Abstract
Fibrosis may be a key factor in sensorimotor dysfunction in patients with chronic overuse-induced musculoskeletal disorders. Using a clinically relevant rodent model, in which performance of a high demand handle-pulling task induces tissue fibrosis and sensorimotor declines, we pharmacologically blocked CCN2 (connective tissue growth factor) with the goal of reducing the progression of these changes. Young adult, female Sprague-Dawley rats were shaped to learn to pull at high force levels (10 min/day, 5 wks), before performing a high repetition high force (HRHF) task for 3 weeks (2 hrs/day, 3 days/wk). HRHF rats were untreated, or treated in task weeks 2 and 3 with a monoclonal antibody that blocks CCN2 (FG-3019), or a control IgG. Control rats were untreated, or received FG-3019, IgG or vehicle (saline) injections. Mean task reach rate and grasp force were higher in 3-week HRHF+FG-3019 rats, compared to untreated HRHF rats. Grip strength declined while forepaw mechanical sensitivity increased in untreated HRHF rats, compared to controls; changes improved by FG-3019 treatment. The HRHF task increased collagen in multiple tissues (flexor digitorum muscles, nerves, and forepaw dermis), which was reduced with FG-3019 treatment. FG-3019 treatment also reduced HRHF-induced increases in CCN2 and TGFbeta in muscles. In tendons, FG-3019 reduced HRHF-induced increases in CCN2, epitendon thickening and cell proliferation. Our findings indicate that CCN2 is critical to the progression of chronic overuse-induced multi-tissue fibrosis and functional declines. FG-3019 treatment may be a novel therapeutic strategy for overuse-induced musculoskeletal disorders.
Keywords: Work-related musculoskeletal disorders (WMSDs), tendinopathy, nerve, muscle, tendon
INTRODUCTION
Chronic overuse-induced musculoskeletal disorders are also known as work-related musculoskeletal disorders, repetitive motion disorders and repetitive strain injuries. They include diagnoses of carpal tunnel syndrome, tendinopathies and myalgias.1 Risk factors include chronic performance of high repetition and high force activities.2 There remains a call for effective treatments for these often debilitating disorders.3
CCN2 (cellular communication network factor 2; CTGF) is a secreted matricellular protein with four modular domains that independently interact with different molecules, such as collagen and proteoglycans in the extracellular matrix.4 CCN1 (Cyr6) and CCN3 (NOV) are two family members that can have similar or opposing functions in fibrogenic processes as CCN2, respectively.5–7 Since fibrosis is thought to distort dynamic properties of tissue due to adherence of adjacent structures, it may also contribute to functional and biomechanical tissue declines.8,9 Down regulation of CCN2 by anti-sense or SiRNA treatment reduces liver fibrosis and limits hypertrophic scarring without affecting wound healing.10,11 Mdx mice (an animal model of Duchenne muscular dystrophy) with a hemizygous CCN2 deletion, or treated with a neutralizing monoclonal antibody that targets the von Willebrand Factor type C (vWC) domain of CCN2 show reduced muscle fibrosis and improved muscle strength.12,13 The CCN2 antibody, known as FG-3019 or Pamrevlumab (Fibrogen Inc), has completed Phase 2 clinical trials for idiopathic pulmonary fibrosis (ClinicalTrials.gov Identifier: NCT01890265 14), and is currently in Phase 2 trials for locally advanced, unresectable pancreatic cancer (NCT02210559 15) and Duchenne muscular dystrophy (NCT02606136).
We have a clinically relevant rodent model of chronic overuse in which rats perform an operant reaching, grasping and lever-pulling task for food reward.16 Performance of this task at high repetition high force (HRHF) levels for 3 to 18 weeks induces progressive musculotendinous and nerve fibrosis and sensorimotor declines,8,17–19 similar to responses seen in patients with upper extremity overuse-induced musculoskeletal disorders.1 Use of ibuprofen or anti-tumor necrosis factor alpha drug reduced tissue inflammation in this model.17,18 However, they did not fully ameliorate task-induced functional declines, or return tissue fibrogenic proteins to control levels in HRHF rats. An initial 5 week shaping period in which rats learn to pull at high force levels also induces elevated levels of extracellular and fibrogenic proteins (periostin-like factor, CCN2 and TGFbeta1), relative to control rats.17,19 Thus, we sought here to determine if a monoclonal antibody that binds human and rodent CCN2 would reduce progression of tissue fibrosis and sensorimotor declines induced early in our operant rat model of overuse-induced musculoskeletal disorders.
MATERIALS AND METHODS
Detailed methods, including ELISA and antibody specifics, are in Supplemental Methods.
Animals
Experiments were approved by Institutional Animal Care and Use Committee in compliance with NIH guidelines. Studies were conducted on young adult female, Sprague-Dawley rats. Rats were food restricted to motivate participation, yet allowed to gain weight over time (Supplemental Figure 1). Task rats first underwent a 5 week shaping period to learn a high force lever pulling task, before performing HRHF task for 3 weeks. HRHF rats were untreated or treated with CCN2 antibody (FG-3019) or control human IgG (hIgG). Reach limbs were examined from untreated 23 3-week HRHF, 10 HRHF+hIgG, and 13 HRHF+FG-3019 rats. Remaining food-restricted rats were untreated or treated with FG-3019, hIgG or saline (29 FRC, 22 FRC+Saline, 5 FRC+hIgG, and 5 FRC+FG-3019 rats, bilaterally). Muscle and serum tissues were also examined from seventeen 0-week HRHF rats (euthanized after shaping).
HRHF Task
Task rats were shaped for 5 weeks to learn a reaching and lever-pulling task at high force loads at no specified reach rate (ramping upwards from naïve, 10 min/day, 5 days/wk). Rats then performed a HRHF reaching and lever-pulling task for 3 weeks (48% maximum pulling force, 4 reaches/min, 2 hrs/day, in 30 min intervals, 3 days/wk) for food reward.16
Pharmacological Treatments
Three-week HRHF rats were untreated, or treated in task weeks 2 and 3 with a human anti-CCN2 monoclonal antibody (FG-3019, FibroGen, San Francisco, CA; 40 mg/kg body wt, i.p.; 2x/wk, 2 wks), or human IgG (hIgG, FibroGen; 50 μl/injection, i.p.). FRC rats were untreated or treated similarly with FG-3019, hIgG or vehicle (100 μl saline, i.p., 2x/wk).
Behavioral Assays
HRHF voluntary reach outcomes were recorded continuously during each session and calculated in task week 3 for twelve HRHF, ten HRHF+FG-3019, and five HFHF+IgG rats. Reflexive grip strength was assayed using a grip strength meter (1027SR-D58, Columbus Instruments, Columbus, Ohio), after onset of food restriction, after shaping (HRHF week 0), and HRHF week 3. Maximum grip strength per trial (5 times/limb) is reported. Forelimb sensitivity to mechanical probing was assayed at end of HRHF task week 3 using monofilaments (Stoelting, IL). Mean number of limb withdrawal responses/10 repeats is reported per monofilament.
ELISAs
Animals were anesthetized and euthanized before blood collection at 36 hours after their last task session. Blood was centrifuged and serum harvested from: 3-week HRHF groups (13 HRHF, 9 HRHF+FG-3019 and 5 HRHF+hIgG), FRC groups (11 FRC, 8 FRC+Saline, 5 FRC+hIgG), and seven 0-week HRHF rats. Serum was assayed using ELISA for Collagen type 1, Collagen type 3, CCN2, transforming growth factor beta 1 (TGFβ1), and inflammatory cytokines (CCL2, CCL3, CXCL2, CXCL5, CXCL10, IL-10, IL-18). Samples were run in duplicate and data reported as pg/ml serum.
Forelimb tissues were collected for ELISA from reach limbs of 3-week HRHF rats (10 HRHF, 4 HRHF+FG-3019 and 5 HRHF+hIgG), one limb per FRC rat (16 FRC, 10 FRC+Saline, 5 FRC+hIgG, and 5 FRC+FG-3019), and seven 0-week HRHF rats. Samples were homogenized in phosphate-buffered saline (PBS) containing proteinase inhibitors, and centrifuged. Supernatants were assayed for collagen types 1 and 3, CCN2 and TGFβ1, as described for serum. Data (pg of protein) were normalized to μg total protein, determined using bicinchoninic acid (BCA, Thermo Fisher Scientific, Waltham, MA).
