Abstract
Background:
Genetic variation at chromosome 9p21 is a recognized risk factor for coronary heart disease (CHD). However, its effect on disease progression and subsequent events is unclear, raising questions about its value for stratification of residual risk.
Methods:
A variant at chromosome 9p21 (rs1333049) was tested for association with subsequent events during follow-up in 103,357 Europeans with established CHD at baseline from the GENIUS-CHD Consortium (73.1% male, mean age 62.9 years). The primary outcome, subsequent CHD death or myocardial infarction (CHD death/MI), occurred in 13,040 of the 93,115 participants with available outcome data. Effect estimates were compared to case/control risk obtained from CARDIoGRAMPlusC4D including 47,222 CHD cases and 122,264 controls free of CHD.
Results:
Meta-analyses revealed no significant association between chromosome 9p21 and the primary outcome of CHD death/MI among those with established CHD at baseline (GENIUS-CHD OR 1.02; 95% CI 0.99–1.05). This contrasted with a strong association in CARDIoGRAMPlusC4D OR 1.20; 95% CI 1.18–1.22; p for interaction <0.001 compared to the GENIUS-CHD estimate. Similarly, no clear associations were identified for additional subsequent outcomes, including all-cause death, although we found a modest positive association between chromosome 9p21 and subsequent revascularization (OR 1.07; 95% CI 1.04–1.09).
Conclusions:
In contrast to studies comparing individuals with CHD to disease free controls, we found no clear association between genetic variation at chromosome 9p21 and risk of subsequent acute CHD events when all individuals had CHD at baseline. However, the association with subsequent revascularization may support the postulated mechanism of chromosome 9p21 for promoting atheroma development.
Journal Subject Terms: Genetic, Association Studies; Coronary Artery Disease; Secondary Prevention; Epidemiology; Risk Factors
Keywords: chromosome 9p21; genetics, association studies; residual risk; prognosis; myocardial infarction; recurrent event; secondary prevention
Introduction
Using a case-control approach, a large number of common genetic variants have now been associated with coronary heart disease (CHD) through genome-wide association studies, in an effort largely led by the CARDIoGRAMPlusC4D consortium.1 Among these variants, the chromosome 9p21 locus was the first to be discovered and the variant with the largest individual effect, and is the most widely replicated genetic risk factor for CHD.2–4 Multiple studies including case-control and prospective cohort studies in general populations have reliably confirmed its effect on risk of CHD among otherwise healthy individuals.5
However, it is uncertain whether variants at the 9p21 locus also affect risk of recurrent or subsequent events, including mortality in those with established CHD. Elucidation of this hypothesis would help to better understand its mechanism and estimate its incremental value for stratification of residual risk. Prior studies have shown conflicting results, although most have been underpowered. A literature based meta-analysis indicated a null association of chromosome 9p21 variants with subsequent CHD events but was based on summary, not individual level data, with varying outcome definitions.6, 7
The new collaborative GENetIcs of sUbSequent Coronary Heart Disease (GENIUS-CHD) consortium, described in this issue of the journal was established to investigate genetic determinants of disease progression following an index CHD event.8
In this paper, we use the GENIUS-CHD resource to: (1) examine the association of variants at the 9p21 locus on risk of subsequent CHD events in individuals with established CHD; (2) compare these to the association between chromosome 9p21 and any CHD observed in the CARDIoGRAMPlusC4D consortium; and, (3) explore the potential impact on these estimates of biases that might affect genetic association studies of disease outcome and prognosis.
Methods
In accordance with Transparency and Openness Promotion (TOP) Guidelines, the data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure. Participating studies received local institutional review board approval and included patients who had provided informed consent at the time of enrolment. The central analysis sites also received waivers from their local institutional review board for collating and analysing summary level data from these individual studies. Details about the GENIUS-CHD consortium and study inclusion criteria have been published separately in this issue of the journal,8 while for this study full details about data sources, genetic variant selection, outcomes and statistical analyses are available in the Supplementary Material.
Results
In total, 49 studies from the GENIUS-CHD consortium contributed to the federated analysis resulting in a sample size of 103,357 individuals of European descent with established CHD and available genotype data at the 9p21 locus. Of these, 93,115 individuals had available data for the primary composite outcome of subsequent CHD death/MI, of whom 13,040 experienced these events. Contributing study details are provided in Table 1. Participant characteristics are representative for populations with established CHD with a weighted mean age of 62.9 years; 73.1% male. As expected, risk factor prevalence was high in this population, including diabetes (24.4%), hypertension (59.1%) and current smoking (25.7%). Statin use at enrollment varied by study, ranging from 5.2%−97.3%, with a median of 61.5% (Table 1).
