Abstract
Aim
To conduct a systematic review and meta-analysis to understand the timing and factors associated with anti-programmed cell death protein-1 (PD-1)/anti-programmed cell death protein-1 ligand (PD-L1) inhibitor-induced Type 1 diabetes.
Methods
We searched MEDLINE, EMBASE, SCOPUS and Cochrane databases (August 2000–2018) for studies of any design on immune checkpoint inhibitors. A total of 71 cases were reviewed from 56 publications. Comparisons were made using Fisher’s exact and Student’s t-tests.
Results
The mean ± sd age at Type 1 diabetes presentation was 61.7±12.2 years, 55% of cases were in men, and melanoma (53.5%) was the most frequent cancer. The median time to Type 1 diabetes onset was 49 (5–448) days with ketoacidosis in 76% of cases. The average ± sd HbA1c concentration was 62 ± 0.3 mmol/mol (7.84±1.0%) at presentation. All cases had insulin deficiency and required permanent exogenous insulin treatment. Half of the cases had Type 1 diabetes-associated antibodies at presentation, and those with antibodies had a more rapid onset (P=0.005) and higher incidence of diabetic ketoacidosis (P=0.02) compared to people without antibodies.
Conclusions
Many people developed Type 1 diabetes within 3 months of initial PD-1/PD-L1 inhibitor exposure. People presenting with Type 1 diabetes-associated antibodies had a more rapid onset and higher incidence of ketoacidosis than those without antibodies. Healthcare providers caring for people receiving these state-of-the-art therapies need to be aware of this potential severe adverse event.
Introduction
Immunotherapy with drugs that block immune checkpoints has emerged as a beneficial treatment for advanced stage cancers. Immune checkpoints include cytotoxic T-lymphocyte antigen 4 (CTLA-4) and its receptors CD80/86, along with programmed cell death protein 1 (PD-1) and its ligand (PD-L1) [1–5]. CTLA-4 and PD-1 are expressed on the surface of activated T lymphocytes and engage their receptors on antigen-presenting cells and various tumours [6]. Upon activation, lymphocytes are rendered unresponsive. Immune checkpoint blockade inhibits negative immune regulation, allowing an immune response directed towards cancer cells.
Monoclonal antibodies directed against CTLA-4, PD-1 and PD-L1 are used in the treatment of many advanced cancers, including melanoma, lung cancer, renal cancer, head and neck cancers, urothelial cancers and others [1]. The clinically approved CTLA-4 inhibitor is ipilimumab, while current PD-1 inhibitors are nivolumab and pembrolizumab, and PD-L1 inhibitors include atezolizumab, avelumab and durvalumab. Inhibition of immune checkpoints can also cause immune-related side effects, however, especially autoimmunity directed towards self-tissues [7]. One such side effect is Type 1 diabetes, which is rare but can present with life-threatening diabetic ketoacidosis and is predominantly linked to anti-PD-1/PD-L1 therapies [8–12]. Type 1 diabetes is now listed as a treatment-related adverse reaction in the prescribing labels for nivolumab, pembrolizumab, avelumab and durvalumab [13–16].
The factors associated with PD-1/PD-L1 inhibitor-induced Type 1 diabetes are not well defined as the incidence is estimated at ~1% from case series at large academic medical centres (27/2960, 0.9%) [17] and the prescribing label of nivolumab reporting 17/1994 (0.9%) [15]. This represents a limited number of new-onset Type 1 diabetes cases following anti-PD-1/PD-L1 therapy; however, the events are clinically significant. To prevent significant morbidity and mortality related to diabetic ketoacidosis in people with advanced cancers, an understanding of the clinical and laboratory measurements surrounding this immune-related adverse event from a larger number of cases is needed. We conducted a systematic review of published cases and case series of new-onset PD-1/PD-L1 inhibitor-induced Type 1 diabetes to analyse time to diabetes onset, presentation with diabetic ketoacidosis, Type 1 diabetes-associated antibodies and other metabolic measurements.
