The adaptive physiological response to acute stress requires the internal milieu of an organism to vary and meet perceived and anticipated demands in the context of a life-threatening situation (i.e. the Fight or Flight Theory). This survival-essential adaptive process is referred as allostasis, in which active homeostasis is rapidly re-established as the acute stressor fades. Incomplete homeostatic rebalancing, especially following repeated stress, leads to long-lasting, maladaptive responses as either psychological and/or physiological dysfunctions. Interestingly, some individuals are able to stay phenotypically stable despite exposure to the same severe, prolonged stress. This phenomenon is termed “resilient” to stress. In resilient individuals, additional neural adaptive mechanisms are recruited to re-establish internal homeostasis, allowing them to stay behaviorally stable and cope with future stressors. At present, much less is known about these recruited “resilient” mechanisms in the brain, in contrast to stress-induced pathology in the stress “susceptible” counterpart.
The Locus coeruleus (LC), the main source of norepinephrine in the brain, is comprised of a cluster of norepinephrine (NE) neurons that are known to be involved in stress and stress-resilience. Many early animal studies have indicated that the LC responds to acute stress, and plays an important role in mediating adaptive homeostatic regulation by antagonizing corticortropin-releasing factor1. In human studies, altered LC-NE activity is observed in some patients with psychiatric disorders, such as major depression and post-traumatic stress disorder. Pharmacological blockade of beta-adrenergic receptors in the amygdala prevents the development of aversive memories2. These studies indicate that the LC and its related neural circuits may play an important role in mediating resilience to stress, while an alteration in the responsiveness of the LC to stress may promote resilience to stress. However, more evidence-based research needs to be performed to further explore the defined mechanism.
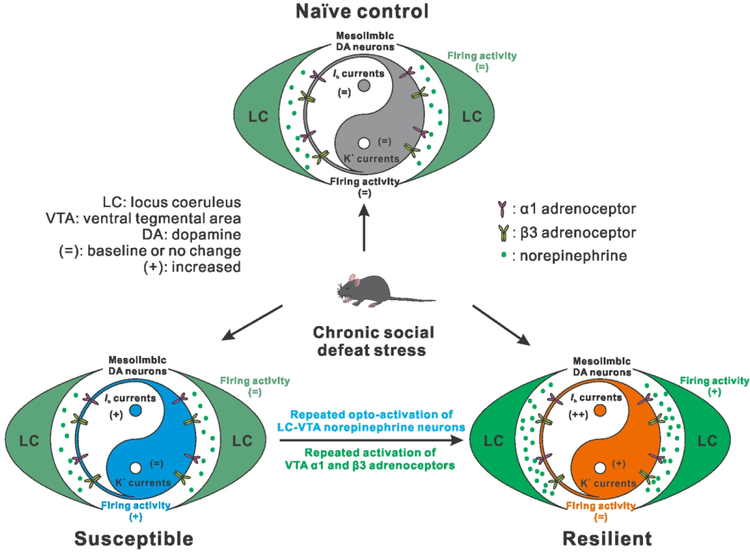
We recently demonstrated that ventral tegmental area (VTA) dopaminergic neurons projecting to the nucleus accumbens (NAc) constitute a neural circuit, in which a resilience-specific homeostasis is established by an intrinsic balance of excitatory /h (hyperpolarization-activated cation channel current) and inhibitory voltage-gated potassium (K+) channel currents to maintain control-like neuronal activity and stable behaviors3. More recently, studies from Bruno Giros4 and our group5 have identified increased activity in the LC-NE neurons projecting to the VTA, in resilient mice, following a repeated social stress model for depression. Further, experimentally activating these neurons induced resilience-like behaviors. More importantly, in our circuit-specific molecular profiling study, we identified the α1 and β3 adrenergic receptors as the synaptic relay between the LC-NE system and the VTA-NAc neural circuit, which provide potential translational molecular targets for the development of resilience-promoting antidepressants5.
In this work, our further pharmacological study proceeded by experimentally activating these receptors, infusing a cocktail of their agonists in the VTA. We then observed a re-establishment of the intrinsic homeostasis within VTA-NAc DA neurons and resilience-like behaviorial phenotypes in previously defined susceptible mice5. For translational purposes, further studies are needed to examine the role of each receptor independently. Moreover, the LC has a widespread, highly collateralized projection system that innervates the entire neuraxis, including stress/depression-related brain regions such as the medial prefrontal cortex (mPFC) and the amygdala. In our in vitro electrophysiological recordings, we observed a promising increased firing activity in LC-NE neurons that project to the mPFC in resilient mice5. Thus, the LC-NE neurons projecting to other brain targets, including the mPFC, might also hold a potential role in mediating resilience to stress.
Figure 1.
Noradrenergic hyperactivity establishes homeostasis in mesolimbic dopamine neurons to maintain or promote resilience to social stress. Following repeated social defeat stress, mice are segregated into susceptible (depressed) or resilient (non-depressed) subpopulations. Within the resilient group, homeostasis is established by an intrinsic balance of excitatory /h and inhibitory K+ currents to maintain control-like firing activity in VTA DA neurons that project to the NAc. The current study further expands on this finding by demonstrating that the resilient group exhibits an increase in firing activity of LC neurons that project to the VTA. Repeated optogenetic stimulation induced hyperactivity of the LC-VTA circuit and was sufficient to promote the resilient phenotype in previously defined susceptible mice by re-establishing the aforementioned homeostatic balance in mesolimbic DA neurons. Reversing susceptibility to promote resilience was mediated by VTA α1 and β3 adrenergic receptors.
References
- 1.Valentino RJ, Foote SL, Page ME. The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann N Y Acad Sei. 1993;697:173–88. [DOI] [PubMed] [Google Scholar]
- 2.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004; 161 (2): 195–216. [DOI] [PubMed] [Google Scholar]
- 3.Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Li X, Dietz DM, Pan N, Vialou VF, Neve RL, Yue Z, Han MH. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344(6181):313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isingrini E, Perret L, Rainer Q, Amilhon B, Guma E, Tanti A, Martin G, Robinson J, Moquin L, Marti F, Mechawar N, Williams S, Gratton A, Giros B. Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat Neurosci. 2016;19(4):560–3. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Chaudhury D, Nectow AR, Friedman AK, Zhang S, Juarez B, Liu H, Pfau ML, Aleyasin H, Jiang C, Crumiller M, Calipari ES, Ku SM, Morel C, Tzavaras N, Montgomery SE, He M, Salton SR, Russo SJ, Nestler EJ, Friedman JM, Cao JL et al. alpha1- and beta3-Adrenergic Receptor-Mediated Mesolimbic Homeostatic Plasticity Confers Resilience to Social Stress in Susceptible Mice. Biol Psychiatry. 2019;85(3):226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]