Table 1:
Overview of studies contributing to chromosome 9p21 analysis and participant characteristics
| Alias | Cohort | Total N genotyped | Study Design | CHD Type | Male (%) | Age (SD) | BMI (SD) | Diabetes (%) | Smoking (%) | Systolic BP (SD) | Total Cholesterol (SD) | Statin use (%) | Creatitine (SD) | Prior Revasc (%) | Prior MI (%) | PubMED ID |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4C | Clinical Cohorts in Coronary disease Collaboration (4C) | 1538 | Cohort | CAD | 62.1 | 62.2 (11.95) | 30.2 (5.67) | 23.4 | - | 133.9 (23.7) | 4.69 (1.10) | 26.4 | 99.3 (83.2) | 22.6 | 15.5 | - |
| AGNES | Arrhythmia Genetics in The Netherlands | 1316 | Cohort | ACS | 79.3 | 57.7 (10.81) | 26.5 (3.87) | 7.6 | 59.3 | - | 5.28 (1.04) | 9.8 | - | - | - | 20622880 |
| ANGES | Angiography and Genes Study | 588 | Cohort | Mixed | 65.5 | 64.1 (9.55) | 28.1 (4.36) | 30.8 | 14.7 | - | 4.84 (0.84) | 69.4 | 83.0 (32.0) | 42.4 | 24.7 | 21640993 |
| ATVB | Italian Atherosclerosis, Thrombosis and Vascular Biology Group | 1465 | Cohort | ACS | 90.4 | 40.0 (4.40) | 26.8 (4.07) | 8.4 | 78.7 | 132.3 (20.6) | 5.76 (1.39) | 56.2 | - | - | - | 21757122 |
| CAB Genomics | Coronary Artery Bypass Genomics | 1542 | Cohort | Mixed | 80.1 | 64.7 (10.08) | 29.7 (5.71) | 10.1 | 11.2 | - | 4.21 (0.95) | 75.2 | - | - | 42.8 | 25649697 |
| CDCS | Coronary Disease Cohort Study | 1800 | Cohort | ACS | 71.5 | 67.5 (11.96) | 27.3 (4.66) | 15.4 | 5.8 | 129.2(21.6) | 5.00(1.09) | 46.5 | 100.5 (40.0) | 26.9 | 30.3 | 20400779 |
| COROGENE | Corogene Study | 1489 | Cohort | ACS | 70.9 | 64.7 (11.87) | 27.6 (4.84) | 18.2 | 34.4 | - | 4.63 (0.99) | 5.2 | 84.0 (44.3) | - | - | 21642350 |
| CTMM | Circulating Cells | 605 | Cohort | Mixed | 68.9 | 63.0 (9.83) | 27.6 (4.45) | 20.7 | 20.7 | 135.4 (19.1) | 4.43(1.05) | - | 86.4 (34.9) | - | 30.1 | 23975238 |
| CURE | Cure-Genetics Study | 4242 | RCT | ACS | 59.3 | 64.7 (10.99) | 27.9 (4.44) | 19.9 | 22.6 | 135.7(21.9) | - | - | 93.0 (33.9) | 13.9 | 31.8 | 11102254 |
| EGCUT | Estonian Biobank | 2408 | Cohort | CAD | 51.0 | 67.1 (10.88) | 28.9(5.16) | 18.7 | 19.2 | 135.6 (18.0) | 5.64(1.17) | 27.3 | - | 15.7 | 36.0 | 24518929 |
| EMORY | Emory Cardiovascular Biobank | 2411 | Cohort | Mixed | 70.1 | 64.5 (11.06) | - | 30.7 | 9.8 | - | 4.49(1.02) | 76.0 | 99.0 (45.1) | 61.7 | 27.9 | 20729229 |
| ERICO | Estrategia de Registro de Insuficiencia Coronariana | 438 | Cohort | ACS | 55.5 | 63.8 (13.36) | 27.0 (5.06) | 39.1 | 31.0 | 99.2 (38.4) | - | 23.8 | - | 11.3 | 25.9 | 23644870 |
| FINCAVAS | Finnish Cardiovascular Study | 1671 | Cohort | Mixed | 69.4 | 60.9 (11.03) | 27.8 (4.35) | 18.4 | 24.3 | 140.2 (22.1) | 4.74 (0.90) | 57.3 | 90.8 (66.8) | 32.6 | 39.0 | 16515696 |
| FRISCH | FRISCH Study | 3106 | RCT | ACS | 69.4 | 66.2 (9.80) | 26.8 (3.87) | 12.7 | 27.1 | 143.3(22.4) | 5.80(1.12) | 12.3 | 90.6 (18.8) | 12.1 | 27.2 | 10475181 |
| GENDEMIP | GENetic DEtermination of Myocardial Infarction in Prague | 1267 | Cohort | ACS | 75.8 | 56.4 (8.63) | 28.6 (4.68) | 18.8 | 60.8 | 137.0 (20.8) | 5.51 (1.17) | 16.6 | - | 29.7 | 41.6 | 23249639 |
| GENEBANK | Cleveland Clinic Genebank Study | 2345 | Cohort | Mixed | 74.3 | 61.5 (11.06) | 29.4 (5.44) | 11.8 | 16.8 | 132.7(21.1) | 4.46 (0.93) | 71.8 | - | 65.3 | 56.1 | 21475195 |
| GENES IS-PRAXY | GENdEr and Sex determinants of cardiovascular disease: From bench to beyond-Premature Acute Coronary Syndrome (GENE SI S-PRAXY) | 784 | Cohort | ACS | 69.2 | 48.3 (5.62) | - | 13.8 | 44.2 | 139.5 (26.5) | 4.85(1.18) | 93.1 | 75.9 (19.7) | 11.3 | 11.4 | 22607849 |
| GENOCOR | Genetic Mapping for Assessment of Cardiovascular Risk | 497 | Cohort | Mixed | 86.7 | 65.2 (8.45) | - | 13.3 | 64.4 | 129.5 (20.3) | 4.70 (0.92) | 72.1 | 94.8 (27.2) | 13.7 | 63.2 | 22717531 |
| GoDARTS incident | Genetics of Diabetes Audit and Research in Tayside Scotland (I) | 1003 | Cohort | CAD | 62.0 | 71.1 (10.62) | 29.7 (5.64) | 77.9 | - | 126.7 (NA) | 4.62 (1.02) | 50.8 | 108.0 (64.6) | 0.2 | 1.3 | - |
| GoDARTS prevalent | Genetics of Diabetes Audit and Research in Tayside Scotland (P) | 2000 | Cohort | CAD | 66.5 | 69.1 (9.20) | 30.3 (5.43) | 77.8 | 14.9 | 136.2 (19.7) | 4.37 (0.83) | 66.8 | 101.6(34.5) | 31.4 | 48.9 | - |
| GRACE_B | Global Registry of Acute Coronary Events - Belgium | 699 | Cohort | ACS | 75.4 | 65.7 (12.01) | 27.0 (4.35) | 81.3 | 49.9 | 138.8 (25.3) | 5.33 (1.19) | 79.3 | 102.8(61.9) | - | 80.1 | 20231156 |
| GRACE_UK | Global Registry of Acute Coronary Events - UK | 1086 | Cohort | ACS | 69.1 | 64.4 (12.04) | 28.0 (5.15) | 15.0 | 69.5 | 137.8 (27.1) | 5.19 (1.29) | 16.8 | 105.0 (40.0) | 20.4 | 32.0 | 20231156 |
| IDEAL | Incremental Decrease in End Points Through Aggressive lipid Lowering (IDEAL) | 6223 | RCT | ACS | 81.8 | 61.2 (9.32) | 27.4 (3.80) | 11.4 | 20.3 | 136.8 (19.8) | 5.03 (0.98) | 76.9 | 100.1 (16.7) | 41.3 | - | 16287954 |
| INTERMOUNTAIN | Intermountain Heart Collaborative Study | 6763 | Cohort | Mixed | 66.7 | 61.2 (11.05) | 29.5 (6.08) | 20.3 | 10.2 | 141.8 (24.4) | 4.96 (1.12) | 38.7 | 99.6 (66.6) | - | 6.6 | 20691829 |