Methods
Data sources and searches
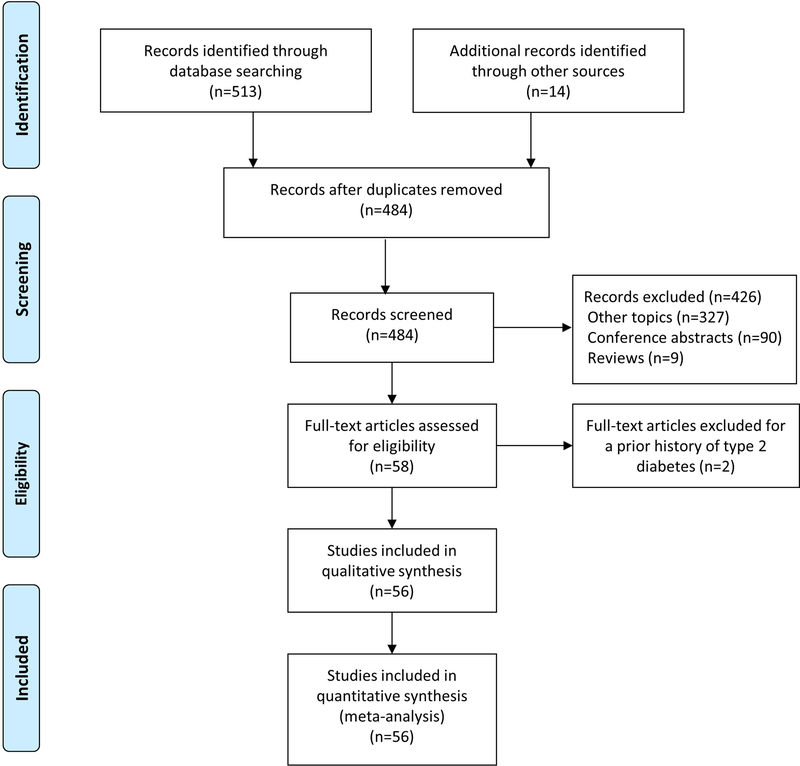
A systematic review and meta-analysis was conducted to answer the clinical question: what are the timing and factors associated with diabetes onset after treatment with immune checkpoint inhibitors? A comprehensive search of databases (Medline In-Process and Other Non-Indexed Citations, MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Scopus) for papers published from 1 August 2000 to 14 August 2018, in English, was conducted based on the terms ‘diabetes’ and ‘immune checkpoint inhibitors’ (Table S1). References in the case reports and series were manually reviewed for additional cases. We contacted one author for missing information (agents administered to patients) from one case series [10]. A total of 71 cases were identified from a total of 56 publications (see references in Supporting Information). A flow diagram for the study is provided in Fig. 1.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram of the study. A total of 56 publications were selected, which resulted in 71 cases of new-onset Type 1 diabetes following immune checkpoint blockade.
Study selection
Inclusion criteria
We included all published case reports and series that reported new-onset diabetes after PD-1/PD-L1 inhibitor therapy. We used the American Diabetes Association criteria for the diagnosis of diabetes and diabetic ketoacidosis including grading for severity (severe, moderate and mild) [18,19]. These data included blood glucose, HbA1c, and other related laboratory measures. Blood glucose and HbA1c levels before exposure to PD-1/PD-L1 were used to define new-onset vs existing diabetes. We collected data regarding serum electrolytes to calculate an anion gap, arterial blood gas, and urine/blood ketone positivity to determine the presentation and severity of diabetic ketoacidosis (Table S2). C-peptide levels were documented as absent or inappropriately low for the concomitant blood glucose. Amylase and lipase levels were extracted to determine exocrine pancreas involvement. Type 1 diabetes-associated antibodies were considered positive if an individual was reported as being positive for these antibodies. All cases reported antibodies measured against glutamic acid decarboxylase (GAD) but not all cases reported results for other antibodies, such as islet cell antibodies, insulin, islet antigen 2 and zinc transporter 8. The presence of one human leukocyte antigen (HLA) allele (DR4, DQ8, DR3, DQ2) was considered high-risk for Type 1 diabetes (Table S2).
Exclusion criteria
Case reports/series with people who were previously diagnosed with Type 2 diabetes prior to the start of anti-PD-1 or PD-L1 therapy were excluded.
Data extraction and quality assessment
We used an adapted version of the Newcastle–Ottawa scale, previously used in systematic reviews and meta-analyses of case reports/series [20], to assess the methodological quality of case reports and case series. Using this approach, we assessed the quality of each case report with regard to four domains: selection; ascertainment; causality; and reporting. We used five criteria in the form of questions, with binary answers (yes/no), as to whether the report met these criteria or not. The questions were as follows:
Did the person(s) represent the whole case(s) of the medical centre?
Was the diagnosis correctly made for new-onset diabetes?
Were all important data cited in the report?
Were other possible diagnoses excluded?
Were factors associated with Type 1 diabetes measured?
