PubMed] [Google Scholar]
- Campanella F, D’Agostini S, Skrap M, & Shallice T (2010). Naming manipulable objects: anatomy of a category specific effect in left temporal tumours. Neuropsychologia, 48(6), 1583–1597. doi: 10.1016/j.neuropsychologia.2010.02.002 [DOI] [PubMed] [Google Scholar]
- Canessa N, Borgo F, Cappa SF, Perani D, Falini A, Buccino G, . . . Shallice T (2008). The different neural correlates of action and functional knowledge in semantic memory: an FMRI study. Cereb Cortex, 18(4), 740–751. doi: 10.1093/cercor/bhm110 [DOI] [PubMed] [Google Scholar]
- Cant JS, & Goodale MA (2007). Attention to form or surface properties modulates different regions of human occipitotemporal cortex. Cereb Cortex, 17(3), 713–731. doi: 10.1093/cercor/bhk022 [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Connolly JD, Monaco S, Figley TD, Milner AD, Schenk T, & Culham JC (2018). Human neuroimaging reveals the subcomponents of grasping, reaching and pointing actions. Cortex, 98, 128–148. doi: 10.1016/j.cortex.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, & Martin A (1999). Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci, 2(10), 913–919. doi: 10.1038/13217 [DOI] [PubMed] [Google Scholar]
- Chao LL, & Martin A (2000). Representation of manipulable man-made objects in the dorsal stream. Neuroimage, 12(4), 478–484. doi: 10.1006/nimg.2000.0635 [DOI] [PubMed] [Google Scholar]
- Chen J, Snow JC, Culham JC, & Goodale MA (2018). What Role Does “Elongation” Play in “Tool-Specific” Activation and Connectivity in the Dorsal and Ventral Visual Streams? Cereb Cortex, 28(4), 1117–1131. doi: 10.1093/cercor/bhx017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Garcea FE, Almeida J, & Mahon BZ (2017). Connectivity-based constraints on category-specificity in the ventral object processing pathway. Neuropsychologia, 105, 184–196. doi: 10.1016/j.neuropsychologia.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Garcea FE, Jacobs RA, & Mahon BZ (2018). Abstract Representations of Object-Directed Action in the Left Inferior Parietal Lobule. Cereb Cortex, 26, 2162–2174. doi: 10.1093/cercor/bhx120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Garcea FE, & Mahon BZ (2016). The Representation of Object-Directed Action and Function Knowledge in the Human Brain. Cereb Cortex, 26(4), 1609–1618. doi: 10.1093/cercor/bhu328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, & Kalaska JF (2010). Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci, 33, 269–298. doi: 10.1146/annurev.neuro.051508.135409 [DOI] [PubMed] [Google Scholar]
- Cloutman LL (2013). Interaction between dorsal and ventral processing streams: where, when and how? Brain Lang, 127(2), 251–263. doi: 10.1016/j.bandl.2012.08.003 [DOI] [PubMed] [Google Scholar]
- Devlin JT, Rushworth MF, & Matthews PM (2005). Category-related activation for written words in the posterior fusiform is task specific. Neuropsychologia, 43(1), 69–74. doi: 10.1016/j.neuropsychologia.2004.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan G, Chen Q, Garcea FE, Mahon BZ, & Jacobs RA (2016). Multisensory Part-based Representations of Objects in Human Lateral Occipital Cortex. J Cogn Neurosci, 28(6), 869–881. doi: 10.1162/jocn_a_00937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud E, Plaut DC, & Behrmann M (2016). ‘What’ Is Happening in the Dorsal Visual Pathway. Trends Cogn Sci, 20(10), 773–784. doi: 10.1016/j.tics.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Freud E, Rosenthal G, Ganel T, & Avidan G (2015). Sensitivity to object impossibility in the human visual cortex: evidence from functional connectivity. J Cogn Neurosci, 27(5), 1029–1043. doi: 10.1162/jocn_a_00753 [DOI] [PubMed] [Google Scholar]
- Gallivan JP, Cant JS, Goodale MA, & Flanagan JR (2014). Representation of object weight in human ventral visual cortex. Curr Biol, 24(16), 1866–1873. doi: 10.1016/j.cub.2014.06.046 [DOI] [PubMed] [Google Scholar]