Gelatin Zymography and Western Blot assays
Aliquots of muscles homogenates (equal amounts of protein ) were assayed for matrix metalloproteinase (MMP) 2 and MMP9 activity using gelatin zymography under nonreducing conditions. Results were normalized to mean FRC and FRC+saline results and ratios compared. Aliquots were also assayed using Western blotting for CCN1, CCN2, CCN3, phosphorylated ERK (pERK) and total ERK using 4–12% Tris-Glycine gels under denaturing conditions. Gels were blotted onto nitrocellulose membranes, blocked in 5% BSA/0.05%Tween-20, and incubated overnight with specific antibodies per protein. After incubation with appropriate secondary antibodies and imaging using Licor and then Image J, normalized bands were compared as described in figure legends.
Immunohistochemistry and Histochemistry
Rats were euthanized, serum and limbs for ELISA removed, and then intracardial perfusion with buffered 4% paraformaldehyde. Reach limbs were collected from 3-week HRHF rats (13 HRHF, 9 HRHF+FG-3019 and 5 HRHF+hIgG), at least one limb per FRC rat (16 FRC, 15 FRC+Saline, and 5 FRC+hIgG), and ten 0-week HRHF rats. A piece of flexor digitorum muscle was removed from mid- to proximal forearm for crossectional slicing; remaining muscle-tendon-nerve mass was sectioned longitudinally (15 μm). Subsets of sections on slides were stained/immunostained with hematoxylin, TUNEL, collagen type 1 and 3, CCN2, PAX7, TGFβ1, anti-human IgG; or were triple-labeled with SMA, PDGFR and tcf4; followed by appropriate secondary antibodies. Forepaws were fixed by immersion, paraffin embedded, sectioned longitudinally (5 μm), placed onto charged slides, and stained with hematoxylin or Masson’s Trichrome.
Histomorphometry
Quantification was performed in 3 fields/tissue by individuals blinded to group assignment, using computerized image analysis systems (Bioquant Corporation, Nashville, TN). In muscles, collagen types 1 and 3, and CCN2 immunostaining were quantified in crosssections using a thresholded pixel count. CCN2 was quantified similarly in longitudinal cryosectioned tendons. CD68, PAX7, TGFβ1, and TUNEL+ cells, and αSMA+/tcf4+/PDGFR+ cells were counted in muscle crossections. Shortest diameters of myofibers (mean circular mil) were assayed in collagen/DAPI immunostained crossections. Longitudinal sections were used to count CD68+ cells within median nerves, epitendon thickness and cellularity, and endotendon cellularity, at wrist level after hematoxylin staining. Cell shape factor analysis (Bioquant) was used to assay if endotendon cells were more spindle-shaped (<0.5) versus rounded (> 0.05). Percent area with collagen staining around median nerves and in digits’ upper dermis was quantified after Masson’s Trichrome staining of paraffin-embedded and longitudinally cut forepaws.20
Statistical Analyses
A power analysis from past work was first performed and showed a minimum of 4 to 5/group was needed.17,20 All data are expressed as mean ± SEM. P values of < 0.05 were considered significant. Unpaired, two-tailed t-tests were used to compare 0-week HRHF and FRC results; one-way ANOVAs were used to compare operant behavior and Western blot densitometry results; and two way ANOVAs were used to analyze grip strength, forepaw mechanical allodynia, ELISA and histological data. Sidak multiple comparison tests were used for planned post hoc assays [untreated HRHF rats versus untreated FRC rats; HRHF+hIgG to FRC+hIgG; HRHF+FG-3019 to FRC+FG-3019 for behavioral or to FRC+saline for tissue data; and HRHF+FG-3019 to both untreated HRHF and HRHF+hIgG rats]. Behavioral results were correlated to collagen staining/levels in tissues using Pearson’s or Spearman’s r tests.
RESULTS
Improved sensorimotor declines
Three week HRHF and HRHF+hIgG rats were unable to meet the target reach rate (Fig. 1A). Mean reach rate increased to target levels in HRHF+FG-3019 rats (Fig. 1A). Mean voluntary grasp force was also higher in HRHF+FG-3019 rats, compared to untreated HRHF rats (Fig 1B).
Figure 1.
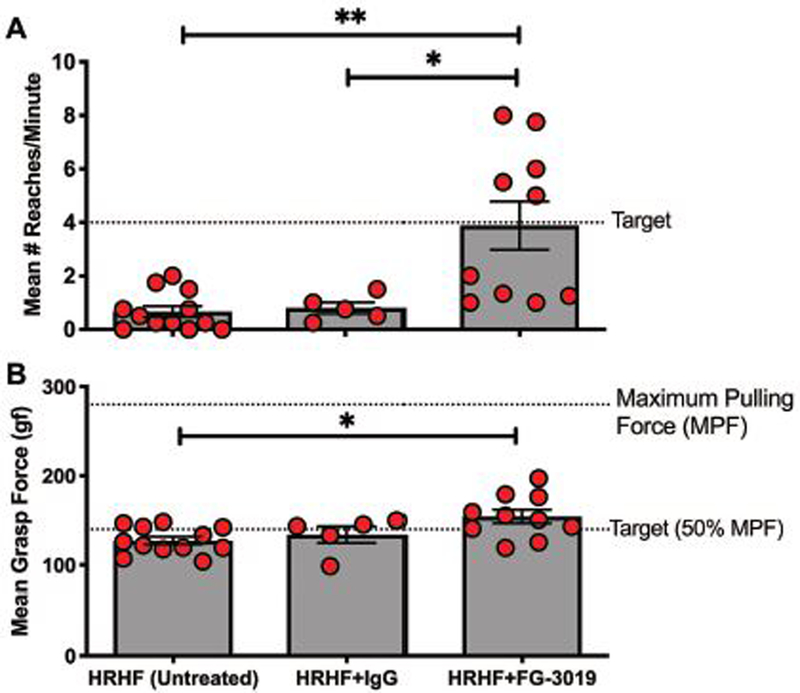
Operant performance behaviors assayed in task week 3 in rats performing a high repetition high force (HRHF) reaching and grasping a lever bar task. A) Target reach rate was 4 reaches/min. B) Mean voluntary grasp force on the lever bar. Symbols: *: p<0.05, and **:p<0.01 compared to groups as shown.
Reflexive grip strength did not differ between FRC groups (Fig. 2A), yet declined immediately after shaping (week 0) in HRHF rats, compared to baseline and FRCs (Fig. 2B). These declines persisted in 3-week HRHF and HRHF+hIgG rats, yet improved in HRHF+FG-3019 rats (Fig 2B). Forepaw mechanical sensitivity was increased in HRHF rats in response to probing with monofilaments sized 3.92 and 9.81 mN, compared to FRCs (Fig. 2C). This hypersensitivity was not present in HRHF+FG-3019 rats.
Figure 2.
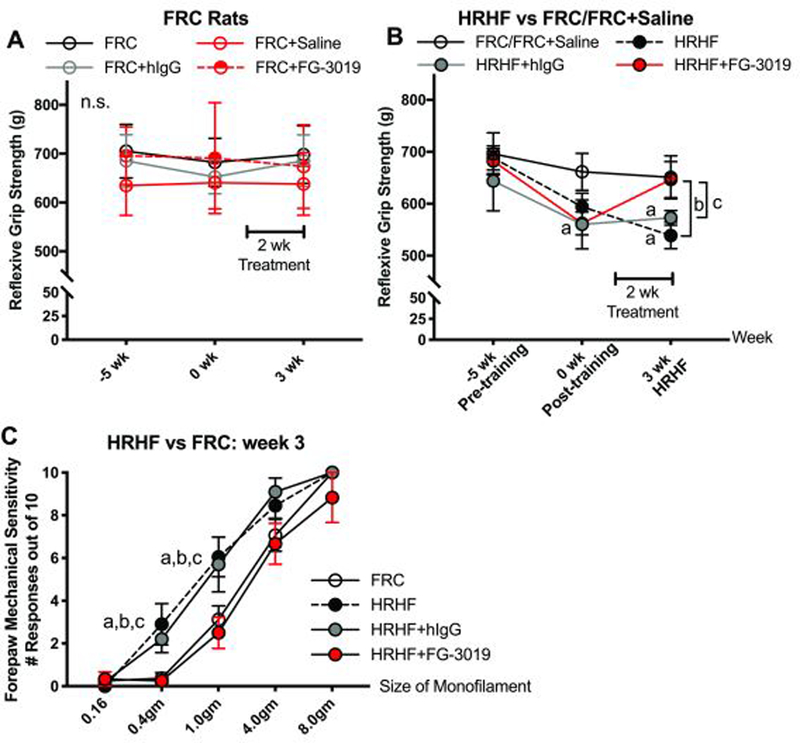
Reflexive grip strength and forepaw mechanical sensitivity. A and B) Reflexive grip strength in food restricted control (FRC) and HRHF task groups. HRHF rats were tested at a naïve timepoint immediately prior to the 5 week shaping period, immediately post shaping (task week 0) and after performing the HRHF task for 3 weeks, with subsets receiving treatments in the final two weeks. FRC rats were tested at similar times. C) Forepaw mechanical sensitivity assayed in task week 3 using nylon monofilaments with milliNewton (mN) sizes shown. Symbols: a: p<0.05, compared to matched FRC group; b: p<0.05, compared to untreated HRHF rats; c: p<0.05, compared to HRHF+hIgG rats; n.s. = not significant. Number of limbs tested per group: HRHF, n=12; HRHF+FG-3019, n=10–12; HRHF+hIgG, n=10; FRC, n=10–25; FRC+Saline, n=10; FRC+hIgG, n=10, and FRC+FG-3019, n=10, bilateral results included for latter two groups.
