Abstract
Importance:
Atopic diseases, including asthma and atopic eczema, are the most common chronic conditions of childhood.
Objective:
To investigate whether an intervention to promote prolonged and exclusive breastfeeding protects against asthma, atopic eczema and low lung function in adolescence.
Design, Setting, and Participants:
Follow-up of a cluster-randomized trial in 30 Belarusian maternity hospitals and affiliated polyclinics; recruitment of 17,046 healthy term infants took place June 1996-December 1997.
Intervention:
Randomization to receive a breastfeeding promotion intervention vs. usual care
Main Outcomes and Measures:
Spirometry and flexural eczema on standardized skin examination by study pediatricians were primary outcomes; secondary outcomes were self-reported asthma diagnosis ever, and wheeze and flexural eczema symptoms in the previous year.
Results:
13,557 (79.5%) participants were followed up September 2012-July 2015. The intervention (n=7064, 79.7%) and control (n=6493, 79.4%) groups were similar at follow-up (50.8%/52.5% male, mean (SD) age 16.2 (0.6)/16.1 (0.5) years). In the intervention group, 0.3% (21/7064) had flexural eczema on skin examination and mean (SD) FEV1/FVC ratio z-score was −0.10 (1.82), compared to 0.7% (43/6493) and 0.35 (1.34) respectively in the control group. In modified intention-to-treat analysis, accounting for clustering by polyclinic, a 54% lower risk of flexural eczema on skin examination was observed in the intervention compared with the control group (OR, 0.46; 95% CI: 0.25, 0.86). Self-reported flexural eczema symptoms in the past year (OR, 0.57; 95% CI: 0.27, 1.18), asthma (OR, 0.76; 95% CI: 0.47, 1.23) and wheezing in the past year (OR, 0.66; 95% CI: 0.37, 1.18) were less frequently reported in the intervention compared with the control group, but confidence intervals were wide and included the null. There was no difference in FEV1/FVC ratio z-score (β −0.15; 95% CI: −0.76, 0.45). All results were similar with additional adjustment for baseline characteristics, on instrumental variable analysis, and with multiple imputation among all 17,046 randomized participants.
Conclusions and Relevance:
A breastfeeding promotion intervention greatly reduced flexural dermatitis risk but had no detectable effect on lung function or questionnaire-derived measures of atopic eczema or asthma in adolescence in a setting where atopic eczema and allergies are rare.
Keywords: Atopic dermatitis, atopic eczema, asthma, lung function, breastfeeding
INTRODUCTION
Many allergy organizations, ministries of health, and the World Health Organization recommend between four and six months of exclusive breastfeeding to aid prevention of allergy and associated illnesses.1 These recommendations are largely based on cross-sectional studies, which have shown contradictory results.2–6 Infancy and early childhood is also a critical period for lung function development, which is linked to asthma. Low lung function at birth is associated with asthma7, and the onset of asthma in childhood is associated with low lung function through adult life.8 There is some evidence that breastfeeding could influence lung development in childhood and subsequent lung function, but results of observational studies have been inconsistent.9
Methodological shortcomings might explain some of these contradictions. Observational studies are prone to confounding, in particular because of substantial differences between mothers who do and do not choose to breastfeed, making it difficult to determine whether the observational associations of breastfeeding duration with child health outcomes are causal or have alternative explanations.
The unbiased effects of breastfeeding can probably only be convincingly demonstrated in a randomized controlled trial (RCT). While it is not feasible to randomize healthy term infants to be breast or bottle fed, it is possible to randomize mother-child pairs to a breastfeeding promotion intervention. The PROmotion of Breastfeeding Intervention Trial (PROBIT, ISRCTN37687716) is a large cluster RCT of breastfeeding promotion carried out in Belarus. The randomization achieved marked differences in breastfeeding exclusivity and duration.10 Follow-up of the PROBIT trial participants thus offers a unique opportunity to test the long-term effects of breastfeeding on childhood outcomes including asthma, lung function, and atopic eczema.
METHODS
Study design
The PROBIT trial design has been described in detail previously10 and the study protocol for the adolescent follow up is found in Supplement 1. In brief, 34 maternity hospitals and one each of their affiliated polyclinics (outpatient clinics where children are followed for routine health care) were paired and randomly assigned to receive either a breastfeeding promotion intervention (experimental group) or continuation of the prevailing maternity hospital and polyclinic practices (control group). Cluster randomization was preferred over individual randomization, because randomizing individual women within the same maternity hospital to different interventions would have led to contamination between the two treatment groups and a consequent dilution of the effect of the intervention. After randomization, two hospitals refused to participate, and a third randomized site was removed from the trial because of documented falsification of outcome data during infant follow-up.11 This left 16 intervention and 15 control sites in the trial. Recruitment for PROBIT began in June 1996 and continued until the end of December 1997. While it was not possible to blind study staff to the status of each site, as they were implementing the intervention, PROBIT participants were blinded to their randomization status.
Participants
Mothers were eligible for participation if they initiated breastfeeding on admission to the postpartum ward, had no illnesses that would contraindicate breastfeeding or severely compromise its success, and had given birth to a healthy singleton infant of at least 37 completed weeks of gestation, 2500 g birth weight, and an Apgar score of 5 at 5 minutes. Study staff estimated that only 1–2% of eligible women declined participation. Since all enrolled women had initiated breastfeeding, the experimental intervention was designed to increase the duration and exclusivity of breastfeeding.
