Abstract
Problem
Neisseria gonorrhoeae (NG) infection has been shown to increase sexual transmission of HIV-1. However, the mechanism of NG induced enhanced HIV-1 transmission is unknown.
Methods
1)The cervical tissues were exposed to NG and cytokine induction was monitored by measuring cytokine proteins in culture supernatants and cytokine m-RNAs in tissues. 2) Transcription and replication of HIV-1 in TZM-bl, U1 and ACH2 cells were measured by Beta-Gal activity and p24 proteins in the supernatant, respectively. 3) HIV-1 transmission was assayed in an organ culture system by measuring transmitted HIV-1 in supernatant and HIV-1 gag m-RNA in the tissues. 4) Transcriptome analysis was done using second generation sequencing.
Results
1) NG induced membrane ruffling of epithelial layer, caused migration of CD3+ cells to the intraepithelial region and induced high levels of inflammatory cytokines IL-1β and TNF-α. 2) NG Induced supernatants (NGIS) increased HIV-1 transcription, induced HIV-1 from latently infected cells, and increased transmission of HIV-1 across cervical mucosa. 3) Transcriptome analysis of the epithelial layer of the tissues exposed to NG and HIV-1 showed significant upregulation of CXCL10 and IL8. IL-1β increased the induction of CXCL10 and IL-8 expression in cervical mucosa with a concomitant increase in HIV-1 transmission.
Conclusion
We present a model in which IL-1β produced from cervical epithelium during NG exposure increases CXCL10 and IL8 in epithelia. This in turn causes upon HIV-1 infection, the migration of HIV-1 target cells towards the subepithelium, resulting in increased HIV-1 transcription in the sub-mucosa and subsequent enhancement of transmission across cervical mucosa.
Keywords: Neisseria gonorrhoeae, HIV, sexual transmission, cytokines
1. INTRODUCTION
Heterosexual transmission is the most common route of HIV-1 infection in women 1, 2. A key co-factor in the transmission of HIV-1 in women is the prior existence of bacterial, viral, and parasitic microbes in the cervix that can alter the cervical environment and thereby influence HIV-1 transmission 3–5. 6–8.
Gonorrhoeae caused by Neisseria gonorrhoeae (NG), a gram-negative diplococci, is one of the most severe and common form of STI 9, 10 that has been shown to increase HIV-1 acquisition10–12. The presence of pro-inflammatory cytokines in the vaginal fluid of NG-infected women and some cell line based studies with NG led to the speculation that NG induced inflammatory cytokines either directly or indirectly could increase HIV-1 transmission 12–16. Additional mechanisms of NG induced enhanced HIV-1 transmission that have been suggested include: recruitment of increased number of endo-cervical CD4+ T cells in NG infected women providing more targets for HIV-117, activation of CD4+T cells by NG 18, epithelial tight junction disruption19 and increased HIV-1 transcription by NG secreted proteins 20.
Currently, the molecular mechanism by which NG enhances HIV-1 /transmission in the female genital tract is still uncertain. Part of the uncertainty is due to lack of a suitable ex vivo model that mimics in vivo situation. HIV-1/NG interaction has been mostly studied in in- vitro cell culture using CD4+T cells, endometrial epithelial cells 15, 18, 21, 22 and immortalized cell lines 15, 23. However, these cell systems do not accurately reflect situationthat occur in human cervix/vaginal tissue. In addition, we do not know the mechanism of HIV-1 transmission through the epithelia of the cervical mucosa, especially when epithelia do not express CD4 and CCR5/CXCR4 24–26. Regardless of how HIV-1 crosses the epithelium, HIV-1 exposure to the epithelial layer or epithelial cells has been shown to induce production of cytokines and chemokines which serve as signaling molecules 27, 28. These signaling molecules may play an important role in HIV-1 transmission by attracting target immune cells to fuel HIV-1 infection in sub-mucosa and hence transmission29, 30.
Here we describe use of a primary cervical tissue based organ culture model of NG infection that provides the natural cervical tissue architecture observed in cervix of NG infected women. Using this organ culture we showed that NG exposure to cervical tissues induced epithelial membrane ruffling and inflammatory cytokine response, reminiscent of in vivo situation. Furthermore, using this model we have shown that NG induces IL-1β from cervical epithelium post exposure and increases the production of epithelial proteins CXCL10 and IL8, two key proteins that may be responsible for HIV-1 transmission, suggesting that increase in CXCL10 and IL-8 production in epithelia may be responsible for NG induced enhanced HIV-1 transmission across cervical mucosa. This study for the first time describes a molecular mechanism of NG induced enhancement of HIV-1 transmission across cervical mucosa.
2. METHODS
The study protocol for the procurement of the cervical tissues from patients undergoing hysterectomy was approved by the Institutional Review Board (IRB) at the University of Pittsburgh.
2.1. Bacterial Cultures
A highly opaque (Opa+) Neisseria gonorrhoeae (NG) phenotype with Pil+/−, a clinical isolate from the clinical lab at the Alleghany county hospital (gift from Dr. Timothy Meitzner, University of Pittsburgh) was used for all the experiments. This NG strain were routinely grown in 5% CO2 at 37°C on gonococcal medium base (GCB) (Difco) or in chocolate agar plates (Remel) for 18 to 24 hours31. This was then selected for Opa positive colonies by choosing the opaque phenotype when the cultured colonies were observed with oblique light under a dissecting microscope 32. The working cultures of each bacteria was generated with two to three colonies from each culture types from the plate, suspended in 10% RPMI with the absorbance adjusted to a concentration of 1× 107 cfu/ml for each experiments. Before using these resuspended colonies for experiment, they were washed and centrifuged at 800g for 5 minutes to remove cytokines.
2.2. Virus stock
HIV-1 BaL strain (cat #510 from NIH AIDS reagent program) was used in all experiments. They were grown in Phytohemagglutinin (PHA)- stimulated CD8 depleted peripheral blood mononuclear cells as described previously 33. The virus containing cell supernatant was filtered using an amicon ultra-15 filter device (Millipore, Billerica, US) to remove the soluble cytokines. The residual levels of cytokines were tested using MSD and were found to be below 10pg/ml. The control culture supernatant was prepared from uninfected cells in similar fasion.
2.3. Cell Cultures
Primary CD8-depleted PBMC were prepared by immune- magnetic depletion of CD8+ T cells from peripheral blood mononuclear cells (PBMC) 34. TZM-bl cells, (NIH AIDS research and reagent program Catalog number 8129, ACH2 cells (Catalog number 349), a human T cell line and U1 cells (Catalog number 165), a promonocytic cell line with minimal constitutive expression of HIV-1 (NIH AIDS research and reagent program) were maintained as described in the AIDS Reagent program. Ectocervix derived epithelium cells (ATCC CRL-2614) E6/E7 cells, were grown and maintained in Keratinocyte media as described by the ATCC.34, 35.
2.4. Organ Culture Model
Ecto-cervical tissues were collected and processed within 2 hours of surgery as described before 36. The ecto-cervical punch biopsies (6mm diameter) were placed into a 12 well transwell (Becton Dickson, NJ, USA)) with the epithelial layer facing up and its edges were sealed with 3% agarose at room temperature. NG at concentrations of 1 × 107 bacteia/ml or cell-free HIV-1 BaL (TCID50 of 106) were added on the epithelial layer of the tissue in upper chamber depending on the experiments 36. Complete 10%RPM1 [RPMI media with 10% fetal bovine serum ] or IL2 media [RPMI media with 10%, fetal bovine serum and interleukin-2 (500 U)] was added to the bottom well. Cultures were incubated at 37oC for different time intervals according to the the experimental set up.
For HIV-1 transmission studies CD8 depleted PBMC s (50,000 cells /ml) were placed at bottom well as indicator cells37. After 4 days in culture, the top wells were removed and tissues were examined for HIV-1 RNA by RTPCR. Bottom wells with CD8 depleted cells were cultured for additional 7 days and transmitted virus was measured by HIV p24 as described previously 37.
2.5. Measurement of inflammatory response and HIV-1 transmission
Total cellular RNA from cells or homogenized tissues were isolated by RNAzol B (TEL-TEST, INC) using the manufacterers protocol and GAPDH was used as a housekeeping gene. The level of proinflammatory cytokines, IL-1β, IL-6, IL-8 and TNF-α messenger RNA (m-RNA) were measured in the NG or control media exposed tissues/cells using RT-PCR with gene specific primers/probes by real time RT PCR using primers and probes as described as described previously38. Secreted cytokines in culture supernatant were measured by MSD multiplex assay according to the manufacturers protocol (MESO SCALE DISCOVERY, Rockville, MD 20850 USA). To measure the transmission of virus across tissues, HIV-Gag 38 m-RNAs were measured in the HIV-1 exposed tissues by RT-PCR and HIV p24 was measured in the supernatants using a commercially available HIV-1 p24 ELISA kit according to manufacturers protocol (SAIC-Fredrick, MD).
2.6. Next generation sequencing
The tissues post exposure to NG and HIV-1 BAL were washed and frozen at −80oC after being embedded in OCT (Thermo Fisher. USA), and then cryo-sectioned (15–30μm thickness). These cryo-sectioned tissues were then subjected to micro-dissection under microscope. Epithelial layer was carefully removed and RNA was extracted from the epithelium with RNA-Bee™ (TEL-TEST, INC, Friendswood, TX). RNA was then either used for whole genome transcriptional profile analysis or RT-PCR to confirm the significantly dys-regulated genes obtained from the ion torrent analysis.
