Abstract
Objectives and Method:
There are growing concerns of tenofovir disoproxil fumarate (TDF)–associated renal toxicity. We evaluated the effect of long-term TDF exposure on renal function in a cohort of HIV-1-infected Nigerians between 2006 and 2015. Multivariate logistic regression was used to identify predictors of renal impairment at different time over 144 weeks of antiretroviral therapy (ART).
Results:
Data of 4897 patients, median age 42 years (interquartile range: 36-49), and 61% females were analyzed. The prevalence of renal impairment increased from 10% at week 24 to 45% at 144 weeks in TDF-exposed participants compared to an increase from 8% at 24 weeks to 14% at 144 weeks in TDF-unexposed participants. Tenofovir disoproxil fumarate exposure predicted the risk of renal impairment at 144 weeks of ART (odds ratio: 2.36; 95% confidence interval: 1.28-4.34).
Conclusion:
Long-term exposure to TDF-based ART significantly increases the likelihood of renal impairment. The continued use of TDF-based regimen in our setting should be reviewed. We recommend the urgent introduction of tenofovir alafenamide-based regimen in the HIV treatment guidelines of Nigeria and other resource-limited countries.
Keywords: HIV, ART, tenofovir, renal impairment, Nigeria
Introduction
Tenofovir disoproxil fumarate (TDF), a potent antiretroviral (ARV) drug was approved for clinical use by the US Food and Drug Administration in 2001, the European Medicines Evaluation Agency in 2002, and was subsequently approved in other countries around the world.1 Tenofovir is one of the preferred backbones for antiretroviral therapy (ART) and is widely used in the management of HIV-1 infection.1-3 Central to the clinical success of TDF has been the low rates of discontinuations due to TDF-related viral resistance or toxicity2,4,5 and its effectiveness in the management of HIV-1 and hepatitis B coinfections.6-8 However, while TDF therapy is generally well tolerated, there are growing concerns about its renal safety. The first case of TDF-induced acute nephrotoxicity was published in 2001,9 and since then there has been increasing evidence from observational studies of the renal toxicity of TDF.1,10-14 Data from a 2010 review and meta-analysis demonstrated greater loss of kidney function and a higher risk of acute renal failure in patients receiving TDF-based therapies versus non-TDF regimens.15 Tenofovir disoproxil fumarate–associated nephrotoxicity may present as proximal tubular dysfunction with preserved renal function or proximal tubular dysfunction associated with decreased renal function.16
Despite mounting evidence of TDF-associated nephrotoxicity, its use in the management of HIV-1 infection is on the rise (over 9 million patient-years),1 especially in resource-limited settings.2,17 In Nigeria, the use of TDF as a component of ART has been scaled up since 2006 when it was first introduced into the National HIV treatment guidelines.18 As part of the preferred first-line ART regimen in Nigeria, it was projected that at least 400 000 of the about 750 000 HIV-infected persons on ART (over 50%) in Nigeria as of 2014 were on TDF-based ART.19-21 There are few reports on the renal safety of TDF in Nigerian patients. Previously, a case report associated nephrotoxicity in a Nigerian patient with the use of TDF-based ART.22 Another earlier observational study in Nigeria evaluated renal function in 186 HIV-1-infected patients on ART and reported that TDF-containing ART was associated with a slight decline in creatinine clearance (CrCl) at 48 weeks.23 However, the study was limited by small sample size and a relatively short duration of follow-up. The paucity of longitudinal data on the renal safety of TDF-based ART in Nigerian patients brings to the fore an overarching need to conduct locally relevant epidemiological studies to quantify the magnitude and trend of TDF-related nephrotoxicity and to generate data to guide programmatic decision nationally and internationally. This study set out to assess the effect of 144-week TDF exposure on the renal function of HIV-infected adult Nigerians.
Method
Study Design, Setting, and Population
We conducted a retrospective cohort analysis in which prospectively collected clinical and laboratory data of patients who accessed ART services between 2006 and 2015 at the AIDS Prevention Initiative in Nigeria (APIN)–supported HIV Centre of the Jos University Teaching Hospital (JUTH) was evaluated. HIV-infected patients ≥18 years of age, treatment naive at ART initiation, and received TDF-based ART were eligible for study inclusion. Patients with only one pharmacy drug pick up, and those with insufficient baseline data such as missing baseline serum creatinine (SCr) were excluded from the study. Also excluded were patients with preexisting renal disorders. The study commencement period of 2006 was selected as it coincided with the period of introduction of TDF into the Nigerian ART treatment program.
The ART program at JUTH has one of the largest ART cohorts in Nigeria with over 26 000 patients cumulatively enrolled since inception. The JUTH ART program started in 2002 as part of the Federal Government of Nigeria ART program. However, in 2004, there was a rapid scale-up of ART services through collaboration between JUTH, the University of Jos and the Harvard T. H. Chan School of Public Health (HSPH)/APIN through funding from the US President’s Emergency Plan for AIDS Relief. Initiation of ART-naive patients on ART was based on eligibility criteria outlined in the Nigerian National HIV Treatment Guidelines18-20 that closely mirrored the World Health Organization (WHO) Guidelines.3,24-26 Beginning in 2004, patients were considered eligible for ART if their CD4 counts dropped to ≤200 cells/mm3 or if symptomatic with CD4 counts ≤350 cells/mm3; from 2010, CD4 count eligibility was raised to ≤350 cells/mm3 regardless of symptoms. The preferred first-line ARV regimen generally included a non-nucleoside reverse transcriptase inhibitor, either nevirapine (NVP) or efavirenz (EFV), in combination with 2 nucleoside reverse transcriptase inhibitors (NRTIs), typically lamivudine (3TC) in combination with either zidovudine (ZDV) or TDF.18-20 Stavudine with 3TC was recommended as an alternate NRTI combination in the 2006 Nigerian HIV treatment guideline18 but was phased out in the 2010 guideline.20 Between 2006 and 2013, most patients initiated ART with the ARV regimen of ZDV/3TC/NVP, except those with anemia or hepatitis B coinfection where TDF/3TC/ EFV was used. There was a major shift toward the use of TDF/3TC/EFV for ART initiation for most patients at the treatment facility beginning in 2013. However, in patients with baseline renal impairment, ZDV or abacavir was used in place of TDF, while in those with a history of psychiatric illness NVP was used instead of EFV.