Two reviewers assessed the methodological quality of the studies and extracted the relevant data for the published cases based on the inclusion/exclusion criteria (D.K and A.S). A third reviewer analysed the results and made a final decision regarding the quality of reports (H.K.A).
Data synthesis and analysis
Statistical analyses were conducted using Stata version 15 (StataCorp LP, College Station, TX, USA). Binary variables (diabetic ketoacidosis and the presence of Type 1 diabetes-associated antibodies) were compared using Fisher’s exact test. Continuous variables (age, duration of diabetes and HbA1c) were compared using Student’s t-test. Unadjusted simple linear regression was carried out using GraphPad Prism 8.0 software to compare time to diabetes onset by HbA1c. A two-tailed P value of <0.05 was taken to indicate statistical significance.
Results
Cases of new-onset diabetes
We identified 58 publications representing 75 cases from 1 August 2000 to 14 August 2018 (Fig. 1). Four cases in two publications were excluded because of a pre-existing diagnosis of diabetes. A total of 71 cases in 56 publications were included in the review. Fifty-six cases had high, 12 had moderate, and three had low methodological quality (Table S3).
The mean (range) age of individuals identified was 61.7 (23–84) years and 55% were men (Table 1). The most common cancer treated was melanoma (53.5%), followed by lung cancer (26.8%). Other cancers comprised <10% of the cases. The most frequently used monoclonal antibodies were those directed towards PD-1 (nivolumab and pembrolizumab) in 90% of cases, while the remaining 10% received anti-PD-L1 therapies (atezolizumab, avelumab or durvalumab). There was no difference among the agents used in terms of diabetes duration as well as comparing metabolic and immunological features at presentation (Table 1), indicating a potential drug class effect for PD-1 and PD-L1 inhibitors to induce Type 1 diabetes; however, more cases, especially with anti-PD-L1 agents, are needed to confirm this observation.
Table 1.
Clinical, metabolic and immunological characteristics of anti-programmed cell death protein-1 or anti-programmed cell death protein-1 ligand inhibitor-induced Type 1 diabetes
| All cases (n=71) |
Nivolumab (n=38) |
Pembrolizumab (n=26) |
anti-PD-L1* (n=7) |
|
|---|---|---|---|---|
| Age, years | ||||
| Mean (sd) | 61.7 (12.2) | 61.4 (12.8) | 61.1 (11.4) | 65.7 (10.8) |
| Median | 62.0 | 62.5 | 61.0 | 66.5 |
| Range | 23–84 | 28–83 | 23–82 | 50–84 |
| Gender | ||||
| Female, % | 45.0 | 50.0 | 38.5 | 42.8 |
| Cancer type, % (n) | ||||
| Melanoma | 53.5 (38) | 50.0 (19) | 73.0 (19) | 0 (0) |