2.7. Histology, Immunohistochemistry and image analysis
To examine the morphology of the mucosal epithelia, hematoxylin and eosin (H&E) staining of the ectocervical tissues was performed. The tissues were washed and embedded in OCT and cryosectioned into 7um thick layers each. They were then stained with hematoxylin and eosin and evaluated as described previously 39. The thickness of epithelial layers was measured in three representative areas of mucosa from the basement membrane up to the surface using the Metamorph software. The images were taken using a EVOS® XL Core Digital Imaging System under 20 x or 40 x objective lenses.
To study the effect of NG on the migration of cells towards the epithelial surface, anti-CD3 antibody and control antibody (diluted 1:100) was added to the NG exposed tissue as well as the control tissues and incubated for 1 hour at room temperature in a moist chamber. These were washed twice for 3 mins in PBS and HRP polymer conjugate from the SuperPicture kit for IHC detection (Invitrogen #87–9263 was added to the tissue and incubated again for 10 minutes at room temperature in the moist chamber. They were washed twice again for 3 mins each and DAB Chromagen, from the SuperPicture kit for IHC detection, was added to the tissue and incubated for 10 minutes at room temperature in the moist chamber. Then the slides were washed for 5 mins in PBS. Images were taken with the Nikon Eclipse E600 microscope using a 20 × or 40 × oil objective.40–42.Rabbit polyclonal-α-CD3 (A0452, Dako, Glostrup, Denmark), mouse polyclonal-α-CD68 (M0814, Dako, Glostrup, Denmark), mouse polyclonal-α-CD20 (M0755, Dako, Glostrup, Denmark), mouse polyclonal- α -DC-SIGN (551249, BD Technologies, NC, USA), and mouse polyclonal p55 (M3567, Dako, Glostrup, Denmark) was used to stain the tissue slides. The CD3+ cells were counted visually in 5 random fields and the number of cells that were stained with the Rabbit polyclonal-α-CD3 were calculated in all of those fields. The total number of cells in the field for the NG exposed tissue as well as control exposed tissue were calculated. The number of positive cells in the NG exposed and control exposed subepithelium regions on the slides were compared and quantitated for migration39.
2.8. Scanning electron microscopy
Human Ecto-cervical tissues were exposed to NG for 24 hours and fixed in 2.5 % glutaraldehyde for 1 hour at room temperature. The biopsies were washed with 0.1 M PBS (pH 7.4) a few times and then the tissues were incubated in 1% OsO4 in 0.1 M PBS for 60 minutes. This was again washed thoroughly 3 times with 0.1 M PBS for 15 minutes each and processed using a protocol from the Center for Biologic imaging (CBI) at the University Of Pittsburgh as previously described43. SEM images were acquired using a (JEOL JEM 1011 TEM (Peabody, MA) at 80kV fitted with a side-mount AMT 2k digital camera (Advanced Microscopy Techniques, Danvers, MA).
2.9. Viral transcription/activation in TZM-bl cells, ACH2 and U1 cells
For measuring HIV-1 transcription in TZM-bl cells, 40,000 cells in a 96 well plate were treated with test samples or control media and then expression of β-galactosidase was determined 48 hour later by Beta glo assay according to the manufacturers protocol(Promega, Madison,WI 53711). Activation of latent HIV-1 in the latently HIV-1 infected U1 and ACH2 cells were measured by monitoring HIV-1 p24 in culture supernatant by ELISA.
2.10. Next generation sequencing using ion torrent technology
i). RNA extraction and Transcriptome analysis
RNA was extracted from micro-dissected (under microscope) epithelial layer of the tissue as described earlier. mRNAs were isolated from the total RNA with a commercially available kit (Dynabeads® mRNA DIRECTTM Micro Purification Kit, Life Technologies). This was followed by cDNA Library construction using Ion Torrent RNA-Seq Kit (Life Technologies) for whole transcriptome libraries. For individual sample Barcodes 1 through 8 were attached using Ion Xpress 1–16 barcoding kits. Quantitation of cDNA libraries was performed using the Ion Library Quantitation Kit (Life Technologies) to evaluate appropriate template dilution factor for subsequent emulsion PCR and sequencing. This was followed by next generation sequencing using the Ion Torrent platform according to manufacturer’s protocols ( Life Technologies, Carlsbad, CA)
ii). Data analysis
Raw sequencing reads were in FastQ format. CLC Genomics Bench 7 was used to assess the quality of raw sequencing reads. Reads were accepted based on the length (longer than 25 nucleotides) and Phred Quality score higher than 20. Then the trimmed reads were mapped to Homo sapiens (hg19) mRNA sequences. To make sure there were sufficient counts for each gene, only genes with mean read counts higher than 10 were retained in the analysis. Bioconductor edgeR in R package was employed to perform the differential expression analysis. Compared with control group, the genes with Benjamini-Hochberg adjusted false discovery rate (FDR) <0.05 and absolute fold change greater than 2 were considered as significant differential expression.
2.11. Statistical Analyses
In most of the cases, if not otherwise mentioned, the data were presented as mean ± standard deviation and was plotted using the PRISM software student’s edition. All the analyses was also done using the same software. For analyzing mRNA expression levels, parametric single sample t-test was used to determine the significance (p=<0.05) for the fold change observed in NG exposed groups relative to controls. This was used because of the relatively small sample size. To determine significance (p=<0.05) in cell numbers of CD3+ cells between NG exposed group and controls, a non parametric paired Wilcoxon signed-rank test was used and the data were represented as mean ± standard error after quantification.
For comparisons of cytokine mRNA levels in the ectocervical tissues treated with NG or HIV-1, T-Test Unequal Variance analysis was performed with the significant level of p=<0.05. In experiemnts of transmission of virus across tissues, the p-value was calculated using unpaired t test of equal variance because of unequal number of bipsy per condition.
3. RESULTS
3.1. NG exposure to cervical tissues induced epithelial membrane ruffling and inflammatory cytokine response, reminiscent of in vivo situation in female cervix
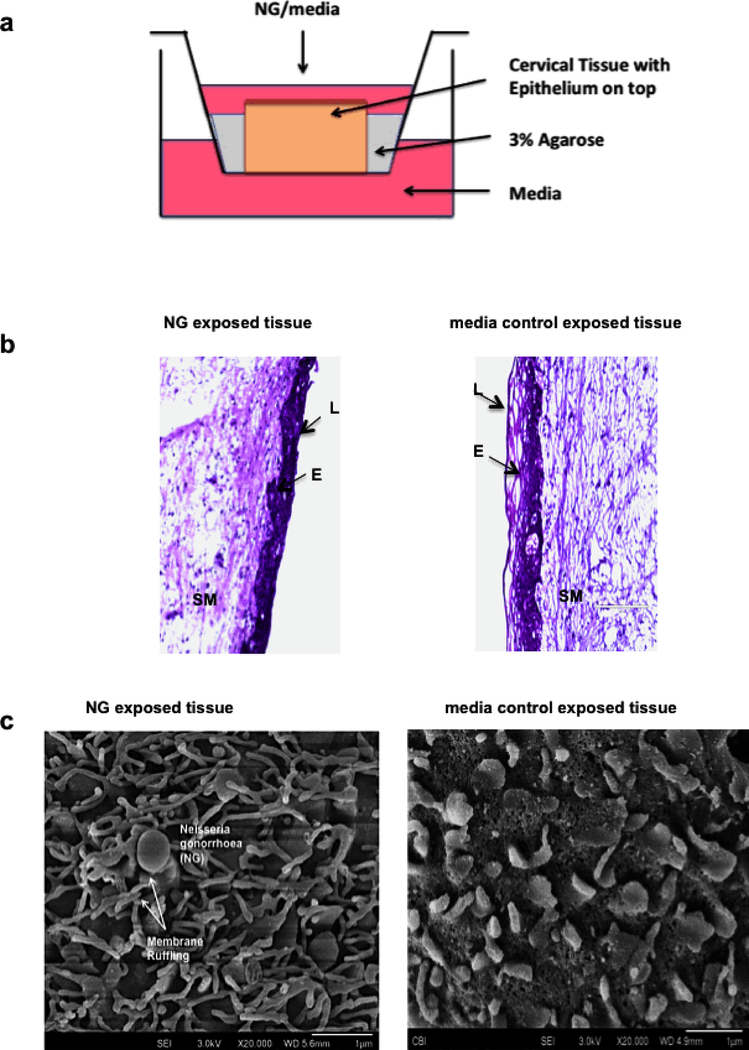
A polarized cervical tissue-based organ culture was set up in a 12 well trans-well system with the epithelial layer of the cervical tissue orientated up as described in Methods (Fig. 1a). NG at a concentration of 1× 107 cells/ml in 300ul were added onto the top of the tissue exposing epithelial layer. Following 24-hour of incubation, culture supernatant from the bottom of the wells and the exposed tissues were saved for measurement of soluble cytokines and intracellular cytokines, repectivly. We found that the tissues exposed to NG 24 hours were 97% viable compared to tissues exposed to media control as determined by MTT assay 44 as well as microscopic examination of H&E stained tissue sections under the light microscope (Fig. 1b). Scanning electron microscopic (SEM) analysis of the epithelial layer of tissues exposed to NG showed signs of epithelial membrane ruffling which is characteristic of the NG infection process in the female cervix (Fig. 1c) and is usually noted in biopsy from patients with cervicitis. We observed no overgrowth of the bacteria on the tissues post 24 hours. Twenty percent of the inoculated NG was found to be adherent to the tissues after 24 hours of exposure as determined by treating them with 1% saponin (Sigma Chemical Co.) solution in PBS for 5 min at 37°C to lyse the cells from the epithelial surface and then releasing the adherent and internalized bacteria. Dilutions of cell lysate were plated on 1% GC agar plates (Remel, San Diego, CA),GC agar to determine the number of viable bacteria. NG did not increase leakiness of the epithelial layer as evidenced by lack of transmission of blue dextran, a 2×106 molecular weight polysaccharide, (Sigma Prod. No. D5751) across cervical mucosa. 36.