As part of the treatment protocol, patients provided written informed consent at enrollment. Only patients who had consented to their clinical data to be used for research were included in this analysis. The protocol and consent forms were reviewed and approved by the institutional review board of HSPH and the JUTH Human Research Ethics Committee (reference number JUTH/DCS/ADM/127/XXVII/803).
Laboratory Assessment
HIV Serology, CD4 Count, and Viral Load.
Sample collection and all analyses were performed in the APIN-supported laboratory at JUTH. The APIN-supported laboratory engages in external quality assurance with UK-NEQAS and the American College of Pathologists; received South African National Accreditation System (SANAS) accreditation in October of 2017. The HIV status of each participant was determined using the rapid immunochromatographic HIV-1/2 test (Abbott Diagnostics) and the Murex HIV antigen/antibody Combination ELISA (Abbott Diagnostics), according to the manufacturer’s instructions. A participant was considered HIV infected if he/she tested positive on the 2 tests, HIV negative if negative for both tests, and discordant if positive for only 1 test, in which case a third test (tiebreaker) was conducted. Participants’ CD4 count was determined via flow cytometry (Partec GmbH), according to the manufacturer’s instructions.
HIV RNA was determined using the Roche Ampliprep Taq-Man (Roche Diagnostics), according to the manufacturer’s protocol. The laboratory parameters included in this analysis were those measured at baseline and 24, 48, 96, and 144 weeks of ART. Baseline clinical assessments or laboratory evaluations for ART naive patients were the closest measurements to, and up to 6 months before ART commencement in most cases or 0.5 months after the ART start date.
Outcome Measure and Exposure Variables
The main outcome measure was renal impairment at week 24, 48, 96, and 144 of ART exposure. Renal impairment was defined as meeting at least 1 of 2 criteria: (i) estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 (calculated using the abbreviated Modification of Diet in Renal Disease [MDRD] equation),27 which constitutes stage 3 or greater renal disease according to the Kidney Disease Outcomes Quality Initiative guidelines28; (ii) a ≥2-fold increase in SCr above baseline or a ≥50% decline in CrCl from baseline on 1 or more measurements, which meets the definition of “renal injury” by the risk, injury, failure, loss, and end-stage kidney criteria.29 For this criterion, CrCl was calculated using the Cockcroft–Gault equation and the patient’s actual body weight.27
Exposure Variable.
The primary exposure variable was TDF exposure baseline. Patients were characterized as TDF-unexposed if they did not receive TDF during the study period. Data on other exposure variables collected included: age, sex, SCr, weight, ART start date, and HIV comorbidities (hepatitis B or C).
Statistical Analysis
Standard descriptive statistical methods were used to describe baseline demographic and clinical characteristics of study participants. Bivariate analysis of demographics and clinical characteristics associated with renal impairment was performed using the χ2 test for categorical variables, and Mann-Whitney U test for continuous variables (due to their skewed distribution determined from initial exploratory analyses). Independent predictors of the binary outcomes of renal impairment at 24, 48, 96, and 144 weeks of ART were evaluated using a multivariate logistic regressions model. We included variables that were significant to a P = .25 in the bivariate analysis in the multivariable model. Variables included in the multivariate model are gender, age at ART initiation, clinical stage of disease, hepatitis B virus status, baseline CD4 count, baseline viral load, and initial ART regimen. Age was included as a continuous variable, WHO HIV clinical stage was categorized as less advanced (WHO stage 1 or 2) or more advanced (WHO stage 3 or 4), while CD4 count was categorized into ≤100 and >100 cells/mm3 with CD4 count ≤100 cells/mm3 representing severe immunosuppression. Multiple imputations of missing values were performed to address potential bias due to missing data. Multiple imputations were done after examination of the pattern of the missing data and verification that data were missing at random. Assuming missing at random, we used Markov chain Monte Carlo method of multiple imputations and the linear regression technique for 5 imputed data set. Statistical significance was defined at an α level of .05. All of the analyses were performed using SPSS for windows version 23 (IBM Corp).
Results
Demographic and Clinical Characteristics of Study Participants
The baseline demographic and clinical characteristics of the 4897 participants stratified by TDF exposure are presented in Table 1. The median age of the study participants was 42 years (interquartile range [IQR]: 36-49) with females in the majority (61%). Tenofovir-unexposed participants were more in proportion, had more females, and were older (median [IQR] age of 42 [36-50] versus 41 [35-48] years for TDF-exposed versus -unexposed; P < .001). A little above a quarter (27%) of the participants were coinfected with hepatitis B or C, with a significantly higher proportion of coinfected patients on TDF-based ART (44% versus 31%). Most participants (68%) had WHO clinical stage 1 or 2 disease at baseline, with a higher proportion of those with WHO stage 3 or 4 disease exposed to TDF, P < .001. Additionally, the majority of participants had HIV viral load ≤10 000 copies/mL (68%) and CD4 count >100 cells/mm3 (72%) prior to ART initiation. Baseline median eGFR were significantly higher in TDF-exposed participants compared to TDF-unexposed participants (102 versus 88 mL/min/1.73 m2 for eGFR; P < .001).
