Abstract
The rise of Zika virus (ZIKV) and its unusual clinical manifestations provided ground for speculative debate. The clinical severity of secondary dengue virus (DENV) infections is associated with antibody-dependent enhancement (ADE), and it was recently suggested that previous exposure to DENV may worsen ZIKV clinical outcomes. In this Opinion article we analyze the relationship among different flaviviruses and ADE. We discuss new evidence obtained in non-human primates and human cohorts demonstrating that there is no correlation to ADE when ZIKV infection occurs in the presence of pre-existing DENV immunity. We propose a redefinition of ADE in the context of complex immunological flavivirus interactions to provide a more objective perspective when translating in vitro or in vivo observations into the clinical setting.
Zika Virus Is a Clinical Outlier Flavivirus
Rising from obscurity 60 years after its discovery, ZIKV caused the first major human epidemic in the Federated States of Micronesia, followed by a major pandemic with its introduction in Brazil sometime in 2013 [1]. Currently, indigenous mosquito-borne ZIKV transmission has been confirmed in 49 countries or territories of the AmericasAppendix A. The introduction and spread of ZIKV in the Americas was marked by the appearance of severe adverse outcomes such as fetal loss [2], congenital Zika syndrome (CZS) (see Glossary) [3], Guillain–Barré syndrome (GBS)[4], and rare cases of encephalopathy [5], meningoencephalitis [6], myelitis [7], uveitis [8], and severe thrombocytopenia [9]. Several hypotheses have been put forward to explain the unprecedented observed pathogenicity of ZIKV infection in the Americas, including prior heterologous flavivirus infection, virulence of the virus, host genetics and environmental factors among others.
ADE: From In Vitro Evidence to Clinical Relevance
Analogous to antibody-dependent enhancement (ADE), during secondary DENV exposure, the scientific community hypothesized that a ZIKV infection following a previous DENV infection may result in increased ZIKV pathogenesis (for example CZS and GBS) in the Americas. ADE in vitro can be considered as a common experimental phenomenon with uncertain clinical relevance, as it has been demonstrated for many viruses (alphaviruses [10], rabies [11], coxsackievirus B3 [12], coronavirus [13], human immunodeficiency virus [14,15], and others) without evidence of worsened disease during secondary infection in mice or in human populations [16]. Such a precise ADE definition is very specific in describing an experimental finding as a fact. In in vitro assays, immune sera from patients exposed to a variety of different flaviviruses, including yellow fever and Japanese encephalitis viruses, will also enhance DENV infection [17]. Even the homotypic serotype responsible for a past DENV infection can induce ADE of DENV, if the serum is diluted to subneutralizing concentrations [18]. However, in contrast to ADE described for other viruses, ADE of DENV in vivo is commonly associated with a worse clinical outcome [19]. Secondary DENV infections result in dramatic clinical impairment along with a cytokine storm characterized by the increase in interleukin-6 (IL-6), IL-8, IL-10, interferon-γ (IFN-γ), IFN-α, and vascular endothelial growth factor (VEGF), combined with tumor necrosis factor-a (TNF-α), indicating a poor prognostic outcome [20] (Figure 1). Because of this, ADE related to flaviviruses should not be seen only as a single biological process of virus–antibody interaction. Defining ADE in the context of pathogenesis, as we usually read the outcome of the biological process, should imply a clinical consequence, including clinical and laboratory evidence of impairment. In this way, ADE would be defined as a common experimental in vitro phenomenon but a rare in vivo occurrence leading to worsening of the clinical presentation usually associated with hemodynamic changes, increased viremia, proinflamatory cytokine profile, and other detectable laboratory alterations.
