Abstract
The prefrontal cortex is central to the orchestrated brain network communication that gives rise to working memory and other cognitive functions. Accordingly, working memory deficits in schizophrenia are increasingly thought to derive from prefrontal cortex dysfunction coupled with broader network disconnectivity. How the prefrontal cortex dynamically communicates with its distal network partners to support working memory and how this communication is disrupted in individuals with schizophrenia remain unclear. Here we review recent evidence that prefrontal cortex communication with the hippocampus and thalamus is essential for normal spatial working memory, and that miscommunication between these structures underlies spatial working memory deficits in schizophrenia. We focus on studies using normal rodents and rodent models designed to probe schizophrenia-related pathology to assess the dynamics of neural interaction between these brain regions. We also highlight recent preclinical work parsing roles for long-range prefrontal cortex connections with the hippocampus and thalamus in normal and disordered spatial working memory. Finally, we discuss how emerging rodent endophenotypes of hippocampal- and thalamo-prefrontal cortex dynamics in spatial working memory could translate into richer understanding of the neural bases of cognitive function and dysfunction in humans.
Keywords: Connectivity, hippocampus, oscillations, prefrontal cortex, schizophrenia, synchrony, thalamus, working memory
Working memory, or the ability to store and manipulate information to guide behaviour on a timescale of seconds, is a central cognitive function (Baddeley, 2012, 1974). Working memory processes are critical for adaptive decision-making and goal-directed action, and their impairment is core to many neuropsychiatric disorders, including schizophrenia (Barch and Ceaser, 2012; Forbes et al., 2009). Although not among the diagnostic criteria for schizophrenia, deficits in working memory and other cognitive functions are predictive of long-term disease prognosis (Fett et al., 2011; Green, 1996; Hofer et al., 2005) and essentially resistant to currently available treatments (Buoli and Altamura, 2015; Lett et al., 2014). As such, the neural bases of normal and disordered working memory remain impactful areas of research.
Spatial working memory (SWM), or the active encoding, maintenance and retrieval of spatial information to direct behaviour in the short term, is a cognitive domain particularly amenable to bridging the preclinical and clinical neuroscience of schizophrenia. SWM is widely studied in humans, non-human primates and rodents using an array of laboratory assays (Bahner and Meyer-Lindenberg, 2017; Dudchenko, 2004; Funahashi, 2017), including spatial delayed-response tasks. In the simple ‘delayed non-match-to-sample’ (DNMS) task, subjects must maintain a memory trace of a recently sampled spatial cue or maze location across an ensuing delay period in order to correctly choose the opposite cue or location to receive a reward (Wikmark et al., 1973). Using these and related tasks, researchers have demonstrated that SWM is markedly impaired in individuals with schizophrenia (Fleming et al., 1997; Glahn et al., 2003; Park and Holzman, 1992; Piskulic et al., 2007). Similar deficits are seen in healthy relatives of those with schizophrenia (Cannon et al., 2000; Park et al., 1995), indicating that these deficits may reflect an underlying genetic predisposition to the disease. Importantly, deficits in SWM are recapitulated in many rodent models for the scientific study of schizophrenia (Gourevitch et al., 2004; Kellendonk et al., 2006; Lipska et al., 2002; Meyer and Feldon, 2009; Verma and Moghaddam, 1996), including mouse models engineered to carry schizophrenia susceptibility genes (Budel et al., 2008; Mukai et al., 2015; Stark et al., 2008).
Since Jacobsen’s (1936) seminal finding that lesions of dorsolateral regions of the prefrontal cortex (PFC) in monkeys impaired their ability to remember the location of a concealed food reward after a brief delay, the PFC has remained the principal known neural substrate of SWM (Funahashi, 2017). From rodents to primates, damage to regions comprising the PFC impairs SWM (Curtis and D’Esposito, 2004; Dias and Aggleton, 2000; Funahashi et al., 1993; Goldman and Rosvold, 1970; Ptito et al., 1995; Rogers et al., 1992; Yoon et al., 2008), despite considerable differences in tasks commonly used to study the cognitive process (e.g. maze-based navigation in rodents vs egocentric visuospatial tasks in primates; Funahashi, 2017) and controversy regarding interspecies homology of the PFC regions most commonly implicated (e.g. medial vs dorsolateral PFC in rodents and primates; Seamans et al., 2008; Uylings and van Eden, 1990). Rodents and primates further show PFC activity patterns during SWM that encode a variety of SWM task–related information and correlate with performance (Bolkan et al., 2017; Driesen et al., 2008; Horst and Laubach, 2012; Jung et al., 1998; Onos et al., 2016; Spellman et al., 2015). For example, sustained activity of individual PFC neurons during the delay period of SWM tasks that encodes spatial information, among other features, has provided an alluring and intuitive neural correlate of a mnemonic trace necessary for SWM maintenance (Funahashi, 2017; Funahashi et al., 1989; Fuster and Alexander, 1971; Goldman-Rakic, 1995; Haller et al., 2017; Kubota and Niki, 1971). Notably, however, refined analyses and modelling of PFC activity during the delay and other SWM task periods suggest that highly dynamic neuronal population coding and trial-by-trial assignment of PFC neuron selectivity shaped by short-term synaptic plasticity better encapsulate SWM processing in the PFC (Bolkan et al., 2017; Lundqvist et al., 2016; Shafi et al., 2007; Stokes, 2015).
In recent decades, it has become clear that SWM, like other high-level cognitive processes, manifests out of orchestrated communication across expansive brain networks (Cohen and D’Esposito, 2016; Dehaene et al., 1998; Fries, 2005). The PFC has been proposed to function as a critical hub in these networks, wherein SWM task–relevant information is dynamically gated and integrated to guide activity in distal brain regions and support contextually tuned behaviour (Cole et al., 2012; Miller and Cohen, 2001). Of the many network partners of the PFC, two with robust long-range anatomical connectivity and functional interactions with the PFC during SWM are the hippocampus (Bahner et al., 2015; Colgin, 2011; Goldman-Rakic et al., 1984; Hoover and Vertes, 2007; Jones and Wilson, 2005) and thalamus (Bolkan et al., 2017; Jones, 2007; Saalmann, 2014). But what is the dynamic nature of their communication with the PFC during SWM? And what information do long-range projections between the PFC and these brain regions convey to support SWM?
PFC dysfunction is central to many theories of schizophrenia pathogenesis (Kraepelin and Robertson, 1919; Lewis et al., 2012; Weinberger and Berman, 1996) and correlates with cognitive deficits of the disorder (Minzenberg et al., 2009; Perlstein et al., 2001). Modern clinical and preclinical imaging and electrophysiology also continue to validate the view that schizophrenia and its cognitive symptoms stem from broad patterns of aberrant neural synchrony and long-range circuit disconnectivity (Barr et al., 2010, 2017; Colgin, 2011; Friston, 1999; Senkowski and Gallinat, 2015; Skatun et al., 2016; Stephan et al., 2009; Uhlhaas, 2013; Zhou et al., 2015). Two brain regions with abnormal connectivity with the PFC in individuals with schizophrenia are the hippocampus (Bahner and Meyer-Lindenberg, 2017) and thalamus (Anticevic et al., 2014; Dawson et al., 2013; Woodward et al., 2012); similar disconnectivity has also been seen in etiologically relevant rodent models (Dickerson et al., 2010; Phillips et al., 2012; Sigurdsson et al., 2010). The physiological dynamics that underlie this abnormal connectivity, and how these dynamics distort the content of hippocampal- and thalamo-PFC communication to impair SWM, remain unknown.
Here we review recent evidence that PFC communication with the hippocampus and thalamus is essential for normal SWM, and that miscommunication between these structures underlies SWM deficits in schizophrenia. We focus on studies using normal rodents and rodent models designed to investigate schizophrenia-related cognitive dysfunction, with emphasis on studies assessing the dynamics of neural communication between these brain regions. We also highlight recent preclinical work parsing roles for long-range PFC connections with the hippocampus and thalamus in normal and disordered SWM.
Hippocampal-PFC communication in SWM
The hippocampus, a brain region with famous roles in spatial navigation, learning and memory (Buzsaki and Moser, 2013; Eichenbaum and Cohen, 2014), is anatomically poised to interact with the PFC via a variety of direct and indirect pathways. Direct hippocampal-PFC connectivity in the rodent derives primarily from unidirectional, monosynaptic projections from the CA1/subiculum subfields of the ventral-most two-thirds of the hippocampus (Hoover and Vertes, 2007; Jay and Witter, 1991; Oh et al., 2014; Swanson, 1981) that innervate both pyramidal cells and interneurons in the medial prefrontal cortex (mPFC; Gabbott et al., 2002; Thierry et al., 2000). The dorsal hippocampus also receives a monosynaptic projection from the anterior cingulate subregion of the mPFC (Rajasethupathy et al., 2015). Many indirect hippocampal-PFC connections exist, most notably via the nucleus reuniens of the thalamus and lateral entorhinal cortex, both of which reciprocally connect to the hippocampus and mPFC (Cassel et al., 2013; Hoover and Vertes, 2012; Moser and Moser, 2010).
Early lesion and inactivation studies in rodents revealed that SWM performance is disrupted by bilaterally interfering with either the hippocampus or the mPFC (Aggleton et al., 1986; Dias and Aggleton, 2000; Hallock et al., 2013; Izaki et al., 2001). ‘Disconnection’ studies impairing the hippocampal function in one hemisphere and PFC function in the other provided early causal evidence of hippocampal-PFC interactions in SWM (Churchwell et al., 2010; Floresco et al., 1997; Lee and Kesner, 2003; Wang and Cai, 2006). Interestingly, such manipulations have implicated various rodent hippocampal and mPFC subregions in these interactions, despite well-documented anatomical and functional demarcations and gradients across the two structures (Barker et al., 2017; Heidbreder and Groenewegen, 2003; Hoover and Vertes, 2007; Strange et al., 2014; Takita et al., 2013).
Insight into the dynamics of hippocampal-PFC interactions in SWM has come from rodent electrophysiological studies assessing how neural activity in the two structures correlates in time during SWM tasks (Sigurdsson and Duvarci, 2015). Many such studies indicate a critical role for long-range coordinated oscillations of neural activity in the theta-frequency band (4–12 Hz), which are known to be generated (in part) within the hippocampus (Buzsaki, 2002; Colgin, 2011). Theta-frequency oscillations in the hippocampus have been shown to entrain the activity of mPFC neurons (Siapas et al., 2005) and to correlate with spatial navigation and cognition (Kahana et al., 1999; Lee et al., 2005; Raghavachari et al., 2001; Vanderwolf, 1969). Recording simultaneously from the rat hippocampus and mPFC, Jones and Wilson (2005) found that correlated neuronal firing in the two structures was enhanced in the ‘choice’ period of an SWM task, when the memory trace is retrieved to guide action execution. Two additional measures of hippocampal-PFC synchrony, the ‘phase locking’ of mPFC neuron firing to theta oscillations of hippocampal local field potentials and theta-phase coherence of mPFC and hippocampal local field potentials, were preferentially enhanced during correct, but not incorrect, choices (see also Hallock et al., 2016; Hyman et al., 2010; Liu et al., 2018; Sigurdsson et al., 2010). Choice period–related increases in hippocampal-PFC theta synchrony have since been reported in both the dorsal and ventral hippocampus of mice (O’Neill et al., 2013) and found to increase with improved performance across training on an SWM task (Sigurdsson et al., 2010; Benchenane et al., 2010). Theta-frequency interactions between the hippocampus and PFC have also been implicated in the delay (maintenance) periods of SWM tasks. Analysis of the lag between mPFC unit firing and dorsal hippocampal theta-frequency oscillations in rats performing a delayed-alternation task revealed that the hippocampal activity preceded the mPFC activity during the delay, suggesting a predominance of hippocampus-to-mPFC information flow and influence in the theta range during this period (Hallock et al., 2016; Liu et al., 2018). Furthermore, a recent study using rats trained on an 8-arm radial maze DNMS task showed that theta power and synchrony between the hippocampus and mPFC peaked towards the end of the delay period, and mPFC neurons that showed a ramp-like increase in firing rate across the delay period showed higher firing rates on good performance trials (Myroshnychenko et al., 2017).
