Abstract
Ricin toxin is a plant-derived, ribosome-inactivating protein (RIP) that is rapidly cleared from circulation by Kupffer cells (KCs) and liver sinusoidal endothelial cells (LSECs)--with fatal consequences. Rather than being inactivated, ricin evades normal degradative pathways and kills both KCs and LSECs with remarkable efficiency. Uptake of ricin by these two specialized cell types in the liver occurs by two parallel routes: a “lactose-sensitive” pathway mediated by ricin’s galactose/N-acetylgalactosamine-specific lectin subunit (RTB), and a “mannose-sensitive” pathway mediated by the mannose receptor (MR; CD206) or other C-type lectins capable of recognizing the mannose-side chains displayed on ricin’s A (RTA) and B subunits. In this report, we investigated the capacity of a collection of ricin-specific mouse monoclonal (MAb) and camelid single-domain (VHH) antibodies to protect KCs and LSECs from ricin-induced killing. In the case of KCs, individual MAbs against RTA or RTB afforded near complete protection against ricin in ex vivo and in vivo challenge studies. In contrast, individual MAbs or VHHs afforded little (<40%) or even no protection to LSECs against ricin-induced death. Complete protection of LSECs was only achieved with MAb or VHH cocktails, with the most effective mixtures targeting RTA and RTB simultaneously. While the exact mechanisms of protection of LSECs remain unknown, evidence indicates that the antibody cocktails exert their effects on the mannose-sensitive uptake pathway without the need for Fcγ receptor involvement. In addition to advancing our understanding of how toxins and small immune complexes are processed by KCs and LSECs, our study has important implications for the development of antibody-based therapies designed to prevent or treat ricin exposure should the toxin be weaponized.
1. INTRODUCTION
Ricin toxin has been classified as a biological threat agent by the Centers for Disease Control and Prevention (CDC) because of its extreme toxicity, the ease by which it can be procured from castor beans, and the absence of any available countermeasures [1]. Continued concerns about ricin are underscored by a recent NATO Biomedical Advisory Council report that ranked ricin at the top of its list of agents with weaponization potential [2]. Ricin is a ~65 kDa heterodimeric glycoprotein consisting of two subunits, RTA and RTB, which are joined via a single disulfide bond. RTB (34 kDa), a bivalent lectin that recognizes galactose (Gal) and N-acetyl galactosamine (GalNAc), promotes ricin attachment to membrane bound glycoproteins and glycolipids on mammalian cells. RTB also facilitates transport of ricin from the plasma membrane to the trans Golgi network (TGN) and endoplasmic reticulum (ER), where RTA is liberated from RTB and translocated into the cytoplasm. RTA (36 kDa) catalyzes the hydrolysis of the sarcin/ricin loop of 28S ribosomal RNA, thereby arresting protein synthesis and triggering programmed cell death pathways [3–5]. Because the addition of exogenous lactose inhibits RTB-mediated attachment to host cells, this pathway is referred to as “lactose-sensitive” pathway [6].
Ricin can also gain entry into certain cells types through a “mannose-sensitive” pathway that was first described on bone marrow-derived macrophages (BDMCs) and later shown to exist on Kupffer cells (KCs) and liver sinusoidal endothelial cells (LSECs) [6–10]. Ricin is mannosylated at three different asparagine residues; one on RTA (position 95) and two on RTB (positions 135 and 280) [11–13]. A number of studies have implicated the mannose receptor (MR:CD206) as being responsible for ricin uptake into BDMC, KCs and LSECs, although most of those studies were conducted years before the full suite of the C-type lectins (CTL) and their roles in pathogen recognition were discovered [14]. Nonetheless, the mannose-sensitive pathway is a highly efficient route by which ricin enters and kills in KCs and LSECs, due in part to increased rates of internalization, as compared to the lactose-sensitive uptake pathway [8–10].
In this report, we sought to revisit the sensitivity of KCs and LSECs to ricin in light of efforts to develop monoclonal antibody (MAb)-based therapies and prophylactics for ricin exposure [15–18]. For example, we recently demonstrated that intravenous delivery of a single MAb, PB10, directed against RTA was able to rescue Rhesus macaques from lethal dose aerosol challenge if administered within a 4 h window [17]. In that model, ricin intoxication is largely restricted to the lung with little systemic involvement [19]. In contrast, following injection, ricin homes to the liver where it concentrates in KCs and LSECs [8–10, 20–24]. Roche and colleagues reported in a mouse model that a protective anti-RTA MAb was beneficial in blunting liver damage following ricin exposure, although the specific interactions between KCs and LSECs were not investigated. LSECs are of particular interest in this regard considering that their importance in scavenging mannosylated proteins from circulation [25, 26] coupled with their central role in promoting the clearance of small immune complexes (SICs) from blood [27, 28].