Intervention
The experimental intervention included 10 steps that maternity hospitals must implement to become certified as ‘Baby-Friendly’.12 Clinical leaders, usually the chief obstetrician and pediatrician from each of the intervention maternity hospitals and polyclinics, received the 18-hour Baby Friendly Hospital Initiative (BFHI) lactation management training course, which was organized by the European Regional Office of the World Health Organization. The course emphasized methods to maintain lactation, promote exclusive and prolonged breastfeeding, and resolve common problems. Full implementation of the experimental intervention required 12–16 months to train midwives, nurses, and physicians in the provision of care to study mothers and infants during labor, delivery, and the postpartum hospital stay, and pediatricians and nurses working at the polyclinics. Monitoring visits were conducted before and during recruitment and follow-up to ensure compliance with and maintenance of the randomized interventions.10
PROBIT follow-up and data quality assurance
Mother-infant pairs were initially followed up for 12 months from the time of birth, including regular skin assessments for atopic eczema. The primary trial outcome was the risk of one or more episodes of gastrointestinal tract infection. The risk of atopic eczema was an important secondary outcome during the initial follow-up and was based on a physical examination at each follow-up visit at the polyclinic affiliated with the maternity hospital. The second follow-up was carried out 2002–2005, when the children were aged 6.5 years, and included the International Study for Asthma and Allergies in Childhood (ISAAC) questionnaire to elicit asthma and atopic eczema symptoms, as well as skin-prick tests. The third follow-up was conducted at 11.5 years of age (2008–2010) but did not include atopy-related outcomes. This current paper focuses on the follow-up at 16 years between September 2012 and July 2015, when atopic eczema was once more assessed through physician-conducted skin examination of all participants (primary outcome), the ISAAC questionnaire was completed for symptoms of asthma and eczema (secondary outcomes), and lung function was measured by spirometry (primary outcome).
The 16-year follow-up was approved by the Belarusian Ministry of Health. Ethical approval was obtained from the McGill University Health Centre Research Ethics Board, the Institutional Review Board at Harvard Pilgrim Health Care, and the Avon Longitudinal Study of Parents and Children Law and Ethics Committee. Parents provided informed consent and children written assent for the adolescent follow up.
Quality assurance was achieved through ongoing data monitoring, as described previously.13 We held an initial workshop during which all participating polyclinic pediatricians were trained in spirometry by the study pediatric pulmonologist (AJH) and in skin examination by the study dermatologist (CF) and then formally examined in a written, skills-based test for the diagnosis of atopic eczema. The performance of spirometry and the accuracy of the pediatricians’ diagnosis of atopic eczema was re-examined halfway through the study through a refresher training workshop, directly followed by a further written skills-based test, which all pediatricians successfully passed.
The quality assurance processes raised concerns about the validity of data collected at the 16-year follow-up from one polyclinic, and the 16-year data from this clinic were therefore not included in the analyses. In the remaining 30 polyclinics (15 in the intervention group, 15 in the control group), the children were seen at the 16-year visits by 36 research pediatricians; one in each of twenty-four polyclinics, and two in each of the remaining 6 high-volume clinics.
Atopic eczema and asthma assessments at 16 years
At the in-person follow-up visit, all children were physically examined for evidence of flexural dermatitis in the following 5 body areas: i) around the eyes; ii) the neck; iii) in front of the elbows; iv) behind the knees; and v) in front of the ankles, using the validated International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two skin examination protocol, which is based on the UK refinement of the Hanifin & Rajka consensus diagnostic criteria.14 Like the ISAAC questions, the UK diagnostic criteria focus on flexural involvement to enhance the specificity of the diagnosis. Many other skin diseases are non-flexural but pruritic, such as scabies and fungal infections, and are frequent in low-income country settings. Participants were categorized as having atopic eczema if they had a typical erythematous rash with surface changes (e.g., fine scaling, vesicles, oozing, crusting or lichenification) in any of the above flexural areas.
In addition to the skin examination, children self-reported their atopic eczema and asthma symptoms in the past 12 months on the ISAAC questionnaire. The instrument was identical to the one used at age 6.5 years in the PROBIT cohort,15 but at 6.5 years the parent was the respondent. The questions relevant to atopic eczema were: “Have you ever had an itchy rash which was coming and going for at least six months?” (yes/no = ‘atopic eczema symptoms ever’), “Have you had this itchy rash at any time in the past 12 months?” (yes/no = ‘atopic eczema symptoms past year’), and “Has this itchy rash at any time affected any of the following places: folds of the elbows, behind the knees, in front of the ankles, around the neck, or eyes?” (yes/no = ‘flexural eczema past year’). Atopic eczema severity was assessed by asking “Has this rash cleared completely at any time during the past 12 months?” and “In the past 12 months, how often, on average, have you been kept awake at night by this itchy rash?” (‘never in the past 12 months’/’less than one night per week’/’one or more nights per week’). Asthma symptoms were sought through responses to the questions: “Have you ever had asthma?” (yes/no = ‘asthma ever’), “Have you had wheezing/whistling in the chest in the past 12 months?” (yes/no = ‘wheezing in the past 12 months’), and “Have you had an attack of asthma in the past 12 months?” (yes/no = ‘asthma attack in the past 12 months’).
Lung function measurements at 16 years
Lung function was measured by spirometry according to standards recommended by the American Thoracic Society/European Respiratory Society task force16 using a Micro 1 handheld spirometer (CareFusion UK 236 Ltd., Basingstoke, United Kingdom). Each spirometer was calibrated at the beginning of each testing session using a 3L calibration syringe according to the manufacturer’s instructions. The calibration procedure was repeated if results differed by more than 3.5% of the calibrated value. If calibration to within these limits could not be achieved, the spirometer was replaced. Spirometry was avoided within 3 weeks of a reported respiratory infection or a course of oral corticosteroids. Participants were asked to omit long-acting bronchodilators for 48 hours and short-acting bronchodilators for 12 hours prior to the study visit. Pediatricians measured the subject’s height to the last completed millimetre using a stadiometer, and weight with an electronic digital scale (Tanita TBF 300GS body-fat analyser, Tanita Inc, Tokyo, Japan). Spirometry was performed in the seated position and participants wore nose clips during each forced expiratory manoeuver. Following a demonstration from the tester, subjects were instructed to fill their lungs and to blow as hard and fast as possible into the mouthpiece with verbal encouragement from the tester to maintain the breath for as long as possible. Up to eight attempts were permitted to achieve three blows that fulfilled the spirometer’s inbuilt start of test, time to peak flow and duration criteria.
Lung function data cleaning and transformation
The results of each accepted blow were analyzed to select the two attempts for each participant with the highest forced vital capacity (FVC) that was reproducible to within 0.15L. Forced expiratory volume in 1 second (FEV1) and FVC were selected from the blow with the higher FVC of the two. Applying these criteria, 1374 results (704 in the intervention and 670 in the control group) were excluded from analysis. Lung function variables (FEV1, FVC and FEV1/FVC ratio) were adjusted for age, height and sex of the participant using Global Lung Initiative (GLI) algorithms17 to derive z-scores for each.
