Abstract
Background:
The ancient and highly evolutionarily conserved Wnt signaling pathway is critical in nearly all tissues and organs for an organism to develop normally from embryo through adult. Wnt signaling is generally parsed into “canonical” or Wnt-β-catenin-dependent or “non-canonical” β-catenin-independent signaling. Even though designating Wnt signaling as either canonical or non-canonical allows for easier conceptual discourse about this signaling pathway, in fact canonical and non-canonical Wnt crosstalk regulates complex nonlinear networks.
Objective:
In this perspective we discuss the integration of canonical and non-canonical Wnt signaling via differential Kat3 (CBP and p300) coactivator usage, thereby regulating and coordinating gene expression programs associated with both proliferation and cellular differentiation and morphogenesis.
Methods:
Pharmacologic inhibitors, cell culture, real-time PCR, chromatin immunoprecipitation, protein immunoprecipitation, Western blotting, reporter-luciferase, protein purification, site-directed mutagenesis, in vitro phosphorylation and binding assays, and immunofluorescence were utilized.
Conclusion:
Coordinated integration between both canonical and non-canonical Wnt pathways appears to be crucial not only in the control of fundamental morphologic processes but also in the regulation of normal as well as pathologic events. Such integration between both canonical and non-canonical Wnt signaling is presumably effected via reversible phosphorylation mechanism (e.g., protein kinase C) to regulate differential β-catenin/Kat3 coactivator usage in order to coordinate proliferation with differentiation and adhesion.
Keywords: Wnt, canonical, non-canonical, CBP, p300, Kat3 coactivator
1. INTRODUCTION
Wnt signaling is an incredibly complicated and critical controller of a myriad of processes in mammals [1]. Nineteen Wnt proteins, binding to two families of receptors, namely, lipoprotein receptor related proteins 5 and 6 (LRP-5/6) and Frizzled receptor proteins (10 different proteins), powerfully regulate cellular proliferation as well as cellular differentiation. Additional modulation of Wnt signaling occurs via ZNRF3 and RNF43 ubiquitylation of Frizzled receptors, leading to endocytosis, which is counter-regulated via ZNRF3 or RNF43 binding to LGR4, 5 or 6 and simultaneous binding to R-spondin [2]. However, a molecular understanding of the divergent outcomes of Wnt signaling is not currently available. “Canonical” Wnt signaling (or Wnt/β-catenin signaling) is associated with ß-catenin accumulation in the nucleus, forming a complex in the classical definition with members of the TCF/LEF family of transcription factors thereby regulating target gene transcription. Wnt/catenin signaling is associated with the ability of stem cells to proliferate without differentiation [3], proliferation of neuronal precursors [4] as well as neuronal differentiation and neural development [5]. There exists a significant body of literature on “non-canonical” Wnt signaling, that is, the subset of Wnt signaling which does not involve ß-catenin accumulation in the nucleus [6]. Non-canonical Wnt signaling has been linked broadly to cellular movement, intercalation, and focused migration resulting in convergence and extension (e.g., along the anterior/posterior axis of the organism) [7, 8]. A key initial observation about non-canonical signaling was its capacity to counter canonical Wnt/catenin signaling. “Non-canonical” Wnt5a overexpression inhibited induction of secondary axis stimulated by ectopically expressed “canonical” Wnt 8 in Xenopus embryos [9]. An array of potential nonexclusive mechanisms to explain these antagonistic effects has been proposed [6, 9–14]; however, an integrated mechanism which explains crosstalk/interaction between the canonical and non-canonical pathways still does not exist.
We originally identified and described that the small molecule antagonist ICG-001 modulates Wnt signaling and decreases the expression of a subset of Wnt/TCF/ß-catenin regulated genes [15–18]. ICG-001 selectively disrupts the ß-catenin/CBP interaction but not the corresponding ß-catenin/p300 interaction [15–18]. Utilizing this novel chemogenomic tool, as well as related compounds which block the ß-catenin/p300 interaction selectively in a variety of cell types, we developed a model [17–20] in which coactivator selection in the Wnt/ß-catenin transcription is the critical branch point in a cell’s decision to proliferate without differentiating i.e. divide symmetrically or to initiate a differentiative pathway i.e. divide asymmetrically [21].
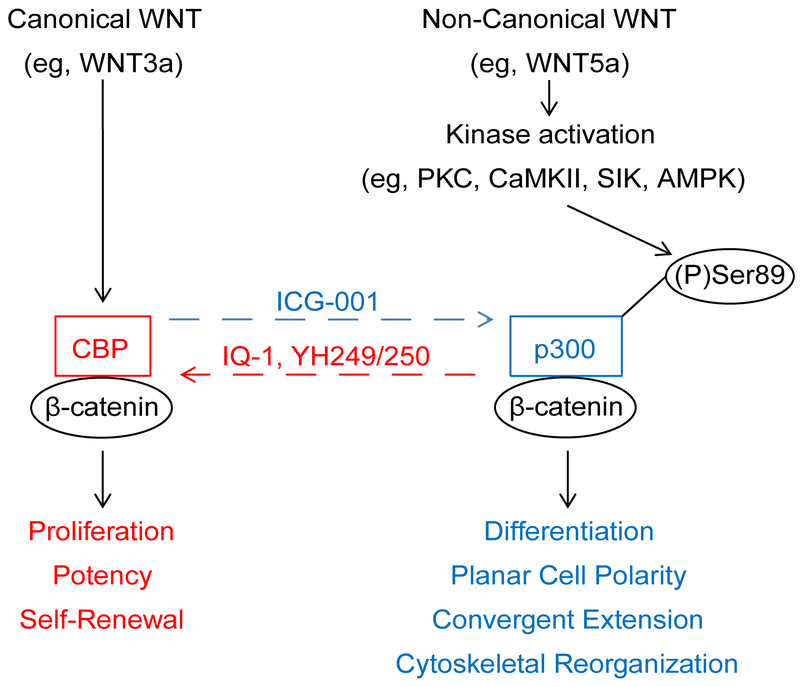
In this perspective, we further discuss and provide additional data on the integration of canonical and non-canonical Wnt signaling via differential Kat3( i.e. CBP and p300) coactivator usage, thereby regulating and coordinating gene expression programs consistent with proliferation and cellular differentiation and morphogenesis (Fig.1) [22].
Fig. 1.
Integration of canonical and non-canonical Wnt signaling via differential CBP and p300 usage. Non-canonical Wnt signaling (represented by WNT5a) “antagonizes” canonical Wnt signaling (represented by WNT3a) by inducing a change in β-catenin coactivator usage and a corresponding switch in gene expression from a proliferative towards a differentiative program. We have demonstrated that WNT5a induces PKC phosphorylation of Ser89 of p300, thereby increasing the affinity of p300 for β-catenin, and we propose this as a mechanism to explain the switch in coactivator usage from CBP/β-catenin to p300/β-catenin. Small molecule ICG-001 specifically blocks the CBP/β-catenin interaction, thus biasing towards p300 usage by β-catenin. In contrast, small molecules IQ-1 (indirect antagonist) and YH249/250 (direct antagonist) block the p300/β-catenin interaction, thus biasing towards CBP usage by β-catenin.
2. MATERIALS AND METHODS
Chemicals
ICG-001 [23] and IQ-1 [24] were synthesized according to previously reported procedures. Go6976, KN93, phorbol-12-myristate-13-acetate (PMA), N-myristoylated protein kinase C (PKC) pseudosubstrate peptide specific PKCα inhibitor (PKCα(20-28)), and Ro31-8425 are commercially available (EMD Millipore).
Cell Lines and Cell Culture
Human colorectal adenocarcinoma cell line SW480 was cultured in DMEM media supplemented with 10% fetal bovine serum, non-essential amino acids, 100 U/mL penicillin G, and 100 U/mL streptomycin. Wild type (WT), heterozygous deficient CBP (+/−), and heterozygous deficient p300 (+/−) mouse embryonic fibroblasts (MEF) cells were cultured in DMEM media supplemented with 10% fetal bovine serum, 4 mM L-glutamine, 100 U/mL penicillin G, and 100 U/mL streptomycin. 3T3 cell were derived from respective MEF cells after passage 24. Human melanoma cell line UACC (4-7) which overexpresses Wnt5a, and UACC (EV) which contains the respective empty vector, were cultured in RPMI-1640 media supplemented with 10% fetal bovine serum, 100 U/mL penicillin G, 100 U/mL streptomycin, and 400 ug/mL G418.
Cell Counting Assay
Cells were seeded into 12-well plates at 5 × 104 cells per well. Cells were harvested and counted 48 h and 96 h later using the trypan blue exclusion assay.
