Abstract
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infection and hospitalization in infants. In spite of the enormous clinical burden caused by RSV infections, there remains no efficacious RSV vaccine. CD8 T cells mediate viral clearance as well as provide protection against a secondary RSV infection. However, RSV-specific CD8 T cells may also induce immunopathology leading to exacerbated morbidity and mortality. Many of the crucial functions performed by CD8 T cells are mediated by the cytokines they produce. IFN-γ and TNF are produced by CD8 T cells following RSV infection and contribute to both the acceleration of viral clearance and the induction of immunopathology. To prevent immunopathology, regulatory mechanisms are in place within the immune system to inhibit CD8 T cell effector functions after the infection has been cleared. The actions of a variety of cytokines, including IL-10 and IL-4, play a critical role in the regulation of CD8 T cell effector activity. Herein, we review the current literature on CD8 T cell responses and the functions of the cytokines they produce following RSV infection. Additionally, we discuss the regulation of CD8 T cell activation and effector functions through the actions of various cytokines.
Keywords: Respiratory syncytial virus, CD8 T cell, cytokines, interferon-γ, tumor necrosis factor, interleukin-10
1. Introduction
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infection in infants and young children [1]. RSV is responsible for a substantial healthcare burden, causing an estimated three-four million hospitalizations and nearly 200,000 deaths annually [1]. In addition to frequently infecting children, RSV is a common cause of severe respiratory disease in immunocompromised and elderly populations [2–5]. RSV is ubiquitous with nearly all children becoming infected by two years of age [6]. However, effective long-term immunity is not conferred by infection with RSV, resulting in frequent reinfections throughout life [6–8]. Despite the immense economic burden caused by RSV-associated disease, there remains no licensed RSV vaccine.
CD8 T cells are induced following RSV infection in both mice and humans and make several important contributions to the host immune response. It is well-established that CD8 T cells are critical for mediating viral clearance following RSV infection [9, 10]. RSV-specific memory CD8 T cells are also capable of providing protection against a secondary RSV infection by reducing lung viral titers through the killing of virus-infected cells [11, 12]. However, CD8 T cells may also be detrimental during RSV infection, as they can induce immunopathology resulting in exacerbated disease [9, 12]. CD8 T cells both produce and are regulated by a variety of cytokines following RSV infection. Interferon-γ (IFN-γ) and tumor necrosis factor (TNF) secreted by CD8 T cells contribute to viral clearance but can also mediate immunopathology following RSV infection [12–15]. In contrast, CD8 T cell activation and effector functions are inhibited by the actions of a variety of cytokines, including interleukin-10 (IL-10) and IL-4 [16–19]. Here we review the current literature on CD8 T cell responses following RSV infection and discuss the role of cytokines produced by CD8 T cells in both protecting against and exacerbating RSV-induced disease. A special emphasis is also placed on the regulation of CD8 T cell responses following RSV infection through the actions of various cytokines.
2. The CD8 T cell response to RSV infection
2.1. CD8 T cell activation
Following RSV infection, dendritic cells (DCs) play a critical role in the activation of naive CD8 T cells. DCs express MHC class I molecules on their cell surface that bind viral antigen for presentation to CD8 T cells. Three main subsets of DCs are recruited to the lung and airways following RSV infection: CD11b+ DCs, CD103+ DCs, and plasmacytoid DCs [20–22]. CD11b+ and CD103+ DCs may be the primary DC subsets responsible for CD8 T cell activation, as both subsets express viral RNA following infection in vivo and induce strong proliferation of CD8 T cells in vitro [21–23]. In contrast, while plasmacytoid DCs are capable of being directly infected in vitro, they are weak inducers of T cell proliferation [24]. In addition to antigen presentation, DCs provide a costimulatory signal to naive CD8 T cells. The interaction between CD80 and CD86 on the surface of DCs and CD28 expressed on T cells is critical for CD8 T cell activation and survival. Both murine and human DCs upregulate CD80 and CD86 costimulatory molecules following RSV infection [23, 25–27]. Additionally, inhibiting costimulation of CD8 T cells through the administration of CD80- and CD86-blocking antibodies results in reduced numbers of RSV-specific CD8 T cells [22, 28]. These results support the critical role of DC-provided costimulatory signals for CD8 T cell activation following RSV infection. Lastly, DCs provide signal 3 cytokines, such as IL-12 and type I IFNs, to CD8 T cells during activation to promote their proliferation, survival, and memory generation [29, 30]. Both murine and human DCs are capable of producing IL-12 and type I IFNs following ex vivo stimulation with RSV, providing evidence that DCs supply signal 3 cytokines to RSV-specific CD8 T cells [20, 26, 31, 32]. Overall, RSV infection promotes pulmonary DCs to provide antigen, costimulation, and signal 3 cytokines to CD8 T cells to induce a robust RSV-specific CD8 T cell response.
2.2. Kinetics and phenotype of the CD8 T cell response
Following activation in mouse models, RSV-specific CD8 T cells expand in both frequency and total number in the lungs and airways [33–35]. Additionally, antigen-specific CD8 T cells can be detected in the lung-draining lymph node, spleen, and peripheral blood [33, 34]. RSV-specific CD8 T cells exhibit an activated phenotype through the upregulation of activation-associated markers, such as CD11a, CD25, CD44, and NKG2a, and the downregulation of the lymphoid homing receptor CD62L (Figure 1a) [36, 37]. CD8 T cells also develop the ability to produce pro-inflammatory cytokines and effector molecules, including the cytokines IFN-γ and TNF [35, 37]. RSV-specific CD8 T cell expansion in the lungs reaches its peak at approximately 8–10 days following RSV infection in mice (Figure 1a) [34, 35, 37, 38]. Due to the lack of defined CD8 T cell epitopes and the difficulty in obtaining serial patient samples following initial RSV exposure, reports examining the detailed kinetics of CD8 T cell responses following a primary infection in humans are limited. The best evidence that is likely most reflective of a primary CD8 T cell response after an initial RSV infection comes from studies evaluating infants. RSV-specific CD8 T cells in the peripheral blood of infants following RSV infection undergo CD8 T cell expansion that peaks at approximately 12 days following symptom onset before declining over time [39, 40]. Similar kinetics are observed in tracheal aspirates of RSV-infected infants, with CD8 T cell frequencies peaking 10–15 days after the onset of respiratory symptoms [41]. As in mice, CD8 T cells from infants exhibit an activated phenotype following an acute RSV infection by upregulating expression of the activation marker HLA-DR and the proliferation marker Ki-67 [39–41]. CD8 T cells from the airways and blood of RSV-infected infants also produce effector cytokines and molecules, including IFN-γ, granzyme B, and perforin [39–41]. Overall, CD8 T cells expand in total number and can exert effector activity following an acute RSV infection in both mice and humans.
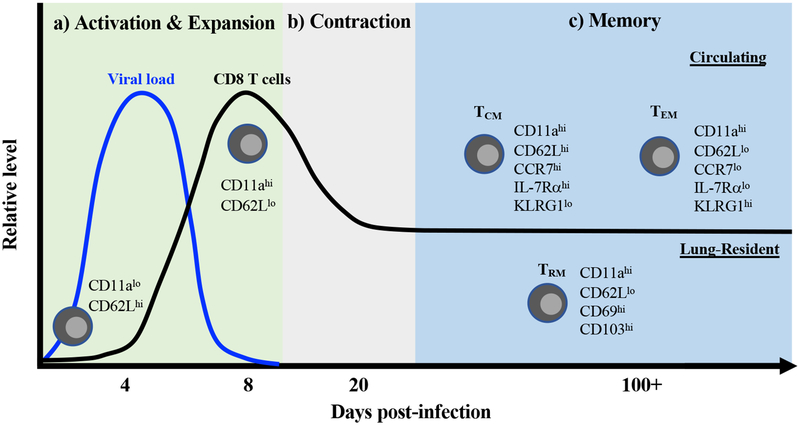
Figure 1. The CD8 T cell response following acute RSV infection.
(A) Following antigen presentation by DCs, naive CD8 T cells become activated, as measured by the upregulation of activation markers, such as CD11a, and the downregulation of the lymphoid homing receptor CD62L. Activated RSV-specific CD8 T cells expand both in frequency and total number in the lung, peaking at approximately 8–10 days following RSV infection. The peak of pulmonary CD8 T cell expansion coincides with complete viral clearance from the lung. (B) After peak expansion, contraction occurs to reduce the total number of RSV-specific CD8 T cells and form a stable memory population. (C) Based on their phenotype and circulatory properties, three primary memory CD8 T cell populations are formed following infection: TCM, TEM, and TRM. TCM (CD62LhiCCR7hiIL-7RαhiKLRG1lo) primarily circulate between the blood and secondary lymphoid organs, while TEM (CD62LloCCR7loIL-7RαloKLRG1hi) predominate within the lung but are also capable of circulating within the blood. In contrast, TRM (CD62LloCD69hiCD103hi) represent a non-circulating, lung-resident population of memory CD8 T cells.
