Abstract
Importance:
Limited empirical research has examined the extent to which cohort-level prevalence of substance use predicts onset of and transitioning into greater involvement in drug use.
Objective:
To use cross-national data to examine time-space variation in cohort-level drug use to predict onset and transitions across stages of drug use, abuse, dependence, and remission.
Design, Setting and Participants:
The World Health Organization World Mental Health Surveys carried out cross-sectional general population surveys in 25 countries using a consistent research protocol and assessment instrument. A total of 90,027 adults from representative household samples were interviewed face-to-face in the community in relation to drug use disorders. The surveys were conducted between 2001 and 2015. Data analysis performed from July 2017 to July 2018.
Main outcomes and Measures:
Data on timing of onset of lifetime drug use, DSM-IV drug use disorders, and remission from these disorders was assessed using the Composite International Diagnostic Interview. Associations of cohort-level alcohol prevalence and drug use prevalence were examined as predictors of these transitions.
Results:
Among the 90,027 respondents (48.1% [SE 0.2%] men; mean [SE] age, 42.1 [0.1] years), one in four respondents (24.8%; SE 0.2) reported either illicit drug use or extra-medical use of prescription drugs at some point in their lifetime, but with substantial time-space variation in this prevalence. Among users, 9.1% (SE 0.2) met lifetime criteria for abuse and 5.0% (SE 0.2) dependence. Individuals with polydrug use had an increased risk of both abuse and dependence and reduced probability of remission from abuse. Birth cohort prevalence of drug use was significantly associated with both initiation and illicit drug use transitions. For example, after controlling for individuals’ experience of substance use and demographics, for each additional 10% of an individual’s cohort using alcohol or drugs, a person’s odds of initiating drug use increased by 28% and 12% respectively (OR 1.28 (95%CI:1.26–1.31) and 1.12 (95%CI:1.11–1.14)).
Conclusions and Relevance:
Birth cohort substance use predicts drug use involvement over and above the effects of individuals’ own history of alcohol and other drug use. This has important implications for understanding the causal pathways into and out of problematic drug use.
Keywords: drugs, abuse, dependence, remission, cohort, discrete-time survival analysis, World Mental Health survey
Introduction
Improved understanding of determinants of drug use disorders (DUDs) and transitions through different levels of involvement is important to assist in identifying critical time periods when specific interventions may be best targeted and shed light on potential factors that may affect such trajectories. Research on trajectories of drug use has most often considered the transition between use and dependence1,2 or focused on specific populations such as people in treatment for DUDs3–5.
The general population studies that have explored the natural history of substance use show that social contextual risk factors have a differential role according to transition stage2,6–10. For example, substance use is linked to social and peer-level variables11; and evidence suggests that the extent to which behaviour is normative may be associated with adverse substance use outcomes (with people engaging in less normative behaviour having a greater likelihood of problematic substance use)12, 13.
Previous studies have found that chronological age, historical period and birth cohort effects are associated with differences in substance use and related problems14–17. Age-related differences in substance use and related problems have often been attributed at least in part to developmental and maturational factors18, especially when cross-sectional comparisons are made between age groups within a sample covering a broad age range of a population 19–21.
However, individuals are also strongly influenced by the broader social context in which they live. Substance use influences (e.g., substance use norms, enforcement of sanctions against drug use, drug availability, and perceptions of risk), have varied widely across geographical locations and in different time periods in history. Cohort effects include the shared social and environmental influences on individuals born at particular times as they mature, experiencing the extant period effects, including changes in period effects over time. There are complex issues involved in distinguishing period and cohort effects22,23, and although there is evidence of both influences, research has shown that substance use behaviours are especially related to cohort effects17,24,25, which may modify period effects, and perhaps have other social influences. Supporting this possibility, we previously used a national study of Australian adults to investigate associations of levels of involvement with alcohol and cannabis use with birth cohort use26 and found that the level of alcohol or cannabis use within an individual’s age cohort predicted risks of progressing further into involvement with alcohol and cannabis use, respectively26.
In the current paper, we present for the first time, country-level data on lifetime prevalence of illicit drug use, DSM-IV drug abuse and dependence, and remission from use disorders. We also conduct the first-ever analyses of the influence of cohort effects on individuals’ drug use cross-nationally using the WHO World Mental Health (WMH) Surveys (www.hcp.med.harvard.edu/WMH)27, a unique database made up of 27 population surveys conducted in 25 countries across the globe. We examine the extent to which an individual’s birth cohort’s use of both alcohol and drugs at various points in the life course predicts the individual transitioning across levels of involvement with drug use net of the effects of the individual’s own history of substance use at that point in time.
Method
Sample
Data come from 27 WMH surveys that assessed DUDs. Six surveys were conducted in countries classified by the World Bank at time of data collection as low or lower-middle income (Colombia [national], Iraq, Nigeria, People’s Republic of China [PRC], Peru, and Ukraine), six in countries classified as upper-middle income (Brazil, Bulgaria, Colombia [Medellin-region], Lebanon, Mexico, and South Africa) and 15 in 14 in countries classified as high income (Argentina, Australia, Belgium, France, Germany, Israel, Italy, Japan, Netherlands, New Zealand, Northern Ireland, Poland, Spain [separate national and regional surveys], and the United States). Most surveys were based on nationally representative household samples. The sample characteristics for all participating surveys are shown in eTable 1. Informed consent was obtained before beginning interviews in all countries. Procedures for obtaining informed consent and protecting human subjects were approved and monitored by the Institutional Review Boards of organisations coordinating surveys in each country. Full details of the WMH surveys have been published previously27–31 and are summarised in the eMethods.
Combining participants from all 27 surveys, 90,093 respondents were administered the drug module. Sixty-six respondents (35 from Israel, 15 from Mexico, 11 from Japan, and 5 from South Africa) provided no valid answers to any drug use question and were excluded. Therefore, a total of 90,027 respondents are included in the analyses described here.
Data Analysis
Age of onset and speed of transition between various drug stages were examined. These stages were use (first time using any drug), DSM-IV abuse, DSM-IV dependence, remission from abuse without dependence (defined as absence of all abuse symptoms for more than 12 months at time of interview) and remission from dependence (absence of all dependence symptoms for more than 12 months at time of interview). To improve cross-national comparability, all survey data was restricted to persons aged 18 and over at time of interview.
All analyses were carried out in SAS® Version 9.432 using weighted data, and accounting for the complex survey design features, namely stratification and clustering. Person weights were used to adjust for probability of selection, nonresponse and post-stratification factors, and, as noted above, Part II data weights adjusted for over-sampling of Part I respondents with mental disorders. These weighting procedures ensured that all samples are representative of the survey region’s population at time of data collection.
Life-table (actuarial) estimates of the survival functions for age of onset and remission were produced using the SAS PROC LIFETEST procedure and are reported as weighted prevalence. Discrete-time logistic regression models were used to investigate the impact of cohort and individual substance use variables on commencement of illicit drug use, transitions from use to disorder (abuse and dependence), and from disorder to remission (among those with a valid age of onset of remission – see eMethods). These analyses were conducted in SAS PROC SURVEYLOGISTIC using person-year as the unit of analysis and a logistic link function.
Person-year datasets were created in which each year in the life of each respondent during which they were at risk of transitioning, from the age of onset of the initial stage up to and including the age of onset of the transition or age at interview (whichever came first), was treated as a separate observational record. The year of transition was coded 1 and earlier years coded 0, on a dichotomous response variable. Survival coefficients and standard errors (SEs) are presented as odds ratios (ORs) and 95% confidence intervals (CIs). Multivariable significance tests were made with Wald χ2 tests using Taylor series design-based coefficient variance-covariance matrices and significance evaluated at 0.05 with two-sided tests.
A country/region-specific contextual variable representing cumulative lifetime prevalence of substance use in the individual’s birth cohort at each year of life was constructed and used to predict transitioning to each drug stage. An individual’s birth cohort was based on their year of birth +/− five years, which created 11- year wide survey-specific cohorts centred around their year of birth. The cohort widths were reduced for those aged between 18 and 22 years to, as close as possible, ensure symmetry around birth year; total band width was of size two for 18-year-olds (18–19), three for 19-year-olds, (18–20), five for 20-year-olds (18–22), seven for 21-year-olds (18–24) and nine for 22-year-olds (18–26). Cohorts were top-coded for those aged 65 or older. The predictor variable was the estimated proportion of people (/10) in the individual’s birth cohort who had used the specific substance (either alcohol or drugs) as of each prior year of age; in this way, it captured the percentage of people in the cohort who had already commenced use at any given age. In order to capture only the most prominent changes in cohort use, cohort use prevalence was set to zero for person years below the age of 12 and top-coded for 30 years and over. Linearity of the cohort use variables were investigated.