Reductions in HRHF-induced increases in muscle collagen
We first examined muscles of 0-week HRHF rats to determine the effects of shaping for 5 weeks to the high force pulling level, and observed increased collagen (immunohistochemically and ELISA assayed) in flexor digitorum muscles, compared to FRCs (p<0.05 each, Supplemental Table 1, Figure 3). Collagen type 1 was further increased in muscles of 3-week HRHF and HRHF+hIgG rats, compared to FRC and FRC+hIgG rats (immunohistochemically and ELISA assayed; Fig. 3A,B). HRHF-induced increases improved in FG-3019 rats (Fig. 3A,B). Increased collagen type 1 was localized around myofibers in HRHF rats, relative to other groups (Fig. 3C). Collagen type 1 was detectable in serum, which showed similar responses as muscles (Fig. 3D). Serum and muscle collagen type 3 did not differ between groups (Table 1).
Figure 3.
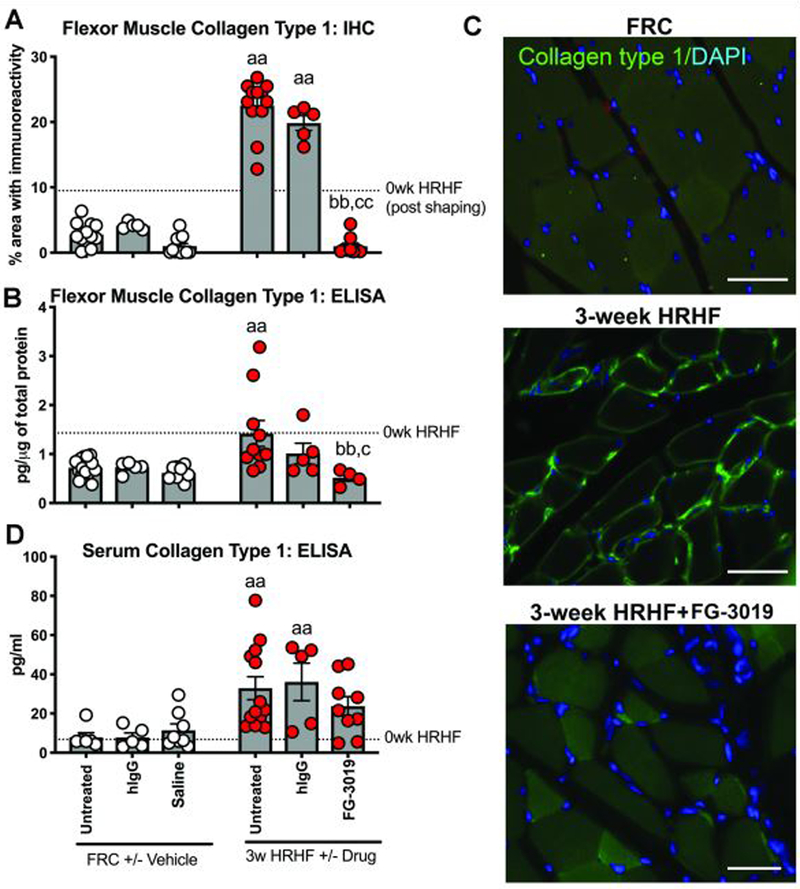
Collagen type 1 in flexor digitorum muscle and serum. A) Percent area with Collagen type 1 immunoreactivity (green) and DAPI nuclear stain (blue) in cross-sectionally cut muscles of FRC, 3-week untreated HRHF and HRHF+FG-3019 treated rats. B) ELISA detected levels in muscles. C) Collagen type 1 serum levels, tested using ELISA. Symbols: aa: p<0.01, compared to matched FRC group; bb: p<0.01, compared to HRHF rats; c: p<0.05, and cc:p<0.01, compared to HRHF+hIgG rats. Scale bars = 50 μm. The 0-week HRHF rat results from Supplemental Table 1 are indicated with dotted lines.
Table 1.
Additional fibrogenic protein and myofiber diameter findings in serum, and flexor digitorum muscle and tendons. Significant changes are bolded.
| Analyte and Tissue | FRC untreated (n=5–9) |
FRC+hIgG (n=5) |
FRC+Saline (n=4–7) |
3-week HRHF untreated (n=8–10) |
3-week HRHF+hIgG (n=5) |
3-week HRHF+FG-3019 (n=4–7) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Serum Collagen type 3, pg/ml1 (n=5–7/gp) | 75.93±9.39 | 92.96±29.38 | 74.32±23.99 | 114.47±18.43 | 106.90±36.41 | 137.12±18.71 | |||
| Muscle Collagen type 3, pg/μg total protein1 (n=4–9/gp) | 0.02±0.003 | 0.03±0.001 | 0.03±0.005 | 0.08±0.03 | 0.05±0.01 | 0.02±0.004 | |||
| Muscle myofibroblasts SMA+PDGFR+tcf4+ cells/field of 0.26mm2 | 0.67±0.67 | NT | 0.17±0.17 | 1.25±0.38a | NT | 0.44±0.24 | |||
| Tendon CCN2, pg/μg total protein1 (n=4–10/gp) | 0.09±0.05 | 0.19±0.03 | 0.26±0.06 | 0.49±0.17a | 0.53±0.03a | 0.20±0.07b,c | |||
| Endotendon # cells/mm2 (n=5–6/gp) | 0.001±0.0006 | 0.001±0.0003 | 0.001±0.0003 | 0.002±0.001 | 0.002±0.001 | 0.001±0.0003 | |||
| Endotendon cell shape factor; spindle-shaped ≤ 0.5 ≥ rounded. (n=5–6/gp) | 0.27±0.01 | 0.22±0.05 | 0.18±0.02 | 0.55±0.17 | 0.61±0.12 | 0.45±0.05 | |||
| Tendon Collagen type 1, pg/μg total protein1 (n=4–8/gp) | 4.51±0.64 | 5.90±0.48 | 5.44±0.79 | 7.81±01.30a | 10.25±0.99a | 10.02±1.18a | |||
| Tendon Collagen type 3, pg/μg total protein1 (n=4–8/gp) | 0.25±0.02 | 0.23±0.03 | 0.19±0.02 | 0.18±0.04 | 0.26±0.01 | 0.24±0.04 | |||
| Myofiber diameters, mcm (n=5–6/gp) | 37.53±2.60 | 37.38±6.27 | 37.73±2.86 | 35.26±3.60 | 37.59±1.89 | 39.03±4.21 | |||
ELISA; NT = not tested.
p<0.05 and aa p<0.01, compared to matched FRC group
p<0.05 and bb p<0.01, compared to untreated HRHF rats
p<0.05, and ccp<0.01, compared to HRHF+hIgG rats
Reductions in HRHF-induced increases in muscle CCN2
We examined using Western blot if FG-3019 treatment affected the ability to immunochemically detect CCN2, using tissues from control rats since CCN2 levels should be similar. No differences in CCN2 levels were seen in tendons and serum of FRC versus FRC+FG-3019 rats (Supplemental Figure 2).
Then, we examined muscles of 0-week HRHF rats to determine the effects of shaping for 5 weeks to high force pulling, and observed increased CCN2 (immunohistochemically and ELISA assayed) in muscles, compared to FRC rats (p<0.05 each, Supplemental Table 1, Fig. 4). We next assayed 3-week HRHF rat muscles, and observed increased CCN2 in HRHF and HRHF+hIgG muscles, compared to FRC and FRC+hIgG rats (Fig. 4A,B). FG-3019 treatment reduced these increases. CCN2 was detectable in serum, which showed similar responses as muscles (Fig. 4C). Increased CCN2 in untreated HRHF muscles was evident within myofibers and in small cells on their perimeter, relative to FRC and HRHF+FG-3019 muscles (Fig. 4E), but not in association with αSMA (Fig. 4D). Using an anti-human IgG antibody, presence of the human FG-3019 agent was detected in small cells on the perimeter of myofibers in HRHF+FG-3019 rat muscles, yet not in untreated HRHF rats (Fig. 4F).