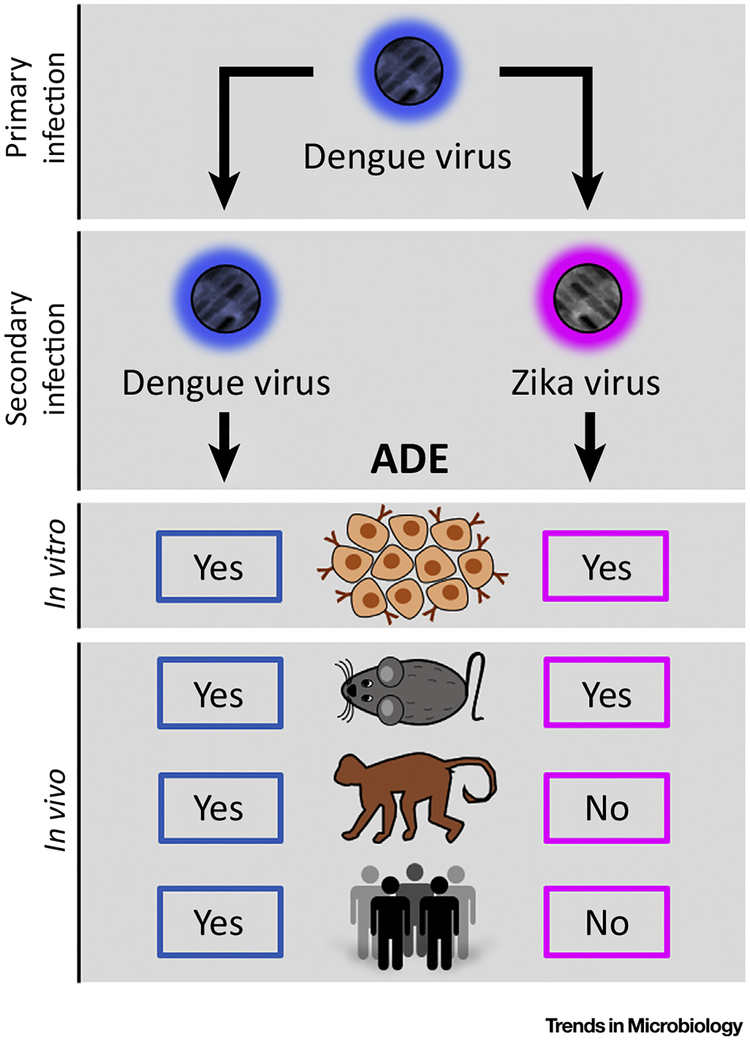
Figure 1. Antibody-Dependent Enhancement (ADE) of Dengue and Zika Virus.
ADE during a secondary heterologous Dengue virus infection has been documented in vitro, in mice, in non-human primates, and in humans playing a key role in the worsening of the clinical presentations. In contrast, ADE of Zika virus by pre-existing immunity to Dengue virus can be induced in vitro, and in immunodeficient mice. However, there is no evidence to support ADE occurring in non-human primates or in humans.
Dengue Induced ZIKV ADE?
The debate of whether ZIKV ADE by flavivirus immune serum has recently increased because of results showing an increase in ZIKV pathogenesis in a mouse model (Stat2−/−) [21]. Using this model, Bardina et al. showed that, by administering DENV and West Nile virus (WNV) immune serum intraperitoneally, in an appropriate concentration before ZIKV infection, this resulted in fever and weight loss with an increased mortality as compared to some of the animals administered serum from flavivirus-naïve individuals [21]. However, results from the same work, in a dose-dependent evaluation of mouse survival and the clinical presentation experiment, revealed that control plasma at the highest concentration could also decrease mice survival by about 40%, similar to the effect of DENV immune plasma at the lowest dilutions. In addition to proving that antibodies induced by prior DENV infection, administered under different concentrations, can amplify or neutralize ZIKV disease manifestations in vivo, Bardina et al. also showed the limited value of ADE in vivo in immunosuppressed mice.
Indeed, ADE of ZIKV by previous flavivirus infection is not a novel concept. Back in 1987 Fabgami et al. demonstrated that ZIKV replication can be enhanced in P388D1 macrophage cell line by subneutralizing concentrations of antibodies in immune ascitic fluids from six other different flaviviruses, including Wesselsbron, Uganda S, WNV, Dakar bat, yellow fever and Potiskum virus [22]. However, the following facts might anticipate the unlikelihood of DEN Vinduced ZIKV ADE (as defined above) in humans: (i) there is no epidemiological or clinical evidence of DENV ADE with any other closely related flavivirus or any other viruses; (ii) before its introduction into the Western hemisphere, ZIKV continuously circulated in flavivirus-endemic areas (such as Africa and Southeast Asia), and an increase in ZIKV pathogenesis has not been reported in these locations; (iii) not all heterologous flavivirus immunity is the same, including the sequence in which infection occurs with different DENV serotypes [18,19].