Data management, statistical analysis and study power
Data management
Audit visits were conducted to assess inter-observer reproducibility of the outcome data, an important step, given that blinding of pediatricians to the experimental vs. control randomized group assignment was not feasible. For each pediatrician in the 24 lower-volume polyclinics, 4 children were randomly selected to return for re-measurement of all variables. For the 6 higher-volume clinics with 2 study pediatricians, 3 children per pediatrician were selected. Thus, a total of 132 children were audited. So that all children seen in follow-up were eligible for the repeated measurements, the selection was carried out after completion of primary data collection, an average of 1.2 years (range, 0.02–2.5) after the initial clinic visit. The audit was carried out by 1 of 3 Minsk-based pediatricians not involved in primary data collection. They were blinded to the measures obtained at the initial visit but not to experimental vs. control status.
Study power
The original sample size for PROBIT was based on power to detect a difference in gastrointestinal tract infections in infancy.10 For this analysis we calculated power based on the available sample size. For the categorical atopic eczema outcome at the 16-year follow-up (flexural eczema on skin examination), the study had 94% power at the 5% significance level to detect a 50% reduction in atopic eczema prevalence between the two study groups, similar to PROBIT I.10 This is a large effect compared to other prevention trials, such as the Barrier Enhancement Eczema Prevention (BEEP) trial, which reported 90% power at the 5% significance level to detect a relative reduction in atopic eczema of 30%; a reduction deemed as clinically significant.18 As for lung function, the minimal detectable difference in FEV1 or FVC at the 1% significance level with 90% power was 0.04 SD units based on the sample size available.
Statistical analysis
Because PROBIT is a randomized trial, the primary analytic approach was by modified intention-to-treat (ITT), excluding one study center (see above). We accounted for possible non-independence of measurements within individual hospitals and their affiliated polyclinic sites (clustering) using mixed effect models. We used the GLIMMIX procedure for binary outcomes to estimate ORs (95% CIs). (SAS version 9.3, SAS Institute, Cary, NC). The results are presented for the simple cluster-adjusted model, as well as after additional adjustment for stratum-level (urban vs. rural and East vs. West Belarus) and for individual-level (child age at follow-up, sex, birth weight, and maternal and paternal education) covariates (pre-specified secondary analyses). For asthma and lung function models we also adjusted for length of gestation. To determine whether results differed in boys versus girls, we conducted mixed models that included multiplicative interaction terms for the sex of the child. In a post hoc sensitivity intention to treat analysis, we used multiple imputation to investigate whether loss to follow up influenced the results, generating plausible values of missing 16-year outcomes for all 17,046 randomized participants. We used SAS multiple imputations (Proc MI) to impute 20 values for each missing observation and combined multivariable modeling estimates using Proc MI ANALYZE in SAS.19
The modified intention-to-treat analysis may underestimate the effect of the true exposures of interest (breastfeeding exclusivity and duration), owing to overlap in breastfeeding between the randomized groups (many intervention mothers did not exclusively breastfeed for 3 or 6 months, and some control mothers did). Therefore, in a pre-specified secondary analysis, we applied instrumental variable methods to estimate the effects of the difference in breastfeeding exclusivity and duration achieved between the two randomized groups (≥3 months vs. <3 months exclusive breastfeeding, as in the other PROBIT phases10) with the study outcomes. Unlike propensity score matching, this approach uses randomization status as an instrument, assuming that randomization status is independent of any confounders of the exposure-outcome relationships and related to the outcome only via the exposure (breastfeeding duration and exclusivity).20 Effects of exclusive breastfeeding for 3 months or longer using instrumental variable analysis was also estimated after accounting for clustering and further adjusting for strata and individual-level covariates. We used the ivprobit procedure in Stata/SE version 14 (Stata Corp) for binary lung function outcomes, and then calculated odds ratios (ORs) to be consistent with the primary modified intention-to-treat analysis by multiplying the probit estimates by 1.6. The validity of this multiplication has been demonstrated both statistically and empirically.21
To assess whether we could reproduce the inverse associations of increased duration and exclusivity of breastfeeding with outcomes reported in previous observational studies, we also conducted observational analyses (i.e., disregarding randomization status) in which we estimated associations of the duration of any or exclusive breastfeeding on the same outcomes, also accounting for clustering and the same baseline characteristics as in the expanded mixed models described above, using multiple logistic regression analysis. Duration of any and exclusive breastfeeding was classified as <3 months (reference) or ≥3 months. We used WHO definitions for this categorization in which infants were considered as exclusively breastfed for 3 or 6 months if they received no solids, nonbreast milk, or water or other liquids (other than vitamins or medications) at all visits up to and including the 3- and 6-month visits, respectively. They were considered predominantly breastfed at these ages if they received no solids or nonbreast milk; juices, water, teas, and other liquids were permitted in this category.
Finally, we carried out a post hoc sensitivity analysis, in which we stratified the results by whether or not the children correctly identified their trial group, to determine whether this knowledge biased any of the measured outcomes. Furthermore, we examined whether those reporting asthma symptoms had reduced lung function to validate the questionnaire-derived findings. We did not conduct any repeated measures analyses, because methods of assessment for outcomes differed over time. We ran additional models adjusted for pregnancy smoking status, child smoking status ever and current, and household smoking. Adding these covariates did not appreciably change the results.
