Abstract
Colorectal cancer is known to arise from multiple tumorigenic pathways; however, the underlying mechanisms remain not completely understood. Metabolomics is becoming an increasingly popular tool in assessing biological processes. Previous metabolomics research focusing on colorectal cancer is limited by sample size and did not replicate findings in independent study populations to verify robustness of reported findings. Here, we performed a ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC-QTOF-MS) screening on EDTA plasma from 268 colorectal cancer patients and 353 controls using independent discovery and replication sets from two European cohorts (ColoCare Study: n=180 patients / n=153 controls; the Colorectal Cancer Study of Austria (CORSA) n=88 patients / n=200 controls), aiming to identify circulating plasma metabolites associated with colorectal cancer and to improve knowledge regarding colorectal cancer aetiology. Multiple logistic regression models were used to test the association between disease state and metabolic features. Statistically significant associated features in the discovery set were taken forward and tested in the replication set to assure robustness of our findings. All models were adjusted for sex, age, BMI and smoking status and corrected for multiple testing using False Discovery Rate. Demographic and clinical data were abstracted from questionnaires and medical records.
A total of 691 metabolic features significantly discriminated between colorectal cancer patients and controls in the discovery stage, of which 97 features remained for replication. Metabolite identification yielded 28 annotated metabolites including: taurine, hypoxanthine, valine, leucine, bilirubin, 1-methylnicotinamide, and several glycerophospholipids. Our study provides novel insights regarding metabolic changes associated with colorectal cancer.
Keywords: Colorectal cancer, Metabolomics, Discovery-Replication approach, UHPLC-QTOF-MS
Background
Colorectal cancer is a major public health concern worldwide, with 1.4 million new cases and an estimated 700,000 deaths annually.1 Colorectal cancer is characterized by a distinct metabolic phenotype and changes in key metabolic pathways such as glycolysis or the tricarboxylic acid (TCA) cycle.2, 3 Yet, underlying mechanisms involved in colorectal carcinogenesis are still unclear4.
Metabolomics is a powerful approach to unravel metabolic changes associated with disease and is gaining momentum in the field of cancer epidemiology.5–7 Compared to other ‘-omics’ techniques, metabolomics is more closely related to a measured clinical phenotype and is increasingly applied as the method of choice to screen for potential metabolites associated with disease status.8, 9 Moreover, metabolomics can help to understand the underlying aetiology of cancer development.10
Differences in metabolic profiles have been reported between colorectal cancer patients and colorectal cancer-free individuals using nuclear magnetic resonance techniques,11 gas chromatography,12–15 and liquid chromatography-mass spectrometry methods.16
Various amino acids, such as aspartic acid, have been shown to be more abundant in cases in different, relatively small, studies, including a study by Nishiumi and colleagues comparing serum metabolite levels of 60 colorectal cancer patients and 60 healthy volunteers using gas-chromatography time-of-flight (TOF) mass spectrometry.13 Similarly, a study by Denkert et al. examined metabolic profiles in colon tissue and normal mucosa samples of 27 colorectal cancer patients and 18 colorectal cancer-free individuals.15 In addition to amino acids, serum taurine was shown to be more abundant among colorectal cancer patients compared to colorectal cancer-free individuals. Another study among 101 newly diagnosed colorectal cancer patients reported a clear difference between serum glutamine, fatty acids, and the urea and TCA cycle metabolites compared to 102 colorectal cancer-free controls.17
The majority of these previous studies have been limited by sample size and did not perform replication of their findings in independent study populations. As metabolomics studies often identified a wide range of metabolites due to the variety of analytical platforms, clinical protocols, and sample handling procedures used, leveraging an independent population for replication using the same platform and similar protocols is essential to ensure robustness of findings. To date, only few studies have used a discovery-replication design to reproduce results in independent study populations.16, 18, 19 Two of these studies investigated metabolic differences between colorectal cancer patients and apparently healthy individuals;16, 18 a third study evaluated metabolomic differences between matched tumour and healthy colon tissue samples from colorectal cancer patients.19 In addition, a very recent study investigating metabolic profiles in adenomas, colorectal cancer cases and controls conducted analysis in two datasets utilizing different metabolomic approaches, but with both sample sets deriving from the same hospital and cohort.20
To complement current research, we utilized a powerful combination of untargeted metabolomics analysis, able to reveal (novel) metabolites, a rigorous discovery-replication design, leveraging samples deriving from two independent study populations, as well as relatively large sample sizes to obtain sufficient statistical power. The overall purpose of our study was to discover, and replicate plasma metabolites associated with colorectal cancer to improve knowledge regarding potential disease aetiology.
Methods
Study populations
We utilized data from two cohort studies embedded in the MetaboCCC Consortium, a consortium of four independent European cohorts to investigate metabolic profiles across the continuum of colorectal carcinogenesis: (1) the Heidelberg site of the international ColoCare Study (ClinicalTrials.gov Identifier: NCT02328677) and (2) the Colorectal Cancer Study of Austria (CORSA). The CORSA and ColoCare studies were selected given the availability of samples from colorectal cancer patients as well as controls. EDTA plasma samples from 621 participants were analysed, consisting of 268 patients with newly diagnosed colorectal cancer and 353 controls. We applied independent discovery (ColoCare Study: n=180 patients / n=153 controls) and replication (CORSA Study: n=88 patients / n=200 controls) sets using an identical metabolomics platform (Supplementary Figure S1).
The ColoCare Study, in Heidelberg initiated in 2010, is an ongoing, international, multi-centre prospective study including women and men newly diagnosed with primary colorectal cancer. Patients are recruited at the University Hospital of Heidelberg and the National Center for Tumor Diseases in Heidelberg, Germany. Participants provided consent prior to tumour resection if they met the following inclusion criteria: newly diagnosed colorectal cancer (both colon (ICD-10 C18) and rectal or recto-sigmoidal cancer (ICD-10 C19/C20)), any stage of the disease, 18+ years at the time of diagnosis, and German-speaking. EDTA blood samples from colorectal cancer patients were collected prior to surgery. Control participants were enrolled in the PRAEVENT Study, a population-based study subjected to similar protocols and procedures, conducted at the National Center for Tumor Diseases in Heidelberg, Germany. All participants consented to take part in this study and EDTA blood samples were collected during a visit at the National Center for Tumor Diseases at recruitment (usually the same day after the consent dialogue and after signing the informed consent form).
In the ongoing CORSA Study participants are recruited in cooperation with the province-wide screening project “Burgenland Prevention Trial of Colorectal Disease with Immunological Testing” (B-PREDICT), since 2003. All inhabitants of the Austrian province Burgenland aged between 40 and 80 years are invited annually to participate in faecal occult blood testing. Positive faecal occult blood tested individuals are subsequently offered a complete colonoscopy, and EDTA blood samples are collected prior to examination. Additional colorectal cancer patients are recruited at the General Hospital of Vienna (Department of Surgery), and at three additional hospitals in Vienna. All colorectal cancer patients included in the CORSA Study are individuals with histologically confirmed, sporadic colorectal cancer. CORSA controls are individuals who received a complete colonoscopy within the B-PREDICT screening but exhibited no pathological findings of disease.