Figure 1: Ecto-cervical tissue based organ culture model to study physical responses and induction of inflammatory cytokines from the cervical tissues in presence of NG.
(a) Transwell with ecto-cervix tissue surrounded with agarose, NG was added to the apical surface of the tissue, with media at the top and bottom well, and incubated for 24 or 48 hours. (b) H&E staining on ecto-cervical tissues exposed to NG or control media in the organ culture for 24 hours. Images were obtained by bright field microscopy (E: Epithelium; L: Lumen of ecto-cervix; SM: Sub-mucosa of the ecto-cervix). Magnification for viewing these ecto-cervical tissue sections was 20X. Each donor had 2–3 control and 2–3 NG exposed biopsies. with 5–10 random images obtained from each biopsy. (c) Membrane ruffling in presence of NG characterized by microvilli projection observed in cervical biopsy post 24 hours exposure to NG under SEM, with a magnification of 20,000X.
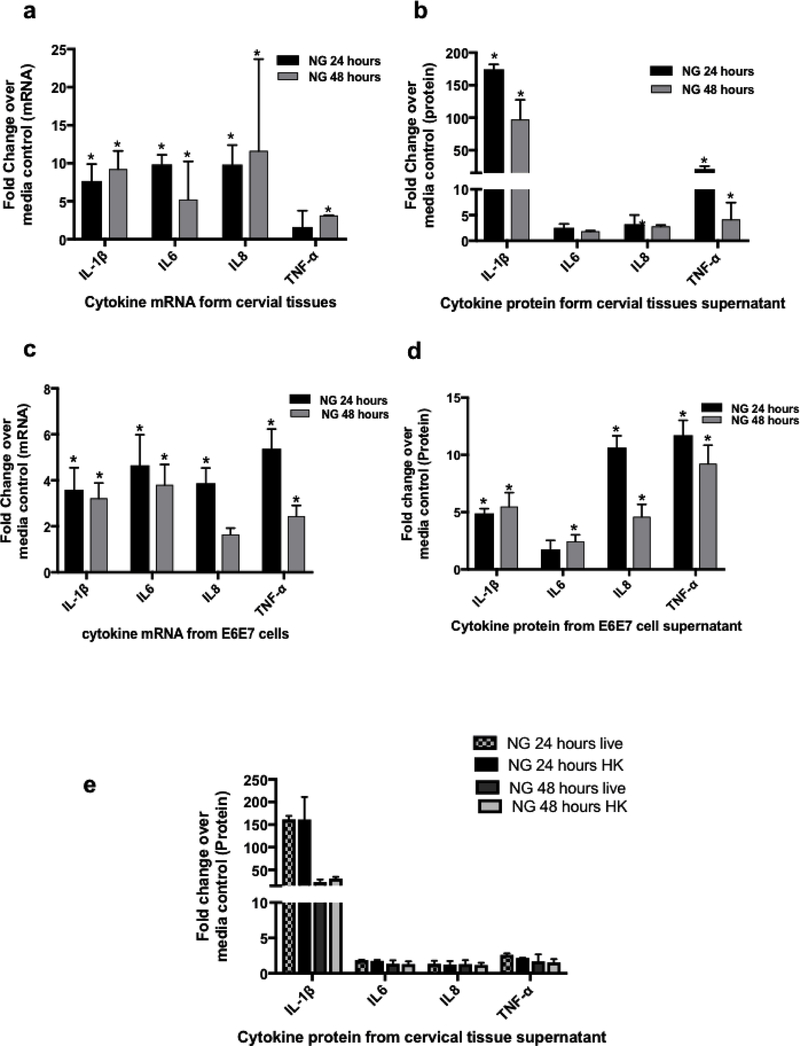
Since pro-inflammatory responses to NG infection in cervix are often observed in NG infected women, we evaluated such responses to NG infection in the cervical tissues in our organ culture. Exposure of cervical tissues to NG (3×106) induced high levels of IL6, IL8, IL-1β and TNF-α at both 24 and 48 hours after NG inoculation, with IL-1β and TNF-α being the highest compared to control tissues exposed to media (2500pg/ml for IL-1β and 500pg/ml for TNF-α in NG exposed compared to 10pg/ml and 17pg/ml in control media respectively). This increase in the cytokine production was noted both at the mRNA level (Fig. 2a) as well as the secreted protein levels (Fig. 2b). A change of 5–10 fold in mRNA and 100–200 fold in protein for IL-1β, and 2–5 fold in mRNA and 5–10 fold in protein for TNF-α was noted. There was a statistically significant (p=<0.05) increase in the level of IL-1β and TNF-α protein in supernatant at both time points although we did not observe a significant increase in TNF-α mRNA at 24 hours. These cytokines were selected on the basis of prior literature, which demonstrated that they were elevated in genital lavages from patients with NG infection as well as studies conducted with NG exposure on epithelium cell lines15, 18, 45. Longer exposure of tissues to NG for 7 days, induced higher elevation of these cytokine mRNA compared to 24 hours (Suppl Fig 1a). As a control, Lactobacillus plantarum did not induce significant levels of cytokine responses upon their exposure to the cervical tissues (data not shown). To determine whether live NG was required for induction of cytokine response, cervical tissues in the same organ culture were exposed to heat-killed (65 0 C for 30 min) NG for 24 hour and 48 hr. No significant difference in the cytokine levels between the heat killed and live NG was observed (Fig. 2e), indicating that live NG was not essential for the inflammatory cytokine response. These results implied that outer membrane structures of NG might be sufficient for inducing inflammatory responses.
Figure 2: Cellular responses induced by NG in cervical tissues and cervical tissue derived cell lines.
Cervical tissue biopsies exposed to NG showed elevation in cytokine levels. Inflammatory cytokines (a)mRNA and (b) protein at 24 hours and 48 hours compared to media control showed high fold changes in IL-1β (5–10 fold in mRNA and 100–200 fold in protein) and TNF-α (2–5 fold in mRNA and 5–10 fold in protein). There was a significant increase in the level of IL-1β and TNF-α protein in supernatant at both time points though we did not observe a significant increase in TNF-α mRNA at 24 hours. The (c) mRNA profile and (d) secreted cytokine protein profile of the E6/E7 Cells upon exposure to NG showed a similar increase in cytokine responses as in the tissues with an significant increase of 3 and 5 folds (IL-1β) and 5 and 10 fold (TNF-α )of cytokines at the mRNA(Fig. 2c) and protein levels respectively. Ecto-Cervical tissue biopsies exposed to either (e) live NG or heat killed NG showed no difference in cytokine response. Bars represent mean ± standard dev of three independent experiments with different donors. Each donor had 2–3 control and 2–3 NG exposed biopsies for each experimental set. Experiemnts with E6/E7 cells were carried out in triplicates. P=<0.05 was considered to be statistically significant compared to the control tissues for these fold changes analyzed by one sample students T test.
Since the epithelial layer exposed to NG seemed to induce pro-inflammatory cytokines, we sought to determine whether exposure of NG to epithelium per se was sufficient for the induction of these pro-inflammatory responses. For this purpose the ectocervix derived epithelial cell line E6/E7 were evaluated for their ability to induce inflammatory responses upon exposure to NG. Like cervical tissues, these epithelial cells, upon exposure to NG, induced a very similar profile of cytokines as observed in the organ culture setup with an increase in the expression of intracellular IL-1β, TNF-α, IL8 and IL6 cytokine mRNAs (Fig. 2c) and secreted IL-1β, TNF-α cytokine proteins (100–250 pg/ml of IL-1β in NG exposed compared to 20–50 pg/ml in control and 100– 500pg/ml of TNF-α in NG exposed compared to 10–50pg/ml in control). This also demonstrated an average change of 3 and 5 folds (IL-1β) and 5 and 10 fold (TNF-α )of cytokines at the mRNA(Fig. 2c) and protein levels, respectively (Fig. 2d) as compared to media controls, which was also in line with earlier studies 46.There was a significant increase in IL-1β and TNF- α (24 hours and 48 hours) both at the m-RNA as well as protein level (p =< 0.05 ).
