Abstract
In animals and people with incomplete spinal cord injury, appropriate operant conditioning of a spinal reflex can improve impaired locomotion. In all previous conditioning studies, the reflex was conditioned during steady-state maintenance of a stable posture; this steady-state protocol sought to change the excitability of the targeted reflex pathway; reflex size gradually changed over 8–10 weeks. The present study introduces a new protocol, a dynamic protocol that seeks to change the functioning of the reflex pathway during a specific phase of a complex movement. Specifically, we down-conditioned the soleus H-reflex during the swing-phase of locomotion in people with hyperreflexia due to chronic incomplete SCI. The swing-phase H-reflex, which is absent or very small in neurologically normal individuals, is abnormally large in this patient population. The results were clear. With swing-phase down-conditioning, the H-reflex decreased much faster and farther than did the H-reflex in all previous animal or human studies with the steady-state protocol, and the decrease persisted for at least 6 months after conditioning ended. The H-reflex decrease was accompanied by improvements in walking speed and in the modulation of locomotor EMG activity in proximal and distal muscles of both legs. These results provide new insight into the factors controlling spinal reflex conditioning; they suggest that conditioning protocols that target reflex function in a specific movement phase provide a promising new opportunity to enhance functional recovery after SCI or in other disorders.
Keywords: spasticity, plasticity, operant conditioning, learning, locomotion, rehabilitation
INTRODUCTION
In animals and humans, an operant conditioning protocol can strengthen or weaken the output of a spinal reflex pathway (Wolpaw & O’Keefe, 1984; Wolpaw, 1987; Chen & Wolpaw, 1995b; Wolf & Segal, 1996; Carp et al., 2006b; Chen et al., 2006a; Thompson et al., 2009a). Because the protocol changes the pathway structurally and functionally (Wolpaw, 1997, 2006; Wolpaw & Chen, 2009; Wolpaw, 2010), it affects behaviors, such as locomotion, that use the pathway (e.g., Chen et al. 2011). This suggested that appropriate conditioning might reduce movement impairments associated with spinal cord injury (SCI), strokes, and other disorders. Animal studies supported this possibility. In rats in which a right lateral column injury had weakened right stance and created a gait asymmetry, up-conditioning of the right soleus H-reflex (electrical analog of the spinal stretch reflex) eliminated the asymmetry and improved locomotion (Chen et al., 2006b); the improvement persisted after conditioning ended (Chen et al., 2014b). These positive results encouraged initial human studies.
In humans, SCI often increases the excitability of spinal stretch reflexes and H-reflexes and impairs their normal modulation over the step cycle (Yang et al., 1991; Stein et al., 1993; Fung & Barbeau, 1994; Boorman et al., 1996; Little et al., 1999; Hiersemenzel et al., 2000; Crone et al., 2003; Nakazawa et al., 2006; Dietz & Sinkjaer, 2007; Nielsen et al., 2007; Thompson et al., 2009b; Thompson et al., 2016). These abnormalities contribute to locomotor impairments in people with spasticity due to SCI (Corcos et al., 1986; Fung & Barbeau, 1989; Hidler & Rymer, 1999, 2000; Khan et al., 2016). In these individuals, down-conditioning of the soleus H-reflex increased walking speed, reduced step asymmetry, and had other functional benefits (Manella et al., 2013; Thompson et al., 2013). Furthermore, the animal and human studies indicate that, by targeting beneficial plasticity to an important spinal pathway, the conditioning protocol triggers wider beneficial plasticity that improves locomotor function in the muscles of both legs (Chen et al., 2011; Thompson et al., 2013; Chen et al., 2014a). These wider beneficial effects are consistent with the negotiated equilibrium model of spinal cord function (Wolpaw, 2010, 2018).
All previous reflex conditioning studies required a defined level of stable pre-stimulus EMG prior to reflex elicitation; and all human studies also required the same static posture (e.g., standing (Thompson et al., 2009a; Thompson et al., 2013; Makihara et al., 2014)). In contrast, the present study conditioned the reflex at a specific phase of a dynamic movement. In people with spastic hyperreflexia due to chronic SCI, we down-conditioned the soleus H-reflex during the late-swing phase of locomotion, when the reflex is abnormally large in these individuals (Yang et al., 1991; Fung & Barbeau, 1994; Thompson et al., 2016). Because hyperreflexia in this phase is likely to contribute to impaired gait (e.g., by exacerbating foot drop and/or clonus), H-reflex down-conditioning was expected to be beneficial. The results confirm this hypothesis. Furthermore, the unprecedented rapidity and magnitude of the reflex decrease provide new insight into reflex conditioning and generate new excitement about its potential clinical applications.
MATERIALS AND METHODS
Ethical Approval
This study was reviewed and approved by the Institutional Review Boards of Helen Hayes Hospital and the Medical University of South Carolina (ethics approval ref. no. Pro00042082). It complied with the ethical guidelines of the Declaration of Helsinki (except for registration in a database). All the participants gave informed consent for the study.
Participants
Thirteen adults with impaired locomotion due to an incomplete SCI 1.5–13 years earlier participated in this study (9 men and 4 women, ages 18–70 years, mean age 49.8(±13.5SD)) (Table 1). A physiatrist or a neurologist determined each prospective participant’s eligibility for the study. The inclusion criteria were: (1) a stable SCI-related locomotor deficit (>1 year after lesion); (2) ability to walk on the treadmill for ≥160 steps without stopping; (3) signs of spasticity (i.e., increased muscle tone, score ≥1 on Modified Ashworth scale) at least unilaterally; (4) presence of a soleus H-reflex in the late-swing phase of locomotion at least unilaterally; (5) a reasonable expectation that current medication would not change over the period of the study (e.g., anti-spasticity medication such as baclofen, diazepam, or tizanidine); and (6) medical clearance to participate. The exclusion criteria were: (1) spinal motoneuron injury; (2) a cardiac condition; (3) another medically unstable condition; (4) cognitive impairment; and/or (5) daily use of functional electrical stimulation to counteract foot drop. In those who exhibited bilateral motor impairments, the soleus H-reflex of the more impaired leg was studied.
Table 1.
Profiles of Down-conditioning (DC) and No-stimulation (NS) control participants.
| Participant | Group | Age | Sex | SCI Cause | SCI Level | AIS | Yrs Post SCI | Baclofen |
|---|---|---|---|---|---|---|---|---|
| 1 | DC | 55 | M | T | C5 | D | 5 | No |
| 2 | DC | 48 | M | T | C1 | D | 2.5 | Yes |
| 3 | DC | 67 | M | T | C8 | D | 2 | Yes |
| 4 | DC | 18 | F | T | C7 | D | 1.5 | Yes |
| 5 | DC | 55 | M | T | C6 | D | 10 | No |
| 6 | DC | 52 | M | T | C4 | D | 2.5 | No |
| 7 | DC | 48 | M | T | T1 | D | 4 | No |
| 8 | NS | 70 | M | NT | T5 | D | 13 | No |
| 9 | NS | 33 | F | T | C7 | D | 10 | Yes |
| 10 | NS | 43 | M | T | C4 | D | 1.5 | Yes |
| 11 | NS | 57 | F | T | C5 | D | 8 | No |
| 12 | NS | 54 | F | NT | C6 | D | 5 | No |
| 13 | NS | 48 | M | T | C7 | D | 1.5 | Yes |
Cause of spinal cord damage (T: trauma, NT: non-trauma).
AIS: American Spinal Injury Association (ASIA) Impairment Scale
The participants were randomly assigned to the Down-Conditioning (DC) group (6 men and 1 woman; ages 18–67 years, mean 49.0(±15.1SD) years; 1.5–10 years since SCI; Participants 1–7 in Table 1) or the No-Stimulation (NS) control group (3 men and 3 women; ages 33–70 years, mean 50.8(±12.7) years); 1.5–13 years since SCI; Participants 8–13 in Table 1). The primary purpose of the NS group was to establish that H-reflex decrease and/or any change in locomotion in the DC group was not due simply to the regularly administered treadmill walking. (H-reflex size does not change in animals or humans with SCI when it is simply measured over days and weeks without feedback on its size (Thompson et al., 2013). Thus, we did not include a stimulation-only control group in the present study.)
The Operant Conditioning Protocol and the Study Schedule
The new operant conditioning protocol used in this study was adapted from that used to condition the soleus H-reflex during standing (i.e., the steady-state protocol) (Thompson et al., 2009a; Thompson et al., 2013; Makihara et al., 2014). The steady-state protocol elicited the H-reflex after the standing participant had maintained soleus EMG activity in a required range for several seconds; the new swing-phase protocol elicited the H-reflex during the late-swing phase of walking. The numbers of conditioning and control sessions, the number of reflex trials per session, and the session schedule were identical to those of the steady-state protocol.
Figure 1 summarizes the swing-phase protocol. In a preliminary session, (1) the locations of the EMG recording electrodes and tibial nerve stimulating electrodes were optimized, (2) the soleus and tibialis anterior (TA) background EMG levels during natural standing were determined, (3) a treadmill speed that the participant found comfortable was selected for use throughout the study, and (4) the participant’s eligibility for participation was confirmed (i.e., ability to walk on the treadmill for ≥160 steps without stopping, a soleus H-reflex evident in the late-swing phase of walking). We also determined the target M-wave size for H-reflex elicitation throughout the subsequent sessions of the DC participants and for H-reflex elicitation during the locomotor assessments of all (DC and NS) participants.
Figure 1.
A: Session view. B: Session schedule. Six baseline sessions were followed by 30 conditioning sessions (in DC participants) or 30 control sessions (in NS participants) sessions, and then by two follow-up sessions. C: Composition of baseline, control, conditioning, and follow-up sessions. D: Visual feedback screens for control and conditioning trials. In all trials, the number of the current trial within its block is displayed. Background EMG panel is present, but the EMG feedback graph remains invisible throughout the trials. Thus, no information on the ongoing EMG activity is provided to the participant. In every other step, tibial nerve stimulation elicits the soleus H-reflex in the late swing phase of the step cycle. In control trials (top), the H-reflex panel is not shown. In conditioning trials (bottom), the shading in the H-reflex panel indicates the rewarded H-reflex range for down-conditioning. The dark horizontal line is the average H-reflex size of the baseline sessions, and the vertical bar is the H-reflex size for the most recent trial. If that H-reflex size falls in the shaded area, the bar is green and the trial is a success. If it exceeds the shaded area, the bar is red and the trial is a failure. The running success rate for the current block is also shown.