All colorectal cancer samples selected for inclusion into the presented study were collected prior to any clinical treatment, including surgery or neo-adjuvant therapy, and did not have a prior history of cancer. Controls included in the study can be considered as ‘cancer-free’; having no prior history of cancer. Patients and controls were 95% of Caucasian origin, recruited within the last fifteen years and selected to be matching according to their recruitment time point. Clinical data, including tumour location, staging, and treatment history were abstracted from medical records. Demographic characteristics (e.g. age, weight, height and smoking status) were assessed by study-specific questionnaires. All clinical and demographic data were harmonized across all cohorts.
Sample collection and analysis
In both cohorts, non-fasted EDTA blood samples were collected and processed within four hours, according to identical processing protocols, and stored at −80°C. Samples at each respective study site were shipped on dry ice to the International Agency for Research on Cancer (IARC) in Lyon, France for analysis. Samples were analysed with a ultra-high performance liquid chromatography- quadrupole time-of-flight mass spectrometry (UHPLC-QTOF-MS) system (Agilent Technologies) consisting of a 1290 Binary LC system, a Jet Stream electrospray ionization (ESI) source, and a 6550 QTOF mass spectrometer. Samples from each study centre were analysed in cohort-specific batches, which consisted of five and six 96-well plates for CORSA and ColoCare, respectively.
A detailed overview of the sample preparation and a complete description of sample analysis by UHPLC-QTOF-MS, pre-processing of metabolomics data can be found in Supplemental File S1. A summary of the data processing workflow is shown in Supplementary Figure S1.
Data Analysis
Features with missing values in >50% of either colorectal cancer patient or control samples in both populations were excluded from analysis. The remaining maximum 50% of missing values were not imputed according to the recommendations of Di Guida et al. 21. Blank adjustment was applied for the ColoCare and CORSA samples separately; features that had a minimum relative mean intensity below the relative mean intensity of blank samples were removed. “Features” were defined as chromatographic peaks formed by specific ions, while “compounds” or “metabolites” referred to a confirmed molecule that can consist of one or more features (adducts, clusters and fragments).
Feature intensities were log transformed using the natural logarithm prior to statistical analysis, to prevent heteroscedasticity.21, 22 Demographic and clinical characteristics are presented as medians with the interquartile range (IQR), or as numbers with corresponding percentages. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). BMI status was categorized based on the recommendations from the World Health Organization (WHO): underweight (<18.5 kg/ m2), normal weight (18.5 – 24.9 kg/m2), overweight (25.0 – 29.9 kg/m2) and obese (≥30.0 kg/m2). Smoking status was categorized as current, former, and never.
Discovery stage
The discovery analysis was conducted in ColoCare samples. Log standardized odds ratios (OR.std) and 95% confidence intervals (CIs) were calculated using multiple logistic regression models with disease state as dependent variable to test the association with feature intensities. The OR.std represents the change in colorectal cancer occurrence when there is a one standard deviation (SD) change in metabolite intensity, allowing comparison of effect sizes between different features. Since odds ratios were standardized, the SD of the controls were used to calculate the OR.std. Sex, age, BMI (continuous), and smoking status were included as covariates in the final model. Features that showed significant differences between colorectal cancer patients and controls after correction for multiple testing, using False Discovery Rate (FDR) correction, in the discovery stage were carried forward to the replication stage. A priori, an FDR p-value <0.05 was considered statistically significant.
Replication stage
The replication stage was conducted in CORSA Study samples. Significant features (FDR p<0.05) from the discovery stage were analysed in the replication stage using the same modelling approach as in the discovery stage. Features were tested if they point in the same direction as the corresponding effects in the discovery stage (one-sided testing). Analyses were checked for any influence by analytical batch, but no marked effect could be identified in both stages. Features with significant test results were selected for identification using authentic chemical standards at IARC. A detailed overview of metabolite identification is explained in Supplemental Table S1. When more than one mass spectrometry feature corresponded with a metabolite, the feature with the highest intensity was selected and presented in the manuscript (Supplementary Table S2).
Spearman correlation analysis was used to identify metabolite-metabolite correlations among all identified metabolites and to understand the intra-relation of metabolites. Spearman correlation coefficients were calculated for all pairs of annotated features for samples from the discovery and replication set to account for deviations from linearity. All statistical analyses were performed in R, version 3.3.3.23
Results
Participant characteristics
Characteristics of the study population are summarized in Table 1. The ColoCare cohort consisted of 63% men in the colorectal cancer group and 38% men in the control group. In addition, ColoCare controls had on average less participants classified as overweight compared to the colorectal cancer patients.
Table 1.
Demographics and clinical characteristics of colorectal cancer patients and controlsa
| Discovery stage: ColoCare Study | Replication stage: CORSA | ||||
|---|---|---|---|---|---|
| CRC patients | Controls | CRC patients | Controls | ||
| Number of participants | 180 | 153 | 88 | 200 | |
| Sex*‡ | Men n(%) | 114 (63.3) | 59 (38.6) | 60 (68.2) | 130 (65.0) |
| Age*¥‡ | Median (IQR) | 66.0 (58.0–73.0) | 51.0 (42.0–63.0) | 70.0 (60.0–76.0) | 64.0 (57.0–74.0) |
| Body mass indexb,*,‡ (kg/m2) | Median (IQR) | 26.4 (24.1–29.2) | 23.5 (22.0–26.7) | 26.1 (23.8–29.4) | 27.0 (24.9–30.1) |
| Underweight, <18.5 n(%) | 2 (1.1) | 19 (12.4) | 0 (0) | 2 (1.0) | |
| Normal weight, 18.5–24.9 n(%) | 59 (32.8) | 3 (2.0) | 26 (29.5) | 47 (23.5) | |
| Overweight, 25–29.9 n(%) | 82 (45.6) | 91 (59.5) | 38 (43.2) | 94 (47.0) | |
| Obese, ≥30 n(%) | 37 (20.6) | 35 (22.9) | 15 (17.0) | 50 (25.0) | |
| Smoking status* n(%) | Current | 30 (16.6) | 25 (16.3) | 20 (22.7) | 25 (12.5) |
| Former | 77 (42.8) | 47 (30.7) | 30 (34.2) | 64 (32.0) | |
| Never | 61 (33.9) | 75 (49.1) | 35 (39.7) | 104 (52.0) | |
| Unknown | 12 (6.7) | 6 (3.9) | 3 (3.4) | 7 (3.5) | |
| Stagec,† n(%) | 0 | 7 (3.9) | - | 0 (0) | - |
| I | 34 (18.9) | - | 30 (34.1) | - | |
| II | 66 (36.7) | - | 17 (19.3) | - | |
| III | 47 (26.1) | - | 18 (20.5) | - | |
| IV | 25 (13.9) | - | 12 (13.6) | - | |
| Unspecified | 1 (0.5) | - | 3 (3.4) | - | |
| Unknown | 0 (0) | - | 8 (9.1) | - | |
| Tumor locationd,† n(%) | Colon – distal | 53 (29.4) | - | 21 (31.8) | - |
| Colon – proximal | 57 (31.7) | - | 33 (28.4) | - | |
| Rectum | 70 (38.9) | - | 34 (39.8) | - | |
Controls are defined as individuals not diagnosed with any colorectal malignancy;
Missing BMI for n= 5, 9 and 7 for ColoCare controls, CORSA CRC patients and controls, respectively;
TNM for colorectal cancer with neo-adjuvant therapy and colon cancers without surgery (28.7%), else pTNM was used (71.3%);
Distal colon: sigmoid colon, descending colon, splenic flexure; Proximal colon: transverse colon, hepatic flexure, ascending colon, cecum, appendix; Rectum: rectum, rectosigmoid junction;
p-value<0.05 between ColoCare CRC and controls
p-value<0.05 between CORSA CRC and controls
p-value<0.05 between CRC
p-value<0.05 between controls
The CORSA cohort consisted of 68% men in the colorectal cancer group and 65% men in the controls group. Control patients from the CORSA cohort had on average slightly more participants categorized as overweight compared to colorectal cancer patients.