Like theta oscillations, gamma oscillations (30–120 Hz) have also been linked to spatial learning and memory (Carr et al., 2012; Yamamoto et al., 2014), coordinate activity in distal brain regions (Engel et al., 1991; Spellman et al., 2015; Steinmann et al., 2017) and contribute to hippocampal-PFC synchrony and SWM performance. Indeed, Spellman et al. (2015) reported heightened phase-locking of mPFC neuron firing to gamma oscillations in the mouse ventral hippocampus during the sample (encoding) period of DNMS trials that was particularly robust on correct trials and implied a hippocampal-to-PFC information flow. Interestingly, gamma synchrony that was consistent with an mPFC-to-dorsal hippocampus directionality of influence and predictive of choice accuracy has been reported in rats at the choice (retrieval) point of a delayed-alternation task (Hallock et al., 2016). These and other findings indicate that the hippocampus and PFC communicate via frequency band–specific and task period–specific synchrony that may support the encoding, maintenance and retrieval of SWM.
Hierarchical relationships between co-occurring theta and gamma oscillations, known as cross-frequency coupling, exist within and between the hippocampus and PFC (Belluscio et al., 2012; Canolty and Knight, 2010; Hyafil et al., 2015; Lisman and Jensen, 2013; Sirota et al., 2008). Local and long-range coupling of the phase and/or power of these oscillations is thought to coordinate activity in neuronal circuits and ensembles important for SWM and other cognitive processes (Axmacher et al., 2010; Canolty et al., 2006; Chaieb et al., 2015; Daume et al., 2017; Li et al., 2012; Lisman and Idiart, 1995; Park et al., 2013; Pastoll et al., 2013; Rajji et al., 2017; Roux and Uhlhaas, 2014; Shirvalkar et al., 2010; Tort et al., 2009). For example, Hallock et al. (2016) showed in rats that the coupling of hippocampal theta phase with PFC gamma power at the choice point of a T-maze significantly correlated with performance on a delayed-alternation task. Increasing the difficulty of a DNMS task also enhanced the phase–power coupling of theta and low gamma oscillations in the mouse hippocampus and PFC selectively during correct trials (Tamura et al., 2017). Interestingly, recent clinical work by Alekseichuk et al., (2016) provides unique support for the contribution of cross-frequency coupling to SWM ability by showing that co-applying theta- and gamma-wave transcranial stimulation to the human PFC boosts SWM only when gamma bursts were phase-locked to peaks of the theta cycle (see also Bilek et al., 2013; Polania et al., 2012).
Emerging evidence also indicates that hippocampal sharp-wave ripples, or characteristic bursts of high-frequency neuronal activity in the hippocampal area CA1 (Buzsaki, 2015), serve as an important conduit for hippocampal-PFC interactions supporting spatial learning and memory processes (Tang and Jadhav, 2018; Yu and Frank, 2015). Often studied as mediators of memory replay and consolidation during slow-wave sleep (Girardeau et al., 2009; Khodagholy et al., 2017; Maingret et al., 2016; Peyrache et al., 2009; Wilson and McNaughton, 1994), sharp-wave ripples can occur during awake immobility and slow movement to coordinate reactivation and stabilisation of ensembles that encode recent spatial experience (Davidson et al., 2009; Diba and Buzsaki, 2007; Foster and Wilson, 2006; Jadhav et al., 2012; Roux et al., 2017) and predict upcoming choices of spatial navigation (Pfeiffer and Foster, 2013; Singer et al., 2013). Awake hippocampal ripples can also profoundly modulate PFC neuron firing and hippocampal-PFC ensembles in a behaviour- and experience-dependent manner (Jadhav et al., 2016; Tang et al., 2017; Yu et al., 2017). Direct tests of the contribution of ripple-induced hippocampal-PFC interactions in explicit tasks of SWM (e.g. delayed-alternation, DNMS) remain to be conducted.
Although hippocampal-PFC synchrony and oscillatory coupling are presumed to optimise information transfer between the two regions (Canolty and Knight, 2010; Colgin, 2011; Gordon, 2011), the exact nature of the information being communicated and the neural substrates supporting this communication remain unclear. Temporally precise causal manipulations of neural activity, enabled by modern optogenetic tools, have refined our understanding of these issues (Kim et al., 2016; Roux et al., 2017; Yamamoto et al., 2014). For example, pathway-specific optogenetic inhibition of inputs from the ventral hippocampus to the mPFC selectively during the sample period, but not the delay or choice periods, impaired performance on a mouse DNMS task (Bolkan et al., 2017; Spellman et al., 2015). This inhibition disrupted the spatial tuning of a subset of mPFC neurons (i.e. preferential firing to one maze location during both the sample and choice periods; Bolkan et al., 2017; Spellman et al., 2015) and reduced the overall strength of sample-period gamma synchrony between the ventral hippocampus and mPFC (Spellman et al., 2015). These findings support a causal role for direct hippocampal-PFC inputs in the frequency-specific communication and spatial cue encoding that guides SWM performance (Figure 1(a)).
Figure 1.
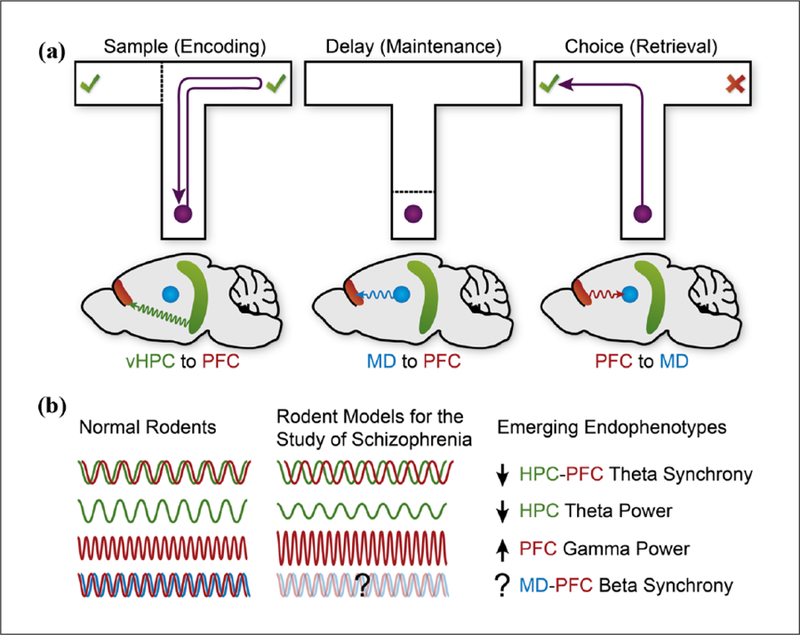
(a) Neural pathway–specific roles in distinct periods of a delayed non-match-to-sample T-maze spatial working memory task. Performance on this task is impaired by optogenetic inhibition of ventral hippocampus (vHPC) inputs to the prefrontal cortex (PFC) during the sample period, mediodorsal thalamus (MD) inputs to the PFC during the delay period, and PFC inputs to the MD during the choice period (Bolkan et al., 2017; Spellman et al., 2015). (b) Schematic noting some emerging electrophysiological endophenotypes of PFC miscommunication with the HPC and MD during spatial working memory in rodent models for the study of schizophrenia (e.g. Dickerson et al., 2010; Hunt and Kasicki, 2013; Sigurdsson et al., 2010).
Thalamo-PFC communication in SWM
The thalamus, a heterogeneous collection of brain nuclei long regarded as a relay between different cortical areas (Jones, 2007; Saalmann, 2014), has a privileged anatomical relationship with the PFC. The mediodorsal thalamus (MD) in particular, among the higher-order thalamic nuclei (Mitchell, 2015), shares dense, direct and reciprocal excitatory projections with various PFC subregions (Groenewegen, 1988; Krettek and Price, 1977; Uylings and van Eden, 1990). This reciprocal connectivity is also notably selective as the MD receives its main cortical input from the PFC, and the PFC receives its main thalamic input from the MD (Jones, 2007; Mitchell, 2015). In addition, the nucleus reuniens of the thalamus reciprocally connects to the PFC and hippocampus (Cassel et al., 2013; Hoover and Vertes, 2012), making it well positioned to influence or mediate the thalamo- and hippocampal-PFC interactions during cognitive functions like SWM (Griffin, 2015; Hallock et al., 2016; Ito et al., 2015).
Reminiscent of the cognitive deficits seen following damage to the mPFC, lesions of the rodent thalamus that include the MD can impair performance in SWM tasks (Alexinsky, 2001; Hunt and Aggleton, 1991, 1998a, 1998b; M’Harzi et al., 1991; Mitchell and Chakraborty, 2013; Stokes and Best, 1990). Functional disconnection of the MD and mPFC by asymmetric pharmacological inhibition similarly impaired performance in a delayed-response radial arm maze task (Floresco et al., 1999). Reversible inactivation of the nucleus reuniens can also impair SWM (Davoodi et al., 2009; Griffin, 2015; Hallock et al., 2016; Hembrook et al., 2012; Layfield et al., 2015; Viena et al., 2018). Interestingly, this manipulation was shown to abolish SWM-related increases in dorsal hippocampal-PFC theta synchrony, underscoring the contribution of this synchrony and its regulation by thalamic structures to normal SWM (Griffin, 2015; Hallock et al., 2016).
Much of what is known about the dynamics of thalamo-PFC communication in SWM comes from electrophysiological studies in non-human primates (Watanabe and Funahashi, 2012). Neurons in the monkey MD, like the dorsolateral prefrontal cortex (DLPFC), show sample-, delay- and choice-period activity, with the majority of task-related neurons showing sustained activity during the delay period (Fuster and Alexander, 1973; Sommer and Wurtz, 2006; Tanibuchi and Goldman-Rakic, 2003). More recently, analogous rodent studies have revealed notable contributions of MD-PFC communication to working memory maintenance and retrieval (Bolkan et al., 2017; Han et al., 2013; Miller et al., 2017; Parnaudeau et al., 2013).
Strong support for MD-PFC communication in SWM maintenance comes from recent work by Bolkan et al. (2017), who simultaneously recorded single mPFC neurons and MD local field potentials during a T-maze DNMS task. Directionality analysis of neural oscillations in the beta frequency (13–30 Hz) revealed that the MD activity preceded the mPFC activity during the delay period, suggesting a predominance of MD-to-PFC information flow and influence during this period. Consistently, optogenetic inhibition of MD-to-PFC terminal function selectively during the delay period impaired SWM performance. Moreover, a subset of mPFC neurons demonstrated elevated delay-phase spiking that did not encode spatial information (see Onos et al., 2016) but correlated with correct SWM performance. Rather than showing sustained activity across the entire delay, these mPFC neurons showed brief bouts of elevated activity at different temporal offsets such that population activity spanned the entire delay period. This activity pattern parallels the sequential activation of cortical neurons seen across other working memory–guided tasks (Baeg et al., 2003; Fujisawa et al., 2008; Harvey et al., 2012; Lundqvist et al., 2016; Schmitt et al., 2017). Importantly, optogenetic inhibition of MD-to-PFC inputs during the delay period suppressed the elevated firing of mPFC neurons seen in middle and late, but not early, time points in the delay period (Bolkan et al., 2017; Schmitt et al., 2017). Moreover, delay period–selective enhancement of MD neural excitability using a stabilised step function opsin improved SWM performance. Although inconsistent with evidence from Miller et al. (2017) that MD neurons in rats performing an operant DNMS task show no delay period–related firing, these findings strikingly align with recent work by Schmitt et al. (2017) using a working memory–guided attention task in which mice had to maintain a sensory-specific rule representation across a brief delay. Like Bolkan and colleagues, these researchers found that optogenetic suppression of MD activity during the delay impaired both the temporal sequencing of delay-period PFC neuron activity and subsequent choice behaviour, and that the enhancement of delay-period MD neural excitability strengthened task rule encoding by PFC neurons and improved performance (Schmitt et al., 2017). Together, these findings support the view that MD inputs to the PFC sustain PFC representations across the delay period to support SWM maintenance (Figure 1(a); Halassa and Kastner, 2017; Parnaudeau et al., 2017).