Each person then completed 6 baseline sessions and 30 control (NS group) or conditioning (DC group) sessions at a rate of 3/week. Each session lasted less than one hour and occurred within the same 2-h daily time window (to prevent the normal diurnal variation in reflex size from affecting the results (Wolpaw & Seegal, 1982; Chen & Wolpaw, 1994; Carp et al., 2006a; Lagerquist et al., 2006)). Ten-meter walking speed, locomotor H-reflexes across the entire step cycle, and locomotor EMG activity were measured in two locomotor assessments, one before and one after the 30 control (NS participants) or conditioning (DC participants) sessions. For the DC group, a follow-up session (identical to the conditioning sessions) occurred 1 month after the final conditioning sessions. Some of the DC group participants were able to come back for additional follow-up sessions 3 and 6 months after the final conditioning session. Each participant wore the same pair of shoes throughout the study. Two participants (one DC and one NS) wore an ankle-foot orthosis (AFO) on the conditioned/tested leg throughout all the study sessions, except for locomotor H-reflex and EMG assessments.
Figure 1C shows the content of each baseline, conditioning, or followup session for the DC and NS groups. In the DC group, each session began with measurement of the H-reflex/M-wave (H-M) recruitment curve while the participant maintained a natural standing posture and the soleus and TA EMG levels defined in the preliminary session (see Electrical Stimulation and EMG Recording below). Then, the participant walked on the treadmill for 30 sec without stimulation. Footswitches in the shoe of the studied leg detected foot-contact or toe-off. The data from this short walk were used to set the stimulus trigger delay from foot-contact or toe-off, so that the H-reflex could be elicited in the late-swing phase of the step cycle. Following these unstimulated steps, the participant completed 3 blocks of walking while the H-reflex was elicited in late-swing (i.e., 85–95% of the way through the step cycle from one ipsilateral foot contact to the next). In each block, the first few steps occurred without stimulation. Then, tibial nerve stimulation elicted the H-reflex every other step until 75 H-reflex trials were obtained. Thus, for each block, the participant took about 160 steps (i.e., ~5 steps before H-reflex trials begin, 75 stimulated steps interspersed with 75 unstimulated steps, ~5 steps after H-reflex trials ended)
In each conditioning session, before the first block of 75 H-reflex trials, the participant did an additional short period of walking to obtain 20 control H-reflex trials. Based on the size of the control reflexes, the reflex criterion value for the first block of 75 conditioned H-reflex trials was determined, as in the original operant conditioning protocol (Thompson et al., 2009a; Thompson et al., 2013). During the three 75-trial conditioning blocks, the participant was asked to decrease H-reflex size and was provided with immediate visual feedback that indicated his or her success in doing so (see Visual Feedback below). In order to maintain the same stimulus strength across all the H-reflex trials of the study, the tibial nerve stimulus was adjusted as needed to keep M-wave size constant for each participant throughout. Follow-up sessions were the same as the conditioning sessions.
In the NS group, each of the 6 baseline and 30 subsequent control sessions consisted of walking on the treadmill for 3 blocks of 160 steps each without H-reflex elicitation and without any special instructions. Thus, the NS group participants walked about as much during each session as did the DC group participants.
Electrical Stimulation and EMG Recording
At the beginning of each baseline, conditioning, or follow-up session for the DC group, and the beginning of each of the two locomotor assessments for both groups, EMG recording and stimulating electrodes were placed over the leg. EMG activity from soleus and its antagonist TA was recorded with surface self-adhesive Ag-AgCl electrodes (2.2 × 3.5 cm, Vermed, Buffalo, NY), amplified, band-pass filtered (10–1000 Hz), digitized (4,000 Hz), and stored. To elicit the H-reflex, the tibial nerve was stimulated in the popliteal fossa, using surface Ag-AgCl electrodes (2.2 × 2.2 cm for the cathode and 2.2 × 3.5 cm for the anode; Vermed) and a Grass S48 stimulator (with a CCU1 constant current unit and an SIU5 stimulus isolation unit; Natus Neurology - Grass, Warwick, RI). The stimulating electrode pair was placed so as to minimize the H-reflex threshold and to avoid stimulating other nerves. This placement was accomplished by monitoring the EMG of soleus and TA and palpating other lower-leg muscles, such as the peroneal muscle group. To avoid session-to-session variability in electrode placement, their locations were mapped in relation to permanent marks on the skin (e.g., scars or moles). The same investigator (AKT) placed the electrodes and conducted all study sessions (including locomotor EMG assessments) that involved EMG recording, for each participant.
At the beginning of each session and the beginning of each locomotor EMG assessment for both DC and NS groups, an H-M recruitment curve was obtained. The tibial nerve was stimulated by a 1-ms square pulse while the participant maintained a natural standing posture with pre-defined levels (see above) of soleus and TA background EMG activity. The stimulus occurred only when the participant had maintained rectified soleus and TA EMG activity within the specified ranges for at least 2 s. Typically, the soleus background EMG level corresponded to 10–20% of a maximum voluntary contraction (Thompson et al., 2009a; Makihara et al., 2014), and the TA level was <7 μV absolute value (i.e., resting level). The minimum inter-stimulus interval was 5 s. Stimulus intensity was varied in increments of 1.25–2.50 mA from below soleus H-reflex threshold, through the intensity that elicited the maximum H-reflex (Hmax), to an intensity just above that needed to elicit the maximum M-wave (Mmax) (Kido et al., 2004a; Makihara et al., 2012). About 10 different intensities were used to obtain each recruitment curve. At each intensity, four EMG responses were averaged to measure the H-reflex and M-wave. The stimulus intensity used for the subsequent H-reflex trials fell on the rising phase of the H-reflex recruitment curve (71±31(SD)% Hmax during the baseline sessions) and produced an M-wave above threshold. In each participant, this M-wave size was maintained for the H-reflex trials of all the sessions.
Visual Feedback
The visual feedback screen presented to the DC participants for the H-reflex trials of the new swing-phase operant conditioning protocol was similar to that of the original steady-state protocol (Thompson et al., 2009a). The screen presented one or two panels, one for soleus background EMG activity and one for H-reflex size. For the H-M recruitment curve measurement during standing, only the background EMG panel was shown: if the participant kept the height of the vertical bar (i.e., soleus background EMG activity level in absolute value) in the specified range for 2 s, and at least 5 s had passed since the last stimulus, a stimulus pulse was delivered. For control or conditioning H-reflex trials during walking (Figure 1D), the background EMG panel was present, but the background EMG bar was invisible (i.e., there was no feedback on background EMG activity). Regardless of the soleus and TA EMG levels, the H-reflex was elicited in the late-swing phase of the studied leg in every other step cycle. The number of the current trial in its block was shown.
During conditioning H-reflex trials, in addition to the empty background EMG panel and the number of the current trial, the H-reflex panel appeared. It showed a heavy horizontal line indicating the participant’s average H-reflex size for the 6 baseline sessions and a shaded area that indicated the H-reflex size range that satisfied the current down-conditioning criterion. The bar indicating the size of the most recent H-reflex was refreshed 200 ms after each stimulus. It was green (indicating success) when H-reflex size fell within the shaded area (i.e., was below the criterion value), and red (indicating failure) when H-reflex size exceeded the criterion. In addition, the current success rate (i.e., percent of trials in the current 75-trial block that were successful) appeared next to the H-reflex bar and was updated after each trial. Thus, for each control trial, the visual feedback provided only the trial number, it gave no information on H-reflex size; in contrast, for each conditioning trial, the visual feedback informed the participant as to whether s/he had succeeded in producing an H-reflex small enough to satisfy the size criterion, and it showed the running success rate for the current block of trials.
In each conditioning session, the criterion value for the first block of 75 conditioning trials was based on the immediately preceding block of 20 control trials, and the criterion values for the second and third blocks of conditioning trials were based on the H-reflexes of the immediately preceding block of 75 conditioning trials. The criterion was selected so that, if H-reflex values for the new block were similar to those for the previous block, 50–60% of the trials would be successful (Chen & Wolpaw, 1995a). For each block, the participant earned a modest extra monetary reward when the success rate exceeded 50%. Further protocol details are provided in Thompson et al. (2009a).
H-reflex measurement for the baseline, conditioning, and follow-up sessions
In the DC participants, the H-reflex and M-wave sizes for each trial were measured as the peak-to-peak values in time windows determined for each participant. A typical time window was 35–47 ms poststimulus for the H-reflex and 6–23 ms poststimulus for the M-wave. For each session of each DC group participant, the average H-reflex size for the 225 trials of the three 75-trial blocks was determined. This value is called the conditioned H-reflex size (regardless of whether the session is a baseline session or a conditioning session). In addition, for each session of each participant, we determined the average H-reflex size for 20 control trials. This value is called the control H-reflex size. For baseline sessions, these 20 control trials were the first 20 trials of the first 75-trial block. For conditioning sessions and follow-up sessions, these 20 control trials were elicited prior to the first block of 75 conditioning trials, as indicated in Figure 1C and described above.
To determine for each DC participant whether the conditioned H-reflex size changed significantly over the 30 conditioning sessions, the average H-reflexes for the 225 trials of the final 6 conditioning sessions (i.e., sessions 25–30) were compared to the average H-reflexes for the 225 trials of the 6 baseline sessions by unpaired t test (one-tailed). To determine the final conditioned H-reflex size for each participant, the average H-reflexes for the 225 trials of the final 3 conditioning sessions were averaged (i.e., the final week of conditioning), and the result was expressed in percentage of the average H-reflex for the 225 trials of the 6 baseline sessions. (Thus, a value of 100% indicated no change.) To determine for each participant the final control H-reflex size, the average H-reflexes for the 20 control trials of the final 3 conditioning sessions were averaged, and the result was expressed in percentage of the average H-reflex for the 20 control trials of the 6 baseline sessions.
