Abstract
Stimulation of group I metabotropic glutamate receptors (mGluRs) by the agonist (S)-dihydroxyphenylglycine (DHPG) in the hippocampus transforms normal neuronal activity into prolonged epileptiform discharges. The conversion is long-lasting in that epileptiform discharges persist following washout of the inducing agonist and serves as a model of epileptogenesis. The group I mGluR model of epileptogenesis took on special significance because epilepsy associated with fragile X syndrome (FXS) may be caused by excessive group I mGluR signaling. At present, the plasticity mechanism underlying the group I mGluR-mediated epileptogenesis is unknown. ImGluR(V), a voltage-gated cationic current activated by group I mGluR agonists in CA3 pyramidal cells in the hippocampus, is a possible candidate. ImGluR(V) activation is associated with group I mGluR agonist-elicited epileptiform discharges. For ImGluR(V) to play a role in epileptogenesis, long-term activation of the current must occur following group I mGluR agonist exposure or synaptic stimulation. We observed that ImGluR(V), once induced by group I mGluR agonist stimulation in CA3 pyramidal cells, remained undiminished for hours following agonist washout. In slices prepared from FXS model mice, repeated stimulation of recurrent CA3 pyramidal cell synapses, effective in eliciting mGluR-mediated epileptiform discharges, also induced long-lasting ImGluR(V) in CA3 pyramidal cells. Similar to group I mGluR-mediated prolonged epileptiform discharges, persistent ImGluR(V) was no longer observed in preparations pretreated with inhibitors of tyrosine kinase, of extracellular signal-regulated kinase (ERK) 1/2, or of mRNA protein synthesis. The results indicate that ImGluR(V) is an intrinsic plasticity mechanism associated with group I mGluR-mediated epileptogenesis.
Keywords: metabotropic glutamate receptor, cation, current, depolarization, epilepsy, epileptiform, seizure, synchronization, plasticity, mRNA, protein synthesis
Introduction
Considerable information on group I mGluR-mediated epileptogenesis has been derived from experiments using the group I mGluR agonist DHPG to stimulate hippocampal slices (Merlin and Wong, 1997; Merlin et al., 1998; Chuang et al., 2001; McNamara et al., 2006). In response to group I mGluR agonist stimulation, rhythmic synchronized discharges of prolonged durations (up to 10 s) appeared in the CA3 neuronal population. Each episode of prolonged synchronized discharge consists of fast membrane oscillations of 12 – 24 Hz with overriding action potential discharges. Blockade of GABA-mediated inhibition is not required for the occurrence of these discharges (Taylor et al., 1995; Zhao et al., 2004). The features of these prolonged synchronized discharges can be compared to ictal discharges recorded during seizures (Lieb et al., 1981). Two notable properties of the group I mGluR-mediated prolonged discharges elicited in hippocampal slices are: (i) prolonged discharges, once induced, persist even when the inducing agonist is washed out (Merlin and Wong, 1997); (ii) induction of the prolonged discharges is dependent on group I mGluR-activated mRNA translation that is mediated via a tyrosine kinase-ERK1/2 signaling cascade (Zhao et al., 2004).
In addition to agonist stimulation, synaptically released glutamate also activates group I mGluRs in the hippocampal CA3 population (Bianchi and Wong, 1995; Lee et al., 2002). However, synaptic stimulation is ineffective at eliciting the mGluR-dependent prolonged synchronized discharges (Lee et al., 2002). This is due to the negative regulation of group I mGluR-activated mRNA translation by the fragile X mental retardation protein (FMRP) (Weiler et al., 1997; Huber et al., 2002; Aschrafi et al., 2005; Chuang et al., 2005; Hou et al., 2006). FMRP is absent in FXS due to a trinucleotide (CGG) repeat expansion of the promoter of the Fmr1 gene (Penagarikano et al., 2007). Under this condition, group I mGluR-dependent protein synthesis is enhanced, enabling the synaptic induction of group I mGluR-mediated prolonged discharges (Chuang et al., 2005). In vivo studies show that FXS model mice have heightened susceptibility to audiogenic seizures mediated by group I mGluR activation, as these seizures are suppressed by group I mGluR antagonist (Yan et al., 2005).
Recordings in the wild-type CA3 pyramidal cells show that a voltage-dependent cationic current, ImGluR(V), was activated accompanying group I mGluR-dependent prolonged discharges (Chuang et al., 2001). ImGluR(V) has an activation threshold (−65 mV) close to the resting potential, a reversal potential of about −10 mV, and shows no inactivation (Chuang et al., 2000, 2001). ImGluR(V) alters the properties of CA3 pyramidal cells by inducing bi-stable resting potentials and by eliciting rhythmic, prolonged trains of action potentials (up to 12 s). Rhythmic prolonged firing in individual CA3 pyramidal cells provides the basic activity pattern which, when synchronized via the recurrent synapses, sustains the prolonged epileptiform discharges in the CA3 population (Wong et al., 2004). In this study, we found that agonist-induced ImGluR(V) is long-lasting and that ImGluR(V) can be induced synaptically in association with the group I mGluR-mediated prolonged epileptiform discharges.
Materials and Methods
Slice Preparation
Fmr1−/− mice and littermates were obtained from the Jackson Laboratories (Bar Harbor, ME). Transverse hippocampal slices (300–400 μm-thick) were prepared from 2–7 wk-old mice or 2–4 wk-old guinea pigs as previously described (Chuang et al., 2005; Bianchi et al., 2006), according to procedures approved by the IACUC of SUNY Downstate Medical Center, Brooklyn, NY. Hippocampi were dissected out in ice-cold solution (containing, in mM: NaCl 124.0, NaHCO3 26.0, KCl 2.5, MgCl2 8.0, CaCl2 0.5, and D-glucose 10.0), glued to the stage of a Lancer Vibratome 1000 (The Vibratome Company, St. Louis, MO) and sliced. For patch-clamp recordings, single slices were submerged in a coverslip-bottomed recording chamber (Luigs & Neumann, Ratingen, Germany) and superfused with artificial cerebro-spinal fluid (aCSF) containing (in mM) NaCl 124, NaHCO3 26, KCl 5, MgCl2 1.6, CaCl2 2, and D-glucose 10, gassed with a 95% O2-5% CO2 (pH 7.4; 31–32°C). The Mn2+/low Ca2+-containing solution (Mn2+ solution) had the same composition as the aCSF except for 0.2 mM CaCl2 and added 1 mM MnCl2. The chamber was on a remotely-controlled stationary stage with mounted micromanipulators (Luigs & Neumann) under an upright microscope equipped with water-immersed objectives and IR-DIC microscopy (BX50WI, Olympus, Middlebush, NJ). Neurons in the slice were visualized through a solid-state camera (Cohu Inc., Electronics Division, San Diego, CA) connected to a video monitor. For intracellular recordings, slices were placed in an interface recording chamber (Fine Science Tools, Vancouver, British Columbia, Canada) and perfused with aCSF (34–36°C).
Electrophysiology
Data shown in Figs. 2–6, S1, and S2A,B are from visually-identified CA3 pyramidal cells recorded in whole-cell voltage-clamp using glass pipettes (3–6 MΩ; World Precision Instruments, Sarasota, FL) and a patch-clamp amplifier (EPC-7; HEKA Instruments Inc., Southboro, MA). Recording pipettes were filled with solution containing (in mM): 119 K+ gluconate, 13 KCl, 10 HEPES, 5 NaCl, 2 CsCl, 2 Mg-ATP, 2 EGTA, and pH was adjusted to 7.3 with KOH. For intracellular application of Cs+, the pipette solution contained (in mM): 119 CsCH3SO3, 10 HEPES, 5 NaCl, 15 CsCl, 2 Mg-ATP, 2 EGTA (pH ~7.3). Signals were sampled at 3 kHz, digitized at 0.1–1 kHz on an Intel-based computer running pCLAMP software (Molecular Devices, Sunnyvale, CA), and simultaneously displayed on an oscilloscope (DSO 400, Gould Instruments, Valley View, OH) and on a chart recorder (TA240, Gould Instruments). Intracellular recordings shown in Figs. 1, 7, and S2C,D were obtained from slices placed in the interface chamber via an Axoclamp 2A amplifier (Molecular Devices). Membrane currents were recorded in single-electrode discontinuous voltage-clamp mode. Thin-walled glass electrodes were filled with potassium acetate (2 M; 30–50 MΩ).