RESULTS
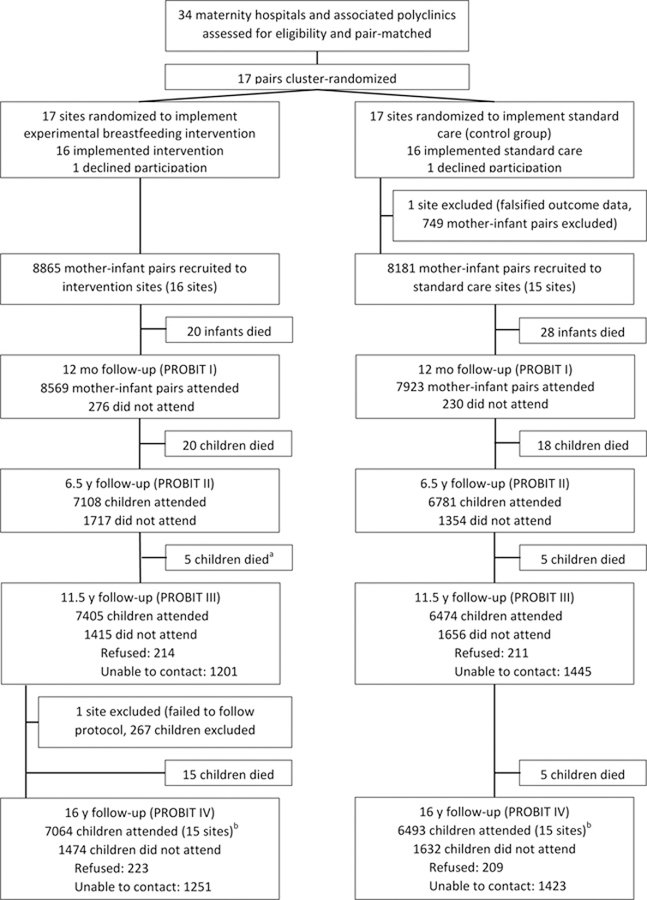
A total of 17,046 mother-infant pairs (n=8865 intervention and n=8181 controls) were enrolled during their postpartum stay. Figure 1 shows the numbers of infant-mother pairs born at maternity hospitals and followed at polyclinics randomized to breastfeeding promotion vs. usual care who participated in the PROBIT IV follow-up. A total of 13,557 adolescents (6981 boys, 51.5%) were examined in the 30 included polyclinics at a median (SD) age of 16.1 (0.54) years (range 14.8–18.9), representing 79.5% of the 17,046 originally randomized. Asthma and atopic eczema outcomes were available for all participants who were followed up at 16 years.
Figure 1.
Flowchart of study sites and participants (CONSORT)
a During PROBIT III, 6 deaths were reported in the intervention arm. Data checking during PROBIT IV found one of these children had been incorrectly reported as deceased and data were amended.
b Of the 13557 seen at PROBIT IV, 12072 were seen at both PROBIT II & III, 274 were not seen at either PROBIT II & III, 449 were seen at PROBIT II but not at PROBIT III, and 762 were seen at PROBIT III but not at PROBIT II.
Cleaning of lung function data resulted in 12,183 participants (n = 6360 intervention and n = 5823 controls) with valid measurements. Of the 3489 participants who were not followed up at 16 years, 116 had died since randomization, 2674 were lost to follow-up, 267 were excluded from one clinic that deviated from the study protocol, and 432 were unable or unwilling to come for their visit (Figure 1). Follow-up rates were similar overall in the experimental (79.7%) and control (79.3%) polyclinics, although they varied by polyclinic from 41% to 98%.
Participant characteristics
The intervention (n=7064, 79.7%) and control (n=6493, 79.4%) groups were similar at follow-up (50.8%/52.5% male, mean (SD) age 16.2 (0.6)/16.1 (0.5) years) (Table 1). Other sociodemographic characteristics were comparable between the two groups, except for over-representation of urban residence in Western Belarus in the intervention group and of advanced secondary/partial university education in the control group (Table 1). Similar results for the population with valid lung function measurements are shown in eTable 1. We also collected information on parental history of allergic diseases (atopic eczema, asthma, and hay fever) during the 12-month follow up of PROBIT. The intervention group had slightly more mothers and fathers who reported atopy vs. the control group (5.2% (463/8865) compared to 3.5% (290/8181), respectively), and this difference persisted into the adolescent follow-up (Chi square p=0.0002). However, adjusting for family history of atopy did not alter the risk estimates.
Table 1.
Characteristics of 13,577 children enrolled in the PROBIT trial with outcome data at 16 years
| N (%) | |||
|---|---|---|---|
| Characteristic | Total (n = 13557) | Intervention (n = 7064) | Control (n = 6493) |
| Measured at child’s birth | |||
| Maternal age, y | |||
| <20 | 1820 (13.4) | 979 (13.9) | 841 (13.0) |
| 20–34 | 11173 (82.4) | 5792 (82.0) | 5381 (82.9) |
| ≥35 | 564 (4.2) | 293 (4.1) | 271 (4.2) |
| Maternal education | |||
| Completed university | 1842 (13.6) | 1002 (14.2) | 840 (12.9) |
| Advanced secondary or partial university | 6925 (51.1) | 3365 (47.6) | 3560 (54.8) |
| Common secondary | 4318 (31.9) | 2406 (34.1) | 1912 (29.4) |
| Incomplete secondary | 472 (3.5) | 291 (4.1) | 181 (2.8) |
| Paternal education | |||
| Completed university | 1737 (12.8) | 936 (13.3) | 801 (12.3) |
| Advanced secondary or partial university | 6205 (45.8) | 2910 (41.2) | 3295 (50.7) |
| Common secondary | 4883 (36.0) | 2828 (40.0) | 2055 (31.6) |
| Incomplete secondary or unknown | 732 (5.4) | 390 (5.5) | 342 (5.3) |
| Stratum-level variable | |||
| East/Urban | 4150 (30.6) | 2215 (31.4) | 1935 (29.8) |
| East/Rural | 2152 (15.9) | 1075 (15.2) | 1077 (16.6) |
| West/Urban | 3524 (26.0) | 2296 (32.5) | 1228 (18.9) |
| West/Rural | 3731 (27.5) | 1478 (20.9) | 2253 (34.7) |
| Number children in household | |||
| 0 | 7707 (56.8) | 4152 (58.8) | 3555 (54.8) |
| 1 | 4717 (34.8) | 2365 (33.5) | 2352 (36.2) |
| 2+ | 1133 (8.4) | 547 (7.7) | 586 (9.0) |
| Maternal smoking during pregnancy | |||
| No | 13287 (98.0) | 6898 (97.7) | 6389 (98.4) |
| Yes | 270 (2.0) | 166 (2.3) | 104 (1.6) |
| Child sex | |||
| Female | 6576 (48.5) | 3474 (49.2) | 3102 (47.8) |
| Male | 6981 (51.5) | 3590 (50.8) | 3391 (52.2) |
| Birthweight, mean (SD), kg | 3.44 (0.42) | 3.44 (0.42) | 3.44 (0.42) |
| Gestation length, mean (SD), wks | 39.4 (1.0) | 39.4 (1.0) | 39.3 (1.0) |
| Measured in child’s first year | |||
| Duration of exclusive breastfeeding, mo | |||