In both cohorts control groups consisted of more participants categorized as never smokers than compared to the colorectal cancer patients. In general, the distributions of covariates were relatively comparable between the discovery and replication cohorts. Controls from the ColoCare cohort were 13 years younger than controls from the CORSA cohort. The majority of participants have a BMI classified as overweight, except for the controls from ColoCare.
Metabolic profiles discriminating between colorectal cancer patients and controls
Metabolomics analysis yielded 10,015 mass spectrometry features, defined as a chromatographic peak formed by specific ions that were identified across all study samples. After data pre-processing, 1,156 and 1,148 features were carried forward for ColoCare and CORSA samples, respectively.
Next, 691 out of 1,156 features were found to be statistically significantly associated with disease state (discovery stage) after FDR correction and adjustment for age, sex, BMI, and smoking status. The 691 significant features were subsequently analysed in the replication dataset, i.e. the CORSA Study samples. Of these features, 97 differed between CORSA patients and controls.
The 97 replicated discriminating mass spectrometry features corresponded to 28 metabolites, defined as a confirmed molecule that can consist of one or more features (adducts, clusters and fragments) (Supplementary Table S2). Six metabolites (taurine, hypoxanthine, valine, leucine, bilirubin, and 1-methylnicotinamide) were identified using authentic standards resulting in a level 1 identification according to the Metabolomics Standards Initiative (MSI), nine compounds (seven lysophosphatidylcholines (LysoPCs) and two lysophosphatidylethanolamines (LysoPEs)) reached MSI level 2 identification, and 14 compounds could not be identified (unknown metabolites, MSI level 4). The intensity of these 15 identified metabolites exhibited significant differences between colorectal cancer patients and controls in both the discovery and replication set (Figure 1). Taurine, hypoxanthine, LysoPE (20:4), and LysoPE (22:6) showed higher relative mean intensity values in the colorectal cancer group compared with controls, representing a OR.std higher than one (Table 2). Valine, leucine, bilirubin, 1-methylnicotinamide, and seven LysoPCs (LysoPC(15:0), LysoPC (16:0), LysoPC(16:0) isomer, LysoPC(P-16:0), LysoPC(16:1), LysoPC(17:0), LysoPC(18:0)) showed higher relative mean intensity values in the control group compared to the colorectal cancer patient group, indicating an OR.std lower than one (Table 3).
Figure 1.
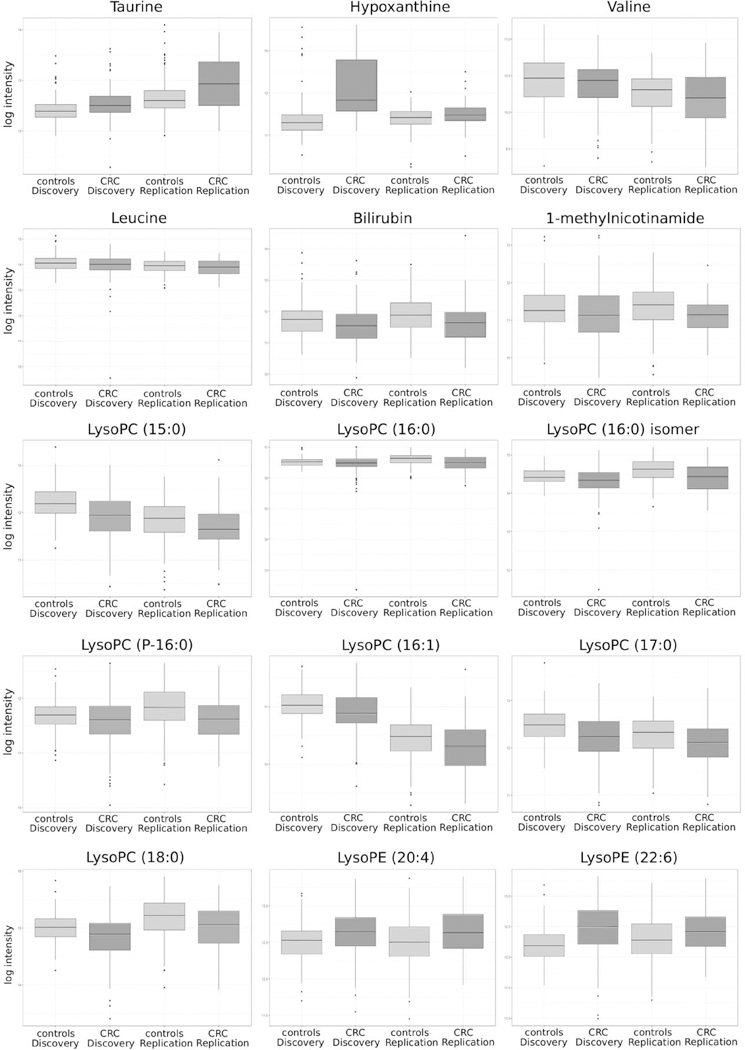
Box plots of the 15 annotated metabolites differentiating colorectal cancer patients (CRC) and controls for the discovery and replication set. The boxplot presents the minimum, first quartile, median, third quartile and maximum log transformed relative intensity values and potential outliers of taurine, hypoxanthine, valine, leucine, bilirubin, 1- methylnicotinamide (MNA), LysoPC (15:0), LysoPC (16:0), LysoPC (16:0) isomer, LysoPC (P-16:0), LysoPC (16:1), LysoPC (17:0), LysoPC (18:0), LysoPE (20:4) and LysoPE (22:6), respectively.
Table 2.