2. MATERIALS AND METHODS
2.1. Chemicals and biological reagents
Ricin toxin (Ricinus communis agglutinin II) was purchased from Vector Laboratories (Burlingame, CA) and was dialyzed to remove sodium azide, as described [29]. Native RTA from Ricinus communis seeds was obtained from BEI Resources (Catalog NR-2619; Manassas, VA) [30]. HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer consisted of 0.22 g/L KCl, 7.7 g/L NaCl, 0.14 g/L Na2HPO4, 1.8 g/L D-glucose, 7.15 g/L HEPES, and 0.001 g/L phenol red in distilled water. Gey’s balanced salt solution (GBSS) contained 0.220 g CaCl2.2H2O, 0.370 g/L KCl, 0.030 g/L KH2PO4, 0.210 g/L MgCl2.6H2O, 0.070 g/L MgSO4.7H2O, 8.00 g/L NaCl, 0.227 g/L NaHCO3, 0.120 g/L Na2HPO4, and 1.00 g/L D-glucose in distilled water. Magnetic-activated cell sorting (MACS) buffer consisted of 0.5% w/v bovine serum albumin (BSA) and 2 mM ethylenediaminetetraacetic acid (EDTA) in phosphate buffer saline (PBS) without Ca2+ and Mg+ (Thermo Fisher, Waltham, MA, USA). Staining/wash buffer (SWB) for flow cytometry contained 2% (w/w) fetal bovine serum (FBS; ThermoFisher) in PBS.
2.2. MAbs and VHHs
The MAbs used in this study are shown in Table S1. The MAbs were purified from hybridoma supernatants by Protein A chromatography at the Dana Farber Cancer Institute (DFCI) Monoclonal Antibody Core facility (Boston, MA) [31]. The murine MAbs against RTA (SyH7, PA1, IB2, WECB2, JD4, and PB10) and RTB (MH3, SylH3, LC5, 24B11, 8B3, and 8A1) have been previously described [29, 31–35]. The VHHs against RTA (JIVF5, V5E1, V1D3, and V13G5) and RTB (JIZB7, V5D1) have also been described, except for V11E10 (manuscript in preparation) [36–39].
2.3. Cell lines and primary cell culture
The macrophage cell line, J774E, originally obtained from Dr. Philip Stahl, Washington University School of Medicine, St Louis, MO, was cultured in DMEM containing 10% (v/v) FBS and was used between passage numbers 6 and 12 [6]. Primary KCs were cultured in medium comprised of DMEM, 10% (v/v) heat-inactivated FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM L-glutamine, 10 ug/mL insulin, and 50 μM 2-mercaptoethanol. Isolated and purified LSECs were cultured in medium containing 44% (v/v) MCDB 131 media (Sigma-Aldrich, St. Louis, MO, USA), 10% heat-inactivated FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM L-glutamine, 1 ng/mL dexamethasone, and 44% (v/v) endothelial cell growth medium-2 microvascular (EGM-2MV) containing SingleQuots™ (Lonza, Walkersville, MD, USA). All cells were cultured on 0.1 mg/mL collagen-coated plates. Plates were coated by adding the collagen solution to the wells for 1 hour at RT, removing, and rinsing one time with calcium and magnesium free (CMF) PBS. Coated plates were stored for up to one month at 4°C. All cells were kept in a humidified incubator at 37°C and 5% atmospheric CO2.