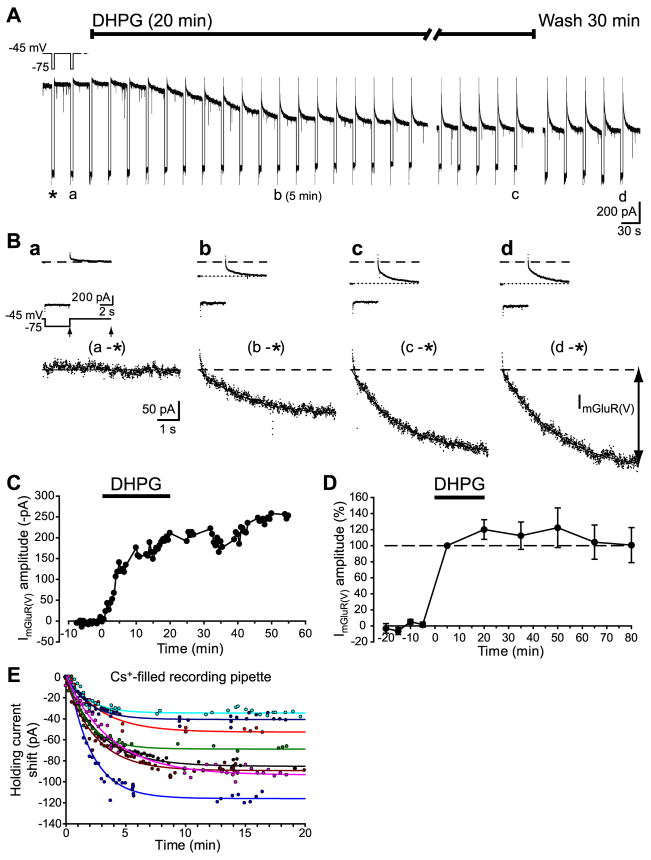
Figure 2.
Long-lasting induction of ImGluR(V) by DHPG. A, Whole-cell patch clamp recordings of responses of a CA3 pyramidal cell to DHPG. Hyperpolarizing voltage steps (inset) were applied every 30 s throughout the recording. B, Current responses to pulses in A are shown expanded in the insets. The net inward current elicited by DHPG (ImGluR(V)) after the pulses (segment between arrows in the voltage protocol in a) was obtained by subtracting the control response (*) from the corresponding traces. C, Time course of the ImGluR(V) amplitude for the experiment shown in A. D, Summary data of ImGluR(V) amplitude normalized to that recorded at 5 min DHPG in 7 cells. E, Shift of holding currents in 8 cells recorded with Cs+-filled pipettes and voltage-clamped at −50 mV. Time 0 is the onset of successful whole-cell patch recording. Plots were well fitted with single exponentials (R2 > 0.91).
Figure 6.
Long-lasting ImGluR(V) is suppressed by inhibitors of tyrosine kinase, ERK1/2, and protein synthesis. A, Current responses of a CA3 pyramidal cell to hyperpolarizing voltage steps from −75 to −45 mV (downward deflections) before, during (filled bar), and after DHPG in the continuous presence of the tyrosine kinase inhibitor genistein (30 μM; hollow bar). ImGluR(V) elicited following the hyperpolarizing pulse was obtained by subtracting the control response (*) from the corresponding indicated responses (Subtracted traces). B, Time course of ImGluR(V) amplitude for the cell shown in A (red circles) and summary data plot for 5 cells (filled circles). C, Mean ImGluR(V) amplitude recorded in control and in DHPG (aCSF; n = 8), in DHPG + genistein (n = 4), in DHPG + PD98059 (n = 3), in DHPG + U0126 (n = 3), in DHPG + cycloeximide (n = 4). In each experiment, agents were added 60 min prior to DHPG. Peak ImGluR(V) activation was observed in all experimental conditions with blockers after 3–10 min of DHPG application.
Figure 1.
DHPG induces rhythmic prolonged intrinsic bursts in CA3 pyramidal cells. A, Intracellular records from synaptically isolated CA3 neurons in CNQX and CPP (20 μM each) before (a) and during DHPG (50 μM; b). Membrane potential values are indicated at the beginning of each current-clamp record. B, Application of hyperpolarizing square-wave pulses (−0.6 nA; 100 ms; 0.33 Hz; arrowheads) to the same cell. A segment of the record in a (dashed line) is expanded in b. The spontaneous depolarization preceding the burst (pacemaker potential, hollow arrow) is suppressed by the pulse (filled arrow). Plateau depolarization during spontaneous bursting is maintained by ImGluR(V). During action potential firing, intracellular Ca2+ accumulates (Bianchi et al., 1999) leading to K+ current activation (Bianchi et al., 1999). When cumulative K+ current activation during the burst exceeds the amplitude of ImGluR(V), net membrane current becomes outward, causing hyperpolarization, deactivation of ImGluR(V), and burst termination. C, Voltage responses to a hyperpolarizing pulse (inset) recorded in CNQX and CPP (a) and after addition of DHPG (b). Note that DHPG induced depolarization of the membrane potential above threshold for action potential firing. Action potentials are clipped by digitization. Sustained activation of ImGluR(V) maintained the resting membrane potential at −45 mV. ImGluR(V) was turned off by hyperpolarization and was activated upon release of the hyperpolarization (arrow). Thus, non-inactivating ImGluR(V) contributes to the DHPG-induced depolarization of the resting membrane potential. In C and D, responses to pulses are superimposed to baseline records. D, Voltage-clamp records obtained in the same conditions as in C. The net inward current elicited by DHPG following the pulse (Dc; ImGluR(V)) was obtained by subtracting the record in Da from that in Db and was fitted with a single exponential (solid line). Dashed line is the current level extrapolated from the fit at the end of the pulse. Zero current level is indicated for each voltage-clamp record.
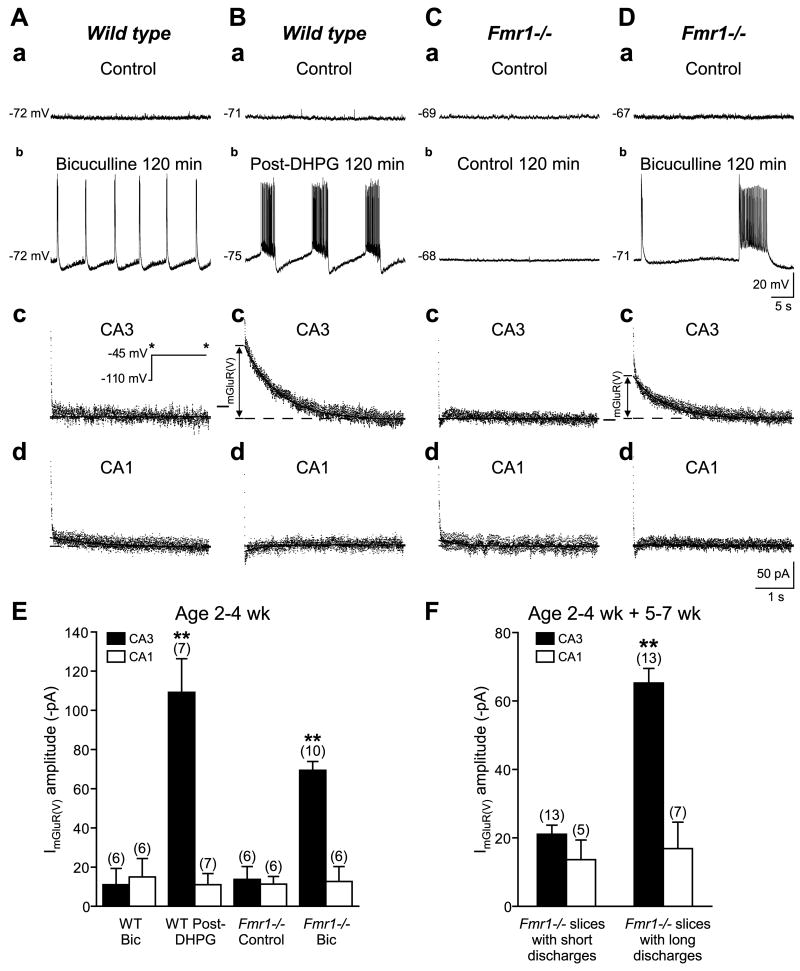
Figure 7.