List of replicated metabolites positively associated with colorectal cancera
| Discovery stage: ColoCare Study |
Replication stage: CORSA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound Name | ID levelb |
CRC Mean ± SDc |
Controls Mean ± SDc |
OR.sthd
[95% CI] |
p valuee |
CRC Mean ± SDc |
Controls Mean ± SDc |
OR.sthd
[95% CI] |
p valuee |
|
| Positively associated | Taurine | 1 | 12.53 ± 0.28 | 12.41 ± 0.24 | 1.44 [1.13; 1.87] | 7.2E-03 | 12.96 ± 0.49 | 12.66 ± 0.34 | 1.95 [1.53; 2.51] | 4.2E-06 |
| Hypoxanthine | 1 | 13.18 ± 1.32 | 11.73 ± 0.91 | 3.07 [2.25; 4.45] | 9.1E-17 | 12.00 ± 0.53 | 11.79 ± 0.49 | 1.43 [1.09; 1.92] | 3.6E-02 | |
| LysoPE(20:4) | 2 | 12.64 ± 0.30 | 12.50 ± 0.26 | 1.37 [1.08; 1.76] | 2.0E-02 | 12.66 ± 0.32 | 12.51 ± 0.32 | 1.51 [1.15; 2.01] | 1.4E-02 | |
| LysoPE(22:6) | 2 | 12.48 ± 0.40 | 12.21 ± 0.29 | 1.80 [1.42; 2.31] | 1.5E-06 | 12.41 ± 0.34 | 12.28 ± 0.36 | 1.60 [1.20; 2.17] | 7.8E-03 | |
| 71.0292@2.8578448 | 4 | 10.32 ± 0.52 | 10.04 ± 0.40 | 1.61 [1.26; 2.08] | 3.4E-04 | 10.2 ± 0.54 | 9.81 ± 0.41 | 2.04 [1.52; 2.81] | 3.5E-05 | |
| 279.1473@2.7668757 | 4 | 11.08 ± 0.83 | 10.59 ± 0.66 | 1.38 [1.09; 1.77] | 1.5E-02 | 10.64 ± 0.84 | 9.97 ± 0.66 | 2.20 [1.62; 3.08] | 1.0E-05 | |
| 428.3615@7.4910417 | 4 | 9.61 ± 0.34 | 9.49 ± 0.26 | 1.49 [1.07; 2.15] | 3.3E-02 | 9.56 ± 0.25 | 9.45 ± 0.18 | 1.59 [1.14; 2.26] | 2.8E-02 | |
Controls are defined as individuals not diagnosed with any colorectal malignancy;
According to MSI;
Log transformed relative intensity values;
OR.std: standardized Odds Ratio, represents the relative change in colorectal cancer (CRC) risk when there is a one standard deviation (SD) change in metabolite intensity. OR.std is based on the SD of the controls;
p-value: FDR-corrected p-value
Table 3.
List of replicated metabolites inversely associated with colorectal cancera
| Discovery stage: ColoCare Study |
Replication stage: CORSA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound Name | ID levelb |
CRC Mean ± SDc |
Controls Mean ± SDc |
OR.sthd
[95% CI] |
p valuee |
CRC Mean ± SDc |
Controls Mean ± SDc |
OR.sthd
[95% CI] |
p valuee | |
| Inversely associated | Leucine | 1 | 13.98 ± 0.49 | 14.07 ± 0.31 | 0.69 [0.53; 0.87] | 4.4E-03 | 13.86 ± 0.32 | 13.93 ± 0.28 | 0.72 [0.55; 0.93] | 4.7E-02 |
| 1-methylnicotinamide | 1 | 11.18 ± 0.68 | 11.33 ± 0.60 | 0.69 [0.53; 0.88] | 6.5E-03 | 11.11 ± 0.45 | 11.36 ± 0.56 | 0.62 [0.46; 0.83] | 6.8E-03 | |
| Valine | 1 | 10.36 ± 0.33 | 10.43 ± 0.33 | 0.74 [0.56; 0.96] | 4.2E-02 | 10.18 ± 0.35 | 10.27 ± 0.27 | 0.69 [0.53; 0.89] | 1.7E-02 | |
| Bilirubin | 1 | 11.54 ± 0.59 | 11.74 ± 0.56 | 0.57 [0.43; 0.75] | 1.5E-04 | 11.66 ± 0.64 | 11.92 ± 0.62 | 0.66 [0.49; 0.87] | 1.5E-02 | |
| LysoPC(16:1) | 2 | 10.90 ± 0.35 | 11.02 ± 0.27 | 0.60 [0.46; 0.77] | 1.2E-04 | 10.31 ± 0.46 | 10.44 ± 0.36 | 0.73 [0.57; 0.93] | 4.4E-02 | |
| LysoPC(P-16:0) | 2 | 11.58 ± 0.42 | 11.68 ± 0.28 | 0.76 [0.61; 0.94] | 2.0E-02 | 11.63 ± 0.42 | 11.81 ± 0.39 | 0.66 [0.50; 0.87] | 1.4E-02 | |
| LysoPC(15:0) | 2 | 11.90 ± 0.49 | 12.19 ± 0.36 | 0.53 [0.40; 0.67] | 2.8E-07 | 11.69 ± 0.48 | 11.83 ± 0.43 | 0.71 [0.55; 0.92] | 4.2E-02 | |
| LysoPC (16:0) | 2 | 16.44 ± 0.38 | 16.52 ± 0.13 | 0.75 [0.61; 0.91] | 5.7E-03 | 16.49 ± 0.26 | 16.61 ± 0.19 | 0.59 [0.46; 0.76] | 3.3E-04 | |
| LysoPC(16:0) isomer | 2 | 14.28 ± 0.45 | 14.44 ± 0.21 | 0.71 [0.55; 0.90] | 8.3E-03 | 14.42 ± 0.38 | 14.59 ± 0.30 | 0.58 [0.42; 0.78] | 2.9E-03 | |
| LysoPC(17:0) | 2 | 12.22 ± 0.47 | 12.47 ± 0.36 | 0.54 [0.41; 0.69] | 8.9E-07 | 12.11 ± 0.47 | 12.27 ± 0.40 | 0.68 [0.52; 0.88] | 1.7E-02 | |
| LysoPC(18:0) | 2 | 14.84 ± 0.39 | 15.00 ± 0.25 | 0.63 [0.50; 0.78] | 5.7E-05 | 15.00 ± 0.42 | 15.18 ± 0.34 | 0.63 [0.49; 0.81] | 2.9E-03 | |
| 57.1107@0.8299292 | 4 | 11.45 ± 0.65 | 11.44 ± 0.61 | 0.69 [0.52; 0.90] | 1.3E-02 | 11.39 ± 0.68 | 11.52 ± 0.66 | 0.67 [0.49; 0.90] | 3.2E-02 | |
| 181.1113@5.475981 | 4 | 10.37 ± 0.51 | 10.75 ± 0.47 | 0.41 [0.30; 0.55] | 5.4E-09 | 10.88 ± 0.42 | 10.95 ± 0.33 | 0.72 [0.55; 0.93] | 4.2E-02 | |
| 190.0061@0.83498704 | 4 | 10.93 ± 0.32 | 11.04 ± 0.24 | 0.61 [0.46; 0.79] | 3.3E-04 | 11.07 ± 0.39 | 11.21 ± 0.32 | 0.66 [0.51; 0.84] | 6.1E-03 | |