2.3. Viral clearance mediated by CD8 T cells
Several studies in mice have firmly established CD8 T cells as being critical for mediating viral clearance following an acute RSV infection. The peak of the RSV-specific CD8 T cell response correlates with complete viral clearance from the lung between 8–10 days following a primary RSV infection (Figure 1a) [15, 18, 34, 35, 42, 43]. Infection of athymic nude mice, which lack T cells, results in a persistent infection in which the virus is detectable for at least 20 days post-infection [43, 44]. However, RSV-infected nude mice efficiently eliminate the virus following the adoptive transfer of RSV-primed splenic CD8 T cells [44]. Similarly, wild-type (WT) mice that receive RSV-primed, in vitro-stimulated splenic CD8 T cells exhibit accelerated viral clearance compared to mice that did not receive a CD8 T cell transfer [14, 45–47]. Recently, two different T cell receptor (TCR) transgenic mouse strains specific to the Kd-restricted RSV-derived M282–90 CD8 T cell epitope were developed [48]. The use of TCR transgenic CD8 T cells is beneficial because they allow for the transfer of RSV-specific CD8 T cells without the need for prior in vitro stimulation. Similar to mice given in vitro-stimulated splenic CD8 T cells, mice receiving TCR transgenic CD8 T cells prior to RSV infection exhibit significantly reduced lung viral titers compared to no transfer controls [10]. Additionally, antibody-mediated depletion of CD8 T cells prior to RSV infection results in significantly delayed viral clearance compared to controls [9]. Together, these studies clearly demonstrate the importance of CD8 T cells in mediating viral clearance following a primary RSV infection in mice.
Although limited studies have been performed to date, CD8 T cells have also been identified as being important for viral control following RSV infection in humans. Similar to studies in immunodeficient mice, immunocompromised children with T cell defects exhibit prolonged viral shedding compared to immunologically normal children following RSV infection [4, 49, 50]. Mean peak viral titers are also significantly elevated in immunodeficient children [4]. Perhaps the greatest example of a CD8 T cell contribution to viral clearance in humans occurred during the evaluation of viral loads in an RSV-infected infant with severe combined immunodeficiency [49]. Following bone marrow transplantation, nasal wash RSV titers were dramatically reduced, which strongly correlated with the engraftment and induction of a CD8 T cell response. Together, these studies support a critical role for CD8 T cells in mediating viral clearance following acute RSV infection in humans.
2.4. Memory CD8 T cell populations following RSV infection
Following expansion, the majority of responding CD8 T cells undergo apoptosis resulting in a decrease in the total number of antigen-specific CD8 T cells to form a stable memory population (Figure 1b). A portion of the memory CD8 T cell population are central memory cells (TCM) that express CD62L, CCR7, and the IL-7 receptor α-chain and primarily circulate between the blood and secondary lymphoid organs (Figure 1c) [51, 52]. In contrast, the vast majority of virus-specific CD8 T cells are located within the lung parenchyma during the memory phase, rather than circulating through the pulmonary vasculature [38]. The populations of memory CD8 T cells within the lung parenchyma are primarily effector memory cells (TEM) and tissue-resident memory cells (TRM). TEM cells are characterized by their expression of KLRG1 and low expression of CD62L and CCR7 (Figure 1c) [52, 53]. Unlike TCM, TEM cells do not circulate through secondary lymphoid organs but can circulate through the blood. TRM are a recently identified population of non-circulating memory CD8 T cells in peripheral organs, including the lung that can be distinguished based on their expression of the canonical tissue-residency markers CD69 and CD103 [54, 55]. As described in other infection models, RSV infection induces the generation of antigen-specific TRM expressing CD69 and CD103 within the lung parenchyma (Figure 1c) [38, 56]. Additionally, RSV-specific TRM within the lung can also be generated by local immunization with RSV-derived antigens [12, 57, 58]. Overall, RSV infection induces robust activation of pulmonary CD8 T cells that form a memory population within the lung tissue following contraction in mice.
While contraction differs slightly from what is observed in murine models, stable memory CD8 T cell populations are also generated in the lung and airways following RSV infection in humans. Contraction of CD8 T cell responses in the peripheral blood of both infants and adults is observed leading to a reduction in the total frequencies of RSV-specific memory CD8 T cells [39, 59]. In contrast, RSV-specific memory CD8 T cell frequencies in the airways of infected infants remain elevated during convalescence and persist for several months after infection [39]. Similar results are observed in the airways of adults following an experimental RSV infection [59]. Like in the airways, an increased frequency of RSV-specific memory CD8 T cells was also observed within lung tissue sections compared to the peripheral blood of an adult patient [60]. Overall, these results suggest that virus-specific memory CD8 T cells are enriched within the lung and airways following RSV infection in humans. Like in mice, human CD8 T cells develop an activated phenotype. RSV-specific peripheral blood memory CD8 T cells from both infants and adults express an effector memory phenotype (CD45RA−CCR7−) and upregulate markers associated with activation and proliferation, including CD27, CD28, CD38, and Ki-67 [39, 59, 61, 62]. A similar phenotype is also observed on RSV-specific memory CD8 T cells in the airways of both infants and adults [39, 59]. As in mice, RSV-specific TRM expressing CD69 and CD103 in the airways following RSV infection have also been described in humans [59]. Together, these studies demonstrate that memory CD8 T cell responses are also induced following RSV infection in humans.
2.5. Protection against secondary infection
In addition to mediating viral clearance following an acute RSV infection, memory CD8 T cells have also been shown to provide protection against secondary RSV exposure. Many studies have evaluated the capacity of memory CD8 T cells to provide protection against RSV infection by immunizing against whole RSV proteins to generate pre-existing RSV-specific CD8 T cells. Viral vector-based approaches engineered to express whole RSV proteins have included recombinant baculoviruses expressing the RSV M2 protein, recombinant murine cytomegaloviruses expressing the RSV M protein, as well as virus-like particles containing both the RSV M and M2 proteins [57, 58, 63]. All of these immunization strategies generated RSV-specific CD8 T cell responses that significantly reduced viral titers in the lung following RSV challenge. However, given that RSV CD4 T cell epitopes are also present within the RSV M and M2 proteins, the protection afforded by these immunization strategies cannot be solely attributed to the RSV-specific memory CD8 T cell response [64, 65]. Alternative approaches have been utilized to specifically address the role of memory CD8 T cells in providing protection in the absence of RSV-specific CD4 T cells and antibodies. One such approach involved challenging RSV-infected mice with a recombinant Listeria monocytogenes (LM) expressing an RSV-derived CD8 T cell epitope such that only RSV-specific CD8 T cells were capable of responding in an antigen-specific manner [33]. Mice previously infected with RSV exhibit significantly enhanced control of LM in the spleen compared to naive mice, suggesting a protective role of memory CD8 T cells. Alternatively, the transfer of sorted airway memory CD8 T cells significantly reduces lung RSV RNA levels following RSV challenge compared to mice receiving either no cells or sorted CD4 T cells [56]. Other studies have used peptide epitope vaccinations to generate pre-existing, systemic RSV-specific memory CD8 T cells that significantly accelerated viral clearance following RSV challenge [11, 12]. Recent studies have demonstrated that TRM play a critical role in providing protection against secondary influenza virus infections [66–68]. Importantly, several reports have also supported a role for RSV-specific TRM in providing protection against RSV infection [12, 56–58]. Together, these studies establish that memory CD8 T cells provide protection against secondary RSV infection.
While well-studied in mouse models, the role of memory CD8 T cells in providing protection against secondary infection in humans remains unclear. The number of pre-existing RSV-specific CD8 T cells in the airways of adults correlates with a reduction in both cumulative nasal viral load and peak viral load in bronchial brushings following an experimental RSV infection [59]. Similarly, the number of pre-existing RSV-specific memory CD8 T cells also correlates with a reduction in symptom illness score. These results support a role for memory CD8 T cell-mediated protection in humans. In contrast, evidence against a role for CD8 T cells in providing protection against secondary infection has also been reported. CD8 T cells in the blood of children are not boosted following a natural reinfection with RSV, however, it is possible that they are boosted within the lung and airways [69]. There is also no difference in the number of pre-existing RSV-specific memory CD8 T cells between experimentally RSV-challenged adults that proceed to have a productive RSV infection and those who do not [59]. These results suggest that while memory CD8 T cells may not prevent an infection from occurring in humans, they are critical for reducing viral load and subsequent disease symptoms in RSV-infected individuals. Overall, much remains to be understood regarding the contribution of memory CD8 T cells in providing protection against RSV in humans and requires further investigation.