| <3 | 9861 (73.2) | 3821 (54.7) | 6040 (93.1) |
| 3–<6 | 3126 (23.2) | 2727 (39.0) | 399 (6.2) |
| ≥6 | 484 (3.6) | 437 (6.3) | 47 (0.7) |
| Duration of any breastfeeding, mo | |||
| <3 | 4692 (34.8) | 2085 (29.8) | 2607 (40.2) |
| 3–<6 | 3105 (23.0) | 1590 (22.7) | 1515 (23.4) |
| ≥6 | 5676 (42.1) | 3315 (47.4) | 2361 (36.4) |
SD = standard deviation
Modified intention-to-treat analyses
Of 7064 children in the intervention group, 21/7064 (0.3%) had signs of flexural eczema on skin examination at the 16-year follow up (primary outcome), compared with 43/6493 (0.7%) in the control group (difference −0.4%, 95% CI: −0.60, −0.16). This difference corresponded to a 54% lower risk in the intervention compared to the control group (cluster-adjusted OR, 0.46; 95% CI: 0.25, 0.86), with a very similar estimate after further adjustment for baseline factors and age at follow-up (adjusted OR, 0.46; 95% CI: 0.25, 0.83, Table 2). The odds ratio was of similar magnitude, but with lower precision, for the questionnaire-derived flexural eczema symptoms (secondary outcomes): flexural eczema in the past year (32/7064 (0.5%) vs. 45/6493 (0.7%); cluster-adjusted OR, 0.57; 95% CI: 0.27, 1.18), persistent flexural eczema in the past year (cluster-adjusted OR, 0.48; 95% CI: 0.22, 1.04), and sleep-disturbed flexural eczema in the past year (cluster adjusted OR, 0.54; 95% CI: 0.23, 1.28). Effect sizes were similar for boys and girls (all interaction p values >0.26).
Table 2.
Modified intention-to-treat analysis comparing atopic eczema and asthma outcomes in intervention vs. control group
| Intervention (n=7064) | Control (n=6493) | Cluster-adjusted | Further adjusted * | |
|---|---|---|---|---|
| Atopic eczema | No. (%) | OR (95% CI) | ||
| Flexural eczema on skin examination | 21 (0.3) | 43 (0.7) | 0.46 (0.25, 0.86) | 0.46 (0.25, 0.83) |
| Flexural eczema past year | 32 (0.5) | 45 (0.7) | 0.57 (0.27, 1.18) | 0.55 (0.27, 1.14) |
| Persistent flexural eczema past year | 10 (0.1) | 19 (0.3) | 0.48 (0.22, 1.04) | 0.47 (0.22, 1.03) |
| Sleep-disturbed flexural eczema past year | 13 (0.2) | 24 (0.4) | 0.54 (0.23, 1.28) | 0.55 (0.22, 1.39) |
| Asthma | ||||
| Ever asthma | 108 (1.5) | 110 (1.7) | 0.76 (0.47, 1.23) | 0.77 (0.47, 1.24) |
| Wheezing in past 12 months | 431 (6.1) | 419 (6.5) | 0.66 (0.37, 1.18) | 0.61 (0.34, 1.07) |
| Asthma attack in past 12 months | 29 (0.4) | 24 (0.4) | 1.01 (0.54, 1.89) | Did not converge |
| Lung function | Mean (SD) | β (95% CI) | ||
| FEV1 z-score | −0.70 (1.57) | −0.13 (1.12) | −0.43 (−0.78, −0.08) | −0.39 (−0.74, −0.04) |
| FVC z-score | −0.45 (1.26) | −0.27 (1.12) | −0.23 (−0.60, 0.13) | −0.19 (−0.56, 0.17 ) |
| FEV1/FVC z-score | −0.10 (1.82) | 0.35 (1.34) | −0.15 (−0.76, 0.45) | −0.16 (−0.76, 0.45) |
| FEV1/FVC x 100 | 0.85 (0.15) | 0.89 (0.09) | −2.03 (−6.34, 2.29) | −2.03 (−6.36, 2.30) |
Adjusted for stratum-level variables (urban vs. rural and East vs. West Belarus), and for child age at follow-up, sex, birthweight, and maternal and paternal education. Asthma and lung function models were also adjusted for gestational age at birth.
There was no evidence of a protective effect of breastfeeding on the secondary asthma outcomes (Table 2). Asthma ever was reported in 1.5% (108/7064) of the intervention group and 1.7% (110/6493) of the control group (cluster-adjusted OR, 0.76; 95% CI: 0.47, 1.23). Wheezing in the past year was less frequently reported in the intervention compared with the control group (cluster-adjusted OR, 0.66; 95% CI: 0.37, 1.18), but the CI was wide and crossed 1.0. There was no difference in reported asthma attacks in the past 12 months between intervention and control groups. The effect estimates for asthma ever and wheezing in the past 12 months were similar after further adjustment for baseline variables.
FEV1, FVC and FEV1/FVC were each lower in the intervention than the control group (Table 2). Mean (SD) FEV1 % predicted was 91.5 (19.0) in the intervention group and 98.4 (13.3) in the control group. Confidence intervals excluded the null in both cluster-adjusted analyses and following additional adjustment for baseline variables, which only marginally changed the effect estimates (Table 2). However, the FEV1/FVC z-score β had a wide confidence interval, including the null (−0.15 (−0.76, 0.45) and −0.16 (−0.76, 0.45) respectively. FEV1 and FVC at audit visits were strongly correlated with the original polyclinic measurements (FEV1; Pearson’s r, 0.84; 95% CI: 0.77, 0.89; FVC; r, 0.90; 95% CI: 0.85, 0.93). Additionally, lung function was correlated with height (FEV1; r, 0.57; FVC; r, 0.67) and showed expected associations with reported asthma categories (eTable 2).