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants Stratified by Tenofovir Exposure.a
| Characteristics | Strata | All Patients, N = 4897 |
TDF-Exposed (a), n = 2104 (43%) |
TDF-Unexposed (b), n = 2793 (57%) |
P Value, (a) versus (b) |
|---|---|---|---|---|---|
| Sex | Female | 2969 (60.63) | 1188 (40.01) | 1781 (59.99) | <.001b |
| Male | 1928 (39.37) | 916 (47.51) | 1012 (52.49) | ||
| Age range | ≤50 | 3715 (75.91) | 1642 (44.2) | 2073 (55.8) | .002b |
| >50 | 1179 (24.09) | 460 (39.02) | 719 (60.98) | ||
| Age, years | Median (IQR) | 42 (36-49) | 41 (35-48) | 42 (36-50) | <.001c |
| Weight, kg | Median (IQR) | 53 (61-70) | 60 (52-70) | 60 (53-69) | .334c |
| Hepatitis B or C | No | 2163 (72.61) | 675 (31.21) | 1488 (68.79) | <.001b |
| Yes | 816 (27.39) | 358 (43.87) | 458 (56.13) | ||
| WHO clinical stage | 1 or 2 | 2168 (68.41) | 638 (29.43) | 1530 (70.57) | <.001b |
| 3 or 4 | 1001 (31.59) | 432 (43.16) | 569 (56.84) | ||
| Viral load, copies/mL | ≤10 000 | 2804 (67.02) | 1092 (38.94) | 1712 (61.06) | .120b |
| >10 000 | 1380 (32.98) | 572 (41.45) | 808 (58.55) | ||
| Log10 | 3.5 (4.6-5.2) | 3.64 (4.59-5.28) | 3.45 (4.52-5.18) | .002c | |
| CD4 cells/mm3 | ≤100 | 1316 (27.04) | 586 (44.53) | 730 (55.47) | .154b |
| >100 | 3550 (72.96) | 1500 (42.25) | 2050 (57.75) | ||
| Median (IQR) | 94 (182-298) | 91 (188-319) | 95 (180-284.25) | .731c | |
| Creatinine (mmol/L) | Median (IQR) | 64 (80-103) | 74.05 (59-92.91) | 83 (66-106) | <.001c |
| CrCl, (mL/min) | Median (IQR) | 62 (82-104) | 97.73 (76.2-126.3) | 87.26 (66.4-112.2) | <.001c |
| eGFR (mL/min/1.73 m2) | Median (IQR) | 70 (92-118) | 102.48 (80.2-130.7) | 88.07 (66.4-112.4) | <.001c |
Abbreviations: CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; IQR, interquartile range; TDF, tenofovir disoproxil fumarate; WHO, World Health Organization.
The number in parenthesis is percentages except indicated otherwise.
The comparison was by χ2 for categorical variables.
Mann-Whitney U test was used for comparison of median values.
Prevalence of Renal Impairment
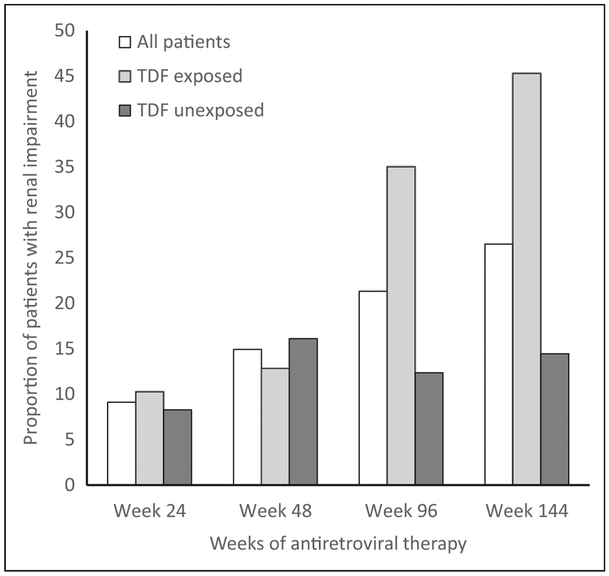
The prevalence of renal impairment over 144 weeks of ART summarized in Table 2 shows that overall 348 (9%) participants of 3806 had renal impairment at 24 weeks of ART. The proportion of patients with renal impairment increased from 9% at 24 weeks to 15%, 21%, and 27% at 48, 96, and 144 weeks of ART, respectively. The prevalence of renal impairment differed significantly between TDF-exposed and TDF-unexposed participants. The trend in renal impairment overall and according to TDF exposure is depicted in Figure 1. It shows a marked increase in the prevalence of renal impairment from 10% at week 24 to 45% at week 144 in TDF-exposed participants, whereas for TDF-unexposed participants there was a moderate increase from 8% at 24 weeks to 14% at 144 weeks.
Table 2.