2.4. Isolation of primary murine KCs and LSECs
Liver perfusions were performed on female Swiss Webster mice 7–12 weeks of age, purchased from Taconic Biosciences (Rensselaer, NY). The mice were kept in a specific-pathogen free environment and treated in accordance to Wadsworth Center’s Institutional Animal Care and Use Committee (IACUC) guidelines. Mice were euthanized by CO2 asphyxiation followed by cervical dislocation. For the liver perfusion, the inferior vena cava was cannulated with a 24G x 0.75 in. closed IV Catheter System with Single Port (BD, Franklin Lakes, NJ, USA) connected to a 20-cc syringe. A total of 20 mL pre-perfusion buffer composed of GBSS without calcium (37°C) was applied with a peristaltic pump (KD Scientific, Holliston, MA, USA) at a flow rate of 5 mL/min. The hepatic portal vein was clipped to allow for fluid drainage. For the enzymatic digestion, 10 mL of 37°C perfusion buffer consisting of GBSS with calcium and 0.0008 g/mL Type IV Collagenase (ThermoFisher) was pumped through the liver at 5 mL/min. Following digestion, the liver was excised, and the gall bladder was removed before it was placed in a 60 × 15 mm cell culture dish containing 10 mL of 0.1% (w/v) bovine serum albumin (BSA; Thermo Fisher) in PBS without Ca2+ and Mg+ (BSA-PBS). In a sterilization hood at room temperature, primary parenchymal and non-parenchymal liver cells were removed from the liver by gentle tearing and shaking. The cell suspension was poured through a 100 µm filter into a 50-mL conical tube. This step was repeated until no more cells were released and the conical tube was filled to a total volume of 40 mL with BSA-PBS. The cell suspension was subject to centrifugation at 50 × g for 3 min two times to remove parenchymal cells, and the supernatants containing non-parenchymal cells were stored on ice. All subsequent steps were performed at 4°C. The non-parenchymal cells were pelleted by centrifuging at 800 x g for 10 min. and the RBCs were lysed by incubating with 7 mL ammonium chloride (Stemcell Technologies, Vancouver, Canada) for 4 min. The RBC lysate was removed by adding 23 mL of BSA-PBS and centrifuging for 5 min. at 800 × g.
2.5. Density gradient centrifugation and MACS
Following parenchymal cell and RBC removal, non-parenchymal cells were resuspended in 5 mL of 17.6% (w/v) Optiprep™ (Cosmo Bio USA, Carlsbad, CA, USA) in a 15-mL conical tube. A 5-mL layer of 8.2% (w/v) Optiprep™ was gently layered on top. Cells were centrifuged at 1400 x g for 17 min. without brake at 4°C. Cells at the interface between both layers (~2 to 3 mL total volume) were carefully removed and placed in a 50-mL conical tube. BSA-PBS was added to a final volume of 30 mL, and the cells were pelleted at 800 x g for 5 min. Cells were resuspended in 170 µL of degassed MACS buffer, and 30 µL of anti-F4/80 microbeads (Miltenyi Biotec, Auburn, CA, USA) were added to positively select for KCs. The cells and beads were incubated for 20 min. at 4°C in the dark. Cells were washed twice with 1 mL MACS buffer and resuspended in 0.5 mL MACS buffer. Cells were separated using a MiniMACS™ kit (Miltenyi Biotec, Auburn, CA, USA). In the presence of a magnetic field, the column was hydrated with 0.5 mL of MACS buffer, the cell suspension was added, and the column was rinsed 3x with 0.5 mL of MACS buffer. The eluted or negatively-selected cells were collected in a 15-mL conical tube, and the positively-selected cells were retrieved by removing the column from the magnetic field, adding 1 mL of MACS buffer, and plunging into a 15-mL conical tube. Cells were seeded on collagen-coated plates, and images were gathered with a phase contrast microscope.
2.6. Flow cytometry
Cells were seeded in a clear, U-bottom, 96-well plate at a density of 1 × 107 cells/mL (0.1 mL per well). Controls included unstained cells and individually stained cells. For KC and LSEC purity assessment, cells were pelleted by centrifugation (1500 rpm for 5 min) and resuspended in 100 µL Fc blocking solution containing TruStain fcXTM (anti-mouse CD16/32) antibody (Biolegend, San Diego, CA, USA) diluted to 1:500 in SWB. Cells were incubated on ice for 15 min in blocking solution and centrifuged at 1500 rpm for 5 min. Cells were incubated with 100 µL of conjugated primary antibodies diluted in SWB (Table S2) on ice for 30 to 45 minutes. For FITC-ricin binding studies, cells were incubated on ice for 30 min with either FITC-ricin alone (3 µg/mL), FITC-ricin and lactose (0.1 M), FITC-ricin and mannan (10 mg/mL), or FITC-ricin and mannan and lactose. Plates were centrifuged at 1500 rpm for 5 min, and the cells were washed twice in 200 µL of SWB. Finally, the plate was spun at 2200 rpm for 5 min, and the cells were resuspended in 400 µL of SWB in 5 mL Falcon tubes. Cells were analyzed immediately with a BD FACSCalibur™ flow cytometer (BD Biosciences). Cell viability was assessed by adding 5 µL of the propidium iodide (PI) staining solution from a FITC Annexin V Apoptosis Detection Kit II (BD Pharmingen, San Diego, CA, USA) to the unstained control.