2.6. CD8 T cell-mediated immunopathology
Despite their importance in mediating viral clearance, CD8 T cells have also been shown to be detrimental following an RSV infection by inducing immunopathology. Weight loss and symptom illness scores are significantly ameliorated in mice that are depleted of CD8 T cells prior to an acute RSV infection compared to controls [9]. Similarly, mice that receive either RSV-primed, in vitro-stimulated CD8 T cells or TCR transgenic CD8 T cells exhibit exacerbated weight loss or mortality following RSV infection, despite a reduction in lung viral titers [10, 14, 45–47]. In addition to inducing immunopathology following a primary RSV infection, we recently showed that memory CD8 T cells can also mediate severe immunopathology following a secondary RSV challenge [12]. In this study, mice received a DC-prime, LM-boost (DC-LM) immunization against an RSV-derived CD8 T cell epitope to generate a large frequency of pre-existing RSV-specific memory CD8 T cells in the absence of RSV-specific CD4 T cells and antibodies. Following RSV challenge, DC-LM-immunized mice exhibited significantly enhanced weight loss, pulmonary dysfunction, and mortality compared to controls. These results were unexpected given that similar immunization approaches to generate high magnitude memory CD8 T cell responses to other respiratory viruses, including influenza virus and SARS coronavirus, did not result in immunopathology following viral challenge [70, 71]. Interestingly, the induction of immunopathology mediated by both primary and memory CD8 T cells is largely driven by cytokine production, particularly IFN-γ and TNF, as will be discussed in detail in sections 3.2 and 3.3 below [12, 14]. The localization of the memory CD8 T cell response is also important in the induction of CD8 T cell-mediated immunopathology. Local immunization generates memory CD8 T cells that are primarily located within the lung parenchyma, while peripheral immunization induces systemic memory CD8 T cells [12]. Importantly, mice immunized locally exhibit significantly ameliorated morbidity and mortality compared to systemically immunized mice. Therefore, memory CD8 T cells within the lung induce significantly reduced immunopathology following RSV challenge compared to peripheral memory CD8 T cells. Together, these studies demonstrate a clear role for CD8 T cells in the induction of immunopathology following RSV infection in mice.
While their role in murine studies has been clearly demonstrated, the extent to which CD8 T cells induce immunopathology following RSV infection in humans remains unclear. CD8 T cell numbers following bone-marrow transplantation in an RSV-infected severe-combined immunodeficiency patient correlated directly with elevated respiratory rates, indicating a worsening of pulmonary function, despite a reduction in viral load [49]. Additionally, children requiring mechanical ventilation due to RSV lower respiratory tract infection exhibit significantly elevated granzymes, which were primarily produced by CD8 T cells, in tracheal aspirates compared to healthy controls [72]. Finally, despite a correlation with declining viral load, peak CD8 T cell activation following experimental RSV infection of adults also correlates with elevated symptom illness scores [59]. In contrast to these results, other studies do not support a role for CD8 T cell-mediated immunopathology following RSV infection in humans. Immunohistochemical staining of pulmonary sections from fatal RSV cases identified a near absence of CD8 T cells, suggesting that CD8 T cells did not contribute to the fatal outcomes [73, 74]. Additionally, gene expression analysis of children with severe RSV infection revealed that CD8 T cell-associated genes are significantly underrepresented compared to children with mild or moderate RSV disease [75]. While these studies do not implicate CD8 T cells, the overall role of CD8 T cells in the induction of immunopathology following RSV infection in humans is unclear and remains an important topic for future investigation.
3. CD8 T cell cytokine production
RSV induces the production of a variety of cytokines in the lungs and airways following infection of both mice and humans. Several pro-inflammatory cytokines are secreted in the lung and airways following RSV infection in mice, including IFN-γ, TNF, IL-4, IL-6, macrophage inflammatory protein-1α (MIP-1α), and MIP-1β [19, 76–78]. Inhibitory cytokines are also detected following RSV infection in mice, most commonly IL-10 [77, 79]. Importantly, these same cytokines are elevated in the nasopharyngeal secretions of infants recently infected with RSV [73, 80–82]. The induction of many pro-inflammatory cytokines has been directly associated with CD8 T cell responses. The adoptive transfer of TCR transgenic CD8 T cells elicits elevated levels of IFN-γ, TNF, IL-6, and MIP-1α protein in the lungs following RSV infection [10]. Additionally, the peak level of IFN-γ, TNF, and IL-6 proteins coincides with the induction of T cell responses following RSV infection [19, 77, 79]. Thus, RSV infection of both mice and humans induces the production of a variety of cytokines as a result of bothvirus-induced inflammation and the host anti-viral response.
Many of the cytokines secreted following RSV infection have been demonstrated to be produced by RSV-specific CD8 T cells (Figure 2a). Several studies have shown that CD8 T cells in the lung, airways, and spleen are capable of producing IFN-γ, TNF, and IL-2 following an acute RSV infection using ex vivo stimulation with either whole RSV proteins or RSV-derived peptides followed by intracellular cytokine staining [34, 35, 37, 42, 83]. Memory CD8 T cells following either primary or secondary RSV infection can also produce IFN-γ, TNF, and IL-2 after ex vivo peptide stimulation [11, 12, 34]. Similarly, CD8 T cells isolated from the peripheral blood of infants or adults exhibit production of IFN-γ, TNF, and IL-2 following stimulation [39, 59, 62]. Alternatively, an in vivo intracellular cytokine staining method has been adopted to allow for the direct detection of cytokines produced by CD8 T cells without the need for ex vivo stimulation [84]. This method has confirmed the production of IFN-γ, TNF, and IL-10 by both effector and memory RSV-specific CD8 T cells [12, 79, 85]. The critical roles played by the cytokines CD8 T cells produce following RSV infection will be discussed in further detail in sections 3.1–3.3
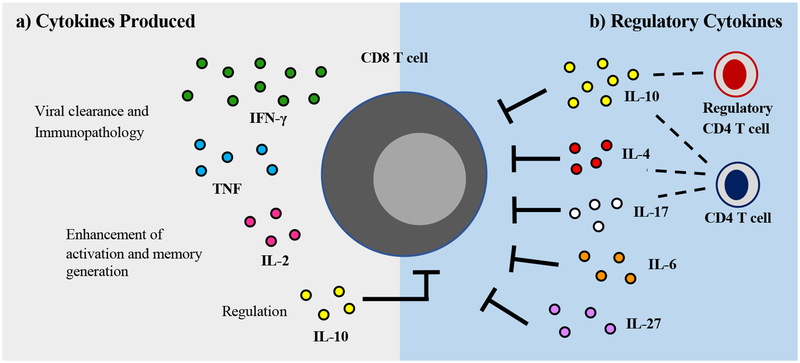
Figure 2. The role of cytokines in the CD8 T cell response following RSV infection.
(A) Activated CD8 T cells in the lung secrete an array of cytokines following RSV infection, including IFN-γ, TNF, and IL-2. IFN-γ and TNF have been established as contributors to accelerating viral clearance as well as inducing immunopathology following infection. IL-2 is critical for the enhancement of CD8 T cell activation, proliferation, and memory generation during RSV infection. Additionally, CD8 T cells are also capable of producing IL-10, which may autoregulate CD8 T cell effector functions. (B) A variety of cytokines contribute to the regulation of CD8 T cell expansion and effector functions to limit immunopathology following RSV infection. IL-10 produced by both regulatory and effector CD4 T cells is the most well-studied cytokine responsible for suppressing RSV-specific CD8 T cell responses. Additionally, IL-4, IL-17, IL-6, and IL-27 may play a role in the inhibition of CD8 T cells following RSV infection.