| 247.0286@3.3104632 | 4 | 12.88 ± 0.43 | 13.14 ± 0.31 | 0.43 [0.31; 0.57] | 2.6E-09 | 12.99 ± 0.40 | 13.13 ± 0.32 | 0.66 [0.50; 0.86] | 1.2E-02 | |
| 445.8809@0.56495804 | 4 | 13.86 ± 0.36 | 13.96 ± 0.11 | 0.76 [0.64; 0.90] | 3.0E-03 | 13.79 ± 0.31 | 13.87 ± 0.16 | 0.77 [0.61; 0.94] | 4.2E-02 | |
| 452.8025@7.3067145 | 4 | 9.77 ± 0.26 | 9.85 ± 0.28 | 0.72 [0.54; 0.96] | 4.4E-02 | 9.88 ± 0.23 | 9.97 ± 0.21 | 0.65 [0.50; 0.86] | 1.1E-02 | |
| 535.2997@7.0497603 | 4 | 11.01 ± 0.41 | 11.16 ± 0.35 | 0.65 [0.49; 0.85] | 3.4E-03 | 11.27 ± 0.38 | 11.37 ± 0.33 | 0.72 [0.55; 0.93] | 4.4E-02 | |
| 545.3467@7.2500143 | 4 | 13.39 ± 0.53 | 13.50 ± 0.31 | 0.79 [0.63; 0.98] | 5.0E-02 | 13.78 ± 0.46 | 14.00 ± 0.39 | 0.61 [0.47; 0.78] | 1.1E-03 | |
| 610.9343@7.191048 | 4 | 11.78 ± 0.43 | 11.94 ± 0.34 | 0.63 [0.49; 0.80] | 4.6E-04 | 11.34 ± 0.40 | 11.51 ± 0.33 | 0.63 [0.48; 0.82] | 3.9E-03 | |
| 638.9663@7.4319897 | 4 | 10.85 ± 0.33 | 10.97 ± 0.28 | 0.68 [0.53; 0.87] | 4.7E-03 | 10.98 ± 0.29 | 11.13 ± 0.24 | 0.58 [0.44; 0.76] | 4.5E-04 | |
Controls are defined as individuals not diagnosed with any colorectal malignancy;
According to MSI;
Log transformed relative intensity values; OR.std: standardized Odds Ratio, represents the relative change in colorectal cancer (CRC) risk when there is a one standard deviation (SD) change in metabolite intensity. OR.std is based on the SD of the controls;
p-value: FDR-corrected p-value
Correlation analysis
Spearman correlation analysis was used to identify potential metabolite-metabolite correlations among all identified metabolites (Figure 2). Correlation patterns demonstrated similar results across the discovery (Figure 2A) and replication stage (Figure 2B). For both stages, all LysoPCs were positively correlated (Spearman correlation coefficient range [rs]: 0.40 – 0.91) but showed only a weak correlation to LysoPE (22:6) and LysoPE (20:4). Valine and leucine were highly correlated (discovery stage rs: 0.73, replication stage rs: 0.78). In addition, the majority of replicated compounds annotated as unknown (n=13) were correlated with each other but showed only weak correlations with the other annotated compounds. Spearman correlation coefficients are shown in Supplementary Table S3.
Figure 2.
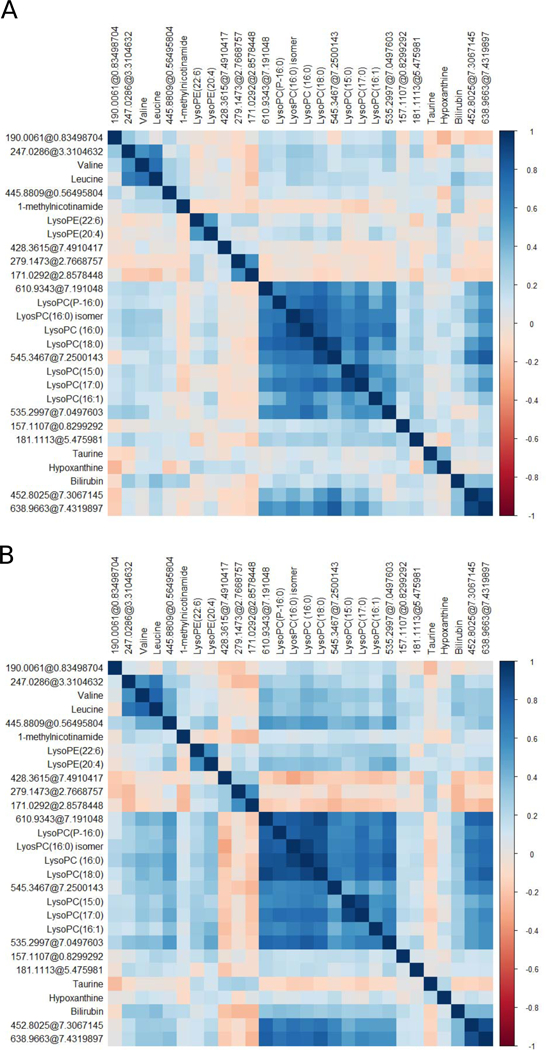
Metabolite-metabolite correlation analysis of replicated metabolites. Positive correlations are highlighted in blue, negative correlations are highlighted in red. Unknown compounds are indicated as monoisotopic mass@retention time. Metabolites are ordered by hierarchical clustering. (A) Spearman correlation analysis plot of the discovery dataset. (B) Spearman correlation analysis plot of the replication dataset.
Discussion
In this study, we identified plasma metabolites that are associated with colorectal cancer and which were replicated in an independent study population. We found 28 metabolites associated with disease state in two independent study cohorts, the ColoCare and CORSA studies. In total, 15 out of 28 metabolites could be identified. Taurine, hypoxanthine, valine, leucine, LysoPCs, and LysoPEs have been reported to be linked with colorectal cancer in previous metabolomics studies. All LysoPCs were positively correlated, valine and leucine were highly correlated, and the majority of unidentified metabolites were correlated with each other. Except for valine and leucine, the identified metabolites were only slightly or not correlated with each other.
Taurine was previously shown to be increased in serum of 60 colorectal cancer patients compared to 60 apparently healthy individuals 13 and in tumour tissue of 16 colorectal cancer patients; 24 which is in agreement with our findings. Recent studies have suggested taurine as a microbiota-associated metabolite playing a mediating role in microbiome-host interactions.25, 26 Given the knowledge that gut microbiota differ between colorectal cancer patients and healthy individuals, and that microbial composition is linked to colorectal cancer risk,27 taurine presents a promising candidate for further investigation.