3.2. NGIS could enhance HIV-1 transcription and replication in latently HIV-1 infected cells
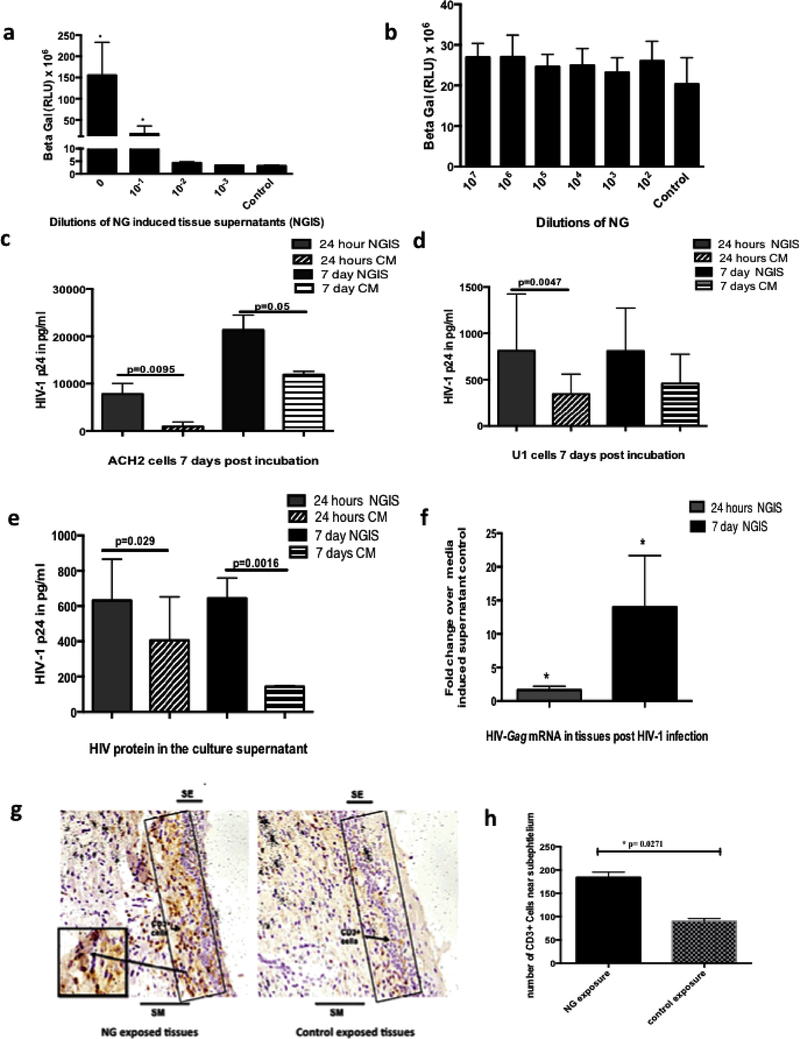
NG infection has been shown to enhance HIV-1 transmission in women. Epidemiological studies suggest that it is not the NG microbe per se, but NG induced cervical milieu may be responsible for increased HIV-1 transmission in women 18, 47. Therefore, we investigated whether culture supernatants from cervical tissues exposed to NG (referred to as NG Induced supernatants, abbreviated as NGIS), reminiscent of NG induced cervical milieu had any effect on the HIV-1 transcription in a TZM-bl cell based HIV-1 LTR driven reporter gene assay. A dose dependent stimulation of HIV-1 LTR activity was observed with a serial tenfold dilution of NGIS, but not with control conditioned media (Fig. 3a). In contrast, NG bacteria alone did not show any significant activation of HIV-1 LTR in TZM-bl cells (Fig. 3b).
Figure 3: Evaluation of the role of NG and NGIS on the replication and transmission of HIV-1.
(a)Dose dependent increase in transcription of HIV-1LTR in the TZM-bl demonstrated by increased beta-galactosidase activity by NGIS compared to CM. (b) NG per se did not show any stimulation of the HIV-LTR activity over control media (CM). NGIS induced higher replication and production of virus particles from latently infected cell lines (c) ACH2 cells and (d) U1 cells compared to CM at both 24 hours and 7 days. All cell studies were carried out in three independent experiments with 3 replicates in each and were analysed using parametric t tests. (e) NGIS increased the transmission of HIV-1 (HIVBAL 300uL of TCID50 of 10 6)across the mucosa as demonstrated by the transmitted virus (HIV p24) and (f) increase in HIV-Gag mRNA in the tissues. The p-value was calculated as significant using unpaired t test of equal variance because of unequal number of bipsy per condition. (g) Increased localization of CD3+ cells observed in the sub-epithelium in NG exposed tissues over CM exposed tissues. Figure is a representative image at 200X magnification. (h) Quantitation of immuno-stained CD3+ cells showed a statistically significant increase in CD3+ T cells on NG exposed tissue compared to CM exposed tissues. p=<0.05 was considered as significant in all cases. For the CD3+ tissue stain, experiments were carried out in 2–3 biopsies from tissues of 3 donors and non-parametric paired Wilcoxon signed ranked test was used in the analysis of this data.
NGIS was further assessed by examining its effect on reactivation of replication competent HIV-1 in latently infected ACH2 (T cell derived) and U1 (monocytic) cells48. NGIS activated high levels of HIV-1 from both ACH2 (Fig. 3c) and U1 cells (Fig. 3d), compared to cells exposed to control conditioned media.
3.3. NGIS enhanced HIV-1 transmission across cervix in an organ culture model
We next examined whether enhancement of HIV-1 replication by NGIS in cells can be translated to enhanced HIV-1 transmission across cervical mucosa. For this purpose we used our standard organ culture for measuring HIV-1 transmission as described previously 36, 38. Undiluted NGIS was added onto the top of the epithelial layer of the cervical tissue and pre-incubated for 24 hours, after which HIV-1 BaL (TCID50 of 1×106) was added onto the tissue. The level of HIV-1 p24 in the bottom well was used to monitor HIV-1 transmission across cervical mucosa. Results indicate a 55% increase (1.65 fold) in the HIV-1 transmission across the cervical tissues with NGIS collected 24 hour after NG exposure and a 350% increase (3.5 fold) in HIV-1 transmission with NGIS collected 7 days after exposure to NG with a significant p value of 0.029 and 0.0061 respectively using an unpaired t test with equal variance. (Fig. 3e) There was also a concomitant increase in intracellular HIV-1 gag mRNA in cervical tissues (2-fold with 24 hour NGIS and 4-fold with 7 day NGIS) ) compared to control media (Fig. 3f).
3.4. NG increased mmigration of CD3+ T cells towards the epithelium in cervical tissue explants
There have been reports that NG exposure recruits CD4+ T cell17, 49, which could increase targets for HIV-1 infection, to the cervices of women infected with NG. These findings raise the possibility that NG infection may increase HIV-1 acquisition by the recruitment of HIV-1 target cells near the epithelium. We tested the possibility of increased recruitment of CD3+ cells by quantifying immune cells in the intraepithelim region upon 24 hour of NG exposure to tissues by imunohistochemistry. As shown in Fig. 3(g,h), significantly higher numbers of CD3+T cells were localized in the intraepithelial layer in the tissue exposed to NG, compared to tissues exposed to control media. During this period we could not detect macrophages or dendritic cells in this region of the tissue (data not shown). This could be due to realtively short NG exposure (24 hr) used in this study. This goes along with our previous observation that HIV-1 infected CD3+ cells are detected in the intraepithelial layer within 6 hr of infection, while HIV-infected macrophge and DC are not detected until 72 hr after infection50.
3.5. CXCL10 and IL8 were differentially upregulated both in NG as well as HIV-1 exposed tissues
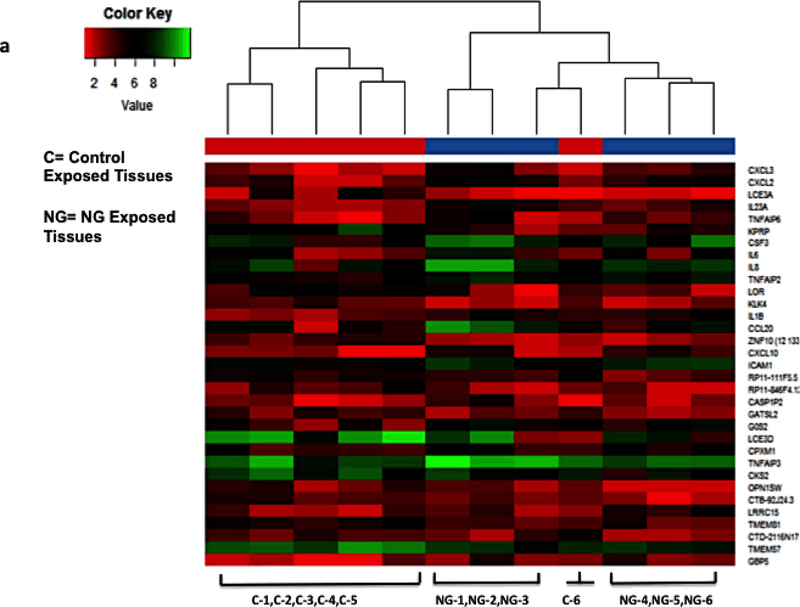
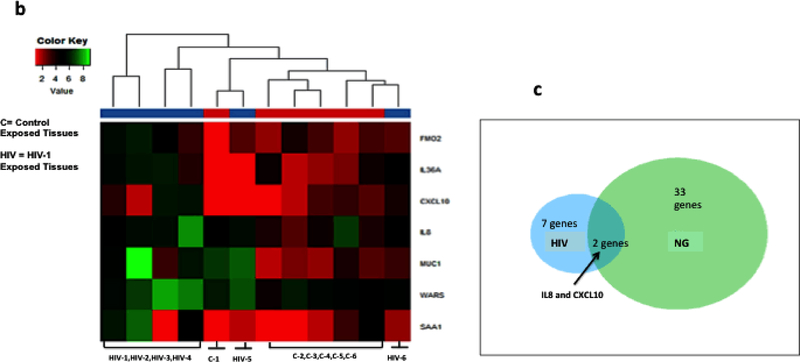
We have shown above that NG exposure to cervical epithelia induces upregulation of inflammatory cytokine m-RNAs and secreted cytokine proteins. To determine how NG induced cytokines may be involved in increased HIV-1 transmission across cervical epithelia, the epithelia exposed to NG and HIV-1 were examined for expression of cellular genes that may be upregulated during exposure to NG and HIV-1. For this purpose, transcriptome analysis using next-generation sequencing was performed on cellular RNA isolated from micro-dissected epithelial layer of the six tissues exposed to NG and HIV-1 separately. The genes which were found to be differentially regulated with high statistical significance after analysis are shown on the right of the heat map with red showing lower expression and green showing higher expression (Fig. 4a,b). Such analysis identified with high statistical significance (p=<0.05) 33 differentially expressed (−3 to 8-fold) genes in NG exposed and 7 differentially expressed (2 to 7-fold) genes in HIV-1 exposed tissues, compared to part of the same tissues exposed to control medium (Suppl Tables 1 and 2).