| INVEST | International Verapamil SR Trandolopril Study Genetic SubstudyNVEST-GENES | 2145 | RCT | CAD | 56.6 | 68.7 (9.38) | - | 23.9 | 12.8 | 148.6 (18.1) | - | 52.8 | - | 47.6 | - | 21372283 |
| JUMC | Krakow-GENIUS-CHD | 704 | Cohort | Mixed | 71.6 | 68.3 (10.25) | 26.3 (4.46) | 36.9 | 27.5 | 148.1 (23.8) | 5.02 (1.06) | 88.3 | 89.9 (37.5) | 50.1 | 39.7 | 28444280 |
| KAROLA | Karola Study | 1147 | Cohort | Mixed | 84.6 | 58.6 (8.13) | 27.0 (3.26) | 18.5 | 32.4 | 119.9 (15.5) | 4.46 (0.84) | 77.4 | 82.4 (26.5) | 42.8 | 21.6 | 24829374 |
| LIFE-Heart | Leipzig (LIFE) Heart Study | 4330 | Cohort | Mixed | 75.5 | 64.0 (11.15) | 29.0 (4.68) | 34.4 | 29.0 | 138.3 (21.8) | 5.24 (1.18) | 38.9 | 87.3 (34.6) | - | 0.1 | 22216169 |
| LURIC | The LUdwigshafen RIsk and Cardiovascular Health Study | 2175 | Cohort | Mixed | 76.5 | 63.8 (9.85) | 27.5 (3.89) | 44.3 | 23.9 | 142.3 (24.1) | 4.94 (0.99) | 58.9 | 88.7 (38.5) | 48.7 | 57.4 | 11258203 |
| NE_POLAND | North East Poland Myocardial Infarction Study | 603 | Cohort | ACS | 75.0 | 62.4 (11.86) | 24.8 (3.79) | 22.2 | 48.1 | 138.9 (27.4) | 5.04 (1.05) | 80.7 | 91.6 (36.3) | 1.3 | 10.6 | 26086777 |
| NEAPOLIS | Neapolis Campania Italia | 1380 | Cohort | Mixed | 74.4 | 67.6 (10.49) | 28.0 (4.18) | 43.0 | 26.8 | 129.4 (14.2) | 4.57 (1.02) | 82.5 | 101.0 (68.1) | 41.5 | 40.8 | 24262617 |
| OHGS | Ottawa Heart Genomics Study | 393 | Cohort | Mixed | 73.0 | 65.3 (11.07) | 28.6 (5.00) | 6.9 | 19.5 | 131.9 (19.0) | 5.53 (1.03) | 92.4 | 89.8 (21.1) | 28.2 | 21.9 | - |
| PLATO | The Study of Platelet Inhibition and Patient Outcomes | 9814 | RCT | ACS | 69.5 | 62.6 (10.95) | 28.2 (4.51) | 22.8 | 35.2 | 135.6 (21.8) | 5.42 (1.23) | 79.7 | 85.6 (26.3) | 15.1 | 20.6 | 19332184 |
| PMI | Post Myocardial Infarction Study | 783 | Cohort | ACS | 78.3 | 62.7 (10.29) | 26.5 (3.82) | 12.0 | 28.2 | 117.0 (15.6) | 5.98 (1.19) | 46.0 | 87.8 (27.8) | - | 17.2 | 12771003 |
| POPular | The POPular study | 997 | RCT | ACS | 74.3 | 63.8 (10.40) | - | 18.9 | 27.5 | 145.0 (22.1) | 4.25 (0.64) | 80.7 | 92.7 (26.8) | 33.1 | 43.7 | 26542508 |
| PROSPER | Prospective Study of Pravastatin in the Elderly at Risk | 439 | RCT | CAD | 69.9 | 75.4 (3.31) | 26.4 (3.87) | 10.3 | 16.2 | 150.0 (21.6) | 5.55 (0.83) | - | 109.4 (23.2) | 26.0 | 85.9 | 10569329 |
| RISCA | Recurrance and Inflammation in the Acute Coronary Syndromes Study | 1052 | Cohort | ACS | 75.9 | 61.9 (11.40) | 27.2 (4.43) | 19.8 | 30.4 | - | - | 46.6 | 100.6 (28.6) | 28.2 | 27.9 | 18549920 |
| SHEEP | Stockholm Heart Epidemiology Program (SHEEP) | 1150 | Cohort | ACS | 70.7 | 59.3 (7.21) | 26.8 (4.02) | 18.2 | 50.0 | 131.8 (20.6) | 6.28 (1.16) | - | - | - | - | 17667644 |
| SMART | Second Manifestations of Arterial Disease | 2485 | Cohort | Mixed | 82.2 | 60.2 (9.26) | 27.3 (3.63) | 16.6 | 24.4 | 137.4 (19.8) | 4.73 (0.96) | 75.7 | 92.3 (22.7) | - | 43.6 | 10468526 |
| STABILITY | Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy trial | 9287 | RCT | Mixed | 82.0 | 64.7 (9.10) | 29.9 (4.97) | 38.4 | 21.4 | 131.7 (16.1) | - | 97.3 | - | 74.6 | 58.6 | 24678955 |
| THI | Texgen | 2729 | Cohort | ACS | 75.3 | 63.6 (10.62) | 29.6 (5.59) | 30.5 | 21.3 | - | - | 57.1 | - | 21.5 | 16.7 | 21414601 |
| TNT | Treating to New T argets | 5104 | RCT | CAD | 81.3 | 61.3 (8.73) | 28.6 (4.59) | 14.8 | 13.4 | 130.9 (16.8) | 4.51 (0.61) | 70.3 | 104.8 (17.3) | - | 57.3 | 15755765 |
| TRIUMPH | Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patient’s Health Status | 1974 | Cohort | ACS | 72.4 | 59.8 (12.04) | 29.5 (5.96) | 28.6 | 37.4 | 117.8 (18.3) | - | 89.0 | 112.5 (76.7) | 27.0 | 18.3 | 21772003 |
| UCORBIO | Utrecht Coronary Biobank | 1073 | Cohort | Mixed | 75.6 | 65.4 (10.26) | 27.2 (4.34) | 21.5 | 23.1 | - | 4.76(1.18) | 64.0 | 91.9(42.9) | - | 28.9 | - |
| UCP | Utrecht Cardiovascular Pharmacogenetics Study | 1500 | Cohort | Mixed | 75.4 | 64.1 (9.96) | - | - | - | 153.4(21.4) | 5.50 (1.10) | 27.1 | 94.7 (24.8) | - | - | 25652526 |
| VHS | Verona Heart Study | 907 | Cohort | CAD | 80.9 | 61.3(9.78) | 26.9 (3.57) | 18.5 | 69.2 | - | 5.37(1.10) | 47.1 | 96.7 (32.2) | 17.4 | 59.6 | 10984565 |
| VIVIT | Vorarlberg Institute for Vascular Investigation and Treatment Study | 1318 | Cohort | CAD | 73.1 | 64.5 (10.44) | 27.4(4.14) | 30.6 | 18.9 | 137.2 (19.2) | 5.45(1.14) | 49.7 | 88.4 (32.7) | 21.1 | 31.0 | 24265174 |
| WARSAW ACS | Warsaw ACS Genetic Registry | 669 | Cohort | ACS | 74.5 | 63.6 (11.72) | 28.1 (4.72) | 21.9 | 42.0 | 127.8 (22.6) | 4.99 (1.07) | - | 93.5(41.4) | - | 18.6 | - |
| WTCCC | WTCCC CAD Study | 1924 | Cohort | Mixed | 79.3 | 60.0(8.13) | 27.6 (4.20) | 11.7 | 12.8 | 143.6 (22.0) | 5.28 (0.98) | 71.6 | - | 67.1 | 72.0 | 17634449 |
Overview of studies contributing to chromosome 9p21 analysis and participant characteristics; alias denotes the abbreviated name of each study used in figures and tables; ACS = acute coronary syndrome, CAD = coronary artery disease; PubMed IDs are provided for individual study descriptions; mean (standard deviation) with proportions (%) are provided unless otherwise stated.