Assessment of locomotor EMG activity, locomotor H-reflex, and walking speed
Before and after the 30 conditioning sessions (DC group) or 30 control sessions (NS group), the locomotor EMG activity and the soleus H-reflex were assessed on the treadmill; and the overground 10-m walking speed was measured. These assessments occurred on non-session days.
First, the participant was asked to walk at his/her fastest comfortable speed on an indoor flat surface that had markers at 0, 2, 12, and 14 m. The 10-m speed was calculated from the time when the toes of the leading foot crossed the 2-m marker to the time when they cross the 12-m marker. The participant repeated this three times, and the average value defined the walking speed. Two participants (1 DC and 1 NS) wore an AFO and three participants (2 DC and 1 NS) used forearm crutches during the 10-m walking tests of both the before and after assessments.
Second, the soleus H-M recruitment curve was obtained while the participant maintained a natural standing posture with pre-defined stable levels of soleus and TA background EMG activity (see Electrical Stimulation and EMG Recording) .
Third, locomotor EMG activity was recorded from the soleus, TA, vastus lateralis (VL), and biceps femoris (BF) muscles of both legs during 3–5 min of treadmill walking at the participant’s self-selected comfortable speed (the same speed was used for both pre and post assessments). Footswitch cells inserted in the participant’s shoes detected foot contact (typically, heel or toe contact). The same investigator (AKT) placed the electrodes for each participant.
Fourth, after a few minutes of rest, the locomotor H-reflex was measured. Single 1-ms square-pulse stimuli were delivered at different points in the step cycle to evaluate phase-dependent H-reflex modulation (Capaday & Stein, 1986; Stein & Capaday, 1988; Ethier et al., 2003; Kido et al., 2004a). The stimulus interval was set to be long enough to have at least one unstimulated step-cycle between successive stimuli. For these measurements, the two participants who wore an AFO were asked to remove it.
To analyze locomotor EMG activity, the step cycle was divided into 12 bins of equal duration (Kido et al., 2004b; Makihara et al., 2012; Thompson et al., 2013). For each leg, the step cycle went from its foot contact to its next foot contact. For each muscle of each participant, the average rectified EMG amplitude (equivalent to absolute value) in each of the 12 bins was determined and expressed in percent of the amplitude in the bin with the highest amplitude. The degree to which each muscle’s activity was modulated during locomotion was determined by calculating its Modulation Index (MI) in percent as: 100 × [(highest bin amplitude - lowest bin amplitude)/highest bin amplitude] (Zehr & Kido, 2001; Zehr & Loadman, 2012). Thus, an MI of 0% indicated that a muscle did not modulate its activity at all over the step cycle.
Similarly, to analyze phase-dependent modulation of the locomotor H-reflex, the step cycle was divided into 12 equal bins. For each bin, the sizes of the H-reflexes accompanied by M-waves of consistent size (i.e., consistent across the bins of the step cycle and across the two assessments) were averaged (Llewellyn et al., 1990; Edamura et al., 1991; Makihara et al., 2014). Typically, about 10 responses were averaged for each bin. To evaluate the extent of H-reflex modulation during walking, the modulation index, [100 × (maximum H-reflex - minimum H-reflex) / maximum H-reflex] (Zehr & Kido, 2001; Kido et al., 2004a; Makihara et al., 2012) was calculated over the step cycle. For comparison of H-reflex size between the two assessments (i.e., pre vs. post), the H-reflex was normalized to the Mmax in each participant, and the normalized values were averaged across the participants.
Statistical Analysis
As mentioned above, to determine for each DC participant whether H-reflex down-conditioning was successful, the average conditioned H-reflexes of the final 6 conditioning sessions were compared to the average H-reflexes of the 6 baseline sessions by unpaired t-test (one-tailed). To determine the group effect of conditioning, a repeated measures ANOVA was used to evaluate conditioned and control H-reflex sizes across successive 6-session blocks (i.e., baseline sessions 1–6 and conditioning sessions 1–6, 7–12, 13–18, 19–24, and 25–30), together with the post-hoc Newman–Keuls test. We also assessed over all sessions the stability of soleus Mmax and M-wave size that accompany the H-reflex using a repeated measures ANOVA (i.e., similar to the evaluation of the group effects on H-reflex size). Soleus Mmax, M-wave for control trials, and M-wave for conditioning trials remained stable across all the sessions (p>0.12 for all, one-way repeated measures ANOVA). This indicated the stability of the EMG recording and nerve stimulation.
To perform pre vs. post comparisons on locomotor EMG activity, locomotor H-reflex, walking speed, and the Mmax and Hmax values during standing in each group, paired two-tailed t-test was used. For between-group comparisons, unpaired two-tailed t-test was used. For all statistical assessments, the α level was set at 0.05.
In addition, to further evaluate the magnitude of H-reflex change with late-swing phase conditioning (in contrast to the magnitude of H-reflex change with standard steady-state conditioning), a two-way repeated measures ANOVA (groups × successive 6-session blocks) was applied to the present swing-phase conditioning data and the previous steady-state standing conditioning data (Thompson et al., 2013), and Hedges’ g (Hedges, 1981), a measure of effect size as [difference in means / pooled and weighted standard deviation] was calculated between the two data sets. Since we anticipated larger H-reflex decreases with swing-phase conditioning (for more synaptic mechanisms would be available to change the swing-phase reflex) than with static standing conditioning, unpaired one-tailed t-test was used to compare the final H-reflex values between the swing-phase conditioning and steady-state standing conditioning groups.
RESULTS
All 13 participants completed the 6 baseline sessions and 30 conditioning or control sessions. As indicated above, the soleus Mmax, and the soleus M-wave size in control and conditioning trials, remained stable across all the sessions in all 7 DC participants.
The results comprise: conditioned and control late-swing phase H-reflex sizes and standing H-reflex sizes (obtained from the M-H recruitment curves) over the course of baseline, conditioning, and follow-up sessions in the DC group; and locomotor EMG activity, locomotor and standing H-reflex sizes, and 10-m overground walking speed before and after the 30 conditioning or control sessions in the DC and NS groups. These data sets are described here.
Swing-phase H-reflex change in DC participants
Swing-phase H-reflex down-conditioning was successful (i.e., the average conditioned H-reflexes for conditioning sessions 25–30 were significantly less than those for the 6 baseline sessions (Thompson et al., 2009a)) in 6 of 7 DC participants. In the remaining DC participant, the H-reflex did not change significantly. The success rate of 6/7 (or 86%) is similar to those for previous, steady-state operant conditioning studies in normal monkeys, rats, and mice (i.e., 75–80%) (Wolpaw et al., 1983; Wolpaw, 1987; Chen & Wolpaw, 1995a; Carp et al., 2006b), in neurologically normal people (i.e., 8/9 or 89% (Thompson et al., 2009a), 7/8 or 88% (Makihara et al., 2014)), and in people with SCI (6/9 or 67%) (Thompson et al., 2013).
Figure 2 shows H-reflexes from a successful DC participant during a baseline session (dashed) and the last conditioning session (solid). Figure 2A illustrates the change in the conditioned H-reflex (i.e., the H-reflex for the three blocks of 75 trials in which, in the conditioning sessions, the participant was encouraged to decrease the H-reflex and provided with immediate feedback as to whether the reflex met the size criterion). Figure 2B illustrates the change in the control H-reflex (i.e., the H-reflex for the first 20 trials of each baseline or conditioning session in which the participant was not asked to decrease the H-reflex and was not provided with feedback as to reflex size). Both the conditioned and control H-reflexes are much smaller after down-conditioning; the decrease is greater in the conditioned H-reflex. As noted above, M-wave size did not change within or across the sessions.
Figure 2.
Average conditioned H-reflexes (left; 225 trials) and control H-reflexes (right; 20 trials) in a baseline session (dashed line) and the last conditioning session (solid line) from a participant whose H-reflex decreased significantly. A small stimulus artifact is present.
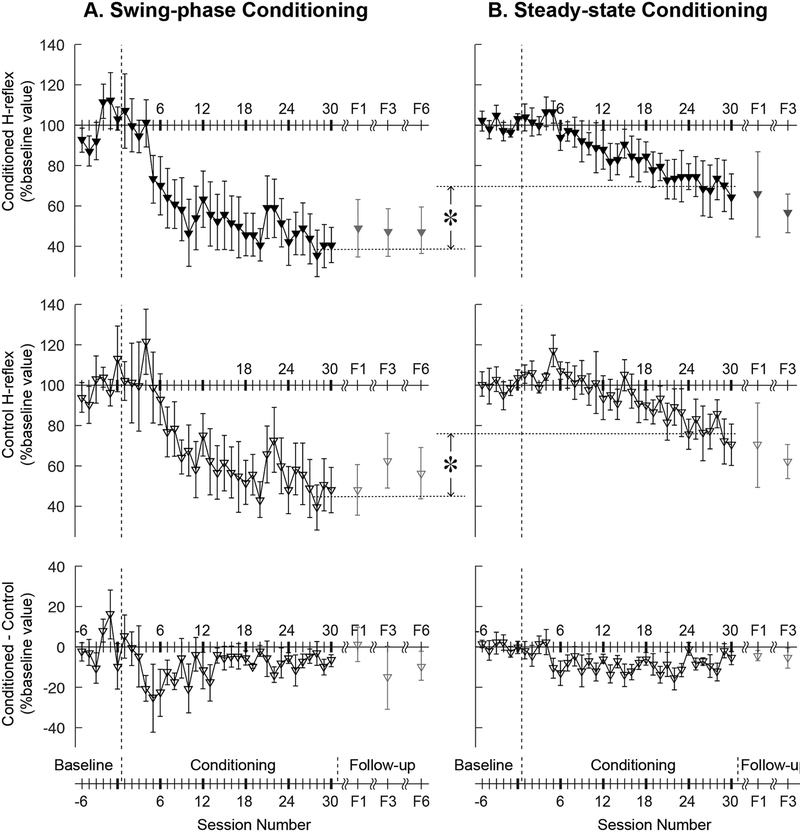
Figure 3 shows the average courses of H-reflex change for the present study’s successfully down-conditioned DC participants with SCI, and it includes for comparison the average courses for successfully down-conditioned participants from our earlier study of H-reflex down-conditioning during standing in people with SCI (Thompson et al., 2013). It shows the courses for the conditioned H-reflex (top), the control H-reflex (middle), and the within-session difference between the conditioned and control H-reflexes (bottom). The within-session difference reflects the task-dependent adaptation that the participants learn to produce when they are asked to decrease H-reflex size; thus, it is present in the conditioning trials but not in the control trials (Thompson et al., 2009a). Task-dependent adaptation is thought to reflect a task-appropriate change in corticospinal tract (CST) influence over the spinal reflex pathway that humans learn to produce typically within 1000 trials (as do monkeys (Wolpaw & O’Keefe, 1984)). Figure 3C shows that, with both steady-state and swing-phase conditioning, it appears after 3–4 conditioning sessions and is evident from then on. Table 2 summarizes the changes in H-reflex size over the course of 30 down-conditioning sessions in successive 6-session blocks.