What Non-human Primates (NHPs) Can Tell Us
NHPs are natural hosts (in the sylvatic transmission cycle) supporting the replication of both DENV and ZIKV. For many years NHPs have been used as a surrogate for human infection in order to understand DENV pathogenesis and to test for vaccine immunogenicity and efficacy [23] – and, more recently, for ZIKV replication and pathogenesis [24–28]. In the past, DENV ADE, in terms of viral replication enhancement, has also been proven in NHPs after secondary DENV infection with DENV 2 [29] or by passive administration of optimal dilutions of human DENV-immune serum to the animals [30], or by using specific concentrations of a monoclonal antibody [31]. In addition to being useful for studying DENV pathogenesis, NHPs are a good model for predicting the behavior of different DENV vaccines in humans and for characterizing specific DENV neutralizing antibodies also occurring naturally in human populations [23]. Because of this, it is plausible to anticipate that data on ZIKV pathogenesis in NHPs can also reproduce or predict what will happen in humans. In a recent study, using a limited number of animals, Pantoja et al. were unable to show ADE of ZIKV in DENV immune macaques [32]. However, results showed that previous immunity to DENV was able to modulate the innate and cellular immune response to ZIKV with a tendency to lower the average ZIKV viremia days, to limit the increase in liver enzymes, and to induce a significant increase in the plasma perforin as evidence of an increased cytotoxic T cell activity [33]. This cellular immune response in NHPs is supported by recent results from human samples showing that prior DENV infection leads to stronger and faster responses to ZIKV in terms of both CD4 and CD8T cell responses, thus providing evidence of a biological outcome [34].
Human Evidence of ZIKV ADE?
Coincident with the report by Pantoja et al., a study on ADE of a human cohort was published[35]. Terzian et al. evaluated a cohort of ZIKV-infected patients and looked for previous DENV exposure and its relationship to viral load, cytokine profile, and clinical symptoms. Despite the suggestions from in vitro studies that ADE could occur in DENV-primed ZIKV-infected patients, the authors found no evidence that the presence of DENV antibodies changes the outcome of ZIKV infection in all tested parameters [35]. Collectively, these observations from NHPs and human cohorts strongly suggest that previous exposure to DENV does not have a deleterious effect in the clinical outcome of ZIKV infection. Supported by these observations, we can propose that the DENV-induced ZIKV ADE in vitro does not exist in vivo or that it is so uncommon that it might be not relevant as an epidemiological phenomenon.
Concluding Remarks
In summary, data from NHPs and humans, and from several serological studies [36,37], do not support the suggestion that ZIKV may be enhanced in vivo by previous exposure to DENV. On the other hand, the few experimental lines of evidence that have addressed ADE of DENV induced by ZIKV-immune serum, as expected, have shown different degrees of in vitro and in vivo increase in DENV replication and pathogenesis respectively [38,39].
Recently George et al. reported that an infection with DENV, after a short period of exposure to ZIKV, can enhance DENV infection in NHPs [40]. This is a very interesting report as it is expected that ZIKV-induced DENV cross-reacting antibodies, induced early after infection, may either neutralize or have no effect in DENV infection outcome. In any case, this report confirms the need for large studies in NHPs and for epidemiological data from the dengue-naïve human population that has been exposed to ZIKV during the recent epidemic.
Lastly, inferences derived from in vitro experiments and from immunologically modified animals will need to be carefully assessed due to the impact they can have on the approaches for ZIKV and DENV vaccines and therapeutics currently under development.
Supplementary Material
Trends.
Zika virus (ZIKV) caused atypical clinical manifestations in areas with previous exposure to other flaviruses.
Different dengue–ZIKV cross-reacting antibodies neutralize or enhance ZIKV in vitro, but the percentage of dengue immune serum neutralizing ZIKV is very low.
Antibody-dependent enhancement (ADE) of ZIKV by dengue and West Nile immune sera has been shown in vitro and induced in immunosuppressed mice by dengue and West Nile immune sera.
No ADE of ZIKV by previous dengue immunity was detected in non-human primates.
No ADE of ZIKV was documented in a human cohort previously exposed to dengue.
ADE needs to be redefined in the context of clinical outcomes.
In vitro and experimental results in small animals need to be carefully weighed when translating results to humans.