Prevalence of Renal Impairmenta according to Tenofovir Exposure at Different Weeks of Antiretroviral Therapy.
| All Patients |
Tenofovir-Exposed Patients |
Tenofovir-Unexposed Patients |
|||||
|---|---|---|---|---|---|---|---|
| Weeks of ART |
Number of Patients with Renal Impairment/ Number of Exposed |
Percentage (95% CI) of Patients with Renal Impairment |
Number of Patients with Renal Impairment/ Number of Exposed |
Percentage (95% CI) of Patients with Renal Impairment (a) |
Number of Patients with Renal Impairment/ Number of Exposed |
Percentage (95% CI) of Patients with Renal Impairment (b) |
P Value (a) versus (b) |
| 24 | 348/3806 | 9.14 (8.22-10.06) | 168/1635 | 10.28 (8.87-11.74) | 180/2171 | 8.29 (7.19-9.53) | .46 |
| 48 | 565/3784 | 14.93 (13.85-16.12) | 176/1370 | 12.85 (11.1-14.82) | 389/2414 | 16.11 (14.71-17.48) | .03 |
| 96 | 597/2802 | 21.31 (19.74-22.91) | 387/1105 | 35.02 (32.4-37.92) | 210/1697 | 12.37 (10.84-14.02) | <.001 |
| 144 | 860/3244 | 26.51 (24.97-28.14) | 574/1267 | 45.3 (42.62-48.38) | 286/1977 | 14.47 (12.85-16.08) | <.001 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; eGFR, estimated glomerular filtration rate; SCr, serum creatinine.
Patient met 1 or more of the defined criteria for renal impairment based on eGFR <60 mL/min/1.73 m2 or a doubling of SCr from baseline.
Figure 1.
Prevalence of renal impairment*, overall, and by tenofovir exposure over 144 weeks of antiretroviral therapy. * denotes patient met 1 or more of the defined criteria for renal impairment based on eGFR <60 mL/min/1.73 m2 or a doubling of serum creatinine from baseline. TDF indicates tenofovir disoproxil fumarate.
Association of Baseline Characteristics with Renal Impairment
Table 3 summarizes the association of participants’ pre-ART characteristics with renal impairment at different time of ART exposure. The proportion of males with renal impairment at 24 and 144 of ART was higher than females (10% versus 8%, at 24 weeks, P < .001, and 30% versus 24%, at 144 weeks, P < .001, for males versus females). Also, the median age of participants with renal impairment was significantly higher compared to those without renal impairment at 24 and 48 weeks of ART, but not at 96 and 144 weeks. A higher proportion of hepatitis B or C coinfected participants compared to non-coinfected participants had renal impairment at 96 and 144 weeks of ART (21% versus 15% and 28% versus 19%, at 96 and 144 weeks of ART, respectively). The WHO clinical stage 3 or 4, as well as pretreatment CD count of ≤100 cells/mm3 were associated with renal impairment at 144 weeks of ART in the bivariate analysis. Furthermore, baseline eGFR was significantly higher in those with renal impairment compared to those without renal impairment (median [IQR] of 96 [75-120] versus 92 [71-114] mL/min/1.73 m2).
Table 3.
Association of Participants’ Pre-ART Characteristics with Renal Impairment over Weeks of Antiretroviral Therapy.
| Renal Impairment at 24 Weeks, N (%) |
Renal Impairment at 48 Weeks, N (%) |
Renal Impairment at 96 Weeks, N (%) |
Renal Impairment at 144 Weeks, N (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Strata | No | Yes | P Value | No | Yes | P Value | No | Yes | P Value | No | Yes | P Value |
| Sex | Female | 2050 (92) | 187 (8) | .045 | 1944 (86) | 321(14) | .110 | 1219 (80) | 314 (20) | .242 | 1407 (76) | 438 (24) | .001 |
| Male | 1408 (90) | 161 (10) | 1275 (84) | 244 (16) | 986 (78) | 283 (22) | 977 (70) | 422 (30) | |||||
| Age, years | ≤50 | 2645 (92) | 237 (8) | .001 | 2451 (87) | 373 (13) | .001 | 1645 (79) | 449 (21) | .762 | 1631 (72) | 645(28) | .001 |
| >50 | 813 (88) | 111 (12) | 768 (80) | 192 (20) | 560 (79) | 148 (21) | 753 (78) | 215 (22) | |||||
| Age, years | Median (IQR) | 44 (38-50) | 45 (38-54) | <.001 | 44 (38-50) | 46 (40-53) | <.001 | 45 (38-51) | 43 (37-50) | .596 | 45 (38-51) | 43 (37-50) | .001 |
| Hepatitis B or C | No | 1502 (90) | 164 (10) | .552 | 1561 (82) | 341 (18) | .634 | 1104 (85) | 198 (15) | .001 | 1553 (81) | 367 (19) | .001 |
| Yes | 638 (89) | 76 (11) | 557 (83) | 115 (17) | 546 (79) | 149 (21) | 54 (72) | 208(28) | |||||
| WHO clinical stage | 1 and 2 | 1545 (91) | 157 (9) | .269 | 1531 (81) | 354 (19) | .014 | 1119 (82) | 244 (18) | .268 | 1555 (81) | 363 (19) | .001 |
| 3 and 4 | 768 (89) | 91 (11) | 735 (85) | 129 (15) | 660 (84) | 126 (16) | 669 (73) | 242 (27) | |||||
| CD4 count cells/mm3 | ≤100 | 976 (91) | 101 (9) | .823 | 898 (86) | 151 (14) | .529 | 621 (79) | 163 (21) | .771 | 632 (71) | 258 (29) | .036 |
| >100 | 2454 (91) | 247 (9) | 2302 (85) | 413 (15) | 1571 (79) | 425 (21) | 1743 (75) | 592 (25) | |||||