Discrete roles for MD-PFC communication in SWM retrieval and action execution have also been identified. In mice trained on a T-maze DNMS task, MD neuron firing showed preferentially enhanced phase-locking to mPFC beta oscillations during the choice period (Parnaudeau et al., 2013). Pharmacogenetic inhibition of MD neurons perturbed this MD-PFC synchrony and impaired task performance (Parnaudeau et al., 2013). In contrast to the MD-to-PFC directionality seen during the delay period of this same task, Bolkan et al. (2017) showed that the mPFC activity led the MD activity during the choice period. Accordingly, optogenetic inhibition of mPFC inputs to the MD during the choice period impaired SWM performance. Complementing the roles for ventral hippocampal and MD inputs to the mPFC in the respective spatial encoding and maintenance of SWM (Bolkan et al., 2017; Spellman et al., 2015), these findings suggest that ‘top-down’ signals from the mPFC to the MD help guide successful SWM retrieval and/or action execution (Figure 1(a)).
Disordered hippocampal-PFC communication and SWM in models for inquiry into schizophrenia
Brain imaging studies of individuals with schizophrenia reliably reveal abnormal hippocampal-PFC functional connectivity (Bahner and Meyer-Lindenberg, 2017; Benetti et al., 2009; Godsil et al., 2013; Meyer-Lindenberg et al., 2001, 2005; Zhou et al., 2008). The relationship between this connectivity and SWM performance, both in healthy subjects and individuals with schizophrenia, remains understudied and controversial (Bahner and Meyer-Lindenberg, 2017). Unlike rodent studies, the majority of clinical imaging studies of normal and disordered working memory employ non-SWM tasks. In a notable functional imaging study, Bahner et al. (2015) employed a virtual reality version of the radial arm maze, on which patients with schizophrenia are markedly impaired (Spieker et al., 2012), to show in healthy subjects that hippocampal-PFC connectivity is high during SWM encoding and retrieval and correlates with SWM ability (see also Kang et al., 2018). Although the precise functional coupling of hippocampal and PFC regions varies across working memory tasks, populations of patients with schizophrenia and high-risk individuals reliably differ from healthy controls in their task-related hippocampal-PFC functional connectivity (Benetti et al., 2009; Cousijn et al., 2015; Meyer-Lindenberg et al., 2001, 2005; Paulus et al., 2014, 2013; Rasetti et al., 2011; Rissman et al., 2008; Wolf et al., 2009).
Rodent models of the neurobiological underpinnings of schizophrenia, although varied in their aetiology, utility and validity, have been critical for establishing altered hippocampal-PFC communication as a key endophenotype of SWM deficits (Figure 1(b); Bahner and Meyer-Lindenberg, 2017). Mirroring the pathogenic complexity of schizophrenia, genetic, environmental, developmental and pharmacological models have been used to probe the neural basis of these cognitive deficits.
The first identified copy number variant associated with schizophrenia, the 22q11.2 microdeletion, increases the risk for developing the disease by a factor of 20–30 (Bassett and Chow, 2008; Karayiorgou et al., 2010). Df(16)A+/− mice carrying a deletion syntenic to the human 22q11.2 locus show impaired SWM (Drew et al., 2011; Stark et al., 2008), phenocopying at least some of the cognitive deficits seen in humans with the deletion (Simon et al., 2005; Sobin et al., 2005). Recording neuronal activity in the hippocampus and PFC during a T-maze DNMS task, Sigurdsson et al. (2010) showed that Df(16)A+/− mice have reduced hippocampal-PFC theta-frequency synchrony, measured by phase-locking of PFC neurons to hippocampal theta oscillations and by coherence of local field potentials in the two structures. Moreover, the magnitude of hippocampal-PFC coherence in Df(16)A+/− mice at training onset predicted the number of trials required to learn the SWM task and increased more slowly across training (Sigurdsson et al., 2010). Mice deficient for a single gene within the 22q11.2 locus, Zdhhc8, mimicked the Df(16)A+/− deficits in hippocampal-PFC synchrony and SWM (Mukai et al., 2015), displayed augmented hippocampal-PFC theta–slow gamma coupling likely to reflect a compensatory mechanism (Tamura et al., 2017) and showed reduced axonal branching of ventral hippocampus inputs to the mPFC (Mukai et al., 2015). Strikingly, developmental inhibition of glycogen synthase kinase-3 (GSK3), which prevented axonal branching deficits in Zdhhc8+/− mice (Mukai et al., 2015), rescued impaired hippocampal-PFC synchrony, SWM performance and mPFC neuronal encoding of spatial representations in Df(16)A+/− mice (Tamura et al., 2016).
Rodent studies of various other genetic risk factors to schizophrenia have drawn links between disordered SWM and hippocampal-PFC communication. Neuregulin-1, encoded by the schizophrenia susceptibility gene, NRG1 (Harrison and Law, 2006), is a cell adhesion molecule that signals through ErbB4 receptors (Mei and Nave, 2014) found nearly exclusively on parvalbumin (PV)-positive interneurons (Fazzari et al., 2010). Deletion of ErbB4 receptors selectively from PV-positive interneurons, long implicated in schizophrenia and cognition-supporting neural oscillations (Cardin et al., 2009; Gonzalez-Burgos et al., 2015; Lewis et al., 2012; Sohal et al., 2009; Uhlhaas and Singer, 2015), impaired SWM ability, reduced resting hippocampal-PFC theta synchrony and increased hippocampal gamma oscillations during exploration (Carlen et al., 2012; Del Pino et al., 2013; Wen et al., 2010; Yin et al., 2013). Mouse models of the schizophrenia-associated 15q13.3 microdeletion have been reported to have blunted auditory-evoked gamma oscillations in the hippocampus and frontal cortex (Fejgin et al., 2014; Stefansson et al., 2008). These mice also showed increased PFC gamma and reduced PFC and hippocampal theta oscillation power, but spared SWM (Fejgin et al., 2014).
Environmental and developmental models also suggest a role for disordered hippocampal-PFC communication in schizophrenia-related deficits in SWM. For example, rodent maternal immune activation models known to produce deficits in SWM (Meyer and Feldon, 2009; Richetto et al., 2013) display reductions in theta- and low-gamma-frequency hippocampal-PFC synchrony (Dickerson et al., 2014, 2010) that are reversed by acute treatment with the atypical antipsychotic, clozapine (Dickerson et al., 2012). Although no direct link has been established between hippocampal-PFC synchrony and the SWM deficits seen using the neonatal ventral hippocampal lesion model (Lipska et al., 2002, 1993), Lee et al. (2012) found that cognitive training during adolescence that reverses interhippocampal synchrony and cognitive deficits induced by the lesion also enhances hippocampal-prefrontal synchrony across a broad frequency range. Another developmental model that produces schizophrenia-like impairments in SWM (Gourevitch et al., 2004) involves gestational exposure to the teratogen, mitotoxin methylazoxymethanol acetate (MAM; Grace, 1998). Recordings from MAM-treated rats show attenuated delta (<4 Hz) and low gamma oscillations in the mPFC (Goto and Grace, 2006), broadly reduced oscillations in the hippocampus (Perreault et al., 2017), blunted fear-conditioned tone-evoked theta and gamma oscillations in the mPFC and gamma oscillations in the ventral hippocampus (Lodge et al., 2009) and hippocampal-prefrontal decoupling during sleep (Phillips et al., 2012).
A key recent hypothesis regarding schizophrenia pathophysiology proposes that the hypofunction of N-methyl-D-aspartate receptors (NMDARs) underlies aspects of the disorder. Commonly studied using pharmacological receptor blockade (Javitt and Zukin, 1991), NMDAR hypofunction is thought to promote aberrant neural oscillations that contribute to cognitive dysfunction (Homayoun and Moghaddam, 2007; Hunt and Kasicki, 2013; Krystal et al., 1994; Uhlhaas and Singer, 2013). Treatment with NMDAR antagonists, such as ketamine, phencyclidine and MK801, induces SWM deficits (Beraki et al., 2009; Castane et al., 2015; Enomoto and Floresco, 2009; Verma and Moghaddam, 1996) and tends to reduce hippocampal theta oscillations, while enhancing PFC and hippocampal delta-, gamma- and high-frequency oscillations (130–180 Hz; Hinman et al., 2013; Hunt and Kasicki, 2013; Kjaerby et al., 2017; Korotkova et al., 2010; Lazarewicz et al., 2010; Moran et al., 2015; Sapkota et al., 2016). Acute and chronic ketamine treatment in rodents also alters the coupling of hippocampal theta phase with gamma- and high-frequency oscillation power (Caixeta et al., 2013; Michaels et al., 2018), reminiscent of the global and fronto-temporal frequency coupling alterations reported in patients with schizophrenia (Allen et al., 2011; Sun et al., 2013; Won et al., 2017). While the effects of NMDAR antagonists on long-range hippocampal-PFC synchrony are less clear (Lee et al., 2017), reports have shown that sub-anaesthetic doses of ketamine increase hippocampal-PFC functional connectivity measured using functional magnetic resonance imaging (fMRI) in rats (Gass et al., 2014) and humans (Grimm et al., 2015).
Unfortunately, few studies using these and other rodent model systems of potential relevance to schizophrenia have assessed the hippocampal-PFC activity dynamics during SWM performance. Moreover, no studies to date have definitively parsed the specific long-range neuronal projections that may mediate, through gain or loss of function, disease-altered hippocampal-prefrontal activity dynamics and SWM.
Disordered thalamo-PFC communication and SWM in models for inquiry into schizophrenia
Classical post-mortem and modern clinical imaging studies indicate that the thalamus, a key hub within cortical–subcortical circuits and an important regulator of cortical activity, is structurally and functionally abnormal in patients with schizophrenia (Buchmann et al., 2014; Glahn et al., 2008; Hazlett et al., 1999; Hazlett et al., 2004; Kemether et al., 2003; Konick and Friedman, 2001; McCarley et al., 1999; Minzenberg et al., 2009; Pakkenberg, 1992). Consistent with their strong reciprocal connectivity, the thalamus and PFC show disordered functional connectivity in patients with the disorder (Anticevic et al., 2015; Cho et al., 2016; Woodward and Heckers, 2016; Woodward et al., 2012). Thalamo-PFC disconnectivity manifests at rest (Anticevic et al., 2014; Giraldo-Chica and Woodward, 2017; Welsh et al., 2010) and during cognitive and working memory testing (Andrews et al., 2006; Bor et al., 2011; Katz et al., 1996; Mitelman et al., 2005; Schneider et al., 2007). Probing the structural basis of this functional disconnectivity, compelling recent work showed in patients with schizophrenia that reductions in thalamo-PFC white matter connectivity correlated with impairments in PFC functional activation and working memory task performance (Giraldo-Chica et al., 2017; Marenco et al., 2012).
Few clinical and rodent studies of genetic predisposition to schizophrenia have shown evidence of altered thalamo-PFC communication, particularly in SWM. Products of the neuregulin-1 gene, NGR1, have been shown in mice to mediate thalamo-cortical axon pathfinding (Lopez-Bendito et al., 2006). This mirrors clinical evidence linking schizophrenia-associated risk variants of the gene with reductions in the structural integrity of the anterior thalamic radiation that connects the MD and anterior thalamic nuclei to the PFC (Barnes et al., 2012; Sprooten et al., 2009). Evidence that neuregulin-1 mutant mice show reduced NMDAR levels in the thalamus (Newell et al., 2013) is particularly intriguing given recent work from Yasuda et al. (2017) showing that the ablation of NMDARs in intralaminar thalamic nuclei attenuates oscillatory power measured by electroencephalography (EEG) in alpha-, beta- and gamma-frequency ranges and induces schizophrenia-like SWM deficits that are rescued by restoring thalamic NMDARs. No preclinical evidence to date indicates disruption in thalamo-PFC communication resulting from the schizophrenia-associated 22q11.2 or 15q13.3 microdeletions, and only mixed evidence of abnormal thalamic structure has been reported in 22q11.2 clinical populations (Bish et al., 2004; Lin et al., 2017). Similarly, environmental and developmental models of potential relevance to schizophrenia have produced limited evidence of disordered thalamo-PFC function or its contribution to SWM deficits, and evidence of altered thalamic volume and neuronal density from these models is modest and mixed (Crum et al., 2017; Matricon et al., 2010; Moore et al., 2006).