- Gallivan JP, & Culham JC (2015). Neural coding within human brain areas involved in actions. Curr Opin Neurobiol, 33, 141–149. doi: 10.1016/j.conb.2015.03.012 [DOI] [PubMed] [Google Scholar]
- Gallivan JP, McLean DA, Valyear KF, & Culham JC (2013). Decoding the neural mechanisms of human tool use. Elife, 2, e00425. doi: 10.7554/eLife.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea FE, Almeida J, Sims MH, Nunno A, Meyers SP, Li YM, . . . Mahon BZ (in press). Domain-Specific Diaschisis: Lesions to Parietal Action Areas Modulate Neural Responses to Tools in the Ventral Stream. Cereb Cortex. doi: 10.1093/cercor/bhy183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea FE, Chen Q, Vargas R, Narayan DA, & Mahon BZ (2018). Task- and domain-specific modulation of functional connectivity in the ventral and dorsal object-processing pathways. Brain Struct Funct, 223, 2589–2607. doi: 10.1007/s00429-018-1641-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea FE, Kristensen S, Almeida J, & Mahon BZ (2016). Resilience to the contralateral visual field bias as a window into object representations. Cortex, 81, 14–23. doi: 10.1016/j.cortex.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea FE, & Mahon BZ (2014). Parcellation of left parietal tool representations by functional connectivity. Neuropsychologia, 60, 131–143. doi: 10.1016/j.neuropsychologia.2014.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea FE, & Mahon BZ (in press). The how and what of object knowledge in the human brain In Schiller NO, & de Zubicaray G (Ed.), The Oxford Handbook of Neurolinguistics. Oxford, UK: Oxford University Press. [Google Scholar]
- Goldenberg G, Hermsdorfer J, Glindemann R, Rorden C, & Karnath HO (2007). Pantomime of tool use depends on integrity of left inferior frontal cortex. Cereb Cortex, 17(12), 2769–2776. doi: 10.1093/cercor/bhm004 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Castillo J, & Bandettini PA (2017). Task-based dynamic functional connectivity: Recent findings and open questions. Neuroimage. doi: 10.1016/j.neuroimage.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Jo HJ, Wallace GL, Saad ZS, Cox RW, & Martin A (2013). Two distinct forms of functional lateralization in the human brain. Proc Natl Acad Sci U S A, 110(36), E3435–3444. doi: 10.1073/pnas.1302581110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Saad ZS, Jo HJ, Wallace GL, Cox RW, & Martin A (2013). The perils of global signal regression for group comparisons: a case study of Autism Spectrum Disorders. Front Hum Neurosci, 7, 356. doi: 10.3389/fnhum.2013.00356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Fadiga L, Arbib MA, & Rizzolatti G (1997). Premotor cortex activation during observation and naming of familiar tools. Neuroimage, 6(4), 231–236. doi: 10.1006/nimg.1997.0293 [DOI] [PubMed] [Google Scholar]
- Hamzei F, Vry MS, Saur D, Glauche V, Hoeren M, Mader I, . . . Rijntjes M (2016). The Dual-Loop Model and the Human Mirror Neuron System: an Exploratory Combined fMRI and DTI Study of the Inferior Frontal Gyrus. Cereb Cortex, 26(5), 2215–2224. doi: 10.1093/cercor/bhv066 [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Terlinden G, Muhlau M, Goldenberg G, & Wohlschlager AM (2007). Neural representations of pantomimed and actual tool use: evidence from an event-related fMRI study. Neuroimage, 36 Suppl 2, T109–118. doi: 10.1016/j.neuroimage.2007.03.037 [DOI] [PubMed] [Google Scholar]
- Hutchison RM, & Gallivan JP (2018). Functional coupling between frontoparietal and occipitotemporal pathways during action and perception. Cortex, 98, 8–27. doi: 10.1016/j.cortex.2016.10.020 [DOI] [PubMed] [Google Scholar]