| Viral load copies/mL | ≤10 000 | 2011 (91) | 200 (9) | .411 | 1925 (84) | 37 (16) | .195 | 1368 (80) | 341 (20) | .967 | 1626 (76) | 520 (24) | .190 |
| >10 000 | 1045 (90) | 115 (10) | 960 (85) | 163 (15) | 663 (80) | 166 (20) | 678 (74) | 244 (26) | |||||
| Baseline serum creatinine | Median (IQR) | 82 (66-102) | 84 (64-114) | .18 | 81 (66-100) | 85 (67-114) | <.001 | 82 (67-103) | 80 (63-96) | .939 | 83 (67-104) | 79 (64-99) | <.001 |
| Baseline CrCl | Median (IQR) | 86 (68-107) | 82 (55-110) | .14 | 86 (68-106) | 84 (60-107) | <.001 | 85 (66-105) | 91 (72-115) | .262 | 85 (66-104) | 91 (71-112) | <.001 |
| Baseline GFR | Median (IQR) | 93 (73-116) | 90 (63-115) | .23 | 93 (74-116) | 85 (65-111) | <.001 | 92 (71-114) | 96 (77-125) | .692 | 92 (71-114) | 96 (75-120) | <.001 |
Abbreviations: ART, antiretroviral therapy; CrCl, creatinine clearance; GFR, glomerular filtration rate; IQR, interquartile range; WHO, World Health Organization.
Predictors of Renal Impairment over 144 Weeks of ART
The results of the multivariable logistic regression analysis to identify independent predictors of renal impairment through 144 weeks of ART are presented in Table 4. Age independently predicted renal impairment at 48 weeks of ART in the complete case analysis, with a 4% increase in the likelihood of renal impairment for every 1-year increment in age. However, after multiple imputations, age was no longer a significant predictor of renal impairment. Similarly, hepatitis B or C coinfection increased the risk of renal impairment by 49% at 96 weeks of ART (odds ratio [OR]: 1.49; 95% confidence interval [CI]: 1.16-1.93) in the complete case analysis, but its effect was not significant after multiple imputations for missing data. At 24 weeks of ART, only TDF exposure predicted the risk of renal impairment, with 47% greater likelihood of renal impairment in participants exposed to TDF. Tenofovir exposure did not, however, predict renal impairment at 48 and 96 weeks of ART but was associated with a 2-fold increase in the odds of renal impairment at 144 weeks of therapy (OR: 2.36; 95% CI: 1.28-4.34).
Table 4.
Multiple Variable Logistic Regression Analysis for Predictors of Renal Failure through 144 Weeks of ART.
| Weeks of ART (Complete Cases/Multiple Imputation) |
Explanatory Variable | Complete Cases |
Multiple Imputation |
||
|---|---|---|---|---|---|
| aOR (95% CI) | P Value | aOR (95% CI) | P Value | ||
| 24 (3806/8303) | Males | 0.86 (0.66-1.13) | .29 | 0.91 (0.59-1.4) | .61 |
| Age, years | 1.04 (1.02-1.05) | <.001 | 1.01 (0.97-1.05) | .52 | |
| WHO stage 3 and 4 | 1.23 (0.96-1.59) | .11 | 0.9 (0.49-1.64) | .66 | |
| CD4 cells/mm3 ≤100 | 1.05 (0.8-1.38) | .71 | 1.17 (0.58-2.36) | .59 | |
| VL >10 000 copies/mL | 1.3 (1-1.67) | .05 | 0.99 (0.74-1.32) | .92 | |
| HBV positive | 1.22 (0.91-1.63) | .19 | 0.95 (0.69-1.31) | .74 | |
| Baseline GFR | 1 (1-1) | .10 | 1 (1-1) | .68 | |
| TDF exposure | 2.34 (1.75-3.13) | <.001 | 1.47 (1.16-1.87) | <.001 | |
| 48 (3784/8303) | Males | 0.92 (0.75-1.12) | .41 | 0.91 (0.67-1.25) | .52 |
| Age, years | 1.04 (1.03-1.05) | <.001 | 1.01 (0.96-1.06) | .55 | |
| WHO stage 3 and 4 | 0.78 (0.64-0.96) | .02 | 0.83 (0.64-1.09) | .16 | |
| CD4 cells/mm3 ≤100 | 0.93 (0.76-1.13) | .46 | 1.08 (0.77-1.51) | .60 | |
| VL >10 000 copies/mL | 0.88 (0.72-1.07) | .19 | 0.88 (0.59-1.31) | .45 | |
| HBV positive | 1.2 (0.95-1.53) | .12 | 1.12 (0.83-1.5) | .41 | |
| Baseline GFR | 1 (1-1) | .12 | 1 (1-1) | .57 | |
| TDF exposure | 1.03 (0.84-1.25) | .79 | 1.25 (0.9-1.75) | .15 | |
| 96 (2802/8303) | Males | 1.01 (0.78-1.31) | .95 | 1.1 (0.48-2.51) | .76 |
| Age, years | 1.01 (1-1.02) | .14 | 1 (0.96-1.05) | .76 | |
| WHO stage 3 and 4 | 0.89 (0.69-1.16) | .40 | 0.94 (0.35-2.48) | .87 | |
| CD4 cells/mm3 ≤100 | 1.18 (0.89-1.56) | .26 | 1.1 (0.18-6.68) | .90 | |
| VL >10 000 copies/mL | 1.02 (0.79-1.34) | .86 | 1.4 (0.66-2.97) | .29 | |
| HBV positive | 1.49 (1.16-1.93) | <.001 | 0.86 (0.32-2.34) | .70 | |
| Baseline GFR | 1 (1-1.01) | .10 | 1 (1-1) | .99 | |
| TDF exposure | 5.42 (3.38-8.7) | <.001 | 0.77 (0.39-1.55) | .37 | |
| 144 (3244/8303) | Males | 1.04 (0.85-1.27) | .68 | 1.08 (0.56-2.1) | .77 |
| Age, years | 1.01 (1-1.02) | .17 | 0.98 (0.92-1.06) | .55 | |
| WHO stage 3 and 4 | 1.33 (1.1-1.61) | <.001 | 1.35 (0.52-3.51) | .43 | |
| CD4 cells/mm3 ≤100 | 1.06 (0.87-1.3) | .56 | 1.24 (0.59-2.62) | .48 | |
| VL >10 000 copies/mL | 0.92 (0.75-1.12) | .41 | 1.1 (0.42-2.87) | .79 | |
| HBV positive | 1.35 (1.1-1.66) | <.001 | 1.18 (0.88-1.58) | .23 | |
| Baseline GFR | 1 (1-1) | .10 | 1 (0.99-1.01) | .47 | |
| TDF exposure | 4.71 (3.72-5.97) | <.001 | 2.36 (1.28-4.34) | .02 | |