To investigate the impact of the individual’s own prior involvement with alcohol on risk of drug transitions, four mutually exclusive, time-varying dummy variables were included as predictors for highest lifetime-to-date level of alcohol involvement (none versus either use, abuse, dependence, or remission from abuse/dependence). In addition, models for transitions after first use considered the types of drugs being used, with indicators for onset of cannabis, cocaine and other drug use (prescription drugs combined with ‘other drugs’ due to small numbers) as well as whether two or more of these drug categories had been used. A total of six models investigating cohort and individual substance involvement were investigated: (1) prevalence of cohort drug use, (2) prevalence of cohort alcohol use, (3) individuals’ level of alcohol involvement, (4) type of drugs, (5) number of drugs, and (6) all cohort and individual substance-related variables. All models adjusted for a wide range of variables (see eMethods). Data analysis was performed from July 2017 to July 2018.
Results
Prevalence of use, abuse, dependence, use disorders and remission
Lifetime prevalence estimates for use of any drug and specific drugs are shown in Table 1. Across countries, 24.8% (SE 0.2) of respondents reported lifetime illicit drug use or extra-medical use of prescription drugs. Within each country income grouping, cannabis was the most commonly used drug of those considered; the United States (42.3%) and New Zealand (41.9%) had the highest lifetime cannabis prevalence. The United States (16.2%) and Murcia (Spain, 7.8%) had the highest lifetime prevalence of cocaine use. Highest estimates of extra-medical prescription drug use were observed in some countries in Europe, whereas Iraq (1.3%), China (5.9%), Lebanon (6.3%), Japan (7.0%) and Bulgaria (7.3%) had the lowest rates of any drug use.
Table 1.
Lifetime prevalence of overall drug use and specific drug use in the World Mental Health Surveys
| Countrya | Nb | Cannabis | Cocaine | Prescription drugsf | Other drugs | Any drugsd,e | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| %c | SE | %c | SE | %c | SE | %c | SE | %c | SE | ||
| Low and Lower-Middle | 18,179 | 5.3 | 0.2 | 2.0 | 0.1 | 4.7 | 0.2 | 0.6 | 0.1 | 10.0 | 0.3 |
| Colombia | 4,426 | 10.8 | 0.6 | 4.0 | 0.4 | 2.2 | 0.3 | 0.9 | 0.2 | 12.7 | 0.7 |
| Iraq | 4,332 | 0.0 | 0.0 | 0.0 | 0.0 | 1.2 | 0.2 | 0.1 | 0.0 | 1.3 | 0.2 |
| Nigeria | 2,143 | 2.7 | 0.5 | 0.1 | 0.1 | 18.7 | 1.3 | 0.5 | 0.2 | 20.4 | 1.3 |
| Peru | 3,930 | 7.9 | 0.4 | 4.8 | 0.2 | 4.3 | 0.3 | 1.0 | 0.1 | 13.3 | 0.5 |
| China | 1,628 | 0.3 | 0.1 | 0.0 | 0.0 | 5.8 | 0.9 | 0.2 | 0.2 | 5.9 | 0.9 |
| Ukraine | 1,720 | 6.4 | 1.0 | 0.1 | 0.0 | 2.4 | 0.6 | 0.8 | 0.2 | 8.4 | 1.2 |
| Upper-Middle | 20,051 | 9.2 | 0.3 | 3.1 | 0.2 | 7.8 | 0.4 | 1.5 | 0.1 | 16.2 | 0.5 |
| Brazil | 5,037 | 11.8 | 0.7 | 5.2 | 0.4 | 6.9 | 0.3 | 2.5 | 0.3 | 17.6 | 0.7 |
| Bulgaria | 2,233 | 1.3 | 0.5 | 0.0 | 0.0 | 6.1 | 0.6 | 0.0 | 0.0 | 7.3 | 0.8 |
| Lebanon | 1,031 | 4.6 | 0.9 | 0.7 | 0.3 | 2.0 | 0.6 | 0.3 | 0.2 | 6.2 | 1.1 |
| Medellin | 1,673 | 21.9 | 1.9 | 6.3 | 0.9 | 2.7 | 0.6 | 1.8 | 0.4 | 22.7 | 1.9 |
| Mexico | 5,767 | 7.8 | 0.5 | 4.0 | 0.4 | 1.8 | 0.3 | 1.0 | 0.2 | 10.1 | 0.5 |
| South Africa | 4,310 | 8.4 | 0.6 | 0.7 | 0.3 | 21.5 | 1.5 | 1.7 | 0.3 | 27.2 | 1.7 |
| High | 51,797 | 24 | 0.3 | 4.4 | 0.1 | 13.6 | 0.2 | 6.2 | 0.2 | 33.3 | 0.3 |
| Argentina | 2,116 | 14.2 | 1.0 | 5.8 | 0.6 | 14.4 | 1.1 | 3.3 | 0.5 | 26.3 | 1.3 |
| Australia | 8,463 | 19.8 | 0.6 | 2.9 | 0.3 | 2.5 | 0.2 | 7.3 | 0.4 | 21.4 | 0.6 |
| Belgium | 1,043 | 10.4 | 1.6 | 1.5 | 0.6 | 43.5 | 3.0 | 2.8 | 0.8 | 47.6 | 2.8 |
| France | 1,436 | 19 | 1.6 | 1.5 | 0.4 | 43.4 | 2.0 | 4.8 | 0.7 | 52.7 | 1.7 |
| Germany | 1,323 | 17.5 | 1.6 | 1.9 | 0.5 | 62.3 | 2.5 | 3.4 | 0.7 | 66.4 | 2.5 |
| Israel | 4,824 | 11.5 | 0.5 | 0.9 | 0.1 | 1.7 | 0.2 | 1.8 | 0.2 | 12.9 | 0.5 |
| Italy | 1,779 | 6.6 | 0.8 | 1.0 | 0.3 | 66.0 | 2.0 | 0.9 | 0.2 | 66.8 | 2.0 |
| Japan | 1,671 | 1.5 | 0.4 | 0.5 | 0.2 | 4.8 | 0.7 | 1.8 | 0.5 | 7.0 | 0.8 |
| Murcia | 1,459 | 23.1 | 1.3 | 7.8 | 1.1 | 0.9 | 0.5 | 3.1 | 0.8 | 24.2 | 1.5 |
| Netherlands | 1,094 | 19.8 | 1.3 | 1.9 | 0.2 | 20.1 | 2.4 | 4.1 | 0.8 | 35.9 | 2.4 |
| New Zealand | 12,790 | 41.9 | 0.7 | 4.3 | 0.3 | 6.6 | 0.3 | 10.2 | 0.4 | 42.9 | 0.7 |
| Northern Ireland | 1,986 | 17.3 | 1.1 | 3.5 | 0.5 | 2.3 | 0.4 | 4.5 | 0.7 | 18.2 | 1.2 |
| Poland | 4,000 | 3.8 | 0.3 | 0.4 | 0.1 | 5.1 | 0.3 | 1.5 | 0.2 | 8.7 | 0.5 |
| Spain | 2,121 | 15.9 | 1.3 | 4.1 | 0.7 | 61.5 | 2.6 | 3.5 | 0.7 | 64.5 | 2.6 |
| United States | 5,692 | 42.3 | 1.0 | 16.2 | 0.6 | 11.3 | 0.5 | 11.1 | 0.6 | 44.2 | 1.1 |
| All Countries | 90,027 | 16.9 | 0.2 | 3.6 | 0.1 | 10.5 | 0.2 | 4.0 | 0.1 | 24.8 | 0.2 |
SE - standard error;
County income group reflects economic development status at time of data collection based on The World Bank country level ranking.
N = The total unweighted number of respondents who responded to illicit drug use question(s) (for the specific drug, where applicable).
Prevalence estimates are based on weighted data.
Used at least one of the drug categories considered; cannabis, cocaine, prescription drugs and other drugs. Any drugs not captured by the first three categories were grouped as ‘other drugs’ (more detail provided in Methods).
Respondents were included in the ‘Any drugs’ category if they provided information relating to the use of at least one drug.
All ESEMED surveys (Belgium, France, Germany, Italy, Netherlands and Spain) asked three separate extra-medical use questions (1. Used without a prescription; 2. Used more than prescribed; and, 3. Used so regularly in a non-medical setting that you couldn’t stop) for each prescription drug category. In contrast, most other surveys asked a single question pertaining to any extra-medical use of specific/any prescription drugs. The more detailed question structure in the ESEMED interviews is likely the reason for the high rates of prescription drug use in these surveys.
Table 2 shows prevalence estimates of lifetime DUDs overall and conditional on ever having used drugs, as well as remission rates overall and among those with the specific use disorders. The lifetime prevalence (SE) of drug abuse and drug dependence in the total sample were 2.2% (0.1) and 1.2% (0.1), respectively (Table 2). Again, there was considerable geographic variation. Around one in seven drug users developed a DUD (14.0%; SE 0.3), with the rate of abuse (9.1%; SE 0.2) higher than dependence (5.0%; SE 0.2). Remission prevalence rates for the entire cohort were 1.8% (0.1) for abuse and 0.9% (<0.1) for dependence. Conditional remission estimates were 78.0% (1.1) for drug abuse and 70.7% (1.7) for drug dependence.