- Ishibashi R, Lambon Ralph MA, Saito S, & Pobric G (2011). Different roles of lateral anterior temporal lobe and inferior parietal lobule in coding function and manipulation tool knowledge: evidence from an rTMS study. Neuropsychologia, 49(5), 1128–1135. doi: 10.1016/j.neuropsychologia.2011.01.004 [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Maloof FR, Newman-Norlund R, Farrer C, Inati S, & Grafton ST (2003). Actions or hand-object interactions? Human inferior frontal cortex and action observation. Neuron, 39(6), 1053–1058. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Newman-Norlund R, & Grafton ST (2005). A distributed left hemisphere network active during planning of everyday tool use skills. Cereb Cortex, 15(6), 681–695. doi: 10.1093/cercor/bhh169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalenine S, & Buxbaum LJ (2016). Thematic knowledge, artifact concepts, and the left posterior temporal lobe: Where action and object semantics converge. Cortex, 82, 164–178. doi: 10.1016/j.cortex.2016.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenbach ML, Brett M, & Patterson K (2003). Actions speak louder than functions: the importance of manipulability and action in tool representation. J Cogn Neurosci, 15(1), 30–46. doi: 10.1162/089892903321107800 [DOI] [PubMed] [Google Scholar]
- Kleineberg NN, Dovern A, Binder E, Grefkes C, Eickhoff SB, Fink GR, & Weiss PH (2018). Action and semantic tool knowledge - Effective connectivity in the underlying neural networks. Hum Brain Mapp. doi: 10.1002/hbm.24188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konen CS, Mruczek RE, Montoya JL, & Kastner S (2013). Functional organization of human posterior parietal cortex: grasping- and reaching-related activations relative to topographically organized cortex. J Neurophysiol, 109(12), 2897–2908. doi: 10.1152/jn.00657.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, & Baker CI (2009). Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci, 12(5), 535–540. doi: 10.1038/nn.2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen S, Garcea FE, Mahon BZ, & Almeida J (2016). Temporal Frequency Tuning Reveals Interactions between the Dorsal and Ventral Visual Streams. J Cogn Neurosci, 28(9), 1295–1302. doi: 10.1162/jocn_a_00969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroliczak G, & Frey SH (2009). A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand-independent level. Cereb Cortex, 19(10), 2396–2410. doi: 10.1093/cercor/bhn261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Brenowitz ND, Voon V, Worbe Y, Vertes PE, Inati SJ, . . . Bullmore ET (2013). Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc Natl Acad Sci U S A, 110(40), 16187–16192. doi: 10.1073/pnas.1301725110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Inati SJ, Evans JW, Luh WM, & Bandettini PA (2012). Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage, 60(3), 1759–1770. doi: 10.1016/j.neuroimage.2011.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW (2006). Cortical networks related to human use of tools. Neuroscientist, 12(3), 211–231. doi: 10.1177/1073858406288327 [DOI] [PubMed] [Google Scholar]
- Lingnau A, & Downing PE (2015). The lateral occipitotemporal cortex in action. Trends Cogn Sci, 19(5), 268–277. doi: 10.1016/j.tics.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Anzellotti S, Schwarzbach J, Zampini M, & Caramazza A (2009). Category-specific organization in the human brain does not require visual experience. Neuron, 63(3), 397–405. doi: 10.1016/j.neuron.2009.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, & Caramazza A (2011). What drives the organization of object knowledge in the brain? Trends Cogn Sci, 15(3), 97–103. doi: 10.1016/j.tics.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Milleville SC, Negri GA, Rumiati RI, Caramazza A, & Martin A (2007). Action-related properties shape object representations in the ventral stream. Neuron, 55(3), 507–520. doi: 10.1016/j.neuron.2007.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A (2007). The representation of object concepts in the brain. Annu Rev Psychol, 58, 25–45. doi: 10.1146/annurev.psych.57.102904.190143 [DOI] [PubMed] [Google Scholar]