Prospective epidemiological and clinical studies are needed to reassure that previous exposure to dengue or other flaviviruses does not increase the pathogenesis of ZIKV.
Outstanding Questions.
What is the role of the time interval between a primary DENV and a subsequent ZIKV infection in the antibody and T cell immune response?
How will this scenario compare to having ZIKV infection following two or more DENV or other flavivirus infections?
How will this timing between infections impact the clinical outcome, if at all?
With the recent ZIKV epidemic there is a substantial population that is ZIKV-positive/DENV-negative. Are these people at risk of having worse clinical presentations or are they partially protected against DENV?
What will be the effect of a ZIKV component in the effectiveness of DENV vaccines currently in clinical trials or the one that has been licensed in some countries?
What is the susceptibility conferred on the population by the DENV and ZIKV vaccines to subsequent heterogonous natural infections with those viruses?
Acknowledgments
The authors’ work on DENV and ZIKV is supported by NIH grants U01 AI115577, R24AI120942 (NV), P40 OD012217 and U42OD021458, (CAS) and FAPESP Grants # 2013/21719–3 and 2016/15021-1 (MNL). We would also like to acknowledge Erick Perez Guzman for expert graphical design on Figure 1. During the review of this manuscript a category 4 hurricane devastated Puerto Rico with an undetermined impact in the research activity. Authors want to deeply thank all collaborators and friends who expressed their sympathy and who were actively engaged in different supporting initiatives.
Glossary
- Antibody-dependent enhancement (ADE)
a mechanism when non-neutralizing antibodies facilitate virus entry into host cells, leading to increased infectivity in the cells and exacerbation of the clinical disease.
- Congenital Zika syndrome (CZS)
a pattern of birth defects found among fetuses and babies infected with ZIKV during pregnancy.
- Guillain–Barré syndrome (GBS)
an autoimmune disorder affecting the peripheral nervous system. Initial symptoms are weakness and numbness in the extremities, which eventually develop into paralysis and, if untreated, death.
Footnotes
Supplemental Information
Supplemental information associated with this article can be found online at https://doi.org/10.1016/j.tim.2017.10.004.
References
- 1.Aliota MT et al. (2017) Zika in the Americas, year 2: what have we learned? What gaps remain? A report from the Global Virus Network. Antiviral Res. 144, 223–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campos GC et al. (2016) Zika virus infection, a new public health challenge. Braz. J. Infect. Dis 20, 227–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brasil P et al. (2016) Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med 375, 2321–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao-Lormeau VM et al. (2016) Guillain–Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet Published online February 29, 2016 10.1016/S0140-6736(16)00562-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roze B et al. (2016) Zika virus detection in cerebrospinal fluid from two patients with encephalopathy, Martinique, February 2016. Euro. Surveill Published online April 21, 2016. 10.2807/1560-7917.ES.2016.21.16.30205 [DOI] [PubMed] [Google Scholar]
- 6.Carteaux G et al. (2016) Zika virus associated with meningoencephalitis. N. Engl. J. Med 374, 1595–1596 [DOI] [PubMed] [Google Scholar]
- 7.Mecharles S et al. (2016) Acute myelitis due to Zika virus infection. Lancet 387, 1481. [DOI] [PubMed] [Google Scholar]
- 8.Furtado JM et al. (2016) Uveitis associated with Zika virus infection. N. Engl. J. Med 375, 394–396 [DOI] [PubMed] [Google Scholar]
- 9.Sharp TM et al. (2016) Zika virus infection associated with severe thrombocytopenia. Clin. Infect. Dis 63, 1198–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peiris JS and Porterfield JS (1982) Antibody-dependent plaque enhancement: its antigenic specificity in relation to Togaviridae. J. Gen. Virol 58, 291–296 [DOI] [PubMed] [Google Scholar]
- 11.King AA et al. (1984) Antibody-mediated enhancement of rabies virus infection in a mouse macrophage cell line (P388D1). J. Gen. Virol 65, 1091–1093 [DOI] [PubMed] [Google Scholar]
- 12.Jarasch-Althof N et al. (2010) Antibody-dependent enhancement of coxsackievirus B3 infection of primary CD19+ B lymphocytes. Viral Immunol. 23, 369–376 [DOI] [PubMed] [Google Scholar]
- 13.Wang SF et al. (2014) Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem. Biophys. Res. Commun 451, 208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fust G (1997) Enhancing antibodies in HIV infection. Parasitology 115 (Suppl), S127–S140 [DOI] [PubMed] [Google Scholar]