Instrumental variable, observational and sensitivity analyses
Instrumental variable (IV) analysis confirmed an inverse association between exclusive breastfeeding for 3 months or longer (vs. exclusive breastfeeding <3 months) and flexural eczema on skin examination (cluster-adjusted OR, 0.34; 95% CI: 0.13, 0.85); additional adjustment for baseline characteristics yielded similar results (OR, 0.34; 95% CI: 0.16, 0.72; Table 3). As in the modified intention-to-treat analysis, the risk estimates for questionnaire-derived flexural eczema symptoms in the past year were less precise (cluster-adjusted OR, 0.55; 95% CI: 0.19, 1.60; after additional adjustment for baseline characteristics: OR, 0.56; 95% CI: 0.22, 1.38; Table 3). The reduced lung function seen in modified ITT, became non-significant in the IV analysis, which also confirmed no evidence of associations between exclusive breastfeeding for ≥3 months (compared with <3 months) and questionnaire-derived asthma outcomes. Wider confidence intervals in IV analyses are expected because of the increased variance introduced by using randomization as a predictor of breastfeeding.
Table 3.
Instrumental variable and observational associations of duration and exclusivity of breastfeeding (≥3 months vs. <3 months) with atopic eczema, lung function and asthma outcomes
| Instrumental variable analysis | Observational analysis | |||
|---|---|---|---|---|
| Exclusive breastfeeding >=3 vs. <3 months (control) | Exclusive breastfeeding >=3 vs. <3 months (control) | |||
| Cluster-adjusted | Further adjusted * | Cluster-adjusted | Further adjusted * | |
| OR (95% CI) | OR (95% CI) | |||
| Flexural eczema on skin examination | 0.34 (0.13, 0.85) | 0.34 (0.16, 0.72) | 0.70 (0.36, 1.36) | 0.64 (0.33, 1.25) |
| Flexural eczema past year | 0.55 (0.19, 1.60) | 0.56 (0.22, 1.38) | 0.89 (0.50, 1.60) | 0.85 (0.47, 1.53) |
| Persistent flexural eczema past year | 0.40 (0.14, 1.12) | 0.39 (0.15, 1.01) | 0.71 (0.29, 1.75) | 0.70 (0.28, 1.72) |
| Sleep disturbed flexural eczema past year (ever) | 0.41 (0.13, 1.23) | 0.46 (0.17, 1.25) | 0.96 (0.41, 2.23) | 0.98 (0.41, 2.30) |
| Wheezing past 12 months | 0.86 (0.44, 1.68) | 0.73 (0.38, 1.40) | 1.02 (0.85, 1.24) | 1.03 (0.85, 1.24) |
| Ever asthma | 0.85 (0.39, 1.85) | 0.82 (0.41, 1.63) | 0.99 (0.70, 1.39) | 0.98 (0.70, 1.38) |
| Asthma attack in past 12 months | 1.14 (0.50, 2.59) | 1.06 (0.51, 2.18) | 1.35 (0.75, 2.44) | Did not converge |
| β (95% CI) | β (95% CI) | |||
| FEV1 z-score | −1.14 (−2.31, 0.02) | −1.00 (−2.17, 0.18) | 0.03 (−0.03, 0.09) | 0.03 (−0.03, 0.10) |
| FVC z-score | −0.62 (−1.56, 0.32) | −0.49 (−1.61, 0.63) | 0.03 (−0.03, 0.08) | 0.03 (−0.02, 0.08) |
| FEV1:FVC ratio z-score | −0.41 (−2.06, 1.25) | −0.41 (−2.30, 1.47) | 0.01 (−0.06, 0.08) | 0.01 (−0.06, 0.08) |
| FEV1:FVC ratio x 100 | −5.34 (−17.76, 7.08) | −5.23 (−19.31, 8.85) | 0.09 (−0.44, 0.62) | 0.10 (−0.43, 0.62) |
Adjusted for stratum-level variables (urban vs. rural and East vs. West Belarus), and for child age at follow-up, sex, birthweight, and both maternal and paternal education. Asthma and lung function models were also adjusted for gestational age at birth.
In the observational analysis (exclusive breastfeeding ≥3 months vs. <3 months, reference), the magnitude of the protective effect was not as large as the ITT effect (flexural eczema on skin examination: cluster-adjusted OR, 0.70; 95% CI: 0.36, 1.36; after additional adjustment for baseline characteristics: OR, 0.64; 95% CI: 0.33, 1.25; flexural eczema symptoms in the past year: cluster-adjusted OR, 0.89; 95% CI: 0.50, 1.60; after additional adjustment for baseline characteristics: OR, 0.85; 95% CI: 0.47, 1.53). We observed no evidence of an association comparing any breastfeeding ≥3 months vs. <3 months (reference) (flexural eczema on skin examination: cluster-adjusted OR, 0.94; 95% CI: 0.56, 1.56; after additional adjustment for baseline characteristics: OR, 0.88; 95% CI: 0.52, 1.47; flexural eczema symptoms in the past year: cluster-adjusted OR, 1.26; 95% CI: 0.77, 2.06; after additional adjustment for baseline characteristics: OR, 1.18; 95% CI: 0.72, 1.93). Similarly, for asthma outcomes, the observational effects were closer to the null than the estimates from the instrumental variables analysis, with no evidence that exclusive breastfeeding ≥3 months was associated with ever asthma (cluster-adjusted OR, 0.99; 95% CI: 0.70, 1.39), wheezing in the past 12 months (cluster-adjusted OR, 1.02; 95% CI: 0.85, 1.24) or asthma attacks in the past 12 months (cluster-adjusted OR, 1.35; 95% CI: 0.75, 2.44). Likewise, observational analyses showed no associations between exclusive breastfeeding ≥3 months and lung function parameters.
Finally, the post hoc sensitivity analysis suggested that the protective effect of prolonged exclusive breastfeeding was unlikely to have been biased by nonblinding of the participating adolescents. In the 9581 (71%) who did not identify their trial group correctly, the protective effect remained large (flexural eczema on skin examination: cluster-adjusted OR, 0.44; 95% CI: 0.22, 0.88; flexural eczema symptoms past year OR, 0.57; 95% CI: 0.28, 1.16). In the 3893 (29%) participants who correctly identified their trial group, the corresponding effect was, if anything, weaker (flexural eczema on skin examination: cluster-adjusted OR, 0.72; 95% CI: 0.18, 2.92; flexural eczema symptoms past year: cluster-adjusted OR, 1.10; 95% CI: 0.24, 5.13). In a logistic regression analysis including intervention (Y/N), correctly identifying trial group (Y/N) and an interaction term between the two, the interaction p values were 0.61 for flexural eczema on skin examination and 0.28 for flexural eczema past year, respectively).