3.1. IL-2
IL-2 has been established as a key T cell growth factor and is critical for the generation of memory CD8 T cell populations [86–88]. Despite the low level of IL-2 production by mouse and human CD8 T cells following RSV infection, IL-2 has been shown to be critical for RSV-specific CD8 T cells both in vitro and in vivo [34, 42, 59, 89]. RSV-specific CD8 T cells isolated from the peripheral blood of adults exhibit significantly enhanced proliferation in vitro when cultured with peptide in addition to IL-2 compared to peptide alone [61]. This enhancement is reversed in the presence of IL-2 receptor-blocking antibodies, highlighting the importance of IL-2 signaling for RSV-specific CD8 T cell proliferation in vitro. IL-2 also plays an important role in vivo following RSV infection by supporting CD8 T cell cytokine production. CD8 T cells from mice which are supplemented with IL-2 in vivo during a primary RSV infection exhibit an increased frequency of IFN-γ production by CD8 T cells following peptide stimulation compared to controls [90]. Additionally, the frequency of RSV-specific CD8 T cells is substantially increased during the memory phase, supporting a role for IL-2 in the generation of memory CD8 T cell populations following RSV infection. Overall, IL-2 plays a critical role in the generation and maintenance of CD8 T cells in RSV infection, as has also been shown with other viral infections.
3.2. IFN-γ
IFN-γ is the primary cytokine produced by CD8 T cells following RSV infection and has been demonstrated to perform a variety of functions. One of the activities attributed to IFN-γ is the acceleration of viral clearance following RSV infection. Infection with a recombinant RSV strain expressing IFN-γ significantly reduces virus replication in both the lungs and nasal turbinates of mice [13]. Similarly, intranasal treatment of mice with recombinant IFN-γ protein accelerates viral clearance in the lungs [91]. Antibody-mediated depletion of IFN-γ prior to RSV infection results in elevated lung viral titers compared to IgG-treated controls [14, 78]. Similar results are observed following the transfer of IFN-γ knockout (KO) splenocytes compared to their WT counterparts [14]. However, regardless of the presence or absence of IFN-γ, all mice are able to completely clear the virus by 8 days post-infection [14, 91]. These results indicate that IFN-γ contributes to RSV clearance in the lung and airways but is not required. In contrast, other studies have found that IFN-γ is not involved in the elimination of virus from the lung following RSV infection. WT and IFN-γ KO mice exhibit similar kinetics of virus replication in the lungs following RSV infection [15]. Similarly, viral control of a secondary RSV challenge by RSV-specific memory CD8 T cells is not affected by antibody-mediated neutralization of IFN-γ [12]. Overall, these results suggest that IFN-γ may play a role in accelerating viral clearance following a primary RSV infection but is likely not the primary mechanism and may not impact clearance of subsequent RSV exposures.
In mouse models of RSV infection, it is well-established that IFN-γ is important for driving CD8 T cell-mediated immunopathology resulting in exacerbated disease. Elevated IFN-γ protein levels correlate with enhanced respiratory disease parameters, including respiratory rate and airway obstruction [78]. RSV infection of IFN-γ KO mice results in significantly reduced airway obstruction compared to WT mice. Additionally, neutralization of IFN-γ prior to the adoptive transfer of RSV-primed splenocytes results in significantly reduced weight loss following RSV infection [14]. Similarly, the adoptive transfer of IFN-γ KO CD8 T cells induces significantly ameliorated disease following RSV infection compared to the adoptive transfer of WT CD8 T cells. RSV challenge of DC-LM-immunized mice results in significantly elevated levels of IFN-γ protein in the lung and serum, which is primarily produced by CD8 T cells in the lung and airways [12]. Neutralization of IFN-γ in DC-LM-immunized mice results in significantly ameliorated weight loss and pulmonary dysfunction as well as complete protection from mortality following RSV challenge. Overall, these studies indicate that IFN-γ, particularly CD8 T cell-derived IFN-γ, drives immunopathology following RSV infection in mice.
In contrast to what is observed in mouse models, the majority of evidence points to a protective role for IFN-γ following RSV infection in humans. RSV infection induces the production of IFN-γ in the nasal passages and serum of infants, and the levels are elevated above what is observed in healthy infants [80, 92]. However, IFN-γ levels in the blood, serum, or nasal washes of infants exhibiting severe RSV disease are substantially reduced compared to infants with only mild RSV disease [80, 82, 93]. One example of this dichotomy is that infants requiring mechanical ventilation following RSV infection exhibit significantly reduced nasal IFN-γ protein levels versus RSV-infected infants not requiring mechanical ventilation [94]. In addition to reduced overall IFN-γ protein levels, infants with severe disease also exhibit fewer CD8 T cells in their peripheral blood producing IFN-γ following in vitro stimulation than infants with mild disease [80]. Also supporting a protective role for IFN-γ during RSV infection is the reduction of IFN-γ-producing CD8 T cells in the peripheral blood of elderly patients, who are more susceptible to RSV disease, compared to younger adults [95]. Together, these results suggest that IFN-γ is protective following RSV infection in humans, as a lack of IFN-γ correlates with more severe disease. Further work is required to evaluate this perceived contradictory role of IFN-γ between human disease and what is observed following infection in murine models.
3.3. TNF
An important function of TNF is its contribution to viral clearance in the lung following RSV infection. Antibody-mediated neutralization of TNF results in significantly elevated lung virus titers at the peak of RSV replication following primary infection in mice [96]. In another study, viral clearance was delayed in mice treated with an anti-TNF neutralizing antibody compared to WT controls, although both groups ultimately achieve complete viral clearance [15]. Mice deficient in either the Fas:FasL or perforin pathways also exhibit significantly delayed viral clearance when TNF is neutralized compared to controls. In addition, TNF may contribute to viral clearance following a secondary RSV infection, as RSV-specific memory CD8 T cells are slightly impaired in their ability to control RSV titers in the lung when TNF is neutralized [12]. In contrast, TNF has no impact on the control of viral titers by adoptively transferred, RSV-primed splenocytes following a primary RSV infection [14]. Together, these results indicate that TNF may contribute to viral clearance by RSV-specific CD8 T cells in cooperation with additional cell death mechanisms, such as the Fas:FasL and perforin pathways.
TNF has also been implicated in the induction of immunopathology following RSV infection in murine models. Mice in which TNF is neutralized exhibit ameliorated weight loss following RSV infection as compared to controls [15, 96]. However, another study demonstrated no difference in weight loss between anti-TNF neutralizing antibody- and control-treated mice [14]. In contrast, mice primed with recombinant vaccinia virus strains expressing either the RSV F or G proteins prior to RSV challenge exhibit significantly reduced weight loss and symptom illness scores in the absence of TNF [97]. Similarly, DC-LM-immunized mice exhibit significantly reduced weight loss, pulmonary dysfunction, and mortality following RSV challenge when administered a TNF-neutralizing antibody compared to IgG-treated controls [12]. These results indicate that while TNF may only play a minor role in the induction of immunopathology following an acute RSV infection, TNF substantially contributes to CD8 T cell-mediated immunopathology following a secondary RSV challenge. Interestingly, the levels of TNF detected following RSV challenge of DC-LM-immunized mice is significantly reduced following antibody-mediated neutralization of IFN-γ [12]. This result indicates that IFN-γ may also be the driving force behind TNF-mediated immunopathology during a secondary RSV infection.
4. Cytokine-mediated regulation of CD8 T cell effector functions
To prevent immunopathology, the immune system has evolved critical mechanisms to regulate the effector activity of pulmonary CD8 T cells following RSV infection. The effector functions of RSV-specific CD8 T cells, including the production of IFN-γ and TNF, are inhibited in the lung following infection [98–100]. The capacity of RSV-specific CD8 T cells in the lung to produce cytokines following ex vivo peptide stimulation is impaired, a phenomenon that does not occur as severely with splenic CD8 T cells [34, 98–100]. These results suggest that regulatory mechanisms are evoked to reduce CD8 T cell effector functions at the site of infection. Indeed, several mechanisms have been defined as being critical regulators of CD8 T cells following RSV infection, including regulatory CD4 T cells [34, 101–103], the PD-1:PD-L1 inhibitory pathway [85, 104, 105], and the actions of various inhibitory cytokines, which will be discussed in further detail in sections 4.1 and 4.2
4.1. IL-10
One of the primary cytokines that has been established as critical for the regulation of pulmonary CD8 T cells following RSV infection is IL-10 (Figure 2b). RSV infection in mice induces IL-10 production in the lung and airways that peaks at approximately 5 days post-infection, prior to the peak of the CD8 T cell response [19, 77, 79]. IL-10 is also detected in the nasopharyngeal secretions of hospitalized RSV-infected infants [73, 81]. T cells are the major cellular source of IL-10, as RSV infection of either CD4 and CD8 T cell-depleted mice or Rag KO mice, which lack T cells, results in little detectable IL-10 in the lung and airways, respectively [19, 79]. Both CD4 and CD8 T cells are capable of making IL-10 in vivo after RSV infection in mice, although CD4 T cells are the primary source [19, 79, 106]. A large proportion of the CD4 T cells producing IL-10 are regulatory CD4 T cells that express the transcription factor Foxp3. Additional conventional CD4 T cell populations producing IL-10 have also been described, including effector CD4 T cells that co-produce IFN-γ and IL-10 and CD4 T cells lacking both IFN-γ production and Foxp3 expression [19, 79, 106]. Within the IL-10-producing population of CD8 T cells, the vast majority co-produce IFN-γ following RSV infection [79, 106]. Human CD4 T cells are also capable of secreting IL-10 following RSV infection in vitro, suggesting that T cells may also be an important cellular source of IL-10 in humans [107].