Hypoxanthine has been previously reported to be increased in tumour tissue of colorectal cancer patients compared to normal tissue of healthy individuals.15 In contrast, a recent study, published by Long et al. reported decreased levels of hypoxanthine in colorectal cancer and polyps compared to controls.20 Like taurine,5 hypoxanthine is an antioxidant and increased levels reported in the current study may be the result of increased oxidative stress,28 which is recognized as an important process in carcinogenesis, including colorectal cancer.29, 30 Inconsistent findings in hypoxanthine levels may be due to the type of specimen analysed, or lack of statistical power because of lower sample numbers included. Furthermore, a possible reason for the inconsistent hypoxanthine levels may be caused by red blood cell haemolysis during the preparation of serum samples utilized in the Long study in contrast to plasma used in the present analysis.31
With respect to branched-chain amino acids (BCAAs), we observed that valine was reduced among colorectal cancer patients compared to controls. This result is consistent with two prior studies; Ma and colleagues compared serum of 30 colorectal cancer patients to 30 colorectal cancer-free controls,12 and Farshidfar et al. investigated metabolomic signatures in colorectal cancer serum of stage I-IV patients.14 Comparable to valine, decreased plasma levels of leucine were also reported in our colorectal cancer patients compared to controls. Decreased blood levels of BCAAs could reflect increased requirement for amino acids due to the high protein turnover in the malignant setting.15, 19, 32
Moreover, seven LysoPCs were detected at lower levels among colorectal cancer patients compared to controls. LysoPC (16:0) and LysoPC (18:0) were reported before to be lower in the plasma of colorectal cancer patients versus control individuals.33, 34 There seems to be a general trend of lower levels of LysoPCs among colorectal cancer patients in existing studies,17, 33, 35 which is in line with the findings reported in our study. This pattern might reflect an increased degradation rate of LysoPCs as a result of the accelerated cell proliferation rate of cancerous cells.36 It has been suggested that decreased levels of LysoPCs could result from weight loss and possibly inflammatory processes related to cancer.37, 38 While the majority of our study participants were classified as overweight, we did not have data on changes in body weight among patients prior to a colorectal cancer diagnosis.
LysoPE (20:4) and LysoPE (22:6) were increased in colorectal cancer patients compared to controls. LysoPEs belong to the group of signalling lipids and are constituents of cell membranes. Recently, serum LysoPEs were found to be elevated among breast cancer patients.39 However, knowledge is limited regarding the role of LysoPEs in healthy and diseased individuals.
We also identified a notable decrease in MNA, an inactive metabolite of nicotinamide,40 among colorectal cancer patients compared to controls. MNA has been reported in vivo to be involved in the COX-2/PGI2 pathway,40 which plays a major role in inflammation and colorectal carcinogenesis.41, 42 In addition, this is the first metabolomics study to report lower plasma bilirubin levels in colorectal cancer patients compared to controls. Previously, a European study analysing genomic alterations in promoter variants involved in bilirubin homeostasis, and another study investigating serum bilirubin levels in a large U.S. population have proposed a protective effect of bilirubin against colorectal carcinogenesis; 43, 44 our metabolomics findings carefully support this hypothesis. The underlying mechanisms of the relationship between bilirubin and colorectal cancer remain unclear.
Untargeted metabolomics is an elegant approach for the discovery of metabolites associated with cancer. However, one may wonder whether the seemingly small differences between colorectal cancer patients and controls are biologically relevant. It is important to keep in mind that findings presented are log transformed relative values. As a consequence, reported results hint towards the direction of the association and quantification of the metabolites is needed to be able to interpret absolute differences. Our results for taurine, hypoxanthine, valine, leucine, bilirubin, and 1-methylnicotinamide suggest future research to investigate the underlying biological mechanism of these metabolites in relation to colorectal cancer.
A strength of the present study is the use of a discovery-replication design leveraging two independent, relatively large, patient cohorts, both including patients of Caucasian origin, from two different countries. In general, untargeted methods typically yield data with high amounts of noise and non-biological information.45 This makes replication of untargeted metabolomics findings within ethnically homogenous cohorts extremely valuable, as it enables the exclusion of features that are not robustly associated with the case-control status.
A limitation of the current study is that due to recruitment procedures we tend to have more early stage colorectal cancer cases (stage I-II) compared to advanced metastatic patients (stage IV). This may indicate that our findings are mostly associated with early metabolic changes in colorectal carcinogenesis rather than with metastatic formation. Furthermore, findings are derived from cross-sectional data. Therefore, it is not possible to explore to which extent metabolites are causally related to cancer or cancer-related changes. Lastly, although our study was performed using a single stringent metabolomics approach across two independent populations, we acknowledge that metabolomics assays can be conducted using a variety of analytical platforms. As such, future studies should include multiple platforms to ensure the highest analytical coverage of the metabolome. Technical progress and the development of more comprehensive metabolite databases will also be needed to improve annotation of unknown compounds, including the unknown metabolites in the current study. Future targeted approaches, allowing the quantitative measurement of metabolites, would allow quantification of their absolute concentrations.46, 47
In summary, this study provides new evidence of associations of colorectal cancer with plasma metabolites and also confirms some evidence of previous findings.
The combination of an untargeted metabolomics approach, a rigorous discovery-replication design utilizing large sample sizes from independent cohorts, led to the identification and replication of 28 metabolites associated with colorectal cancer, including 15 metabolites that could be identified. These 15 identifiable metabolites should be carried forward as candidates for targeted analysis in prospective cohort studies, preferably derived from a colorectal cancer screening program, to verify their discriminating or potential predicting properties. Our study provides important leads for further studies focusing on metabolic differences between colorectal cancer-free individuals, and patients with different stages of colorectal cancer. Together, our findings emphasize the power of metabolomics as a strong molecular approach for gaining novel insights regarding metabolic changes associated with colorectal cancer.
Supplementary Material
Novelty and Impact.
Colorectal cancer is characterized by distinct changes in key metabolic pathways. However, underlying mechanisms involved in colorectal carcinogenesis are still unclear. Therefore, a metabolomics discovery-replication approach was conducted to investigate circulating plasma metabolites in colorectal cancer within two European cohorts to verify robustness of the findings. This study reports 28 metabolites associated with colorectal cancer. These findings highlight the potential of metabolomic approaches to gain novel insights regarding metabolic changes associated with colorectal cancer.
Acknowledgments.
The authors would like to acknowledge the contribution of all ColoCare and CORSA Study participants. ColoCare ColoCare samples were stored by the liquid biobank of the National Center for Tumor Diseases according to the SOPs of the Biomaterialbank Heidelberg. Lin Zielske, Anett Brendel, Renate Skatula, Marita Wenzel supported biobanking at the National Center for Tumor Diseases. In addition, we would like to thank Rifraz Farook and Werner Diehl for their data management support and the ColoCare team, specifically Torsten Kölsch, Clare Abbenhardt-Martin, Susanne Jakob, and Judith Kammer for patient recruitment and follow-up. CORSA We kindly thank Michael Micksche (Institute of Cancer Research, Department of Medicine I, Medical University of Vienna), Karl Mach, Azita Deutinger-Permoon (KRAGES, Austria), and their co-workers for supporting CORSA recruitment. In addition, we would like to acknowledge Elisabeth Feik, Cornelia Zöchmeister, Ricarda Staudinger, Anna Doralt, and Marie Luise Brunner for patient recruitment, sample processing, and follow-up.