- Martin M, Beume L, Kummerer D, Schmidt CS, Bormann T, Dressing A, . . . Weiller C (2016). Differential Roles of Ventral and Dorsal Streams for Conceptual and Production-Related Components of Tool Use in Acute Stroke Patients. Cereb Cortex, 26(9), 3754–3771. doi: 10.1093/cercor/bhv179 [DOI] [PubMed] [Google Scholar]
- Milner AD (2017). How do the two visual streams interact with each other? Exp Brain Res, 235(5), 1297–1308. doi: 10.1007/s00221-017-4917-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U, Price CJ, Penny WD, & Friston KJ (2006). Two distinct neural mechanisms for category-selective responses. Cereb Cortex, 16(3), 437–445. doi: 10.1093/cercor/bhi123 [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Orban GA, & Caruana F (2014). The neural basis of human tool use. Front Psychol, 5, 310. doi: 10.3389/fpsyg.2014.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelgrims B, Olivier E, & Andres M (2011). Dissociation between manipulation and conceptual knowledge of object use in the supramarginalis gyrus. Hum Brain Mapp, 32(11), 1802–1810. doi: 10.1002/hbm.21149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, & Ralph MA (2010). Amodal semantic representations depend on both anterior temporal lobes: evidence from repetitive transcranial magnetic stimulation. Neuropsychologia, 48(5), 1336–1342. doi: 10.1016/j.neuropsychologia.2009.12.036 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, & Matelli M (2003). Two different streams form the dorsal visual system: anatomy and functions. Exp Brain Res, 153(2), 146–157. doi: 10.1007/s00221-003-1588-0 [DOI] [PubMed] [Google Scholar]
- Rumiati RI, Weiss PH, Shallice T, Ottoboni G, Noth J, Zilles K, & Fink GR (2004). Neural basis of pantomiming the use of visually presented objects. Neuroimage, 21(4), 1224–1231. doi: 10.1016/j.neuroimage.2003.11.017 [DOI] [PubMed] [Google Scholar]
- Saad ZS, Reynolds RC, Jo HJ, Gotts SJ, Chen G, Martin A, & Cox RW (2013). Correcting brain-wide correlation differences in resting-state FMRI. Brain Connect, 3(4), 339–352. doi: 10.1089/brain.2013.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI, Wurm MF, Wittmann MK, & von Cramon DY (2014). Objects tell us what action we can expect: dissociating brain areas for retrieval and exploitation of action knowledge during action observation in fMRI. Front Psychol, 5, 636. doi: 10.3389/fpsyg.2014.00636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim EJ, Helbig HB, Graf M, & Kiefer M (2015). When Action Observation Facilitates Visual Perception: Activation in Visuo-Motor Areas Contributes to Object Recognition. Cereb Cortex, 25(9), 2907–2918. doi: 10.1093/cercor/bhu087 [DOI] [PubMed] [Google Scholar]
- Simmons WK, & Martin A (2012). Spontaneous resting-state BOLD fluctuations reveal persistent domain-specific neural networks. Soc Cogn Affect Neurosci, 7(4), 467–475. doi: 10.1093/scan/nsr018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, & Tournoux P (1988). Co-planar Stereotaxic Atlas of the Human Brain 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme Medical Publishers. [Google Scholar]
- Valyear KF, & Culham JC (2010). Observing learned object-specific functional grasps preferentially activates the ventral stream. J Cogn Neurosci, 22(5), 970–984. doi: 10.1162/jocn.2009.21256 [DOI] [PubMed] [Google Scholar]
- van Polanen V, & Davare M (2015). Interactions between dorsal and ventral streams for controlling skilled grasp. Neuropsychologia, 79(Pt B), 186–191. doi: 10.1016/j.neuropsychologia.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vry MS, Tritschler LC, Hamzei F, Rijntjes M, Kaller CP, Hoeren M, . . . Weiller C (2015). The ventral fiber pathway for pantomime of object use. Neuroimage, 106, 252–263. doi: 10.1016/j.neuroimage.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Watson CE, & Buxbaum LJ (2015). A distributed network critical for selecting among tool-directed actions. Cortex, 65, 65–82. doi: 10.1016/j.cortex.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