Real-time PCR Analysis
Cells were harvested for RNA extraction using Qiagen RNeasy Kit (Qiagen). 1 ug of RNA was then subjected to cDNA synthesis using TaqMan RT (Roche). Real-time PCR was performed using SYBR Green (Perkin Elmer). Real-time PCR analysis was performed using Bio-Rad iCycler (Bio-Rad). The primers used for mouse were: Fz-1: forward 5’-ACC CTG CGA ACC CAC CAA GGT TTA-3’, reverse 5’-CCC GTC CTC TGC AAA CTT GTC GTT-3’; Fz-2: forward 5’-TCC TGC CAA GCC TAG TCA CTC GTA-3’, reverse 5’-TCG GAG CGA GGG CTA GAG-3’; Fz-3: forward 5’-TAG CAA TGG AGC CCT TCC ACC-3’, reverse 5’-CTC CAT ATC TTC AGG CCA CGG-3’; Fz-4: forward 5’-GCT TGT GCT ATG TTG GGA ACC CAC-3’, reverse 5’-ACA GGT TGC AGG AAC CGT-3’; Fz-5: forward 5’-GTC TGT GCT GTG CTT CAT C-3’, reverse 5’-AGT GAC ACA CAC AGG TAG CA-3’; Fz-6: forward 5’-TGA AGG AGA GAA GCA ATG GAT C-3’, reverse 5’-TGA ACA GGC AGA GAT GTG GAG-3’; Fz-7: forward 5’-GGC CAT CGA GGC CAA CTC GCA-3’, reverse 5’-CGC AAT CGA TCC ACA CTA GAC-3’; Fz-8: forward 5’-CTA CTC GCA GTA CTT CCA CC-3’, reverse 5’-GCG GAT CAT GAG TTT TTC TA-3’; Fz-9: forward 5’-AAA TCT TCA TGT CTT GGT G-3’, reverse 5’-ATG TTC TAG AGG TGT GTG GG -3’; Fz-10: forward 5’-TGT CCG GTT GCT ACA CCA TGG GCT-3’, reverse 5’-GCC AGG AAC CAG GTG AGG-3’; c-myc: forward 5’-ACC AAC AGG AAC TAT GAC CTC-3’, reverse 5’-AAG GAC GTA GCG ACC GCA AC-3’; cyclin D1: forward 5’-CAC AAC GCA CTT TCT TTC CA-3’, reverse 5’-GAC CAG CCT CTT CCT CCA C-3’; survivin: forward 5’-GTT TGA GTC GTC TTG GCG GAG GTT GTG GTG ACG CCA TC-3’, reverse CTC AGG TCC AAG TTA TCT CAG CAA AGG CTC AGC A-3’; β-actin: forward 5’ -GGC TGT ATT CCC CTC CAT CG-3’, reverse 5’-CCA GTT GGT AAC AAT GCC ATG T-3’. Primers for human: axin 2: forward 5’-GTG CAA ACT TTC GCC AAC CG-3’, reverse 5’-GCT GGT GCA AAG ACA TAG CC-3’; c-myc: forward 5’-TGA AAG GCT CTC CTT GCA GC-3’, reverse 5’-GCT GGT AGA AGT TCT CCT CC-3’; c-jun: forward 5’-AAC CTC AGC AAC TTC AAC CC-3’, reverse 5’-CTT CCT TTT TCG GCA CTT GG-3’; Wnt5a: forward 5’-ATT CTT GGT GGT CGC TAG GTA-3’, reverse 5’-CGC CTT CTC CGA TGT ACT GC-3’; survivin: forward 5’-AGC CCT TTC AAG GAC CAC-3’, reverse 5’-GCA CTT TCT TCG CAG TTT CC-3’; hNKD (NKD1): forward 5’-GCA GCT GAA GTT TGA AGA GC-3’, reverse 5’-ATC TAC GCA ATG GTG GTA GC-3’; cyclin D1: forward 5’-ATG TGT GCA GAA GGA GGT CC-3’, reverse 5’-CTT AGA GGC CAC GAA CAT GC-3’; FRA-1 (FOSL1): forward 5’-GCA TCA ACA CCA TGA GTG GC-3’, reverse 5’-GAA GTC GGT CAG TTC CTT CC-3’; MITF: forward 5’-AAA CCC CAC CAA GTA CCA CA-3’, reverse 5’-ACA TGG CAA GCT CAG GAC-3’; β-actin: forward 5’-AGG AGC ACC CCG TGC TGC TGA-3’, reverse 5’-CTA GAA GCA TTT GCG GTG GAC-3’. Data were analyzed by the cycle threshold (Ct) comparative method, by which the 2−ΔΔCt value was calculated, where ΔCt=CtGene of Interest–Ctβ–actin, and ΔΔCt=ΔCtSample–ΔCtReference.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay in SW480 cells was performed as previously described [25]. Antibodies for ChIP: CBP (AC26, a generous gift from Prof. David Livingston, Harvard University [26]); p300 (C-20, Santa Cruz Biotechnology). PCR primers for c-myc promoter: forward primer: 5’-TGG TAG GCG CGC GTA GTT A-3’, reverse primer: 5’-GGG CGG AGA TTA GCG AGA G-3’. These ChIP PCR primers are for the region around the TCF binding element 2 (TBE2) of a MYC 5’ Wnt responsive DNA element (WRE).
Generation of Conditioned Media
Conditioned media for Wnt3a and Wnt5a were generated by culturing L-cells which were stably transfected with either Wnt3a or Wnt5a. The conditioned media were harvested and centrifuged to remove debris.
Transfection and Luciferase Assay
Cells were transfected with 0.5 ug of TOPFlash (β-catenin/TCF luciferase reporter) plasmid or pGL3-6270Luc (survivin promoter luciferase reporter) plasmid using Lipofectamine reagent as per manufacturer’s protocol (Invitrogen). After 3 h of incubation, the medium was replaced with fresh serum containing medium. Cells were treated as indicated and incubated for 24 h prior to assay. Luciferase activity was determined using the Promega assay system per manufacturer’s protocol (Promega).
Immunoprecipitation and Western Blotting
Cells were harvested and extracted for nuclear protein on ice using NE-PER kit (Pierce). Protease inhibitor cocktail (Roche) and 1 mM sodium ortho-vanadate were added to lysis buffers. Protein extracts were quantitated using Bio-Rad Protein Assay kit (Bio-Rad). 100 ug protein was diluted in CoIP buffer (20 mM Tris-HCl pH8.0, 75 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 0.5% NP-40, 10% glycerol) supplemented with 1 mM DTT and protease inhibitor cocktail before use. After preclearing of the samples using 50% slurry protein A agarose, 2 ug of respective antibody were added to samples and incubated overnight at 4°C. Then, 50 uL of 50% slurry protein A agarose was added to samples to pull down antibody complexes, after which the bead-antibody complexes were washed with CoIP buffer. Antibodies for immunoprecipitation unless otherwise indicated: CBP (A-22, Santa Cruz Biotechnology); p300 (C-20, Santa Cruz Biotechnology). The control antibody used is a normal rabbit IgG from Santa Cruz, catalog #: sc-2027.
For Western blot, samples were run out on SDS-PAGE Tris-glycine Novex gels and transferred onto PVDF. The membrane was probed with antibodies and then was visualized using the ECL system (Amersham). Antibodies for Western blotting unless otherwise indicated: CBP (A-22, Santa Cruz Biotechnology); p300 (C-20, Santa Cruz Biotechnology); phospho-PKCα/βII (Cell Signaling Technology); mouse monoclonal β-catenin (BD Biosciences); α-tubulin (Calbiochem). In addition, a phosphoserine 89-specific antiserum was produced from immunization of rabbits with a p300-derived oligopeptide containing phosphoserine 89 (CLS ELL RSG SpSP NLN) by Alpha Diagnostic International.
Protein Purification
His-tagged versions of the N-terminal region (amino acid 1-111) of CBP (C1), of the N-terminal region (amino acid 1-111) of p300 (P1), and of the N-terminal region (amino acid 1-111) of a Serine 89 to Alanine point mutant of p300 (mt P1(S89A)) were expressed in E. coli (BL21(DE3)pLysS competent cells) and purified by Ni-NTA agarose (Qiagen) chromatography.
Site-Directed Mutagenesis
In vitro site-directed mutagenesis of P1 to create point mutant P1 (mt P1(S89A)) was performed by using the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene) based upon a previously published protocol [27]. The forward and reverse mutagenic primers were: 5’-AGA ATT GCT GCG ATC TGG TGC TTC CCC TAA CCT CAA TAT GG-3’ and 5’-CCA TAT TGA GGT TAG GGG AAG CAC CAG ATC GCT GCA ATT CT-3’, respectively. PCR was carried out in a 50 μL mixture using 50 ng template plasmid DNA (pTriEX-4-P1), 125 ng of each primer, 10 nmol of dNTPs, 2.5 U of Pfu DNA polymerase (Stratagene) in Pfu polymerase reaction buffer. The thermal cycler was programmed as follows: initial denaturation at 95°C for 1 min; 18 cycles at 95°C for 50 s, 60°C for 50 s and 68°C for 5 min (P1); post-incubation at 68°C for 7 min. One microliter (20 U) of DpnI (Stratagene) was added to the sample (50 μL), and incubated at 37°C for 1 h. Two microliters of the final sample were used to transform E. coli (DH5α competent cells).
In Vitro Phosphorylation
Purified p300 (P1) and purified point mutant of p300 (mt P1(S89A)) were phosphorylated by recombinant PKCα. The phosphorylation reaction was carried out at 30°C for 20 min in kinase buffer (20 mM HEPES pH 7.4, 10 mM magnesium acetate, 1 mM dithiothreitol, 100 uM ATP) supplemented with 0.5 mM CaCl2, 100 ng/mL phorbol-12-myristate-13-acetate, and 100 ug/mL phosphatidylserine, based on a previously published protocol [28].
In Vitro Binding Assay
Cells were lysed with M-Per Mammalian Protein Extraction Reagent (Pierce) in the presence of protease inhibitor cocktail (Roche) for 20 min on ice and were cleared by centrifugation. Whole cell lysates were incubated with β-catenin monoclonal antibody pre-bound to Protein A agarose beads (Roche) for 4 h at 4°C and beads were washed several times in protein binding buffer (20 mM Tris HCl pH 8.0, 75 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 0.5% NP40, 10% glycerol, 1 mM dithiothreitol). The wild type P1 or point mutant P1 (mt P1(S89A)), in vitro phosphorylated or not phosphorylated, were incubated with β-catenin immobilized Protein A agarose beads for 4 hours at 4°C. After several washings with protein binding buffer, protein eluted from beads was subjected to Western blotting.
Immunofluorescence
E14.5 mouse cochlea cells were placed into culture based upon a previously published protocol [29]. After treatment for 6 days with ICG-001 and/or IQ-1 versus vehicle control, cells were fixed and stained for myosin 7A (FITC) or phalloidin (TRITC) (Sigma). Slide images were acquired by laser scanning confocal microscopy.
Data analysis
Numerical data were expressed as the means ±S.E.M. unless otherwise noted. Student’s t-test or One-way ANOVA with post-hoc Tukey test was performed as appropriate. p-values < 0.05 were considered significant.
3. RESULTS
3.1. Differential Regulation and Coactivator Utilization by TCF/ß-catenin Target Genes
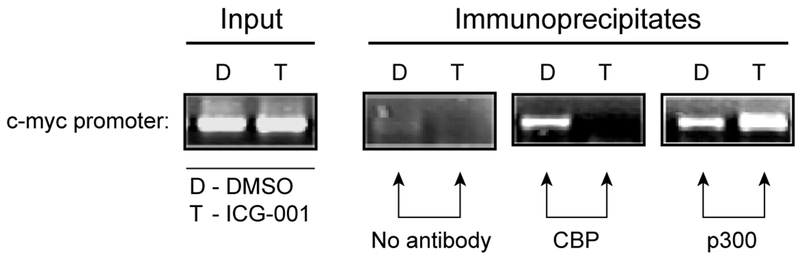
Cyclin D1 is expressed aberrantly in numerous types of tumors, and has been reported to be a Wnt/ß-catenin direct target [30, 31]. We previously demonstrated that ICG-001, by blocking the CBP/ß-catenin interaction down-regulated both cyclin D1 and survivin/BIRC5 mRNA [15, 16] in cancer cells. RT-PCR confirmed that ICG-001 reduced the mRNA levels for both axin 2 and human naked cuticle (hnkd) (data not shown), two additional genes previously identified as TCF/ß-catenin targets [32]. However, contrary to the reduction of cyclin D1, survivin, axin 2 and hnkd, ICG-001 in the same cells, increased mRNA expression of c-myc, fra and c-jun (data not shown), known to be TCF/ß-catenin regulated genes [33, 34]. The finding that ICG-001 inhibited the expression of a subset of TCF/ß-catenin genes (i.e. cyclin D1, survivin, axin 2, hnkd) yet increased the expression of other well-known TCF/ß-catenin signaling target genes (i.e. c-myc, jun, fra), combined with the inherent binding specificity of the small molecule ICG-001 for CBP but not p300, lead us to ask whether potential usage of p300 at the c-myc promoter allowed c-myc expression to avoid being repressed by ICG-001. To investigate Kat3 coactivator utilization at the c-myc promoter, we performed chromatin immunoprecipitation (ChIP) analysis in SW480 cells treated with either ICG-001 (25μM) or vehicle only (control DMSO). As depicted in Fig. 2, both coactivators CBP and p300 occupy the c-myc promoter in vehicle only treated cells. Treatment with ICG-001 entirely and specifically inhibited CBP usage at the c-myc promoter and simultaneously increased p300 usage at the c-myc promoter. Similarly to the c-myc promoter, ICG-001 treatment entirely and specifically blocked CBP usage at the cyclin D1 promoter [15]. However, p300 could not substitute for CBP in binding to the cyclin D1 promoter in these cells [15]. We concluded that ICG-001 selectively reduces the association of CBP but not p300 at TCF/ß-catenin-regulated gene promoters, yet can increase p300 coactivator occupancy which drives transcription at promoters that are permissive for or prefer p300 (e.g. c-myc, EphB2) [16, 35]. Interestingly, c-myc seems to play a critical role in modulating between differentiation and self-renewal in stem cells, and c-myc expression is up-regulated with differentiation [36, 37].