- 15.Morens DM (1994) Antibody-dependent enhancement of infection and the pathogenesis of viral disease. Clin. Infect. Dis 19, 500–512 [DOI] [PubMed] [Google Scholar]
- 16.Miner JJ and Diamond MS (2017) Dengue antibodies, then Zika: a fatal sequence in mice. Immunity 46, 771–773 [DOI] [PubMed] [Google Scholar]
- 17.Halstead SB et al. (1980) Enhancement of dengue virus infection in monocytes by flavivirus antisera. Am. J. Trop. Med. Hyg 29, 638–642 [DOI] [PubMed] [Google Scholar]
- 18.de Alwis R et al. (2014) Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera. PLoS Pathog. 10, e1004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzman MG et al. (2013) Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol 158, 1445–1459 [DOI] [PubMed] [Google Scholar]
- 20.Srikiatkhachorn A et al. (2017) Immune-mediated cytokine storm and its role in severe dengue. Semin. Immunopathol 39, 563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bardina SV et al. (2017) Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 356, 175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagbami AH et al. (1987) Cross-infection enhancement among African flaviviruses by immune mouse ascitic fluids. Cytobios 49, 49–55 [PubMed] [Google Scholar]
- 23.Sariol CA and White LJ (2014) Utility, limitations, and future of non-human primates for dengue research and vaccine development. Front. Immunol 5, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudley DM et al. (2016) A rhesus macaque model of Asianlineage Zika virus infection. Nat. Commun 7, 12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haddow AD et al. (2017) High infection rates for adult macaques after intravaginal or intrarectal inoculation with Zika virus. Emerg. Infect. Dis 23, 1274–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch AJ et al. (2017) Zika virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 13, e1006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen SM et al. (2017) Highly efficient maternal–fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 13, e1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osuna CE et al. (2016) Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat. Med 22, 1448–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halstead SB et al. (1973) Studies on the pathogenesis of dengue infection in monkeys. II. Clinical laboratory responses to heterologous infection. J. Infect. Dis 128, 15–22 [DOI] [PubMed] [Google Scholar]
- 30.Halstead SB (1979) In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J. Infect. Dis 140, 527–533 [DOI] [PubMed] [Google Scholar]
- 31.Goncalvez AP et al. (2007) Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl. Acad. Sci. U. S. A 104, 9422–9427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petraleigh Pantoja EXP-G et al. (2016) Secondary Zika Virus Infection Do Not Support Evidences of Antibody-Dependent Enhancement in vivo in Dengue Pre-exposed Rhesus Macaques, pp. 1–16, University of Puerto Rico Medical Sciences Campus Caribbean Research Center [Google Scholar]
- 33.Pantoja P et al. (2017) Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat. Commun 8, 15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grifoni A et al. (2017) Prior Dengue virus exposure shapes T cell immunity to Zika virus in humans. J. Virol Published online October 4, 2017. 10.1128/jvi.01469-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terzian ACB et al. (2017) Viral load and cytokine response profile does not support antibody-dependent enhancement in dengue-primed Zika-infected patients. Clin. Infect. Dis 65, 1260–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins MH et al. (2017) Lack of durable cross-neutralizing antibodies against Zika virus from Dengue virus infection. Emerg. Infect. Dis 23, 773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swanstrom JA et al. (2016) Dengue virus envelope dimer epitope monoclonal antibodies isolated from dengue patients are protective against Zika virus. mBio Published online July 19, 2016 10.1128/mBio.01123-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawiecki AB and Christofferson RC (2016) Zika virus-induced antibody response enhances Dengue virus serotype 2 replication in vitro. J. Infect. Dis 214, 1357–1360 [DOI] [PubMed] [Google Scholar]
- 39.Stettler K et al. (2016) Specificity, cross-reactivity and function of antibodies elicited by Zika virus infection. Science 353, 823–826 [DOI] [PubMed] [Google Scholar]
- 40.George J et al. (2017) Prior exposure to Zika virus significantly enhances peak Dengue-2 viremia in Rhesus macaques. Sci. Rep 7, 10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.