Abbreviations: aOR, adjusted odds ratio; ART, antiretroviral therapy; CI, confidence interval; GFR, glomerular filtration rate; HBV, hepatitis B virus; TDF, tenofovir disoproxil fumarate; VL, viral load; WHO, World Health Organization.
Discussion
This study utilized longitudinal data from a large cohort of HIV-1-infected adults on ART to examine the risk of renal impairment in patients exposed and unexposed to TDF-based ART over 144 weeks. We found that exposure to TDF-based ART doubled the likelihood of renal impairment at 144 weeks of ART after adjusting for age, sex, and other baseline risk factors for renal impairment.
We found only a marginal association between older age and renal impairment probably because our study population was predominantly young adults. Evidence of increased risk of renal impairment with older age has been reported in previous studies. 13,14,30-32 Furthermore, a pharmacokinetic study suggested that older age was independently associated with greater TDF exposure, which increases the risk of renal toxicity.30
In this study, coinfection with hepatitis B virus was not associated with increased odds of renal impairment. This finding is similar to that of a recent observational data from a multicenter Italian study of 6984 HIV-1-infected adults, which did not find an increased risk of renal impairment with hepatitis B serum antigen positivity.33
A notable finding of this study is the increased risk of renal function decline with longer exposure to TDF. Noteworthy because, unlike the 14% renal impairment at 144 weeks observed among patient unexposed to TDF, the proportion of those with renal impairment among TDF-exposed patients rose from 13% at 48 weeks to 35% and 45% at 96 and 144 weeks of ART, respectively. Consistent with data from previous studies,14,33-35 this finding highlights an increased risk of renal function decline with longer exposure to TDF. In a study that evaluated kidney function in 10 841 HIV-infected patients, each year of exposure to TDF was associated with 34% increased risk of proteinuria (95% CI: 25%-45%; P < .0001), 11% increased risk of rapid eGFR decline (3%-18%; P = .0033), and 33% increased risk of chronic kidney disease (CKD; 18-51%; P < .0001).14
The high prevalence of renal impairment among patients exposed to TDF observed in this study and the reported poor outcomes among patients exposed to TDF in other multicenter observational studies in Nigeria is a source of concern.36,37 There is thus an urgent need to review the continued use of TDF as one of the preferred first-line ARV drugs for HIV in Nigeria and other resource-constrained settings, as evidence exists to show that continued exposure of patients with mild renal impairment to TDF progressively leads to CKD.33,34
This study had some limitations that should be considered in the interpretation and application of the results. A major limitation of this study was that it was a retrospective and nonrandomized study, not designed to explore the causality of the relationship observed. However, the large sample size and the long duration of follow-up generated longitudinal data that provided useful information of a “real life” setting. Moreover, several observational studies 12,14,31,33-35,38 support the result of our study with regard to increased risk of renal impairment over time in patients exposed to TDF. Also, there exists the possibility of residual confounders that were not evaluated which should be considered when interpreting the results of this study. For instance, we had no data on concomitant medication use or comorbidities such as diabetes or hypertension and limited data on intercurrent illness(s). Additionally, we were unable to evaluate the impact of proteinuria on the results of this study as we did not routinely test for proteinuria at the commencement of ART in the earlier years of the program, as this was not recommended in the guidelines. Proteinuria is the most important factor in the development and progression of CKD.39 Furthermore, eGFR was estimated in our study using the MDRD equation instead of the CKD-EPI equation. In comparison to CKD-EPI, the MDRD equation tends to underestimate measured GFR at the higher range, around 60 mL/min/1.73 m2, leading to misclassification to a lower category and overdiagnosis of CKD.40 However, aside from the fact that MDRD has been shown to be valid in similar populations,41 our study used more than 1 criteria to define renal impairment. Finally, further studies involving time-to-event analysis is recommended to account for those who started with non-TDF-based regimens but later switched to TDF-based regimens or vice versa.