Agonist- or synaptically-induced ImGluR(V) underlies prolonged epileptiform discharges. A, Intracellular recordings from a CA3 pyramidal cell in control solution (aCSF; a) and 120 min after addition of bicuculline 50 μM (b). The burst firing represent short duration (300–500 ms) synchronized discharges of the CA3 population. The same cell was recorded in CNQX and CPP following the bicuculline treatment (c). ImGluR(V) amplitude at −45 mV, activated following a hyperpolarizing pulse to −110 mV, was measured under voltage-clamp. In this condition, ImGluR(V) was negligible. A CA1 cell in the same slice was recorded under the same condition (d). B, Prolonged epileptiform discharges induced by DHPG persisted following agonist washout (b). Addition of CNQX and CPP blocked the synchronized discharges (c). ImGluR(V) amplitude activated in the same cell was measured under voltage-clamp. A CA1 cell in the same slice was recorded under the same condition (d). C and D, CA3 pyramidal cells from Fmr1−/− mice. C, In aCSF, ImGluR(V) was negligible in both CA3 (c) and CA1 (d) cells. D, Bicuculline induced group I mGluR-mediated prolonged epileptiform discharges (b). In the same cell, recorded in CNQX and CPP to suppress the epileptiform discharges, ImGluR(V) was observed in CA3 (c) but not in CA1 (d) cells. E, Summary data of ImGluR(V) amplitude under the different conditions in (A–Dc,d). Data are from 2–4 week-old (young) mice. Numbers of cells are indicated in parenthesis (** P < 0.01). F, Mean ImGluR(V) amplitude in CA3 and CA1 cells recorded in slices isolated from Fmr1−/− mice that showed interictal (short; < 1.5 s) and prolonged epileptiform (long;≥ 1.5 s) discharges. Data are pooled from young and older (5–7 week) mice.
Data Analysis
The amplitude of ImGluR(V) was obtained from the term ‘a’ in the equation y = y0 + ae−bx of the best fitting function (R > 0.9). Fits and plots were obtained using SigmaPlot 8 (Systat Software, Point Richmond, CA). Average data were expressed as mean ± SEM. Student’s t-test and ANOVA with post-hoc Newman-Keuls test were used for statistical comparisons with significance set at P = 0.05 (GB STAT, Dynamic Microsystems, Silver Spring, MD).
Pharmacological Agents
Agents were stored in stock solutions at −80°C for no more than 1–2 weeks and diluted into the perfusing solution at the indicated final concentrations at the time of the experiments. (S)-dihydroxyphenylglycine (DHPG), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), (RS)-3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP), (S)-a-methyl-4-carboxyphenylglycine (MCPG), 2-Methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP), (S)-(+)-a-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385), and cycloheximide were purchased from Tocris Bioscience (Ellisville, MO). Genistein, 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene (U0126), and 2-amino-3-methoxyflavone (PD98059) were obtained from Calbiochem (San Diego, CA). All the other chemicals were from Sigma-Aldrich (St. Louis, MO). We note that CNQX can have excitatory actions on a subset of interneurons (though not on excitatory neurons; McBain et al., 1992; Brickley et al., 2001; Maccaferri et al., 2002; Hashimoto et al., 2004; see also Menuz et al., 2007). To evaluate possible actions of CNQX on our recorded cells, we measured, in current clamp, membrane potential (Vm) and input resistance (Rin) of 5 CA3 pyramidal cells before (Vm −65.7 ± 1.0 mV; Rin 48.4 ± 8.0 MΩ) and after (Vm −65.8 ± 0.9 mV; Rin 46.3 ± 6.9 MΩ) CNQX and CPP application. The results show that these membrane parameters are not significantly affected by CNQX (Vm, P = 0.49; Rin, P = 0.60).
Results
Cellular responses of CA3 pyramidal cells to DHPG
All data were obtained from intracellular recordings of CA3 pyramidal cells in hippocampal slices isolated from guinea pigs (Figs. 1–6, S1) and from wild type and knockout mice (Figs. 7, S2). Data obtained from mouse preparations are indicated in the figure legends.
Figure 1 shows the effects of the group I mGluR agonist DHPG (50 μM) on CA3 pyramidal cells. To characterize the effect of DHPG on single CA3 pyramidal cells, independent of recurrent synaptic activity, ionotropic glutamate receptor (iGluR) antagonists (CNQX + CPP; 20 μM each) were added to the perfusing solution to suppress excitatory synaptic transmission and to block synchronized discharges. Intracellular recordings show that DHPG application elicited prolonged rhythmic depolarizations (5 – 20 s) with overriding action potentials (prolonged bursts) in single CA3 neurons (Fig. 1Ab,Ba). Prolonged bursts occurred at regular intervals of 15 – 50 s. During the interval, gradual depolarizations (pacemaker potentials) were recorded that reached threshold and elicited another phase of prolonged bursting. Membrane hyperpolarization applied through intracellular current injection during the pacemaker potential reset the membrane potential to a hyperpolarized level (Fig. 1Bb). In addition, hyperpolarization applied during the prolonged burst could arrest the burst and terminate the underlying depolarization (Fig. 1B).
At more depolarized membrane potentials (> −55 mV), DHPG exposure elicited tonic depolarization of CA3 pyramidal cells (Fig. 1Cb). Hyperpolarizing responses elicited by current injection at these membrane potentials (more depolarized than −55 mV) followed a significantly slower time course than that observed in control condition, before the addition of DHPG (cf. Fig. 1Ca with 1Cb). The voltage response is slow presumably because the hyperpolarizing pulse now turned off an inward current, ImGluR(V), that was elicited by DHPG and active at membrane potentials more depolarized than −55 mV (ImGluR(V) threshold: about −55 mV; see Fig. 4Ac). The gradual turn-off of the current, due to the slow deactivation time constant of ImGluR(V) (about 0.54 s at −45 mV; Chuang et al., 2000) contributed to the slower hyperpolarizing response elicited by the current pulse. Upon release of the injected hyperpolarizing current, a fast repolarization of the membrane potential was followed by a secondary, more gradual depolarization (the start of which is indicated by a filled arrow in Fig. 1Cb) to the pre-hyperpolarizing pulse level. The slow depolarization can be compared to the pacemaker potential shown in Fig. 1B.
Figure 4.
Activation properties of long-lasting ImGluR(V). A, Voltage-gated calcium currents were suppressed (a) in a solution containing low Ca2+ (0.2 mM), Mn2+ (1 mM), TTX (1 μM), and Cs+ (5 mM). Under this condition (Control), a linear current response was obtained with a depolarizing ramp from −70 mV to −5 mV (Ab, bottom). With the addition of DHPG, an inward ‘sag’ developed in the current response to the same ramp (b). I–V plots (c) were obtained by subtracting the control current response (blue record) from the indicated responses. The I–V plot provided values for the peak amplitude (arrow) and voltage (Vpeak), threshold (Vthr), and reversal potentials (Vrev) of ImGluR(V). B, Plots of ImGluR(V) amplitude (a), and of Vpeak, Vthr, and Vrev (b), for the current (inset) recorded before (i), during (ii), and after (iii) DHPG application in a CA3 pyramidal cell. C, Summary data from 11 CA3 pyramidal cells.
DHPG responses elicited in CA3 pyramidal cells were examined under voltage-clamp. The time course of the current response was examined following hyperpolarizing pulses (−100 mV; 1.5 s) applied at a holding potential of −45 mV. Termination of the hyperpolarizing pulse was followed by a slowly developing inward current (Fig. 1Db, arrow). The inward current brought the holding current back to the level preceding the hyperpolarizing pulse. The data is consistent with the finding that DHPG activates an inward current (ImGluR(V)) in CA3 pyramidal cells. The current is voltage-sensitive in that its amplitude increases with membrane depolarization (Chuang et al., 2000, 2001). In Fig. 1Db, ImGluR(V) was tonically activated at the holding potential of −45 mV. The hyperpolarizing pulse deactivated ImGluR(V). Immediately following the hyperpolarization pulse, when the membrane potential jumped from −100 to −45 mV, the instantaneous ImGluR(V) conductance remained at the −100 mV level. With time, ImGluR(V) developed, following its activation time constant, to the level appropriate for −45 mV. The gradual activation of ImGluR(V) caused the gradual depolarization indicated by the arrow in the current-clamp recording in Fig. 1Cb. In subsequent experiments, the time course of ImGluR(V) activation from hyperpolarized levels to −45 mV (post-hyperpolarization current response) was used to indicate the amplitude of ImGluR(V) activated in recorded cells (Fig. 1 Dc).