| Lung | 26.8 (19) | 34.2 (13) | 15.4 (4) | 28.5 (2) |
| Renal cell | 5.7 (4) | 10.6 (4) | 0 (0) | 0 (0) |
| Head and neck | 7.0 (5) | 2.6 (1) | 7.7 (2) | 28.5 (2) |
| Urothelial carcinoma | 4.2 (3) | 0 (0) | 0 (0) | 43.0 (3) |
| Other | 2.8 (2) | 2.6 (1) | 3.9 (1) | 0 (0) |
| Duration to diabetes, days | ||||
| Mean (sd) | 83.5 (88.5) | 98.0 (102.1) | 65.9 (72.1) | 75.0 (39.7) |
| Median | 49 | 73.5 | 42 | 84 |
| Range | 5–448 | 5–448 | 14–365 | 21–126 |
| Diabetic ketoacidosis, % (n) | ||||
| Present | 76.0 (54) | 71.0 (27) | 80.7 (21) | 85.7 (6) |
| Severe | 38.9 (21) | 33.3 (9) | 52.4 (11) | 16.6 (1) |
| Moderate | 20.4 (11) | 22.2 (6) | 14.3 (3) | 33.3 (2) |
| Mild | 11.1 (6) | 3.7 (1) | 14.3 (3) | 33.3 (2) |
| Not able to assess | 29.6 (16) | 40.8 (11) | 19.0 (4) | 16.6 (1) |
| Blood glucose, mg/dl | ||||
| Mean (sd) | 601.7 (207.4) | 609.0 (217.5) | 612.9 (170.4) |
529.1 (248.7) |
| Median | 580 | 571 | 616 | 411 |
| Range | 246–1211 | 246–1211 | 270–909 | 326–1015 |
| HbA1c, % | ||||
| Mean (sd) | 7.84 (1.0) | 7.64 (1.0) | 8.0 (1.0) | 8.2 (1.2) |
| Median | 7.7 | 7.3 | 7.85 | 8.2 |
| Range | 5.8–10.7 | 5.8–10.2 | 5.8–10.7 | 6.4–9.8 |
| Type 1 diabetes-associated antibodies, % (n) | ||||
| Present | 50.7 (36) | 44.7 (17) | 57.7 (15) | 57.1 (4) |
| Absent | 49.3 (35) | 55.3 (21) | 42.3 (11) | 42.9 (3) |
| Type 1 diabetes risk HLA genes†, % (n) | ||||
| Present | 38.0 (27) | 39.5 (15) | 38.5 (10) | 28.5 (2) |
| Absent | 7.0 (5) | 13.1 (5) | 0 (0) | 0 (0) |
| Not Reported | 55.0 (39) | 47.4 (18) | 61.5 (16) | 71.5 (5) |
| Ipilimumab (anti-CTLA-4) use‡, % (n) | ||||
| Present | 31.0 (22) | 31.5 (12) | 38.5 (10) | 0 (0) |
| Absent | 69.0 (49) | 68.5 (26) | 61.5 (16) | 100.0 (7) |
HLA, human leukocyte antigen; PD-1, programmed cell death protein; PD-L1, programmed cell death protein-1 ligand.
Atezolizumab, avelumab and durvalumab.
HLA-DQ-DR risk genes include DR3, DR4, DQ2, DQ8.
Prior or concurrent use of ipilimumab with anti-PD-1/PD-L1 treatment.
Clinical and laboratory features
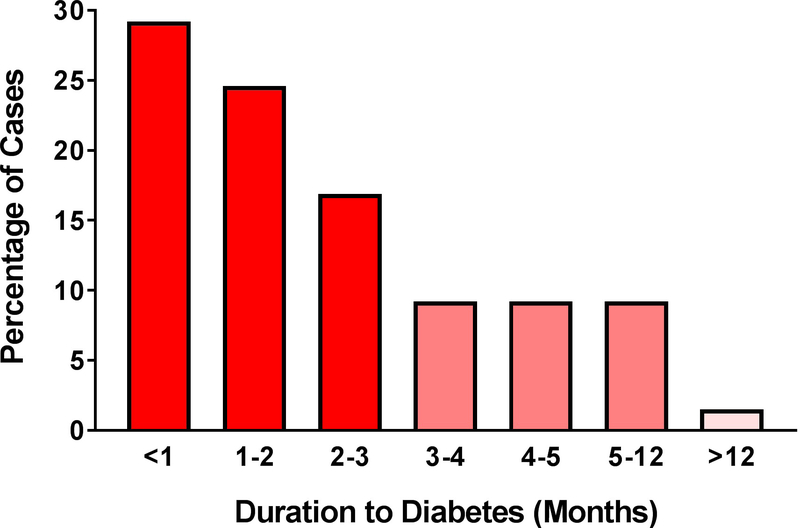
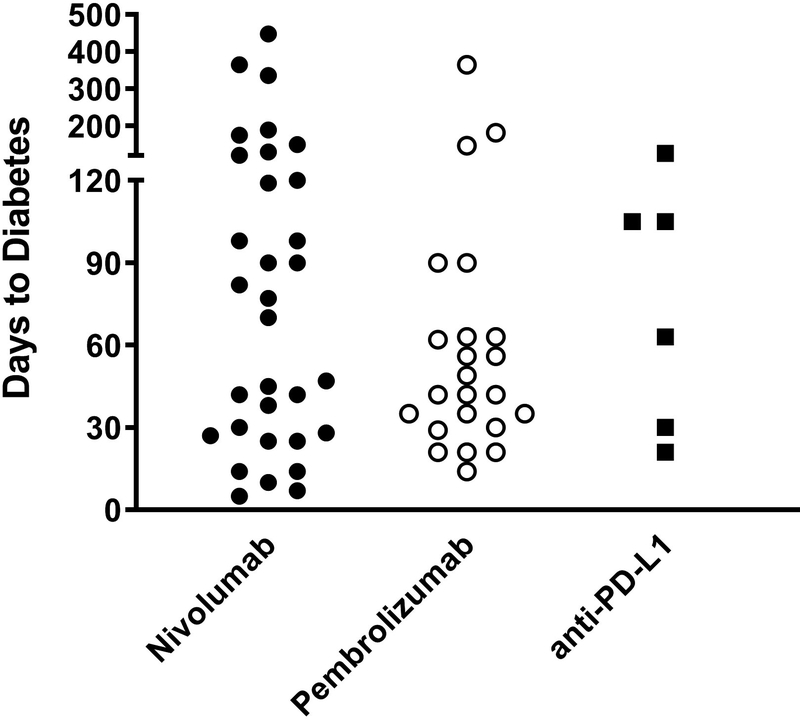
The median duration until diabetes onset after the start of anti-PD1/PD-L1 treatment was 49 days. The earliest time to onset was 5 days and the longest duration was reported at 448 days. Remarkably, 71% of the cases developed new-onset Type 1 diabetes within 3 months after the first exposure to anti-PD-1/PD-L1 treatment (Fig. 2a). When examining the agents individually, the median duration to diabetes was 42 days for pembrolizumab, 73.5 days for nivolumab, and 84 days for the anti-PD-L1 agents (Fig. 2b).