Table 2.
Conditional lifetime prevalence of DSM-IV drug use disorders and remission in the World Mental Health surveysa
| Countryb | Nd | Prevalence | Conditional prevalenceg | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abusec | Dependence | Remission from abusec |
Remission from dependence |
Abusec among users |
Dependence among users |
Any drug use disorder among users |
Remission among LT abuse casesf |
Remission among LT dependence cases |
|||||||||||
| %e | SE | %e | SE | %e | SE | %e | SE | %d | SE | %d | SE | %d | SE | %d | SE | %d | SE | ||
| Low and Lower-Middle | 18,179 | 0.6 | 0.1 | 0.3 | 0.0 | 0.5 | 0.1 | 0.2 | 0 | 6.1 | 0.6 | 3.2 | 0.5 | 9.3 | 0.9 | 75 | 5.5 | 56.4 | 6.7 |
| Colombia | 4,426 | 0.9 | 0.2 | 0.8 | 0.2 | 0.7 | 0.1 | 0.4 | 0.1 | 6.8 | 1.3 | 6.4 | 1.3 | 13.2 | 2.1 | 75 | 8.3 | 54.7 | 7.6 |
| Iraq | 4,332 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 11.4 | 7.6 | 0.9 | 0.9 | 12.3 | 7.6 | - | - | - | - |
| Nigeria | 2,143 | 1.0 | 0.2 | 0.0 | 0.0 | 0.8 | 0.2 | 0.0 | 0.0 | 5.0 | 1.1 | 0.1 | 0.1 | 5.1 | 1.1 | - | - | - | - |
| Peru | 3,930 | 0.8 | 0.1 | 0.3 | 0.1 | 0.7 | 0.1 | 0.1 | 0.1 | 5.8 | 0.8 | 2.1 | 0.8 | 8.0 | 1.2 | - | - | - | - |
| China | 1,628 | 0.4 | 0.2 | 0.0 | 0.0 | 0.4 | 0.2 | 0.0 | 0.0 | 7.5 | 3.2 | 0.2 | 0.2 | 7.7 | 3.2 | - | - | - | - |
| Ukraine | 1,720 | 0.4 | 0.2 | 0.6 | 0.2 | 0.2 | 0.1 | 0.4 | 0.2 | 5.0 | 2.5 | 6.7 | 1.9 | 11.7 | 2.6 | - | - | - | - |
| Upper-Middle | 20,051 | 1.7 | 0.1 | 0.8 | 0.1 | 1.1 | 0.1 | 0.5 | 0.1 | 10.4 | 0.7 | 4.9 | 0.6 | 15.3 | 0.9 | 67.7 | 3.2 | 64.1 | 4.4 |
| Brazil | 5,037 | 1.5 | 0.2 | 1.4 | 0.3 | 1.3 | 0.2 | 0.9 | 0.2 | 8.6 | 1.0 | 7.9 | 1.6 | 16.5 | 1.8 | 83.1 | 5.2 | 62.4 | 6.3 |
| Bulgaria | 2,233 | 0.2 | 0.1 | - | - | 0.2 | 0.1 | 0.0 | 0.0 | 2.3 | 1.2 | 0.0 | 0.0 | 2.3 | 1.2 | - | - | - | - |
| Lebanon | 1,031 | 0.3 | 0.3 | 0.1 | 0.1 | 0.3 | 0.3 | 0.0 | 0.0 | 5.6 | 4.6 | 2.3 | 1.5 | 7.8 | 3.8 | - | - | - | - |
| Medellin | 1,673 | 3.4 | 0.5 | 1.9 | 0.4 | 2.9 | 0.5 | 1.1 | 0.3 | 14.9 | 2.2 | 8.2 | 1.7 | 23.1 | 2.8 | 84.9 | 4.6 | 57.3 | 10.1 |
| Mexico | 5,767 | 0.9 | 0.2 | 0.5 | 0.1 | 0.7 | 0.1 | 0.3 | 0.1 | 9.1 | 1.5 | 4.9 | 1.1 | 14.0 | 1.6 | 76.6 | 6.6 | - | - |
| South Africa | 4,310 | 3.4 | 0.3 | 0.6 | 0.2 | 1.6 | 0.2 | 0.5 | 0.1 | 12.4 | 1.4 | 2.3 | 0.6 | 14.7 | 1.8 | 48.2 | 5.6 | - | - |
| High | 51,797 | 3.0 | 0.1 | 1.7 | 0.1 | 2.4 | 0.1 | 1.3 | 0.1 | 9.1 | 0.3 | 5.2 | 0.2 | 14.3 | 0.3 | 80.5 | 1.2 | 72.9 | 1.9 |
| Argentina | 2,116 | 3.0 | 0.5 | 1.2 | 0.3 | 2.1 | 0.3 | 0.7 | 0.2 | 11.4 | 1.7 | 4.4 | 1.1 | 15.9 | 1.9 | 68.2 | 7 | 63.9 | 8.5 |
| Australia | 8,463 | 4.6 | 0.2 | 2.9 | 0.3 | 4.0 | 0.2 | 2.3 | 0.3 | 21.6 | 1.2 | 13.5 | 1.4 | 35.1 | 1.7 | 86.2 | 1.8 | 79.8 | 3.6 |
| Belgium | 1,043 | 3.4 | 0.7 | 1.1 | 0.6 | 2.3 | 0.5 | 0.4 | 0.2 | 7.1 | 1.4 | 2.3 | 1.3 | 9.4 | 1.8 | 69.2 | 13.1 | - | - |
| France | 1,436 | 2.6 | 0.3 | 0.9 | 0.3 | 2.2 | 0.2 | 0.6 | 0.3 | 5.0 | 0.5 | 1.7 | 0.5 | 6.6 | 0.9 | 84.3 | 2.7 | - | - |
| Germany | 1,323 | 2.4 | 0.5 | 0.5 | 0.3 | 2.2 | 0.4 | 0.2 | 0.1 | 3.6 | 0.7 | 0.7 | 0.4 | 4.4 | 0.8 | 90 | 6.1 | - | - |
| Israel | 4,824 | 1.4 | 0.2 | 0.3 | 0.1 | 1.1 | 0.2 | 0.3 | 0.1 | 10.8 | 1.3 | 2.3 | 0.6 | 13.1 | 1.4 | 81.6 | 4.8 | - | - |
| Italy | 1,779 | 2.1 | 0.4 | 0.4 | 0.1 | 1.8 | 0.4 | 0.3 | 0.1 | 3.1 | 0.6 | 0.6 | 0.2 | 3.7 | 0.6 | 87.4 | 5.9 | - | - |
| Japan | 1,671 | 0.2 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 3.0 | 1.4 | 0.7 | 0.5 | 3.7 | 1.5 | - | - | - | - |
| Murcia | 1,459 | 2.4 | 0.7 | 1.2 | 0.4 | 1.7 | 0.7 | 0.6 | 0.2 | 10.0 | 2.4 | 5.2 | 1.5 | 15.2 | 2.5 | - | - | - | - |
| Netherlands | 1,094 | 1.0 | 0.3 | 1.1 | 0.6 | 0.9 | 0.3 | 1.0 | 0.6 | 2.9 | 0.9 | 3.0 | 1.7 | 5.8 | 1.8 | - | - | - | - |
| New Zealand | 12,790 | 3.1 | 0.2 | 2.5 | 0.2 | 2.6 | 0.2 | 1.6 | 0.1 | 7.2 | 0.5 | 5.8 | 0.4 | 13.0 | 0.6 | 82.8 | 2.3 | 65.2 | 3.4 |
| Northern Ireland | 1,986 | 2.7 | 0.4 | 0.6 | 0.2 | 1.5 | 0.3 | 0.4 | 0.1 | 14.8 | 2.3 | 3.5 | 0.9 | 18.4 | 2.5 | 54.2 | 8.6 | - | - |
| Poland | 4,000 | 1.2 | 0.2 | 0.2 | 0.1 | 0.4 | 0.1 | 0.1 | 0.0 | 13.5 | 1.7 | 2.8 | 0.9 | 16.2 | 2.0 | 36.8 | 7.1 | - | - |
| Spain | 2,121 | 3.8 | 0.5 | 0.3 | 0.1 | 3.2 | 0.6 | 0.3 | 0.1 | 5.9 | 0.9 | 0.5 | 0.1 | 6.3 | 0.9 | 84.8 | 5.7 | - | - |
| United States | 5,692 | 4.9 | 0.3 | 3.5 | 0.2 | 4.1 | 0.2 | 2.9 | 0.2 | 11.1 | 0.7 | 7.8 | 0.5 | 18.9 | 0.9 | 83.1 | 1.7 | 83.5 | 2.6 |
| All Countries | 90,027 | 2.2 | 0.1 | 1.2 | 0.1 | 1.8 | 0.1 | 0.9 | 0.0 | 9.1 | 0.2 | 5.0 | 0.2 | 14.0 | 0.3 | 78 | 1.1 | 70.7 | 1.7 |
SE - standard error; LT – lifetime;
Empty cells (−) indicate the estimates were not provided due to small sample sizes (n<30).