The rs1333049 single nucleotide polymorphism (SNP) was genotyped in 42 studies, with the remaining 7 studies using highly correlated proxies (R-squared > 0.90); rs10757278 (4 studies) or rs4977574 (3 studies) when the primary SNP was unavailable. Genotyping details are provided in Supplementary Table 1. For rs1333049, the average risk allele frequency across the participating studies was 0.518 ranging from 0.453 to 0.587 (Supplementary Figure 1).
From CARDIOGRAMplusC4D, after excluding 6 cohorts which had contributed data to both consortia, data were available for association with chromosome 9p21 from 41 studies, including 47,222 cases with CHD and 122,264 controls free of any CHD. Power to detect different effect sizes, including the effect size identified in CARDIoGRAMplusC4D, using a two-sided alpha of 0.05, are provided in Supplementary Table 2
Chromosome 9p21 Association with Subsequent CHD Events
Study-specific results for the association between chromosome 9p21 and risk of the primary outcome of CHD death or myocardial infarction among individuals with established CHD at baseline, adjusted for age and sex are presented in Supplementary Figure 2.
The per-allele odds ratio for the primary outcome during follow-up was 1.02 (95%CI 0.99–1.05). The effect estimate again for the primary outcome, based on a time to event analysis and using a Cox regression model, was also similar with a HR of 1.02 (95%CI 0.99–1.04, Supplementary Figure 3).
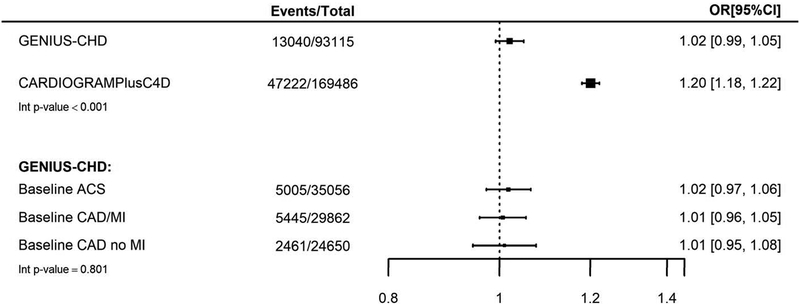
In contrast, a meta-analysis of CARDIOGRAMIplusC4D data (excluding studies also contributing data to GENIUS-CHD), revealed a per-allele odds ratio for a CHD event similar to that reported previously (OR 1.20; 95% CI 1.18–1.22). There was evidence of statistical heterogeneity between the estimates (Interaction p<0.001), Figure 1.
Figure 1:
Association between chromosome 9p21 and subsequent CHD events in all participants with baseline CHD (GENIUS-CHD) compared to association in CHD cases and CHD-free controls (CARDIoGRAMPlusC4D)
For the CARDIoGRAMPlusC4D meta-analysis estimate, 6 studies [LURIC, LIFE-Heart, GoDARTS, OHGS, PROSPER, WTCCC] were excluded as they were also included in GENIUS-CHD.
Estimates for GENIUS-CHD are also presented by subtype of CHD at baseline including acute coronary syndrome (ACS), stable coronary artery disease without prior MI (CAD/no MI) and Stable CAD with prior MI (CAD/MI). All estimates were adjusted for age and sex.
Subgroup Analyses
We found minimal evidence for heterogeneity in effect estimates when stratifying by CHD subtype at baseline (interaction p-value 0.801), with no clear evidence for an effect of chromosome 9p21 genetic variation on subsequent CHD death or MI in individuals enrolled with ACS (OR 1.02; 95% CI 0.97–1.06), those with coronary artery disease with a prior MI (OR 1.01; 95% CI 0.96–1.05) and those with coronary artery disease without prior MI (OR 1.01; 95% CI 0.95–1.08, Figure 1).
We further examined the effect of chromosome 9p21 on the primary outcome in prespecified subgroup analyses. We noted a borderline nominally significant interaction with sex, suggesting a greater risk among women with the chromosome 9p21 risk allele, for subsequent CHD death/MI (interaction p value = 0.04), while non-significant trends were noted for greater risk in those without hypertension (p value = 0.08) or without renal impairment (p value = 0.17). There were minimal differences in effect estimates by other patient level characteristics including age and diabetes, or according to statin or anti-platelet use or LV impairment at baseline (Supplementary Figure 4).
Similarly, when stratified by study level features, we observed minimal evidence for heterogeneity in effect estimates by study size, geographical region, study design or length of follow up (Supplementary Figure 5). However, when ordered by date of first enrollment, there was no evidence for variation in effect by time of enrollment (Supplementary Figure 2).