FIGURE 2.
Percentage of cases presenting with new-onset Type 1 diabetes stratified by duration from initial anti-programmed cell death protein-1 (PD-1)or anti-programmed cell death protein-1 ligand (PD-L1) exposure. (a) Graph showing that 71% of the cases developed Type 1 diabetes in the first 3 months; n=65 cases reporting time to diabetes onset. (b) Duration to diabetes onset by treatment agent.
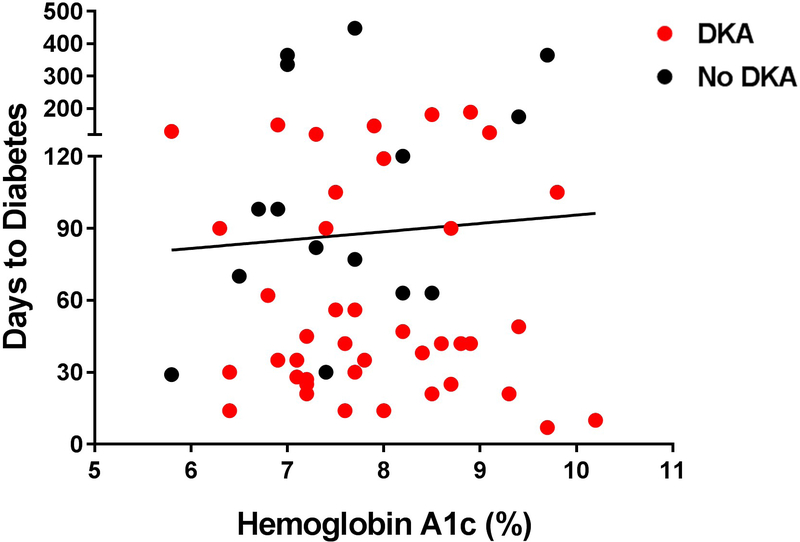
The majority (76%) of the people presented with diabetic ketoacidosis, which is associated with elevated risk of morbidity and mortality based on severity (Table 1). Among the cases with available criteria by which to assess diabetic ketoacidosis severity, 38.9% presented with severe, 20.4% with moderate and 11.1% mild ketoacidosis. Blood glucose levels at presentation were elevated, with a mean ± sd (range) of 33.4 ± 11.5 (13.7–67.3) mmol/l [602 ± 207 (246–1211) mg/dl]. Average (range) HbA1c, a measure of average blood glucose over the preceding 3 months, was 62 ± 0.3 (40–93) mmol/mol [7.8 ± 1.0 (5.8–10.7)%; Table 1]. Interestingly, there was no correlation between HbA1c and time to new-onset Type 1 diabetes (r2 = 0.001, P=0.78), potentially indicating a short time period of significantly elevated blood glucose prior to diabetes onset (Fig. 3a).
FIGURE 3.