Figure 4.
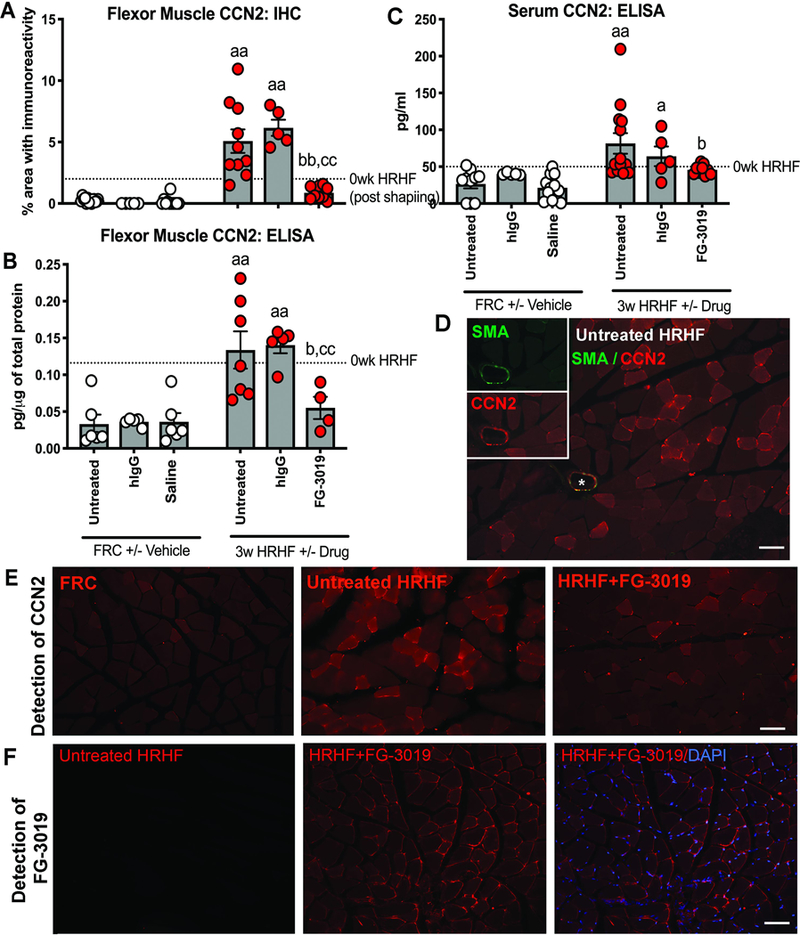
CCN2 in flexor digitorum muscles. A-C) Immunoexpression and ELISA detected levels in muscles and serum. D) An untreated 3-week HRHF muscle probed for both SMA and CCN2 immunoexpression. Only the arteriole shown in the insets shows co-localization. E) Location of CCN2 immunoexpression in muscle cross-sections. F) Anti-human IgG detection of the FG-3019 agent in muscles of HRHF+FG-3019 rats; staining absent in untreated HRHF rats. Symbols: a: p<0.05 and aa: p<0.01, compared to matched FRC group; b: p<0.05 and bb: p<0.01, compared to HRHF rats; cc:p<0.01, compared to HRHF+hIgG rats. Scale bars = 50 μm. The 0-week HRHF rat results from Supplemental Table 1 are indicated with dotted lines.
We also examined the effects of task and treatment on CCN1 and CCN3. CCN1 was unchanged with task or treatment (Supplemental Figure 3). while CCN3 was downregulated in both HRHF and HRHF+FG-3019 rats, significantly so in HRHF rat muscles, compared to FRCs (Fig. 5).
Figure 5.
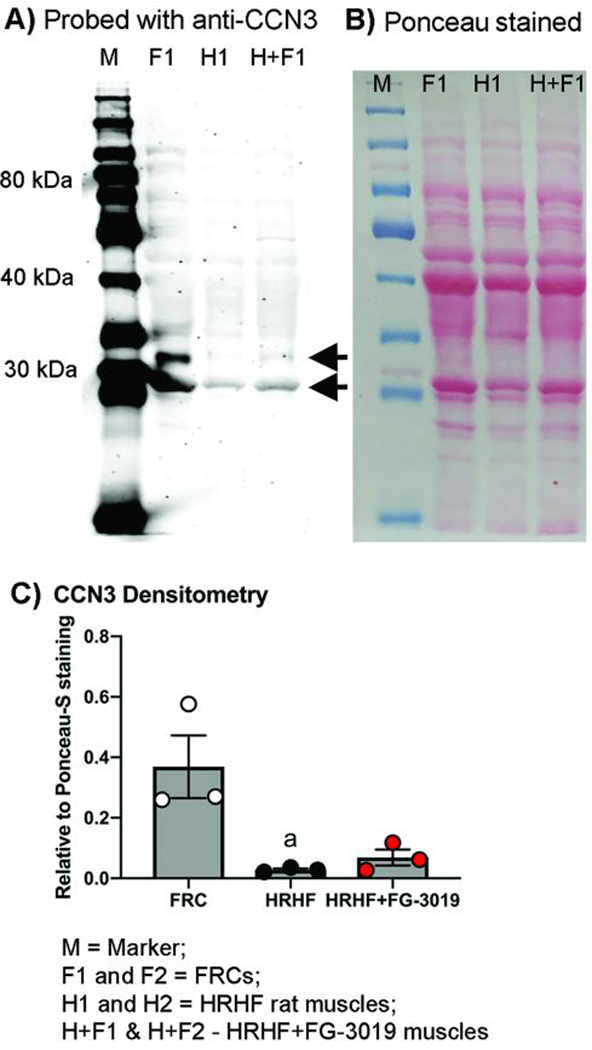
Anti-CCN3/NOV probed Western blots showing truncated forms of CCN3 with approximate molecular weights of 32 and 28 kDa (arrows). (A) Western blot showing flexor digitorum muscle samples from food restricted control rats (F; FRC), untreated high repetition high force rats (H; HRHF), and HRHF rats treated with the FG-3019 agent (H+F; HRHF+FG-3019). (B) Ponceau S red staining of same membrane shown in panel A. (C) Densitometry results in which CCN3 bands were compared to the total protein loaded per lane, determined from Ponceau-S red stained membranes. Gels were repeated until 3 different samples per group were assayed. a: p<0.05, compared to FRC levels.
Reductions in HRHF-induced increases in muscle TGFβ1
TGFβ1 immunostaining and levels increased in muscles of HRHF and HRHF+hIgG rats, compared to matched FRCs, and decreased in HRHF+FG-3019 rats, compared to HRHF rats (Fig. 6A,B). Increased TGFβ1 was seen in small cells on the perimeter of myofibers and in the cytoplasm of some myofibers (Fig. 6C).
Figure 6.
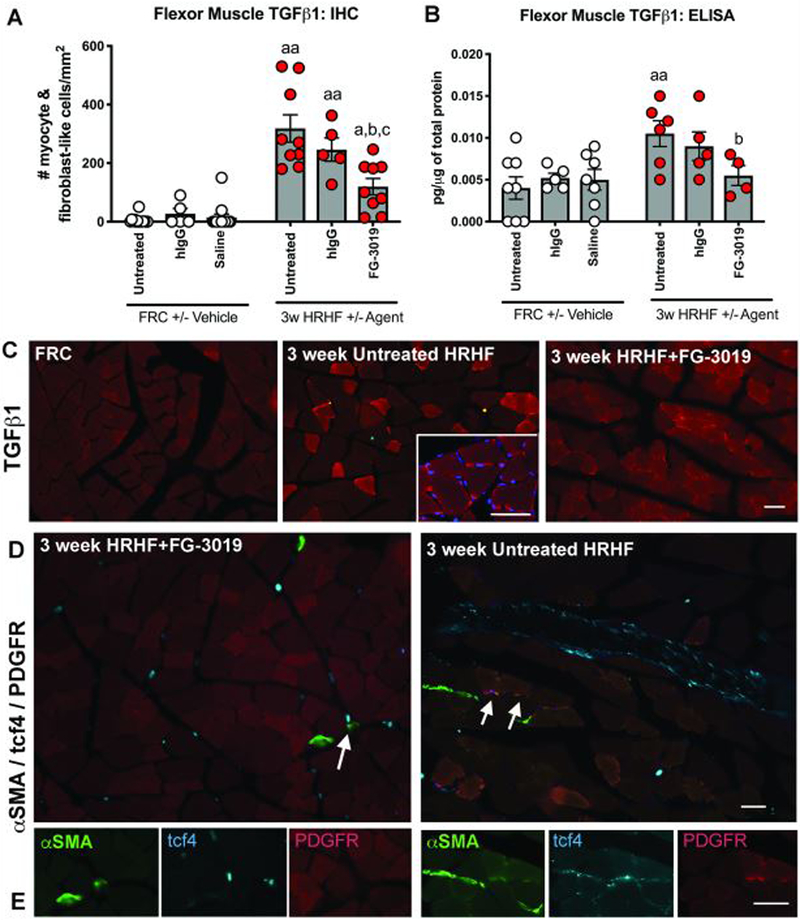
TGFß1 in flexor digitorum muscles. A,B) Immunoexpression and ELISA detected levels. Symbols: a: p<0.05 and aa: p<0.01, compared to matched FRC group; b: p<0.05, compared to HRHF rats; c:p<0.05, compared to HRHF+hIgG rats. C) Location of TGFß1 immunoexpression in muscle cross-sections. D) Presence and location of αSMA+/tcf4+/PDGFR+ and αSMA+/tcf4+/PDGFR- cells in HRHF+FG-3019 and HRHF rat muscles. A few triple labeled cells were found on the perimeter of myofibers in 3-week HRHF rat muscles (arrows). These cells are shown at higher magnification in Panel E, right side. Left side of panel E shows examples of αSMA+/tcf4+/PDGFR- cells (i.e., double labeled only) in the endomysium. Scale bars = 50 μm.