Fig. 2.
ICG-001 blocks the association of CBP with the c-myc promoter and concomitantly increases the level of p300 associated with the c-myc promoter. To evaluate coactivator usage at the endogenous c-myc promoter, chromatin immunoprecipitation (ChIP) analysis was performed on SW480 cells treated with either ICG-001 (25 μM) or control DMSO. The c-myc promoter is occupied by both coactivators CBP and p300 in control DMSO treated cells. However, treatment with ICG-001 completely and selectively blocks the association of CBP with the c-myc promoter and concomitantly increases the level of p300 associated with this promoter. D, DMSO (control) treatment. T, treatment with ICG-001.
3.2. Endogenous Coactivator Switch Controlling Mechanisms
Our data, which demonstrated that ICG-001 can pharmacologically induce a switch to p300 coactivator usage at selected TCF/ß-catenin driven promoters, prompted us to begin to investigate endogenous mechanisms for coactivator selection. In particular, our attention was drawn to the non-canonical Wnt pathway, as this pathway has previously been shown to counter-regulate many of the effects of the canonical pathway [6].
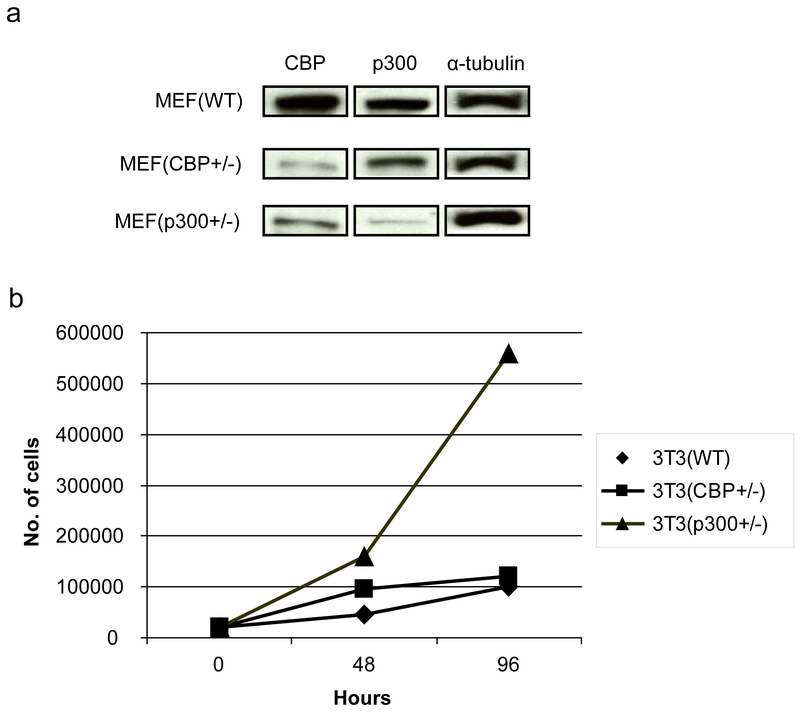
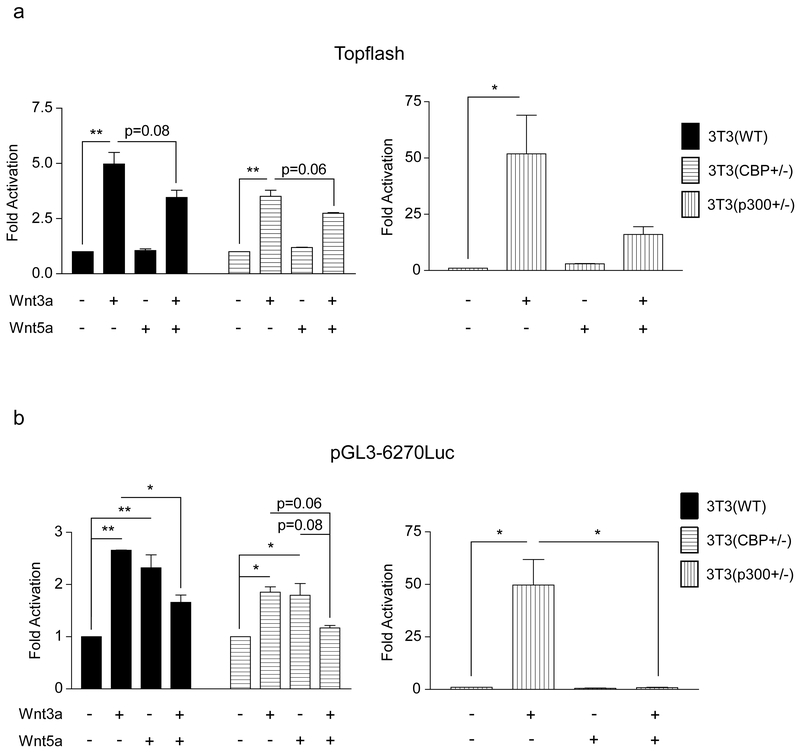
To initiate these investigations, we generated 3T3 cells from MEF cells that are heterozygous deficient for either CBP or p300 [38]. As shown in Fig. 3a CBP (+/−) cells express significantly less CBP than either wild type 3T3 (wt) or p300 heterozygous p300 (+/−). Conversely, the p300 (+/−) cells express considerably less p300 than the other two cell types. Interestingly, and consistent with the model depicted in Fig. 1 and the results of Rebel et al. [38] on CBP (−/−) and p300 (−/−) hematopoietic stem cells, the p300 (+/−) 3T3 cells had a significantly steeper growth curve than either the wt or CBP (+/−) 3T3 cells (Fig. 3b). Next, we characterized the expression pattern of Fz receptors in the 3 cells lines, as it has been demonstrated that differences in canonical versus non-canonical Wnt signaling are not totally intrinsic, and are regulated at least in part by differential Fz receptor usage [39]. With the exception of Fz2, Fz4 and Fz9, message for all of the other Fz receptors was relatively abundant in the wt 3T3 cells. The expression of the Fz receptors across the 3 cell lines was relatively uniform with the exception of increased Fz1 in the CBP (+/−) cells (data not shown). When stimulated with Wnt3a conditioned media, the 3T3 wt and CBP (+/−) cells exhibited a small but reproducible induction in TOPFlash reporter activity (Fig. 4a), however the p300 (+/−) cells had a significantly larger increase in activity (Fig. 4a). As anticipated, Wnt5a alone had almost no effect on basal TOPFlash reporter gene activity in all 3 cell lines (Fig. 4a). However, co-treatment of the cells with Wnt3a and Wnt5a provided significantly different responses in the 3 cell lines. In wt 3T3 cells, the co-administration of Wnt5a and Wnt3a resulted in an ~20% reduction in TOPFlash activity, a similar effect was noted in the CBP (+/−); however, p300 (+/−) cells showed a dramatic reduction in TOPFlash activity under the same treatment conditions. The TOPFlash reporter gene construct permits usage of either of the Kat3 coactivators (i.e., CBP or p300), whereas the survivin promoter luciferase construct pGL3-6270Luc activity is more CBP dependent [16, 20]. Wnt3a caused an ~2.5 fold increase in survivin luciferase activity in 3T3 wt cells, a reduced but still significant ~2 fold increase in 3T3 CBP (+/−) cells and a dramatic ~50 fold increase in the p300 (+/−) cells (Fig. 4b). Wnt5a conditioned media elicited a similar minimal response above control media in the wt and CBP (+/−) cells, yet had essentially no effect on the p300 (+/−) cells. Co-administration of Wnt3a and Wnt5a conditioned media led to modest reductions in survivin luciferase activity in the wt and CBP (+/−) cells compared to Wnt3a or 5a alone, however co-administration of Wnt3a and 5a dramatically reduced survivin luciferase activity in the p300 (+/−) cells compared to Wnt3a alone (Fig. 4b).
Fig. 3.
Endogenous CBP and p300 expression in and the growth of wild type, CBP (+/−), and p300 (+/−) cells. 3T3 cells were generated from MEF cells that are heterozygous deficient for either CBP or p300. (a) Heterozygous CBP (+/−) MEFs express significantly less CBP than either wild type (WT) or p300 heterozygous p300 (+/−) cells as demonstrated by Western blot. Conversely, p300 (+/−) cells express considerably less p300 than the other two cell types. (b) p300 (+/−) 3T3 cells had a significantly steeper growth curve than either the WT or CBP (+/−) cells.
Fig. 4.
p300 (+/−) versus wild type and CBP (+/−) 3T3 cells display heightened response to canonical Wnt3a and negligible response to non-canonical Wnt5a. (a) When stimulated with Wnt3a conditioned media, 3T3 wild type (WT) and CBP (+/−) cells exhibited a small activation of the TOPFlash reporter gene construct; however, p300 (+/−) cells had a significantly larger increase in TOPFlash activity. Wnt5a alone had almost no effect on basal TOPFlash activity in the 3 cell lines. In WT and CBP (+/−) 3T3 cells, co-administration of Wnt5a and Wnt3a versus Wnt3a alone resulted in an ~20% reduction in TOPFlash activity, whereas a dramatic reduction in TOPFlash activity was observed in the p300 (+/−) cells. n ≥ 2, *p < 0.05, **p < 0.01. (b) To gain additional perspective, our studies were extended to include the survivin promoter luciferase construct pGL3-6270Luc whose activity is more CBP-dependent than the TOPFlash construct. Wnt3a caused an ~2.5-fold increase in survivin luciferase activity in 3T3 WT cells, an ~2-fold increase in 3T3 CBP (+/−) cells, and a dramatic ~50-fold increase in the p300 (+/−) cells. Wnt5a elicited a similar minimal response above control media in the WT and CBP (+/−) cells, yet had essentially no effect on the p300 (+/−) cells. Co-administration of Wnt3a and Wnt5a conditioned media led to modest reductions in survivin luciferase activity in the WT and CBP (+/−) cells compared to Wnt3a or 5a alone, whereas co-administration of Wnt3a and 5a dramatically reduced survivin luciferase activity in the p300 (+/−) cells compared to Wnt3a alone. n ≥ 2, *p < 0.05, **p < 0.01.