Figure 3.
Average (±SE) H-reflex values for baseline, conditioning, and follow-up sessions for the present DC (swing-phase conditioning) participants with SCI (A, N=6, filled symbols) and for the previous DC (standing, steady-state conditioning) participants (B, N=6, open symbols, from Thompson et al., 2013) in whom the H-reflex decreased significantly. Of the present group of participants, all 6 completed 1 month follow-up session (F1) and 4 completed 3 and 6 months (F3 and F6) follow-up sessions. Of the Thompson et al. (2013) participants, 4 completed 1 and 3 months follow-up (F1 and F3) sessions. Top: Average conditioned H-reflex size. Middle: Average control H-reflex size. Bottom: Average of conditioned H-reflex size minus control H-reflex size (i.e., task-dependent adaptation; for details see (Thompson et al., 2009a)). The asterisks between swing-phase conditioning and steady-state conditioning indicate significant differences (p<0.05) between the two conditioning protocols in the final conditioned H-reflex size and the final control H-reflex size.
Table 2.
Changes in the soleus H-reflex with down-conditioning during standing (i.e., steady-state conditioning) (Thompson et al., 2013) and down-conditioning during the late swing phase of walking (i.e., swing-phase conditioning) (present study).
| standing | 102.1±12.0 | 92.1±15.2 | 84.5±20.1 | 75.4±18.6 * | 69.7±27.9 * |
| walking | 91.0±25.1 | 58.0±34.7 * | 51.8±34.2 * | 49.9±28.5 * | 42.5±25.0 * |
| standing | 103.5±9.3 | 100.6±13.9 | 94.8±16.8 | 85.5±13.3 | 77.4±23.0 * |
| walking | 102.4±33.8 | 69.7±24.6 * | 57.8±32.0 * | 57.1±27.5 * | 49.6±35.7 * |
| standing | −1.4±15.2 | −8.4±7.0 | −10.3±8.5 | −10.0±8.1 | −7.7±6.9 |
| walking | −11.4±13.5 | −11.7±14.0 | −6.7±8.3 | −7.3±7.4 | −7.1±6.1 |
All values are in % of baseline value ±SD.
For the conditioned and control reflexes, asterisks indicate significant differences from the 6 baseline sessions (p<0.05, Newman-Keuls test for post hoc).
These courses of change in the conditioned and control H-reflexes for swing-phase H-reflex down-conditioning are strikingly different from those found in previous human studies, in which the H-reflex was conditioned during standing. First, the final average value (i.e., the average of the last 3 conditioning sessions) of the swing-phase conditioned H-reflex size was 38±23(SD)% of the baseline value, much smaller than the final average values of standing conditioned H-reflex size in the previous conditioning study in people with SCI (69±27% (Thompson et al., 2013)) (p=0.03, unpaired t test). Thus, swing-phase conditioning decreased the conditioning H-reflex more than did steady-state conditioning.
Second, the final value of the control H-reflex (i.e., the average of the last 3 conditioning sessions) was smaller with swing-phase conditioning (i.e., 45±27(SD)% of baseline) than with steady-state conditioning (i.e., 76±22%) (p=0.03, unpaired t test). Thus, conditioning during the late-swing phase of walking decreased the control H-reflex more than conditioning during standing.
Third, as Table 2 shows, both the conditioned and control H-reflex decreased more rapidly with swing-phase conditioning than with conditioning during standing. With swing-phase conditioning, the average values of the conditioned and control H-reflexes for conditioning sessions 7–12 were significantly less than those for the baseline sessions (p<0.05 for both, one-way repeated measured ANOVA and post-hoc Newman–Keuls test). This is in clear contrast to steady-state conditioning: in people with SCI (Thompson et al., 2013), the H-reflex did not become significantly smaller than baseline until sessions 19–24 (for the conditioned reflex) and 25–30 (for the control reflex).
Table 3 summarizes the statistical comparisons of H-reflex size changes for swing-phase conditioning vs. steady-state conditioning (Thompson et al., 2013). For both the conditioned H-reflex and the control H-reflex, larger Hedges’ g values (i.e., larger differences between the two protocol groups) were commonly observed in conditioning sessions 7 through 24, consistent with the Figure-3 data indicating that H-reflex change occurred earlier and was greater with swing-phase conditioning than with steady-state conditioning.
Table 3.
Comparisons of H-reflex sizes across successive 6-session blocks (i.e., baseline sessions 1–6 and conditioning sessions 1–6, 7–12, 13–18, 19–24, and 25–30) for steady-state conditioning (Thompson et al., 2013) vs. swing-phase conditioning (present study). Hedges’ g values >0.8 imply large differences (indicated by asterixes).
| Comparison of steady-state conditioning vs. swing-phase conditioning | ANOVA (F and p values) | Hedges’ g (Hedges, 1981) | ||||||
|---|---|---|---|---|---|---|---|---|
| Group effect | Session effect | Group × Session | C1–6 | C7–12 | C13–18 | C19–24 | C25–30 | |
| Conditioned reflex during standing (2013 study) vs. during walking (present study) | F=4.34 p=0.06 | F=17.81 p<.0001 | F=2.47 p=0.04 | 0.48 | 1.13* | 1.03* | 0.92* | 0.90* |
| Control reflex during standing (2013 study) vs. during walking (present study) | F=6.15 p=0.03 | F=10.86 p<.0001 | F=2.80 p=0.03 | 0.12 | 1.26* | 1.33* | 1.22* | 0.59 |
| Within-session difference between conditioned and control reflexes (2013 study vs. present study) | F=0.11 p=0.75 | F=2.41 p=0.05 | F=1.18 p=0.33 | 0.62 | 0.27 | −0.39 | −0.32 | −0.08 |
| Control Reflex during standing (2013 study vs. present study) | F=5.29 p=0.04 | F=9.13 p<.0001 | F=1.83 p=0.13 | 1.00* | 1.12* | 1.17* | 1.20* | 0.40 |
Fourth, in contrast to their striking differences in the rapidity and magnitude of conditioned and control reflex change, people with SCI conditioned while walking (present study) or while standing (Thompson et al., 2013) displayed task-dependent adaptation that was similar in onset session (i.e., sessions 3–4) and average magnitude (6±6(SD)% with swing-phase conditioning, 7±7% with steady-state conditioning).
All 6 successful DC participants completed the 1-month follow-up session, and 4 completed the 3- and 6-month follow-up sessions. The conditioned H-reflex remained small in every participant’s follow-up sessions, averaging 49±35(SD)% of baseline value at 1 month, 47±24% at 3 months, and 47±28% at 6 months. The control H-reflex also remained small, averaging 48±30% at 1 month, 62±27% at 3 months, and 56±26% at 6 months.
In summary, both the conditioned and the control H-reflexes decreased much faster and much more with swing-phase conditioning than with steady-state conditioning; and they remained small for at least 6 months after conditioning ended. At the same time, in people with SCI, swing-phase conditioning and steady-state conditioning were similar in the onset time and average magnitude of task-dependent adaptation.
The locomotor H-Reflex across the step-cycle
Before and after the 30 conditioning (DC participants) or control sessions (NS participants), the locomotor H-reflex was measured throughout the step cycle. The step cycle was divided into 12 bins of equal duration and average H-reflex size for each bin was determined. The average of these 12 values was defined as the average locomotor H-reflex. In addition, the averages of bins 4–6 and bins 10–12 were defined as the mid-late stance phase H-reflex (henceforth referred to as the “stance-phase H-reflex”) and mid-late-swing phase H-reflex (henceforth referred to as the “swing-phase H-reflex”), respectively.
The Modulation Index (MI) of the locomotor H-reflex over the step cycle (Zehr & Kido, 2001; Kido et al., 2004a; Makihara et al., 2012) was high before the 30 conditioning sessions (DC participants: 90±8(SD)%) or the 30 control sessions (NS participants: 93±13%), and did not change significantly after the 30 sessions (DC participants: 91±9%; NS participants: 91±13%). The fact that the locomotor H-reflex MI was high even before conditioning, as it is in neurologically normal individuals (Kido et al., 2004a; Makihara et al., 2014), was expected, since the participants in this study were community ambulators (baseline 10-m walking speed: 1.02±0.43(SD) m/s; 3.7 km/h). However, the phase dependence of their modulation was not normal: the H-reflex was abnormally large during the swing phase of walking, during which the H-reflex is very small or absent in normal individuals (i.e., <5% Mmax (Makihara et al., 2012, 2014)).