Muscle myofibroblast numbers were examined by triple-labeling with αSMA, tcf4 and PDGFR.21 Small but significant increases in αSMA+/ tcf4+/ PDGFR+ triple-labeled cells were seen at the edges of myofibers in untreated HRHF rat muscles, compared to HRHF+FG-3019 and FRC rat muscles (Fig. 6D,E; Table 1). HRHF+FG-3019 also showed presence of αSMA+/ tcf4+, yet PDGFR- cells in the endomysium (Fig. 6C,E), at similar levels as untreated HRHF rat muscles, although more than in FRC rat (data not shown).
Improved HRHF-induced epitendon changes
CCN2 levels increased in HRHF and HRHF+hIgG tendons, compared to HRHF+FG-3019, FRC or FRC+hIgG rat tendons (Fig. 7A; Table 1). The FG-3019 agent was detectable in tendons of HRHF+FG-3019 rats, yet not in untreated HRHF rats (Fig. 7B). HRHF and HRHF+hIgG rats showed increased epitendon cellularity and thickness, compared to the other groups (Fig. 7C–E), but no endotendon changes (Table 1). Collagen type 1 levels increased in tendons of each task group, compared to FRCs; collagen type 3 levels did not alter with task or treatment (Table 1).
Figure 7.
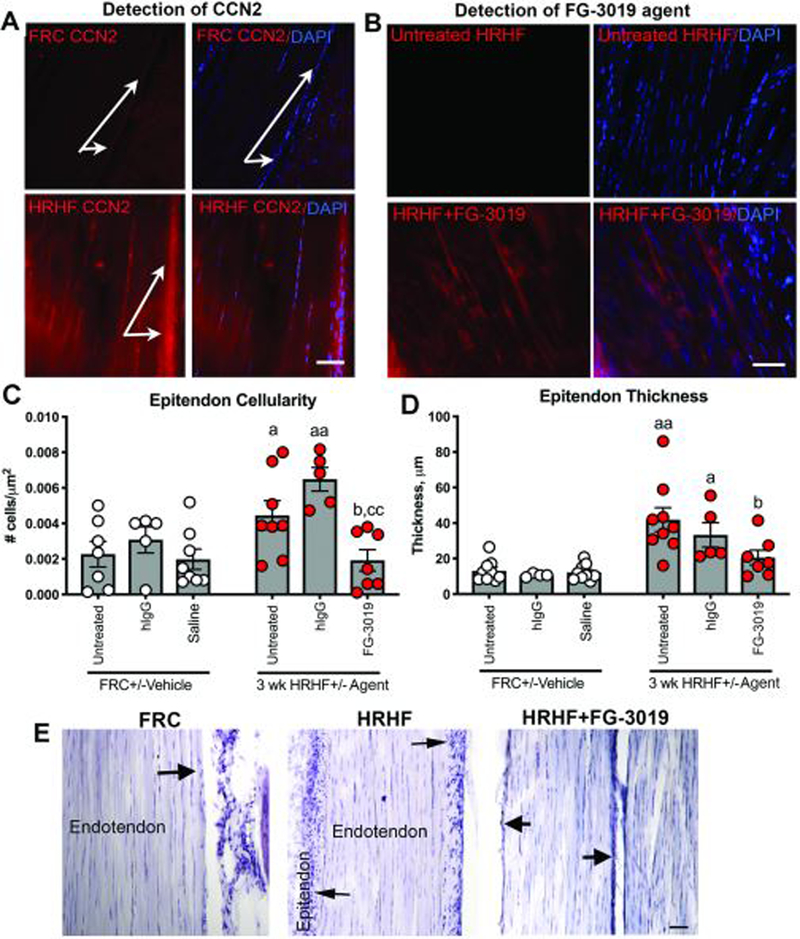
Task and treatment induced changes in flexor digitorum tendons at wrist level. A) Location of CCN2 staining in tendons showing increases in tenocytes and epitendon cells of HRHF rats (arrows). B) Anti-human IgG detection of FG-3019 agent in tendons of HRHF+FG-3019 rats; staining absent in untreated HRHF rats. C,D) Quantification of epitendon cellularity and thickness. E) Representative images of H&E stained tendons. Arrows indicate epitendon region. Symbols: a: p<0.05 and aa: p<0.01, compared to matched FRC group; b: p<0.05, compared to untreated HRHF rats; cc: p<0.01, compared to HRHF+hIgG rats. Scale bars = 50 μm.
Reduced HRHF-induced nerve and dermal fibrosis
Increased collagen deposition (Masson’s Trichrome staining) was observed around median nerves at wrist level and in the dermis of forepaw digits in HRHF rats, compared to FRC and HRHF+FG-3019 rats (Fig. 8A,B). Increased collagen was also evident in dermal papillae regions containing nerves (Fig. 8B). Quantification confirmed these observations (Fig. 8C,D).
Figure 8.
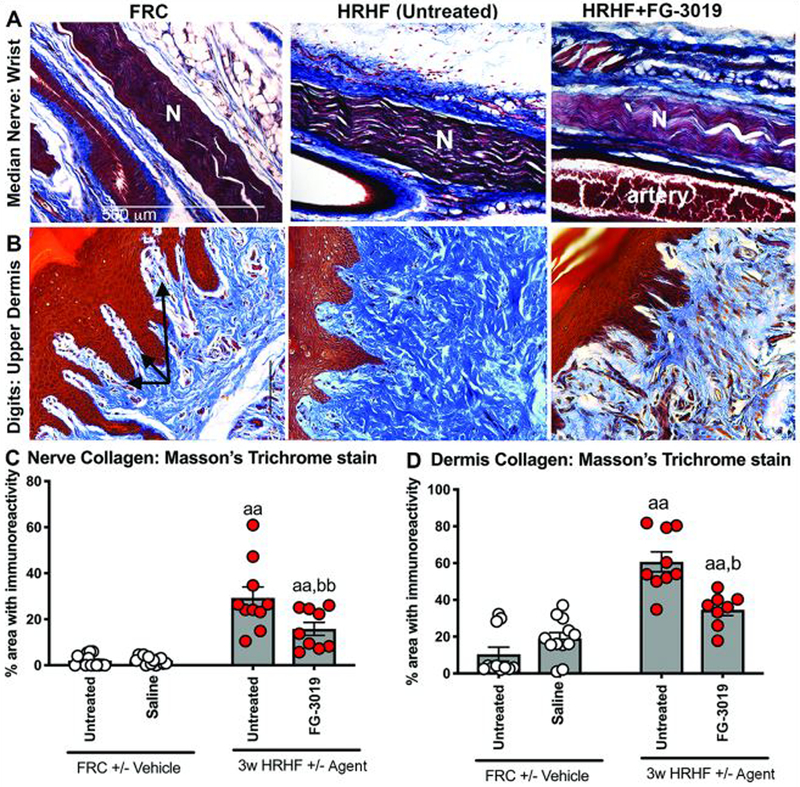
Masson’s Trichrome staining around the median nerve branches and in upper dermis of forepaw digits. A) Masson’s trichrome staining with collagen stained blue around the median nerve (N) at wrist level in longitudinal sections. B) Masson’s trichrome staining in the upper dermis. Arrows indicate regions containing nerves within dermal papillae. C) Quantification of blue-stained collagen in paraneural regions (i.e., areas surrounding nerve) at wrist level. D) Quantification of blue-stained collagen in the upper dermis. Symbols: aa: p<0.01, compared to matched FRC group; b:p<0.05 and bb: p<0.01, compared to untreated HRHF rats.