The role of group I mGluR subtypes, mGluR1 and mGluR5, in ImGluR(V) activation was examined using the specific blockers LY367385 and MPEP, respectively. Blockers were added 10 min before DHPG application. Amplitude of ImGluR(V) elicited by DHPG was −116 ± 16 pA in 15 CA3 pyramidal cells. In slices pretreated with LY367385 (100 μM) or MPEP (50 μM), ImGluR(V) amplitude elicited by DHPG was significantly reduced. ImGluR(V) amplitude elicited in the presence of the mGluR1 antagonist was 24% of control value (−28 ± 8 pA; n = 5; P < 0.01). In the presence of the mGluR5 antagonist, ImGluR(V) amplitude was 33% of the control value (−38 ± 11 pA; n = 5; P < 0.05). When both antagonists were applied, ImGluR(V) amplitude was −5 ± 4 pA (n = 5), not significantly different from that measured in control (i.e. without DHPG, −6 ± 3 pA; n = 15; P = 0.97). The data suggest that DHPG elicited ImGluR(V) via activation of both mGluR1 and mGluR5. These results are consistent with a previous report demonstrating that ImGluR(V) elicited by DHPG was significantly attenuated in CA3 neurons from mGluR1 knockout mice (Chuang et al., 2002). Synergistic action of mGluR1 and mGluR5 on the activation of a DHPG-elicited inward current in CA1 pyramidal cells has also been reported (Rae and Irving, 2004).
Persistent activation of ImGluR(V)
To isolate ImGluR(V) from voltage-gated intrinsic Na+ and K+ currents, slices were perfused in tetrodotoxin (TTX; 1 μM) and Cs+ (5 mM). In addition, voltage-clamp recordings were carried out using Cs+-filled (134 mM) pipettes. Following the onset of a whole-cell patch recording using Cs+-containing pipettes, an inward current developed with a time constant in the range of 1.7 – 3.7 min (n = 8; Fig. 2E). Holding currents became stable after about 10 min. DHPG was routinely applied 30 min after the onset of whole-cell recording mode, a time span during which baseline holding current was stable.
Cells were held at −45 mV. DHPG (50 μM; 20 min) elicited an inward current in CA3 pyramidal cells (Fig. 2A). Periodic hyperpolarizing pulses (−75 mV) were applied during DHPG application. The post-hyperpolarization current response was monitored before (control) and during DHPG application. ImGluR(V) amplitude was obtained by subtracting the control post-hyperpolarization current response (Fig. 2A, *) from those obtained in the presence of DHPG (Fig. 2Bb,c).
The amplitudes of the holding current and of peak ImGluR(V) were not altered by DHPG washout (cf. Fig. 2Bc and d). Fig. 2D shows the average peak ImGluR(V) amplitude recorded in 7 cells during DHPG application and followed for 1 h after washout. The data indicate that ImGluR(V) amplitude remained stable throughout the washout period.
Properties of persistent ImGluR(V)
The properties of ImGluR(V) elicited during DHPG exposure and following DHPG washout were examined using step depolarization and ramp depolarization command voltages.
Depolarizing steps from −90 to −35 mV were applied in 15 mV-increments (Fig. 3). The perfusing solution contained TTX and Cs+, and Cs+-filled pipettes were used for whole-cell patch recordings. ImGluR(V) was activated by the depolarizing pulses. The time course of the current during the depolarization (Fig. 3A) was obtained by subtracting the current responses recorded before DHPG (Control) from those recorded during (Fig. 3 Aa) or after (Fig. 3Ab) DHPG exposure. ImGluR(V) was not activated at voltages more hyperpolarized than −65 mV. At −50 mV and above, the current increased in amplitude with increased depolarization. The amplitudes of ImGluR(V) activated by DHPG at different levels of depolarization were not altered by DHPG washout (Fig. 3A,B).
Figure 3.
Long-lasting ImGluR(V) is activated by depolarization. A, ImGluR(V) amplitude activated by depolarizing voltage steps (bottom) recorded during (a) and 45 min after (b) DHPG washout. Records were obtained by subtraction of indicated current responses. TTX (1 μM) and Cs+ (5 mM) were present throughout the recording, carried out with Cs+-filled pipettes. B, Average amplitude of ImGluR(V) activated by depolarizing pulses to the indicated levels during (filled bars) and 45 min after DHPG (hollow bars) in 6 cells. The amplitude of ImGluR(V) was maintained following DHPG washout (* P < 0.01; ** P < 0.05).
To explore ImGluR(V) properties over a more extended membrane potential range, a ramp voltage command from −70 to −5 mV was applied (Fig. 4). As before, intrinsic Na+ current was suppressed by TTX and K+ current by Cs+ in the extracellular and intracellular solutions. In addition, voltage-dependent Ca+ currents were suppressed using a Mn2+ (1 mM)/low Ca2+ (0.2 mM) solution (Mn2+ solution). Fig. 4Aa shows the initial activation of the Ca2+ current by the ramp command and the subsequent gradual suppression of the Ca2+ current during the wash-in of the Mn2+ solution. The Ca2+ current, with peak activation around −10 mV (Fig. 4Ac), was completely suppressed following 23 min of perfusion with the Mn2+ solution. Following the Ca2+ current suppression, responses to the ramp depolarization became linear over the voltage range tested (Fig. 4Aa,b, Control). DHPG elicited non-linear responses with the activation of a U-shaped current profile at depolarized voltages (Fig. 4Ab, DHPG). Subtraction of the control current responses from those elicited in DHPG provided the current-voltage (I–V) relationship and amplitude of the DHPG-activated current (ImGluR(V); Fig. 4Ac). Maximum ImGluR(V) amplitude, its activation threshold, its reversal potential and peak activation voltage were measured from the I–V curve and these parameters were all distinct from those recorded for the Ca2+ current. Fig. 4B,C shows these measures for ImGluR(V) activated in the presence of DHPG and during 1.5 h of washout. The data show that the properties of ImGluR(V) were not altered by DHPG washout, further indicating that long-lasting ImGluR(V) is induced by transient DHPG exposure.
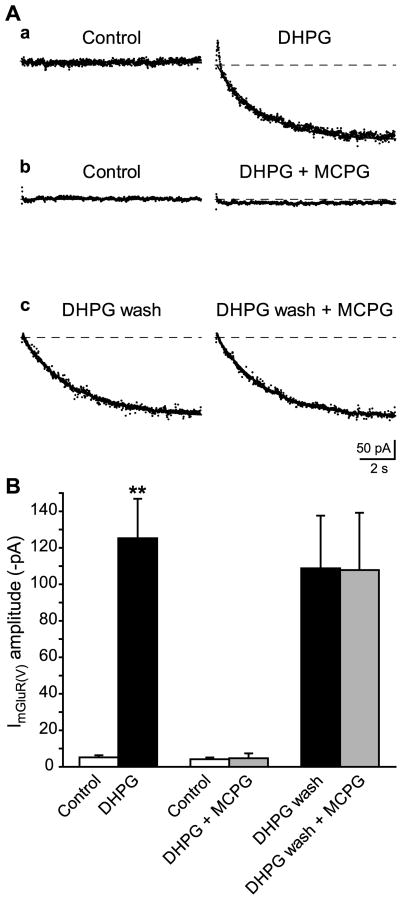
The sensitivity of persistent ImGluR(V) to the competitive group I mGluR antagonists MCPG was examined (Fig. 5). In the first set of experiment, slices were pretreated with MCPG (250 μM) for 10 min. In the presence of MCPG, recording from CA3 pyramidal cells show that application of DHPG (50 μM) did not elicit ImGluR(V) (Fig. 5Ab and B). In a second set of experiment, persistent ImGluR(V) was recorded following DHPG washout (Fig. 5Ac, DHPG wash). MCPG (250 μM) was then introduced into the perfusion solution. The amplitude of persistent ImGluR(V) was not affected by the blocker (Fig. 5Ac and B).