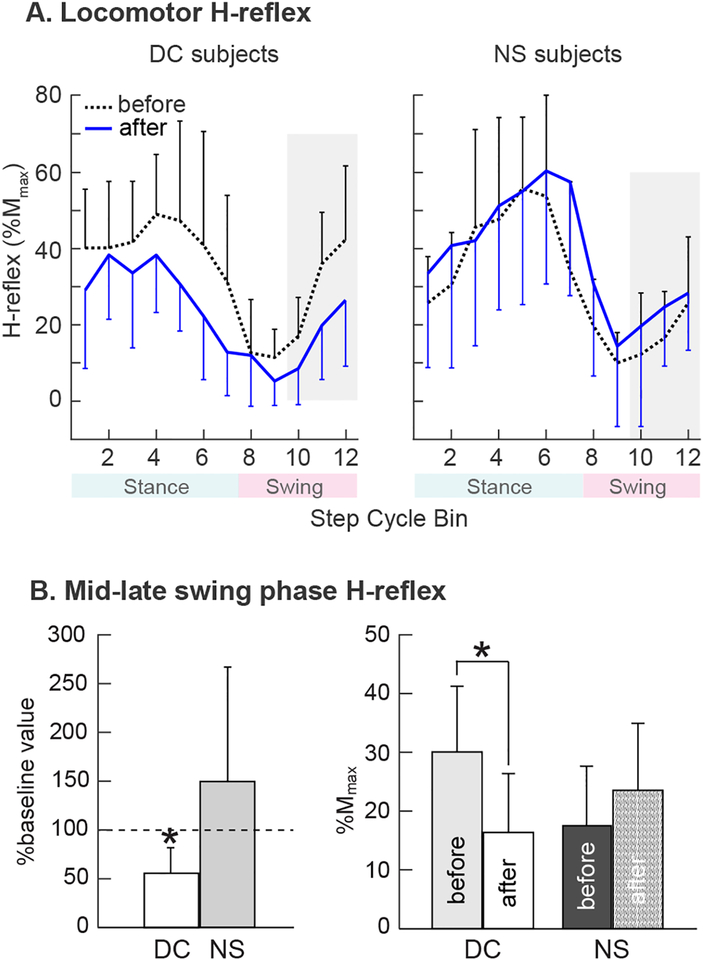
Figure 4A shows average H-reflex modulation over the step cycle in successful DC participants and NS participants before and after the 30 conditioning or control sessions. In the successful DC participants, the average locomotor H-reflex over the entire step cycle decreased by 29% (from 34±14(SD)% Mmax to 24±11% Mmax)(p=0.007 by paired t test); the average stance-phase H-reflex decreased by 34% (from 47±22(SD)% Mmax to 31±13% Mmax) (p=0.04); and the average swing-phase H-reflex decreased by 43% (from 30±11(SD)% Mmax to 17±10% Mmax)(p=0.02, Fig. 4B). The sizes of the M-waves (in %Mmax) associated with the H-reflexes did not change (p>0.39 for all comparisons). Thus, while down-conditioning of the swing-phase H-reflex had its greatest effect on the swing-phase H-reflex, it also reduced the H-reflex elsewhere in the step cycle.
Figure 4.
A: Locomotor H-reflex (mean±SD) in DC participants in whom the H-reflex decreased significantly (N=6, left) and NS participants (N=6, right) before and after 30 conditioning or control sessions. The step cycle is divided into 12 equal bins, starting from foot contact. Thus, bins 1–7 are for the stance phase and bins 8–12 are for the swing phase. In DC participants, H-reflex decreased in the swing phase and also in the stance phase. Such decreases were not present in NS participants. B: H-reflex in the mid-late swing phase (i.e., bins 10–12, highlighted in A) measured before and after 30 conditioning or control sessions. An asterisk indicates a significant difference (p<0.05, t-test) from the baseline value (left) or between the measurements (right).
In contrast, the locomotor H-reflexes of the NS participants did not change significantly: the average H-reflex over the step cycle increased by 19% (from 31±11(SD)%Mmax to 37±15% Mmax) (p=0.09); the average stance-phase H-reflex increased by 6% (from 52±24(SD)%Mmax to 55±29% Mmax) (p=0.21); and the average swing-phase H-reflex increased by 35% (from 17±11(SD)% Mmax to 23±15% Mmax) (p=0.17) (Fig. 4B). The sizes of the M-waves (in %Mmax) associated with the H-reflexes did not change (p>0.19 for all comparisons). Thus, the decreases in the locomotor H-reflex observed in successful DC participants cannot be attributed to simply walking on the treadmill for 30 sessions over 10 weeks.
The H-Reflex during standing
In the DC group, H-M recruitment curves were measured at each session (including the locomotor assessment sessions); and in the NS group they were measured in the locomotor assessment sessions before and after the 30 control sessions. These curves were obtained with the participant standing and providing fixed levels of ongoing soleus and TA EMG activity. These data allowed us to assess the impact of the 30 conditioning or control sessions on Mmax and Hmax, and, for the DC participants, on the size of the standing H-reflex (Hs) elicited by a stimulus that produced an M-wave of the same size produced by the stimulus used to elicit the H-reflex during locomotion. Mmax did not change significantly in either group: in the DC group, Mmax was 6.0±1.4(SD) mV before and 6.2±1.4 mV after (p=0.34 by paired t-test); in the NS group, it averaged 5.2±1.6 mV before and 4.7±1.7 mV after (p=0.18).
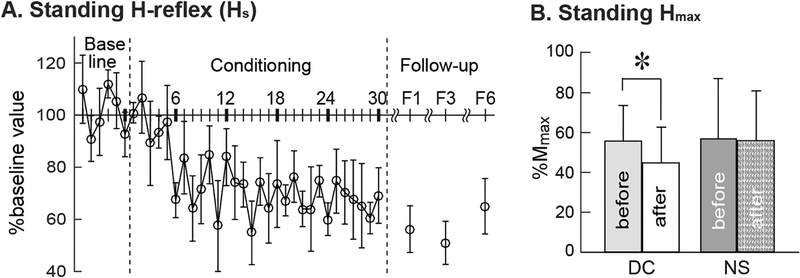
Figure 5B shows Hmax during standing measured before and after the 30 conditioning or control sessions. In the successful DC participants, Hmax fell to 80±19(SD)% of its pre-conditioning value (from 56±18(SD)%Mmax before to 45±18% Mmax after) (p=0.03 by paired t test). In the NC group, the Hmax during standing did not change significantly after 30 control sesssions; it was 58±30(SD)% Mmax before the sessions and 56±25% Mmax after (p=0.45 by paired t test).
Figure 5.
A: Average (±SE) standing H-reflex values for baseline, conditioning, and follow-up sessions for the present DC group participants with SCI (N=6) in whom the late-swing phase H-reflex decreased significantly. Follow-up data are from all 6 participants for 1 month follow-up (F1) and from 4 for 3 and 6 months (F3 and F6) follow-up sessions. B: The Hmax during standing (mean±SD) was measured before and after 30 conditioning or control sessions.
Figure 5A shows the course of change in the H-reflex elicited during standing (i.e., Hs) over the baseline sessions, the 30 conditioning sessions, and the follow-up sessions for the successful DC participants. The stimulus level used to obtain these standing H-reflexes was the same as that used during the swing-phase H-reflex trials (i.e., they produced M-waves of very similar size). Soleus and TA background EMG during these Hs trials remain stable throughout the study (p>0.69 and 0.22, by repeated measures ANOVA). Similar to the swing-phase control reflex, this standing control H-reflex started to decrease rapidly around conditioning session 6, and was significantly decreased for conditioning sessions 7–12, 13–18, 19–24, 25–30 (p<0.05 for each, one-way repeated measured ANOVA and Newman–Keuls test as post hoc). However, it did not appear to decrease as much as the swing-phase control reflex: final Hs size was 65±15(SD)% of baseline versus 45±27% for the swing-phase control H-reflex (p=0.15 by paired t-test).
Walking speed and locomotor EMG Activity
In the 6 successful DC participants, 10-m walking speed increased significantly over the 30 conditioning sessions; the final value was 112±9(SD)% of baseline, and represented an average increase of 0.12±0.08(SD) m/s (from 1.04 to 1.16 m/s, p=0.02, paired t test). The speed increase ranged from 0.04 m/s to 0.26 m/s across participants. For 10-m walking speed in individuals with SCI similar to the present participants, several groups have defined values for the smallest real difference (SRD) of 0.05–0.10 m/s and values for the minimally clinically important difference (MCID) of 0.10–0.15 m/s (Lam et al., 2008; Forrest et al., 2014; Musselman & Yang, 2014; Yang et al., 2014). Five of the six successful DC participants met or exceeded the SRD range, and three met or exceeded the MCID range. Walking speed increase was not correlated with the final H-reflex size (correlation coefficient r=0.33). In contrast, in the NS participants, walking speed did not change; the final value was 99±8(SD)% of baseline, reflecting an average change of 0.01±0.04 m/s (from 0.896 to 0.902 m/s, p=0.89).
To further assess possible changes in walking in the DC and NS participants, locomotor EMG activity was recorded from the soleus, TA, vastus lateralis (VL), and biceps femoris (BF) muscles of both legs before and after the 30 conditioning or control sessions, and each muscle’s Modulation Index (MI) was calculated as described in the Methods.
MI values varied across participants and across the 8 muscles of each participant. In the 6 DC participants in whom the H-reflex decreased, the average MI of the conditioned leg rose from 84±9(SD)% to 87±7% and the average MI of the contralateral leg changed from 86±10% to 88±7%. The average MI across both legs rose significantly from 85±9% to 88±7% (p=0.003, paired t-test). This improvement in EMG modulation is similar to our previous study of steady-state down-conditioning in people with SCI (Thompson et al., 2013). Thus, successful H-reflex down-conditioning was associated with significant increase in the degree to which ankle and knee flexor and extensor muscles of both legs modulated their activity in synchrony with the step cycle. In the NS participants, the average MI across the 8 muscles did not change (81±14% before and 81±14% after, p=0.47).