Studies using pharmacology to model aspects of schizophrenia, particularly those modelling NDMAR hypofunction, provide the most insight to date into disease-related dynamics of thalamo-PFC communication (Pratt et al., 2017). Acute and chronic administrations of NMDAR antagonists that induce SWM deficits (Enomoto and Floresco, 2009; Verma and Moghaddam, 1996) enhance activity-related gene expression in the thalamus (Castane et al., 2015), but exert mixed effects on the firing of thalamic neurons (Celada et al., 2013; Furth et al., 2017; Santana et al., 2011). Using 2-deoxyglucose imaging in rats, Dawson et al. (2013) showed that acute and chronic (Dawson et al., 2014) ketamine induced respective hypo- and hypermetabolism in the thalamus and PFC, as well as abnormal thalamo-PFC connectivity, paralleling that seen in humans (Langsjo et al., 2004). Interestingly, the same study showed increased functional connectivity between the PFC and thalamic reticular nucleus (Dawson et al., 2013), a structure that inhibits and exerts oscillatory influence over other thalamic nuclei (Mitrofanis and Guillery, 1993; Pratt et al., 2017). Neural oscillations in thalamo-cortical circuits are particularly modulated by NMDAR antagonists, showing preferential increases in power at higher frequency ranges that have been implicated in cognitive functions like SWM (Pratt et al., 2017; Uhlhaas and Singer, 2013; Zhang et al., 2012). For example, recent work by Furth et al. (2017) showed in rats that ketamine increased power within a comparable gamma-frequency range in the mPFC and MD thalamus, but was without effect on thalamo-PFC synchrony. Ketamine also induced contrasting neural activity dynamics in the mPFC and MD, increasing the rate and gamma phase-locking of neuron firing in the mPFC, but decreasing both measures in MD neurons (Furth et al., 2017). Lower frequency oscillations, including delta oscillations, have also been shown to be altered by NMDAR antagonism (Buzsaki, 1991; Zhang et al., 2009), as they are in schizophrenia (Boutros et al., 2008; Clementz et al., 1994; Fehr et al., 2001). For example, systemic and local NMDAR blockade within the MD (but not the mPFC) increased delta-frequency power in the mPFC (Kiss et al., 2011a, 2011bb), supporting a role for thalamic NMDAR hypofunction in thalamo-PFC activity dynamics. Interestingly, infusion of ketamine into the nucleus reuniens of the thalamus increased delta oscillations locally and in hippocampal CA1, and inhibition of the reuniens prevented this increase in hippocampal delta (but not gamma) oscillations following systemic NMDAR antagonism (Duan et al., 2015; Zhang et al., 2012). Supporting the behavioural relevance of these thalamus-derived delta oscillations, optogenetic activation of inputs from the reuniens to the dorsal hippocampus at a 3 Hz (delta) frequency impaired SWM performance (Duan et al., 2015).
To date, very few studies of schizophrenia-related models have assessed the thalamo-PFC activity during SWM performance and therefore do not inform how disordered thalamo-PFC communication may dynamically vary as the information processing requirements move from encoding to maintenance to retrieval across a given trial (Figure 1; Bolkan et al., 2017; Miller et al., 2017). Fewer studies yet have probed task period–specific and frequency-specific roles for discrete long-range neuronal projections that connect the thalamus and PFC, directly or indirectly, in disordered SWM.
Outstanding questions and future directions
Considerable advances have been made towards an understanding of the dynamic communication of the PFC with its hippocampal and thalamic network partners that supports SWM. Modern preclinical work has revealed that long-range projections between these structures contribute to inter-regional neural synchrony and convey contextually tuned information to facilitate effective SWM (Figure 1(a)). Evidence indicates that the hippocampus and PFC are functionally coupled at various stages of SWM, and the direction of this coupling may transition from a predominantly hippocampus-to-PFC information flow during encoding and maintenance to a ‘top-down’ flow during retrieval (see also Eichenbaum, 2017; Place et al., 2016). Theta-frequency interactions between the hippocampus and PFC may provide a conduit for information pertaining to the maintenance and retrieval of encoded spatial representations in SWM (see also Kang et al., 2018). Gamma-based functional coupling of the hippocampus and PFC may play a privileged role in the encoding of spatial representations, and direct inputs from the ventral hippocampus to the PFC appear to subserve this coupling. Thalamic-PFC communication seems to complement the roles of hippocampal-PFC interactions in SWM. Thalamic influence over PFC activity may be strongest during SWM maintenance, perhaps serving to amplify and sustain cortical representations of the memory trace (Halassa and Kastner, 2017; Parnaudeau et al., 2017), and this influence may reverse during SWM retrieval and action execution. This bidirectional interaction, which may be mediated via oscillations in the beta frequency, requires the function of direct reciprocal thalamo-PFC projections.
Disordered prefrontal connectivity and communication with the hippocampus and thalamus appear to be associated with SWM deficits seen in schizophrenia and its related rodent models (Figure 1(b)). Genetic risk models provide the strongest evidence that impaired hippocampal-PFC theta synchrony underlies schizophrenia-related impairments in SWM. Other models provide general support for altered oscillatory signatures in the two structures, particularly in the theta- and gamma-frequency ranges. A less clear description of disordered thalamo-PFC communication derives from these models. Indeed, although they show altered functional connectivity and reveal opposing disease-related alterations in neural activity between the thalamus and PFC, few of these models provide evidence of altered thalamo-PFC neural synchrony, particularly in the beta-frequency ranges implicated in normal SWM. Notably, the anatomical and functional heterogeneity of juxtaposed thalamic nuclei, coupled with the regional diversity of PFC and hippocampal cells and circuits, likely contributes to variability in the literature.
More importantly, however, very few studies of schizophrenia-related models involve simultaneous recordings from the PFC, thalamus and hippocampus during SWM performance. Rigorous analysis of dynamic changes in the strength and directionality of inter-regional coherence as animals encode, maintain and retrieve SWM representations stands to reveal more nuanced and predictive electrophysiological endophenotypes of schizophrenia. Furthermore, studies attempting to normalise these endophenotypes with established and emerging treatment strategies will serve as powerful within-subject tests of their validity and help identify those warranting deeper mechanistic study.
Tests of causality are critical to meaningfully advance the understanding of the neural basis of normal and disordered SWM. Loss- and gain-of-function manipulations with the temporal and spatial resolution afforded by modern optogenetics are required to not only parse the discrete cells and projections that mediate SWM function and dysfunction, but also to test the causal relevance of their discrete activity patterns. Open- and closed-loop interventions that replicate, strengthen, weaken or clamp specific oscillatory patterns by the informed and real-time manipulation of discrete cells and projections are beginning to be employed (Grosenick et al., 2015; Jadhav et al., 2012; Padilla-Coreano et al., 2017; Roux et al., 2017; Siegle and Wilson, 2014). Such approaches will help reveal the hippocampal- and thalamo-PFC network dynamics that promote or impair SWM, and advance our understanding of these processes beyond the realm of correlation.
Finally, SWM is a cognitive domain that is ideally suited for translational research, as it is testable in rodents and primates, and its deficits in schizophrenia are recapitulated in many models. Future efforts to parse the neural circuit bases of normal and disordered SWM should prioritise neural synchrony and connectivity phenotypes that are shared between these species, and between the clinical condition and its associated models. Increased clinical application of traditionally preclinical SWM tasks (e.g. Bahner et al., 2015) will aid in identifying phenotypes with the clearest translational utility. Of course, meaningful comparison of these phenotypes requires continued efforts to relate diverse electrical and metabolic measures of neural synchrony and connectivity (Logothetis, 2015), sober consideration of anatomical, functional and behavioural homology across species (Seamans et al., 2008; Uylings and van Eden, 1990) and critical evaluation of the validity of animal models of potential relevance to schizophrenia (Cope et al., 2016; Nestler and Hyman, 2010). Therefore, as rodent systems continue to reveal fundamental neural substrates and principles of network function underlying SWM, concurrent development of non-human primate models of schizophrenia predisposition (Jennings et al., 2016) will enable testing of these findings in animals with brains and behaviours that more closely resemble our own.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Aggleton JP, Hunt PR and Rawlins JN (1986) The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behavioural Brain Research 19(2): 133–146. [DOI] [PubMed] [Google Scholar]
- Alekseichuk I, Turi Z, Amador de Lara G, et al. (2016) Spatial working memory in humans depends on theta and high gamma synchronization in the prefrontal cortex. Current Biology 26(12): 1513–1521. [DOI] [PubMed] [Google Scholar]
- Alexinsky T (2001) Differential effect of thalamic and cortical lesions on memory systems in the rat. Behavioural Brain Research 122(2): 175–191. [DOI] [PubMed] [Google Scholar]
- Allen EA, Liu J, Kiehl KA, et al. (2011) Components of cross-frequency modulation in health and disease. Frontiers in Systems Neuroscience 5: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J, Wang L, Csernansky JG, et al. (2006) Abnormalities of thalamic activation and cognition in schizophrenia. American Journal of Psychiatry 163(3): 463–469. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Repovs G, et al. (2014) Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cerebral Cortex 24(12): 3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Haut K, Murray JD, et al. (2015) Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry 72(9): 882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Henseler MM, Jensen O, et al. (2010) Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proceedings of the National Academy of Sciences of the United States of America 107(7): 3228–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD (2012) Working memory: Theories, models, and controversies. Annual Review of Psychology 63: 1–29. [DOI] [PubMed] [Google Scholar]
- Baddeley AD and Hitch G (1974) Working Memory New York: Academic Press. [Google Scholar]
- Baeg EH, Kim YB, Huh K, et al. (2003) Dynamics of population code for working memory in the prefrontal cortex. Neuron 40(1): 177–188. [DOI] [PubMed] [Google Scholar]
- Bahner F and Meyer-Lindenberg A (2017) Hippocampal-prefrontal connectivity as a translational phenotype for schizophrenia. European Neuropsychopharmacology 27(2): 93–106. [DOI] [PubMed] [Google Scholar]
- Bahner F, Demanuele C, Schweiger J, et al. (2015) Hippocampal-dorsolateral prefrontal coupling as a species-conserved cognitive mechanism: A human translational imaging study. Neuropsychopharmacology 40(7): 1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM and Ceaser A (2012) Cognition in schizophrenia: Core psychological and neural mechanisms. Trends in Cognitive Sciences 16(1): 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Banks PJ, Scott H, et al. (2017) Separate elements of episodic memory subserved by distinct hippocampal-prefrontal connections. Nature Neuroscience 20(2): 242–250. [DOI] [PubMed] [Google Scholar]
- Barnes A, Isohanni M, Barnett JH, et al. (2012) Neuregulin-1 genotype is associated with structural differences in the normal human brain. Neuroimage 59(3): 2057–2061. [DOI] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Tran LC, et al. (2010) Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory. Schizophrenia Research 121(1–3): 146–152. [DOI] [PubMed] [Google Scholar]
- Barr MS, Rajji TK, Zomorrodi R, et al. (2017) Impaired theta-gamma coupling during working memory performance in schizophrenia. Schizophrenia Research 189: 104–110. [DOI] [PubMed] [Google Scholar]
- Bassett AS and Chow EW (2008) Schizophrenia and 22q11.2 deletion syndrome. Current Psychiatry Reports 10(2): 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio MA, Mizuseki K, Schmidt R, et al. (2012) Cross-frequency phase-phase coupling between θ and γ oscillations in the hippocampus. Journal of Neuroscience 32(2): 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, et al. (2010) Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron 66(6): 921–936. [DOI] [PubMed] [Google Scholar]
- Benetti S, Mechelli A, Picchioni M, et al. (2009) Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain 132(Pt 9): 2426–2436. [DOI] [PubMed] [Google Scholar]
- Beraki S, Diaz-Heijtz R, Tai F, et al. (2009) Effects of repeated treatment of phencyclidine on cognition and gene expression in C57BL/6 mice. International Journal of Neuropsychopharmacology 12(2): 243–255. [DOI] [PubMed] [Google Scholar]
- Bilek E, Schafer A, Ochs E, et al. (2013) Application of high-frequency repetitive transcranial magnetic stimulation to the DLPFC alters human prefrontal-hippocampal functional interaction. Journal of Neuroscience 33(16): 7050–7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bish JP, Nguyen V, Ding L, et al. (2004) Thalamic reductions in children with chromosome 22q11.2 deletion syndrome. Neuroreport 15(9): 1413–1415. [DOI] [PubMed] [Google Scholar]