Correlation of collagen with behavioral outcomes
Pearson’s r tests (Table 2) showed significant inverse relationships between: 1) reaches per minute and % collagen in muscles and collagen levels in tendons; 2) voluntary grasp force and collagen staining and levels in muscle; 3) reflexive grip strength and collagen in all tissues examined. Spearman’s r tests showed increasing forepaw mechanical sensitivity with increasing nerve/dermal collagen, epitendon cellularity and thickness.
Table 2.
Pearson and Spearman’s r correlations between behavioral outcomes and collagen.
| Tissue analyses: | Reaches/minute (Pearson r) |
Grasp Force (Pearson r) |
Reflexive grip strength (Pearson r) |
Forepaw mechanical sensitivity (3.92 mN filament; Spearman r) |
|---|---|---|---|---|
| Muscle: % area collagen type 1 (immunohistochemistry) | r = - 0.58, p=0.004 | r = - 0.46, p=0.030 | r = - 0.44, p=0.002 | r = - 0.06, p=0.79 |
| Muscle Collagen type 1 (ELISA) | r = - 0.39, p=0.10 | r = - 0.56, p=0.01 | r = - 0.43, p=0.002 | r = 0.16, p=0.49 |
| Tendon Collagen type 1 (ELISA) | r = - 0.75, p=0.002 | r = - 0.37, p=0.19 | r = - 0.48, p=0.01 | r = 0.63, p=0.08 |
| Epitendon: #cells/μm2 | r = - 0.34, p=0.20 | r = - 0.24, p=0.27 | r = 0.23, p=0.24 | r = 0.39, p=0.02 |
| Epitendon: thickness (μm) | r = - 0.40, p=0.12 | r = - 0.35, p=0.18 | r = - 0.13, p=0.52 | r = 0.44, p=0.01 |
| Nerve: % area collagen staining around median nerve at wrist | r = - 0.39, p=0.09 | r = - 0.20, p=0.39 | r = - 0.46, p=0.002 | r = 0.50, p=0.01 |
| Dermis: % area collagen staining in digits’ upper dermis | r = - 0.33, p=0.19 | r = - 0.38, p=0.139 | r = - 0.58, p=0.001 | r = 0.68, p=0.001 |
Significant moderate to strong correlations are bolded.
Reduced HRHF-induced inflammation
CD68+ cells were counted in flexor digitorum muscles and median nerves. Increases were observed in HRHF rats, compared to HRHF+FG-3019 and FRC rats (Table 2). CCL2 and CXCL10 increased in serum of HRHF rats, compared to FRCs, as did IL-10, an anti-inflammatory cytokine (Table 2). Their levels were reduced in HRHF+FG-3019 rats. Serum levels of CCL3, CXCL2, CXCL5 and IL-18 were not altered (Table 2).
Neither task nor treatment altered myofiber diameters, PAX7, TUNEL, MMP or ERK
There were no effects of task or treatment in muscles on: mean myofiber diameters (Table 1); numbers of PAX7+ or TUNEL+ cells in muscles, tendons or nerves (data not shown); muscle MMP2 or MMP9 activity (Supplemental Fig. 4); pERK or total ERK levels (Supplemental Fig. 5).
DISCUSSION
We previously reported muscle, tendon and median nerve fibrosis, characterized by increased tissue levels of collagen type 1, CCN2 and TGFβ1 in rats performing the HRHF lever pulling task for 6 to 18 weeks.8,17,22,23 The initial 5–6 week shaping period in which rats learn to pull the lever bar at high force levels also enhances CCN2 and TGFβ1 production, relative to control levels 17,19 (results confirmed and expanded here to show increased collagen type 1 production). Therefore, we chose an antibody that blocks CCN2 signaling (FG-3019) 14,24, since CCN2 plays essential roles in mediating excessive matrix deposition in overexpression experiments.13,25 We administered this agent in weeks 2 and 3 of HRHF performance to determine if CCN2 contributes to the progression of fibrosis in this model. We found here that FG-3019 treatment reduced: collagen deposition in multiple tissues (skeletal muscle, dermis, around nerves, and serum), CCN2 production in multiple tissues (muscles, tendons and serum), TGFβ1 production in muscles, and epitendon cellularity and thickening, each induced by shaping and then performance of the HRHF task for 3 weeks. These reduced tissue changes were associated with improvement in sensorimotor declines.
Rest, ice, compression and elevation are used to treat acute injuries, but have proved less effective for chronic musculoskeletal disorders. Surgical release or steroid injections are common treatments for fibrotic nerve compression, yet are also not consistently effective.26 We postulate that the multi-tissue involvement of these disorders hinders the effectiveness of interventions focused on improving only one involved tissue. NSAIDS are also commonly used treatments for acute and chronic musculoskeletal pain,27,28 yet are also not completely effective29 and may reduce tissue healing.30 NSAIDs may fail because they treat algesic symptoms but not the key underlying cause, which we and others believe are fibrotic adhesions tethering tissues and compressing nerves.9,31 The FG-3019 antibody used here targets domain II of CCN2, the vWC domain, located within the amino-terminal half of the full length protein.4,32 This domain is thought to stimulate collagen synthesis and bind proteoglycans in the extracellular matrix (other specific associations are being clarified).4 Systemic administration of FG-3019 was used to block fibrogenic processes occurring in all upper extremity tissues involved in performing this chronic repetitive high force task, since collagen production increases in skeletal muscle, tendons, dermis and extraneural connective tissues, and CCN2 increases in myofibers and small cells on their perimeter, as well as tenocytes, epitendon, mast and Schwann cells.8,23 The effects of blocking other domains of CCN2, such as domains III and IV shown to elicit cell signaling activity,4 may also reduce the observed multi-tissue fibrosis, although this remains to be tested since to our knowledge no antibodies exist that block only specific domain functions.
An early treatment timepoint was chosen to tease out whether CCN2 plays a key role in the progression of fibrosis in this chronic overuse model, or if persistent mechanical loading alone increases collagen production through TGFβ−1 signaling pathways independent of CCN2.33 As indicated above, increased muscle levels of CCN2 and TGFβ−1 are already present in 0-week HRHF rats,17 yet, indices of central sensitization (i.e., spinal cord changes associated with chronic pain) are not detectable until HRHF week 6.34 This earlier time point allowed us to avoid central nervous system changes that would confound interpretation of behavioral findings.
Sensorimotor behavioral declines in untreated HRHF rats suggest they have either discomfort or reduced tissue function. Fibrosis within and around tissues can reduce dynamic tissue biomechanical function (muscle fibrosis can biomechanically reduce muscle contractions35,36). Improved reach rate, voluntary grasp force and reflexive grip strength with FG-3019 treatment could be due to the reduced tissue collagen, an idea supported by the inverse relationship between motor function and tissue collagen. Fibrotic compression of median nerves in the carpal tunnel leads to reduced nerve conduction, degraded myelin and neuritis.23 FG-3019 treatment of HRHF task rats improved their forepaw mechanical allodynia, in parallel with reduced collagen around nerves at the wrist and forepaw digits. Short-term FG-3019 treatment also improves function in irradiated mouse lungs and reduce mRNA expression of fibrogenic-related factors and extracellular matrix production.37,38 It has been shown to reduce skeletal muscle fibrosis and improve muscle performance in a mouse model of amyotrophic lateral sclerosis 39 and a mdx mouse model,12 and lung function in human patients with idiopathic pulmonary fibrosis.14
Although the use of a targeted monoclonal antibody to a protein has the potential to reduce the ability to detect said protein in tissues, we did not observe differences in CCN2 levels in tendons and serum of untreated controls versus controls treated with FG-3019. This may be because tissues were collected five days after its last injection. Although this should be considered cautiously due to this aforementioned limitation, CCN2 levels were reduced in tissues of FG-3019 treated HRHF rats. This finding matches results of an irradiation-induced lung fibrosis study,37 although differing from another showing that FG-3019 has no effect on skeletal muscle levels of CCN2.39 CCN2 levels and signaling responses are known to be context dependent, vary with cell type and matrix in which it is functioning, and environmental stimulants.40
We examined muscles for two other members of the CCN family, CCN1/Cyrc61 and CCN3/Nov. A truncated form of CCN1 was observed (~32kDa), close in molecular weight to the ~28kDa form representing its NH2-terminal end.41,42 CCN1 shows rapid and transient alterations with mechanical loading (<1–6 hr).7,43,44 However, in this study, muscle CCN1 levels did not alter with task or treatment, likely because tissues were not collected until 36 hours after the last task session in order to avoid activity-induced changes in inflammatory cytokines. This later collection point is also the most probable reason why no changes were observed in MMP2 and pERK levels. CCN1 has a multimodular structure (as do CCN2 and CCN3) that is thought to be the underlying means by which it exhibits diverse activities dependent on environmental context.42 Truncated forms of CCN3 were also observed (~32 kDa and 28 kDa), matching the molecular weight of previously identified truncated forms of CCN3.45,46 CCN3 is anti-fibrogenic, antagonizes the fibrogenic effects of CCN2, and has an inverse expression pattern than CCN2.5,6 Its down-regulation in task animals when CCN2 levels are increasing warrants further examination in fibrogenic musculoskeletal disorders since provision of CCN3 or derived peptides has been postulated as a viable anti-fibrotic treatment for diabetic nephropathy.6
TGFβ1 levels increase in muscles of HRHF rats after shaping and remained increased with continued performance of the HRHF task for up to 18 weeks.8,17 It is well known that TGFβ1 is a potent inducer of CCN2 production.47 Yet, in the context of this model, blocking CCN2 reduced TGFβ1 production. This agrees with results of a study of diabetic rats where inhibition of CTGF gene expression inhibited TGFβ2 in the retina.48 Since mechanical loading and increased TGFβ1 levels are thought to increase myofibroblast numbers in muscles, we examined the flexor digitorum muscles for αSMA+/ tcf4+/ PDGFR+ triple-labeled cells.21 Only small numbers were seen at the edges of myofibers in untreated HRHF rat muscles (Table 1). These numbers were several fold lower than reported previously in mdx mutant mouse studies.21 Thus, although the increases observed in HRHF rat muscles relative to the other groups were statistically significant, this increase is likely not biologically relevant in the context of this study.