The role of IL-10 in the regulation of CD8 T cell responses has been primarily studied through the use of either IL-10 KO mice or treatment with IL-10 receptor blocking antibodies in vivo. Deficiency of IL-10 in either model results in significantly increased weight loss and pulmonary dysfunction following RSV infection compared to controls [19, 79, 106]. Additionally, the lung pathology score by histological analysis and levels of the pro-inflammatory cytokines IFN-γ, TNF, and IL-6 in the lung and airways are significantly elevated in mice lacking functional IL-10 [19, 79, 106]. Given the prominent role of CD8 T cells in exacerbating disease following RSV infection, these results suggest that IL-10 may regulate CD8 T cell responses. Indeed, mice lacking IL-10 accumulate significantly increased numbers of CD8 T cells in their lungs and lung-draining lymph nodes compared to their WT counterparts [19, 106]. CD8 T cell effector functions are also elevated following RSV infection in the absence of IL-10. Mice lacking functional IL-10 exhibit an increased number of pulmonary CD8 T cells capable of producing IFN-γ or both IFN-γ and TNF after ex vivo stimulation [19, 106]. Similarly, enhanced IFN-γ production was also observed by pulmonary CD8 T cells in mice treated with IL-10 receptor blocking antibody [79]. While IL-10 is thought to primarily target cells of the myeloid lineage, Sun et al. demonstrated that CD8 T cells are capable of being regulated directly by IL-10 [79, 108]. Purified CD8 T cells stimulated with anti-CD3 in vitro exhibited significantly reduced IFN-γ production in the presence of IL-10, but this effect was completely reversed with the inclusion of IL-10 receptor blocking antibodies [79]. Additionally, elevated levels of IFN-γ were secreted in mice with a CD8 T cell-specific deletion of the IL-10 receptor compared to controls in which the IL-10 receptor was expressed on all cell types. Together, these results suggest that IL-10 can directly regulate CD8 T cell responses following RSV infection. Additionally, autocrine regulation of CD8 T cells co-producing IL-10 and IFN-γ may also be possible (Figure 2). Overall, these studies demonstrate that IL-10 is a critical cytokine by which CD8 T cell numbers and effector functions are regulated following RSV infection.
While regulation by IL-10 has not been extensively studied following RSV infection in humans, there is evidence correlating reduced IL-10 expression to exacerbated disease. Children with a severe, natural RSV infection exhibit a reduced expression of genes associated with IL-10 signaling pathways in peripheral blood samples [75]. Similarly, the peripheral blood of elderly individuals who are more susceptible to RSV-induced respiratory disease contain a decreased number of CD4 T cells capable of producing IL-10 compared to younger adults [107]. These data suggest that IL-10 may also play a regulatory role in human RSV infection.
4.2. Additional cytokines regulating CD8 T cell responses
While IL-10 is the most well-studied, several other cytokines have been identified in contributing to the regulation of RSV-specific CD8 T cell responses (Figure 2b). The Th2 cytokine IL-4 is primarily associated with exacerbating RSV immunopathology but can also function as a regulator of RSV-specific CD8 T cell effector functions [109, 110]. Infection with a recombinant RSV strain expressing IL-4 generates reduced numbers of total CD8 T cells, RSV-specific CD8 T cells, and CD8 T cells producing IFN-γ in the lung compared to WT RSV after peptide stimulation [111, 112]. Additionally, the in vitro cytolytic activity of RSV-specific CD8 T cells from mice supplemented with IL-4 during RSV infection is significantly less than that of CD8 T cells from mice infected with WT RSV [16, 111]. Importantly, the cytolytic activity is restored with the addition of neutralizing antibodies against IL-4 [16]. Similarly, antibody-mediated neutralization of IL-4 in vivo results in enhanced cytolytic activity of RSV-specific CD8 T cells compared to PBS-treated controls [110]. Together, these results support a role for IL-4-mediated regulation of CD8 T cell responses during RSV infection.
Several additional cytokines that act to suppress CD8 T cell numbers and cytokine production have been identified utilizing antibody-mediated neutralization. Antibody-mediated blockade of IL-17 in vivo enhances the total numbers of RSV-specific CD8 T cells and CD8 T cells producing IFN-γ or granzyme B in the lung following RSV infection [17]. IL-27 also suppresses CD8 T cell cytokine production in the airways, as the numbers of CD8 T cells producing IFN-γ and both IFN-γ and TNF are significantly increased in mice treated with an IL-27 neutralizing antibody [18]. Blockade of IL-6 also significantly elevates the total number of pulmonary RSV-specific CD8 T cells compared to IgG-treated controls [18]. Interestingly, IL-6 blockade prior to day 3 post-infection has a more profound effect on the enhancement of RSV-specific CD8 T cell numbers than later antibody treatment. IL-6 protein levels in the lung and airways are elevated early after infection and then decline within a few days [18, 77]. These results suggest that the early burst of IL-6 following RSV infection is critical for dampening the CD8 T cell response. Overall, a variety of cytokines play a role in regulating RSV-specific CD8 T cells in addition to IL-10.
5. Conclusions
CD8 T cells play a critical role following RSV infection by mediating viral clearance and providing protection against secondary RSV infection. The cytokines produced by RSV-specific CD8 T cells play an important part in exerting these vital functions. While IFN-γ and TNF produced by CD8 T cells may contribute to viral clearance, the exact mechanisms governing CD8 T cell-mediated viral control following RSV infection remain unknown. Identifying the mechanisms most responsible for viral clearance by CD8 T cells will provide valuable knowledge to the development of a future CD8 T cell-focused vaccine. While CD8 T cells are vital for viral control, they can also induce immunopathology following RSV infection. IFN-γ and TNF produced by CD8 T cells play a critical role in the induction of immunopathology following RSV infection. This is especially true in murine models, where IFN-γ and TNF have been demonstrated to initiate immunopathology following either a primary or secondary RSV infection. Interestingly, IFN-γ produced by memory CD8 T cells in DC-LM-immunized mice was determined to be the primary driver of CD8 T cell-mediated immunopathology following a secondary RSV challenge. The role of IFN-γ in driving exacerbated disease included the induction of TNF in the lung following challenge, which also independently mediated exacerbated morbidity and mortality in this model. These results indicate that the IFN-γ production by CD8 T cells induced by vaccination needs to be carefully regulated to avoid excess immunopathology. It has been shown that multiple cytokines can function to suppress the magnitude of IFN-γ production by RSV-specific CD8 T cells in the lung, including IL-10. A future CD8 T cell-mediated RSV vaccine with the ability to induce one or more regulatory cytokines following subsequent RSV exposure could provide necessary inhibitory signals to dampen CD8 T cell responses and prevent their prolonged activation. Overall, the induction of CD8 T cell responses by a future RSV vaccine will require careful consideration to balance CD8 T cell-mediated protection and immunopathology. In particular, the secretion of the pro-inflammatory cytokines IFN-γ and TNF by virus-specific CD8 T cells will need to be controlled. Combining the induction of pro-inflammatory cytokine-producing CD8 T cells with the actions of inhibitory cytokines may assist in achieving this balance and provide an effective RSV vaccine.