- Bolkan SS, Stujenske JM, Parnaudeau S, et al. (2017) Thalamic projections sustain prefrontal activity during working memory maintenance. Nature Neuroscience 20(7): 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor J, Brunelin J, Sappey-Marinier D, et al. (2011) Thalamus abnormalities during working memory in schizophrenia. An fMRI study. Schizophrenia Research 125(1): 49–53. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Arfken C, Galderisi S, et al. (2008) The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Research 99(1–3): 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann A, Dentico D, Peterson MJ, et al. (2014) Reduced mediodorsal thalamic volume and prefrontal cortical spindle activity in schizophrenia. Neuroimage 102(Pt 2): 540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budel S, Padukkavidana T, Liu BP, et al. (2008) Genetic variants of Nogo-66 receptor with possible association to schizophrenia block myelin inhibition of axon growth. Journal of Neuroscience 28(49): 13161–13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buoli M and Altamura AC (2015) May non-antipsychotic drugs improve cognition of schizophrenia patients? Pharmacopsychiatry 48(2): 41–50. [DOI] [PubMed] [Google Scholar]
- Buzsaki G (1991) The thalamic clock: Emergent network properties. Neuroscience 41(2–3): 351–364. [DOI] [PubMed] [Google Scholar]
- Buzsaki G (2002) Theta oscillations in the hippocampus. Neuron 33(3): 325–340. [DOI] [PubMed] [Google Scholar]
- Buzsaki G (2015) Hippocampal sharp wave-ripple: A cognitive bio-marker for episodic memory and planning. Hippocampus 25(10): 1073–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G and Moser EI (2013) Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature Neuroscience 16(2): 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caixeta FV, Cornelio AM, Scheffer-Teixeira R, et al. (2013) Ketamine alters oscillatory coupling in the hippocampus. Scientific Reports 3: 2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Bearden CE, Hollister JM, et al. (2000) Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: A prospective cohort study. Schizophrenia Bulletin 26(2): 379–393. [DOI] [PubMed] [Google Scholar]
- Canolty RT and Knight RT (2010) The functional role of cross-frequency coupling. Trends in Cognitive Sciences 14(11): 506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, et al. (2006) High gamma power is phase-locked to theta oscillations in human neocortex. Science 313(5793): 1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, et al. (2009) Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459(7247): 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Siegle JH, et al. (2012) A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Molecular Psychiatry 17(5): 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MF, Karlsson MP and Frank LM (2012) Transient slow gamma synchrony underlies hippocampal memory replay. Neuron 75(4): 700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel JC, de Vasconcelos AP, Loureiro M, et al. (2013) The reuniens and rhomboid nuclei: Neuroanatomy, electrophysiological characteristics and behavioral implications. Progress in Neurobiology 111: 34–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castane A, Santana N and Artigas F (2015) PCP-based mice models of schizophrenia: Differential behavioral, neurochemical and cellular effects of acute and subchronic treatments. Psychopharmacology 232(21–22): 4085–4097. [DOI] [PubMed] [Google Scholar]
- Celada P, Llado-Pelfort L, Santana N, et al. (2013) Disruption of thalamo-cortical activity in schizophrenia models: Relevance to antipsychotic drug action. International Journal of Neuropsychopharmacology 16(10): 2145–2163. [DOI] [PubMed] [Google Scholar]
- Chaieb L, Leszczynski M, Axmacher N, et al. (2015) Theta-gamma phase-phase coupling during working memory maintenance in the human hippocampus. Cognitive Neuroscience 6(4): 149–157. [DOI] [PubMed] [Google Scholar]
- Cho KI, Shenton ME, Kubicki M, et al. (2016) Altered thalamo-cortical white matter connectivity: Probabilistic tractography study in clinical-high risk for psychosis and first-episode psychosis. Schizophrenia Bulletin 42(3): 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Musso ND, et al. (2010) Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiology of Learning and Memory 93(3): 415–421. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sponheim SR, Iacono WG, et al. (1994) Resting EEG in first-episode schizophrenia patients, bipolar psychosis patients, and their first-degree relatives. Psychophysiology 31(5): 486–494. [DOI] [PubMed] [Google Scholar]
- Cohen JR and D’Esposito M (2016) The segregation and integration of distinct brain networks and their relationship to cognition. Journal of Neuroscience 36(48): 12083–12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Yarkoni T, Repovs G, et al. (2012) Global connectivity of prefrontal cortex predicts cognitive control and intelligence. Journal of Neuroscience 32(26): 8988–8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL (2011) Oscillations and hippocampal-prefrontal synchrony. Current Opinion in Neurology 21(3): 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope ZA, Powell SB and Young JW (2016) Modeling neurodevelopmental cognitive deficits in tasks with cross-species translational validity. Genes, Brain and Behavior 15(1): 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn H, Tunbridge EM, Rolinski M, et al. (2015) Modulation of hippocampal theta and hippocampal-prefrontal cortex function by a schizophrenia risk gene. Human Brain Mapping 36(6): 2387–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum WR, Sawiak SJ, Chege W, et al. (2017) Evolution of structural abnormalities in the rat brain following in utero exposure to maternal immune activation: A longitudinal in vivo MRI study. Brain, Behavior, and Immunity 63: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE and D’Esposito M (2004) The effects of prefrontal lesions on working memory performance and theory. Cognitive, Affective, and Behavioral Neuroscience 4(4): 528–539. [DOI] [PubMed] [Google Scholar]
- Daume J, Gruber T, Engel AK, et al. (2017) Phase-amplitude coupling and long-range phase synchronization reveal frontotemporal interactions during visual working memory. Journal of Neuroscience 37(2): 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TJ, Kloosterman F and Wilson MA (2009) Hippocampal replay of extended experience. Neuron 63(4): 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodi FG, Motamedi F, Naghdi N, et al. (2009) Effect of reversible inactivation of the reuniens nucleus on spatial learning and memory in rats using Morris water maze task. Behavioural Brain Research 198(1): 130–135. [DOI] [PubMed] [Google Scholar]
- Dawson N, Morris BJ and Pratt JA (2013) Subanaesthetic ketamine treatment alters prefrontal cortex connectivity with thalamus and ascending subcortical systems. Schizophrenia Bulletin 39(2): 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson N, Xiao X, McDonald M, et al. (2014) Sustained NMDA receptor hypofunction induces compromised neural systems integration and schizophrenia-like alterations in functional brain networks. Cerebral Cortex 24(2): 452–464. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Kerszberg M and Changeux JP (1998) A neuronal model of a global workspace in effortful cognitive tasks. Proceedings of the National Academy of Sciences of the United States of America 95(24): 14529–14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pino I, Garcia-Frigola C, Dehorter N, et al. (2013) Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron 79(6): 1152–1168. [DOI] [PubMed] [Google Scholar]
- Dias R and Aggleton JP (2000) Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: Differential involvement of the prelimbicinfralimbic and anterior cingulate cortices in providing behavioural flexibility. European Journal of Neuroscience 12(12): 4457–4466. [DOI] [PubMed] [Google Scholar]
- Diba K and Buzsaki G (2007) Forward and reverse hippocampal place-cell sequences during ripples. Nature Neuroscience 10(10): 1241–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson DD, Overeem KA, Wolff AR, et al. (2014) Association of aberrant neural synchrony and altered GAD67 expression following exposure to maternal immune activation, a risk factor for schizophrenia. Translational Psychiatry 4: e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson DD, Restieaux AM and Bilkey DK (2012) Clozapine administration ameliorates disrupted long-range synchrony in a neurodevelopmental animal model of schizophrenia. Schizophrenia Research 135(1–3): 112–115. [DOI] [PubMed] [Google Scholar]
- Dickerson DD, Wolff AR and Bilkey DK (2010) Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. Journal of Neuroscience 30(37): 12424–12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Crabtree GW, Markx S, et al. (2011) The 22q11.2 microdeletion: Fifteen years of insights into the genetic and neural complexity of psychiatric disorders. International Journal of Developmental Neuroscience 29(3): 259–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen NR, Leung HC, Calhoun VD, et al. (2008) Impairment of working memory maintenance and response in schizophrenia: Functional magnetic resonance imaging evidence. Biological Psychiatry 64(12): 1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan AR, Varela C, Zhang Y, et al. (2015) Delta frequency optogenetic stimulation of the thalamic nucleus reuniens is sufficient to produce working memory deficits: Relevance to schizophrenia. Biological Psychiatry 77(12): 1098–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA (2004) An overview of the tasks used to test working memory in rodents. Neuroscience and Biobehavioral Reviews 28(7): 699–709. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2017) Prefrontal-hippocampal interactions in episodic memory. Nature Reviews Neuroscience 18(9): 547–558. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H and Cohen NJ (2014) Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron 83(4): 764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Konig P, Kreiter AK, et al. (1991) Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science 252(5009): 1177–1179. [DOI] [PubMed] [Google Scholar]
- Enomoto T and Floresco SB (2009) Disruptions in spatial working memory, but not short-term memory, induced by repeated ketamine exposure. Progress in Neuro-Psychopharmacology & Biological Psychiatry 33(4): 668–675. [DOI] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, et al. (2010) Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature 464(7293): 1376–1380. [DOI] [PubMed] [Google Scholar]
- Fehr T, Kissler J, Moratti S, et al. (2001) Source distribution of neuromagnetic slow waves and MEG-delta activity in schizophrenic patients. Biological Psychiatry 50(2): 108–116. [DOI] [PubMed] [Google Scholar]
- Fejgin K, Nielsen J, Birknow MR, et al. (2014) A mouse model that recapitulates cardinal features of the 15q13.3 microdeletion syndrome including schizophrenia- and epilepsy-related alterations. Biological Psychiatry 76(2): 128–137. [DOI] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, et al. (2011) The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neuroscience and Biobehavioral Reviews 35(5): 573–588. [DOI] [PubMed] [Google Scholar]
- Fleming K, Goldberg TE, Binks S, et al. (1997) Visuospatial working memory in patients with schizophrenia. Biological Psychiatry 41(1): 43–49. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Braaksma DN and Phillips AG (1999) Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial arm maze. Journal of Neuroscience 19(24): 11061–11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK and Phillips AG (1997) Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial arm maze tasks with or without a delay. Journal of Neuroscience 17(5): 1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes NF, Carrick LA, McIntosh AM, et al. (2009) Working memory in schizophrenia: A meta-analysis. Psychological Medicine 39(6): 889–905. [DOI] [PubMed] [Google Scholar]
- Foster DJ and Wilson MA (2006) Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440(7084): 680–683. [DOI] [PubMed] [Google Scholar]
- Fries P (2005) A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends in Cognitive Sciences 9(10): 474–480. [DOI] [PubMed] [Google Scholar]
- Friston KJ (1999) Schizophrenia and the disconnection hypothesis. Acta Psychiatrica Scandinavica 395: 68–79. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, et al. (2008) Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nature Neuroscience 11(7): 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S (2017) Working memory in the prefrontal cortex. Brain Sciences 7(5): 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ and Goldman-Rakic PS (1989) Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. Journal of Neurophysiology 61(2): 331–349. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ and Goldman-Rakic PS (1993) Dorsolateral prefrontal lesions and oculomotor delayed-response performance: Evidence for mnemonic ‘scotomas’. Journal of Neuroscience 13(4): 1479–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth KE, McCoy AJ, Dodge C, et al. (2017) Neuronal correlates of ketamine and walking induced gamma oscillations in the medial prefrontal cortex and mediodorsal thalamus. PLoS ONE 12(11): e0186732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM and Alexander GE (1971) Neuron activity related to short-term memory. Science 173(3997): 652–654. [DOI] [PubMed] [Google Scholar]
- Fuster JM and Alexander GE (1973) Firing changes in cells of the nucleus medialis dorsalis associated with delayed response behavior. Brain Research 61: 79–91. [DOI] [PubMed] [Google Scholar]
- Gabbott P, Headlam A and Busby S (2002) Morphological evidence that CA1 hippocampal afferents monosynaptically innervate PV-containing neurons and NADPH-diaphorase reactive cells in the medial prefrontal cortex (Areas 25/32) of the rat. Brain Research 946(2): 314–322. [DOI] [PubMed] [Google Scholar]