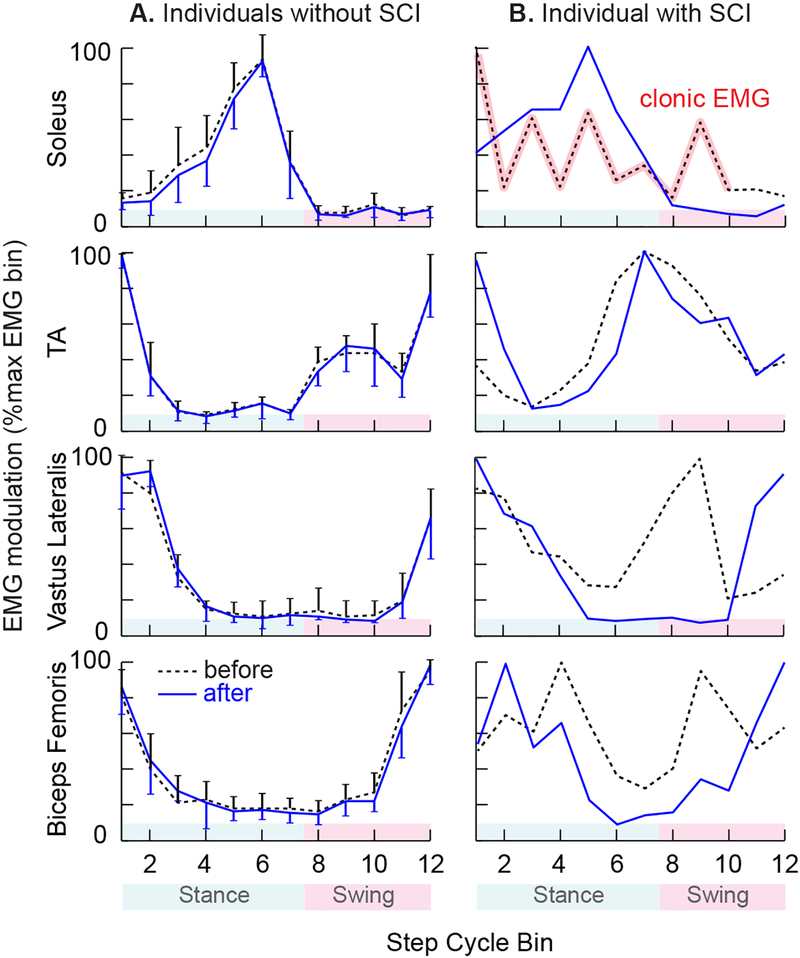
Figure 6 shows average locomotor EMG modulation in the muscles of the conditioned leg in normal participants (N=7) before and after successful steady-state H-reflex down-conditioning during standing (from Makihara et al., (2014)) and in a DC participant before and after successful swing-phase H-reflex down-conditioning. As discussed in Makihara et al. (2014), successful H-reflex down-conditioning does not disturb normal locomotor EMG activity in normal participants (Fig. 6A). In contrast, in a participant with SCI in whom late-swing phase H-reflex down-conditioning was successful, EMG modulation over the step cycle improved in both distal and proximal muscles (e.g., Fig. 6B). Notably, the clonic soleus activity was replaced by an almost normal locomotor burst. Furthermore, TA activity increased around foot contact, which would help to stabilize the ankle; and the activity of the two proximal muscles became nearly normal. These improvements were still present 6 months after conditioning ended (not shown in the figure).
Figure 6.
Average locomotor EMG activity from soleus, tibialis anterior (TA), vastus lateralis, and biceps femoris of the conditioned leg, before (dashed) and after (solid) the 30 soleus H-reflex down-conditioning sessions. For each muscle, EMG amplitude in each of the 12 equal bins was normalized to the amplitude in the bin with the highest amplitude. A: Locomotor EMG in normal participants (N=7) in whom H-reflex conditioning during standing was successful (modified from Makihara et al., 2014). As discussed in Makihara et al. (2014), successful H-reflex down-conditioning does not disturb normal locomotor EMG activity in normal participants. An error bar indicates 1SD for each bin. B: Locomotor EMG in a participant with SCI in whom swing-phase H-reflex down-conditioning was successful. EMG modulation over the step cycle improved after conditioning in both distal and proximal muscles (compare to normal participants). The clonic soleus activity was replaced by an almost normal locomotor burst, TA activity at the onset of stance increased (i.e., helping to stabilize the ankle around foot contact), and vastus lateralis and biceps femoris activity became much more normal.
DISCUSSION
Earlier studies used steady-state operant conditioning protocols to change the excitability of a spinal reflex pathway. In contrast, this study used a swing-phase operant conditioning protocol to change the participation of the reflex pathway in the swing-phase of locomotion. Thus, while the original protocol simply sought to change the reflex excitability, the new protocol described here is more focused; it seeks to change the functioning of the reflex pathway in a specific phase of a dynamic movement. We studied people with spastic hyperreflexia and moderately impaired locomotion due to chronic spinal cord injury. They were an appropriate study population because hyperreflexia during the swing-phase was likely to contribute to their impairment (Yang et al., 1991; Fung & Barbeau, 1994; Thompson et al., 2016) and because they walked well enough to be able to attend to the conditioning protocol while walking on a treadmill.
The results are clear. Swing-phase down-conditioning was successful in 6 of the 7 DC participants (86%), indicating the feasibility of operantly conditioning a reflex in a specific phase of a dynamic movement. Furthermore, the swing-phase conditioning protocol reduced the swing-phase soleus H-reflex faster and farther than the steady-state down-conditioning protocol decreased the standing H-reflex in people with SCI (Thompson et al., 2013). Indeed, the rate and magnitude of reflex decrease with the swing-phase protocol were greater than those with the steady-state protocol in neurologically normal people, monkeys, rats, or mice (reviewed in (Thompson & Wolpaw, 2014)). It is unlikely that such faster and greater reflex change was simply a corrective response to the perturbation caused by H-reflex elicitation, since the swing-phase H-reflex did not decrease over the 6 baseline sessions in which the participant received no instructions, visual feedback, or encouragement to reduce H-reflex size. The H-reflex only began to decrease with the down-conditioning sessions, in which such instructions, feedback, and encouragement were provided.
Furthermore, in contrast to steady-state conditioning, in which the control H-reflex decrease is much slower and smaller than the conditioned H-reflex decrease (Thompson et al., 2013), swing-phase conditioning decreased the conditioned and control H-reflexes almost in parallel. The control H-reflex decrease lagged only ~2 sessions beyond the conditioned H-reflex decrease and was nearly as large (Fig. 3). At the same time, the two protocols were similar in the onset time and magnitude of task-dependent adaptation (i.e., within-session difference between the control and conditioned H-reflexes) (Thompson et al., 2013).
In the present study, the treadmill-walking inclusion criterion ensured that the participants could attend to the operant conditioning task while walking on the treadmill. Thus, they walked faster than the people studied in Thompson et al. (2013b) (initial walking speed: 1.02±0.12(SE) m/s (present study) vs. 0.39±0.22 m/s (2013 study)). This initial difference in locomotor function is unlikely to account for the difference in speed and magnitude of H-reflex change. The H-reflex decrease produced here by the swing-phase protocol is not only faster and greater than the reflex decreases with the steady-state protocol in previous studies of people with SCI (Segal & Wolf, 1994; Manella et al., 2013; Thompson et al., 2013), it is also faster and greater than the decreases with the steady-state protocol in people or animals with a fully intact CNS (Wolf & Segal, 1996; Thompson et al., 2009a; Makihara et al., 2014; Mrachacz-Kersting & Kersting, 2016). Furthermore, neither in this study nor in Thompson et al. (2013b) did final H-reflex decrease correlate with initial walking speed (r<0.1, p>0.85 for each). Thus, the greater rate and magnitude of reflex decrease with swing-phase conditioning cannot simply be ascribed to milder SCI. Their differences in the speed and magnitude of H-reflex decrease suggest that swing-phase and steady-state conditioning have differences in mechanism.
Mechanisms of H-Reflex Decrease with Swing-Phase Conditioning
In the present context, the mechanisms that might account for the H-reflex decrease with swing-phase conditioning fall into two categories: (1) mechanisms that can occur without changes in posture or in background soleus and TA EMG levels; and (2) mechanisms that involve such changes. The first category is available to both the swing-phase protocol and the steady-state protocol; the second is available to the swing-phase protocol but not to the steady-state protocol.
Studies in rats and non-human primates indicate that the smaller H-reflex produced by steady-state conditioning is largely attributable to a positive shift in motoneuron firing threshold (Carp & Wolpaw, 1994); it is associated with increases in GABAergic input to the motoneuron (Wang et al., 2006; Wang et al., 2012) and in the number of GABAergic interneurons in the ventral horn (Wang et al., 2009). The present finding that swing-phase conditioning also decreases the standing H-reflex (i.e., Fig. 5A) suggests that it too accesses this first category of mechanisms.
Because the steady-state protocol requires maintenance of the same stable posture and the same background soleus and TA EMG levels throughout baseline and conditioning sessions ((Thompson & Wolpaw, 2014, 2015) for review) it cannot access the second category: mechanisms for changing H-reflex size that would also change ongoing EMG levels. In contrast, the swing-phase protocol does not require stable and specific EMG levels or posture prior to reflex elicitation. Thus, it can access mechanisms such as changes in reciprocal inhibition from the antagonist muscle, autogenic Ib inhibition, recurrent inhibition, and cutaneous and joint afferent inputs (Stein, 1995; Windhorst, 1996; Brooke et al., 1997; Zehr & Stein, 1999; Pierrot-Deseilligny & Burke, 2012). These category-2 mechanisms, added to the category-1 mechanisms evident in Fig. 5A, could help explain the unprecedently rapid and large decrease in the swing-phase H-reflex.
At the same time, while the combination of category-1 and category-2 mechanisms might explain the speed and magnitude of decrease in the swing-phase H-reflex, it cannot explain the finding that the decrease in the standing H-reflex was similarly rapid and nearly as large (i.e., Fig. 5A). A possible explanation is provided by recent studies indicating that concurrent aerobic exercise enhances motor learning (Roig et al., 2013; Roig et al., 2016; Singh et al., 2016). Also important may be the fact that, in the context of the swing-phase protocol, a small swing-phase H-reflex is doubly adaptive – it earns a reward on the screen and it is associated with better walking. This explanation is consistent with the observation that the steady-state protocol decreases the control H-reflex significantly more in people with incomplete SCI than in people who are neurologically normal (Thompson et al., 2013). In people with SCI, the decrease in the hyperactivity of the reflex pathway improves walking; in people without SCI, in whom the reflex functions normally, a general decrease of the reflex excitability may necessitate compensatory plasticity to preserve normal walking (Makihara et al., 2014). This explanation is also consistent with the finding in rats with SCI that appropriate H-reflex change continues to progress after conditioning ends, presumably because it benefits walking (Chen et al., 2014b) (Wolpaw (Wolpaw, 2018) for discussion). In sum, the doubly adaptive nature of a small swing-phase H-reflex in the swing-phase protocol may drive category-1 mechanisms more effectively than does the steady-state protocol, thereby accounting for the difference between Figure 5A and Figure 3B (middle panel) in the rapidity and magnitude of H-reflex decrease.