Figure 5.
Long-lasting ImGluR(V) is not blocked by the competitive mGluR antagonist MCPG. A, Currents were elicited by depolarizing steps from −80 to −45 mV. The currents shown were obtained by subtracting the corresponding control (i.e. before DHPG application) traces from the responses recorded in the indicated conditions (a, DHPG: following 5 min application of DHPG 50 μM; b, DHPG + MCPG: following 5 min DHPG 50 μM applied in the presence of MCPG 250 μM; c, DHPG wash: after 30 min of DHPG washout; and c, DHPG wash + MCPG: after application of MCPG 250 μM for 40 min subsequent to DHPG washout, in the same group of cells shown as DHPG wash). As in Fig. 1Dc, subtracted traces were fitted with single exponentials (solid lines). B, Summary data of ImGluR(V) amplitude in the different series of experiments indicated. Significant activation of ImGluR(V) (DHPG −125.2 ± 21.6 pA vs Control −5.2 ± 1.1 pA; n = 6; ** P < 0.01, Student’s paired t-test) was prevented in the presence of MCPG 250 μM (DHPG + MCPG −4.6 ± 2.7 pA, vs Control−4.1 ± 1.0 pA; n = 5; P = 0.90). In contrast, ImGluR(V) persisting after DHPG washout was not affected by the same concentration of the antagonist (DHPG wash + MCPG −107.8 ± 31.5 pA, vs DHPG wash −108.8 ± 28.9 pA; n = 6; P = 0.85).
Signaling mechanisms underlying ImGluR(V) induction
Previous studies showed that the induction of group I mGluR-mediated prolonged epileptiform discharges requires activation of mRNA translation through a tyrosine kinase-ERK1/2 signaling pathway (Zhao et al., 2004; Chuang et al., 2005). We evaluated whether the induction of ImGluR(V) by DHPG also involves a similar signaling mechanism.
Slices were pre-incubated in the tyrosine kinase inhibitor genistein (30 μM) for at least 1 h. Application of DHPG (50 μM; 20 min) under this condition elicited a transient inward current that lasted for a few minutes (3–15 min; n = 5) and faded in the presence of DHPG (Fig. 6A,B). Hyperpolarizing steps applied during the transient inward current elicited inward tail currents (post-hyperpolarization current responses) indicative of ImGluR(V) activation (Fig. 6A,B).
Similar to genistein, pre-incubation of inhibitors of ERK1/2 phosphorylation (PD98059, 50 μM; or U0126, 20 μM) or of protein synthesis (cycloheximide, 60 μM) resulted in the activation of transient inward currents that faded in the presence of DHPG. In addition, inward current responses were not detected following DHPG washout under any of these conditions (Fig. 6C).
These results indicate that the translational protein synthesis mediated via tyrosine kinase-ERK1/2 signaling is involved in ImGluR(V) induction. These data parallel the results showing that induction of DHPG-activated prolonged epileptiform discharges was also suppressed by inhibitors of mRNA translation, tyrosine kinase, or ERK1/2 activation (Zhao et al., 2004; Chuang et al., 2005).
Synaptic induction of ImGluR(V)
While group I mGluR-mediated prolonged epileptiform discharges are activated by the agonist DHPG in the wild type preparation, stimulation of the glutamatergic recurrent synapses is ineffective in activating the group I mGluR-mediated prolonged discharges (Lee et al., 2002). In contrast, group I mGluR-mediated prolonged discharges are synaptically induced in FXS mouse model preparations (Chuang et al., 2005). We examined whether synaptic stimulation was also effective in activating ImGluR(V) in the FXS preparations.
Experiments were first carried out in the wild type preparations to measure ImGluR(V) activation in association with the long-lasting DHPG-induced discharges. Persistent group I mGluR-mediated discharges were recorded for at least 2 h after DHPG washout (Fig. 7Bb). Antagonists of iGluRs were then added to the perfusing solution to suppress synchronized prolonged discharges and to facilitate voltage-clamp studies of individual CA3 or CA1 pyramidal cells. Hyperpolarizing pulses to −110 mV from a holding potential of −45 mV were used to measure ImGluR(V) activation. Post-hyperpolarization current responses (Fig. 7Bc) were elicited in 7 CA3 cells recorded from 6 slices that exhibited group I mGluR-mediated prolonged discharges prior to the addition of iGluR blockers (Fig. 7E). Under the same condition, post-hyperpolarization current responses were not detected in CA1 neurons recorded in the same slices (Fig. 7Bd,E). The latter result is consistent with previous findings that ImGluR(V) can only be elicited in CA3, but not in CA1, pyramidal cells (Chuang et al., 2002).
Addition of bicuculline in wild type preparations elicited short duration, interictal-like synchronized discharges in the pyramidal cell population (Fig. 7Ab). Each interictal-like discharge is sustained by the simultaneous activation of recurrent glutamatergic synapses between CA3 pyramidal cells (Traub and Wong, 1982). Voltage-clamp studies carried out in CA3 or CA1 pyramidal cells following at least 2 h of interictal-like discharges showed absence of the post-hyperpolarization current responses (Fig. 7Ac,d), i.e. hyperpolarizing pulses did not elicit detectable inward current tails in either CA3 or CA1 cells (Fig. 7E).
In another set of experiments, slices from 2–4 week-old FXS (Fmr1−/−) mice were treated with bicuculline. Interictal-like discharges (~500 ms) were first detected, as observed in the wild type slices. Following a period (25 – 60 min) of interictal discharge activity, prolonged (> 1.5 s) discharges appeared in Fmr1−/− slices (Fig. 7Db). Slices exhibiting group I mGluR-mediated prolonged discharges (Fig. 7Db) were then perfused in a solution containing iGluR blockers which suppressed the prolonged discharges. Measurement of post-hyperpolarization current responses showed that ImGluR(V) amplitudes recorded in CA3 neurons of Fmr1−/− slices that displayed prolonged discharges (Fig. 7Dc,E) were significantly larger than those recorded in CA3 neurons from Fmr1−/− slices that were not treated with bicuculline and did not display epileptiform discharges (Fig. 7Cc,E). ImGluR(V) was essentially not detected in CA1 neurons of Fmr1−/− slices either treated with bicuculline (Fig. 7Dd,E) or in control conditions (Fig. 7Cd,E).
The data above indicated that extended exposure to bicuculline induced group I mGluR-mediated prolonged epileptiform discharges in all slices isolated from 2–4 week-old Fmr1−/− mice (7 out of 7 slices; Fig. 7D,E). Additional experiments were carried out to examine Fmr1−/− preparations from older (5–7 week) mice. These preparations exhibited a decreased susceptibility to bicuculline-induced epileptiform discharge prolongation (this data parallel the in vivo finding that susceptibility for audiogenic seizure peaked in younger, 2–4 week-old, FXS mice; Yan et al., 2005). In older preparations, prolonged (≥ 1.5 s) discharges were elicited in 4 out of 12 slices, whereas short (< 1.5 s), interictal-like discharges continued in the remaining 8 slices during bicuculline exposures of up to 120 min. In CA3 neurons of Fmr1−/− slices that displayed prolonged discharges, ImGluR(V) amplitude was significantly larger than the current recorded in CA3 neurons from Fmr1−/− slices that only exhibited interictal-like discharges (Fig. 7F). Thus, significant ImGluR(V) was induced in all CA3 cells from slices that exhibited prolonged discharges in both age groups, whereas negligible ImGluR(V) was recorded in CA1 neurons of both groups of slices (Fig. 7F).
Discussion
The data of the present study point to ImGluR(V) – a depolarization-activated inward current – as the cellular mechanism for group I mGluR-mediated epileptogenesis. To define a cellular event as a mechanism for epileptogenesis, at least three criteria must be satisfied: (i) the emergence of a form of epileptiform discharges is associated with the activation of this event; (ii) the cellular event, once induced, must be long-lasting to sustain persistent epileptiform discharges; (iii) the cellular event must be activatable by integrative activities of the neuronal circuit and must serve as a candidate for a form of epilepsy. Our data show that ImGluR(V) activation satisfies all these criteria. The role of ImGluR(V) in prolonged epileptiform discharges can be further defined by showing that blockade of ImGluR(V) also suppresses the epileptiform discharges, as indicated by our initial data (Fig. S2). In addition, it remains to be determined whether ImGluR(V) activation alone is sufficient to sustain this epileptiform activity. In this case, specific induction of ImGluR(V) alone would be sufficient to sustain epileptiform discharges.