Figure 4: Transcriptome analysis of cervical epithelium exposed to NG and HIV-1 identified up-regulated cellular genes.
Heat Map showing transcriptome analysis of epithelial layer of the cervix using next- generation sequencing of tissue epithelium exposed to (a)NG (n=6) and (b) HIV-1 (n=6). NG depicts Neisseria numbered 1–6, HIV-1 is also numbered 1–6. C depicts tissues exposed to control supernatant and numbered as 1–6. Figure shows only the 6 tissues which were subjected to transcriptome analysis and genes which were found to be upregulated. Ven-diagram showing (c) common genes CXCL10 and IL8 expressed by NG and HIV-1 exposure on the cervical epithelium. The expression of the genes in (a) NG exposed and (b) HIV-1 exposed ectocervical epithelia was at least 2-fold difference with false discovery rate (FDR) <0.05 compared to the controls.
Venn diagram analysis of these differentially expressed genes in HIV-1 and NG exposed epithelia indicated that only two genes CXCL10 and IL8 were found to be common upregulated genes between tissues exposed to HIV-1 and NG groups (Fig. 4c).
To confirm the RNA seq analysis of NG exposed tissues, we examined expression of seven genes (CXCL10, CXCL3, CXCL20, TNFA1P6, IL8, IL6, IL-1β) by RTPCR in 10 tissues exposed to NG or control supernatant. Results shown in Table 1 indicate upregulation of these seven genes by more than 5 fold with high significance (p =< 0.05) compared to tissues exposed to control supernatant. Similarly, RT-PCR analysis of the seven differentially expressed genes from RNA sequence analysis from a total of 14 HIV-1 exposed tissues also showed that six (CXCL10, MUC1, IL36A, FMO2, IL8, WARS) out of seven genes were found to be up-regulated (3 to 44 fold) with high statistical significance(p=<0.05) in all tissues compared to tissue exposed to media control (Table 2). A comparison of the RTPCR data of the transcripts between the NG and HIV-1 exposed tissues also confirmed that CXCL10 and IL-8 were the only two genes common between these two groups that were significantly elevated (11.44 and 4.24 in HIV-1 exposed tissues, respectively and 9 and 29 fold in NG exposed tissues, respectively). Elevated levels of CXCL10 and IL8 proteins were also detected in NGIS collected at both at 24 hours as well as 7 days post exposure of NG (Suppl Fig 1b).
Table 1:
Fold changes of DEGs (Differentially expressed genes) up-regulated upon NG exposure on cervical tissues compared to tissues exposed to media control
| Sample Number | CXCL10 | CXCL3 | CCL20 | TNFAIP6 | IL8 | IL6 | IL-1β |
|---|---|---|---|---|---|---|---|
| E-9202 (NG) | 3.39 | 3.32 | 2.38 | 9.32 | 1.6 | 2.46 | 1.68 |
| E-9235 (NG) | 0.18 | 0.73 | 0.74 | 0.79 | 0.57 | 0.59 | 0.75 |
| E-9249 (NG) | 6.06 | 5.35 | 17.63 | NA | NA | NA | NA |
| E-9326 (NG) | 5.82 | 61.82 | 113.77 | 44.63 | 174.85 | 106.15 | 30.91 |
| E-9395 (NG) | 7.26 | 5.82 | 26.72 | 8.63 | 8.51 | 5.24 | 4.29 |
| E-9431 (NG) | 19.56 | 4 | 2.79 | 14.12 | 4.66 | 4.29 | 2.89 |
| E-9318 (NG) | 26.17 | 3.41 | 4.89 | 7.46 | 7.67 | 4.32 | 8.4 |
| E-9457 (NG) | 13.83 | 5.94 | 10.41 | 3.07 | 42.22 | 3.48 | 8.17 |
| E-9727 (NG) | 4.29 | 15.78 | 6.59 | 2.91 | 6.06 | 10.85 | 22.94 |
| E-9761 (NG) | 3.81 | 3.12 | 34.54 | 3.39 | 15.24 | 5.13 | 6.96 |
| Average Fold Change (n=10) | 9.04 * | 10.93 * | 22.05 * | 10.48 * | 29.04 * | 15.84 * | 9.67 * |
| p value | 0.0041 | 0.0039 | 0.0039 | 0.0078 | 0.0117 | 0.0078 | 0.0078 |
The upregulated genes found in the transcriptome analysis was verified using RT-PCR on additional tissues (n=10). Numbers indicate fold changes of DEGs upon NG exposure on cervical tissues compared to tissues exposed to media control.
All the genes confirmed to be upregulated were statistically significant. A non parametric paired t test was used for calculating the statistical significance.
Table 2:
Fold changes of of DEGs (Differentially expressed genes)up-regulated upon HIV-1 exposure on cervical tissues compared to tissues exposed to media control
| Sample Number | CXCL10 | SAA1/SAA2 | MUC1 | IL36A | FMO2 | IL8 | WARS |
|---|---|---|---|---|---|---|---|
| E-8776 (HIV) | 11.16 | 8.75 | 6.54 | 3.29 | 5.06 | 3.51 | 4 |
| E-7688 (HIV) | 40.22 | 2.22 | 2.83 | 0.99 | 2.6 | 1.56 | 9.45 |
| E-8901 (HIV) | 5.7 | 0.72 | 1.32 | 0.84 | 0.8 | 0.99 | 1.51 |
| E-8916 (HIV) | 19.7 | 16 | 580.04 | 235.57 | 34.06 | 34.78 | 5.31 |
| E-9274 (HIV) | 12.64 | 4.11 | 3.76 | 2.95 | 3.41 | 5.39 | 9.45 |
| E-9301 (HIV) | 1.41 | 0.26 | 0.37 | 1.41 | 0.79 | 2.16 | 1.03 |
| E-9323 (HIV) | 9.19 | 4.06 | 2.57 | 1.61 | 1.3 | 0.75 | 3.66 |
| E-9387 (HIV) | 15.67 | 1.57 | 0.79 | 0.58 | 1.09 | 1.42 | 1.69 |
| E-9483 (HIV) | 3.56 | 0.93 | 1.75 | 2.53 | 2.19 | 2.19 | 2.11 |
| E-9493 (HIV) | 2.51 | 1.2 | 0.68 | 1.57 | 1.59 | 1.12 | 1.69 |
| E-9564 (HIV) | 15.24 | 1.8 | 10.27 | NA | 9.65 | 3.16 | 10.34 |
| E-9571 (HIV) | 2.57 | 0.2 | 1.04 | 0.38 | 0.47 | 0.29 | 1.09 |
| E-9769 (HIV) | 7.26 | 0.74 | 0.18 | 2.04 | 0.8 | 0.26 | 0.33 |
| E-9864 (HIV) | 13.27 | 1.21 | 5.39 | NA | 8.51 | 1.79 | 11.16 |
| Average Fold Change (n=14) | 11.44 * | 3.13 | 44.11 * | 21.15 * | 5.17 * | 4.24 * | 4.49 * |
| p value | 0.0001 | 0.104 | 0.0245 | 0.05 | 0.0157 | 0.0245 | 0.0009 |
The upregulated genes found in the transcriptome analysis was verified using RT-PCR on additional tissues (n=14). Numbers denote fold changes of DEGs in tissues exposed to HIV-1 compared to tissues exposed to media control.
Six genes out of the seven genes were found to be statistically upregulated by RTPCR. A non parametric paired t test was used for calculating the statistical significance.