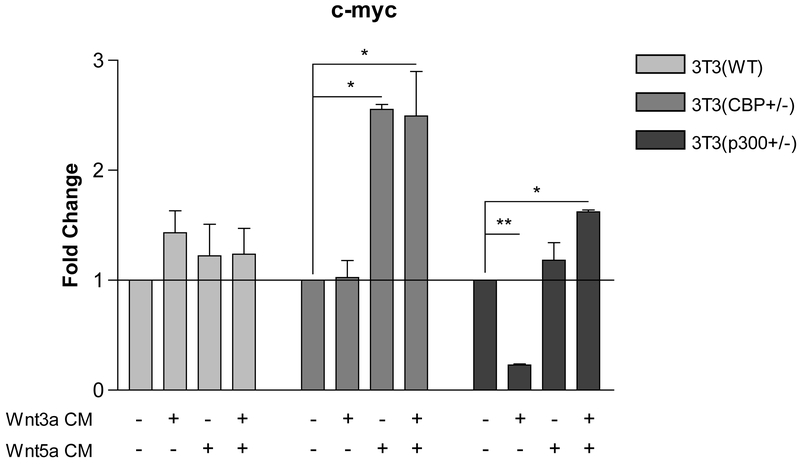
We next examined the same set of stimuli on the endogenous expression of c-myc in the same three cell lines. Based upon the ChIP assay data for the c-myc promoter, we anticipated that c-myc transcription could be effectively sustained utilizing either coactivator, or in fact might be increased when relying primarily on p300. As can be seen in Fig. 5, in wt. 3T3cells treatment with Wnt3a, Wnt5a and Wnt3a+Wnt5a led to a modest increase in c-myc message compared to control media. However, in CBP (+/−) cells in which there is a significantly increased ratio of p300 to CBP, treatment with Wnt5a or Wnt3a+Wnt5a led to increased c-myc expression, which was not observed with Wnt3a stimulation alone. Even more dramatically, Wnt3a reduced c-myc message in p300 (+/− cells) whereas Wnt5a and Wnt3a+Wnt5a treatment maintained c-myc expression at similar levels to media control treated cells. We concluded that TCF/β-catenin gene expression in the 3 cell lines depends significantly on Kat3 coactivator (i.e., CBP or p300) level and that differential coactivator usage after Wnt3a/5a treatment can critically and differentially regulate TCF/β-catenin-dependent gene transcription in a promoter specific manner [16].
Fig. 5.
Wnt3a suppresses c-myc expression in p300 (+/−) 3T3 cells, whereas Wnt5a induces c-myc expression in CBP (+/−) cells. In CBP (+/−) cells in which there is a significantly increased ratio of p300 to CBP, treatment with Wnt5a or Wnt3a+Wnt5a conditioned media (CM) led to increased c-myc mRNA expression, which was not observed with Wnt3a alone, as assessed by real-time PCR. In contrast, Wnt3a dramatically reduced c-myc message in p300 (+/−) cells, whereas Wnt5a and Wnt3a+Wnt5a treatment did not greatly change c-myc expression compared to control media-treated cells. n ≥ 2, *p < 0.05, **p < 0.01.
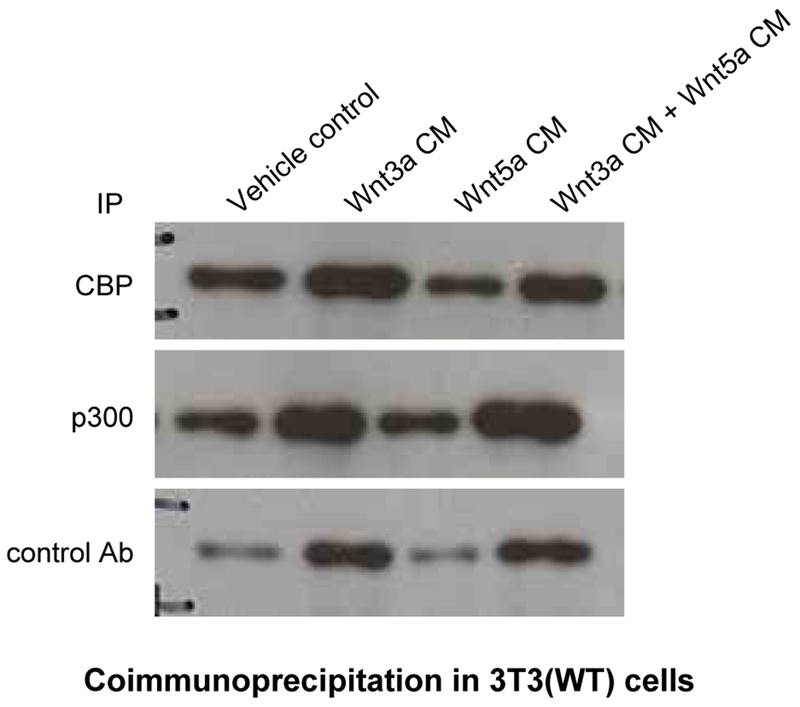
Next, we examined the interaction of nuclear β-catenin with the coactivators CBP or p300 after Wnt stimulation. 3T3 cells were treated either with Wnt3a, Wnt5a or both conditioned media (Fig. 6). Wnt3a treatment significantly increased the coimmunoprecipitation of β-catenin with both CBP and p300 compared to untreated cells (compare lanes 1 and 2). Wnt5a conditioned media alone, as anticipated, caused no increase in the amount of β-catenin associated with p300. However, a significant decrease in the amount of β-catenin associated with CBP compared to media control (compare lanes 1 and 3) was observed. Co-treatment with Wnt3a and Wnt5a conditioned media caused a decrease in the amount of β-catenin associated with CBP compared to treatment with Wnt3a alone (compare lanes 2 and 4). Yet a strikingly selective increase in p300 associated β-catenin was observed under these conditions. We concluded that simultaneous stimulation of both the “canonical” and “non-canonical” Wnt pathway leads to differential coactivator utilization by nuclear β-catenin leading to preferential interaction between β-catenin and p300.
Fig. 6.
Simultaneous stimulation of both canonical and non-canonical Wnt signaling leads to differential coactivator utilization by nuclear β-catenin leading to preferential interaction between β-catenin and p300. To examine the interaction of nuclear β-catenin with the coactivators CBP or p300 after Wnt stimulation, wild type (WT) 3T3 cells were treated with Wnt3a, Wnt5a, or Wnt3a+Wnt5a conditioned media (CM) versus control for 24 h, after which nuclear lysates were prepared and subjected to immunoprecipitation (IP) with CBP, p300, or control antibody, followed by immunoblot for β-catenin. Wnt3a-only conditioned media markedly increased the coimmunoprecipitation of β-catenin with both CBP and p300 compared to control treated cells (compare lanes 1 and 2). Whereas Wnt5a-only conditioned media caused no increase in the amount of β-catenin associated with p300, a marked decrease in the amount of β-catenin associated with CBP compared to control (compare lanes 1 and 3) was observed. Co-treatment with Wnt3a and Wnt5a conditioned media caused a decrease in the amount of β-catenin associated with CBP compared to treatment with Wnt3a alone, while a conspicuous increase in p300 associated β-catenin was observed under these conditions. Ab, antibody.
3.3. PKC Activation via the Non-canonical Pathway Affects TCF/β-catenin Coactivator Usage
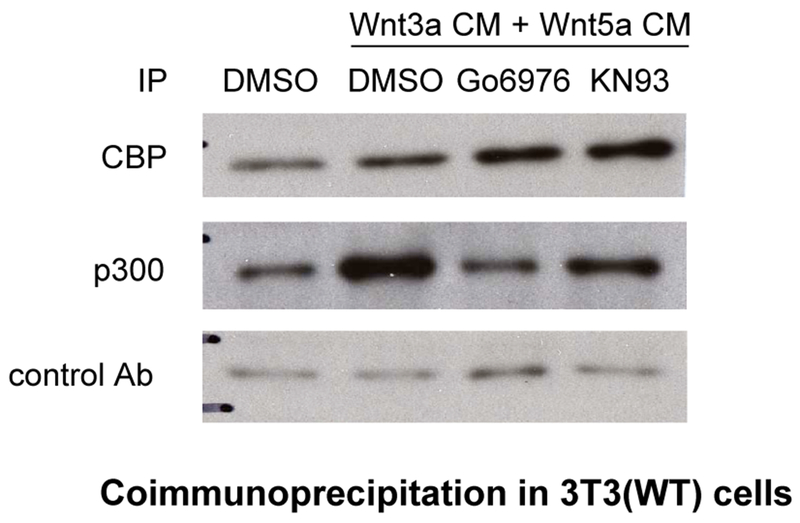
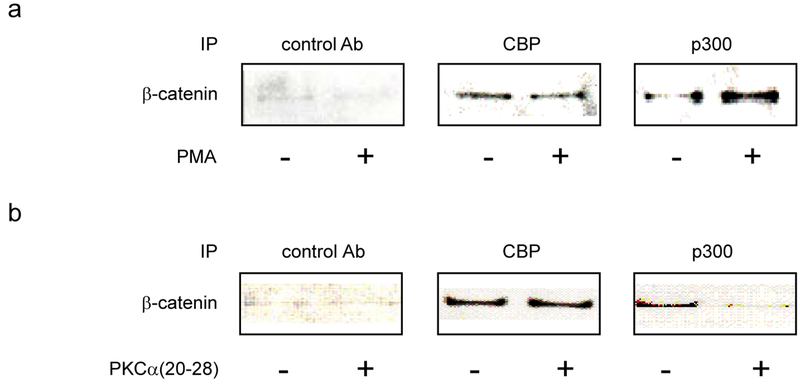
Overexpression of Wnt5a in Xenopus embryos activates calcium/calmodulin-dependent kinases (CaMKII) [40] and protein kinase C (PKC) [41]. PKC activity is also significantly enhanced in UACC1273 melanoma cells in response to stable transfection with Wnt5a [42]. A PKC phosphorylation site has been mapped to Ser-89 in the N-terminal region of CBP/p300 [43] to which the C-terminus of β-catenin binds [15]. We previously reported that phosphorylation at Ser-89 enhances the p300/β-catenin interaction in mES cells [19]. Additionally, simultaneous with differentiation of the alveolar epithelial cell [22], prototypical “non-canonical” Wnt5a induces phosphorylation at Ser-89 of p300 and thereby enhances the p300/β-catenin interaction in a manner dependent on PKC. This research identified a previously unknown mechanistic link between non-canonical and canonical Wnt pathways in adult progenitor cell differentiation [22]. To further examine the role of PKC activation on differential coactivator usage, we treated 3T3 cells with both Wnt3a and 5a conditioned media in the presence of the selective small molecule PKC inhibitor Go6976. As previously observed (Fig. 6), co-administration of Wnt3a and 5a led to a substantial increase in p300 coactivator associated nuclear β-catenin (Fig. 7, compare lanes 1 and 2) with a substantial preference for β-catenin binding to p300. Treatment with Go6976 (1 uM) dramatically and selectively decreased the amount of β-catenin associated with p300 (Fig. 7, compare lanes 2 and 3), while increasing the amount of β-catenin associated with CBP. Similar results were obtained utilizing the selective CaMKII inhibitor KN93 (Fig. 7 lanes 2 and 4). PMA is a well-known activator of PKC [44]. Treatment of 3T3 cells with PMA (1μM) led to a modest, yet highly coactivator selective increase in β-catenin associated with p300 (Fig. 8a). To confirm that PKC was being activated in the 3T3 cells, we treated the cells with either Wnt5a conditioned media or PMA (1μM) and immunoblotted for phosphorylated activated PKCα/βII [42]. Both treatments resulted in significant phosphorylation and activation of PKC α/βII compared to control treated cells (data not shown). Having confirmed that minimally, the PKC isotypes PKCα and βII were significantly activated in 3T3 cells stimulated with non-canonical Wnt5a and to overcome concerns about the non-specific effects of small molecule kinase inhibitors [45], we investigated the effects of an N-myristoylated PKC pseudosubstrate peptide specific PKCα inhibitor (PKCα(20-28)) [46] on β-catenin coactivator binding. 3T3 cells were treated with Wnt3a and 5a conditioned media in the presence or absence of 20μM N-myristoylated PKC (20-28). The peptide, similarly to the small molecule inhibitor Go6976, selectively inhibited the coimmunoprecipitation of β-catenin with p300 (Fig. 8b). We concluded that non-canonical Wnt activation of PKC in 3T3 cells specifically enhances the interaction of β-catenin with the coactivator p300.