Disorder and remission diagnoses are for any drug.
Country income group reflects economic development status at time of data collection based on The World Bank country level ranking.
Excludes those persons with lifetime drug dependence.
N = The total unweighted number of respondents.
Prevalence estimates are based on weighted data.
Remission from abuse excludes those persons with lifetime drug dependence.
Inclusion in the denominator is conditional on persons having met a certain level of drug involvement.
Age of onset and time to transition across stages of involvement
Figure 1 shows the cumulative age of onset (AOO) curves for onset of illicit drug use, abuse, dependence, remission from abuse and remission from dependence (left) and the cumulative time to transition between drug stages (right). Onset of drug use largely occurred during the late teenage years (median AOO of 19 years). For DUDs, the median AOO was slightly earlier for abuse (20 years) compared to dependence (21 years). This was similar for remission, with the median AOO of remitting from abuse one year younger than the median AOO of dependence remission (28 vs. 29 years).
Figure 1. Age of onset (left) and transition times between (right) drug use, use disorders and remission.
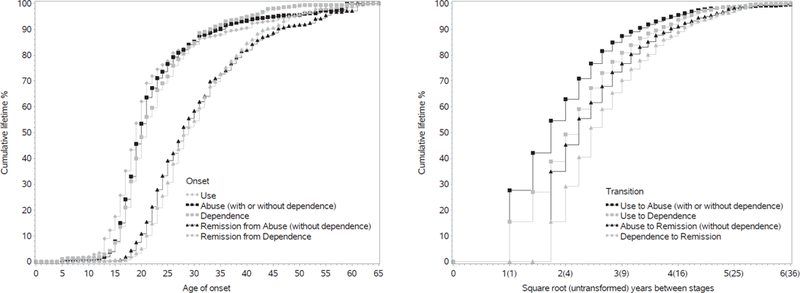
The left panel shows the cumulative age of onset curves for illicit drug use, abuse (without hierarchy), dependence, remission from abuse and remission from dependence. Each curve includes respondents with and without the specific diagnosis, where age of onset for the latter is censored at age of interview. Estimates were scaled up to reach 100%. The right panel shows the cumulative curves for time to transition between various drug stages. Each curve includes only those respondents with a diagnosis of the second stage. For left and right panels, persons with missing age of onset of remission were excluded from associated curves (N=147 – remission from abuse; N=104 – remission from dependence).
The transition from initial use to DUD onset was often quite fast, with over half of all users who developed abuse doing so within three years of first use. Median time-to-dependence was slightly longer at five years from first use. Among those that eventually remitted, time with the disorder was slightly longer for dependence than abuse at six and five years, respectively.
Predictors of transitions between stages of drug involvement
Table 3 summarises the results from five models investigating the association of each substance-related variable with transitions between stages of drug involvement, with adjustment for all socio-demographic variables (complete set of results are shown in eTables 2–7).
Table 3.
Association of each substance-related variable (excluding all others) with transitions between stages of lifetime illicit drug use, use disorders and remission, adjusted for all sociodemographic variablesa
| Transition 1: | Transition 2: | Transition 3: | Transition 4: | Transition 5: | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Commencing Use | Use to abuse (with and without dependence) |
Use to dependence | Remission from abuse (without dependence)e |
Remission from dependencee | ||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Model I | ||||||||||
| Percentage of age cohort already using drugsb | 1.31* | (1.29–1.33) | 1.11* | (1.07–1.16) | 1.10* | (1.03–1.18) | 1.65* | (1.54–1.77) | 1.65* | (1.44–1.88) |
| X21 [p] | 1389.01** | [<0.001] | 24.84** | [<0.001] | 7.16** | [0.007] | 197.06** | [<0.001] | 53.87** | [<0.001] |
| Model II | ||||||||||
| Percentage of age cohort already using alcoholb | 1.51* | (1.49–1.54) | 1.13* | (1.07–1.19) | 1.08 | (0.99–1.18) | 1.44* | (1.30–1.61) | 1.67* | (1.12–2.50) |
| X21 [p] | 2043.51** | [<0.001] | 21.85** | [<0.001] | 2.94 | [0.086] | 44.00** | [<0.001] | 6.22** | [0.013] |
| Model III | ||||||||||
| Highest level of individual’s alcohol involvemente | ||||||||||
| Use | 4.64* | (4.39–4.90) | 1.50* | (1.26–1.80) | 1.39 | (0.99–1.96) | 1.39* | (1.11–1.72) | 1.94* | (1.19–3.18) |
| X21 [p] | 3087.76** | [<0.001] | 19.60** | [<0.001] | 3.53 | [0.060] | 8.61** | [0.003] | 6.95** | [0.008] |
| Abuse | 10.78* | (9.38–12.40) | 5.52* | (4.40–6.92) | 3.80* | (2.50–5.78) | 1.36* | (1.07–1.72) | 2.18* | (1.32–3.62) |
| X21 [p] | 1120.27** | [<0.001] | 219.64** | [<0.001] | 39.03** | [<0.001] | 6.38** | [0.012] | 9.14** | [0.003] |
| Dependence | 12.81* | (10.29–15.94) | 6.48* | (4.94–8.50) | 6.33* | (4.12–9.73) | 1.49* | (1.12–1.99) | 1.76* | (1.09–2.84) |
| X21 [p] | 522.38** | [<0.001] | 182.37** | [<0.001] | 71.06** | [<0.001] | 7.59** | [0.006] | 5.30** | [0.021] |
| Remission | 4.08* | (3.20–5.21) | 2.59* | (1.78–3.78) | 2.01* | (1.20–3.37) | 2.25* | (1.67–3.02) | 3.28* | (1.99–5.40) |
| X21 [p] | 128.18** | [<0.001] | 24.48** | [<0.001] | 7.08** | [0.008] | 28.88** | [<0.001] | 21.70** | [<0.001] |
| Joint test of all four indicators - X24 [p] | 3251.41** | [<0.001] | 465.08** | [<0.001] | 150.16** | [<0.001] | 30.29** | [<0.001] | 42.15** | [<0.001] |
| Model IV | ||||||||||
| Type of drug(s) already used by individual | ||||||||||
| Cannabis | 3.10* | (2.53–3.81) | 2.53* | (1.76–3.63) | 1.68* | (1.36–2.08) | 1.65* | (1.20–2.27) | ||
| X21 [p] | 116.54** | [<0.001] | 25.02** | [<0.001] | 22.50** | [<0.001] | 9.56** | [0.002] | ||
| Cocaine | 3.22* | (2.79–3.72) | 3.54* | (2.83–4.42) | 1.1 | (0.96–1.26) | 0.88 | (0.73–1.06) | ||
| X21 [p] | 255.96** | [<0.001] | 123.08** | [<0.001] | 1.98 | [0.159] | 1.74 | [0.187] | ||
| Other | 2.62* | (2.28–3.01) | 3.25* | (2.61–4.04) | 0.80* | (0.71–0.89) | 0.92 | (0.75–1.12) | ||
| X21 [p] | 184.01** | [<0.001] | 112.36** | [<0.001] | 16.55** | [<0.001] | 0.67 | [0.412] | ||
| Joint test of all three indicators - X23 [p] | 696.02** | [<0.001] | 379.52** | [<0.001] | 43.05** | [<0.001] | 12.99** | [0.005] | ||
| Model V | ||||||||||
| Individual used 2+ drug types | 5.17* | (4.66–5.73) | 5.99* | (5.02–7.16) | 0.86* | (0.76–0.98) | 0.91 | (0.74–1.13) | ||
| X21 [p] | 976.45** | [<0.001] | 390.67** | [<0.001] | 5.45** | [0.020] | 0.74 | [0.389] | ||
| Total sample size (Nf) | 90,022 | 23,073 | 23,073 | 2,088 | 1,167 | |||||
OR - odds ratio; CI - confidence interval;
All transitions are based on weighted person-year data. Each model (I-V, for all transitions) was estimated with only the one drug-related variable entered at a time as predictor of each transition controlling for:
Transition 1 – person-year age groups; sex; education level; major depressive episode; broad bipolar disorder; number of anxiety disorders; and survey.
Transition 2 – Included all controls specified for Transition 1 as well as age tertile of commencing alcohol usec.
Transition 3 – Included all controls specified for Transition 2 as well as drug abuse.
Transition 4 and 5 – Included all controls specified in Transition 2 as well as speed to transition from use to disorderd and years with disorder.
Percentage (/10) of +/−5-yr specific cohort who had used the substance by the prior person year.