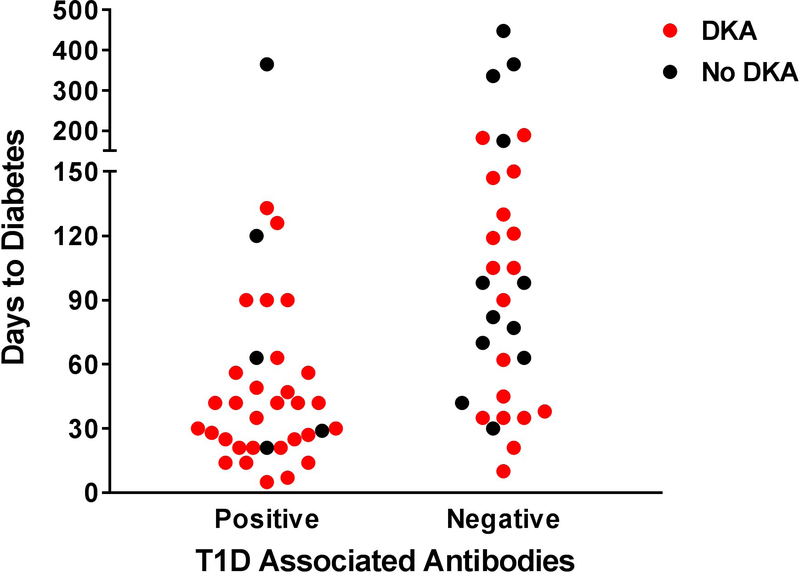
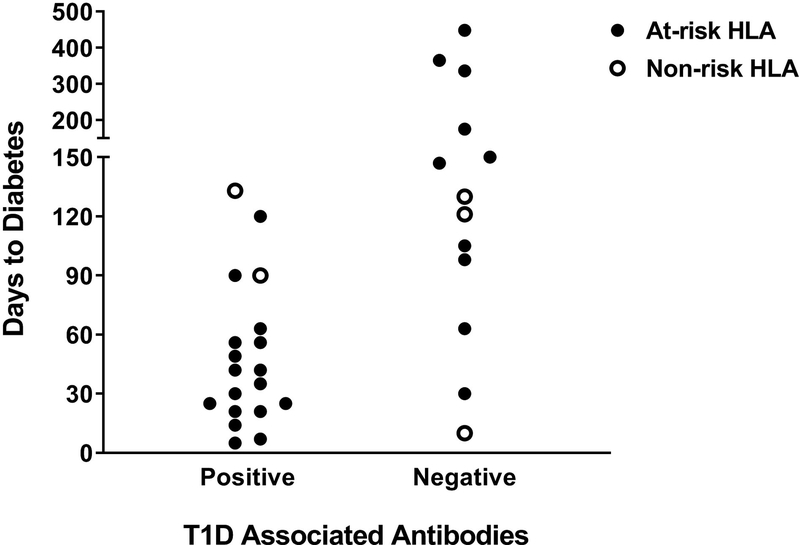
Comparison of duration to diabetes onset to HbA1c, Type 1 diabetes associated antibodies at presentation, and human leukocyte antigen (HLA) following anti-programmed cell death protein-1 (PD-1) or anti-programmed cell death protein-1 ligand (PD-L1) inhibitor treatment. (a) Duration to Type 1 diabetes onset by HbA1c. (b) Duration to diabetes onset by Type 1 diabetes associated antibody positivity. Cases with a shorter duration to diabetes were more likely to present with diabetic ketoacidosis. (c) Duration to Type 1 diabetes onset by antibody positivity and the presence or absence of at-risk HLA genes.
As expected, residual β-cell function, as measured by C-peptide which is secreted in a 1:1 ratio with insulin, was absent or inappropriately low for given blood glucose in all 55 cases for which levels were reported (Table S2). This insulin deficiency was permanent as all cases required continued treatment with exogenous insulin, and none of the people were reported to have come off insulin. Lipase and/or amylase were elevated in eight of 17 cases that reported these values, which indicates that pancreas inflammation was present in a subset of people with immune checkpoint inhibitor-induced Type 1 diabetes.
Immunological features
Half of the cases had detectable Type 1 diabetes-associated antibodies at presentation, with antibodies directed towards GAD being the most commonly reported. In contrast, ~90% of the people with prototypical childhood-onset Type 1 diabetes have one or more antibodies at clinical diagnosis [21,22]. Cases with positive Type 1 diabetes-associated antibodies had a shorter duration to diabetes onset (55 vs 117 days [95% CI 33.25–76.75 vs 78.03–155.51]; P=0.005). Those with antibodies also had a higher incidence of presentation with diabetic ketoacidosis compared to people without traditional Type 1 diabetes-associated antibodies [30/35 (86%) vs 18/30 (60%); P=0.02 (Fig. 3b)].