Fig. 7.
Inhibition of PKC attenuates p300/β-catenin interaction while enhancing CBP/β-catenin interaction. To examine the role of PKC activation on differential coactivator usage, wild type 3T3 cells were treated with combined Wnt3a and 5a conditioned media (CM) in the presence or absence of selective small molecule PKC inhibitor Go6976 or CaMKII inhibitor KN93 for 24 h. Nuclear lysates were prepared and subjected to immunoprecipitation (IP) with CBP, p300, or control antibody, followed by immunoblot for β-catenin. Compared with DMSO-only control, co-administration of Wnt3a and 5a led to a substantial increase in p300-associated nuclear β-catenin (compare lanes 1 and 2). Treatment with Go6976 (1 uM) dramatically and selectively decreased the amount of β-catenin associated with p300 (compare lanes 2 and 3), while increasing the amount of β-catenin associated with CBP. Similar results were obtained utilizing the selective CaMKII inhibitor KN93 (5 uM) (compare lanes 2 and 4). Ab, antibody.
Fig. 8.
Activation of PKC enhances the interaction of β-catenin with p300. After respective treatments (as described below), nuclear lysates of wild type 3T3 cells were prepared and subjected to immunoprecipitation with CBP, p300, or control antibody, followed by immunoblot for β-catenin. (a) Treatment of 3T3 cells with specific activator of PKC PMA (100 μM) for 1 h led to a modest, yet highly coactivator selective increase in β-catenin associated with p300. (b) 3T3 cells were treated with Wnt3a and 5a conditioned media in the presence (20 μM) or absence of N-myristoylated PKC pseudosubstrate peptide specific PKCα inhibitor PKCα(20-28) for 24 h. PKCα(20-28) selectively inhibited the coimmunoprecipitation of β-catenin with p300.
3.4. Phosphorylation of p300 Ser89 Increases binding to β-catenin
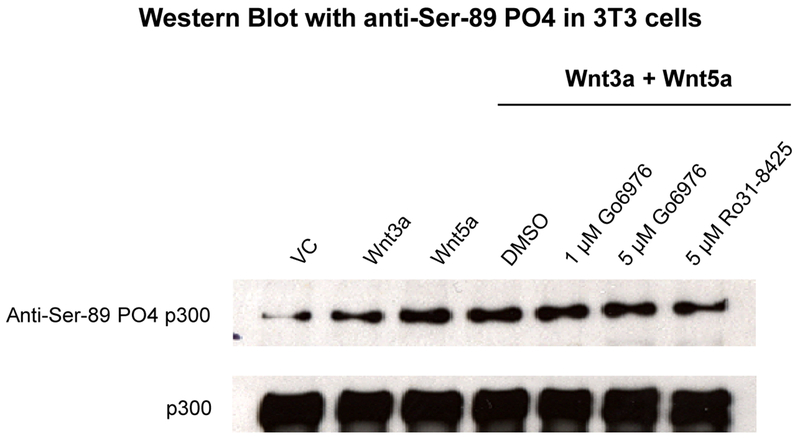
PKC has been previously reported to phosphorylate p300 Ser89 [19, 22, 43]. To determine if non-canonical Wnt activation of PKC was inducing PKC phosphorylation of p300 Ser89 and thereby increasing the β-catenin/p300 interaction, we performed the following series of experiments. 3T3 cells were treated as before with Wnt3a, Wnt5a and Wnt3a+Wnt5a in the presence or absence of PKC inhibitor. Nuclear lysates were prepared and immunoblotted for p300 and for phospho-Ser89 p300 [19, 43]. As can be clearly seen (Fig. 9), although the levels of total p300 remain essentially unchanged, the level of phospho-Ser89 p300 was increased upon treatment with Wnt5a conditioned media and the phosphorylation could be blocked utilizing a small molecule PKC inhibitor (either Go6976 or Ro31-8425).
Fig. 9.
Non-canonical Wnt activation of PKC induces PKC phosphorylation of p300. 3T3 cells were treated with Wnt3a, Wnt5a, or Wnt3a+Wnt5a in the presence or absence of PKC inhibitor for 24 h. Nuclear lysates were prepared and immunoblotted for total p300 and for phospho-Ser89 p300 (using anti-Ser-89 PO4 p300 antibody). Although the levels of total p300 remained essentially unchanged, the levels of phospho-Ser89 p300 were markedly increased upon treatment with conditioned media containing Wnt5a, and the phosphorylation could be blocked by small molecule PKC inhibitor (either Go6976 or Ro31-8425). VC, vehicle control. p300 antibody (N-15, Santa Cruz Biotechnology) used to assess total p300.
To further investigate the biochemical effects of PKC phosphorylation of p300 on binding to β-catenin, we expressed a His-tagged version of the N-terminal region (1-111aa) p300 in E. coli and purified it on a Ni-NTA column. Lysates from NIH3T3 cells were prepared and incubated with p300 (1-111). As can be clearly seen in Fig. 10, p300 (1-111) immunoprecipitated with β-catenin. Next, we in vitro phosphorylated the purified p300 (1-111) with PKCα. Phosphorylation of p300 with PKCα significantly enhanced the binding to β-catenin. To confirm that PKCα phosphorylation of Ser89 was the critical modification leading to enhanced binding, we constructed a point mutated p300 (1-111 S89A) (referred to as mt P1(S89A) in Fig. 10). The mutant p300 bound with somewhat less avidity to β-catenin, whereas the in vitro phosphorylated p300 mutant was significantly less effective in binding to β-catenin than the phosphorylated wt p300. We conclude that phosphorylation of Ser89 in p300 by PKC (as well as other S/T kinases [47], AMPK [48], SIK2 [49]) selectively increases the affinity of p300 for β-catenin. This post-translational modification of p300 S89 is involved in a diverse array of biologic effects including both activation and inhibition of transcription [48, 50], inhibition of the histone acetyl transferase activity [51], regulation of insulin/glucagon signaling [49], and differentiation of mES cells [19] and adult progenitor cells [22]. This led us to subsequently generate p300 S89A knockin mice. These mice, although born at a sub-Mendelian ratio, appear to be relatively normal at birth. However, they exhibit a number of metabolic and immunologic differences compared with their wild type counterparts in multiple organs systems. Details concerning these mice will be communicated separately in due course.
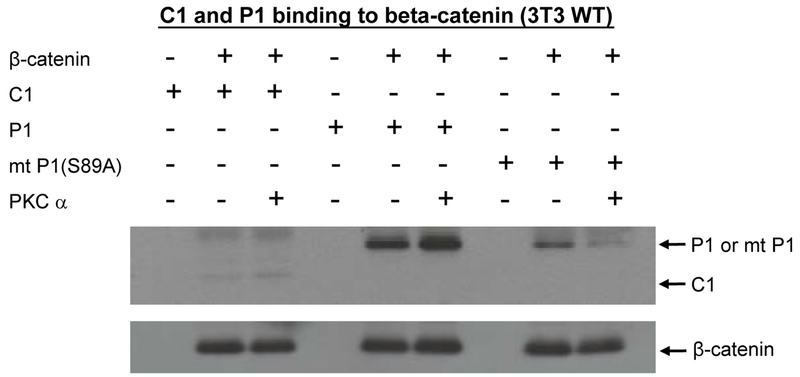
Fig. 10.
Phosphorylation of p300 Ser89 increases binding to β-catenin. To further investigate the biochemical effects of PKC phosphorylation of p300 on binding to β-catenin, His-tagged versions of the N-terminal regions (amino acid 1-111) of wild type p300 (P1), of a Ser89Ala point-mutated p300 (mt P1(S89A)), and of CBP (C1) were expressed in E. Coli and purified on a Ni-NTA column. Lysates from wild type (WT) 3T3 cells were prepared and incubated with P1, mt P1(S89A), or C1. Wild type p300 immunoprecipitated with β-catenin. In vitro phosphorylation of wild type p300 with PKCα markedly enhanced binding to β-catenin. Mutant p300 bound with somewhat less avidity to β-catenin, whereas the in vitro phosphorylated mutant p300 was dramatically less effective in binding to β-catenin than the phosphorylated wild type p300.
3.5. Wnt5a Overexpression in UACC 1273 Melanoma Cells Is Associated with Differential Wnt/β-catenin-Regulated Gene Expression
UACC 1273 melanoma cells normally have low Wnt5a expression and low in vitro invasive activity [52]. On the contrary, UACC 1273(4-7) cells, which stably express Wnt5a, exhibit significantly different cell morphology and are significantly more adherent and invasive [42]. Wnt5a overexpression in these cells does not affect β-catenin expression but significantly augments PKC activation [42].