Individuals’ age of commencing drug use is split into survey-specific tertiles among all those who ever used illicit drugs.
Individuals’ speed of transition from drug use to disorder is split into survey-specific tertiles.
Individuals with a missing age of onset of remission were excluded from the model (N=147 for remission from abuse and N=104 for remission from dependence).
N = The total unweighted number of respondents included in model conditioning on initial stage.
Cohort-level substance use as predictors
In the transition models that considered prevalence of drug use in an individual’s age cohort (Model I), an increase in an individual’s cohort’s drug use was associated with an increased individual risk of commencing drug use, developing a DUD and remitting from those disorders. With the exception of transitions to dependence, similar results were also observed when examining the prevalence of cohort alcohol use (Model II).
Individual-level substance use history as predictors
At the individual level, having already developed alcohol abuse or dependence, or remitted from either disorder, were all strongly associated with an increased risk of starting drug use, transitioning to DUDs, but also remitting from DUDs (Model III). Considering the types of drugs used (Model IV), cocaine and other drugs both increase risks of transitioning to drug dependence; people with a history of cannabis use were also more likely to remit from both drug abuse and drug dependence than those who had not used cannabis. When considering only the number of drugs used (Model V), the use of two or more drug types increased the odds of transitioning to abuse and dependence and reduced the odds of remitting from abuse.
Including both individual and cohort substance use history
Table 4 presents the results obtained when including all individual and cohort-level substance use variables considered above in the same model (also adjusting for sociodemographic variables). Once adjusting for an individual’s own prior substance involvement, an increase in their cohort’s drug use and alcohol use was associated with an increased individual risk of commencing drug use and remitting from DUDs but was no longer associated with developing DUDs. Most other effects observed in the separate models described above remained significant. Analyses at the country income level were also investigated, the results of which are shown in eTables 8–13. Findings were largely consistent between country income group analyses, and the pooled analyses presented here.
Table 4.
Associations of all substance-related variables with transitions between stages of lifetime illicit drug use, use disorders and remission, adjusted for all sociodemographic variablesa
| Model VI | Transition 1: | Transition 2: | Transition 3: | Transition 4: | Transition 5: | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Commencing Use | Use to abuse (with and without dependence) |
Use to dependence | Remission from abuse (without dependence)e |
Remission from dependencee | ||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Percentage of age cohort already using drugsb | 1.12* | (1.11–1.14) | 1.04 | (0.99–1.09) | 1.04 | (0.96–1.13) | 1.57* | (1.46–1.69) | 1.59* | (1.39–1.83) |
| X21 [p] | 205.46** | [<0.001] | 2.19 | [0.139] | 1.04 | [0.308] | 138.99** | [<0.001] | 44.36** | [<0.001] |
| Percentage of age cohort already using alcoholb | 1.28* | (1.26–1.31) | 0.99 | (0.94–1.05) | 0.94 | (0.85–1.04) | 1.11* | (1.00–1.22) | 1.19 | (0.86–1.64) |
| X21 [p] | 639.15** | [<0.001] | 0.07 | [0.792] | 1.28 | [0.258] | 4.22** | [0.040] | 1.07 | [0.300] |
| Highest level of individual’s alcohol involvemente | ||||||||||
| Use | 3.59* | (3.40–3.80) | 1.22* | (1.02–1.47) | 1.17 | (0.81–1.70) | 1.00 | (0.79–1.27) | 1.68* | (1.03–2.74) |
| X21 [p] | 2027.16** | [<0.001] | 4.54** | [0.033] | 0.74 | [0.390] | 0.00 | [0.995] | 4.33** | [0.037] |
| Abuse | 8.20* | (7.16–9.38) | 3.76* | (2.98–4.74) | 2.56* | (1.65–3.97) | 0.92 | (0.71–1.20) | 1.90* | (1.15–3.15) |
| X21 [p] | 933.29** | [<0.001] | 125.44** | [<0.001] | 17.43** | [<0.001] | 0.37 | [0.545] | 6.21** | [0.013] |
| Dependence | 9.77* | (7.90–12.08) | 4.33* | (3.27–5.74) | 4.19* | (2.67–6.59) | 1.11 | (0.82–1.49) | 1.5 | (0.93–2.42) |
| X21 [p] | 441.06** | [<0.001] | 104.69** | [<0.001] | 38.54** | [<0.001] | 0.43 | [0.510] | 2.78 | [0.096] |
| Remission | 3.10* | (2.43–3.95) | 1.76* | (1.21–2.57) | 1.39 | (0.81–2.40) | 1.59* | (1.18–2.14) | 2.78* | (1.69–4.55) |
| X21 [p] | 84.01** | [<0.001] | 8.58** | [0.003] | 1.41 | [0.235] | 9.14** | [0.003] | 16.43** | [<0.001] |
| Joint test of all four indicators - X24 [p] | 2238.03** | [<0.001] | 304.38** | [<0.001] | 99.35** | [<0.001] | 26.23** | [<0.001] | 35.78** | [<0.001] |
| Type of drug(s) used by individual | ||||||||||
| Cannabis | 0.84 | (0.62–1.13) | 1.07 | (0.66–1.73) | 2.06* | (1.61–2.64) | 1.65* | (1.16–2.34) | ||
| X21 [p] | 1.36 | [0.244] | 0.07 | [0.790] | 32.54** | [<0.001] | 7.62** | [0.006] | ||
| Cocaine | 1.42* | (1.14–1.76) | 2.35* | (1.78–3.10) | 1.28* | (1.08–1.52) | 0.83 | (0.67–1.03) | ||
| X21 [p] | 9.75** | [0.002] | 36.01** | [<0.001] | 7.91** | [0.005] | 2.99 | [0.084] | ||
| Other | 0.82 | (0.64–1.04) | 1.48* | (1.06–2.07) | 1.06 | (0.88–1.28) | 0.99 | (0.77–1.29) | ||
| X21 [p] | 2.67 | [0.102] | 5.31** | [0.021] | 0.43 | [0.514] | <0.01 | [0.969] | ||
| Joint test of all three indicators - X23 [p] | 35.36** | [<0.001] | 48.37** | [<0.001] | 43.82** | [<0.001] | 11.82** | [0.008] | ||
| Individual used 2+ drug types | 4.69* | (3.56–6.18) | 2.90* | (1.97–4.29) | 0.58* | (0.46–0.74) | 0.83 | (0.58–1.18) | ||
| X21 [p] | 120.52** | [<0.001] | 28.74** | [<0.001] | 19.96** | [<0.001] | 1.07 | [0.300] | ||
| Total sample size (Nf) | 90,022 | 23,073 | 23,073 | 2,088 | 1,167 | |||||
OR - odds ratio; CI - confidence interval;
All transitions are based on weighted person-year data. Models were estimated with all drug-related variables entered as predictors of each transition (excluding type and number of drugs in commencing use) controlling for:
Transition 1 – person-year age groups; sex; education level; major depressive episode; broad bipolar disorder; number of anxiety disorders; and survey.
Transition 2 – Included all controls specified for Transition 1 as well as age tertile of commencing alcohol usec.
Transition 3 – Included all controls specified for Transition 2 as well as drug abuse.
Transition 4 and 5 – Included all controls specified for Transition 2 as well as speed to transition from use to disorderd and years with disorder.
Percentage (/10) of +/−5-yr specific cohort who had used the substance by the prior person year.
Individuals’ age of commencing drug use is split into survey-specific tertiles among all those who ever used illicit drugs.
Individuals’ speed of transition from drug use to disorder is split into survey-specific tertiles.
Individuals with a missing age of onset of remission were excluded from the model (N=147 for remission from abuse and N=104 for remission from dependence).
N = The total unweighted number of respondents included in model conditioning on initial stage.
Discussion
The primary aim of the present study was to provide cross-national data on the epidemiology of drug use, abuse and dependence, and use a unique cross-national dataset to examine transitions across levels of involvement with drug use, and the extent to which alcohol and other drug use in an individual’s birth cohort predicted an individual’s risk of these transitions, in addition to that person’s own prior involvement in alcohol and drug use. At an individual level, extent of involvement with both alcohol and drug use strongly predicted risks of transitioning into drug abuse and drug dependence, consistent with previous findings33,34. Even after having remitted from alcohol use disorder, individuals remained at increased risk of beginning drug use and transitioning to DUDs. Interestingly, individuals who had previously remitted from alcohol use disorder also had a higher likelihood of remitting from DUDs than those who never used alcohol.
But net of these associations, extent of illicit drug use in an individual’s birth cohort was associated with significantly increased risk of the individual beginning drug use and remitting from DUDs. Cohort alcohol use also positively predicted commencement of illicit drug use and remission from drug abuse. That is, the more people in an individual’s cohort who had a history of using those substances, the greater the likelihood of the individual remitting from the DUD after developing this disorder.