Therapeutic Implications
The present results add to the data indicating that spinal reflex conditioning can enhance functional recovery after SCI (Manella et al., 2013; Thompson et al., 2013). In people with spastic hyperreflexia due to chronic incomplete SCI, swing-phase down-conditioning triggered wider change: locomotor EMG modulation improved in proximal and distal leg muscles of both legs and walking speed increased. While statistically significant, the improvements were relatively modest. This probably reflected the fact that the participants already walked fairly well, considerably better than the participants of Thompson et al. (2013) in whom steady-state down-conditioning greatly improved locomotion. Nevertheless, all but one of the successful DC participants in the present study met or exceeded the range for the smallest real difference (SRD) in 10-m walking speed, and half met or exceeded the range for the minimally clinically important difference (MCID) (Lam et al., 2008; Forrest et al., 2014; Musselman & Yang, 2014; Yang et al., 2014).
The ability of a targeted beneficial change in one key reflex pathway to trigger wider beneficial change in locomotion is consistent with the negotiated equilibrium model of spinal cord function (Wolpaw, 2010, 2018). Acquisition of an appropriate new behavior (e.g., a smaller swing-phase H-reflex) improves the ongoing negotiation among behaviors that determines spinal neuronal and synaptic properties. After SCI and standard rehabilitation, the spinal cord typically reaches a suboptimal local minimum in the multi-dimensional space defined by spinal neuronal and synaptic properties (e.g., reflex hyperactivity persists). By targeting beneficial change in a key reflex pathway, H-reflex down-conditioning enables the spinal cord to escape this local minimum; it thereby triggers a new negotiation in which the new behavior (i.e., a smaller H-reflex) and old behaviors (e.g., locomotion) can act synergistically to reach a superior new equilibrium. Locomotor EMG activity (and kinematics (Chen et al., 2011; Chen et al., 2014a)) change bilaterally and locomotion improves.
The most exciting finding of this study is that the swing-phase protocol decreased the H-reflex much faster and farther than the steady-state protocol (Fig. 3 and Tables 2 and 3). Furthermore, the decrease persisted for at least 6 months after conditioning ended. These results imply that the number of conditioning sessions could be considerably reduced, thereby enhancing the clinical practicality and appeal of spinal reflex conditioning. At the same time, as noted above, the demands of treadmill walking during conditioning made the swing-phase protocol inaccessible to participants with more severe locomotor impairments. Initial animal data suggest that the rapid reflex change found here might also occur in participants who undergo steady-state conditioning trials in close proximity to locomotor practice (Chen et al., 2017). This would enable more rapid conditioning even in people with more impaired locomotion. The present results also encourage development of reflex conditioning protocols that target beneficial changes in reflex function in specific phases of other movements (e.g., reach and grasp).
Conclusions
In people with hyperreflexia due to chronic incomplete SCI, an operant down-conditioning protocol applied to the H-reflex during the swing-phase of locomotion decreased the reflex much faster and farther than did the steady-state operant conditioning protocol used in previous studies of animal or humans with or without SCI. The rapid large decrease in the swing-phase H-reflex was accompanied by rapid decrease in the standing H-reflex and in the H-reflex elsewhere in the step cycle. It was also associated with faster walking speed and improved modulation of locomotor EMG activity in the proximal and distal leg muscles. H-reflex decrease persisted for at least 6 months after conditioning ended. The results illuminate the factors affecting the rate and magnitude of spinal reflex conditioning; they thereby indicate how the efficacy and efficiency of this novel therapeutic method might be further enhanced. Protocols that target reflex function in a specific movement phase offer a focused and flexible new approach to improving functional recovery after SCI or in other disorders.
KEY POINTS SUMMARY.
In people or animals with incomplete spinal cord injury (SCI), changing a spinal reflex through an operant conditioning protocol can improve locomotion.
All previous studies conditioned the reflex during steady-state maintenance of a specific posture. In contrast, this new study down-conditioned the reflex during the swing-phase of locomotion in people with hyperreflexia due to chronic incomplete SCI; it sought to modify the functioning of the reflex in a specific phase of a dynamic movement.
This novel swing-phase conditioning protocol decreased the reflex much faster and farther than did the steady-state protocol in people or animals with or without SCI, and it improved locomotion.
The reflex decrease persisted for at least 6 months after conditioning ended.
The results suggest that conditioning reflex function in a specific phase of a dynamic movement offers a new approach to enhancing and/or accelerating recovery after SCI or in other disorders.
Acknowledgments
We thank Dr. Elizabeth Winter Wolpaw for helpful comments on the manuscript, Dr. Wayne Feng and the late Dr. Ferne Pomerantz for participant screening, Ms. Jodi Brangaccio, Ms. Gina Fiorenza, Ms. Stephanie Pudlik, and Ms. Rachel Cote for assistance in data collection and analysis, and Ms. Laura Tenteromano and Ms. Christina Gill for assistance in participant recruitment.
Funding
This work was supported in part by the National Institutes of Health [NS069551 to AKT, NS022189 and P41-EB018783 to JRW, NS061823 to JRW and Xiang Yang Chen]; the South Carolina Spinal Cord Injury Research Fund (AKT); and a National Institutes of Health (NIGMS) Institutional Development Award (IDeA) U54-GM104941 (Binder-MacLeod).
Footnotes
Competing interests
AKT and JRW hold several patents related to operant conditioning of spinal reflexes (US patent number 8862236, 9138579, and 9545515). These patents are not providing income in any form.
References
- Boorman GI, Lee RG, Becker WJ & Windhorst UR. (1996). Impaired “natural reciprocal inhibition” in patients with spasticity due to incomplete spinal cord injury. Electroencephalography and Clinical Neurophysiology 101, 84–92. [DOI] [PubMed] [Google Scholar]
- Brooke JD, Cheng J, Collins DF, McIlroy WE, Misiaszek JE & Staines WR. (1997). Sensori-sensory afferent conditioning with leg movement: gain control in spinal reflex and ascending paths. Progress in Neurobiology 51, 393–421. [DOI] [PubMed] [Google Scholar]
- Capaday C & Stein RB. (1986). Amplitude modulation of the soleus H-reflex in the human during walking and standing. Journal of Neuroscience 6, 1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp JS, Tennissen AM, Chen XY & Wolpaw JR. (2006a). Diurnal H-reflex variation in mice. Exp Brain Res 168, 517–528. [DOI] [PubMed] [Google Scholar]
- Carp JS, Tennissen AM, Chen XY & Wolpaw JR. (2006b). H-reflex operant conditioning in mice. J Neurophysiol 96, 1718–1727. [DOI] [PubMed] [Google Scholar]
- Carp JS & Wolpaw JR. (1994). Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. Journal of Neurophysiology 72, 431–442. [DOI] [PubMed] [Google Scholar]
- Chen XY, Chen L, Chen Y & Wolpaw JR. (2006a). Operant conditioning of reciprocal inhibition in rat soleus muscle. J Neurophysiol 96, 2144–2150. [DOI] [PubMed] [Google Scholar]
- Chen XY & Wolpaw JR. (1994). Circadian rhythm in rat H-reflex. Brain Res 648, 167–170. [PubMed] [Google Scholar]
- Chen XY & Wolpaw JR. (1995a). Operant conditioning of H-reflex in freely moving rats. J Neurophysiol 73, 411–415. [DOI] [PubMed] [Google Scholar]
- Chen XY & Wolpaw JR. (1995b). Operant conditioning of H-reflex in freely moving rats. Journal of Neurophysiology 73, 411–415. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen L, Liu R, Wang Y, Chen XY & Wolpaw JR. (2014a). Locomotor impact of beneficial or nonbeneficial H-reflex conditioning after spinal cord injury. J Neurophysiol 111, 1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen L, Wang Y, Wolpaw JR & Chen XY. (2011). Operant conditioning of rat soleus H-reflex oppositely affects another H-reflex and changes locomotor kinematics. J Neurosci 31, 11370–11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen L, Wang Y, Wolpaw JR & Chen XY. (2014b). Persistent beneficial impact of H-reflex conditioning in spinal cord-injured rats. J Neurophysiol 112, 2374–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen L, Yang X, Wang Y, Wolpaw JR & Chen X. (2017). Effects of combining H-reflex conditioning and locomotor training on locomotor recovery in rats with lateral column transection: Initial study. In Neuroscience 2017, pp. 780.709. Society for Neuroscience, Washington, DC. [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT & Wolpaw JR. (2006b). Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci 26, 12537–12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcos DM, Gottlieb GL, Penn RD, Myklebust B & Agarwal GC. (1986). Movement deficits caused by hyperexcitable stretch reflexes in spastic humans. Brain 109 ( Pt 5), 1043–1058. [DOI] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering-Sorensen F & Nielsen JB. (2003). Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain 126, 495–507. [DOI] [PubMed] [Google Scholar]
- Dietz V & Sinkjaer T. (2007). Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol 6, 725–733. [DOI] [PubMed] [Google Scholar]
- Edamura M, Yang JF & Stein RB. (1991). Factors that determine the magnitude and time course of human H-reflexes in locomotion. Journal of Neuroscience 11, 420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier C, Imbeault MA, Ung V & Capaday C. (2003). On the soleus H-reflex modulation pattern during walking. Exp Brain Res 151, 420–425. [DOI] [PubMed] [Google Scholar]
- Forrest GF, Hutchinson K, Lorenz DJ, Buehner JJ, Vanhiel LR, Sisto SA & Basso DM. (2014). Are the 10 meter and 6 minute walk tests redundant in patients with spinal cord injury? PloS one 9, e94108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung J & Barbeau H. (1989). A dynamic EMG profile index to quantify muscular activation disorder in spastic paretic gait. Electroencephalogr Clin Neurophysiol 73, 233–244. [DOI] [PubMed] [Google Scholar]