Post hoc multiple imputation analyses
The multiple imputation analyses, based on the sample of 17,046 participants originally enrolled at birth, yielded similar results to those of the modified intention-to-treat analyses presented above, although the lung function results lost statistical significance in the fully adjusted model ( eTable 3).
DISCUSSION
There was an approximate 50% reduction in the odds of flexural eczema on skin examination at 16 years of age in adolescents born to mothers and infants who attended maternity hospitals and polyclinics randomized to the intervention, compared to those who received standard care. In contrast, no evidence was found for an association between the intervention and self-reported atopic eczema symptoms in the past year or with asthma outcomes (asthma ever or symptoms in the past 12 months). For lung function, there was a negative association between the intervention and FEV1, FVC and FEV1/FVC in modified ITT analysis, which lost statistical significance in the multiple imputation, IV, and observational analyses. The conclusions were similar after using instrumental variable and multiple imputation analyses for all other outcomes, and the results for atopic eczema are in keeping with the findings previously reported for the first year of life.10
The strengths of the PROBIT study include the cluster randomized design, reducing vulnerability to bias and confounding, compared to observational studies. The follow-up rate of nearly 80% at 16 years from randomization at birth is high compared to other long-term follow-up studies and makes attrition (selection) bias unlikely, which is also underlined by the similarity of characteristics at follow-up between the two randomized groups and the comparable findings in the multiple imputation analysis. Another strength of the study is the use of lung function testing and physician skin examination for atopic eczema using validated, standardized protocols, rather than relying on questionnaire-derived outcomes alone, which results in the misclassification of some atopic eczema cases and consequently weakens associations, as confirmed here. In addition, those who reported wheezing in the past year and a diagnosis of asthma had reduced lung function, providing additional validity to the questionnaire-based outcomes. The robustness of the 16 year follow up phase with regard to atopic eczema is strengthened further by the stronger inverse association observed in instrumental variable analysis, which accounts for nonadherence to the intervention. In addition, the sensitivity analysis shows that bias due to nonblinding of participants to the randomized trial group is a very unlikely explanation of these findings.
Limitations of the study include the remaining possibility that pediatricians’ knowledge of the treatment allocation may have led to unconscious bias in their skin examination. One study center had to be excluded because of concerns about the validity of the data collected. Although the overall follow up rates were similar between the intervention and the control groups (79.7% and 79.3% respectively), there were differences between the polyclinics, ranging between 41% and 98%. Importantly, however, this was equally the case in the intervention and control groups. In addition, the multiple imputation results were consistent with the modified intention-to-treat analysis, and all analyses account for clustering within clinics.
A further limitation was the inability to conduct comprehensive quality assurance of measured lung function variables due to technical limitations of the equipment used in the field. However, repeated training was provided throughout the fieldwork, in addition to quality control visits, and by applying a strict threshold of acceptability of lung function results. Lung function could also have been influenced by growth patterns in early childhood,22 but no association was found between lung function measured in adolescence and contemporaneous body mass index (BMI); the latter also being positively associated with rapid early growth.
In contrast to the results for atopic eczema, no evidence was found to support an association of breastfeeding promotion with asthma, which confirms the previously reported findings from the PROBIT study at age 6.5 years. Some studies that reported a protective association of breastfeeding with asthma have suggested this may be stronger for non-atopic asthma,23 but previous results from PROBIT did not support an association between the study intervention and allergic sensitization at age 6.5 years.15
The contrast of the current results with previously reported protective associations with asthma could also arise from misclassification of wheezing in early life as asthma. Wheeze is common in early childhood and often associated with viral respiratory infections, but the majority of preschool wheezing illness does not evolve into asthma.25 An influence of breastfeeding on reducing the frequency of viral infections in early life could have a protective influence on viral induced wheezing. It is conceivable that diagnosing preschool wheezing as asthma is less likely in settings such as Belarus that have a low prevalence of asthma in childhood.
In addition to the duration and exclusivity of breastfeeding, the specific foods that infants are being weaned to might also have an impact on atopic eczema, asthma and lung function risk; this is not something that was investigated in PROBIT. However, a very recent RCT comparing the sequential introduction of 6 allergenic foods from three months of age (with partial breastfeeding) versus exclusive breastfeeding for 6 months did not show a difference in risk of atopic eczema or asthma up to three years of age.26 Finally, some of the questionnaire-based outcomes relied on participant recall, such as ‘asthma diagnosis ever’ and ‘itchy rash in the past 12 months that involves the flexures’. However, those reporting a diagnosis of asthma or symptoms of flexural atopic eczema showed reduced lung function and very strong correlation with atopic eczema on skin examination respectively, supporting the validity of the questionnaire-derived outcomes.
Although basic health services and sanitary conditions in Belarus are quite similar to those in North America and Western Europe, some aspects of the Belarusian health care system may limit the generalizability of the findings. For instance, the highly centralized Belarusian health care system undoubtedly helped in the implementation of the experimental intervention, resulting in substantial changes in the exclusivity and duration of breastfeeding in the intervention hospitals and polyclinics within a brief pre-recruitment period (12–16 months). It is also important to note that the prolonged (6–7 days) postpartum stay for routine vaginal deliveries far exceeds those currently found in the West and may have helped to establish good breastfeeding practices and instil maternal confidence.
Furthermore, atopic eczema is much less common in Belarus compared to more developed settings, such as North America and Western Europe. These prevalence differences are likely driven by a range of environmental risk factors linked to an affluent lifestyle, including hygiene-related exposures27, which may overcome and counteract the protective effect of exclusive breastfeeding found in Belarus.
CONCLUSIONS
Breastfeeding has many undisputed health benefits. However, most evidence is derived from observational studies and long-term follow up data are sparse. A cluster RCT with a breastfeeding promotion intervention had a large protective effect on flexural dermatitis risk but no detectable effect on lung function or questionnaire-derived measures of atopic eczema or asthma in adolescence in a setting where atopic eczema and allergies are rare.
Supplementary Material
Key Points.
Question:
Does prolonged and exclusive breastfeeding reduce the risk of asthma, atopic eczema and improve lung function in adolescence?