These findings speak to the social context in which substance use occurs. One of the most consistent findings in substance use research is that substance use of one’s peers predicts a greater likelihood of involvement with substance use for an individual35. Here, we have further shown that this is a generalised pattern, whereby it is not only substance use among one’s friends, but in one’s peer cohort more generally. This may be through multiple mechanisms, such as impacts on perceived drug use norms36 and increased opportunities to use substances37. Furthermore, cohort substance use was shown not only to be associated with greater involvement with drugs, but even stronger associations are observed for transitions to remission from DUDs. This may reflect the fact that individuals exposed to higher cohort-level prevalence also have greater access to treatment services than individuals exposed to lower cohort-level prevalence, or perhaps that as cohort substance use increases those who are transitioning to these disorders may be less problematic or use disorder prone at the individual level and, as a result, remit from those disorders at a higher rate. These findings also suggest that the risk for commencing drug use and remission from problems is not constant but varies, in this case according to the extent to which substance use is occurring among one’s age peers.
Although higher rates of use in an individual’s cohort was associated with an increased likelihood the individual will start using drugs, there was no independent effect of cohort use on the transition to abuse or dependence once use had begun. This suggests that while higher rates of use in an individual’s cohort increases the likelihood that the individual will start using drugs, the propensity to transition to problematic use is not affected by such external variables; by contrast, we found that it was affected by their own prior substance use history. Therefore, any intervention aiming to reduce substance use within a cohort might also reduce individual-level risk for transitioning into greater levels of involvement with drug use. The type of substance such interventions should target warrants further investigation, especially considering cohort alcohol use had a stronger effect on commencing drug use than cohort drug use, but implementation would ideally be early in life and before opportunities to use either substance arise (see eTable 16). If this occurred, the smaller group of individuals who nonetheless developed DUDs despite the decrease in prevalence of use within that cohort would be more refractive cases.
Limitations
This study provided detail regarding the prevalence and timing of various stages across the full trajectory of both alcohol and illicit drug use, with clinically valid diagnoses and inclusion of contextual predictors not previously accounted for within the literature. Data on age of onset for each stage were obtained via retrospective self-report and may be subject to ‘forward telescoping’, whereby participants are more likely to report events as closer to the point of interview than is accurate38,39. However, this literature does not suggest that the order of recalled events will be altered.
Investigating the interactive effects of the personal and contextual variables on risk of transitioning involvement with illicit drugs was beyond the scope of this paper. However, future work should investigate whether conditional relationships exist between individual-level predictors (substance use, history of mental disorder) and cohort contextual variables which impact individuals’ risk of commencing use and transitioning to greater involvement with drugs.
The WMH surveys have several important limitations. There is not full representation of all countries, regions, country income levels or other country characteristics. There was variation in response rates across countries, the year in which the studies were administered, and possibly cross-national differences in willingness to disclose personal information about drug use and problems. Respondent information is subject to the limitations of recall inherent in retrospective reporting, leading to potential underestimates in lifetime prevalence. Survival bias may also contribute to downward bias in lifetime estimates.
In addition to these general limitations, there are some limitations specific to the assessment of DUDs. The WMH surveys are household surveys, which have limitations when used to assess less common and more stigmatised behaviours. Illicit drug use can be a rare occurrence and geographically concentrated, and surveys such as the WMH surveys that rely on stratified sampling methods are poorly suited to capturing concentrated geographic ‘pockets’ of drug use. Furthermore, the use of households as the primary sampling unit will not capture marginalised groups who do not live in traditional household contexts (e.g. homeless, prison, hospital, or other non-household accommodation). These factors mean that prevalence rates presented here should be considered lower-bound estimates; “true” lifetime prevalence of DUDs may be substantially higher.
Transition times to drug use disorders (DUDs) have been shown to differ widely depending on substance class40. As most surveys assessed DUDs at the general illicit drug level, it was not possible to evaluate transition times at the drug-specific level. The estimates presented here therefore represent averages of first transitions across all (single and multi-type) illicit drug users.
Due to the way in which symptom onset and recency is assessed in the CIDI, it was only possible to assess remission at the time of interview. Given the chronic nature of DUDs, if we had information on lifetime remission (i.e. any period in life with an absence of symptoms for more than 12 months) we may have found other variables were associated with remission from DUDs.
Conclusion
We have found, across countries, that an individual’s personal risk of transitioning to greater involvement with drug use is impacted by their history of involvement with drugs and alcohol, and the substance use histories of their age cohort. These variables predict transitioning into and out of problematic drug use, when considering them together, in addition to a range of other sociodemographic correlates. These findings have important implications for our understanding of the causal pathways into and out of problematic substance use.
Supplementary Material
Key Points.
Question:
Does the extent to which alcohol and other drugs are used in an individual’s birth cohort impact an individual’s risk of commencing drug use, transitioning to problematic use, and remitting?
Findings:
Using cross-national data from the World Mental Health Surveys, an individual’s personal risk of transitioning to greater involvement with drug use is impacted by the substance use histories of their age cohort, as well as their own history of involvement with drugs and alcohol. Results were statistically significant after controlling for socio-demographics and were consistent across country income levels.
Meaning:
Any intervention to reduce substance use within a cohort would also reduce individual-level risk for transitioning into greater levels of involvement with drug use.
Funding Acknowledgements
The World Health Organization World Mental Health (WMH) Survey Initiative is supported by the United States National Institute of Mental Health (NIMH; R01 MH070884), the John D. and Catherine T. MacArthur Foundation, the Pfizer Foundation, the United States Public Health Service (R13-MH066849, R01-MH069864, and R01 DA016558), the Fogarty International Center (FIRCA R03-TW006481), the Pan American Health Organization, Eli Lilly and Company, Ortho-McNeil Pharmaceutical Inc., GlaxoSmithKline, and Bristol-Myers Squibb. This work was supported by an Australian National Health and Medical Research Council (NHMRC) project grant (no. 1081984). Dr Degenhardt is supported by a NHMRC Senior Principal Research Fellowship (no. 1135991) and NIDA NIH grant R01 DA044170–02. We thank the staff of the WMH Data Collection and Data Analysis Coordination Centres for assistance with instrumentation, fieldwork, and consultation on data analysis. None of the funders had any role in the design, analysis, interpretation of results, or preparation of this paper. The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of the World Health Organization, other sponsoring organizations, agencies, or governments.
The 2007 Australian National Survey of Mental Health and Wellbeing is funded by the Australian Government Department of Health and Ageing. The Argentina survey -- Estudio Argentino de Epidemiología en Salud Mental (EASM) -- was supported by a grant from the Argentinian Ministry of Health (Ministerio de Salud de la Nación). The São Paulo Megacity Mental Health Survey is supported by the State of São Paulo Research Foundation (FAPESP) Thematic Project Grant 03/00204–3. The Bulgarian Epidemiological Study of common mental disorders EPIBUL is supported by the Ministry of Health and the National Center for Public Health Protection. The Chinese World Mental Health Survey Initiative is supported by the Pfizer Foundation. The Colombian National Study of Mental Health (NSMH) is supported by the Ministry of Social Protection. The Mental Health Study Medellín – Colombia was carried out and supported jointly by the Center for Excellence on Research in Mental Health (CES University) and the Secretary of Health of Medellín. The ESEMeD project is funded by the European Commission (Contracts QLG5–1999-01042; SANCO 2004123, and EAHC 20081308), (the Piedmont Region (Italy)), Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spain (FIS 00/0028), Ministerio de Ciencia y Tecnología, Spain (SAF 2000–158-CE), Departament de Salut, Generalitat de Catalunya, Spain, DIUE de la Generalitat de Catalunya (2017 SGR 452; 2014 SGR 748), Instituto de Salud Carlos III (CIBER CB06/02/0046, RETICS RD06/0011 REM-TAP), and other local agencies and by an unrestricted educational grant from GlaxoSmithKline. Implementation of the Iraq Mental Health Survey (IMHS) and data entry were carried out by the staff of the Iraqi MOH and MOP with direct support from the Iraqi IMHS team with funding from both the Japanese and European Funds through United Nations Development Group Iraq Trust Fund (UNDG ITF). The Israel National Health Survey is funded by the Ministry of Health with support from the Israel National Institute for Health Policy and Health Services Research and the National Insurance Institute of Israel. The World Mental Health Japan (WMHJ) Survey is supported by the Grant for Research on Psychiatric and Neurological Diseases and Mental Health (H13-SHOGAI-023, H14-TOKUBETSU-026, H16-KOKORO-013, H25-SEISHIN-IPPAN-006) from the Japan Ministry of Health, Labour and Welfare. The Lebanese Evaluation of the Burden of Ailments and Needs Of the Nation (L.E.B.A.N.O.N.) is supported by the Lebanese Ministry of Public Health, the WHO (Lebanon), National Institute of Health / Fogarty International Center (R03 TW006481–01), anonymous private donations to IDRAAC, Lebanon, and unrestricted grants from, Algorithm, AstraZeneca, Benta, Bella Pharma, Eli Lilly, Glaxo Smith Kline, Lundbeck, Novartis, OmniPharma, Pfizer, Phenicia, Servier, UPO. The Mexican National Comorbidity Survey (MNCS) is supported by The National Institute of Psychiatry Ramon de la Fuente (INPRFMDIES 4280) and by the National Council on Science and Technology (CONACyT-G30544- H), with supplemental support from the Pan American Health Organization (PAHO). Te Rau Hinengaro: The New Zealand Mental Health Survey (NZMHS) is supported by the New Zealand Ministry of Health, Alcohol Advisory Council, and the Health Research Council. The Nigerian Survey of Mental Health and Wellbeing (NSMHW) is supported by the WHO (Geneva), the WHO (Nigeria), and the Federal Ministry of Health, Abuja, Nigeria. The Northern Ireland Study of Mental Health was funded by the Health & Social Care Research & Development Division of the Public Health Agency. The Peruvian World Mental Health Study was funded by the National Institute of Health of the Ministry of Health of Peru. The Polish project Epidemiology of Mental Health and Access to Care –EZOP Project (PL 0256) was supported by Iceland, Liechtenstein and Norway through funding from the EEA Financial Mechanism and the Norwegian Financial Mechanism. EZOP project was co-financed by the Polish Ministry of Health. The South Africa Stress and Health Study (SASH) is supported by the US National Institute of Mental Health (R01-MH059575) and National Institute of Drug Abuse with supplemental funding from the South African Department of Health and the University of Michigan. The Psychiatric Enquiry to General Population in Southeast Spain – Murcia (PEGASUS-Murcia) Project has been financed by the Regional Health Authorities of Murcia (Servicio Murciano de Salud and Consejería de Sanidad y Política Social) and Fundación para la Formación e Investigación Sanitarias (FFIS) of Murcia. The Ukraine Comorbid Mental Disorders during Periods of Social Disruption (CMDPSD) study is funded by the US National Institute of Mental Health (RO1-MH61905). The US National Comorbidity Survey Replication (NCS-R) is supported by the National Institute of Mental Health (NIMH; U01-MH60220) with supplemental support from the National Institute of Drug Abuse (NIDA), the Substance Abuse and Mental Health Services Administration (SAMHSA), the Robert Wood Johnson Foundation (RWJF; Grant 044708), and the John W. Alden Trust. Dr Andrade is supported by the Brazilian Council for Scientific and Technological Development (CNPq Grant # 307784/2016–9). Dr Stein is supported by the Medical Research Council of South Africa (MRC).