Emergence of group I mGluR-mediated epileptiform discharges is associated with ImGluR(V) activation
In addition to ImGluR(V) activation, DHPG also increases the cell’s input resistance. In PLCβ1−/− preparations, DHPG only increased the input resistance, but did not elicit ImGluR(V). In the absence of ImGluR(V), prolonged epileptiform discharges were also not generated in PLCβ1−/− preparations (Chuang et al., 2001). Incorporation of ImGluR(V) as an intrinsic current in CA3 pyramidal cells modified the firing pattern of single cells from spontaneous action potential firing to rhythmic prolonged periods of firing of up to 10 s (Fig. 1). Prolonged epileptiform discharges differ from single cell firing in that the former consist of synchronized firing of the whole population activity. In our studies on single cell properties, prolonged epileptiform discharges were desynchronized by applying iGluR blockers (Fig. 1). The data indicate that, once prolonged single cell firing was elicited by DHPG, prolonged epileptiform discharges were generated via synaptic excitation mediated by iGluRs, which synchronized the activity of the CA3 pyramidal cell population. The role of iGluR-dependent recurrent excitation between CA3 pyramidal cells in the generation of short epileptiform activities resembling interictal spikes has been well described (Traub and Wong, 1982). By prolonging single cell firing, ImGluR(V) provided the basic activity pattern which, when synchronized, produced the prolonged epileptiform discharges. ImGluR(V) is an example of a transmitter-activated inward current that underlies intrinsic rhythmic bursting and contributes to network activities (Swensen and Marder, 2001).
Previous studies show that DHPG-induced prolonged epileptiform discharges can be suppressed by intervention on the signaling process downstream from group I mGluR activation. Specifically, inhibitors of tyrosine kinase, ERK1/2, and mRNA translation are each effective in preventing the induction of prolonged epileptiform discharges (Merlin et al., 1998; Zhao et al., 2004). We now show that each of these inhibitors also prevented the induction of ImGluR(V) in CA3 pyramidal cells upon DHPG stimulation. In the presence of these blockers, DHPG elicited a transient inward current response which faded even in the presence of the agonist (Fig. 6C). Other long-lasting DHPG-elicited responses – such as long-term synaptic depression (LTD) and persistent spike afterhyperpolarization suppression – are also transiently activated when protein synthesis is inhibited (Huber et al., 2000; Young et al., 2008). Apparently, protein synthesis is only required to maintain the persistency of the responses.
The association of ImGluR(V) with prolonged epileptiform discharges is further indicated by our initial results (Fig. S2): lidocaine blocked the generation of prolonged epileptiform discharges, presumably via a specific suppression of ImGluR(V), suggesting a direct role of ImGluR(V) in prolonged epileptiform discharge generation.
ImGluR(V), once induced, is long-lasting
The fact that DHPG-induced prolonged epileptiform discharges are long-lasting and the finding that ImGluR(V) plays a fundamental role in patterning prolonged epileptiform discharges suggest that long-lasting ImGluR(V) occurs to sustain the prolonged epileptiform discharges. The data show that ImGluR(V), once induced in CA3 pyramidal cells, became a component of the intrinsic current which defined the cell’s basic membrane properties. Activation of ImGluR(V) following its induction was solely determined by trans-membrane voltage and was dissociated from group I mGluR activation. Figs. 2 and 4 show that the amplitude of ImGluR(V) remained undiminished over hours of washout. Prolonged epileptiform discharges induced by DHPG are also maintained for hours following agonist application (Merlin and Wong, 1997). These robust plastic changes induced by DHPG are those that define the DHPG responses as ‘epileptogenic’ and ImGluR(V) as a mechanism associated with epileptogenesis.
Persistency of the plastic change brings into question as to why ImGluR(V) was not detectable in CA3 cells before DHPG stimulation, especially in view of data showing that group I mGluRs are stimulated by synaptic activities (Fig. 6Dc). The data show that induction of ImGluR(V) – similar to that of the group I mGluR-mediated prolonged epileptiform discharges (Chuang et al., 2005) – is tightly controlled by the repressive action of FMRP, such that normal synaptic stimulation of group I mGluRs is ineffective in activating the mRNA translation necessary for ImGluR(V) induction (Fig. 8).
Figure 8.
Schematic representation of the effects of group I mGluR stimulation in hippocampal CA3 pyramidal cells. Group I mGluRs activated by DHPG and by synaptic glutamate are depicted separately for clarity. DHPG is likely to activate extra-synaptic and synaptic group I mGluRs. Model of persistent activation of ImGluR(V) – DHPG action (left side of the scheme). DHPG activates ImGluR(V) via stimulation of mGluR1 and mGluR5 through phospholipase C β1 (PLCβ1)-mediated signaling (Chuang et al., 2001). Stimulation of group I mGluRs also activates protein synthesis via Src tyrosine kinase-ERK1/2 signaling-dependent mRNA translation. The group I mGluR-mediated protein synthesis is required to induce long-lasting ImGluR(V) (thick arrow). FMRP regulation of synaptic group I mGluR-mediated mRNA translation – Synaptic glutamate (right side of the scheme). Synaptic stimulation of group I mGluRs is elicited by spontaneous synchronized discharges of CA3 pyramidal cells in the presence of bicuculline (Chuang et al., 2005). Prolonged periods of synaptic stimulation of group I mGluRs induced persistent ImGluR(V) in Fmr1−/− preparations, whereas similar pattern of stimulation was ineffective in ImGluR(V) activation in wild type preparations. Presumably, ImGluR(V) induction is prevented by FMRP in the wild type preparation. Whereas synaptic induction of ImGluR(V) is prevented in the wild type by FMRP, we note that DHPG is effective in the induction of ImGluR(V) in the same preparation. There are at least two possible explanations for the difference in synaptic- vs agonist-induced responses in the wild type preparation. (1) Only synaptic group I mGluRs are regulated by FMRP, whereas DHPG activates also non-synaptic group I mGluRs which are not subject to FMRP regulation. Evidence for the inhibitory role of FMRP on synaptically-activated prolonged epileptiform discharges is from data in Fig. 7, showing that synaptic activation of group I mGluRs can elicit epileptogenesis in the absence of FMRP (Fmr1−/−preparations) but not in the presence of functional FMRP (wild type preparations). (2) Stimulation of group I mGluRs by DHPG leads to down-regulation of FMRP function, possibly via activation of the ubiquitin-proteasome system (Hou et al., 2006), whereas synaptic stimulation does not affect FMRP levels.
ImGluR(V) is activated by synchronized synaptic activity and is implicated in epilepsy associated with fragile X syndrome
The relevance of experiments showing DHPG-induced epileptiform discharges to the study of epileptogenesis is indicated by the data obtained in the FXS model mouse. Here, prolonged epileptiform activity was induced by synaptically-released glutamate. Furthermore, it has been shown that enhanced audiogenic seizures in FXS mice is prevented by specific mGluR5 antagonism (Yan et al., 2005). To examine whether ImGluR(V) could be induced synaptically in a CA3 pyramidal cell, we studied the effect of simultaneous activation of all the recurrent synapses of neighboring CA3 pyramidal cells onto that cell by taking advantage of a property of the CA3 circuit. CA3 pyramidal cells are densely interconnected via glutamatergic synapses. One CA3 pyramidal cell can receive as many as 650 synaptic inputs from neighboring cells (Wittner and Miles, 2007). The recurrent connections are sufficiently strong that firing in one neuron activates a few postsynaptic cells and, in the absence of inhibitory control (such as in the presence of bicuculline), cell firing spreads through the entire CA3 population within ~200 ms and results in a synchronized discharge (Miles and Wong, 1983). Within a synchronized discharge, each pyramidal cell is stimulated by the recurrent synapses from all the projecting presynaptic cells (Traub and Wong, 1982). Each synaptic input also activates both postsynaptic iGluRs and group I mGluRs. The data from wild type preparation show that synaptic activation of group I mGluRs (without added DHPG) did not elicit ImGluR(V) (Fig. 6Bc). In contrast, in the FXS model mouse, synaptic stimulation in a similar manner elicited prolonged epileptiform discharges in a majority of the preparations (Fig. 6Db) and prominent ImGluR(V) in CA3 pyramidal cells of preparations exhibiting prolonged epileptiform discharges (Fig. 6Dc). The data is consistent with previous findings suggesting that group I mGluR-dependent responses are exaggerated in FXS preparations because of the absence of the repressive control by FMRP on the group I mGluR-stimulated mRNA translation (Fig. 8; Bear et al., 2004).