In conclusion, exposure to TDF-based ART significantly increased the likelihood of renal impairment over 144 weeks of therapy in our patient cohort. The continued use of TDF-based regimen in this setting needs urgent review, considering that over 40% of the patients are currently on TDF-based ART and 90% of new patients initiate treatment with TDF-containing regimen as per the Nigerian HIV treatment guidelines. We recommend the urgent introduction of tenofovir alafenamide–based regimen in the HIV treatment guidelines of Nigeria and other resource-limited countries. In the meantime, adherence to the estimation of SCr and eGFR before initiation of TDF-containing ART as we implement the HIV “test and start” strategy is important.
What Do We Already Know about This Topic?
Long-term nephrotoxicity associated with the use of tenofovir disoproxil fumarate (TDF); a potent antiretroviral drug is poorly described in HIV-1 positive Nigerians on TDF-based antiretroviral therapy (ART).
How Does Your Research Contribute to the Field?
Analysis of longitudinal data from a large cohort of HIV-1-infected adult Nigerians revealed that exposure to TDF-based ART doubled the likelihood of renal impairment at 144 weeks of ART.
What Are Your Research’s Implications toward Theory, Practice, or Policy?
Long-term use of TDF-based ART raises significant renal safety concerns among HIV-1-infected Nigerians and its use as initial ART regimen for adults and adolescents as per the Nigerian HIV treatment guidelines should be reconsidered, especially in settings were renal function monitoring cannot be guaranteed.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The U.S. Department of Health and Human Services, Health Resources and Services Administration (U51HA02522) and the Centers for Disease Control and Prevention (CDC) through a cooperative agreement with APIN (PS 001058) funded patient care, in part. The Fogarty International Center and National Institute of Mental Health, of the National Institutes of Health under Award Number D43 TW010130 and D43 TW010543, supported research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Ray AS, Fordyce MW, Hitchcock MJM. Tenofovir alafenamide: a novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res. 2016;125:63–70. doi: 10.1016/j.antiviral.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Brennan AT, Maskew M, Ive P, et al. Increases in regimen durability associated with the introduction of tenofovir at a large public-sector clinic in Johannesburg, South Africa. J Int AIDS Soc. 2013;16:1–12. doi: 10.7448/IAS.16.1.18794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368(9534):505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 4.Abgrall S, Ingle SM, May MT, et al. Durability of first ART regimen and risk factors for modification, interruption or death in HIV-positive patients starting ART in Europe and North America 2002–2009. AIDS. 2013;27(5):803–813. doi: 10.1097/QAD.0b013e32835cb997. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett JA, Chen S-S, Quinn JB. Comparative efficacy of nucleoside/nucleotide reverse transcriptase inhibitors in combination with efavirenz: results of a systematic overview. HIV Clin Trials. 2007;8(4):221–226. doi: 10.1310/hct0804-221. [DOI] [PubMed] [Google Scholar]
- 6.Dore GJ, Cooper DA, Pozniak AL, et al. Efficacy of tenofovir disoproxil fumarate in antiretroviral therapy—naive and—experienced patients coinfected with HIV-1 and hepatitis B virus. J Infect Dis. 2010;189(7):1185–1192. [DOI] [PubMed] [Google Scholar]
- 7.Bottecchia M, Garcia-Samaniego J, Soriano V. The implications of antiviral drugs with activity against hepatitis B virus and HIV. Curr Opin Intern Med. 2008;7(1):57–64. doi: 10.1097/QCO.0b013e3282f1e022. [DOI] [PubMed] [Google Scholar]
- 8.Matthews G The management of HIV and hepatitis B coinfection. Curr Opin Infect Dis. 2007;20(1):16–21. doi: 10.1097/QCO.0b013e328012c5aa. [DOI] [PubMed] [Google Scholar]
- 9.Verhelst D, Monge M, Meynard J-L, et al. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am J Kidney Dis. 2002;40(6):1331–1333. doi: 10.1053/ajkd.2002.36924. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell EP, Scarsi KK, Darin KM, Gerzenshtein L, Postelnick MJ, Palella FJ. Low incidence of renal impairment observed in tenofovir-treated patients. J Antimicrob Chemother. 2011;66(5): 1120–1126. doi: 10.1093/jac/dkr039. [DOI] [PubMed] [Google Scholar]
- 11.Kyaw NTT, Harries AD, Chinnakali P, et al. Low incidence of renal dysfunction among HIV-infected patients on a tenofovir-based first line antiretroviral treatment regimen in Myanmar. PLoS One. 2015;10(8):1–11. doi: 10.1371/journal.pone.0135188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quesada PR, Esteban LL, García JR, et al. Incidence and risk factors for tenofovir-associated renal toxicity in HIV-infected patients. Int J Clin Pharm. 2015;37(5):865–872. doi: 10.1007/s11096-015-0132-1. [DOI] [PubMed] [Google Scholar]
- 13.De Waal R, Cohen K, Fox MP, et al. Changes in estimated glomerular filtration rate over time in South African HIV-1-infected patients receiving tenofovir: a retrospective cohort study. J Int AIDS Soc. 2017;20(1):1–8. doi: 10.7448/IAS.20.01/21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26(7):867–875. doi: 10.1097/QAD.0b013e328351f68f.Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51(5):496–505. doi: 10.1086/655681. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, et al. Tenofovir nephrotoxicity: 2011 update. AIDS Res Treat. 2011; 2011:354908. doi: 10.1155/2011/354908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mudzviti T, Mudzongo NT, Gavi S, Chimbetete C, Maponga CC, Morse GD. A time to event analysis of adverse drug reactions due to tenofovir, zidovudine and stavudine in a cohort of patients receiving antiretroviral treatment at an outpatient clinic in Zimbabwe. Pharmacol Pharm. 2015;6(3):201–206. doi: 10.4236/pp.2015.63021. [DOI] [Google Scholar]
- 18.Federal Ministry of Health Nigeria. National Guidelines for HIV and AIDS Treatment and Care in Adolescents and Adults. 2007. http://www.who.int/hiv/amds/Nigeria_adult_2007.pdf. Accessed August 15, 2017.