Secondary outcomes
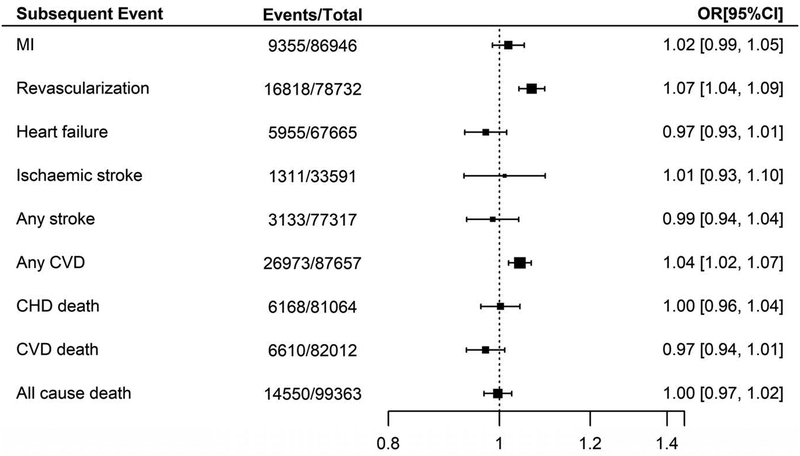
We additionally examined the association between chromosome 9p21 and other subsequent events available for this analysis within the GENIUS-CHD Consortium, listed in Supplementary Table 3, with summary estimates provided in Figure 2. Of note, the per-allele effect of risk variants at chromosome 9p21 on subsequent revascularization during follow up was 1.07 (95% CI 1.04–1.09). The effect on the composite outcome of any CVD, which includes revascularization, was also significant at 1.04 (95% CI 1.02–1.07). However, there was no clear evidence of association for the remaining secondary outcomes, with only a marginal trend to protection for both subsequent heart failure (OR 0.97; 95% CI 0.93–1.01) and CVD death (OR 0.97; 95% CI 0.94–1.01), as shown in Figure 2.
Figure 2:
Association between chromosome 9p21 and secondary outcomes in participants with baseline CHD, within GENIUS-CHD. All meta-analysis estimates were adjusted for age and sex.
Selection Bias
To explore the potential for index event bias we looked for differences in associations between chromosome 9p21 and known cardiovascular risk factors in the UKB, among the subset of participants with established CHD, compared to the full UKB cohort (Supplementary Table 4). While there were differences between the groups in the prevalence or values of the tested risk factors, we did not find clear evidence to indicate a distortion in associations between chromosome 9p21 and age, blood pressure, diabetes or smoking. There was, however, a small difference for BMI, with a greater statistical association between the chromosome 9p21 risk allele and lower BMI identified in those with established CHD than in the general population (nominal interaction p value 0.02, Supplementary Table 4).
We also observed that the chromosome 9p21 risk allele frequency in those surviving with CHD, both in UKB (0.529) and in GENIUS-CHD (0.518, Supplementary Figure 1), was higher than the general population in the UKB (0.481) and European reference populations from the 1000 Genomes (Phase 3),9 (0.472). This difference in frequency confirms the association of chromosome 9p21 with CHD and also indicated absence of a crude survival bias with loss of large numbers of risk allele carriers to fatal events prior to entry into CHD cohorts. We did however observe a trend to an age association in those with established CHD as well as the general population in the UKB, with lower chromosome 9p21 risk allele frequencies with advancing age, relative to younger carriers (Supplementary Figure 6).
Discussion
In this study, we examined the effect of genetic variation at the chromosome 9p21 locus on risk of subsequent events in 103,357 individuals with established CHD using the newly formed GENIUS-CHD consortium.8 We found that: (1) in contrast to the known strong association with CHD observed in CARDIoGRAMPlusC4D, there was a markedly attenuated and non-significant association with subsequent CHD events in GENIUS-CHD; (2) effect estimates in GENIUS-CHD were broadly consistent in stratified analyses based on features related to study design, patient characteristics and type of index CHD event and (3) exploratory analyses suggested that selection biases were unlikely to explain the discrepancy. However, we did find evidence of an association between these variants and a secondary outcome of future revascularization events. Our findings, taken together with those from others, support the view that chromosome 9p21 promotes CHD through progressive stable atheroma rather than through development of an unstable phenotype.
The chromosome 9p21 locus is the most widely-replicated genetic risk locus for CHD identified to date, with an estimated 15–35% increased risk in carriers of the variant allele in prospective population and case-control studies.5 However, studies examining the effect on subsequent CHD events in people with known CHD at baseline have reported conflicting results.10–14 Our group previously examined this in a literature-based meta-analysis, based on 15 studies with median sample size of 1,750 individuals, accruing 25,163 cases of established CHD, and reported no clear evidence of an effect of variants at chromosome 9p21 on the risk of subsequent events.15 An analysis by the CHARGE consortium of 2,953 MI survivors also reported no association with subsequent mortality.7 However, the limited size of most prior studies and the limitations of literature meta-analyses indicate that many possible explanations, including errors in risk allele coding and selection biases, could not be adequately explored, precluding meaningful interpretations for any mechanistic or clinical implications.
The emergence of the GENIUS-CHD Consortium has now permitted a robust evaluation of the role of chromosome 9p21 in subsequent CHD event risk, revealing a clear lack of association with a common composite coronary endpoint. This is in marked contrast to findings from studies comparing cases to CHD-free controls, as confirmed through meta-analysis of CARDIoGRAMPlusC4D data. Furthermore, we were able to add to previous findings by showing that the type of CHD at baseline, whether acute coronary syndrome or stable CHD with or without prior MI, does not alter this association. We also interrogated several widely-proposed explanations that could account for our findings through pre-specified subgroup analyses, and confirmed that most of these, specifically older age, medication use at baseline (statin or antiplatelet), study size or follow up duration, did not appreciably alter the association findings. Our finding of a possible interaction with sex, warrants further investigation, but should be considered hypothesis-generating given the borderline evidence of an interaction.