Although ImGluR(V) was elicited by the simultaneous activation of recurrent synapses during a synchronized discharge, our data also reveal that the current was not detectable in CA3 pyramidal cells of the FXS preparation in the absence of such synchronized discharges (Fig. 6F). The simplest explanation for this observation is that, in the absence of synchronized discharges, the amplitude of ImGluR(V) elicited in a CA3 pyramidal cell by the stochastic firing of the presynaptic population was small and was not detected by our somatic recording. Only with simultaneous synaptic activation of the entire population, ImGluR(V) became sufficiently large that it could be detected at the somatic site. Thus, in this extreme case, the consequence of a synaptically-induced plastic change was no longer confined to the specific synapse, but was extended to affect the response of the entire neuron. Our data indicate that the induction of ImGluR(V), as a form of synaptic plasticity mechanism underlying epileptogenesis, is not a synapse-specific event, but rather leads to modifications that cause global changes in the neuron properties.
The exaggerated group I mGluR-mediated responses recorded in the FXS preparation is paralleled by the propensity for epileptogenesis in FXS model mice. FXS model mice showed increased seizure vulnerability in response to audio stimuli (Musumeci et al., 2000; Chen and Toth, 2001). This form of audiogenic seizures is group I mGluR-mediated as it is suppressed by the group I mGluR subtype blocker MPEP (Yan et al., 2005). In addition, audiogenic seizure susceptibility shows an age-dependency with vulnerability peaking at 20–21 days of age (Yan et al., 2005). Our data show a similar age-dependent pattern in the efficacy of synaptic induction of ImGluR(V) in the in vitro preparation (Fig. 6E,F). Finally, experimental data in vitro and in vivo strongly suggest that, in the absence of FMRP regulation, age-dependent increased propensity for epilepsy in FXS patient (Wisniewski et al., 1991) may be caused by dysregulation of group I mGluR-stimulated mRNA translation. Accordingly, activation of ImGluR(V) may constitute a cellular mechanism for epileptogenesis in FXS patients.
Supplementary Material
Activation properties of ImGluR(V) recorded with K+-filled pipettes. Voltage-clamp recordings were obtained with 119 mM potassium gluconate- filled pipettes from CA3 pyramidal cells perfused with a solution containing low Ca2+ (0.2 mM), Mn2+ (1 mM), TTX (1 μM), and Cs+ (5 mM). A, I–V plots of currents obtained with depolarizing ramps from −70 mV to −5 mV and following subtraction of control responses from those recorded before DHPG (black record) and after DHPG (red record). B, ImGluR(V) peak amplitude (a, K+: −188.1 ± 30.2 pA; n = 8; Cs+: −190.3 ± 28.2 pA; n = 11), peak voltage (b, Vpeak, K+: −28.0 ± 1.7 mV; Cs+: − 28.9 ± 1.0 mV), threshold (b, Vthr, K+: −54.8 ± 2.1 mV; Cs+: −54.5 ± 1.6 mV), and reversal potentials (b, Vrev, K+: − 13.1 ± 1.9 mV; Cs+: −15.7 ± 1.6 mV) were not different from those measured from recordings obtained with Cs+-filled pipettes.
Lidocaine 10 μM blocked ImGluR(V) and the prolonged epileptiform discharges. Data obtained from wild type mouse preparations. Traces in Aa and Ba are responses to depolarizing steps from −75 to −45 mV, shown after subtraction of the respective control responses. A, Inclusion of QX-314 (5 mM; an impermeant lidocaine-derivative) in the recording pipette prevented activation of ImGluR(V) by DHPG. The mean response amplitude before DHPG was −13.2 ± 6.1 pA (b, white bar) and after 3–6 min DHPG was −17.1 ± 7.6 pA (b, black bar; n = 5; P = 0.24). B, DHPG-induced ImGluR(V) was blocked by 10 μM lidocaine. ImGluR(V) after 20–30 min of DHPG was −118.2 ± 4.1 pA (b, black bar) and after 15–40 min lidocaine was −26.4 ± 4.3 pA (b, gray bar; n = 5; *** P < 0.001). C, Lidocaine (10 μM; DHPG + Lidocaine, 45 min) suppressed DHPG-induced prolonged epileptiform discharges (a). In 4 experiments, the duration of prolonged discharges was significantly shortened from a range of 2.8 – 4.6 s (b, DHPG, black symbols) to a range of 0.21 – 1.2 s (b, DHPG + Lidocaine, gray symbols; in all cases, *** P < 0.001, Student’s paired t-test). After lidocaine application, interictal, short duration bursts persisted, similar to previous data indicating that DHPG-induced short duration bursts are resistant to blockers of Src tyrosine kinase, ERK1/2, and mRNA translation (Zhao et al., 2004). D, Short synchronized bursts induced by 50 μM bicuculline (a, Bicuculline, 25 min) and recorded after addition of lidocaine 10 μM to the perfusing solution (a, Bicuculline + Lidocaine, 45 min). Lidocaine 10 μM did not significantly affect the duration of short synchronized bursts induced by bicuculline (b, Bicuculline: range, 0.37 – 0.61 s; Bicuculline + Lidocaine: range, 0.37 – 0.63 s). Other studies showed that lidocaine, at this concentration, did not affect the neuronal firing properties (Raley-Susman et al., 2001).
Acknowledgments
This work has been supported by the FRAXA Research Foundation and the NIH grant NS35481.