Funding. This study was supported by the Austrian Science Fund (FWF, Austria; project no. I1578-B19), the Federal Ministry of Education and Research (BMBF, Germany; project no. 01KT1512), the National Cancer Institute (INCa, France; project no. 2014–007), The Research Council of Norway (RCN, Norway; project no. 236564/H10), the Dutch Cancer Society (KWF Kankerbestrijding, the Netherlands; project no. UW2013–6397), and the Netherlands Organization for Health Research and Development (ZonMw, the Netherlands) coordinated by the ERA-NET, JTC 2012 call on Translational Cancer Research (TRANSCAN). In addition, researchers involved in this work were funded by the ERA-NET, TRANSCAN project 01KT1503, the National Cancer Institute project R01 CA189184, R01 CA207371, and U01 CA206110, the Huntsman Cancer Foundation, as well as the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 HG008962 from the National Human Genome Research Institute.
List of abbreviations
- BCAA
Branched chain amino acid
- BMI
Body mass index
- CI
Confidence interval
- CORSA
Colorectal Cancer Study of Austria
- ESI
Electrospray ionization
- FDR
False Discovery Rate
- FIT
Faecal Immunochemical Testing
- IARC
International Agency for Research on Cancer
- IQR
Interquartile range
- LysoPEs
Lysophosphatidylethanolamines
- LysoPCs
Lysophosphatidylcholines
- MNA
1-methylnicotinamide
- MS
mass spectrometry
- MSI
the Metabolomics Standards Initiative
- OR.std
Log standardized odds ratios
- (Q)TOF
(Quadrupole) time-of-flight
- SD
Standard deviation
- TCA
Tricarboxylic acid
- UHPLC
Ultra-high performance liquid chromatography
- WHO
World Health Organization
Footnotes
Ethical approval and consent to participate. Written informed consent was obtained from all participants. The ColoCare Study has been approved by the ethics committee of the Medical Faculty at the University of Heidelberg. The CORSA Study was approved by the ethical review committee of the Medical University of Vienna (1160/2016), by the “Ethikkommission der Stadt Wien” (06–150-VK) and by the institutional review board “Ethikkommission Burgenland”. The study was also approved by the International Agency for Research on Cancer ethics committee.
Competing Interests. Michael M. Bergman is founder of the biotechnology company Vacthera BioTech GmbH (Vienna, Austria) and received a consultant fee (<2000€) from Dr. Falk GmbH (Vienna, Austria). Cornelia M. Ulrich (Director of the Comprehensive Cancer Center at Huntsman Cancer Institute, Salt Lake City, USA) oversees research funded by several pharmaceutical companies but has not received funding directly herself.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136: E359–86. [DOI] [PubMed] [Google Scholar]
- 2.Wishart DS, Mandal R, Stanislaus A, Ramirez-Gaona M. Cancer Metabolomics and the Human Metabolome Database. Metabolites 2016;6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seyfried TN, Flores RE, Poff AM, D’Agostino DP. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis 2014;35: 515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams MD, Reeves R, Resar LS, Hill HH. Metabolomics of colorectal cancer: past and current analytical platforms. Anal Bioanal Chem 2013;405: 5013–30. [DOI] [PubMed] [Google Scholar]
- 5.Liesenfeld DB, Habermann N, Owen RW, Scalbert A, Ulrich CM. Review of mass spectrometry-based metabolomics in cancer research. Cancer Epidemiol Biomarkers Prev 2013;22: 2182–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang F, Zhang Y, Zhao W, Deng K, Wang Z, Yang C, Ma L, Openkova MS, Hou Y, Li K. Metabolomics for biomarker discovery in the diagnosis, prognosis, survival and recurrence of colorectal cancer: a systematic review. Oncotarget 2017;8: 35460–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armitage EG, Barbas C. Metabolomics in cancer biomarker discovery: current trends and future perspectives. J Pharm Biomed Anal 2014;87: 1–11. [DOI] [PubMed] [Google Scholar]
- 8.Xia J, Broadhurst DI, Wilson M, Wishart DS. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics 2013;9: 280–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang A, Sun H, Yan G, Wang P, Wang X. Metabolomics for Biomarker Discovery: Moving to the Clinic. BioMed Research International 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nambiar PR, Gupta RR, Misra V. An “Omics” based survey of human colon cancer. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2010;693: 3–18. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig C, Ward D, Martin A, Viant M, Ismail T, Johnson P, Wakelam M, Günther U. Fast targeted multidimensional NMR metabolomics of colorectal cancer. Magn Reson Chem 2009;47: S68–S73. [DOI] [PubMed] [Google Scholar]
- 12.Ma Y, Zhang P, Wang F, Liu W, Yang J, Qin H. An integrated proteomics and metabolomics approach for defining oncofetal biomarkers in the colorectal cancer. Ann Surg 2012;255: 720–30. [DOI] [PubMed] [Google Scholar]
- 13.Nishiumi S, Kobayashi T, Ikeda A, Yoshie T, Kibi M, Izumi Y, Okuno T, Hayashi N, Kawano S, Takenawa T. A novel serum metabolomics-based diagnostic approach for colorectal cancer. PLoS One 2012;7: e40459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farshidfar F, Weljie AM, Kopciuk KA, Hilsden R, McGregor SE, Buie WD, MacLean A, Vogel HJ, Bathe OF. A validated metabolomic signature for colorectal cancer: exploration of the clinical value of metabolomics. Br J Cancer 2016;115: 848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denkert C, Budczies J, Weichert W, Wohlgemuth G, Scholz M, Kind T, Niesporek S, Noske A, Buckendahl A, Dietel M, Fiehn O. Metabolite profiling of human colon carcinoma--deregulation of TCA cycle and amino acid turnover. Mol Cancer 2008;7: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritchie SA, Ahiahonu PW, Jayasinghe D, Heath D, Liu J, Lu Y, Jin W, Kavianpour A, Yamazaki Y, Khan AM, Hossain M, Su-Myat KK, et al. Reduced levels of hydroxylated, polyunsaturated ultra long-chain fatty acids in the serum of colorectal cancer patients: implications for early screening and detection. BMC Med 2010;8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan B, Qiu Y, Zou X, Chen T, Xie G, Cheng Y, Dong T, Zhao L, Feng B, Hu X. Metabonomics identifies serum metabolite markers of colorectal cancer. J Proteome Res 2013;12: 3000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowling P, Hughes DJ, Larkin AM, Meiller J, Henry M, Meleady P, Lynch V, Pardini B, Naccarati A, Levy M. Elevated levels of 14–3-3 proteins, serotonin, gamma enolase and pyruvate kinase identified in clinical samples from patients diagnosed with colorectal cancer. Clin Chim Acta 2015;441: 133–41. [DOI] [PubMed] [Google Scholar]