Findings:
In this adolescent follow-up of a cluster-randomized trial in Belarus, which assessed the effect of a breastfeeding promotion intervention vs. usual care among 13,557 participants, there was a 54% reduction in atopic eczema on skin examination but no significant effect on lung function (spirometry) and self-reported asthma diagnosis and symptoms of atopic eczema and wheezing in the past year.
Meaning:
Promotion of prolonged and exclusive breastfeeding reduces atopic eczema risk in adolescence.
Acknowledgments
Funding/Support:
This work was supported by: European Union, Early Nutrition Programming Long-term Efficacy and Safety (FOOD-DT-2005–007036); Canadian Institutes of Health Research (MOP-53155); the USA National Institutes of Health (R01 HD050758, K24 HD069408). G.D.S. and R.M.M. work in the Integrative Epidemiology Unit (IEU) supported by the United Kingdom Medical Research Council (MRC) and the University of Bristol (Grant Code: MC_UU_12013/1–9). The NIHR Bristol Nutrition Biomedical Research Unit is funded by the National Institute for Health Research (NIHR) and is a partnership between the University Hospitals Bristol NHS Foundation Trust and the University of Bristol. CF is funded through a NIHR Career Development Fellowship (CDF-2014–07-037) and also supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NIH, the EU, the Canadian Institutes of Health Research, the UK National Health Service, the UK NIHR, MRC or the UK Department of Health. The funders had no role in the conduct or reporting of the study.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Trial Registration: ClinicalTrials.gov Identifier: NCT01561612
Conflict of interest disclosures: None of the authors have a conflict of interest.
References
- 1.Fewtrell M, Wilson DC, Booth I, Lucas A. Six months of exclusive breast feeding: how good is the evidence? BMJ 2010;342:c5955. [DOI] [PubMed] [Google Scholar]
- 2.Fergusson DM, Horwood LJ. Early solid food diet and eczema in childhood: a 10-year longitudinal study. Pediatr Allergy Immunol 1994;5(6 Suppl):44–47. [DOI] [PubMed] [Google Scholar]
- 3.Forsyth JS, Ogston SA, Clark A, Florey CD, Howie PW. Relation between early introduction of solid food to infants and their weight and illnesses during the first two years of life. BMJ 1993;306(6892):1572–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sears MR, Greene JM, Willan AR, et al. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet 2002;360(9337):901–907. [DOI] [PubMed] [Google Scholar]
- 5.Yang YW, Tsai CL, Lu CY. Exclusive breastfeeding and incident atopic dermatitis in childhood: a systematic review and meta-analysis of prospective cohort studies. Br J Dermatol 2009;161(2):373–383. [DOI] [PubMed] [Google Scholar]
- 6.Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol 2014;179(10):1153–1167. [DOI] [PubMed] [Google Scholar]
- 7.Bisgaard HJS, Bonnelykke K. . Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med 2012;185(11):1183–1189. [DOI] [PubMed] [Google Scholar]
- 8.Tai A, Tran H, Roberts M, et al. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol 2014;133(6):1572–1578 e1573. [DOI] [PubMed] [Google Scholar]
- 9.Waidyatillake NT, Allen KJ, Lodge CJ, et al. The impact of breastfeeding on lung development and function: a systematic review. Expert Rev Clin Immunol 2013;9(12):1253–1265. [DOI] [PubMed] [Google Scholar]
- 10.Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA 2001;285(4):413–420. [DOI] [PubMed] [Google Scholar]
- 11.Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of breastfeeding intervention trial (PROBIT): a cluster-randomized trial in the Republic of Belarus. Design, follow-up, and data validation. Adv Exp Med Biol 2000;478:327–345. [PubMed] [Google Scholar]
- 12.WHO/UNICEF: Protecting, Promoting and Supporting Breastfeeding: The Special Role of Maternity Services Geneva, Switzerland: World Health Organization; 1989. [Google Scholar]
- 13.Guthrie LB, Oken E, Sterne JA, et al. Ongoing monitoring of data clustering in multicenter studies. BMC Med Res Methodol 2012;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiland SK, Bjorksten B, Brunekreef B, et al. Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): rationale and methods. Eur Respir J 2004;24(3):406–412. [DOI] [PubMed] [Google Scholar]
- 15.Kramer MS, Matush L, Vanilovich I, et al. Effect of prolonged and exclusive breast feeding on risk of allergy and asthma: cluster randomised trial. BMJ 2007;335(7624):815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- 17.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40(6):1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.2017 BEEP protocol 2016; http://www.nottingham.ac.uk/research/groups/cebd/documents/methodological-resources/beep-protocol-final-version-5.0-26october2016-signed.pdf.
- 19.White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342:d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scosyrev E Identification of causal effects using instrumental variables in randomized trials with stochastic compliance. Biom J 2013;55(1):97–113. [DOI] [PubMed] [Google Scholar]
- 21.Rassen JA, Schneeweiss S, Glynn RJ, Mittleman M, Brookhart MA. Instrumental variable analysis for estimation of treatment effects with dichotomous outcomes. Am J Epidemiol 2009;169:273–284. [DOI] [PubMed] [Google Scholar]
- 22.den Dekker HT, Sonnenschein-van der Voort AM, de Jongste JC, et al. Early growth characteristics and the risk of reduced lung function and asthma: A meta-analysis of 25,000 children. J Allergy Clin Immunol 2016;137(4):1026–1035. [DOI] [PubMed] [Google Scholar]
- 23.Nagel G, Buchele G, Weinmayr G, et al. Effect of breastfeeding on asthma, lung function and bronchial hyperreactivity in ISAAC Phase II. Eur Respir J 2009;33(5):993–1002. [DOI] [PubMed] [Google Scholar]
- 24.Ziyab AH, Mukherjee N, Ewart S, et al. Filaggrin gene loss-of-function variants modify the effect of breast-feeding on eczema risk in early childhood. Allergy 2016;71(9):1371–1373. [DOI] [PubMed] [Google Scholar]
- 25.Morgan WJ, Stern DA, Sherrill DL, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med 2005;172(10):1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkin MR, Logan K, Tseng A, et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N Engl J Med 2016;374(18):1733–1743. [DOI] [PubMed] [Google Scholar]
- 27.Flohr C, Mann J. New insights into the epidemiology of atopic dermatitis. Allergy 2014;69:3–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.