Selection bias (i.e., index event bias or collider-stratification bias) could potentially explain reversed or attenuated associations in disease progression studies like this, operating by inducing relationships between (otherwise independent) risk factors through the selection of individuals with disease.16, 17 Specifically, individuals surviving a first event consequent on exposure to a particularly strong risk factor may have lower levels of exposure to other individually weaker, independent risk factors, which can then attenuate the association of the risk factor of interest with subsequent events. However, the distribution of common risk factors by chromosome 9p21 genotype did not differ when compared between the general population and the subset with CHD in the UKB, using interaction tests. The only exception was for BMI, a potentially differential association with chromosome 9p21 in those with CHD compared to the general population was noted. However, the effect size was small in both populations and on its own is unlikely to indicate presence of substantial index event bias.
Selection bias may also theoretically occur by focussing on subjects surviving a first event, where chromosome 9p21 risk allele carriers at risk of fatal CHD events are lost prior to enrollment into CHD cohorts, thereby diluting the future impact of the variant on subsequent CHD events. In this scenario, we would expect a lower risk-allele frequency in those surviving CHD and entering CHD cohorts, but we found no evidence for this. Among those with CHD in the UKB, and among the whole UKB cohort, we did find a progressive loss of risk allele carriers with increasing age, consistent with prior findings of a greater association with CHD, among younger individuals in case-control studies.5 Given patients with CHD are generally older, it is possible that a subtle survival bias may still be influencing our findings, although all analyses were adjusted for age. However, based on simulation modelling, sample size and projected SNP effect size, we and others have previously estimated that selection biases are only minimally operating in this context and would be unlikely to account for our observed findings.18, 19 Although our findings potentially argue against important selection biases in the analysis for the primary outcome, they are relatively insensitive assessments and may not fully elucidate such biases.
Possible biological explanations could also exist for our findings. Pathological studies indicate differences between chronic stable atherosclerotic plaques that cause ischemia through progressive vessel occlusion and vulnerable plaques with thin caps, prone to sudden plaque rupture, unheralded MI and coronary deaths.20 In a seminal study dissecting the phenotype of CHD, a lack of effect for chromosome 9p21 and MI was noted, when both cases and controls had underlying atherosclerosis.21 Our group and others have in parallel shown that chromosome 9p21 robustly associates with atherosclerotic phenotypes,22 while functional studies have also implicated this region with molecular activity that drives atheroma.23 Furthermore, in this study we show that the only outcome positively associated with chromosome 9p21 is incident revascularization, perhaps reflecting more severe atherosclerosis burden. Collectively these data support the concept that chromosome 9p21 promotes progressive atheroma formation and does not confer risk via plaque rupture.
In this context, it is worth noting that chromosome 9p21 associates more robustly with CHD in case-control studies than in prospective cohort studies.7 The difference, as proposed by others, could hypothetically be accounted for by incidence–prevalence bias, with chromosome 9p21 carriers more likely to survive a CHD event and thus be over represented among CHD cases (the opposite to survival bias described above).7 This becomes more likely as stated above if chromosome 9p21 drives a more progressive and stable atheroma phenotype. If this holds true then among survivors with established CHD, one might expect that chromosome 9p21 carriers could hold a small favourable advantage over those who experience CHD in its absence, due instead to other more dangerous or vulnerable characteristics, and despite undergoing more subsequent revascularization, these chromosome 9p21 carriers do not experience more dangerous or fatal events.
These findings have important implications. Clinically, they indicate that a degree of caution should be applied when considering or evaluating patients for chromosome 9p21 in order to predict disease progression or residual risk. They also highlight the need to appreciate important biases that may inflate or attenuate association findings in the setting of subsequent events for individuals with established disease. Mechanistically, these findings support existing and emerging efforts seeking to elucidate the mechanism of the most robust genetic discovery for CHD in recent decades.
There are important limitations to consider. First, among individuals in GENIUS with established CHD, the timing of the first CHD event or age of onset was often unknown so we could not account for this variable in our analyses. However, the lack of association in the ACS studies, which had documented timing of the first event, suggests this did not impact the findings. Second, we had limited information on whether subsequent revascularization events were late staged procedures, which would count as part of the index CHD event or unplanned and symptom driven and thereby a true subsequent event, which may have diluted the effect estimate. Third, while we did not observe a specific interaction for statin or aspirin use, we cannot rule out an effect of combined or additional medication usage attenuating the association signal, given the high prevalence of secondary prevention drug use in this setting compared to general population cohorts. Fourth, our analyses were restricted to participants of European descent as most of the included studies only recruited these individuals and so we were markedly underpowered to explore associations in other ethnic groups. Unfortunately, this remains a wider problem of genetic research and global efforts are ongoing to address this imbalance. Finally, variability of follow-up duration across studies is an analytical challenge and could have impacted our findings, through misclassification. However, a sensitivity analysis stratifying on the follow-up duration of individual studies (<5 or 5≥ years) revealed minimal evidence (P=0.62) of heterogeneity in effect estimates (Supplemental Figure 5), suggesting that this is unlikely to have influenced our findings significantly as effect estimates were concordant across studies with different lengths of follow-up. Our major strengths however include the size of the study and the large number and types of subsequent events and an effort to examine for selection biases. We also sought to mitigate potential miscoding of the risk allele, given rs1333049 is a palindromic SNP, and also the risk allele C changes from being a minor allele in population cohorts to the major allele in CHD cohorts. Finally, this analysis benefitted from the collective expertise and input of over 170 investigators and analysts, many of whom have previously reported on chromosome 9p21.
In conclusion, using the newly formed GENIUS-CHD consortium, we demonstrate that variation at chromosome 9p21 shows no clear association with risk of subsequent CHD events when all individuals have established CHD at baseline. This is in marked contrast to prior case-control studies examining odds of CHD presence compared to disease-free controls. We could not account for the attenuation of effect in terms of selection biases or subgroup effects. However, we did find a greater risk for incident revascularization in those with established CHD and although residual bias may be at play, our findings collectively support the view that chromosome 9p21 promotes CHD through progressive stable atheroma rather than through development of an unstable phenotype.
Supplementary Material
Acknowledgments:
The GENIUS CHD collaborators would like to express their immense gratitude to all patients who participated in each of the individual studies as well as the many personnel who helped with recruitment, collection, curation, management and processing of the samples and data. We also thank the CARDIoGRAMPlusC4D steering committee for providing the summary data after excluding the cohorts already in GENIUS-CHD.