- 19.Qiu Y, Cai G, Zhou B, Li D, Zhao A, Xie G, Li H, Cai S, Xie D, Huang C. A distinct metabolic signature of human colorectal cancer with prognostic potential. Clin Cancer Res 2014: clincanres. 1939.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long Y, Sanchez-Espiridion B, Lin M, White L, Mishra L, Raju GS, Kopetz S, Eng C, Hildebrandt MAT, Chang DW, Ye Y, Liang D, et al. Global and targeted serum metabolic profiling of colorectal cancer progression. Cancer 2017;123: 4066–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Guida R, Engel J, Allwood JW, Weber RJM, Jones MR, Sommer U, Viant MR, Dunn WB. Non-targeted UHPLC-MS metabolomic data processing methods: a comparative investigation of normalisation, missing value imputation, transformation and scaling. Metabolomics 2016;12: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics 2006;7: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bratt O Hereditary prostate cancer: clinical aspects. The Journal of urology 2002;168: 906–13. [DOI] [PubMed] [Google Scholar]
- 24.Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res 2009;69: 4918–25. [DOI] [PubMed] [Google Scholar]
- 25.Levy M, Thaiss CA, Zeevi D, Dohnalova L, Zilberman-Schapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig Y. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 2015;163: 1428–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGarr SE, Ridlon JM, Hylemon PB. Diet, anaerobic bacterial metabolism, and colon cancer: a review of the literature. J Clin Gastroenterol 2005;39: 98–109. [PubMed] [Google Scholar]
- 27.Hibberd AA, Lyra A, Ouwehand AC, Rolny P, Lindegren H, Cedgard L, Wettergren Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenteroly 2017;4: e000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams MD, Zhang X, Park JJ, Siems WF, Gang DR, Resar LM, Reeves R, Hill HH, Jr. Characterizing metabolic changes in human colorectal cancer. Anal Bioanal Chem 2015;407: 4581–95. [DOI] [PubMed] [Google Scholar]
- 29.Perše M Oxidative Stress in the Pathogenesis of Colorectal Cancer: Cause or Consequence? BioMed Research International 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leufkens AM, van Duijnhoven FJ, Woudt SH, Siersema PD, Jenab M, Jansen EH, Pischon T, Tjonneland A, Olsen A, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, et al. Biomarkers of oxidative stress and risk of developing colorectal cancer: a cohort-nested case-control study in the European Prospective Investigation Into Cancer and Nutrition. American Journal of Epidemology 2012;175: 653–63. [DOI] [PubMed] [Google Scholar]
- 31.Harkness RA. Hypoxanthine, xanthine and uridine in body fluids, indicators of ATP depletion. Journal of Chromatography B: Biomedical Sciences and Applications 1988;429: 255–78. [DOI] [PubMed] [Google Scholar]
- 32.Delphan M, Lin T, Liesenfeld DB, Nattenmüller J, Böhm JT, Gigic B, Habermann N, Zielske L, Schrotz-King P, Schneider M. Associations of branched-chain amino acids with parameters of energy balance and survival in colorectal cancer patients: results from the ColoCare study. Metabolomics 2018;14: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Z, Xiao Y, Elson P, Tan H, Plummer SJ, Berk M, Aung PP, Lavery IC, Achkar JP, Li L. Plasma lysophosphatidylcholine levels: potential biomarkers for colorectal cancer. J Clin Oncol 2007;25: 2696–701. [DOI] [PubMed] [Google Scholar]
- 34.Kuhn T, Floegel A, Sookthai D, Johnson T, Rolle-Kampczyk U, Otto W, von Bergen M, Boeing H, Kaaks R. Higher plasma levels of lysophosphatidylcholine 18:0 are related to a lower risk of common cancers in a prospective metabolomics study. BMC Med 2016;14: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shu X, Xiang YB, Rothman N, Yu D, Li HL, Yang G, Cai H, Ma X, Lan Q, Gao YT. Prospective study of blood metabolites associated with colorectal cancer risk. Int J Cancer 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 2008;7: 11–20. [DOI] [PubMed] [Google Scholar]
- 37.Kuliszkiewicz-Janus M, Janus W, Baczyński S. Application of 31P NMR spectroscopy in clinical analysis of changes of serum phospholipids in leukemia, lymphoma and some other non-haematological cancers. Anticancer Res 1996;16: 1587–94. [PubMed] [Google Scholar]
- 38.Taylor LA, Arends J, Hodina AK, Unger C, Massing U. Plasma lyso-phosphatidylcholine concentration is decreased in cancer patients with weight loss and activated inflammatory status. Lipids Health Dis 2007;6: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui M, Wang Q, Chen GCBMCR. Serum metabolomics analysis reveals changes in signaling lipids in breast cancer patients. Biomed Chromatogr 2016;30: 42–7. [DOI] [PubMed] [Google Scholar]
- 40.Chlopicki S, Swies J, Mogielnicki A, Buczko W, Bartus M, Lomnicka M, Adamus J, Gebicki J. 1‐Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti‐thrombotic activity mediated by a cyclooxygenase‐2/prostacyclin pathway. Br J Pharmacol 2007;152: 230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricciotti E, FitzGerald GA. Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol 2011;31: 986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan Z, Khan N, P Tiwari R, K Sah N, Prasad G, S Bisen P. Biology of Cox-2: an application in cancer therapeutics. Curr Drug Targets 2011;12: 1082–93. [DOI] [PubMed] [Google Scholar]
- 43.Zucker SD, Horn PS, Sherman KE. Serum bilirubin levels in the US population: gender effect and inverse correlation with colorectal cancer. Hepatology 2004;40: 827–35. [DOI] [PubMed] [Google Scholar]
- 44.Jiraskova A, Novotny J, Novotny L, Vodicka P, Pardini B, Naccarati A, Schwertner HA, Hubacek JA, Puncocharova L, Smerhovsky Z, Vitek L. Association of serum bilirubin and promoter variations in HMOX1 and UGT1A1 genes with sporadic colorectal cancer. Int J Cancer 2012;131: 1549–55. [DOI] [PubMed] [Google Scholar]
- 45.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nature reviews Molecular cell biology 2016;17: 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scalbert A, Brennan L, Fiehn O, Hankemeier T, Kristal BS, van Ommen B, Pujos-Guillot E, Verheij E, Wishart D, Wopereis S. Mass-spectrometry-based metabolomics: limitations and recommendations for future progress with particular focus on nutrition research. Metabolomics 2009;5: 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowen BP, Northen TR. Dealing with the Unknown: Metabolomics and Metabolite Atlases. J Am Soc Mass Spectrom 2010;21: 1471–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