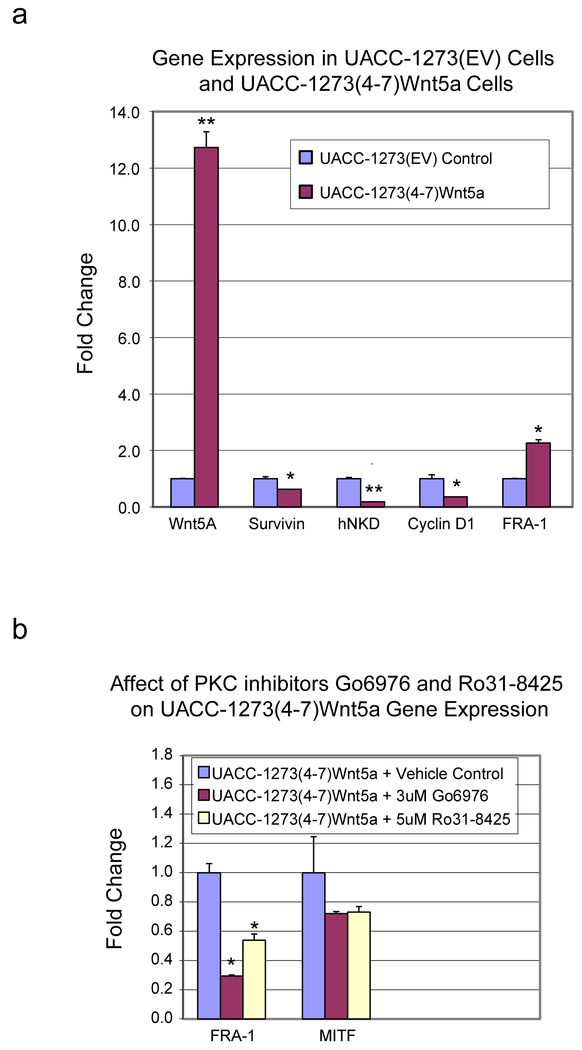
We selected the UACC 1273(4-7) to examine a series of genes that are known to be Wnt/β-catenin regulated and that we have previously used to examine coactivator selectivity. UACC 1273(EV) empty vector control or UACC 1273(4-7) cells were evaluated for the expression of survivin, cyclin D1, hnkd, and fra-1. We had previously found that survivin, cyclin D1 and hnkd to be TCF/β-catenin regulated genes that required the coactivator CBP for their expression. Therefore, we anticipated that in the UACC 1273(4-7), which have significantly elevated PKC activity compared with the UACC 1273(EV) cells, thereby increasing the p300/β-catenin interaction, that survivin, cyclin D1, and hnkd message levels would decrease. Conversely, we anticipated increased or at least similar level of fra-1 expression, which is also regulated in a TCF/β-catenin fashion, however can use either coactivator or prefers p300 at its promoter. In the UACC 1273(4-7) which demonstrated a ~13-fold increase in Wnt5a expression, message for survivin, cyclin D1, and hnkd all decreased significantly, and as anticipated, fra-1 message was increased (greater than 2 fold), (Fig. 11a). Treatment of the UACC 1273(4-7) cells with a PKC inhibitor demonstrated that PKC activation played a critical role in coactivator usage and the increased expression of fra-1 (Fig. 11b). Interestingly, MITF (microphthalmia associated transcription factor), which regulates melanocyte development, proliferation, and survival and is often aberrantly regulated in melanoma, was also decreased in UACC-1273 (4-7) cells after PKC inhibition (Fig. 11b).
Fig. 11.
Wnt5a overexpression in UACC 1273 melanoma cells is associated with differential Wnt/β-catenin-regulated gene expression. (a) UACC 1273(4-7) melanoma cells which stably express Wnt5a versus UACC 1273(EV) empty vector control cells were evaluated by real-time PCR for mRNA expression of Wnt5a, survivin, cyclin D1, hnkd, and fra-1. In UACC 1273(4-7) cells which demonstrated a ~13-fold increase in Wnt5a expression, mRNA levels for survivin, cyclin D1, and hnkd all decreased significantly while levels for fra-1 message increased significantly. n ≥ 2, *p < 0.05, **p < 0.01. (b) Treatment of UACC 1273(4-7) cells with PKC inhibitor (either 3 uM Go6976 or 5 uM Ro31-8425) demonstrated suppression of mRNA expression of fra-1 and MITF as assessed by real-time PCR. n ≥ 2, *p < 0.05.
3.6. Planar Cell Polarity (PCP) Is a p300 Dependent Event
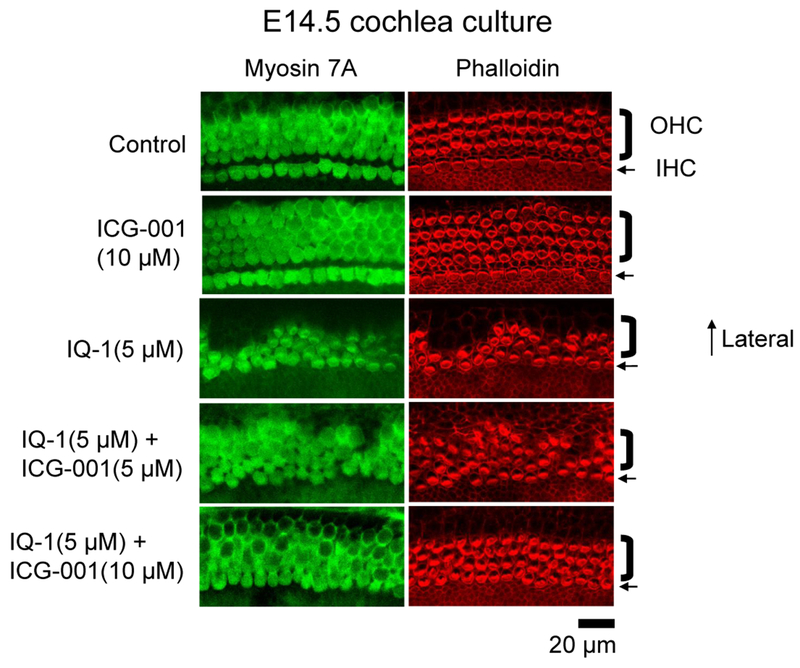
The generation of planar cell polarity (PCP) is fundamentally required for normal, vertebrate embryonic development. PCP refers to the structural alignment of cells in the same orientation within an epithelial plane. PCP is a key feature of the non-canonical Wnt pathway at the tissue or organism level [53]. A classic example of PCP exists in the mechanosensory hair cells of the organ of Corti in the mammalian cochlea. All cochlear stereociliary bundles are aligned in the direction of the cochlear duct’s outer border [54]. This orientation is critical to provide for normal levels of hearing sensitivity [55–59]. Non-canonical Wnt5a has been shown to play an important role in stereocilia orientation and cochlear extension [60]. Therefore, to investigate the effects of differential Kat3 coactivator usage on PCP, we chose to look at cochlear hair cell development during mouse embryonic development ex vivo. E14.5 mouse cochleae were placed into culture and treated for 6 days with vehicle control, the indirect p300/β-Catenin antagonist IQ-1 [19, 61, 62], or the direct CBP/β-Catenin antagonist ICG-001. As can be clearly seen (Fig. 12), whereas CBP/β-Catenin inhibition with ICG-001 did not have any deleterious effects on cochlear development and stereocilia orientation, inhibition of the p300/β-Catenin interaction by IQ-1 disrupted PCP and cochlear hair cell orientation. Importantly, in a dose dependent fashion, the CBP/β-Catenin antagonist ICG-001 reversed the deleterious effects of IQ-1 on PCP.
Fig. 12.
Non-canonical planar cell polarity is p300-dependent. To investigate the effects of differential p300 versus CBP coactivator usage on planar cell polarity (PCP), we examined cochlear hair cell development during mouse embryonic development ex vivo. E14.5 mouse cochleae were placed into culture and treated for 6 days, with indirect p300/β-catenin antagonist IQ-1 or direct CBP/β-catenin antagonist ICG-001 versus vehicle control. Whereas inhibition of CBP/β-catenin interaction did not have any deleterious effects on cochlear development and stereocilia orientation, inhibition of the p300/β-catenin interaction disrupted PCP and cochlear hair cell orientation. ICG-001, in a dose dependent fashion, reversed the deleterious effects of IQ-1 on PCP. OHC, outer hair cell; IHC, inner hair cell.
4. DISCUSSION
An extensive wealth of knowledge has been garnered concerning the canonical Wnt or Wnt/β-Catenin signaling pathway over the past 40 years [1, 63, 64]. More recently, more is becoming known about the non-canonical Wnt pathway, which signals independently of translocating β-catenin [6, 65]. A key initial observation about non-canonical signaling was that “non-canonical” Wnt5a overexpression inhibited induction of secondary axis stimulated by ectopically expressed “canonical” Wnt 8 in Xenopus embryos [9], thereby “antagonizing” the effects of canonical Wnt signaling. An array of potential nonexclusive mechanisms to explain these antagonistic effects has been proposed [6, 9–14]; however, an integrated mechanism which explains crosstalk/interaction between the canonical and non-canonical pathways still does not exist. We now provide a mechanism coupling the two pathways, whereby non-canonical Wnt signaling “antagonizes” canonical Wnt signaling by inducing a change in TCF/β-catenin Kat3 coactivator utilization which switches gene expression to a differentiative, migratory and adhesive program from a proliferative program [66].
A complex set of modifications, both transcriptional and non-transcriptional, governs a cell’s switch to a differentiative program from a proliferative, non-differentiative program [66]. Wnt signaling plays a central role in the regulation of such differentiative and non-differentiative programs in numerous types of cells throughout animal development [66]. Cells change their shape and start moving to specific coordinates within the developing embryo [66]. Orchestrating the myriad of cellular players in these events involves regulation of specific gene programs and coupling with reorganization of the cytoskeleton [66]. The non-canonical Wnt pathway obviously plays a crucial role in this process given that overexpression of either Wnt5 or Wnt11 interferes with this process [9] [66]. Indeed, coordinating non-canonical and canonical Wnt signaling is crucial in regulating cell fate determination and movement throughout various developmental stages [67] [66]. Rho, Rac and CDC42 (small GTPases) have previously been shown to directly link Wnt signaling to the cytoskeleton [68–71] [66]. Evidence on the antagonizing effects of several Wnts has existed for more than two decades [9] [66]. We now provide additional evidence that suggests that non-canonical Wnt antagonizes the canonical Wnt pathway and effects a corresponding shift in transcriptional programs due to differential Kat3 coactivator usage and a switch from β-catenin/CBP to β-catenin/p300. We have shown that one mechanism accounting for this alteration in β-catenin/coactivator usage depends on phosphorylation of p300 Ser89 by PKC whose activation is induced by non-canonical Wnt signaling [66]. Indeed, phosphorylation at Ser89 of p300 by PKC augments p300’s affinity for β-catenin both in vitro and in vivo [66]. Suppression of phosphorylation at Ser89 of p300 by inhibitors [66], site directed mutagenesis (Ser89Ala) [66], or point mutant knockin decreases p300’s affinity for β-catenin (T. Sasaki and M. Kahn unpublished results).
We have previously shown that the small molecule specific inhibitor of CBP/β-catenin interaction, ICG-001, interferes with expression of a subset of TCF/β-catenin targets (i.e. survivin, cyclin D1, axin2, hnkd) whereas it does not disrupt or up-regulates the expression of other TCF/β-catenin targets (i.e. c-myc, c-jun, fra-1) [15–18] [66]. We have also shown that such chemical induction of a switch to β-catenin/p300 from β-catenin/CBP is associated with an altered gene expression profile changing to the initiation of a differentiative program from proliferation without differentiation (i.e. symmetric division)) [15, 17–19, 21, 72] [66]. Such observations led us to explore endogenous mechanisms which influence this switch. Numerous cellular signaling processes rely on the reversibility of phosphorylation in proteins at serine, threonine and tyrosine residues [66]. Such reversibility is dependent on the coordinated activities of protein kinases and protein phosphatases, both existing as large families of proteins [66]. In addition to its role in the initiation of cytoskeletal reorganization, the non-canonical Wnt pathway activates several protein kinase families (i.e. PKC, CaMK, and MAPK) [11, 73, 74] [66]. Whereas the canonical Wnt/β-catenin pathway is associated with stem cell/progenitor cell proliferation (i.e. symmetric division) [3, 75–77], the non-canonical Wnt signaling pathway is associated with the differentiation of these cell types (i.e. asymmetric division) [78–80]. Additionally, Koyanagi et al. showed that non-canonical Wnt11- or PMA-mediated PKC activation enhances differentiation of cardiac progenitor cells [78] [66].