Acknowledgements
This work was supported by funds by the Department of Microbiology and Immunology at the University of Iowa (to SMV) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers R01AI124093 (to SMV) and T32AI007485 (to MES). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- [1].Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H, Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis, Lancet 375(9725) (2010) 1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Falsey AR, Cunningham CK, Barker WH, Kouides RW, Yuen JB, Menegus M, Weiner LB, Bonville CA, Betts RF, Respiratory syncytial virus and influenza A infections in the hospitalized elderly, Infect Dis 172(2) (1995) 389–94. [DOI] [PubMed] [Google Scholar]
- [3].Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE, Respiratory syncytial virus infection in elderly and high-risk adults, N Engl J Med 352(17) (2005) 1749–59. [DOI] [PubMed] [Google Scholar]
- [4].Hall CB, Powell KR, MacDonald NE, Gala CL, Menegus ME, Suffin SC, Cohen HJ, Respiratory syncytial viral infection in children with compromised immune function, N Engl J Med 315(2) (1986) 77–81. [DOI] [PubMed] [Google Scholar]
- [5].Jansen AG, Sanders EA, Hoes AW, van Loon AM, Hak E, Influenza- and respiratory syncytial virus-associated mortality and hospitalisations, Eur Respir J 30(6) (2007) 1158–66. [DOI] [PubMed] [Google Scholar]
- [6].Glezen WP, Taber LH, Frank AL, Kasel JA, Risk of primary infection and reinfection with respiratory syncytial virus, Am J Dis Child 140(6) (1986) 543–6. [DOI] [PubMed] [Google Scholar]
- [7].Hall CB, Walsh EE, Long CE, Schnabel KC, Immunity to and frequency of reinfection with respiratory syncytial virus, Infect Dis 163(4) (1991) 693–8. [DOI] [PubMed] [Google Scholar]
- [8].Henderson FW, Collier AM, Clyde WA Jr., Denny FW, Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children, N Engl J Med 300(10) (1979) 530–4. [DOI] [PubMed] [Google Scholar]
- [9].Graham BS, Bunton LA, Wright PF, Karzon DT, Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice, J Clin Invest 88(3) (1991) 1026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Morabito KM, Erez N, Graham BS, Ruckwardt TJ, Phenotype and Hierarchy of Two Transgenic T Cell Lines Targeting the Respiratory Syncytial Virus KdM282–90 Epitope Is Transfer Dose-Dependent, PLoS One 11(1) (2016) e0146781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee S, Stokes KL, Currier MG, Sakamoto K, Lukacs NW, Celis E, Moore ML, Vaccine-elicited CD8+ T cells protect against respiratory syncytial virus strain A2-line19F-induced pathogenesis in BALB/c mice, J Virol 86(23) (2012) 13016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schmidt ME, Knudson CJ, Hartwig SM, Pewe LL, Meyerholz DK, Langlois RA, Harty JT, Varga SM, Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection, PLoS Pathog 14(1) (2018) e1006810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bukreyev A, Whitehead SS, Bukreyeva N, Murphy BR, Collins PL, Interferon gamma expressed by a recombinant respiratory syncytial virus attenuates virus replication in mice without compromising immunogenicity, Proc Natl Acad Sci U S A 96(5) (1999) 2367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ostler T, Davidson W, Ehl S, Virus clearance and immunopathology by CD8(+) T cells during infection with respiratory syncytial virus are mediated by IFN-gamma, Eur J Immunol 32(8) (2002) 2117–23. [DOI] [PubMed] [Google Scholar]
- [15].Rutigliano JA, Graham BS, Prolonged production of TNF-alpha exacerbates illness during respiratory syncytial virus infection, J Immunol 173(5) (2004) 3408–17. [DOI] [PubMed] [Google Scholar]
- [16].Aung S, Tang YW, Graham BS, Interleukin-4 diminishes CD8(+) respiratory syncytial virus-specific cytotoxic T-lymphocyte activity in vivo, J Virol 73(11) (1999) 8944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mukherjee S, Lindell DM, Berlin AA, Morris SB, Shanley TP, Hershenson MB, Lukacs NW, IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease, Am J Pathol 179(1) (2011) 248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pyle CJ, Uwadiae FI, Swieboda DP, Harker JA, Early IL-6 signalling promotes IL-27 dependent maturation of regulatory T cells in the lungs and resolution of viral immunopathology, PLoS Pathog 13(9) (2017) e1006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Weiss KA, Christiaansen AF, Fulton RB, Meyerholz DK, Varga SM, Multiple CD4+ T cell subsets produce immunomodulatory IL-10 during respiratory syncytial virus infection, J Immunol 187(6) (2011) 3145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guerrero-Plata A, Kolli D, Hong C, Casola A, Garofalo RP, Subversion of pulmonary dendritic cell function by paramyxovirus infections, J Immunol 182(5) (2009) 3072–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lukens MV, Kruijsen D, Coenjaerts FE, Kimpen JL, van Bleek GM, Respiratory syncytial virus-induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung-draining lymph node, J Virol 83(14) (2009) 7235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ruckwardt TJ, Malloy AM, Morabito KM, Graham BS, Quantitative and qualitative deficits in neonatal lung-migratory dendritic cells impact the generation of the CD8+ T cell response, PLoS Pathog 10(2) (2014) e1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ruckwardt TJ, Morabito KM, Bar-Haim E, Nair D, Graham BS, Neonatal mice possess two phenotypically and functionally distinct lung-migratory CD103(+) dendritic cell populations following respiratory infection, Mucosal Immunol 11(1) (2018) 186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang H, Peters N, Schwarze J, Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection, J Immunol 177(9) (2006) 6263–70. [DOI] [PubMed] [Google Scholar]
- [25].de Graaff PM, de Jong EC, van Capel TM, van Dijk ME, Roholl PJ, Boes J, Luytjes W, Kimpen JL, van Bleek GM, Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate CD4 T cells, J Immunol 175(9) (2005) 5904–11. [DOI] [PubMed] [Google Scholar]
- [26].Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, Garofalo RP, Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus, Am J Respir Cell Mol Biol 34(3) (2006) 320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rothoeft T, Fischer K, Zawatzki S, Schulz V, Schauer U, Korner Rettberg C, Differential response of human naive and memory/effector T cells to dendritic cells infected by respiratory syncytial virus, Clin Exp Immunol 150(2) (2007) 263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Malloy AM, Ruckwardt TJ, Morabito KM, Lau-Kilby AW, Graham BS, Pulmonary Dendritic Cell Subsets Shape the Respiratory Syncytial Virus-Specific CD8+ T Cell Immunodominance Hierarchy in Neonates, J Immunol 198(1) (2017) 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF, Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation, J Immunol 174(8) (2005) 4465–9. [DOI] [PubMed] [Google Scholar]
- [30].Gately MK, Wolitzky AG, Quinn PM, Chizzonite R, Regulation of human cytolytic lymphocyte responses by interleukin-12, Cell Immunol 143(1) (1992) 127–42. [DOI] [PubMed] [Google Scholar]
- [31].Bartz H, Turkel O, Hoffjan S, Rothoeft T, Gonschorek A, Schauer U, Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-gamma in naive T cells, Immunology 109(1) (2003) 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rudd BD, Luker GD, Luker KE, Peebles RS, Lukacs NW, Type I interferon regulates respiratory virus infected dendritic cell maturation and cytokine production, Viral Immunol 20(4) (2007) 531–40. [DOI] [PubMed] [Google Scholar]
- [33].Fulton RB, Weiss KA, Pewe LL, Harty JT, Varga SM, Aged mice exhibit a severely diminished CD8 T cell response following respiratory syncytial virus infection, J Virol 87(23) (2013) 12694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ruckwardt TJ, Bonaparte KL, Nason MC, Graham BS, Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities, J Virol 83(7) (2009) 3019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rutigliano JA, Ruckwardt TJ, Martin JE, Graham BS, Relative dominance of epitope-specific CD8+ T cell responses in an F1 hybrid mouse model of respiratory syncytial virus infection, Virology 362(2) (2007) 314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chang J, Srikiatkhachorn A, Braciale TJ, Visualization and characterization of respiratory syncytial virus F-specific CD8(+) T cells during experimental virus infection, J Immunol 167(8) (2001) 4254–60. [DOI] [PubMed] [Google Scholar]
- [37].Lukens MV, Claassen EA, de Graaff PM, van Dijk ME, Hoogerhout P, Toebes M, Schumacher TN, van der Most RG, Kimpen JL, van Bleek GM, Characterization of the CD8+ T cell responses directed against respiratory syncytial virus during primary and secondary infection in C57BL/6 mice, Virology 352(1) (2006) 157–68. [DOI] [PubMed] [Google Scholar]