In the past three years, Dr Degenhardt has received investigator-initiated untied educational grants for studies of opioid medications in Australia from Indivior, Mundipharma and Seqirus. Dr Kessler received support for his epidemiological studies from Sanofi Aventis; was a consultant for Johnson & Johnson Wellness and Prevention, Sage Pharmaceuticals, Shire, Takeda; served on an advisory board for the Johnson & Johnson Services Inc. Lake Nona Life Project; and being a co-owner of DataStat, Inc., a market research firm that carries out healthcare research. Dr Demyttenaere has served on advisory boards for Boehringer Ingelheim, Eli Lilly, Lundbeck, Johnson&Johnson, Livanova, Servier, and has research grants from Eli Lilly, foundation ‘ga voor geluk’, Fonds voor Wetenschappelijk Onderzoek Vlaanderen. Dr Stein has received research grants and/or consultancy honoraria from AMBRF, Biocodex, Cipla, Lundbeck, National Responsible Gambling Foundation, Novartis, Servier, and Sun.
The views expressed in this report are those of the authors and should not be construed to represent the views or policies of the WHO, other sponsoring organisations, agencies, or governments, and do not necessarily represent the views, official policy, or position of the US. Department of Health and Human Services or any of its affiliated institutions or agencies. Dr Glantz’s role on this study is through his involvement as a Science Officer on U01-MH60220. He had no involvement in the other cited grants.
A complete list of all within-country and cross-national WMH publications can be found at http://www.hcp.med.harvard.edu/wmh/.
Footnotes
Conflict of Interest
Other authors have no financial or personal conflicts of interest to disclose.
Contributor Information
Louisa Degenhardt, National Drug and Alcohol Research Centre (NDARC), UNSW Sydney, Australia.
Chrianna Bharat, National Drug and Alcohol Research Centre (NDARC), UNSW Sydney, Australia.
Meyer D. Glantz, Department of Epidemiology, Services, and Prevention Research (DESPR), National Institute on Drug Abuse (NIDA), National Institute of Health (NIH), Bethesda, Maryland, USA.
Nancy A. Sampson, Department of Health Care Policy, Harvard Medical School, Boston, Massachusetts, USA.
Ali Al-Hamzawi, College of Medicine, Al-Qadisiya University, Diwaniya governorate, Iraq.
Jordi Alonso, Health Services Research Unit, IMIM-Hospital del Mar Medical Research Institute; Pompeu Fabra University (UPF); and CIBER en Epidemiología y Salud Pública (CIBERESP), Barcelona, Spain.
Laura H. Andrade, Section of Psychiatric Epidemiology - Laboratórios de Investigação Médica No. 23, Institute of Psychiatry, University of São Paulo Medical School, São Paulo, Brazil.
Brendan Bunting, School of Psychology, Ulster University, Londonderry, United Kingdom.
Alfredo Cia, Anxiety Disorders Center, Buenos Aires, Argentina.
Giovanni De Girolamo, Unit of Epidemiological and Evaluation Psychiatry, Istituti di Ricovero e Cura a Carattere Scientifico (IRCCS)-St. John of God Clinical Research Centre, Via Pilastroni 4, Brescia, Italy.
Peter De Jonge, Developmental Psychology, Department of Psychology, Rijksuniversiteit Groningen, Groningen, NL; Interdisciplinary Center Psychopathology and Emotion Regulation, Department of Psychiatry, University Medical Center Groningen, Groningen, NL.
Koen Demyttenaere, Department of Psychiatry, University Hospital Gasthuisberg, Katholieke Universiteit Leuven, Leuven, Belgium.
Oye Gureje, Department of Psychiatry, University College Hospital, Ibadan, Nigeria.
Josep Maria Haro, Parc Sanitari Sant Joan de Déu, CIBERSAM, Universitat de Barcelona, Sant Boi de Llobregat, Barcelona, Spain.
Meredith G. Harris, School of Public Health, The University of Queensland; Queensland Centre for Mental Health Research, The Park Centre for Mental Health, QLD, Australia.
Yanling He, Shanghai Mental Health Center, Shanghai Jiao Tong University, School of Medicine, Shanghai, China.
Hristo Hinkov, National Center of Public Health and Analyses, Sofia, Bulgaria.
Aimee Nasser Karam, Institute for Development, Research, Advocacy & Applied Care (IDRAAC), Beirut, Lebanon.
Elie G. Karam, Department of Psychiatry and Clinical Psychology, Faculty of Medicine, Balamand University, Beirut, Lebanon; Department of Psychiatry and Clinical Psychology, St George Hospital University Medical Center, Beirut, Lebanon; Institute for Development Research Advocacy and Applied Care (IDRAAC), Beirut, Lebanon.
Andrzej Kiejna, Wroclaw Medical University; University of Lower Silesia, Wroclaw, Poland.
Viviane Kovess-Masfety, Ecole des Hautes Etudes en Santé Publique (EHESP), EA 4057, Paris Descartes University, Paris, France.
Victor Lasebikan, Department of Psychiatry, College of Medicine, University of Ibadan, Nigeria.
Sing Lee, Department of Psychiatry, Chinese University of Hong Kong, Tai Po, Hong Kong.
Daphna Levinson, Mental Health Services, Ministry of Health, Jerusalem, Israel.
Maria Elena Medina-Mora, , National Institute of Psychiatry Ramón de la Fuente Muñiz, Mexico City, Mexico.
Zeina Mneimneh, Survey Research Center, Institute for Social Research, University of Michigan, Ann Arbor, Michigan, USA.
Fernando Navarro-Mateu, Subdirección General de Planificación, Innovación y Cronicidad, Servicio Murciano de Salud. IMIB-Arrixaca. CIBERESP-Murcia, Murcia, Spain.
Marina Piazza, Universidad Peruana Cayetano Heredia, Lima, Peru.
José Posada-Villa, Colegio Mayor de Cundinamarca University, Faculty of Social Sciences, Bogota, Colombia.
Kate Scott, Department of Psychological Medicine, University of Otago, Dunedin, Otago, New Zealand.
Dan J. Stein, Dept of Psychiatry & Mental Health and South African Medical Council Research Unit on Risk and Resilience in Mental Disorders, University of Cape Town and Groote Schuur Hospital, Cape Town, Republic of South Africa.
Hisateru Tachimori, National Institute of Mental Health, National Center for Neurology and Psychiatry, Kodaira, Tokyo, Japan.