In addition to its roles in PCP and stem cell/progenitor cell differentiation, non-canonical Wnt also regulates convergent extension [53] [66], the process by which tissue converges (i.e., narrows) along an axis and extends (i.e., elongates) in a perpendicular axis [81]. Indeed, treatment of embryonic zebrafish with indirect p300/β-Catenin antagonist IQ-1 caused significant deleterious effects on convergent extension and overall development in the animals (J. Teo and M. Kahn unpublished results).
The switch from a β-catenin/CBP to a β-catenin/p300 driven expression cassette appears to be a fundamental mechanism in a cell’s decision to proliferate or initiate a differentiative process that dates back approximately 450 million years to the beginning of the vertebrate radiation [21]. We now demonstrate that in addition to the cytoskeletal rearrangements that are part of activation of non-canonical Wnt signaling, a change in Kat3 coactivator usage by β-catenin is part of the program that is initiated by non-canonical Wnt signaling and is at least in part controlled via differential phosphorylation of Ser89 of the coactivator p300 by PKC. Many potential phosphorylation sites are present in the amino terminal regions of p300 and CBP which interact with β-catenin [66]. Besides PKC, other protein kinases which are activated by non-canonical Wnt signaling have been shown to phosphorylate sites in this region of p300/CBP [47] [66]. Potentially, activation of a myriad of kinases through alternative signal transduction pathways (e.g., growth factor receptors, non-Frizzled G-protein coupled receptors, ion channels, etc.) may control differential coactivator utilization by β-catenin via differential phosphorylation of either the coactivators or β-catenin itself [66].
CONCLUSION
As opposed to pharmacological treatment with ICG-001, which essentially induces an irreversible switch from β-catenin/CBP to β-catenin/p300 [82, 83], protein phosphorylation is reversible and regulated by protein phosphatases [84]. In this regard, we previously demonstrated that the Ser/Thr phosphatase PP2A plays a crucial role in controlling Wnt/β-catenin differential coactivator utilization in stem cells [66], likely via dephosphorylation of p300S90, which inhibits the phosphorylation of p300S89 [19, 22]. Coordinated integration between both canonical and non-canonical Wnt pathways appears to be crucial not only in the control of fundamental morphologic processes (e.g., convergent extension and PCP) but also in the regulation of normal (e.g., wound healing) as well as pathologic (e.g., metastatic spread of cancer) events. Such integration between both canonical and non-canonical Wnt signaling is presumably effected via the aforementioned reversible phosphorylation mechanism (e.g., PKC) to regulate differential β-catenin/Kat3 coactivator usage in order to coordinate proliferation with differentiation and adhesion. Further investigations to explore differential Kat3 coactivator utilization in the context of these processes are in progress and will be reported in due course.
ACKNOWLEDGEMENTS
Grant support: K.K.Y. Lai has been supported by NIH K08AA025112. M. Kahn has been supported by NIH P30CA014089, R01CA166161, R21NS074392, R21AI105057, and R01HL112638. George Wang, of the City of Hope Internship Program, is thanked for providing comments on the manuscript.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Nelson WJ; Nusse R Convergence of Wnt, beta-catenin, and cadherin pathways. Science, 2004, 303, 1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].de Lau W; Peng WC; Gros P; Clevers H The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev, 2014, 28, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Reya T; Duncan AW; Ailles L; Domen J; Scherer DC; Willert K; Hintz L; Nusse R; Weissman IL A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature, 2003, 423, 409–414. [DOI] [PubMed] [Google Scholar]
- [4].Chenn A; Walsh CA Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science, 2002, 297, 365–369. [DOI] [PubMed] [Google Scholar]
- [5].Hirabayashi Y; Itoh Y; Tabata H; Nakajima K; Akiyama T; Masuyama N; Gotoh Y The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development, 2004, 131, 2791–2801. [DOI] [PubMed] [Google Scholar]
- [6].Veeman MT; Axelrod JD; Moon RT A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell, 2003, 5, 367–377. [DOI] [PubMed] [Google Scholar]
- [7].Keller R Shaping the vertebrate body plan by polarized embryonic cell movements. Science, 2002, 298, 1950–1954. [DOI] [PubMed] [Google Scholar]
- [8].Mlodzik M Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet, 2002, 18, 564–571. [DOI] [PubMed] [Google Scholar]
- [9].Torres MA; Yang-Snyder JA; Purcell SM; DeMarais AA; McGrew LL; Moon RT Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J. Cell Biol, 1996, 133, 1123–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ishitani T; Ninomiya-Tsuji J; Nagai S; Nishita M; Meneghini M; Barker N; Waterman M; Bowerman B; Clevers H; Shibuya H; Matsumoto K The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature, 1999, 399, 798–802. [DOI] [PubMed] [Google Scholar]
- [11].Ishitani T; Kishida S; Hyodo-Miura J; Ueno N; Yasuda J; Waterman M; Shibuya H; Moon RT; Ninomiya-Tsuji J; Matsumoto K The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol. Cell. Biol, 2003, 23, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Park M; Moon RT The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat. Cell Biol, 2002, 4, 20–25. [DOI] [PubMed] [Google Scholar]
- [13].Topol L; Jiang X; Choi H; Garrett-Beal L; Carolan PJ; Yang Y Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J. Cell Biol, 2003, 162, 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Westfall TA; Brimeyer R; Twedt J; Gladon J; Olberding A; Furutani-Seiki M; Slusarski DC Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin activity. J. Cell Biol, 2003, 162, 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Emami KH; Nguyen C; Ma H; Kim DH; Jeong KW; Eguchi M; Moon RT; Teo JL; Oh SW; Kim HY; Moon SH; Ha JR; Kahn M A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc. Natl. Acad. Sci. U S A, 2004, 101, 12682–12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ma H; Nguyen C; Lee KS; Kahn M Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene, 2005, 24, 3619–3631. [DOI] [PubMed] [Google Scholar]
- [17].Teo JL; Ma H; Nguyen C; Lam C; Kahn M Specific inhibition of CBP/beta-catenin interaction rescues defects in neuronal differentiation caused by a presenilin-1 mutation. Proc. Natl. Acad. Sci. U S A, 2005, 102, 12171–12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McMillan M; Kahn M Investigating Wnt signaling: a chemogenomic safari. Drug Discov. Today, 2005, 10, 1467–1474. [DOI] [PubMed] [Google Scholar]
- [19].Miyabayashi T; Teo JL; Yamamoto M; McMillan M; Nguyen C; Kahn M Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc. Natl. Acad. Sci. U S A, 2007, 104, 5668–5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Higuchi Y; Nguyen C; Yasuda SY; McMillan M; Hasegawa K; Kahn M Specific Direct Small Molecule p300/β-Catenin Antagonists Maintain Stem Cell Potency. Curr. Mol. Pharmacol, 2016, 9, 272–279. [DOI] [PubMed] [Google Scholar]
- [21].Thomas PD; Kahn M Kat3 coactivators in somatic stem cells and cancer stem cells: biological roles, evolution, and pharmacologic manipulation. Cell Biol. Toxicol, 2016, 32, 61–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rieger ME; Zhou B; Solomon N; Sunohara M; Li C; Nguyen C; Liu Y; Pan JH; Minoo P; Crandall ED; Brody SL; Kahn M; Borok Z p300/β-Catenin Interactions Regulate Adult Progenitor Cell Differentiation Downstream of WNT5a/Protein Kinase C (PKC). J. Biol. Chem, 2016, 291, 6569–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Eguchi M; Nguyen C; Lee SC; Kahn M ICG-001, a novel small molecule regulator of TCF/beta-catenin transcription. Med. Chem, 2005, 1, 467–472. [DOI] [PubMed] [Google Scholar]
- [24].Miyabayashi T; Yamamoto M Cell differentiation inhibiting agent, cell culture method using the same, culture medium, and cultured cell line. U.S. Patent 10, 875, 194, July 25, 2005.