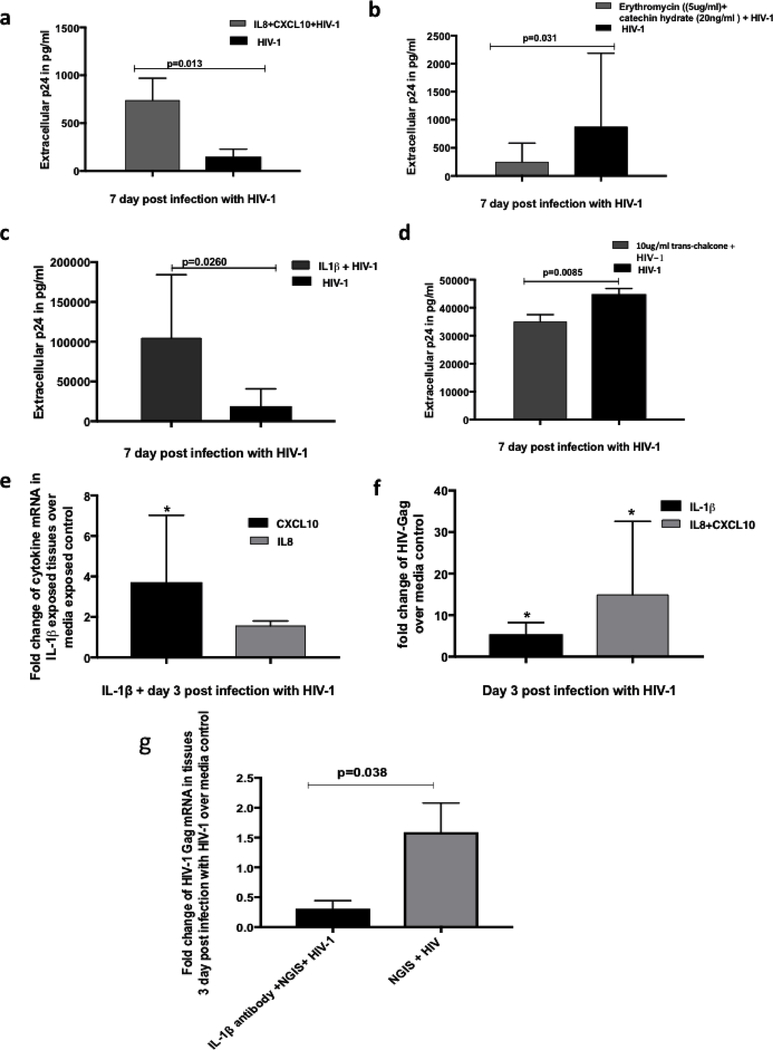
3.6. IL8 and CXCL10 present in NGIS together could enhance HIV-1 transmission across cervical explants
To determine the role of CXCL10 and IL8 directly in HIV-1 transmission across epithelia, tissues were incubated for 24 hour with IL8 and CXCL10 (200 ng/ml and 1000 pg/ml respectively at concentrations present in the NGIS ) and then exposed to HIV-1. Results shown in Fig. 5a showed a significant enhancement (5–8 fold) of HIV-1 transmission across cervical mucosa with a concomitant increase in HIV-1 transcription in the cervical tissues, findings which are in line with prior literature 51–53 (Fig. 5f). Furthermore, addition of IL-8 inhibitory drug erythromycin (5ug/ml) and CXCL10 inhibitory drug catechin hydrate (20ng/ml) 54–56 showed a significant decrease (56%) in the HIV-1 transmission (Fig. 5b).
Figure 5: Effect of IL-1β on the transmission of HIV-1 across cervical mucosa.
(a) IL8 (200 ng/ml) and CXCL10 (1000 pg/ml) at concentrations present in the 24 hour NGIS increased the transmission of HIV-1 across cervical mucosa in the organ culture. (b) Inhibitory drugs erythromycin (5ug/ml) against IL-8 and catechin-hydrate (20ng/ml) against CXCL10 decreased the transmission of HIV-1 across the mucosa. (c) IL-1β (2500pg/ml) in concentration present in the 24 hour NGIS increases the transmission of HIV-1 across cervical mucosa in the organ culture. (d) Addition of IL-1β inhibitor (10ug/ml trans-chalcone) decreased the transmission of HIV-1. (e) Addition of the IL-1β (2500pg/ml) on the cervical tissues increased the CXCL10 and IL8 in the tissues at the mRNA level. (f) IL-1β as well as a combination of CXCL10 and IL8 increased the HIV-1 transcription in the tissues compared to media exposed control. (g) Monoclonal antibodies against IL-1β (10ug/ml) in the NGIS along with HIV-1 decreased transmission compared to NGIS and HIV-1 alone. P=<0.05 was considered as significant and either parametric t tests or T-Test Unequal Variance was used to calculate for the p values.
It has been shown that in intestinal epithelium cells IL-1β induces CXCL10 57, 58 and IL-858. We therefore investigated the effect of IL-1β on the production of CXCL10 and IL-8 and their effect on HIV-1 transmission in cervical tissues. As shown in Fig. 5c, addition of IL-1β to cervical tissues at concentrations (2500pg/ml) detected in the NGIS showed a significant increase in HIV-1 transmission across cervical mucosa (Fig. 5c) with a concomitant slight increase (1.58 fold) in the expression of IL8 and significant increase in CXCL10 mRNA in tissue (Fig. 5e). Furthermore, treatment of tissues with trans-chalcone, an inhibitor of IL-1β 59, showed a statistically significant decrease in the transmission of HIV-1 across the cervical epithelium (Fig. 5d). Monoclonal antibodies against IL-1β (10ug/ml) added to NGIS significantly decreased the HIV-1 transmission across the mucosa when compared to NGIS alone (Fig. 5g).
4. DISCUSSION
Despite the worldwide problem of increased HIV-1 transmission driven by NG, the molecular mechanism associated with NG induced enhanced HIV-1 transmission is largely unknown because of the unavailability of a suitable in vitro model. We described here a cervical tissue-based organ culture model that mimics several aspects of cervical tissues in vivo. This includes: the minimal toxicity of NG to cervical tissue, NG-induced membrane ruffling, and induction of pro-inflammatory cytokines with the highest being that of IL-1β and TNF-α. However, such cytokine responses were not dependent on live NG, therefore could be elicited by the bacterial outer membrane structure.
The data reported here affirm previous epidemiological presumptions in NG infected women that it was not NG per se but NGIS, analogous to cervical/vaginal milieu, that induced expression of the HIV-1 LTR and replication competent HIV-1 from latently infected lymphocytic and pro-monocytic cells. This is in accord with recent data showing heptose monophosphate (HMP) secreted from NG-infected cells activate the HIV-1 LTR in CD4+ T cells 20. Most importantly, NGIS enhanced HIV-1 transmission across cervical mucosa, most likely by increasing HIV-1 transcription in the sub-mucosa. All of these data together indicate that this cervical tissue based organ culture system could be an appropriate model to study NG induced enhanced HIV-1 transmission in cervical tissues.
Another problem in investigating molecular determinants of NG-driven increased HIV-1 acquisition in the female genital tract is due to our lack of a clear understanding of cellular factors that are responsible for HIV-1 transmission through epithelia of the cervical mucosa, especially when cervical epithelia do not express significant amount of CD4 and CCR5/CXCR4 24, 25. The other parts of the female reproductive tract especially the uterine epithelium have however been shown to express HIV-1 receptors/co-receptors60. We have reasoned that pro-inflammatory cytokines often present in genital secretions of patients with NG 12–15 interact with cellular factors responsible for HIV-1 transmission and thereby enhance HIV-1 transmission. Here we provided evidence using transcriptome analysis of cervical epithelium exposed to NG or HIV-1, that NG infection induced in cervical epithelia two cellular proteins CXCL10 and IL-8 that were also induced during HIV-1 transmission across cervical mucosa. Furthermore, the role of CXCL10 and IL-8 on HIV-1 transmission has been confirmed by the finding enhanced HIV-1 transmission with direct addition of CXCL10 and IL8, and inhibition of HIV-1 transmission by inhibitors of CXCL10 and IL-8.