In addition to Type 1 diabetes-associated antibodies, genes within the HLA region are known to predispose to significant risk of developing childhood-onset Type 1 diabetes [23]. In particular, the class II DR and DQ genes (e.g. DR4–DQ8 and DR3–DQ2) confer the greatest susceptibility [24,25]. HLA typing was reported in 32 cases, with 27 out of 32 (85%) having at least one Type 1 diabetes risk DR or DQ allele (Table 1). This is comparable to childhood-onset Type 1 diabetes where ~90% of people have at least one of these risk genes [24]. There appears to be a cohort of people with positive Type 1 diabetes-associated antibodies and at-risk HLA that develops diabetes rapidly (Fig. 3C).
Prior or concomitant use of another immunotherapy has the potential to increase Type 1 diabetes risk. To investigate this possibility, use of ipilimumab, a CTLA-4 inhibitor which was clinically approved prior to anti-PD-1/PD-L1 therapies, was evaluated in our cases. The use of ipilimumab was reported in only 22 cases (Table 1). There was no association among cases receiving ipilimumab compared to those without for our measured outcomes. Interestingly, none of our reported cases with new-onset Type 1 diabetes following immune checkpoint inhibition occurred with the use of ipilimumab as a single agent.
Discussion
This is the largest and most comprehensive systematic review of case reports and series reporting duration to Type 1 diabetes onset following immune checkpoint inhibitor therapy. As this immune-related adverse event commonly presents with insulin deficiency and life-threatening diabetic ketoacidosis, there is a need to understand the clinical, metabolic and immunological features on presentation. We identified a critical window for disease development within the first 3 months of starting therapy in which 71% of the reviewed cases developed Type 1 diabetes. All of the cases were insulin-deficient at presentation and required permanent treatment with exogenous insulin.
Those people with Type 1 diabetes-associated antibodies, which were predominantly directed against GAD, were more likely to present within a shorter timeframe and with diabetic ketoacidosis compared to those without antibodies at presentation. Slightly more than half of the cases were associated with traditional Type 1 diabetes antibodies, which is similar to a recent case series reporting a rate of 10/25 (40%), along with a faster onset to diabetes with antibodies at presentation [17]. It is unknown whether these antibodies preceded the start of therapy or developed after exposure to immune checkpoint inhibitors. A subset of people may have pre-existing Type 1 diabetes-associated antibodies as disease developed within a few days to weeks after the start of therapy [26]. More research is needed to evaluate all of the traditional Type 1 diabetes-associated antibodies and those directed against post-translationally modified β-cell proteins. Future research also needs to evaluate HLA genes and other potential genetic risk factors, as a limited number of people have had HLA typing performed to date.
The incidence of immune checkpoint inhibitor-induced Type 1 diabetes is estimated at 1% [13–17]. With this frequency, prospective clinical trials assessing PD-1/PD-L1 inhibitor-induced Type 1 diabetes will be challenging, therefore, there is a need to form registries and rigorously review cases reported in the literature. There is evidence from the WHO database of individual case safety reports (VigiBase) that indicates an increased reporting of immune checkpoint inhibitor-associated diabetes [27]. This could be attributable to the increased use of anti-PD-1 and anti-PD-L1 therapies across cancers or differences in patient populations between clinical trials and those treated in clinical practice. Notably, most phase II and III clinical trials excluded people with an existing autoimmune disorder [26].
The majority of cases developing Type 1 diabetes were treated for melanoma, which probably reflects the fact that this was the first indication for PD-1 inhibitors and more people with melanoma have been treated than those with other malignancies. With the encouraging cancer response rates for immune checkpoint inhibition, it is anticipated that many more patients with varying cancers will be treated with these therapies. Additionally, combination therapy with CTLA-4 and PD-1 inhibition is being evaluated [28,29], which may increase the frequency of Type 1 diabetes and other immune-related adverse events.
Taken together, these lines of evidence indicate clinical practice guidelines are needed to screen and monitor for this adverse event [30]. Healthcare providers managing patients being treated with these therapies need to be aware of Type 1 diabetes presenting with diabetic ketoacidosis. Patients should be educated about the signs and symptoms of hyperglycaemia and diabetic ketoacidosis, along with monitoring blood glucose. Our results indicate blood glucose levels at presentation were substantially elevated and the time to Type 1 diabetes onset did not correlate to HbA1c, implying a short timeframe of significant hyperglycaemia. Routine self-monitoring of blood glucose or the use of continuous glucose monitoring may be needed to identify the initial elevations in blood glucose. Additionally, assays that evaluate specific immune cells involved in Type 1 diabetes pathogenesis are needed to understand the mechanisms of immune checkpoint inhibitor-induced Type 1 diabetes and provide a framework in which to test therapies to prevent this unwanted side effect.