Sources of Funding: The funder(s) of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Within GENIUS-CHD, all participating investigators and sponsors who contributed data and analyses are acknowledged irrespective of academic or industry affiliations. Specific funding statements: Dr Patel is funded by a British Heart Foundation Intermediate Fellowship (FS/14/76/30933). This research was also supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.; Dr Schmidt is funded by BHF grant PG/18/5033837; Dr Holmes works in a unit that receives funding from the UK Medical Research Council and is supported by a British Heart Foundation Intermediate Clinical Research Fellowship (FS/18/23/33512) and the National Institute for Health Research Oxford Biomedical Research Centre.; The AGNES study was supported by research grants from the Netherlands Heart Foundation (2001D019, 2003T302, 2007B202 and the PREDICT project (CVON 2012–10)), the Leducq Foundation (grant 05-CVD) and the Center for Translational Molecular Medicine (CTMM COHFAR).; The Cleveland Clinic Genebank Study was supported in part by NIH grants R0133169, R01ES021801, R01MD010358, and R01ES025786, R01HL103866, R01DK106000, R01HL126827, P20HL113452., P01HL098055, P01HL076491, and R01HL103931; The Clinical Cohorts in Coronary disease Collaboration (4C) study was supported in part by NIHR and Barts Charity; The Corogene study was supported by grants from Aarno Koskelo Foundation, Helsinki University Central Hospital special government funds (EVO #TYH7215, #TKK2012005, #TYH2012209, #TYH2014312), and Finnish Foundation for Cardiovascular research; CABGenomics was supported by Stanton Shernan, C. David Collard, Amanda A. Fox/R01 HL 098601 NHLBI; The CDCS and PMI studies were funded by the Health Research Council and Heart Foundation of New Zealand; Dr Samman-Tahnan is supported by the National Institutes of Health/National Institutes of Aging grant AG051633; Dr Sandesara is supported by the Abraham J. & Phyllis Katz Foundation (Atlanta, GA, USA); The Emory Cardiovascular Biobank is supported by National Institutes of Health (NIH) grants 5P01HL101398–02, 1P20HL113451–01, 1R56HL126558–01, 1RF1AG051633–01, R01 NS064162–01, R01 HL89650–01, HL095479–01, 1U10HL110302–01, 1DP3DK094346–01, 2P01HL086773–06A1; This Estonian Biobank was funded by EU H2020 grant 692145, Estonian Research Council Grant IUT20–60, IUT24–6, PUT1660, PUT735 and European Union through the European Regional Development Fund Project No.2014–2020.4.01.15–0012 GENTRANSMED, NIH – GIANT, ERA-CVD grant Detectin-HF and 2R01DK075787–06A1.; GENESIS-PRAXY is funded by the Canadian Institutes of Health Research and Heart and Stroke Foundations of Alberta, NWT & Nunavut, British Columbia and Yukon, Nova Scotia, Ontario, and Quebec (HSFC); The GENDEMIP study was supported by Project (MH, Czech Republic) No. 00023001 (ICEM, Prague); GoDARTS was funded by the Wellcome Trust (072960/Z/03/Z, 084726/Z/08/Z, 084727/Z/08/Z, 085475/Z/08/Z, 085475/B/08/Z) and as part of the EU IMI-SUMMIT programme. C.N.A.P. has received grant funding from the Wellcome Trust to develop the GoDARTS cohort.; Dr Mordi is supported by an NHS Education of Scotland/Chief Scientist Office Postdoctoral Clinical Lectureship (PCL 17/07); the GENECOR stuidy was supported in part by the Italian Ministry of Research’s Fund for Basic Research (FIRB 2005); GRACE UK was supported in part by an Educational Grant from Sanofi Aventis; Award from Chief Scientist Office, Scotland; INVEST-GENES was supported by the National Institute of Health Pharmacogenomics Research Network grant U01-GM074492, NIH R01 HL074730, University of Florida Opportunity Fund, BASF Pharma and Abbott Laboratories.; IATVB was supported by Epidemiologia e Genetica della Morte Improvvisa in Sardegna; The KAROLA study has received financial support by the German Ministry of Education and Research (01GD9820/0 and 01ER0814), by the Willy-Robert-Pitzer Foundation, and by the Waldburg-Zeil Clinics Isny.; The KRAKOW GENIUS Study was supported by a grant from the Polish Ministry of Science and Higher Education, no. NN402083939 and the National Science Centre, no. 2013/09/B/NZ5/00770; LIFE-Heart was funded by the Leipzig Research Center for Civilization Diseases (LIFE). LIFE is an organizational unit affiliated to the Medical Faculty of the University of Leipzig. LIFE is funded by means of the European Union, by the European Regional Development Fund (ERDF) and by funds of the Free State of Saxony within the framework of the excellence initiative.; The LURIC study was supported by the 7th Framework Program (AtheroRemo, grant agreement number 201668 and RiskyCAD, grant agreement number 305739) of the European Union; The NEAPOLIS CAMPANIA study was suppported by European Research Council Advanced Grant (CardioEpigen, #294609);Italian Ministry of Health (PE-2013–02356818);Italian Ministry of Education, University and Research (2015583WMX); The North East Poland Myocardial Infarction Study was supported by grant N N 402 529139 from the National Science Center (Poland); Dr Vilmundarson is supported by a graduate fellowship of the University of Ottawa Heart Institute; OHGS was funded in part by a Heart and Stroke Foundation grant; Dr Stott was supported in part by an investgator initiated grant from Bristol Myers Squibb USA; The PROSPER study was supported by an investigator initiated grant obtained from Bristol-Myers Squibb. Prof. Dr. J. W. Jukema is an Established Clinical Investigator of the Netherlands Heart Foundation (grant 2001 D 032). Support for genotyping was provided by the seventh framework program of the European commission (grant 223004) and by the Netherlands Genomics Initiative (Netherlands Consortium for Healthy Aging grant 050–060-810).; The RISCA study ws supported in part by FRSQ, HSFC, Merck Frost Canada, Pfizer Canada; The SHEEP study was supported by grants from the Swedish Council for Work Life and Social Research, and the Stockholm County Council.; The TNT trial was sponsored by Pfizer who granted access to data, Genotyping of the samples was funded in part by grants from Genome Canada and Genome Quebec and the Canadian Institutes of Health Research (CIHR).; Dr Arsenault holds a junior scholar award from the Fonds de recherche du Quebec- Sante (FRQS); Dr. Cresci is supported, in part, by the National Institutes of Health (Cresci R01 NR013396). The TRIUMPH study was sponsored by the National Institutes of Health: Washington University School of Medicine SCCOR Grant P50 HL077113; The UCP studies were funded by the Netherlands Heart Foundation and the Dutch Top Institute Pharma Mondriaan Project; The Verona Heart Study was supported by the Cariverona Foundation; Veneto Region; Italian Ministry of Education, University, and Research (MIUR); LURM (Laboratorio Universitario di Ricerca Medica) Research Center, University of Verona; The Warsaw ACS Registry is supported by grant N R13 0001 06 from The National Centre for Research and Development (NCBiR), Statutory Grant from Medical University of Warsaw; Dr Nelson is funded by the British Heart Foundation; Prof. Samani is funded by the British Heart Foundation and is a NIHR Senior Investigator. Prof Hingorani is a NIHR Senior Investigator; Prof Asselbergs is supported by UCL Hospitals NIHR Biomedical Research Centre, EU/EFPIA Innovative Medicines Initiative 2 Joint Undertaking BigData@Heart grant n° 116074, the European Union’s Horizon 2020 research and innovation programme under the ERA-NET Co-fund action N°01KL1802 (Druggable-MI-gene) jointly funded by the Dutch Heart Foundation and Netherlands Organization for Health Research and Development (ZonMw).