- [38].Knudson CJ, Weiss KA, Hartwig SM, Varga SM, The pulmonary localization of virus-specific T lymphocytes is governed by the tissue tropism of infection, J Virol 88(16) (2014) 9010–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Heidema J, Lukens MV, van Maren WW, van Dijk ME, Otten HG, van Vught AJ, van der Werff DB, van Gestel SJ, Semple MG, Smyth RL, Kimpen JL, van Bleek GM, CD8+ T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections, J Immunol 179(12) (2007) 8410–7. [DOI] [PubMed] [Google Scholar]
- [40].Lukens MV, van de Pol AC, Coenjaerts FE, Jansen NJ, Kamp VM, Kimpen JL, Rossen JW, Ulfman LH, Tacke CE, Viveen MC, Koenderman L, Wolfs TF, van Bleek GM, A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants, J Virol 84(5) (2010) 2374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Heidema J, Rossen JW, Lukens MV, Ketel MS, Scheltens E, Kranendonk ME, van Maren WW, van Loon AM, Otten HG, Kimpen JL, van Bleek GM, Dynamics of human respiratory virus-specific CD8+ T cell responses in blood and airways during episodes of common cold, J Immunol 181(8) (2008) 5551–9. [DOI] [PubMed] [Google Scholar]
- [42].Ruckwardt TJ, Luongo C, Malloy AM, Liu J, Chen M, Collins PL, Graham BS, Responses against a subdominant CD8+ T cell epitope protect against immunopathology caused by a dominant epitope, J Immunol 185(8) (2010) 4673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Taylor G, Stott EJ, Hayle AJ, Cytotoxic lymphocytes in the lungs of mice infected with respiratory syncytial virus, J Gen Virol 66 (Pt 12) (1985) 2533–8. [DOI] [PubMed] [Google Scholar]
- [44].Cannon MJ, Stott EJ, Taylor G, Askonas BA, Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells, Immunology 62(1) (1987) 133–8. [PMC free article] [PubMed] [Google Scholar]
- [45].Alwan WH, Record FM, Openshaw PJ, CD4+ T cells clear virus but augment disease in mice infected with respiratory syncytial virus. Comparison with the effects of CD8+ T cells, Clin Exp Immunol 88(3) (1992) 527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Alwan WH, Kozlowska WJ, Openshaw PJ, Distinct types of lung disease caused by functional subsets of antiviral T cells, J Exp Med 179(1) (1994) 81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cannon MJ, Openshaw PJ, Askonas BA, Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus, J Exp Med 168(3) (1988) 1163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bar-Haim E, Erez N, Malloy AM, Graham BS, Ruckwardt TJ, CD8+ TCR transgenic strains expressing public versus private TCR targeting the respiratory syncytial virus K(d)M2(82–90) epitope demonstrate similar functional profiles, PLoS One 9(6) (2014) e99249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].El Saleeby CM, Suzich J, Conley ME, DeVincenzo JP, Quantitative effects of palivizumab and donor-derived T cells on chronic respiratory syncytial virus infection, lung disease, and fusion glycoprotein amino acid sequences in a patient before and after bone marrow transplantation, Clin Infect Dis 39(2) (2004) e17–20. [DOI] [PubMed] [Google Scholar]
- [50].Fishaut M, Tubergen D, McIntosh K, Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity, J Pediatr 96(2) (1980) 179–86. [DOI] [PubMed] [Google Scholar]
- [51].Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R, Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells, Nat Immunol 4(12) (2003) 1191–8. [DOI] [PubMed] [Google Scholar]
- [52].Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A, Two subsets of memory T lymphocytes with distinct homing potentials and effector functions, Nature 401(6754) (1999) 708–12. [DOI] [PubMed] [Google Scholar]
- [53].Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R, Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates, J Exp Med 205(3) (2008) 625–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR, Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus, Nat Immunol 10(5) (2009) 524–30. [DOI] [PubMed] [Google Scholar]
- [55].Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, Farber DL, Lung niches for the generation and maintenance of tissue-resident memory T cells, Mucosal Immunol 7(3) (2014) 501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kinnear E, Lambert L, McDonald JU, Cheeseman HM, Caproni LJ, Tregoning JS, Airway T cells protect against RSV infection in the absence of antibody, Mucosal Immunol 11(1) (2018) 249–256. [DOI] [PubMed] [Google Scholar]
- [57].Morabito KM, Ruckwardt TR, Redwood AJ, Moin SM, Price DA, Graham BS, Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung, Mucosal Immunol 10(2) (2017) 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schwarz B, Morabito KM, Ruckwardt TJ, Patterson DP, Avera J, Miettinen HM, Graham BS, Douglas T, Viruslike Particles Encapsidating Respiratory Syncytial Virus M and M2 Proteins Induce Robust T Cell Responses, ACS Biomater Sci Eng 2(12) (2016) 2324–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, Almond M, Wong EH, Sykes A, Maybeno M, Del Rosario J, Trujillo-Torralbo MB, Mallia P, Sidney J, Peters B, Kon OM, Sette A, Johnston SL, Openshaw PJ, Chiu C, RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection, Nat Commun 6 (2015) 10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].de Bree GJ, van Leeuwen EM, Out TA, Jansen HM, Jonkers RE, van Lier RA, Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung, J Exp Med 202(10) (2005) 1433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].de Bree GJ, Heidema J, van Leeuwen EM, van Bleek GM, Jonkers RE, Jansen HM, van Lier RA, Out TA, Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons, J Infect Dis 191(10) (2005) 1710–8. [DOI] [PubMed] [Google Scholar]
- [62].Heidema J, de Bree GJ, De Graaff PM, van Maren WW, Hoogerhout P, Out TA, Kimpen JL, van Bleek GM, Human CD8(+) T cell responses against five newly identified respiratory syncytial virus-derived epitopes, J Gen Virol 85(Pt 8) (2004) 2365–74. [DOI] [PubMed] [Google Scholar]
- [63].Lee JY, Chang J, Recombinant baculovirus-based vaccine expressing M2 protein induces protective CD8(+) T-cell immunity against respiratory syncytial virus infection, J Microbiol 55(11) (2017) 900–908. [DOI] [PubMed] [Google Scholar]
- [64].Liu J, Ruckwardt TJ, Chen M, Johnson TR, Graham BS, Characterization of respiratory syncytial virus M- and M2-specific CD4 T cells in a murine model, J Virol 83(10) (2009) 4934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].McDermott DS, Knudson CJ, Varga SM, Determining the breadth of the respiratory syncytial virus-specific T cell response, J Virol 88(6) (2014) 3135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].McMaster SR, Wilson JJ, Wang H, Kohlmeier JE, Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-gamma Production, J Immunol 195(1) (2015) 203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Slutter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, Harty JT, Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity, Sci Immunol 2(7) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, Cauley LS, Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection, J Leukoc Biol 95(2) (2014) 215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bont L, Versteegh J, Swelsen WT, Heijnen CJ, Kavelaars A, Brus F, Draaisma JM, Pekelharing-Berghuis M, van Diemen-Steenvoorde RA, Kimpen JL, Natural reinfection with respiratory syncytial virus does not boost virus-specific T-cell immunity, Pediatr Res 52(3) (2002) 363–7. [DOI] [PubMed] [Google Scholar]
- [70].Channappanavar R, Fett C, Zhao J, Meyerholz DK, Perlman S, Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection, J Virol 88(19) (2014) 11034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Slutter B, Pewe LL, Kaech SM, Harty JT, Lung airway-surveilling CXCR3(hi) memory CD8(+) T cells are critical for protection against influenza A virus, Immunity 39(5) (2013) 939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bem RA, Bos AP, Bots M, Wolbink AM, van Ham SM, Medema JP, Lutter R, van Woensel JB, Activation of the granzyme pathway in children with severe respiratory syncytial virus infection, Pediatr Res 63(6) (2008) 650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, Velozo L, Jafri H, Chavez-Bueno S, Ogra PL, McKinney L, Reed JL, Welliver RC Sr., Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses, J Infect Dis 195(8) (2007) 1126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Welliver TP, Reed JL, Welliver RC Sr., Respiratory syncytial virus and influenza virus infections: observations from tissues of fatal infant cases, Pediatr Infect Dis J 27(10 Suppl) (2008) S92–6. [DOI] [PubMed] [Google Scholar]
- [75].Mejias A, Dimo B, Suarez NM, Garcia C, Suarez-Arrabal MC, Jartti T, Blankenship D, Jordan-Villegas A, Ardura MI, Xu Z, Banchereau J, Chaussabel D, Ramilo O, Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection, PLoS Med 10(11) (2013) e1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Aung S, Rutigliano JA, Graham BS, Alternative mechanisms of respiratory syncytial virus clearance in perforin knockout mice lead to enhanced disease, J Virol 75(20) (2001) 9918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Jafri HS, Chavez-Bueno S, Mejias A, Gomez AM, Rios AM, Nassi SS, Yusuf M, Kapur P, Hardy RD, Hatfield J, Rogers BB, Krisher K, Ramilo O, Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice, J Infect Dis 189(10) (2004) 1856–65. [DOI] [PubMed] [Google Scholar]