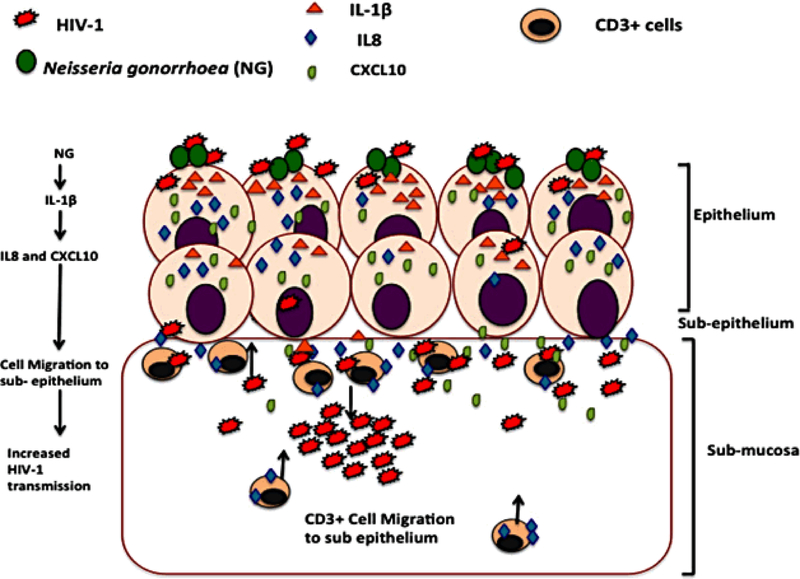
To decipher how NG infection might be involved in up-regulation of CXCL10 and IL-8, we noticed that IL-1β secreted by NG has been shown to induce CXCL10 and IL-8 in intestinal epithelial cells. Our data in cervical tissue also showed that IL-1β induced CXCL10 and IL-8 expression with a concomitant increase in HIV-1 transmission across cervical mucosa. In addition, NG infection causes migration of CD3+T cells towards the intraepithelial region which is in line with known chemo-attractant properties of CXCL10 and IL-8 for CD3+T cells and macrophages that have been predicted from the IPA analysis of differentially expressed genes in HIV-1 and NG exposed epithelia. Furthermore, it has been shown that CD4+ cells are the first cells that become infected within 24 hr of infection during HIV-1 transmission across cervical mucosa33, 61. Altogether these data are consistent with a model (Fig. 6) for NG-induced enhancement of HIV-1 transmission: NG infection leads to secretion of IL-1β, which induces production of epithelial CXCL10 and IL-8. Upon HIV-1 infection, increase of these cytokines cause migration of the CD3+ T cells towards the intraepithelial region which fuels higher HIV-1 replication in the sub-mucosa and consequently enhances HIV-1 transmission. Identification of IL-1β and its target cellular proteins in NG-induced enhanced HIV-1 transmission could be useful in development of drugs that impede the HIV-1 transmission. The explant models have a few disadvantages to using a full animal model. The cellular circulation system here is limited, therefore it does not help study the mechanism of migration of cells to different areas of the body. Inspite of this,the system is well equipped to study the basic immediate impact of HIV-1 infection and its transmission across the mucosa along with the migration of cells from the basal to the apical end of the tissue explants 33. Another limitation of this study is the small sample size. This is primarily because of the low availability of cervical tissues to work with and thus we could not use more than 3 tissues for any experiment. There is also a difference in age, race and the menstrual cycle among different tissues obtained which may affect the susceptibility to infection. This may be the reason why some tissues transmitted more easily and produced more cytokine response than the others when exposed to HIV-1 or NG. Like in our case, one tissue exposed to HIV-1 during the cytokine upregulation study showed very high cytokine response compared to others. This was a more susceptible tissue. The higher sample size (n=14) in this study (Table.2) helped in determining the significance of the study. Future work in nonhuman primates or humanized mouse models could provide further information on the in vivo role of CXCL10 and IL-8 in HIV-1 transmission and its modulation by NG secreted proteins like IL-1β. There has already been some work which has shown that NG infection in HIV-1 infected humanized mouse increases HIV-1 shedding in the genitalia 62.
Figure 6. Schematic representation of NG induced enhanced HIV-1 transmission across the ecto-cervical tissues.
NG infection of the epithelial surface leads to secretion of IL-1β, which induces production of epithelial CXCL10 and IL-8 upon HIV-1 infection. These cytokines being chemo-attractants for immune cells, cause migration of higher HIV-1 target CD3+ T cells to the periphery of the epithelium and the intraepithelial region which then fuels the increase in the HIV-1 replication at the sub-mucosa and consequently enhances HIV-1 transmission.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Mohit Dhir for procuring cervical tissues through the pathology tissue bank of the University of Pittsburgh Medical Center, Lori Caruso and Beth Fallert for the excellent technical assistance, Jonathon Frank and Dr Donna Stalz from the Center for Biologic Imaging (CBI) University of Pittsburgh for their technical assistance and expertise with SEM and Dr Ansuman Chattopadhyay for the help with the genetic analysis softwares. We also thank Drs Debjani Guha, Ronald Montelaro and Jodi Craigo for helpful discussion and editing the paper. This project was supported by the NIH RO1 grant HD072693(P.G). A.S and S.S were supported by the Fogarty International HIV Research and Training Porgram of the National Institutes of Health (D43TW00103).
Footnotes
Authors’ declaration.: The authors declare no conflict of interest. This article is a direct contribution from a member of the American Academy of Microbiology.
REFERENCE
- 1.Hansasuta P, Rowland-Jones SL: HIV-1 transmission and acute HIV-1 infection. Br Med Bull 2001;58:109–127. [DOI] [PubMed] [Google Scholar]
- 2.Ramjee G, Daniels B: Women and HIV in Sub-Saharan Africa. AIDS Res Ther 2013;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J: Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. The Journal of infectious diseases 1999;180:1863–1868. [DOI] [PubMed] [Google Scholar]
- 4.Mirmonsef P, Krass L, Landay A, Spear GT: The role of bacterial vaginosis and trichomonas in HIV transmission across the female genital tract. Curr HIV Res 2012;10:202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salas JT, Chang TL: Microbiome in human immunodeficiency virus infection. Clin Lab Med 2014;34:733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anzala AO, Simonsen JN, Kimani J, Ball TB, Nagelkerke NJ, Rutherford J, Ngugi EN, Bwayo JJ, Plummer FA: Acute sexually transmitted infections increase human immunodeficiency virus type 1 plasma viremia, increase plasma type 2 cytokines, and decrease CD4 cell counts. The Journal of infectious diseases 2000;182:459–466. [DOI] [PubMed] [Google Scholar]
- 7.Plummer FA, Simonsen JN, Cameron DW, Ndinya-Achola JO, Kreiss JK, Gakinya MN, Waiyaki P, Cheang M, Piot P, Ronald AR, et al. : Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. The Journal of infectious diseases 1991;163:233–239. [DOI] [PubMed] [Google Scholar]
- 8.Galvin SR, Cohen MS: The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2004;2:33–42. [DOI] [PubMed] [Google Scholar]
- 9.Page KR, Moore RD, Wilgus B, Gindi R, Erbelding EJ: Neisseria gonorrhoeae and Chlamydia trachomatis among human immunodeficiency virus-infected women. Sexually transmitted diseases 2008;35:859–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mlisana K, Naicker N, Werner L, Roberts L, van Loggerenberg F, Baxter C, Passmore JA, Grobler AC, Sturm AW, Williamson C, Ronacher K, Walzl G, Abdool Karim SS: Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. The Journal of infectious diseases 2012;206:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarvis GA, Chang TL: Modulation of HIV transmission by Neisseria gonorrhoeae: molecular and immunological aspects. Curr HIV Res 2012;10:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards JL, Apicella MA: The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev 2004;17:965–981, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fichorova RN, Desai PJ, Gibson FC 3rd, Genco CA: Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun 2001;69:5840–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedges SR, Sibley DA, Mayo MS, Hook EW 3rd, Russell MW: Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. The Journal of infectious diseases 1998;178:742–751. [DOI] [PubMed] [Google Scholar]
- 15.Dobson-Belaire WN, Cochrane A, Ostrowski MA, Gray-Owen SD: Differential response of primary and immortalized CD4+ T cells to Neisseria gonorrhoeae-induced cytokines determines the effect on HIV-1 replication. PLoS One 2011;6:e18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh M, Shen Z, Fahey JV, Crist SG, Patel M, Smith JM, Wira CR: Pathogen recognition in the human female reproductive tract: expression of intracellular cytosolic sensors NOD1, NOD2, RIG-1, and MDA5 and response to HIV-1 and Neisseria gonorrhea. Am J Reprod Immunol 2013;69:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine WC, Pope V, Bhoomkar A, Tambe P, Lewis JS, Zaidi AA, Farshy CE, Mitchell S, Talkington DF: Increase in endocervical CD4 lymphocytes among women with nonulcerative sexually transmitted diseases. The Journal of infectious diseases 1998;177:167–174. [DOI] [PubMed] [Google Scholar]
- 18.Ding J, Rapista A, Teleshova N, Mosoyan G, Jarvis GA, Klotman ME, Chang TL: Neisseria gonorrhoeae enhances HIV-1 infection of primary resting CD4+ T cells through TLR2 activation. J Immunol 2010;184:2814–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Tirado C, Maisey K, Rodriguez FE, Reyes-Cerpa S, Reyes-Lopez FE, Imarai M: Neisseria gonorrhoeae induced disruption of cell junction complexes in epithelial cells of the human genital tract. Microbes Infect 2012;14:290–300. [DOI] [PubMed] [Google Scholar]
- 20.Malott RJ, Keller BO, Gaudet RG, McCaw SE, Lai CC, Dobson-Belaire WN, Hobbs JL, St Michael F, Cox AD, Moraes TF, Gray-Owen SD: Neisseria gonorrhoeae-derived heptose elicits an innate immune response and drives HIV-1 expression. Proc Natl Acad Sci U S A 2013;110:10234–10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Li G, Bafica A, Pantelic M, Zhang P, Broxmeyer H, Liu Y, Wetzler L, He JJ, Chen T: Neisseria gonorrhoeae enhances infection of dendritic cells by HIV type 1. J Immunol 2005;174:7995–8002. [DOI] [PubMed] [Google Scholar]