- Fung J & Barbeau H. (1994). Effects of conditioning cutaneomuscular stimulation on the soleus H-reflex in normal and spastic paretic subjects during walking and standing. J Neurophysiol 72, 2090–2104. [DOI] [PubMed] [Google Scholar]
- Hedges LV. (1981). Distribution Theory for Glass’s Estimator of Effect Size and Related Estimators. Journal of Educational Statistics 6, 107–128. [Google Scholar]
- Hidler JM & Rymer WZ. (1999). A simulation study of reflex instability in spasticity: origins of clonus. IEEE Trans Rehabil Eng 7, 327–340. [DOI] [PubMed] [Google Scholar]
- Hidler JM & Rymer WZ. (2000). Limit cycle behavior in spasticity: analysis and evaluation. IEEE Trans Biomed Eng 47, 1565–1575. [DOI] [PubMed] [Google Scholar]
- Hiersemenzel LP, Curt A & Dietz V. (2000). From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology 54, 1574–1582. [DOI] [PubMed] [Google Scholar]
- Khan AS, Patrick SK, Roy FD, Gorassini MA & Yang JF. (2016). Training-Specific Neural Plasticity in Spinal Reflexes after Incomplete Spinal Cord Injury. Neural Plast 2016, 6718763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido A, Tanaka N & Stein RB. (2004a). Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol 82, 238–248. [DOI] [PubMed] [Google Scholar]
- Kido A, Tanaka N & Stein RB. (2004b). Spinal reciprocal inhibition in human locomotion. J Appl Physiol 96, 1969–1977. [DOI] [PubMed] [Google Scholar]
- Lagerquist O, Zehr EP, Baldwin ER, Klakowicz PM & Collins DF. (2006). Diurnal changes in the amplitude of the Hoffmann reflex in the human soleus but not in the flexor carpi radialis muscle. Exp Brain Res 170, 1–6. [DOI] [PubMed] [Google Scholar]
- Lam T, Noonan VK, Eng JJ & Team SR. (2008). A systematic review of functional ambulation outcome measures in spinal cord injury. Spinal Cord 46, 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JW, Ditunno JF Jr., Stiens SA & Harris RM. (1999). Incomplete spinal cord injury: neuronal mechanisms of motor recovery and hyperreflexia. Archives of Physical Medicine and Rehabilitation 80, 587–599. [DOI] [PubMed] [Google Scholar]
- Llewellyn M, Yang JF & Prochazka A. (1990). Human H-reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp Brain Res 83, 22–28. [DOI] [PubMed] [Google Scholar]
- Makihara Y, Segal RL, Wolpaw JR & Thompson AK. (2012). H-reflex modulation in the human medial and lateral gastrocnemii during standing and walking. Muscle Nerve 45, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makihara Y, Segal RL, Wolpaw JR & Thompson AK. (2014). Operant conditioning of the soleus H-reflex does not induce long-term changes in the gastrocnemius H-reflexes and does not disturb normal locomotion in humans. J Neurophysiol 112, 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manella KJ, Roach KE & Field-Fote EC. (2013). Operant conditioning to increase ankle control or decrease reflex excitability improves reflex modulation and walking function in chronic spinal cord injury. J Neurophysiol 109, 2666–2679. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kersting N & Kersting UG. (2016). Operant conditioning of the human soleus short latency stretch reflex and implications for the medium latency soleus stretch reflex. In Converging Clinical and Engineering Research on Neurorehabilitation: Proceedings of the 3rd International Conference on NeuroRehabilitation (ICNR2016), ed. Ibáñez, González-Vargas J, Azorín JA, Akay A & Pons JL, pp. 59–63. Springer International Publishing AG, Cham, Switzerland. [Google Scholar]
- Musselman KE & Yang JF. (2014). Spinal Cord Injury Functional Ambulation Profile: a preliminary look at responsiveness. Phys Ther 94, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Kawashima N & Akai M. (2006). Enhanced stretch reflex excitability of the soleus muscle in persons with incomplete rather than complete chronic spinal cord injury. Arch Phys Med Rehabil 87, 71–75. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Crone C & Hultborn H. (2007). The spinal pathophysiology of spasticity--from a basic science point of view. Acta Physiol (Oxf) 189, 171–180. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E & Burke D. (2012). The Circuitry of the Human Spinal Cord: Spinal and Corticospinal Mechanisms of Movement. Cambridge University Press. [Google Scholar]
- Roig M, Nordbrandt S, Geertsen SS & Nielsen JB. (2013). The effects of cardiovascular exercise on human memory: a review with meta-analysis. Neurosci Biobehav Rev 37, 1645–1666. [DOI] [PubMed] [Google Scholar]
- Roig M, Thomas R, Mang CS, Snow NJ, Ostadan F, Boyd LA & Lundbye-Jensen J. (2016). Time-Dependent Effects of Cardiovascular Exercise on Memory. Exerc Sport Sci Rev 44, 81–88. [DOI] [PubMed] [Google Scholar]
- Segal RL & Wolf SL. (1994). Operant conditioning of spinal stretch reflexes in patients with spinal cord injuries. Exp Neurol 130, 202–213. [DOI] [PubMed] [Google Scholar]
- Singh AM, Neva JL & Staines WR. (2016). Aerobic exercise enhances neural correlates of motor skill learning. Behav Brain Res 301, 19–26. [DOI] [PubMed] [Google Scholar]
- Stein RB. (1995). Presynaptic inhibition in humans. Progress in Neurobiology 47, 533–544. [DOI] [PubMed] [Google Scholar]
- Stein RB & Capaday C. (1988). The modulation of human reflexes during functional motor tasks. Trends in Neurosciences 11, 328–332. [DOI] [PubMed] [Google Scholar]
- Stein RB, Yang JF, Belanger M & Pearson KG. (1993). Modification of reflexes in normal and abnormal movements. Progress in Brain Research 97, 189–196. [DOI] [PubMed] [Google Scholar]
- Thompson AK, Chen XY & Wolpaw JR. (2009a). Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci 29, 5784–5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Estabrooks KL, Chong S & Stein RB. (2009b). Spinal reflexes in ankle flexor and extensor muscles after chronic central nervous system lesions and functional electrical stimulation. Neurorehabil Neural Repair 23, 133–142. [DOI] [PubMed] [Google Scholar]
- Thompson AK, Mrachacz-Kersting N, Sinkjær T & Andersen JB. (2016). Soleus stretch reflex modulation during walking in people with chronic incomplete spinal cord injury. In Society for Neuroscience 46th Annual Meeting, pp. Program No. 158.102. San Diego, CA. [Google Scholar]
- Thompson AK, Pomerantz FR & Wolpaw JR. (2013). Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci 33, 2365–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK & Wolpaw JR. (2014). Operant conditioning of spinal reflexes: from basic science to clinical therapy. Front Integr Neurosci 8, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK & Wolpaw JR. (2015). Targeted neuroplasticity for rehabilitation. Prog Brain Res 218, 157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen Y, Chen L, Wolpaw JR & Chen XY. (2012). Cortical stimulation causes long-term changes in H-reflexes and spinal motoneuron GABA receptors. J Neurophysiol 108, 2668–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Pillai S, Wolpaw JR & Chen XY. (2006). Motor learning changes GABAergic terminals on spinal motoneurons in normal rats. Eur J Neurosci 23, 141–150. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pillai S, Wolpaw JR & Chen XY. (2009). H-reflex down-conditioning greatly increases the number of identifiable GABAergic interneurons in rat ventral horn. Neurosci Lett 452, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windhorst U (1996). On the role of recurrent inhibitory feedback in motor control. Prog Neurobiol 49, 517–587. [DOI] [PubMed] [Google Scholar]
- Wolf SL & Segal RL. (1996). Reducing human biceps brachii spinal stretch reflex magnitude. J Neurophysiol 75, 1637–1646. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. (1987). Operant conditioning of primate spinal reflexes: the H-reflex. Journal of Neurophysiology 57, 443–459. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. (1997). The complex structure of a simple memory. Trends Neurosci 20, 588–594. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. (2006). The education and re-education of the spinal cord. Prog Brain Res 157, 261–280. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. (2010). What can the spinal cord teach us about learning and memory? Neuroscientist 16, 532–549. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. (2018). The negotiated equilibrium model of spinal cord function. J Physiol 596, 3469–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, Braitman DJ & Seegal RF. (1983). Adaptive plasticity in primate spinal stretch reflex: initial development. J Neurophysiol 50, 1296–1311. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR & Chen XY. (2009). Operant conditioning of reflexes. In Encyclopedia of Neuroscience, ed. Squire LR, pp. 225–233. Oxford: Academic Press. [Google Scholar]
- Wolpaw JR & O’Keefe JA. (1984). Adaptive plasticity in the primate spinal stretch reflex: evidence for a two-phase process. J Neurosci 4, 2718–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR & Seegal RF. (1982). Diurnal rhythm in the spinal stretch reflex. Brain Res 244, 365–369. [DOI] [PubMed] [Google Scholar]
- Yang JF, Fung J, Edamura M, Blunt R, Stein RB & Barbeau H. (1991). H-reflex modulation during walking in spastic paretic subjects. Canadian Journal of Neurological Sciences 18, 443–452. [DOI] [PubMed] [Google Scholar]
- Yang JF, Musselman KE, Livingstone D, Brunton K, Hendricks G, Hill D & Gorassini M. (2014). Repetitive mass practice or focused precise practice for retraining walking after incomplete spinal cord injury? A pilot randomized clinical trial. Neurorehabil Neural Repair 28, 314–324. [DOI] [PubMed] [Google Scholar]
- Zehr EP & Kido A. (2001). Neural control of rhythmic, cyclical human arm movement: task dependency, nerve specificity and phase modulation of cutaneous reflexes. Journal of Physiology 537, 1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP & Loadman PM. (2012). Persistence of locomotor-related interlimb reflex networks during walking after stroke. Clin Neurophysiol 123, 796–807. [DOI] [PubMed] [Google Scholar]
- Zehr EP & Stein RB. (1999). What functions do reflexes serve during human locomotion? Progress in Neurobiology 58, 185–205. [DOI] [PubMed] [Google Scholar]