Disclosures: Dr Patel has received speaker fees and honoraria from Amgen, Sanofi and Bayer and research grant funding from Regeneron; Dr Holmes has collaborated with Boehringer Ingelheim in research, and in accordance with the policy of The Clinical Trial Service Unit and Epidemiological Studies Unit (University of Oxford), did not accept any personal payment; Dr Akerblom has received institutional research grant and speakers fee from AstraZeneca, institutional research grant from Roche Diagnostics.; Dr James has received grants from AstraZeneca, The Medicines Company, Swedish heart and lung foundation, Swedish research council, Janssen; personal fees from Bayer.; Dr Hagstrom declares being an expert committee member, lecture fees, and institutional research grant from Sanofi, and Amgen; institutional research grants from AstraZeneca, and GlaxoSmithKline; expert committee member and lecture fees NovoNordisk and Behringer.; Dr Held declares institutional research grant, advisory board member and speaker’s bureau from AstraZeneca; institutional research grants from Bristol-Myers Squibb Merck & Co, GlaxoSmithKline, Roche Diagnostics. Advisory board for Bayer and Boehringer Ingelheim; Dr Lindholm has received institutional research grants from AstraZeneca, and GlaxoSmithKline; Speaker fees from AstraZeneca, Speaker fees from AstraZeneca; Dr Siegbahn has received institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Roche Diagnostics, GlaxoSmithKline.; Dr. J.M. ten Berg reports receiving fees for board membership from AstraZeneca, consulting fees from AstraZeneca, Eli Lilly, and Merck, and lecture fees from Daiichi Sankyo and Eli Lilly, AstraZeneca, Sanofi and Accumetrics; Prof Wallentin reports institutional research grants, consultancy fees, lecture fees, and travel support from Bristol-Myers Squibb/Pfizer, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim; institutional research grants from Merck & Co, Roche Diagnostics; consultancy fees from Abbott; and holds a patent EP2047275B1 licensed to Roche Diagnostics, and a patent US8951742B2 licensed to Roche Diagnostics; Dr Claes reports lecture fees, and an institutional research grant from Sanofi, and Amgen; institutional research grants from AstraZeneca, and GlaxoSmithKline; and lecture fees from NovoNordisk and AstraZeneca. Prof Asselbergs has received research funding from Regeneron, Pfizer, Sanofi.
References:
- 1.Nikpay M, et al. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helgadottir A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–3. [DOI] [PubMed] [Google Scholar]
- 3.McPherson R, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samani NJ, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palomaki GE, et al. Association between 9p21 genomic markers and heart disease: a meta-analysis. JAMA. 2010;303:648–56. [DOI] [PubMed] [Google Scholar]
- 6.Patel RS, et al. Genetic variants at chromosome 9p21 and risk of first versus subsequent coronary heart disease events: a systematic review and meta-analysis. J Am Coll Cardiol. 2014;63:2234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehghan A, et al. Genome-Wide Association Study for Incident Myocardial Infarction and Coronary Heart Disease in Prospective Cohort Studies: The CHARGE Consortium. PLoS One. 2016;11:e0144997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel RS, et al. Subsequent Event Risk in Individuals with Established Coronary Heart Disease: Design and Rationale of the GENIUS-CHD Consortium Circ Genom Precis Med. 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genomes Project C, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis KL, et al. A common variant at chromosome 9P21.3 is associated with age of onset of coronary disease but not subsequent mortality. Circ Cardiovasc Genet. 2010;3:286–93. [DOI] [PubMed] [Google Scholar]
- 11.Gong Y, et al. Chromosome 9p21 haplotypes and prognosis in white and black patients with coronary artery disease. Circ Cardiovasc Genet. 2011;4:169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horne BD, et al. Association of variation in the chromosome 9p21 locus with myocardial infarction versus chronic coronary artery disease. Circ Cardiovasc Genet. 2008;1:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virani SS, et al. Chromosome 9p21 single nucleotide polymorphisms are not associated with recurrent myocardial infarction in patients with established coronary artery disease. Circ J. 2012;76:950–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wauters E, et al. Influence of 23 coronary artery disease variants on recurrent myocardial infarction or cardiac death: the GRACE Genetics Study. Eur Heart J. 2013;34:993–1001. [DOI] [PubMed] [Google Scholar]
- 15.Patel RS, et al. Genetic variants at chromosome 9p21 and risk of first versus subsequent coronary heart disease events: A systematic review and meta-analysis. J Am Coll Cardiol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahabreh IJ, et al. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA. 2011;305:822–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole SR, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2010;39:417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson CD, et al. The effect of survival bias on case-control genetic association studies of highly lethal diseases. Circ Cardiovasc Genet. 2011;4:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu YJ, et al. Impact of Selection Bias on Estimation of Subsequent Event Risk. Circ Cardiovasc Genet. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falk E, et al. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013;34:719–28. [DOI] [PubMed] [Google Scholar]
- 21.Reilly MP, et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet. 2011;377:383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan K, et al. Association between the chromosome 9p21 locus and angiographic coronary artery disease burden: a collaborative meta-analysis. J Am Coll Cardiol. 2013;61:957–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visel A, et al. Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature. 2010;464:409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.