FG-3019 treatment also reduced epitendon fibroblast proliferation and thickening, known to increase in humans and animals with repetitive overuse.22,49 The latter findings match those showing decreased fibroblast and mesothelioma cell line proliferation after FG-3019 treatment.50 However, in that study, FG-3019 treatment also increased cell apoptosis, a response not observed in this study.
Effects of CCN2 on immune cell infiltrates and inflammatory cytokines that contribute to fibrogenic responses are largely unexplored. We were unable to separate out effects of the FG-3019 agent on inflammation versus those produced by fibrotic tethering of tissues in this in vivo model. What we can say is that CD68+ macrophage numbers and serum levels of several chemokines were reduced in HRHF+FG-3019 relative to HRHF rats. A two-week treatment with FG-3019 reduced macrophage gene signatures and mRNA levels of CCL2 and CXCL10 in mice with radiation therapy-induced lung injury and fibrosis.37 FG-3019 pretreatment prevented leukocyte influx into lungs after irradiation, while post-treatment reversed the influx of mast cells and macrophages into lungs when the antibody administration begins 16 weeks after the irradiation.38 Inhibition of CCN2 also reduced CD45+ leukocytes in an Angiotensin II-induced mouse model of skin fibrosis.33 Although more studies are needed, CCN2 may be a key modulator of inflammatory responses.
We have several limitations. We are unable to tease out inflammatory versus collagen deposition-induced changes, since these changes occur simultaneously during early phases of our model.17 We postulate that inflammation and fibrosis are interrelated in a fibrotic-inflammatory complex. Perhaps the strength of the FG-3019 agent was its ability to ameliorate both responses. We cannot address down-stream signaling changes in this in vivo model, if we also hope to avoid activity-induced changes by collecting tissues at 36 hours after the last task session. Lastly, we assessed effects of FG-3019 only in early fibrogenic processes. Studies are nearly complete examining its abilities at reversing established tissue fibrosis in this model.
In conclusion, we evaluated for the first time, the effectiveness of FG-3019 treatment in reducing the early progression of fibrosis in an operant model of overuse-induced musculoskeletal disorders. Our findings indicate that CCN2 and its downstream product, collagen, increase early with performance of highly repetitive and forceful tasks. We found that systemic injections of a human monoclonal antibody directed against CCN2 reduced the progression of multi-tissue fibrosis. Importantly, sensorimotor declines improved, supporting our hypothesis that these behavioral declines are the result of tissue tethering and compression. The effectiveness of this agent in our model of overuse-induced musculoskeletal disorders shows promise for the treatment of related disorders in human subjects
Supplementary Material
Table 3.
Inflammatory responses in flexor digitorum muscle, median nerve and serum. Significant changes are bolded.
| Analyte and Tissue | FRC/FRC+Saline (n=5–16) |
3-week HRHF Untreated (n=6–12) |
3-week HRHF+FG-3019 (n=4–9) |
|---|---|---|---|
| Muscle CD68-immunopositive cells, #cells/mm2 (n=7–11/gp) | 0.20±0.08 | 0.62±0.06aa | 0.33±0.03bb |
| Nerve CD68-immunopositive cells, #cells/mm2 (n=9–16/gp) | 6.01±0.88 | 20.91±2.92aa | 11.02±2.65b |
| Serum CCL2/MCP-1, pg/ml (n=4–6/gp) | 92.85±58.06 | 494.0±52.91aa | 156.2±68.39bb |
| Serum CCL3/MIP1a, pg/ml (n=4–6/gp) | 13.6±2.43 | 8.07±2.03 | 9.12±1.01 |
| Serum CXCL2/MIP2, pg/ml (n=4–6/gp) | 10.62±2.77 | 9.25±3.89 | 10.74±3.24 |
| Serum CXCL5/LIX, pg/ml (n=4–6/gp) | 778.5±93.42 | 1069.0±127.40 | 874.4±60.20 |
| Serum CXCL10/IP-10, pg/ml (n=4–6/gp) | 172.4±33.24 | 345.8±65.80a | 120.5±12.05b |
| Serum IL-10, pg/ml (n=4–6/gp) | 0.00±0.00 | 112.30±45.41a | 1.48±1.47b |
| Serum IL-18, pg/ml (n=4–6/gp) | 20.76±6.16 | 20.16±5.22 | 12.82±7.08 |
p<0.05 and
p<0.01, compared to matched FRC group
p<0.05 and
p<0.01, compared to untreated HRHF rats
ACKNOWLEDGEMENTS:
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR056019, and by American Society of Bone and Mineral Research Gap Award 1025. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The FG-3019 monoclonal antibody was provided by FibroGen, Inc.
Footnotes
Financial conflicts of interest, disclaimers: The authors have no financial conflicts of interest or disclaimers to declare.