References
- Aschrafi A, Cunningham BA, Edelman GM, Vanderklish PW. The fragile X mental retardation protein and group I metabotropic glutamate receptors regulate levels of mRNA granules in brain. Proc Natl Acad Sci U S A. 2005;102:2180–2185. doi: 10.1073/pnas.0409803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Wong RKS. Excitatory synaptic potentials dependent on metabotropic glutamate receptor activation in guinea-pig hippocampal pyramidal cells. J Physiol. 1995;487 (Pt 3):663–676. doi: 10.1113/jphysiol.1995.sp020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi R, Young SR, Wong RKS. Group I mGluR activation causes voltage-dependent and -independent Ca2+ rises in hippocampal pyramidal cells. J Neurophysiol. 1999;81:2903–2913. doi: 10.1152/jn.1999.81.6.2903. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Chuang SC, Wong RKS. Pharmacology of a slowly inactivating outward current in hippocampal CA3 pyramidal neurons. J Neurophysiol. 2006;96:1116–1123. doi: 10.1152/jn.00465.2006. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Farrant M, Swanson GT, Cull-Candy SG. CNQX increases GABA-mediated synaptic transmission in the cerebellum by an AMPA/kainate receptor-independent mechanism. Neuropharmacology. 2001;41:730–736. doi: 10.1016/s0028-3908(01)00135-6. [DOI] [PubMed] [Google Scholar]
- Chen L, Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103:1043–1050. doi: 10.1016/s0306-4522(01)00036-7. [DOI] [PubMed] [Google Scholar]
- Chuang SC, Bianchi R, Wong RKS. Group I mGluR activation turns on a voltage-gated inward current in hippocampal pyramidal cells. J Neurophysiol. 2000;83:2844–2853. doi: 10.1152/jn.2000.83.5.2844. [DOI] [PubMed] [Google Scholar]
- Chuang SC, Bianchi R, Kim D, Shin HS, Wong RKS. Group I metabotropic glutamate receptors elicit epileptiform discharges in the hippocampus through PLCβ1 signaling. J Neurosci. 2001;21:6387–6394. doi: 10.1523/JNEUROSCI.21-16-06387.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SC, Zhao W, Young SR, Conquet F, Bianchi R, Wong RKS. Activation of group I mGluRs elicits different responses in murine CA1 and CA3 pyramidal cells. J Physiol. 2002;541:113–121. doi: 10.1113/jphysiol.2001.013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RKS. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci. 2005;25:8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Miyakawa H, Kudo Y, Inoue M. 6-Cyano-7-nitroquinoxaline-2,3- dione (CNQX) increases GABAA receptor-mediated spontaneous postsynaptic currents in the dentate granule cells of rat hippocampal slices. Neurosci Lett. 2004;358:33–36. doi: 10.1016/j.neulet.2003.12.083. [DOI] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Wong RKS, Chuang SC, Shin HS, Bianchi R. Role of synaptic metabotropic glutamate receptors in epileptiform discharges in hippocampal slices. J Neurophysiol. 2002;88:1625–1633. doi: 10.1152/jn.2002.88.4.1625. [DOI] [PubMed] [Google Scholar]
- Lieb JP, Engel J, Jr, Gevins A, Crandal PH. Surface and deep EEG correlates of surgical outcome in temporal lobe epilepsy. Epilepsia. 1981;22:515–538. doi: 10.1111/j.1528-1157.1981.tb04124.x. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Dingledine R. Complex effects of CNQX on CA1 interneurons of the developing rat hippocampus. Neuropharmacology. 2002;43:523–529. doi: 10.1016/s0028-3908(02)00161-2. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Eaton JV, Brown T, Dingledine R. CNQX increases spontaneous inhibitory input to CA3 pyramidal neurones in neonatal rat hippocampal slices. Brain Res. 1992;592:255–260. doi: 10.1016/0006-8993(92)91683-6. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE. 2006;2006:re12. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- Menuz K, Stroud RM, Nicoll RA, Hays FA. TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists. Science. 2007;318:815–817. doi: 10.1126/science.1146317. [DOI] [PubMed] [Google Scholar]
- Merlin LR, Wong RKS. Role of group I metabotropic glutamate receptors in the patterning of epileptiform activities in vitro. J Neurophysiol. 1997;78:539–544. doi: 10.1152/jn.1997.78.1.539. [DOI] [PubMed] [Google Scholar]
- Merlin LR, Bergold PJ, Wong RKS. Requirement of protein synthesis for group I mGluR-mediated induction of epileptiform discharges. J Neurophysiol. 1998;80:989–993. doi: 10.1152/jn.1998.80.2.989. [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RKS. Single neurones can initiate synchronized population discharge in the hippocampus. Nature. 1983;306:371–373. doi: 10.1038/306371a0. [DOI] [PubMed] [Google Scholar]
- Musumeci SA, Bosco P, Calabrese G, Bakker C, De Sarro GB, Elia M, Ferri R, Oostra BA. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia. 2000;41:19–23. doi: 10.1111/j.1528-1157.2000.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- Rae MG, Irving AJ. Both mGluR1 and mGluR5 mediate Ca2+ release and inward currents in hippocampal CA1 pyramidal neurons. Neuropharmacology. 2004;46:1057–1069. doi: 10.1016/j.neuropharm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Raley-Susman KM, Kass IS, Cottrell JE, Newman RB, Chambers G, Wang J. Sodium influx blockade and hypoxic damage to CA1 pyramidal neurons in rat hippocampal slices. J Neurophysiol. 2001;86:2715–2726. doi: 10.1152/jn.2001.86.6.2715. [DOI] [PubMed] [Google Scholar]
- Swensen AM, Marder E. Modulators with convergent cellular actions elicit distinct circuit outputs. J Neurosci. 2001;21:4050–4058. doi: 10.1523/JNEUROSCI.21-11-04050.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GW, Merlin LR, Wong RKS. Synchronized oscillations in hippocampal CA3 neurons induced by metabotropic glutamate receptor activation. J Neurosci. 1995;15:8039–8052. doi: 10.1523/JNEUROSCI.15-12-08039.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Wong RKS. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982;216:745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci U S A. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski KE, Segan SM, Miezejeski CM, Sersen EA, Rudelli RD. The Fra(X) syndrome: neurological, electrophysiological, and neuropathological abnormalities. Am J Med Genet. 1991;38:476–480. doi: 10.1002/ajmg.1320380267. [DOI] [PubMed] [Google Scholar]
- Wittner L, Miles R. Factors defining a pacemaker region for synchrony in the hippocampus. J Physiol. 2007;584:867–883. doi: 10.1113/jphysiol.2007.138131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RKS, Chuang SC, Bianchi R. Plasticity mechanisms underlying mGluR-induced epileptogenesis. Adv Exp Med Biol. 2004;548:69–75. doi: 10.1007/978-1-4757-6376-8_5. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Young SR, Bianchi R, Wong RKS. Signaling mechanisms underlying group I mGluR-induced persistent AHP suppression in CA3 hippocampal neurons. J Neurophysiol. 2008;99:1105–1118. doi: 10.1152/jn.00435.2007. [DOI] [PubMed] [Google Scholar]
- Zhao W, Bianchi R, Wang M, Wong RKS. Extracellular signal-regulated kinase 1/2 is required for the induction of group I metabotropic glutamate receptor-mediated epileptiform discharges. J Neurosci. 2004;24:76–84. doi: 10.1523/JNEUROSCI.4515-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Activation properties of ImGluR(V) recorded with K+-filled pipettes. Voltage-clamp recordings were obtained with 119 mM potassium gluconate- filled pipettes from CA3 pyramidal cells perfused with a solution containing low Ca2+ (0.2 mM), Mn2+ (1 mM), TTX (1 μM), and Cs+ (5 mM). A, I–V plots of currents obtained with depolarizing ramps from −70 mV to −5 mV and following subtraction of control responses from those recorded before DHPG (black record) and after DHPG (red record). B, ImGluR(V) peak amplitude (a, K+: −188.1 ± 30.2 pA; n = 8; Cs+: −190.3 ± 28.2 pA; n = 11), peak voltage (b, Vpeak, K+: −28.0 ± 1.7 mV; Cs+: − 28.9 ± 1.0 mV), threshold (b, Vthr, K+: −54.8 ± 2.1 mV; Cs+: −54.5 ± 1.6 mV), and reversal potentials (b, Vrev, K+: − 13.1 ± 1.9 mV; Cs+: −15.7 ± 1.6 mV) were not different from those measured from recordings obtained with Cs+-filled pipettes.
Lidocaine 10 μM blocked ImGluR(V) and the prolonged epileptiform discharges. Data obtained from wild type mouse preparations. Traces in Aa and Ba are responses to depolarizing steps from −75 to −45 mV, shown after subtraction of the respective control responses. A, Inclusion of QX-314 (5 mM; an impermeant lidocaine-derivative) in the recording pipette prevented activation of ImGluR(V) by DHPG. The mean response amplitude before DHPG was −13.2 ± 6.1 pA (b, white bar) and after 3–6 min DHPG was −17.1 ± 7.6 pA (b, black bar; n = 5; P = 0.24). B, DHPG-induced ImGluR(V) was blocked by 10 μM lidocaine. ImGluR(V) after 20–30 min of DHPG was −118.2 ± 4.1 pA (b, black bar) and after 15–40 min lidocaine was −26.4 ± 4.3 pA (b, gray bar; n = 5; *** P < 0.001). C, Lidocaine (10 μM; DHPG + Lidocaine, 45 min) suppressed DHPG-induced prolonged epileptiform discharges (a). In 4 experiments, the duration of prolonged discharges was significantly shortened from a range of 2.8 – 4.6 s (b, DHPG, black symbols) to a range of 0.21 – 1.2 s (b, DHPG + Lidocaine, gray symbols; in all cases, *** P < 0.001, Student’s paired t-test). After lidocaine application, interictal, short duration bursts persisted, similar to previous data indicating that DHPG-induced short duration bursts are resistant to blockers of Src tyrosine kinase, ERK1/2, and mRNA translation (Zhao et al., 2004). D, Short synchronized bursts induced by 50 μM bicuculline (a, Bicuculline, 25 min) and recorded after addition of lidocaine 10 μM to the perfusing solution (a, Bicuculline + Lidocaine, 45 min). Lidocaine 10 μM did not significantly affect the duration of short synchronized bursts induced by bicuculline (b, Bicuculline: range, 0.37 – 0.61 s; Bicuculline + Lidocaine: range, 0.37 – 0.63 s). Other studies showed that lidocaine, at this concentration, did not affect the neuronal firing properties (Raley-Susman et al., 2001).