- 22.Simpson SD, Ho Y, Rice PA, Wetzler LM: T lymphocyte response to Neisseria gonorrhoeae porin in individuals with mucosal gonococcal infections. The Journal of infectious diseases 1999;180:762–773. [DOI] [PubMed] [Google Scholar]
- 23.Chen A, Boulton IC, Pongoski J, Cochrane A, Gray-Owen SD: Induction of HIV-1 long terminal repeat-mediated transcription by Neisseria gonorrhoeae. AIDS 2003;17:625–628. [DOI] [PubMed] [Google Scholar]
- 24.Shattock RJ, Moore JP: Inhibiting sexual transmission of HIV-1 infection. Nature reviews Microbiology 2003;1:25–34. [DOI] [PubMed] [Google Scholar]
- 25.Miller CJ, Shattock RJ: Target cells in vaginal HIV transmission. Microbes Infect 2003;5:59–67. [DOI] [PubMed] [Google Scholar]
- 26.Hladik F, Hope TJ: HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep 2009;6:20–28. [DOI] [PubMed] [Google Scholar]
- 27.Ray P, Yang L, Zhang DH, Ghosh SK, Ray A: Selective up-regulation of cytokine-induced RANTES gene expression in lung epithelial cells by overexpression of IkappaBR. J Biol Chem 1997;272:20191–20197. [DOI] [PubMed] [Google Scholar]
- 28.Mattsby-Baltzer I, Platz-Christensen JJ, Hosseini N, Rosen P: IL-1beta, IL-6, TNFalpha, fetal fibronectin, and endotoxin in the lower genital tract of pregnant women with bacterial vaginosis. Acta Obstet Gynecol Scand 1998;77:701–706. [PubMed] [Google Scholar]
- 29.Haase AT: Targeting early infection to prevent HIV-1 mucosal transmission. Nature 2010;464:217–223. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT: Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009;458:1034–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng LK, Martin IE: The laboratory diagnosis of Neisseria gonorrhoeae. Can J Infect Dis Med Microbiol 2005;16:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeVan A, Zimmerman LI, Mahle AC, Swanson KV, DeShong P, Park J, Edwards VL, Song W, Stein DC: Construction and characterization of a derivative of Neisseria gonorrhoeae strain MS11 devoid of all opa genes. J Bacteriol 2012;194:6468–6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta P, Collins KB, Ratner D, Watkins S, Naus GJ, Landers DV, Patterson BK: Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol 2002;76:9868–9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Rinaldo C, Gupta P: A semiquantitative assay for CD8+ T-cell-mediated suppression of human immunodeficiency virus type 1 infection. Clin Diagn Lab Immunol 1997;4:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Dampf D, Chen M, Kulka K, Volsky DJ, Saha K, Gupta P: Dependence of CD8+ T-cell-mediated suppression of HIV type 1 on viral phenotypes and mediation of phenotype-dependent suppression by viral envelope gene and not by beta-chemokines. AIDS Res Hum Retroviruses 2000;16:117–124. [DOI] [PubMed] [Google Scholar]
- 36.Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P: Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med 2000;6:475–479. [DOI] [PubMed] [Google Scholar]
- 37.Soto-Rivera J, Patterson BK, Chen Y, Shen C, Ratner D, Ding M, Tumne A, Gupta P: Study of HIV-1 transmission across cervical mucosa to tonsil tissue cells using an organ culture. Am J Reprod Immunol 2013;69:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta P, Ratner D, Ding M, Patterson B, Rohan LC, Reinhart TA, Ayyavoo V, Huang X, Patton DL, Ramratnam B, Cole AM: Retrocyclin RC-101 blocks HIV-1 transmission across cervical mucosa in an organ culture. J Acquir Immune Defic Syndr 2012;60:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fallert BA, Reinhart TA: Improved detection of simian immunodeficiency virus RNA by in situ hybridization in fixed tissue sections: combined effects of temperatures for tissue fixation and probe hybridization. J Virol Methods 2002;99:23–32. [DOI] [PubMed] [Google Scholar]
- 40.Qin S, Fallert Junecko BA, Trichel AM, Tarwater PM, Murphey-Corb MA, Kirschner DE, Reinhart TA: Simian immunodeficiency virus infection alters chemokine networks in lung tissues of cynomolgus macaques: association with Pneumocystis carinii infection. Am J Pathol 2010;177:1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin S, Junecko BA, Lucero CM, Klamar CR, Trichel AM, Murphey-Corb MA, Tarwater PM, Kirschner DE, Reinhart TA: Simian immunodeficiency virus infection potently modulates chemokine networks and immune environments in hilar lymph nodes of cynomolgus macaques. J Acquir Immune Defic Syndr 2013;63:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berendam SJ, Fallert Junecko BA, Murphey-Corb MA, Fuller DH, Reinhart TA: Isolation, characterization, and functional analysis of ferret lymphatic endothelial cells. Vet Immunol Immunopathol 2015;163:134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davina JH, Stadhouders AM, Lamers GE, van Haelst UJ, Kenemans P: Surface pattern differentiation of the epithelial cells of the human uterine ectocervix. Scan Electron Microsc 1981:37–48. [PubMed] [Google Scholar]
- 44.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ: Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol 2000;74:5577–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramsey KH, Schneider H, Cross AS, Boslego JW, Hoover DL, Staley TL, Kuschner RA, Deal CD: Inflammatory cytokines produced in response to experimental human gonorrhea. The Journal of infectious diseases 1995;172:186–191. [DOI] [PubMed] [Google Scholar]
- 46.Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR: Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. J Immunol 2002;168:2424–2432. [DOI] [PubMed] [Google Scholar]
- 47.de Jong MA, de Witte L, Oudhoff MJ, Gringhuis SI, Gallay P, Geijtenbeek TB: TNF-alpha and TLR agonists increase susceptibility to HIV-1 transmission by human Langerhans cells ex vivo. J Clin Invest 2008;118:3440–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cannon P, Kim SH, Ulich C, Kim S: Analysis of Tat function in human immunodeficiency virus type 1-infected low-level-expression cell lines U1 and ACH-2. J Virol 1994;68:1993–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plant LJ, Jonsson AB: Type IV pili of Neisseria gonorrhoeae influence the activation of human CD4+ T cells. Infect Immun 2006;74:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sankapal S, Gupta P, Ratner D, Ding M, Shen C, Sanyal A, Stolz D, Cu-Uvin S, Ramratnam B, Chen Y: HIV Exposure to the Epithelia in Ectocervical and Colon Tissues Induces Inflammatory Cytokines Without Tight Junction Disruption. AIDS Res Hum Retroviruses 2016;32:1054–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stiles LN, Liu MT, Kane JA, Lane TE: CXCL10 and trafficking of virus-specific T cells during coronavirus-induced demyelination. Autoimmunity 2009;42:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoneyama H, Narumi S, Zhang Y, Murai M, Baggiolini M, Lanzavecchia A, Ichida T, Asakura H, Matsushima K: Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J Exp Med 2002;195:1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamagna C, Aurrand-Lions M, Imhof BA: Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol 2006;80:705–713. [DOI] [PubMed] [Google Scholar]
- 54.Kohyama T, Takizawa H, Kawasaki S, Akiyama N, Sato M, Ito K: Fourteen-member macrolides inhibit interleukin-8 release by human eosinophils from atopic donors. Antimicrob Agents Chemother 1999;43:907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hosokawa Y, Hosokawa I, Ozaki K, Nakanishi T, Nakae H, Matsuo T: Catechins inhibit CXCL10 production from oncostatin M-stimulated human gingival fibroblasts. J Nutr Biochem 2010;21:659–664. [DOI] [PubMed] [Google Scholar]
- 56.Hosokawa Y, Hosokawa I, Shindo S, Ozaki K, Nakanishi T, Nakae H, Matsuo T: Black tea polyphenol inhibits CXCL10 production in oncostatin M-stimulated human gingival fibroblasts. Int Immunopharmacol 2011;11:670–674. [DOI] [PubMed] [Google Scholar]
- 57.Yeruva S, Ramadori G, Raddatz D: NF-kappaB-dependent synergistic regulation of CXCL10 gene expression by IL-1beta and IFN-gamma in human intestinal epithelial cell lines. Int J Colorectal Dis 2008;23:305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sunil Y, Ramadori G, Raddatzc D: Influence of NFkappaB inhibitors on IL-1beta-induced chemokine CXCL8 and −10 expression levels in intestinal epithelial cell lines: glucocorticoid ineffectiveness and paradoxical effect of PDTC. Int J Colorectal Dis 2010;25:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahapatra DK, Bharti SK, Asati V: Chalcone Derivatives: Anti-inflammatory Potential and Molecular Targets Perspectives. Curr Top Med Chem 2017;17:3146–3169. [DOI] [PubMed] [Google Scholar]
- 60.Yeaman GR, Howell AL, Weldon S, Demian DJ, Collins JE, O’Connell DM, Asin SN, Wira CR, Fanger MW: Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology 2003;109:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hladik F, McElrath MJ: Setting the stage: host invasion by HIV. Nat Rev Immunol 2008;8:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu SX, Leontyev D, Kaul R, Gray-Owen SD: Neisseria gonorrhoeae co-infection exacerbates vaginal HIV shedding without affecting systemic viral loads in human CD34+ engrafted mice. PLoS One 2018;13:e0191672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.