REFERENCES
- 1.Gold JE, Hallman DM, Hellstrom F, et al. 2016. Systematic review of biochemical biomarkers for neck and upper-extremity musculoskeletal disorders. Scand J Work Environ Health 42:103–124. [DOI] [PubMed] [Google Scholar]
- 2.Gallagher S, Heberger JR. 2013. Examining the interaction of force and repetition on musculoskeletal disorder risk: a systematic literature review. Hum Factors 55:108–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Beek AJ, Dennerlein JT, Huysmans MA, et al. 2017. A research framework for the development and implementation of interventions preventing work-related musculoskeletal disorders. Scand J Work Environ Health 43:526–539. [DOI] [PubMed] [Google Scholar]
- 4.Kaasboll OJ, Gadicherla AK, Wang JH, et al. 2018. Connective tissue growth factor (CCN2) is a matricellular preproprotein controlled by proteolytic activation. J Biol Chem 293:17953–17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riser BL, Najmabadi F, Perbal B, et al. 2009. CCN3 (NOV) is a negative regulator of CCN2 (CTGF) and a novel endogenous inhibitor of the fibrotic pathway in an in vitro model of renal disease. The American journal of pathology 174:1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leask A 2015. Yin and Yang revisited: CCN3 as an anti-fibrotic therapeutic? J Cell Commun Signal 9:97–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schild C, Trueb B. 2004. Three members of the connective tissue growth factor family CCN are differentially regulated by mechanical stress. Biochim Biophys Acta 1691:33–40. [DOI] [PubMed] [Google Scholar]
- 8.Fisher PW, Zhao Y, Rico MC, et al. 2015. Increased CCN2, substance P and tissue fibrosis are associated with sensorimotor declines in a rat model of repetitive overuse injury. J Cell Commun Signal:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zugel M, Maganaris CN, Wilke J, et al. 2018. Fascial tissue research in sports medicine: from molecules to tissue adaptation, injury and diagnostics. Br J Sports Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George J, Tsutsumi M. 2007. siRNA-mediated knockdown of connective tissue growth factor prevents N-nitrosodimethylamine-induced hepatic fibrosis in rats. Gene Ther 14:790–803. [DOI] [PubMed] [Google Scholar]
- 11.Sisco M, Kryger ZB, O’Shaughnessy KD, et al. 2008. Antisense inhibition of connective tissue growth factor (CTGF/CCN2) mRNA limits hypertrophic scarring without affecting wound healing in vivo. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 16:661–673. [DOI] [PubMed] [Google Scholar]
- 12.Morales MG, Gutierrez J, Cabello-Verrugio C, et al. 2013. Reducing CTGF/CCN2 slows down mdx muscle dystrophy and improves cell therapy. Hum Mol Genet. [DOI] [PubMed] [Google Scholar]
- 13.Morales MG, Cabello-Verrugio C, Santander C, et al. 2011. CTGF/CCN-2 over-expression can directly induce features of skeletal muscle dystrophy. The Journal of pathology 225:490–501. [DOI] [PubMed] [Google Scholar]
- 14.Raghu G, Scholand MB, de Andrade J, et al. 2016. FG-3019 anti-connective tissue growth factor monoclonal antibody: results of an open-label clinical trial in idiopathic pulmonary fibrosis. Eur Respir J 47:1481–1491. [DOI] [PubMed] [Google Scholar]
- 15.Picozzi VJ, Pipas JM, Koong AC, et al. 2017. FG-3019, A Human Monoclonal Antibody to Connective Tissue Growth Factor, Combined with Chemotherapy in Patients with Locally Advanced or Metastatic Pancreatic Ductal Adenocarcinoma. Journal of Cancer Clinical Trials 2. [Google Scholar]
- 16.Barbe MF, Gallagher S, Massicotte VS, et al. 2013. The interaction of force and repetition on musculoskeletal and neural tissue responses and sensorimotor behavior in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet Disord 14:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdelmagid SM, Barr AE, Rico M, et al. 2012. Performance of repetitive tasks induces decreased grip strength and increased fibrogenic proteins in skeletal muscle: role of force and inflammation. PLoS One 7:e38359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rani S, Barbe MF, Barr AE, et al. 2010. Role of TNF alpha and PLF in bone remodeling in a rat model of repetitive reaching and grasping. J Cell Physiol 225:152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rani S, Barbe MF, Barr AE, et al. 2009. Induction of periostin-like factor and periostin in forearm muscle, tendon, and nerve in an animal model of work-related musculoskeletal disorder. J Histochem Cytochem 57:1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bove GM, Delany SP, Hobson L, et al. 2019. Manual therapy prevents onset of nociceptor activity, sensorimotor dysfunction, and neural fibrosis induced by a volitional repetitive task. Pain 160:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contreras O, Rebolledo DL, Oyarzun JE, et al. 2016. Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: relevance in fibroblast-myofibroblast differentiation and skeletal muscle fibrosis. Cell Tissue Res 364:647–660. [DOI] [PubMed] [Google Scholar]
- 22.Fedorczyk JM, Barr AE, Rani S, et al. 2010. Exposure-dependent increases in IL-1beta, substance P, CTGF, and tendinosis in flexor digitorum tendons with upper extremity repetitive strain injury. J Orthop Res 28:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark BD, Al Shatti TA, Barr AE, et al. 2004. Performance of a high-repetition, high-force task induces carpal tunnel syndrome in rats. J OrthopSports PhysTher 34:244–253. [DOI] [PubMed] [Google Scholar]
- 24.Brenner MC, Krzyzanski W, Chou JZ, et al. 2016. FG-3019, a Human Monoclonal Antibody Recognizing Connective Tissue Growth Factor, is Subject to Target-Mediated Drug Disposition. Pharm Res 33:1833–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi-wen X, Pennington D, Holmes A, et al. 2000. Autocrine overexpression of CTGF maintains fibrosis: RDA analysis of fibrosis genes in systemic sclerosis. Experimental cell research 259:213–224. [DOI] [PubMed] [Google Scholar]
- 26.Shi Q, MacDermid JC. 2011. Is surgical intervention more effective than non-surgical treatment for carpal tunnel syndrome? A systematic review. J Orthop Surg Res 6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anthony MJ, Martin EG, Avery AM, et al. 2010. Self care and health-seeking behavior of migrant farmworkers. J Immigr Minor Health 12:634–639. [DOI] [PubMed] [Google Scholar]
- 28.Krause N, Scherzer T, Rugulies R. 2005. Physical workload, work intensification, and prevalence of pain in low wage workers: results from a participatory research project with hotel room cleaners in Las Vegas. Am J Ind Med 48:326–337. [DOI] [PubMed] [Google Scholar]
- 29.Kietrys DM, Barr AE, Barbe MF. 2011. Exposure to repetitive tasks induces motor changes related to skill acquisition and inflammation in rats. J Mot Behav 43:465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connizzo BK, Yannascoli SM, Tucker JJ, et al. 2013. The Detrimental Effects of Systemic Ibuprofen Delivery on Tendon Healing Are Time-Dependent. Clinical orthopaedics and related research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavan PG, Stecco A, Stern R, et al. 2014. Painful connections: densification versus fibrosis of fascia. Curr Pain Headache Rep 18:441. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Usinger W, Nichols B, et al. 2011. Cooperative interaction of CTGF and TGF-beta in animal models of fibrotic disease. Fibrogenesis & tissue repair 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makino K, Makino T, Stawski L, et al. 2017. Anti-connective tissue growth factor (CTGF/CCN2) monoclonal antibody attenuates skin fibrosis in mice models of systemic sclerosis. Arthritis Res Ther 19:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xin DL, Hadrevi J, Elliott ME, et al. 2017. Effectiveness of conservative interventions for sickness and pain behaviors induced by a high repetition high force upper extremity task. BMC Neurosci 18:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stauber WT, Knack KK, Miller GR, et al. 1996. Fibrosis and intercellular collagen connections from four weeks of muscle strains. Muscle Nerve 19:423–430. [DOI] [PubMed] [Google Scholar]
- 36.Driscoll M, Blyum L. 2011. The presence of physiological stress shielding in the degenerative cycle of musculoskeletal disorders. Journal of bodywork and movement therapies 15:335–342. [DOI] [PubMed] [Google Scholar]
- 37.Sternlicht MD, Wirkner U, Bickelhaupt S, et al. 2018. Radiation-induced pulmonary gene expression changes are attenuated by the CTGF antibody Pamrevlumab. Respir Res 19:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bickelhaupt S, Erbel C, Timke C, et al. 2017. Effects of CTGF Blockade on Attenuation and Reversal of Radiation-Induced Pulmonary Fibrosis. J Natl Cancer Inst 109. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez D, Rebolledo DL, Correa LM, et al. 2018. The inhibition of CTGF/CCN2 activity improves muscle and locomotor function in a murine ALS model. Hum Mol Genet 27:2913–2926. [DOI] [PubMed] [Google Scholar]
- 40.Lipson KE, Wong C, Teng Y, et al. 2012. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis & tissue repair 5 Suppl 1:S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pendurthi UR, Tran TT, Post M, et al. 2005. Proteolysis of CCN1 by plasmin: functional implications. Cancer Res 65:9705–9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi J, Lin A, Shrier E, et al. 2013. Degradome products of the matricellular protein CCN1 as modulators of pathological angiogenesis in the retina. J Biol Chem 288:23075–23089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kivela R, Kyrolainen H, Selanne H, et al. 2007. A single bout of exercise with high mechanical loading induces the expression of Cyr61/CCN1 and CTGF/CCN2 in human skeletal muscle. J Appl Physiol (1985) 103:1395–1401. [DOI] [PubMed] [Google Scholar]
- 44.Grote K, Bavendiek U, Grothusen C, et al. 2004. Stretch-inducible expression of the angiogenic factor CCN1 in vascular smooth muscle cells is mediated by Egr-1. J Biol Chem 279:55675–55681. [DOI] [PubMed] [Google Scholar]
- 45.Kyurkchiev S, Yeger H, Bleau AM, et al. 2004. Potential cellular conformations of the CCN3(NOV) protein. Cell Commun Signal 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perbal B 2001. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol 54:57–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnott JA, Lambi AG, Mundy C, et al. 2011. The role of connective tissue growth factor (CTGF/CCN2) in skeletogenesis. Crit Rev Eukaryot Gene Expr 21:43–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang H, Huang Y, Chen X, et al. 2010. The role of CTGF in the diabetic rat retina and its relationship with VEGF and TGF-beta(2) , elucidated by treatment with CTGFsiRNA. Acta Ophthalmol 88:652–659. [DOI] [PubMed] [Google Scholar]
- 49.Ettema AM, Amadio PC, Zhao C, et al. 2004. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am 86-A:1458–1466. [DOI] [PubMed] [Google Scholar]
- 50.Ohara Y, Chew SH, Misawa N, et al. 2018. Connective tissue growth factor-specific monoclonal antibody inhibits growth of malignant mesothelioma in an orthotopic mouse model. Oncotarget 9:18494–18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