In conclusion, the rapid onset of Type 1 diabetes can result when manipulating the immune system to target tumour cells with the use of immune checkpoint inhibitors. Future prospective clinical studies are warranted to screen patients receiving these therapies for risk factors associated with Type 1 diabetes onset, which will help lessen the risk of life-threatening diabetic ketoacidosis.
Supplementary Material
What’s new?
This systematic review showed that the majority of people developed immune checkpoint inhibitor-induced Type 1 diabetes within 3 months of initial treatment.
People presenting with Type 1 diabetes-associated antibodies had a more rapid onset and higher incidence of diabetic ketoacidosis at presentation.
Healthcare providers caring for people receiving these state-of-the-art therapies need to be aware of this potential life-threatening adverse event.
Funding sources
This work was supported by grants from the NIH DK108868, DK110845, and DK032083.
Footnotes
Competing interests
None declared.
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359:1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 2007; 19:813–824. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest 2015; 125:3384–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas A Releasing the Brakes on Cancer Immunotherapy. N Engl J Med 2015; 373:1490–1492. [DOI] [PubMed] [Google Scholar]
- 5.Ribas A Tumor immunotherapy directed at PD-1. N Engl J Med 2012; 366:2517–2519. [DOI] [PubMed] [Google Scholar]
- 6.Zarour HM. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin Cancer Res 2016; 22:1856–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018; 378:158–168. [DOI] [PubMed] [Google Scholar]
- 8.Clotman K, Janssens K, Specenier P, Weets I, De Block CEM. Programmed cell death-1 (PD-1) inhibitor induced type 1 diabetes mellitus: mini-review. J Clin Endocrinol Metab 2018; 103(9):3144–3154 [DOI] [PubMed] [Google Scholar]
- 9.Hughes J, Vudattu N, Sznol M, Gettinger S, Kluger H, Lupsa B et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care 2015; 38:e55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellati M, Eaton KD, Brooks-Worrell BM, Hagopian WA, Martins R, Palmer JP et al. Anti-PD-1 and Anti-PDL-1 Monoclonal Antibodies Causing Type 1 Diabetes. Diabetes Care 2015; 38:e137–138. [DOI] [PubMed] [Google Scholar]
- 11.Akturk HK, Michels AW. Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018; 378:1163–1164. [DOI] [PubMed] [Google Scholar]
- 12.Zaied AA, Akturk HK, Joseph RW, Lee AS. New-onset insulin-dependent diabetes due to nivolumab. Endocrinol Diabetes Metab Case Rep 2018; pii: 17–0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avelumab label information. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761049s000lbl.pdf. Last accessed February 2019.
- 14.Durvalumab label information. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761069s000lbl.pdf. Last accessed February 2019.
- 15.Pembrolizumab label information. Available at https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf.). Last accessed February 2019.
- 16.Nivolumab label information. Available at https://packageinserts.bms.com/pi/pi_opdivo.pdf. Last accessed February 2019.
- 17.Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes 2018; 67:1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009; 32:1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018; 41:S13–S27. [DOI] [PubMed] [Google Scholar]
- 20.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018; 23:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bingley PJ. Clinical applications of diabetes antibody testing. J Clin Endocrinol Metab 2010; 95:25–33. [DOI] [PubMed] [Google Scholar]
- 22.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med 2009; 360:1646–1654. [DOI] [PubMed] [Google Scholar]
- 23.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014; 383:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 2008; 57:1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu X, Deutsch AJ, Lenz TL, Onengut-Gumuscu S, Han B, Chen WM et al. Additive and interaction effects at three amino acid positions in HLA-DQ and HLA-DR molecules drive type 1 diabetes risk. Nat Genet 2015; 47:898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akturk HK, Alkanani A, Zhao Z, Yu L, Michels AW. PD-1 Inhibitor Immune-Related Adverse Events in Patients with Preexisting Endocrine Autoimmunity. J Clin Endocrinol Metab 2018; 103(10):3589–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright JJ, Salem JE, Johnson DB, Lebrun-Vignes B, Stamatouli A, Thomas JW et al. Increased Reporting of Immune Checkpoint Inhibitor-Associated Diabetes. Diabetes Care 2018; 41:e150–e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015; 373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2017; 377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akturk HK, Michels AW. Adverse Events Associated with Immune Checkpoint Inhibitors. JAMA 2019;321:1219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.