- [25].Spencer VA; Sun JM; Li L; Davie JR Chromatin immunoprecipitation: a tool for studying histone acetylation and transcription factor binding. Methods, 2003, 31, 67–75. [DOI] [PubMed] [Google Scholar]
- [26].Eckner R; Ludlow JW; Lill NL; Oldread E; Arany Z; Modjtahedi N; DeCaprio JA; Livingston DM; Morgan JA Association of p300 and CBP with simian virus 40 large T antigen. Mol. Cell. Biol, 1996, 16, 3454–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Weiner MP; Costa GL; Schoettlin W; Cline J; Mathur E; Bauer JC Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene, 1994, 151, 119–123. [DOI] [PubMed] [Google Scholar]
- [28].Johnson JE; Edwards AS; Newton AC A putative phosphatidylserine binding motif is not involved in the lipid regulation of protein kinase C. J. Biol. Chem, 1997, 272, 30787–30792. [DOI] [PubMed] [Google Scholar]
- [29].Basch ML; Ohyama T; Segil N; Groves AK Canonical Notch signaling is not necessary for prosensory induction in the mouse cochlea: insights from a conditional mutant of RBPjkappa. J. Neurosci, 2011, 31, 8046–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shtutman M; Zhurinsky J; Simcha I; Albanese C; D’Amico M; Pestell R; Ben-Ze’ev A The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. U S A, 1999, 96, 5522–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tetsu O; McCormick F Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature, 1999, 398, 422–426. [DOI] [PubMed] [Google Scholar]
- [32].Yan D; Wiesmann M; Rohan M; Chan V; Jefferson AB; Guo L; Sakamoto D; Caothien RH; Fuller JH; Reinhard C; Garcia PD; Randazzo FM; Escobedo J; Fantl WJ; Williams LT Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc. Natl. Acad. Sci. U S A, 2001, 98, 14973–14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].He TC; Sparks AB; Rago C; Hermeking H; Zawel L; da Costa LT; Morin PJ; Vogelstein B; Kinzler KW Identification of c-MYC as a target of the APC pathway. Science, 1998, 281, 1509–1512. [DOI] [PubMed] [Google Scholar]
- [34].Mann B; Gelos M; Siedow A; Hanski ML; Gratchev A; Ilyas M; Bodmer WF; Moyer MP; Riecken EO; Buhr HJ; Hanski C Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc. Natl. Acad. Sci. U S A, 1999, 96, 1603–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kumar SR; Scehnet JS; Ley EJ; Singh J; Krasnoperov V; Liu R; Manchanda PK; Ladner RD; Hawes D; Weaver FA; Beart RW; Singh G; Nguyen C; Kahn M; Gill PS Preferential induction of EphB4 over EphB2 and its implication in colorectal cancer progression. Cancer Res, 2009, 69, 3736–3745. [DOI] [PubMed] [Google Scholar]
- [36].Wilson A; Murphy MJ; Oskarsson T; Kaloulis K; Bettess MD; Oser GM; Pasche AC; Knabenhans C; Macdonald HR; Trumpp A c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev, 2004, 18, 2747–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Quinn LM; Secombe J; Hime GR, Myc in stem cell behaviour: insights from Drosophila. Adv. Exp. Med. Biol, 2013, 786, 269–285. [DOI] [PubMed] [Google Scholar]
- [38].Rebel VI; Kung AL; Tanner EA; Yang H; Bronson RT; Livingston DM Distinct roles for CREB-binding protein and p300 in hematopoietic stem cell self-renewal. Proc. Natl. Acad. Sci. U S A, 2002, 99, 14789–14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Holmen SL; Salic A; Zylstra CR; Kirschner MW; Williams BO A novel set of Wnt-Frizzled fusion proteins identifies receptor components that activate beta -catenin-dependent signaling. J. Biol. Chem, 2002, 277, 34727–34735. [DOI] [PubMed] [Google Scholar]
- [40].Kühl M; Sheldahl LC; Malbon CC; Moon RT Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J. Biol. Chem, 2000, 275, 12701–12711. [DOI] [PubMed] [Google Scholar]
- [41].Sheldahl LC; Park M; Malbon CC; Moon RT Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr. Biol, 1999, 9, 695–698. [DOI] [PubMed] [Google Scholar]
- [42].Weeraratna AT; Jiang Y; Hostetter G; Rosenblatt K; Duray P; Bittner M; Trent JM Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell, 2002, 1, 279–288. [DOI] [PubMed] [Google Scholar]
- [43].Yuan LW; Gambee JE Phosphorylation of p300 at serine 89 by protein kinase C. J. Biol. Chem, 2000, 275, 40946–40951. [DOI] [PubMed] [Google Scholar]
- [44].Szallasi Z; Smith CB; Pettit GR; Blumberg PM Differential regulation of protein kinase C isozymes by bryostatin 1 and phorbol 12-myristate 13-acetate in NIH 3T3 fibroblasts. J. Biol. Chem, 1994, 269, 2118–2124. [PubMed] [Google Scholar]
- [45].Davies SP; Reddy H; Caivano M; Cohen P Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J, 2000, 351, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gupta KP; Ward NE; Gravitt KR; Bergman PJ; O’Brian CA Partial reversal of multidrug resistance in human breast cancer cells by an N-myristoylated protein kinase C-alpha pseudosubstrate peptide. J. Biol. Chem, 1996, 271, 2102–2111. [DOI] [PubMed] [Google Scholar]
- [47].Corcoran EE; Joseph JD; MacDonald JA; Kane CD; Haystead TA; Means AR Proteomic analysis of calcium/calmodulin-dependent protein kinase I and IV in vitro substrates reveals distinct catalytic preferences. J. Biol. Chem, 2003, 278, 10516–10522. [DOI] [PubMed] [Google Scholar]
- [48].Yang W; Hong YH; Shen XQ; Frankowski C; Camp HS; Leff T Regulation of transcription by AMP-activated protein kinase: phosphorylation of p300 blocks its interaction with nuclear receptors. J. Biol. Chem, 2001, 276, 38341–38344. [DOI] [PubMed] [Google Scholar]
- [49].Liu Y; Dentin R; Chen D; Hedrick S; Ravnskjaer K; Schenk S; Milne J; Meyers DJ; Cole P; Yates J; Olefsky J; Guarente L; Montminy M A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature, 2008, 456, 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gusterson RJ; Yuan LW; Latchman DS Distinct serine residues in CBP and p300 are necessary for their activation by phenylephrine. Int. J. Biochem. Cell Biol, 2004, 36, 893–899. [DOI] [PubMed] [Google Scholar]
- [51].Yuan LW; Soh JW; Weinstein IB Inhibition of histone acetyltransferase function of p300 by PKCdelta. Biochim. Biophys. Acta, 2002, 1592, 205–211. [DOI] [PubMed] [Google Scholar]
- [52].Bittner M; Meltzer P; Chen Y; Jiang Y; Seftor E; Hendrix M; Radmacher M; Simon R; Yakhini Z; Ben-Dor A; Sampas N; Dougherty E; Wang E; Marincola F; Gooden C; Lueders J; Glatfelter A; Pollock P; Carpten J; Gillanders E; Leja D; Dietrich K; Beaudry C; Berens M; Alberts D; Sondak V Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature, 2000, 406, 536–540. [DOI] [PubMed] [Google Scholar]
- [53].Yan D; Wallingford JB; Sun TQ; Nelson AM; Sakanaka C; Reinhard C; Harland RM; Fantl WJ; Williams LT Cell autonomous regulation of multiple Dishevelled-dependent pathways by mammalian Nkd. Proc. Natl. Acad. Sci. U S A, 2001, 98, 3802–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dabdoub A; Kelley MW Planar cell polarity and a potential role for a Wnt morphogen gradient in stereociliary bundle orientation in the mammalian inner ear. J. Neurobiol, 2005, 64, 446–457. [DOI] [PubMed] [Google Scholar]
- [55].Fujita H; Orita Y An inner ear anomaly in golden hamsters. Am. J. Otolaryngol, 1988, 9, 224–231. [DOI] [PubMed] [Google Scholar]
- [56].Comis SD; Pickles JO; Osborne MP; Pepper CB Tip-link organization in anomalously-oriented hair cells of the guinea pig cochlea. Hear. Res, 1989, 40, 205–211. [DOI] [PubMed] [Google Scholar]
- [57].Fujita H Mutant golden hamsters with an abnormal outer hair cell stereociliary arrangement. Hear. Res, 1990, 44, 63–69. [DOI] [PubMed] [Google Scholar]
- [58].Furness DN; Hackney CM; Hynd AN Rotated stereociliary bundles and their relationship with the tectorial membrane in the guinea pig cochlea. Acta Otolaryngol, 1990, 109, 66–75. [DOI] [PubMed] [Google Scholar]
- [59].Yoshida N; Liberman MC Stereociliary anomaly in the guinea pig: effects of hair bundle rotation on cochlear sensitivity. Hear. Res, 1999, 131, 29–38. [DOI] [PubMed] [Google Scholar]
- [60].Qian D; Jones C; Rzadzinska A; Mark S; Zhang X; Steel KP; Dai X; Chen P Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol, 2007, 306, 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sasaki T; Hwang H; Nguyen C; Kloner RA; Kahn M The small molecule Wnt signaling modulator ICG-001 improves contractile function in chronically infarcted rat myocardium. PLoS One, 2013, 8, e75010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sasaki T; Kahn M Inhibition of β-catenin/p300 interaction proximalizes mouse embryonic lung epithelium. Transl. Respir. Med, 2014, 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Huelsken J; Behrens J The Wnt signalling pathway. J. Cell Sci, 2002, 115, 3977–3978. [DOI] [PubMed] [Google Scholar]
- [64].Steinhart Z; Angers S Wnt signaling in development and tissue homeostasis. Development, 2018, 145, (11). [DOI] [PubMed] [Google Scholar]
- [65].May-Simera HL; Kelley MW Cilia, Wnt signaling, and the cytoskeleton. Cilia, 2012, 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Teo JL; Kahn M The Wnt signaling pathway in cellular proliferation and differentiation: A tale of two coactivators. Adv. Drug Deliv. Rev, 2010, 62, 1149–1155. [DOI] [PubMed] [Google Scholar]
- [67].Cavodeassi F; Carreira-Barbosa F; Young RM; Concha ML; Allende ML; Houart C; Tada M; Wilson SW Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/beta-catenin pathway. Neuron, 2005, 47, 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Winter CG; Wang B; Ballew A; Royou A; Karess R; Axelrod JD; Luo L Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell, 2001, 105, 81–91. [DOI] [PubMed] [Google Scholar]
- [69].Habas R; Kato Y; He X Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell, 2001, 107, 843–854. [DOI] [PubMed] [Google Scholar]
- [70].Habas R; Dawid IB; He X Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev, 2003, 17, 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Penzo-Mendèz A; Umbhauer M; Djiane A; Boucaut JC; Riou JF Activation of Gbetagamma signaling downstream of Wnt-11/Xfz7 regulates Cdc42 activity during Xenopus gastrulation. Dev. Biol, 2003, 257, 302–314. [DOI] [PubMed] [Google Scholar]
- [72].Manegold P; Lai KKY; Wu Y; Teo JL; Lenz HJ; Genyk YS; Pandol SJ; Wu K; Lin DP; Chen Y; Nguyen C; Zhao Y; Kahn M Differentiation Therapy Targeting the β-Catenin/CBP Interaction in Pancreatic Cancer. Cancers (Basel), 2018, 10, (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yamanaka H; Moriguchi T; Masuyama N; Kusakabe M; Hanafusa H; Takada R; Takada S; Nishida E JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep, 2002, 3, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kühl M; Sheldahl LC; Park M; Miller JR; Moon RT The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet, 2000, 16, 279–283. [DOI] [PubMed] [Google Scholar]
- [75].Sato N; Meijer L; Skaltsounis L; Greengard P; Brivanlou AH Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med, 2004, 10, 55–63. [DOI] [PubMed] [Google Scholar]
- [76].Boland GM; Perkins G; Hall DJ; Tuan RS Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J. Cell. Biochem, 2004, 93, 1210–1230. [DOI] [PubMed] [Google Scholar]
- [77].Mohammed MK; Shao C; Wang J; Wei Q; Wang X; Collier Z; Tang S; Liu H; Zhang F; Huang J; Guo D; Lu M; Liu F; Liu J; Ma C; Shi LL; Athiviraham A; He TC; Lee MJ Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis, 2016, 3, 11–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Koyanagi M; Haendeler J; Badorff C; Brandes RP; Hoffmann J; Pandur P; Zeiher AM; Kühl M; Dimmeler S Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J. Biol. Chem, 2005, 280, 16838–16842. [DOI] [PubMed] [Google Scholar]
- [79].Schulte G; Bryja V; Rawal N; Castelo-Branco G; Sousa KM; Arenas E Purified Wnt-5a increases differentiation of midbrain dopaminergic cells and dishevelled phosphorylation. J. Neurochem, 2005, 92, 1550–1553. [DOI] [PubMed] [Google Scholar]
- [80].Xiao Q; Chen Z; Jin X; Mao R The many postures of noncanonical Wnt signaling in development and diseases. Biomed. Pharmacother, 2017, 93, 359–369. [DOI] [PubMed] [Google Scholar]
- [81].Wallingford JB; Fraser SE; Harland RM Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev. Cell, 2002, 2, 695–706. [DOI] [PubMed] [Google Scholar]
- [82].Zhao Y; Masiello D; McMillian M; Nguyen C; Wu Y; Melendez E; Smbatyan G; Kida A; He Y; Teo JL; Kahn M CBP/catenin antagonist safely eliminates drug-resistant leukemia-initiating cells. Oncogene, 2016, 35, 3705–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kim YM; Gang EJ; Kahn M CBP/Catenin antagonists: Targeting LSCs’ Achilles heel. Exp. Hematol, 2017, 52, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Graves JD; Krebs EG Protein phosphorylation and signal transduction. Pharmacol. Ther, 1999, 82, 111–121. [DOI] [PubMed] [Google Scholar]