- [78].van Schaik SM, Obot N, Enhorning G, Hintz K, Gross K, Hancock GE, Stack AM, Welliver RC, Role of interferon gamma in the pathogenesis of primary respiratory syncytial virus infection in BALB/c mice, J Med Virol 62(2) (2000) 257–66. [DOI] [PubMed] [Google Scholar]
- [79].Sun J, Cardani A, Sharma AK, Laubach VE, Jack RS, Muller W, Braciale TJ, Autocrine regulation of pulmonary inflammation by effector T-cell derived IL-10 during infection with respiratory syncytial virus, PLoS Pathog 7(8) (2011) e1002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chen ZM, Mao JH, Du LZ, Tang YM, Association of cytokine responses with disease severity in infants with respiratory syncytial virus infection, Acta Paediatr 91(9) (2002) 914–22. [DOI] [PubMed] [Google Scholar]
- [81].Christiaansen AF, Syed MA, Ten Eyck PP, Hartwig SM, Durairaj L, Kamath SS, Varga SM, Altered Treg and cytokine responses in RSV-infected infants, Pediatr Res 80(5) (2016) 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Thwaites RS, Coates M, Ito K, Ghazaly M, Feather C, Abdulla F, Tunstall T, Jain P, Cass L, Rapeport G, Hansel TT, Nadel S, Openshaw PJ, Reduced Nasal Viral Load and IFN Responses in Infants with RSV Bronchiolitis and Respiratory Failure, Am J Respir Crit Care Med (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ostler T, Ehl S, Pulmonary T cells induced by respiratory syncytial virus are functional and can make an important contribution to long-lived protective immunity, Eur J Immunol 32(9) (2002) 2562–9. [DOI] [PubMed] [Google Scholar]
- [84].Liu F, Whitton JL, Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections, J Immunol 174(10) (2005) 5936–40. [DOI] [PubMed] [Google Scholar]
- [85].Yao S, Jiang L, Moser EK, Jewett LB, Wright J, Du J, Zhou B, Davis SD, Krupp NL, Braciale TJ, Sun J, Control of pathogenic effector T-cell activities in situ by PD-L1 expression on respiratory inflammatory dendritic cells during respiratory syncytial virus infection, Mucosal Immunol 8(4) (2015) 746–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Feau S, Arens R, Togher S, Schoenberger SP, Autocrine IL-2 is required for secondary population expansion of CD8(+) memory T cells, Nat Immunol 12(9) (2011) 908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Malek TR, The biology of interleukin-2, Annu Rev Immunol 26 (2008) 453–79. [DOI] [PubMed] [Google Scholar]
- [88].Williams MA, Tyznik AJ, Bevan MJ, Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells, Nature 441(7095) (2006) 890–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Krampera M, Vinante F, Tavecchia L, Morosato L, Chilosi M, Romagnani S, Zanolin ME, Pizzolo G, Progressive polarization towards a T helper/cytotoxic type-1 cytokine pattern during age-dependent maturation of the immune response inversely correlates with CD30 cell expression and serum concentration, Clin Exp Immunol 117(2) (1999) 291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chang J, Choi SY, Jin HT, Sung YC, Braciale TJ, Improved effector activity and memory CD8 T cell development by IL-2 expression during experimental respiratory syncytial virus infection, J Immunol 172(1) (2004) 503–8. [DOI] [PubMed] [Google Scholar]
- [91].Eichinger KM, Empey KM, Data describing IFNgamma-mediated viral clearance in an adult mouse model of respiratory syncytial virus (RSV), Data Brief 14 (2017) 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kim CK, Callaway Z, Koh YY, Kim SH, Fujisawa T, Airway IFN-gamma production during RSV bronchiolitis is associated with eosinophilic inflammation, Lung 190(2) (2012) 183–8. [DOI] [PubMed] [Google Scholar]
- [93].Aberle JH, Aberle SW, Dworzak MN, Mandl CW, Rebhandl W, Vollnhofer G, Kundi M, Popow-Kraupp T, Reduced interferon-gamma expression in peripheral blood mononuclear cells of infants with severe respiratory syncytial virus disease, Am J Respir Crit Care Med 160(4) (1999) 1263–8. [DOI] [PubMed] [Google Scholar]
- [94].Bont L, Heijnen CJ, Kavelaars A, van Aalderen WM, Brus F, Draaisma JM, Pekelharing-Berghuis M, van Diemen-Steenvoorde RA, Kimpen JL, Local interferon-gamma levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity, J Infect Dis 184(3) (2001) 355–8. [DOI] [PubMed] [Google Scholar]
- [95].Cherukuri A, Patton K, Gasser RA Jr., Zuo F, Woo J, Esser MT, Tang RS, Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein, Clin Vaccine Immunol 20(2) (2013) 239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Tregoning JS, Pribul PK, Pennycook AM, Hussell T, Wang B, Lukacs N, Schwarze J, Culley FJ, Openshaw PJ, The chemokine MIP1alpha/CCL3 determines pathology in primary RSV infection by regulating the balance of T cell populations in the murine lung, PLoS One 5(2) (2010) e9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hussell T, Pennycook A, Openshaw PJ, Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology, Eur J Immunol 31(9) (2001) 2566–73. [DOI] [PubMed] [Google Scholar]
- [98].Chang J, Braciale TJ, Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract, Nat Med 8(1) (2002) 54–60. [DOI] [PubMed] [Google Scholar]
- [99].DiNapoli JM, Murphy BR, Collins PL, Bukreyev A, Impairment of the CD8+ T cell response in lungs following infection with human respiratory syncytial virus is specific to the anatomical site rather than the virus, antigen, or route of infection, Virol J 5 (2008) 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Fulton RB, Olson MR, Varga SM, Regulation of cytokine production by virus-specific CD8 T cells in the lungs, J Virol 82(16) (2008) 7799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Durant LR, Makris S, Voorburg CM, Loebbermann J, Johansson C, Openshaw PJ, Regulatory T cells prevent Th2 immune responses and pulmonary eosinophilia during respiratory syncytial virus infection in mice, J Virol 87(20) (2013) 10946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Fulton RB, Meyerholz DK, Varga SM, Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection, J Immunol 185(4) (2010) 2382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Liu J, Ruckwardt TJ, Chen M, Nicewonger JD, Johnson TR, Graham BS, Epitope-specific regulatory CD4 T cells reduce virus-induced illness while preserving CD8 T-cell effector function at the site of infection, J Virol 84(20) (2010) 10501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Erickson JJ, Gilchuk P, Hastings AK, Tollefson SJ, Johnson M, Downing MB, Boyd KL, Johnson JE, Kim AS, Joyce S, Williams JV, Viral acute lower respiratory infections impair CD8+ T cells through PD-1, J Clin Invest 122(8) (2012) 2967–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Telcian AG, Laza-Stanca V, Edwards MR, Harker JA, Wang H, Bartlett NW, Mallia P, Zdrenghea MT, Kebadze T, Coyle AJ, Openshaw PJ, Stanciu LA, Johnston SL, RSV-induced bronchial epithelial cell PD-L1 expression inhibits CD8+ T cell nonspecific antiviral activity, J Infect Dis 203(1) (2011) 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Loebbermann J, Schnoeller C, Thornton H, Durant L, Sweeney NP, Schuijs M, O’Garra A, Johansson C, Openshaw PJ, IL-10 regulates viral lung immunopathology during acute respiratory syncytial virus infection in mice, PLoS One 7(2) (2012) e32371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Lee FE, Walsh EE, Falsey AR, Liu N, Liu D, Divekar A, Snyder-Cappione JE, Mosmann TR, The balance between influenza- and RSV-specific CD4 T cells secreting IL-10 or IFNgamma in young and healthy-elderly subjects, Mech Ageing Dev 126(11) (2005) 1223–9. [DOI] [PubMed] [Google Scholar]
- [108].Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A, Interleukin-10 and the interleukin-10 receptor, Annu Rev Immunol 19 (2001) 683–765. [DOI] [PubMed] [Google Scholar]
- [109].Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC 3rd, Murphy BR, Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10, J Virol 68(8) (1994) 5321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Tang YW, Graham BS, Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus, J Clin Invest 94(5) (1994) 1953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bukreyev A, Belyakov IM, Prince GA, Yim KC, Harris KK, Berzofsky JA, Collins PL, Expression of interleukin-4 by recombinant respiratory syncytial virus is associated with accelerated inflammation and a nonfunctional cytotoxic T-lymphocyte response following primary infection but not following challenge with wild-type virus, J Virol 79(15) (2005) 9515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Harker J, Bukreyev A, Collins PL, Wang B, Openshaw PJ, Tregoning JS, Virally delivered cytokines alter the immune response to future lung infections, J Virol 81(23) (2007) 13105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]