- Gass N, Schwarz AJ, Sartorius A, et al. (2014) Sub-anesthetic ketamine modulates intrinsic BOLD connectivity within the hippocampal-prefrontal circuit in the rat. Neuropsychopharmacology 39(4): 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo-Chica M and Woodward ND (2017) Review of thalamocortical resting-state fMRI studies in schizophrenia. Schizophrenia Research 180: 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo-Chica M, Rogers BP, Damon SM, et al. (2017) Prefrontal-thalamic anatomical connectivity and executive cognitive function in schizophrenia. Biological Psychiatry 53(6): 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, et al. (2009) Selective suppression of hippocampal ripples impairs spatial memory. Nature Neuroscience 12(10): 1222–1223. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, et al. (2008) Meta-analysis of gray matter anomalies in schizophrenia: Application of anatomic likelihood estimation and network analysis. Biological Psychiatry 64(9): 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Therman S, Manninen M, et al. (2003) Spatial working memory as an endophenotype for schizophrenia. Biological Psychiatry 53(7): 624–626. [DOI] [PubMed] [Google Scholar]
- Godsil BP, Kiss JP, Spedding M, et al. (2013) The hippocampal-prefrontal pathway: The weak link in psychiatric disorders? European Neuropsychopharmacology 23(10): 1165–1181. [DOI] [PubMed] [Google Scholar]
- Goldman PS and Rosvold HE (1970) Localization of function within the dorsolateral prefrontal cortex of the rhesus monkey. Experimental Neurology 27(2): 291–304. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1995) Cellular basis of working memory. Neuron 14(3): 477–485. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD and Schwartz ML (1984) Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience 12(3): 719–743. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Cho RY and Lewis DA (2015) Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biological Psychiatry 77(12): 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA (2011) Oscillations and hippocampal-prefrontal synchrony. Current Opinion in Neurology 21(3): 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y and Grace AA (2006) Alterations in medial prefrontal cortical activity and plasticity in rats with disruption of cortical development. Biological Psychiatry 60(11): 1259–1267. [DOI] [PubMed] [Google Scholar]
- Gourevitch R, Rocher C, Le Pen G, et al. (2004) Working memory deficits in adult rats after prenatal disruption of neurogenesis. Behavioural Pharmacology 15(4): 287–292. [DOI] [PubMed] [Google Scholar]
- Grace AA and Moore H (1998) Regulation of Information Flow in the Nucleus Accumbens: A Model for the Pathophysiology of Schizophrenia Washington, DC: American Psychological Association Press. [Google Scholar]
- Green MF (1996) What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry 153(3): 321–330. [DOI] [PubMed] [Google Scholar]
- Griffin AL (2015) Role of the thalamic nucleus reuniens in mediating interactions between the hippocampus and medial prefrontal cortex during spatial working memory. Frontiers in Systems Neuroscience 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm O, Gass N, Weber-Fahr W, et al. (2015) Acute ketamine challenge increases resting state prefrontal-hippocampal connectivity in both humans and rats. Psychopharmacology 232(21–22): 4231–4241. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ (1988) Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience 24(2): 379–431. [DOI] [PubMed] [Google Scholar]
- Grosenick L, Marshel JH and Deisseroth K (2015) Closed-loop and activity-guided optogenetic control. Neuron 86(1): 106–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM and Kastner S (2017) Thalamic functions in distributed cognitive control. Nature Neuroscience 20(12): 1669–1679. [DOI] [PubMed] [Google Scholar]
- Haller MCJ, Crone NE, Chang EF, et al. (2018) Persistent neuronal activity in human prefrontal cortex links perception and action. Nature Human Behaviour 2: 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Arreola AC, Shaw CL, et al. (2013) Dissociable roles of the dorsal striatum and dorsal hippocampus in conditional discrimination and spatial alternation T-maze tasks. Neurobiology of Learning and Memory 100: 108–116. [DOI] [PubMed] [Google Scholar]
- Hallock HL, Wang A and Griffin AL (2016) Ventral midline thalamus is critical for hippocampal-prefrontal synchrony and spatial working memory. Journal of Neuroscience 36(32): 8372–8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lee JH, Kim MJ, et al. (2013) Neural activity in mediodorsal nucleus of thalamus in rats performing a working memory task. Frontiers in Neural Circuits 7: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ and Law AJ (2006) Neuregulin 1 and schizophrenia: Genetics, gene expression, and neurobiology. Biological Psychiatry 60(2): 132–140. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Coen P and Tank DW (2012) Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 484(7392): 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Byne W, et al. (1999) Three-dimensional analysis with MRI and PET of the size, shape, and function of the thalamus in the schizophrenia spectrum. American Journal of Psychiatry 156(8): 1190–1199. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Kemether E, et al. (2004) Abnormal glucose metabolism in the mediodorsal nucleus of the thalamus in schizophrenia. American Journal of Psychiatry 161(2): 305–314. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA and Groenewegen HJ (2003) The medial prefrontal cortex in the rat: Evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neuroscience and Biobehavioral Reviews 27(6): 555–579. [DOI] [PubMed] [Google Scholar]
- Hembrook JR, Onos KD and Mair RG (2012) Inactivation of ventral midline thalamus produces selective spatial delayed conditional discrimination impairment in the rat. Hippocampus 22(4): 853–860. [DOI] [PubMed] [Google Scholar]
- Hinman JR, Penley SC, Escabi MA, et al. (2013) Ketamine disrupts theta synchrony across the septotemporal axis of the CA1 region of hippocampus. Journal of Neurophysiology 109(2): 570–579. [DOI] [PubMed] [Google Scholar]
- Hofer A, Baumgartner S, Bodner T, et al. (2005) Patient outcomes in schizophrenia II: The impact of cognition. European Psychiatry 20(5–6): 395–402. [DOI] [PubMed] [Google Scholar]
- Homayoun H and Moghaddam B (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. Journal of Neuroscience 27(3): 11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB and Vertes RP (2007) Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure & Function 212(2): 149–179. [DOI] [PubMed] [Google Scholar]
- Hoover WB and Vertes RP (2012) Collateral projections from nucleus reuniens of thalamus to hippocampus and medial prefrontal cortex in the rat: A single and double retrograde fluorescent labeling study. Brain Structure & Function 217(2): 191–209. [DOI] [PubMed] [Google Scholar]
- Horst NK and Laubach M (2012) Working with memory: Evidence for a role for the medial prefrontal cortex in performance monitoring during spatial delayed alternation. Journal of Neurophysiology 108(12): 3276–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt MJ and Kasicki S (2013) A systematic review of the effects of NMDA receptor antagonists on oscillatory activity recorded in vivo. Journal of Psychopharmacology 27(11): 972–986. [DOI] [PubMed] [Google Scholar]
- Hunt PR and Aggleton JP (1991) Medial dorsal thalamic lesions and working memory in the rat. Behavioral and Neural Biology 55(2): 227–246. [DOI] [PubMed] [Google Scholar]
- Hunt PR and Aggleton JP (1998a) An examination of the spatial working memory deficit following neurotoxic medial dorsal thalamic lesions in rats. Behavioural Brain Research 97(1–2): 129–141. [DOI] [PubMed] [Google Scholar]
- Hunt PR and Aggleton JP (1998b) Neurotoxic lesions of the dorsomedial thalamus impair the acquisition but not the performance of delayed matching to place by rats: A deficit in shifting response rules. Journal of Neuroscience 18(23): 10045–10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyafil A, Giraud AL, Fontolan L, et al. (2015) Neural cross-frequency coupling: Connecting architectures, mechanisms, and functions. Trends in Neurosciences 38(11): 725–740. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, et al. (2010) Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Frontiers in Integrative Neuroscience 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Zhang SJ, Witter MP, et al. (2015) A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nature 522(7554): 50–55. [DOI] [PubMed] [Google Scholar]
- Izaki Y, Maruki K, Hori K, et al. (2001) Effects of rat medial prefrontal cortex temporal inactivation on a delayed alternation task. Neuroscience Letters 315(3): 129–132. [DOI] [PubMed] [Google Scholar]
- Jacobsen CF (1936) Studies of cerebral function in primates. Comparative Psychology Monographs 13(3): 1–60. [Google Scholar]
- Jadhav SP, Kemere C, German PW, et al. (2012) Awake hippocampal sharp-wave ripples support spatial memory. Science 336(6087): 1454–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Rothschild G, Roumis DK, et al. (2016) Coordinated excitation and inhibition of prefrontal ensembles during awake hippocampal sharp-wave ripple events. Neuron 90(1): 113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC and Zukin SR (1991) Recent advances in the phencyclidine model of schizophrenia. American Journal of Psychiatry 148(10): 1301–1308. [DOI] [PubMed] [Google Scholar]
- Jay TM and Witter MP (1991) Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. Journal of Comparative Neurology 313(4): 574–586. [DOI] [PubMed] [Google Scholar]
- Jennings CG, Landman R, Zhou Y, et al. (2016) Opportunities and challenges in modeling human brain disorders in transgenic primates. Nature Neuroscience 19(9): 1123–1130. [DOI] [PubMed] [Google Scholar]
- Jones EG (2007) The Thalamus New York: Cambridge University Press. [Google Scholar]
- Jones MW and Wilson MA (2005) Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biology 3(12): e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, Qin Y, McNaughton BL, et al. (1998) Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cerebral Cortex 8(5): 437–450. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, et al. (1999) Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399(6738): 781–784. [DOI] [PubMed] [Google Scholar]
- Kang SS, MacDonald AW 3rd, Chafee MV, et al. (2018) Abnormal cortical neural synchrony during working memory in schizophrenia. Clinical Neurophysiology 129(1): 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Simon TJ and Gogos JA (2010) 22q11.2 microdeletions: Linking DNA structural variation to brain dysfunction and schizophrenia. Nature Reviews Neuroscience 11(6): 402–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M, Buchsbaum MS, Siegel BV, et al. (1996) Correlational patterns of cerebral glucose metabolism in never-medicated schizophrenics. Neuropsychobiology 33(1): 1–11. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, et al. (2006) Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 49(4): 603–615. [DOI] [PubMed] [Google Scholar]
- Kemether EM, Buchsbaum MS, Byne W, et al. (2003) Magnetic resonance imaging of mediodorsal, pulvinar, and centromedian nuclei of the thalamus in patients with schizophrenia. Archives of General Psychiatry 60(10): 983–991. [DOI] [PubMed] [Google Scholar]
- Khodagholy D, Gelinas JN and Buzsaki G (2017) Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science 358(6361): 369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Jeong H, Lee J, et al. (2016) Distinct roles of parvalbumin- and somatostatin-expressing interneurons in working memory. Neuron 92(4): 902–915. [DOI] [PubMed] [Google Scholar]
- Kiss T, Hoffmann WE and Hajos M (2011a) Delta oscillation and short-term plasticity in the rat medial prefrontal cortex: Modelling NMDA hypofunction of schizophrenia. International Journal of Neuropsychopharmacology 14(1): 29–42. [DOI] [PubMed] [Google Scholar]
- Kiss T, Hoffmann WE, Scott L, et al. (2011b) Role of thalamic projection in NMDA receptor-induced disruption of cortical slow oscillation and short-term plasticity. Frontiers in Psychiatry 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerby C, Hovelso N, Dalby NO, et al. (2017) Phencyclidine administration during neurodevelopment alters network activity in prefrontal cortex and hippocampus in adult rats. Journal of Neurophysiology 118(2): 1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konick LC and Friedman L (2001) Meta-analysis of thalamic size in schizophrenia. Biological Psychiatry 49(1): 28–38. [DOI] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, et al. (2010) NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 68(3): 557–569. [DOI] [PubMed] [Google Scholar]
- Kraepelin E and Robertson GM (1919) Dementia Praecox and Paraphrenia Edinburgh: Livingstone. [Google Scholar]
- Krettek JE and Price JL (1977) The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. Journal of Comparative Neurology 171(2): 157–191. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, et al. (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of General Psychiatry 51(3): 199–214. [DOI] [PubMed] [Google Scholar]
- Kubota K and Niki H (1971) Prefrontal cortical unit activity and delayed alternation performance in monkeys. Journal of Neurophysiology 34(3): 337–347. [DOI] [PubMed] [Google Scholar]
- Langsjo JW, Salmi E, Kaisti KK, et al. (2004) Effects of subanesthetic ketamine on regional cerebral glucose metabolism in humans. Anesthesiology 100(5): 1065–1071. [DOI] [PubMed] [Google Scholar]