Nathan Tintle, Department of Mathematics, Statistics and Computer Science, Dordt College, Sioux Center, Iowa, USA.
Yolanda Torres, Center for Excellence on Research in Mental Health, CES University, Medellin, Colombia.
Ronald C. Kessler, Department of Health Care Policy, Harvard Medical School, Boston, Massachusetts, USA..
References
- 1.Lopez-Quintero C, de los Cobos JP, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug and Alcohol Dependence 2011; 115(1–2): 120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butterworth P, Slade T, Degenhardt L. Factors associated with the timing and onset of cannabis use and cannabis use disorder: results from the 2007 Australian National Survey of Mental Health and Well-Being. Drug Alcohol Rev 2014; 33(5): 555–64. [DOI] [PubMed] [Google Scholar]
- 3.Behrendt S, Wittchen H-U, Höfler M, Lieb R, Beesdo K. Transitions from first substance use to substance use disorders in adolescence: is early onset associated with a rapid escalation? Drug and alcohol dependence 2009; 99(1): 68–78. [DOI] [PubMed] [Google Scholar]
- 4.Coffey C, Carlin JB, Lynskey M, Li N, Patton GC. Adolescent precursors of cannabis dependence: findings from the Victorian Adolescent Health Cohort Study. Br J Psychiatry 2003; 182(4): 330–6. [DOI] [PubMed] [Google Scholar]
- 5.Larance B, Gisev N, Cama E, et al. Predictors of transitions across stages of heroin use and dependence prior to treatment-seeking among people in treatment for opioid dependence. Drug Alcohol Depend 2018; 191: 145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suliman S, Seedat S, Williams DR, Stein DJ. Predictors of Transitions Across Stages of Alcohol Use and Alcohol-Use Disorders in South Africa*. Journal of studies on alcohol and drugs 2010; 71(5): 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalaydjian A, Swendsen J, Chiu WT, et al. Sociodemographic predictors of transitions across stages of alcohol use, disorders, and remission in the National Comorbidity Survey Replication. Comprehensive Psychiatry 2009; 50(4): 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silveira CM, Viana MC, Siu ER, de Andrade AG, Anthony JC, Andrade LH. Sociodemographic Correlates of Transitions from Alcohol Use to Disorders and Remission in the Sao Paulo Megacity Mental Health Survey, Brazil. Alcohol and Alcoholism 2011; 46(3): 324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdin E, Subramaniam M, Vaingankar JA, Chong SA. The Role of Sociodemographic Factors in the Risk of Transition from Alcohol Use to Disorders and Remission in Singapore. Alcohol and Alcoholism 2014; 49(1): 103–8. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Guo WJ, Tsang A, et al. Associations of cohort and socio‐demographic correlates with transitions from alcohol use to disorders and remission in metropolitan China. Addiction 2009; 104(8): 1313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall WD, Patton G, Stockings E, et al. Why young people’s substance use matters for global health. Lancet Psychiatry 2016; 3(3): 265–79. [DOI] [PubMed] [Google Scholar]
- 12.Weiss RD, Mirin SM, Griffin ML, Michael JL. Psychopathology in cocaine abusers. Changing trends. J Nerv Ment Dis 1988; 176(12): 719–25. [DOI] [PubMed] [Google Scholar]
- 13.Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biol Psychiatry 2004; 55(1): 69–76. [DOI] [PubMed] [Google Scholar]
- 14.O’Malley PM, Bachman JG, Johnston LD. Period, age, and cohort effects on substance use among young Americans: a decade of change, 1976–86. American journal of public health 1988; 78(10): 1315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anthony JC, Warner L, Kessler R. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Exp Clinical Psychopharmacol 1994; 2(3): 244–68. [Google Scholar]
- 16.Grant BF. Prevalence and correlates of drug use and DSM-IV drug dependence in the United States: Results of the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse 1996; 8(2): 195–210. [DOI] [PubMed] [Google Scholar]
- 17.Rice JP, Neuman RJ, Saccone NL, et al. Age and birth cohort effects on rates of alcohol dependence. Alcohol Clin Exp Res 2003; 27(1): 93–9. [DOI] [PubMed] [Google Scholar]
- 18.Hall W, Patton G, Stockings E, et al. Why young people’s substance use matters for global health (invited paper). The Lancet Psychiatry 2016; 10.1016/S2215-0366(16)00013-4. [DOI] [PubMed] [Google Scholar]
- 19.Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: Results of the National Longitudinal Alcohol Epidemiologic Survey. Journal of Studies on Alcohol 1997; 58(5): 464–73. [DOI] [PubMed] [Google Scholar]
- 20.Hasin D, Grant B. The co-occurrence of DSM-IV alcohol abuse in DSM-IV alcohol dependence: NESARC results on heterogeneity that differs by population subgroup. Arch Gen Psychiatry 2004; 61: 891–6. [DOI] [PubMed] [Google Scholar]
- 21.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51(1): 8–19. [DOI] [PubMed] [Google Scholar]
- 22.Johnson RA, Gerstein DR. Age, period, and cohort effects in marijuana and alcohol incidence: United States females and males, 1961–1990. Subst Use Misuse 2000; 35(6–8): 925–48. [DOI] [PubMed] [Google Scholar]
- 23.Kerr WC, Greenfield TK, Bond J, Ye Y, Rehm J. Age–period–cohort modelling of alcohol volume and heavy drinking days in the US National Alcohol Surveys: divergence in younger and older adult trends. Addiction 2009; 104(1): 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keyes KM, Schulenberg JE, O’malley PM, et al. The social norms of birth cohorts and adolescent marijuana use in the United States, 1976–2007. Addiction 2011; 106(10): 1790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grucza RA, Bucholz KK, Rice JP, Bierut LJ. Secular trends in the lifetime prevalence of alcohol dependence in the United States: a re-evaluation. Alcohol Clin Exp Res 2008; 32(5): 763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degenhardt L, Glantz M, Bharat C, et al. The impact of cohort substance use upon likelihood of transitioning through stages of alcohol and cannabis use and use disorder: Findings from the Australian National Survey on Mental Health and Wellbeing. Drug Alcohol Rev 2018; 37(4): 546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res 2004; 13(2): 93–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haro JM, Arbabzadeh-Bouchez S, Brugha TS, et al. Concordance of the Composite International Diagnostic Interview Version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO World Mental Health surveys. Int J Methods Psychiatr Res 2006; 15(4): 167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kessler RC, Ustun T. The WHO Mental Health Surveys. Global perspectives on the epidemiology of mental disorders 2008. [Google Scholar]
- 30.Lago L, Glantz MD, Kessler RC, et al. Substance dependence among those without symptoms of substance abuse in the World Mental Health Survey. Int J Methods Psychiatr Res 2017; 26(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degenhardt L, Torres Y, Hinkov H, Have Mt, Glantz MD. Drug-Use Disorders In: Stein DJ, Scott KM, de Jonge P, Kessler RC, eds. Mental Disorders Around the World: Facts and Figures from the WHO World Mental Health Surveys. Cambridge: Cambridge University Press; 2018: 243–62. [Google Scholar]
- 32.SAS Institute Inc. Cary, NC, USA. [Google Scholar]
- 33.Compton WM, Dawson DA, Conway KP, Brodsky M, Grant BF. Transitions in illicit drug use status over 3 years: a prospective analysis of a general population sample. Am J Psychiatry 2013; 170(6): 660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Florez-Salamanca L, Secades-Villa R, Hasin DS, et al. Probability and predictors of transition from abuse to dependence on alcohol, cannabis, and cocaine: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Am J Drug Alcohol Abuse 2013; 39(3): 168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Degenhardt L, Stockings E, Patton G, Hall WD, Lynskey M. The increasing global health priority of substance use in young people. Lancet Psychiatry 2016; 3(3): 251–64. [DOI] [PubMed] [Google Scholar]
- 36.Pollard JW, Freeman JE, Ziegler DA, Hersman MN, Goss CW. Predictions of Normative Drug Use by College Students. Journal of College Student Psychotherapy 2000; 14(3): 5–12. [Google Scholar]
- 37.Wells JE, Haro JM, Karam E, et al. Cross-national comparisons of sex differences in opportunities to use alcohol or drugs, and the transitions to use. Subst Use Misuse 2011; 46(9): 1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shillington AM, Woodruff SI, Clapp JD, Reed MB, Lemus H. Self-Reported Age of Onset and Telescoping for Cigarettes, Alcohol, and Marijuana Across Eight Years of the National Longitudinal Survey of Youth. J Child Adolesc Subst Abuse 2012; 21(4): 333–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson EO, Schultz L. Forward telescoping bias in reported age of onset: an example from cigarette smoking. Int J Methods Psychiatr Res 2005; 14(3): 119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridenour TA, Lanza ST, Donny EC, Clark DB. Different lengths of times for progressions in adolescent substance involvement. Addict Behav 2006; 31(6): 962–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