- 19.Federal Ministry of Health Abuja N. National Guidelines for HIV Prevention Treatment and Care, National AIDS and STI’s Control Programme; 2016. [Google Scholar]
- 20.Federal Ministry of Health Nigeria. National Guidelines for HIV and AIDS Treatment and Care in Adolescents and Adults. 2010. http://www.who.int/hiv/pub/guidelines/nigeria_art.pdf. Accessed August 15, 2017.
- 21.National Agency for the Control of AIDS. Global AIDS Response Country Progress Report—Nigeria GARPR 2015. Abuja, Nigeria: National Agency for the Control of AIDS (NACA); 2015. doi: 10.1016/S0140-6736(73)92790-6. [DOI] [Google Scholar]
- 22.Agbaji O, Abene E, Johnson S, et al. Tenofovir-induced fanconi syndrome in an HIV infected nigerian: a case report. Trop J Nephrol. 2011;6(2):121–125. [Google Scholar]
- 23.Agbaji OO, Agaba PA, Idoko JA, et al. Temporal changes in renal glomerular function associated with the use of. West Afr J Med. 2011;30(3):164–168. http://www.ncbi.nlm.nih.gov/pubmed/22120479. Accessed August 15, 2016. [PubMed] [Google Scholar]
- 24.World Health Organization. WHO∣Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2013. http://www.who.int/hiv/pub/guidelines/arv2013/download/en/. Accessed August 18, 2015. [PubMed]
- 25.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2nd ed. France, Europe: World Health Organization; 2016. http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf. Accessed September 3, 2016. [PubMed] [Google Scholar]
- 26.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach. Vol 4911; 2010. http://www.who.int/hiv/pub/arv/adult2010/en/index.html. Accessed August 15, 2016. [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Kidney Foundation–Disease Outcomes Quality Initiative. NKF-DOQI clinical practice guidelines for hemodialysis adequacy. Am J Kidney Dis. 1997;30(3): S15–S66. doi: 10.1016/S0272-6386(97)70027-1. [DOI] [PubMed] [Google Scholar]
- 29.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4): R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baxi SM, Greenblatt RM, Bacchetti P, et al. Common clinical conditions—age, low BMI, ritonavir use, mild renal impairment—affect tenofovir pharmacokinetics in a large cohort of HIV-infected women. AIDS. 2014;28(1):59–66. doi: 10.1097/QAD.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poizot-Martin I, Solas C, Allemand J, et al. Renal impairment in patients receiving a tenofovir-cART regimen. J Acquir Immune Defic Syndr. 2013;62(4):375–380. doi: 10.1097/QAI.0b013e31827ce4ee. [DOI] [PubMed] [Google Scholar]
- 32.Yombi JC, Pozniak A, Boffito M, et al. Antiretrovirals and the kidney in current clinical practice. AIDS. 2014;28(5):621–632. doi: 10.1097/QAD.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 33.Lapadula G, Bernasconi DP, Casari S, et al. Risk of chronic kidney disease among patients developing mild renal impairment during tenofovir-containing antiretroviral treatment. PLoS One. 2016;11(9):1–11. doi: 10.1371/journal.pone.0162320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryom L, Mocroft A, Kirk O, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D Study. J Infect Dis. 2013;207(March 2012):5–8. doi: 10.1093/infdis/jit043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jotwani V, Scherzer R, Estrella MM, et al. Cumulative tenofovir disoproxil fumarate exposure is associated with biomarkers of tubular injury and fibrosis in HIV-infected men. J Acquir Immune Defic Syndr. 2016;73(2):177–181. doi: 10.1097/QAI.0000000000001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalhatu I, Onotu D, Odafe S, et al. Outcomes of Nigeria’s HIV/ AIDS treatment program for patients initiated on antiretroviral treatment between 2004–2012. PLoS One. 2016;11(11):1–25. doi: 10.1371/journal.pone.0165528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odafe S, Torpey K, Khamofu H, et al. The pattern of attrition from an antiretroviral treatment program in Nigeria. PLoS One. 2012;7(12):1–7. doi: 10.1371/journal.pone.0051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horberg M, Tang B, Towner W, et al. Impact of tenofovir on renal function in HIV-infected, antiretroviral-naive patients. J Acquir Immune Defic Syndr. 2010;53(1):62–69. doi: 10.1097/QAI.0b013e3181be6be2. [DOI] [PubMed] [Google Scholar]
- 39.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010; 303(5):423. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 40.Stevens LA, Li S, Kurella Tamura M, et al. Comparison of the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) study equations: risk factors for and complications of CKD and mortality in the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2011;57(3 suppl 2):1–17. doi: 10.1053/j.ajkd.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the Modification of Diet in Renal Disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18(10):2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]