- Layfield DM, Patel M, Hallock H, et al. (2015) Inactivation of the nucleus reuniens/rhomboid causes a delay-dependent impairment of spatial working memory. Neurobiology of Learning and Memory 125: 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarewicz MT, Ehrlichman RS, Maxwell CR, et al. (2010) Ketamine modulates theta and gamma oscillations. Journal of Cognitive Neuroscience 22(7): 1452–1464. [DOI] [PubMed] [Google Scholar]
- Lee H, Dvorak D, Kao HY, et al. (2012) Early cognitive experience prevents adult deficits in a neurodevelopmental schizophrenia model. Neuron 75(4): 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Simpson GV, Logothetis NK, et al. (2005) Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron 45(1): 147–156. [DOI] [PubMed] [Google Scholar]
- Lee I and Kesner RP (2003) Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. Journal of Neuroscience 23(4): 1517–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Hudson MR, O’Brien TJ, et al. (2017) Local NMDA receptor hypofunction evokes generalized effects on gamma and high-frequency oscillations and behavior. Neuroscience 358: 124–136. [DOI] [PubMed] [Google Scholar]
- Lett TA, Voineskos AN, Kennedy JL, et al. (2014) Treating working memory deficits in schizophrenia: A review of the neurobiology. Biological Psychiatry 75(5): 361–370. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, et al. (2012) Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in Neurosciences 35(1): 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bai W, Liu T, et al. (2012) Increases of theta-low gamma coupling in rat medial prefrontal cortex during working memory task. Brain Research Bulletin 89(3–4): 115–123. [DOI] [PubMed] [Google Scholar]
- Lin A, Ching CRK, Vajdi A, et al. (2017) Mapping 22q11.2 gene dosage effects on brain morphometry. Journal of Neuroscience 37(26): 6183–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Aultman JM, Verma A, et al. (2002) Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology 27(1): 47–54. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE and Weinberger DR (1993) Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: A potential animal model of schizophrenia. Neuropsychopharmacology 9(1): 67–75. [DOI] [PubMed] [Google Scholar]
- Lisman JE and Idiart MA (1995) Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science 267(5203): 1512–1515. [DOI] [PubMed] [Google Scholar]
- Lisman JE and Jensen O (2013) The theta-gamma neural code. Neuron 77(6): 1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Bai W, Xia M, et al. (2018) Directional hippocampal-prefrontal interactions during working memory. Behavioural Brain Research 338: 1–8. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM and Grace AA (2009) A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. Journal of Neuroscience 29(8): 2344–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK (2015) Neural-event-triggered fMRI of large-scale neural networks. Current Opinion in Neurology 31: 214–222. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Cautinat A, Sanchez JA, et al. (2006) Tangential neuronal migration controls axon guidance: A role for neuregulin-1 in thalamocortical axon navigation. Cell 125(1): 127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist M, Rose J, Herman P, et al. (2016) Gamma and beta bursts underlie working memory. Neuron 90(1): 152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M’Harzi M, Jarrard LE, Willig F, et al. (1991) Selective fimbria and thalamic lesions differentially impair forms of working memory in rats. Behavioral and Neural Biology 56(3): 221–239. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, et al. (1999) MRI anatomy of schizophrenia. Biological Psychiatry 45(9): 1099–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret N, Girardeau G, Todorova R, et al. (2016) Hippocampo-cortical coupling mediates memory consolidation during sleep. Nature Neuroscience 19(7): 959–964. [DOI] [PubMed] [Google Scholar]
- Marenco S, Stein JL, Savostyanova AA, et al. (2012) Investigation of anatomical thalamo-cortical connectivity and FMRI activation in schizophrenia. Neuropsychopharmacology 37(2): 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matricon J, Bellon A, Frieling H, et al. (2010) Neuropathological and Reelin deficiencies in the hippocampal formation of rats exposed to MAM; differences and similarities with schizophrenia. PLoS ONE 5(4): e10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L and Nave KA (2014) Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron 83(1): 27–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U and Feldon J (2009) Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behavioural Brain Research 204(2): 322–334. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, et al. (2001) Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. American Journal of Psychiatry 158(11): 1809–1817. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, et al. (2005) Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Archives of General Psychiatry 62(4): 379–386. [DOI] [PubMed] [Google Scholar]
- Michaels TI, Long LL, Stevenson IH, et al. (2018) Effects of chronic ketamine on hippocampal cross-frequency coupling: Implications for schizophrenia pathophysiology. European Journal of Neuroscience 10.1111/ejn.13822. [DOI] [PubMed]
- Miller EK and Cohen JD (2001) An integrative theory of prefrontal cortex function. Annual Review of Neuroscience 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Miller RLA, Francoeur MJ, Gibson BM, et al. (2017) Mediodorsal thalamic neurons mirror the activity of medial prefrontal neurons responding to movement and reinforcement during a dynamic DNMTP task. Eneuro 4(5): pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, et al. (2009) Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry 66(8): 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS (2015) The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neuroscience and Biobehavioral Reviews 54: 76–88. [DOI] [PubMed] [Google Scholar]
- Mitchell AS and Chakraborty S (2013) What does the mediodorsal thalamus do? Frontiers in Systems Neuroscience 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman SA, Byne W, Kemether EM, et al. (2005) Metabolic disconnection between the mediodorsal nucleus of the thalamus and cortical Brodmann’s areas of the left hemisphere in schizophrenia. American Journal of Psychiatry 162(9): 1733–1735. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J and Guillery RW (1993) New views of the thalamic reticular nucleus in the adult and the developing brain. Trends in Neurosciences 16(6): 240–245. [DOI] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, et al. (2006) A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: Implications for the neuropathology of schizophrenia. Biological Psychiatry 60(3): 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran RJ, Jones MW, Blockeel AJ, et al. (2015) Losing control under ketamine: Suppressed cortico-hippocampal drive following acute ketamine in rats. Neuropsychopharmacology 40(2): 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI and Moser M-B (2010) Entorhinal Cortex Oxford: Oxford University Press. [Google Scholar]
- Mukai J, Tamura M, Fenelon K, et al. (2015) Molecular substrates of altered axonal growth and brain connectivity in a mouse model of schizophrenia. Neuron 86(3): 680–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myroshnychenko M, Seamans JK, Phillips AG, et al. (2017) Temporal dynamics of hippocampal and medial prefrontal cortex interactions during the delay period of a working memory-guided foraging task. Cerebral Cortex 27(11): 5331–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ and Hyman SE (2010) Animal models of neuropsychiatric disorders. Nature Neuroscience 13(10): 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell KA, Karl T and Huang XF (2013) A neuregulin 1 transmembrane domain mutation causes imbalanced glutamatergic and dopaminergic receptor expression in mice. Neuroscience 248: 670–680. [DOI] [PubMed] [Google Scholar]
- O’Neill PK, Gordon JA and Sigurdsson T (2013) Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. Journal of Neuroscience 33(35): 14211–14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, et al. (2014) A mesoscale connectome of the mouse brain. Nature 508(7495): 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onos KD, Francoeur MJ, Wormwood BA, et al. (2016) Prefrontal neurons encode actions and outcomes in conjunction with spatial location in rats performing a dynamic delayed non-match to position task. PLoS ONE 11(2): e0149019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Coreano NC, Teboul E, Garcia-Garcia A, et al. (2017) Frequency-Specific Facilitation of Hippocampal-Prefrontal Transmission Increases Anxiety-Like Behavior Washington, DC: Society for Neuroscience. [Google Scholar]
- Pakkenberg B (1992) The volume of the mediodorsal thalamic nucleus in treated and untreated schizophrenics. Schizophrenia Research 7(2): 95–100. [DOI] [PubMed] [Google Scholar]
- Park JY, Jhung K, Lee J, et al. (2013) Theta-gamma coupling during a working memory task as compared to a simple vigilance task. Neuroscience Letters 532: 39–43. [DOI] [PubMed] [Google Scholar]
- Park S and Holzman PS (1992) Schizophrenics show spatial working memory deficits. Archives of General Psychiatry 49(12): 975–982. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS and Goldman-Rakic PS (1995) Spatial working memory deficits in the relatives of schizophrenic patients. Archives of General Psychiatry 52(10): 821–828. [DOI] [PubMed] [Google Scholar]
- Parnaudeau S, Bolkan SS and Kellendonk C (2017) The mediodorsal thalamus: An essential partner of the prefrontal cortex for cognition. Biological Psychiatry 83(8): 648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaudeau S, O’Neill PK, Bolkan SS, et al. (2013) Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron 77(6): 1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoll H, Solanka L, van Rossum MC, et al. (2013) Feedback inhibition enables θ-nested γ oscillations and grid firing fields. Neuron 77(1): 141–154. [DOI] [PubMed] [Google Scholar]
- Paulus FM, Bedenbender J, Krach S, et al. (2014) Association of rs1006737 in CACNA1C with alterations in prefrontal activation and fronto-hippocampal connectivity. Human Brain Mapping 35(4): 1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus FM, Krach S, Bedenbender J, et al. (2013) Partial support for ZNF804A genotype-dependent alterations in prefrontal connectivity. Human Brain Mapping 34(2): 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, et al. (2001) Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. American Journal of Psychiatry 158(7): 1105–1113. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Fan T, Banasikowski TJ, et al. (2017) The atypical dopamine receptor agonist SKF 83959 enhances hippocampal and prefrontal cortical neuronal network activity in a rat model of cognitive dysfunction. European Journal of Neuroscience 46(4): 2015–2025. [DOI] [PubMed] [Google Scholar]
- Peyrache A, Khamassi M, Benchenane K, et al. (2009) Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nature Neuroscience 12(7): 919–926. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE and Foster DJ (2013) Hippocampal place-cell sequences depict future paths to remembered goals. Nature 497(7447): 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KG, Bartsch U, McCarthy AP, et al. (2012) Decoupling of sleep-dependent cortical and hippocampal interactions in a neurodevelopmental model of schizophrenia. Neuron 76(3): 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskulic D, Olver JS, Norman TR, et al. (2007) Behavioural studies of spatial working memory dysfunction in schizophrenia: A quantitative literature review. Psychiatry Research 150(2): 111–121. [DOI] [PubMed] [Google Scholar]
- Place R, Farovik A, Brockmann M, et al. (2016) Bidirectional prefrontal-hippocampal interactions support context-guided memory. Nature Neuroscience 19(8): 992–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polania R, Nitsche MA, Korman C, et al. (2012) The importance of timing in segregated theta phase-coupling for cognitive performance. Current Biology 22(14): 1314–1318. [DOI] [PubMed] [Google Scholar]
- Pratt J, Dawson N, Morris BJ, et al. (2017) Thalamo-cortical communication, glutamatergic neurotransmission and neural oscillations: A unique window into the origins of ScZ? Schizophrenia Research 180: 4–12. [DOI] [PubMed] [Google Scholar]
- Ptito A, Crane J, Leonard G, et al. (1995) Visual-spatial localization by patients with frontal-lobe lesions invading or sparing area 46. Neuroreport 6(13): 1781–1784. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, et al. (2001) Gating of human theta oscillations by a working memory task. Journal of Neuroscience 21(9): 3175–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasethupathy P, Sankaran S, Marshel JH, et al. (2015) Projections from neocortex mediate top-down control of memory retrieval. Nature 526(7575): 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajji TK, Zomorrodi R, Barr MS, et al. (2017) Ordering information in working memory and modulation of gamma by theta oscillations in humans. Cerebral Cortex 27(2): 1482–1490. [DOI] [PubMed] [Google Scholar]
- Rasetti R, Sambataro F, Chen Q, et al. (2011) Altered cortical network dynamics: A potential intermediate phenotype for schizophrenia and association with ZNF804A. Archives of General Psychiatry 68(12): 1207–1217. [DOI] [PubMed] [Google Scholar]
- Richetto J, Calabrese F, Meyer U, et al. (2013) Prenatal versus postnatal maternal factors in the development of infection-induced working memory impairments in mice. Brain, Behavior, and Immunity 33: 190–200. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A and D’Esposito M (2008) Dynamic adjustments in prefrontal, hippocampal, and inferior temporal interactions with increasing visual working memory load. Cerebral Cortex 18(7): 1618–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DC, Wright PW, Roberts JC, et al. (1992) Photothrombotic lesions of the frontal cortex impair the performance of the delayed non-matching to position task by rats. Behavioural Brain Research 49(2): 231–235. [DOI] [PubMed] [Google Scholar]
- Roux F and Uhlhaas PJ (2014) Working memory and neural oscillations: Alpha-gamma versus theta-gamma codes for distinct WM information? Trends in Cognitive Sciences 18(1): 16–25. [DOI] [PubMed] [Google Scholar]
- Roux L, Hu B, Eichler R, et al. (2017) Sharp wave ripples during learning stabilize the hippocampal spatial map. Nature Neuroscience 20(6): 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB (2014) Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Frontiers in Systems Neuroscience 8: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N, Troyano-Rodriguez E, Mengod G, et al. (2011) Activation of thalamocortical networks by the N-methyl-D-aspartate receptor antagonist phencyclidine: Reversal by clozapine. Biological Psychiatry 69(10): 918–927. [DOI] [PubMed] [Google Scholar]
- Sapkota K, Mao Z, Synowicki P, et al. (2016) GluN2D N-methyl-d-aspartate receptor subunit contribution to the stimulation of brain activity and gamma oscillations by ketamine: Implications for schizophrenia. Journal of Pharmacology and Experimental Therapeutics 356(3): 702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt LI, Wimmer RD, Nakajima M, et al. (2017) Thalamic amplification of cortical connectivity sustains attentional control. Nature 545(7653): 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Habel U, Reske M, et al. (2007) Neural correlates of working memory dysfunction in first-episode schizophrenia patients: An fMRI multi-center study. Schizophrenia Research 89(1–3): 198–210. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Lapish CC and Durstewitz D (2008) Comparing the prefrontal cortex of rats and primates: Insights from electrophysiology. Neurotoxicity Research 14(2–3): 249–262. [DOI] [PubMed] [Google Scholar]
- Senkowski D and Gallinat J (2015) Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biological Psychiatry 77(2): 1010–1019. [DOI] [PubMed] [Google Scholar]
- Shafi M, Zhou Y, Quintana J, et al. (2007) Variability in neuronal activity in primate cortex during working memory tasks. Neuroscience 146(3): 1082–1108. [DOI] [PubMed] [Google Scholar]
- Shirvalkar PR, Rapp PR and Shapiro ML (2010) Bidirectional changes to hippocampal theta-gamma comodulation predict memory for recent spatial episodes. Proceedings of the National Academy of Sciences of the United States of America 107(15): 7054–7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV and Wilson MA (2005) Prefrontal phase locking to hippocampal theta oscillations. Neuron 46(1): 141–151. [DOI] [PubMed] [Google Scholar]
- Siegle JH and Wilson MA (2014) Enhancement of encoding and retrieval functions through theta phase-specific manipulation of hippocampus. Elife 3: e03061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T and Duvarci S (2015) Hippocampal-prefrontal interactions in cognition, behavior and psychiatric disease. Frontiers in Systems Neuroscience 9: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, et al. (2010) Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature 464(7289): 763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Bearden CE, Mc-Ginn DM, et al. (2005) Visuospatial and numerical cognitive deficits in children with chromosome 22q11.2 deletion syndrome. Cortex 41(12): 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AC, Carr MF, Karlsson MP, et al. (2013) Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Neuron 77(6): 1163–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, et al. (2008) Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60(4): 683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skatun KC, Kaufmann T, Tonnesen S, et al. (2016) Global brain connectivity alterations in patients with schizophrenia and bipolar spectrum disorders. Journal of Psychiatry & Neuroscience 41(5): 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Daniels S, et al. (2005) Neuropsychological characteristics of children with the 22q11 deletion syndrome: A descriptive analysis. Child Neuropsychol 11(1): 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, et al. (2009) Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459(7247): 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA and Wurtz RH (2006) Influence of the thalamus on spatial visual processing in frontal cortex. Nature 444(7117): 374–377. [DOI] [PubMed] [Google Scholar]
- Spellman T, Rigotti M, Ahmari SE, et al. (2015) Hippocampal-prefrontal input supports spatial encoding in working memory. Nature 522(7556): 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieker EA, Astur RS, West JT, et al. (2012) Spatial memory deficits in a virtual reality eight-arm radial maze in schizophrenia. Schizophrenia Research 135(1–3): 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprooten E, Lymer GK, Maniega SM, et al. (2009) The relationship of anterior thalamic radiation integrity to psychosis risk associated neuregulin-1 variants. Molecular Psychiatry 14(3): 233; 237–238. [DOI] [PubMed] [Google Scholar]
- Stark KL, Xu B, Bagchi A, et al. (2008) Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nature Genetics 40(6): 751–760. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, et al. (2008) Large recurrent microdeletions associated with schizophrenia. Nature 455(7210): 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann S, Leicht G, Andreou C, et al. (2017) Auditory verbal hallucinations related to altered long-range synchrony of gamma-band oscillations. Scientific Reports 7(1): 8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ and Frith CD (2009) Dysconnection in schizophrenia: From abnormal synaptic plasticity to failures of self-monitoring. Schizophrenia Bulletin 35(3): 509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes KA and Best PJ (1990) Mediodorsal thalamic lesions impair ‘reference’ and ‘working’ memory in rats. Physiology & Behavior 47(3): 471–476. [DOI] [PubMed] [Google Scholar]
- Stokes MG (2015) ‘Activity-silent’ working memory in prefrontal cortex: A dynamic coding framework. Trends in Cognitive Sciences 19(7): 394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, et al. (2014) Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience 15(10): 655–669. [DOI] [PubMed] [Google Scholar]
- Sun L, Castellanos N, Grutzner C, et al. (2013) Evidence for dysregulated high-frequency oscillations during sensory processing in medication-naive, first episode schizophrenia. Schizophrenia Research 150(2–3): 519–525. [DOI] [PubMed] [Google Scholar]
- Swanson LW (1981) A direct projection from Ammon’s horn to prefrontal cortex in the rat. Brain Research 217(1): 150–154. [DOI] [PubMed] [Google Scholar]
- Takita M, Fujiwara SE and Izaki Y (2013) Functional structure of the intermediate and ventral hippocampo–prefrontal pathway in the prefrontal convergent system. Journal of Physiology 107(6): 441–447. [DOI] [PubMed] [Google Scholar]
- Tamura M, Mukai J, Gordon JA, et al. (2016) Developmental inhibition of Gsk3 rescues behavioral and neurophysiological deficits in a mouse model of schizophrenia predisposition. Neuron 89(5): 1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M, Spellman TJ, Rosen AM, et al. (2017) Hippocampal-prefrontal theta-gamma coupling during performance of a spatial working memory task. Nature Communications 8(1): 2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W and Jadhav SP (2018) Sharp-wave ripples as a signature of hippocampal-prefrontal reactivation for memory during sleep and waking states. Neurobiology of Learning and Memory [DOI] [PMC free article] [PubMed]
- Tang W, Shin JD, Frank LM, et al. (2017) Hippocampal-prefrontal reactivation during learning is stronger in awake compared with sleep states. Journal of Neuroscience 37(49): 11789–11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanibuchi I and Goldman-Rakic PS (2003) Dissociation of spatial-, object-, and sound-coding neurons in the mediodorsal nucleus of the primate thalamus. Journal of Neurophysiology 89(2): 1067–1077. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Degenetais E, et al. (2000) Hippocampo-prefrontal cortex pathway: Anatomical and electrophysiological characteristics. Hippocampus 10(4): 411–419. [DOI] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, et al. (2009) Theta-gamma coupling increases during the learning of item-context associations. Proceedings of the National Academy of Sciences of the United States of America 106(49): 20942–20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ (2013) Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Current Opinion in Neurology 23(2): 283–290. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ and Singer W (2013) High-frequency oscillations and the neurobiology of schizophrenia. Dialogues in Clinical Neuroscience 15(3): 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ and Singer W (2015) Oscillations and neuronal dynamics in schizophrenia: The search for basic symptoms and translational opportunities. Biological Psychiatry 77(12): 1001–1009. [DOI] [PubMed] [Google Scholar]
- Uylings HB and van Eden CG (1990) Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Progress in Brain Research 85: 31–62. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH (1969) Hippocampal electrical activity and voluntary movement in the rat. Electroencephalography and Clinical Neurophysiology 26(4): 407–418. [DOI] [PubMed] [Google Scholar]
- Verma A and Moghaddam B (1996) NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: Modulation by dopamine. Journal of Neuroscience 16(1): 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viena TD, Linley SB and Vertes RP (2018) Inactivation of nucleus reuniens impairs spatial working memory and behavioral flexibility in the rat. Hippocampus 28(4): 297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GW and Cai JX (2006) Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behavioural Brain Research 175(2): 329–336. [DOI] [PubMed] [Google Scholar]
- Watanabe Y and Funahashi S (2012) Thalamic mediodorsal nucleus and working memory. Neuroscience and Biobehavioral Reviews 36(1): 134–142. [DOI] [PubMed] [Google Scholar]
- Weinberger DR and Berman KF (1996) Prefrontal function in schizophrenia: Confounds and controversies. Philosophical Transactions of the Royal Society of London: Series B, Biological Sciences 351(1348): 1495–1503. [DOI] [PubMed] [Google Scholar]
- Welsh RC, Chen AC and Taylor SF (2010) Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophrenia Bulletin 36(4): 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Lu YS, Zhu XH, et al. (2010) Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proceedings of the National Academy of Sciences of the United States of America 107(3): 1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikmark RG, Divac I and Weiss R (1973) Retention of spatial delayed alternation in rats with lesions in the frontal lobes: Implications for a comparative neuropsychology of the prefrontal system. Brain, behavior and evolution 8(5): 329–339. [DOI] [PubMed] [Google Scholar]
- Wilson MA and McNaughton BL (1994) Reactivation of hippocampal ensemble memories during sleep. Science 265(5172): 676–679. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Vasic N, Sambataro F, et al. (2009) Temporally anticorrelated brain networks during working memory performance reveal aberrant prefrontal and hippocampal connectivity in patients with schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry 33(8): 1464–1473. [DOI] [PubMed] [Google Scholar]
- Won GH, Kim JW, Choi TY, et al. (2017) Theta-phase gamma-amplitude coupling as a neurophysiological marker in neuroleptic-naive schizophrenia. Psychiatry Research 260: 406–411. [DOI] [PubMed] [Google Scholar]
- Woodward ND and Heckers S (2016) Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biological Psychiatry 79(12): 1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H and Heckers S (2012) Thalamocortical dysconnectivity in schizophrenia. American Journal of Psychiatry 169(10): 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Suh J, Takeuchi D, et al. (2014) Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell 157(4): 845–857. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Hayashi Y, Yoshida T, et al. (2017) Schizophrenia-like phenotypes in mice with NMDA receptor ablation in intralaminar thalamic nucleus cells and gene therapy-based reversal in adults. Translational Psychiatry 7(2): e1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin DM, Chen YJ, Lu YS, et al. (2013) Reversal of behavioral deficits and synaptic dysfunction in mice overexpressing neuregulin 1. Neuron 78(4): 644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Okada J, Jung MW, et al. (2008) Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learning & Memory 15(3): 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY and Frank LM (2015) Hippocampal-cortical interaction in decision making. Neurobiology of Learning and Memory 117: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY, Kay K, Liu DF, et al. (2017) Distinct hippocampal-cortical memory representations for experiences associated with movement versus immobility. Elife 6: e27621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Llinas RR and Lisman JE (2009) Inhibition of NMDARs in the nucleus reticularis of the thalamus produces delta frequency bursting. Frontiers in Neural Circuits 3: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yoshida T, Katz DB, et al. (2012) NMDAR antagonist action in thalamus imposes delta oscillations on the hippocampus. Journal of Neurophysiology 107(11): 3181–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fan L, Qiu C, et al. (2015) Prefrontal cortex and the dysconnectivity hypothesis of schizophrenia. Neuroscience Bulletin 31(2): 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Shu N, Liu Y, et al. (2008) Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophrenia Research 100(1–3): 120–132. [DOI] [PubMed] [Google Scholar]