2.7. Ricin cytotoxicity and neutralization assays
White, flat-bottom 96-well plates were coated with Type I rat tail collagen (0.1 mg/mL; ThermoFisher Scientific) diluted in PBS. For ricin toxin dose optimization, LSECs and J774E cells were seeded at 2 × 105, 5 × 105, and 1 × 106 cells/mL, and KCs were seeded at 1 × 105, 2 × 105, and 5 × 105 cells/mL. Cells were treated with ricin diluted 2-fold starting at 100 ng/mL for 18 hours. At this point, the ricin was removed, the cells were washed with HEPES buffer, and regular media was placed in the well. After 24 h, cell viability was assessed using CellTiter-GLO reagent (Promega, Madison, WI) and a Spectramax L Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). For the 2 h pulse ricin treatment, cells were treated with ricin diluted 2-fold starting at 400 ng/mL right after an 18 h attachment period, and cell viability was assessed 24 h later. For plant-based RTA treatment, cells were seeded at 5 × 105 cells/mL and treated overnight with RTA diluted 2-fold starting at 400 ng/mL. All treatments were performed in triplicate, and 100% viability was defined as the average value obtained from wells in which cells were treated solely with cell medium.
The ricin toxin neutralization assays with murine MAbs and VHHs followed an 18 h treatment time-course incorporating ricin at a dose corresponding to the EC90 from the ricin cytotoxicity curves: LSECs and J774E cells were treated with 10 ng/mL of ricin, and KCs were treated with 1 ng/mL of ricin. The MAbs were titrated into the wells containing ricin starting at 20 µg/mL. As a positive control, cells were treated with pooled antisera from BALB/c mice that had been hyper-immunized with RiVax [40]. Dose response curves for all three cell types were also generated for this antiserum in the presence of ricin. Cell viability was assessed as described above. Cell viability was normalized to cells treated with medium only, and background noise was reduced by subtracting the average of wells treated only with ricin at a dose equivalent to the EC90 value.
2.8. MR and CLEctin binding assays
Immulon 4HBX flat bottom microtiter plates (Thermo Scientific, Rochester, NY) were coated overnight at 4°C with ricin (1 µg/ml), washed with PBS plus 0.05% (v/v) Tween-20 (PBS-T), then blocked for 2 h with PBS-T containing 2% (v/v) goat serum (Gibco, MD, USA). Samples were prepared in a separate PVC plate. Ten-fold serial dilutions of 0.1M mannan (Sigma-Aldrich, St. Louis, MO) were mixed with recombinant mouse MR (R&D Systems, Minneapolis, MN) or CLECtin (R&D Systems) at concentrations equivalent to their relative EC90 values (~10 µg/ml). The mannan-MR or CLECtin mixtures were incubated for 1 h at RT and then applied to the microtiter plate coated with ricin, in duplicate, and incubated for 1 h. Control wells contained only MR. Plates were then washed and probed with horseradish peroxidase (HRP)- conjugated anti-His tag (R&D Systems, Minneapolis, MN) for 45 minutes and developed. For inhibition assays, five-fold serial dilutions of MAbs (starting at 500 µg/ml for PB10 or SyH7 separately, 250 µg/ml of each when combined) were mixed with recombinant mouse MR, incubated for 1 h at RT and then applied to the microtiter plate coated with ricin, as described above.
2.9. Lactose and mannan inhibition studies
Based on cytotoxicity results for the 2 h pulse treatment with ricin toxin, cells were seeded at 5 × 105 cells/mL in collagen-coated 96-well plates and were treated with ricin at a dose approximately equal to the EC90 value on the ricin dose curves. Approximately 18 h after seeding, LSECs and KCs were treated with 50 ng/mL of ricin, and J774E cells were treated with 100 ng/mL of ricin for 2 h. Concurrently, cells were treated with either 1 mg/mL of yeast α-mannan (Sigma-Aldrich), 0.1 M lactose (Thermo Fisher), or a mixture of yeast α-mannan and lactose. Cell viability was assessed 24 h later using CellTiter-GLO reagent and a Spectramax L Microplate reader. Cell viability was normalized to cells treated with medium only and background noise was reduced by subtracting the average of wells treated only with ricin at a dose equivalent to the EC90 value.
2.10. In vivo ricin toxin neutralization assays
Mice were challenged intraperitoneally (IP) with 0.6 µg or intravenously (IV) with 2 µg ricin toxin in PBS pre-mixed with 20 µg of total MAb(s). Controls included mice treated solely with ricin toxin or PBS. After 18 h, the livers were perfused, and the cells were separated down to a KC/LSEC mixture using density gradient centrifugation, as described above. Flow cytometry was performed on this mixture to assess KC depletion in each treatment group. During the same experiments, livers and spleens were harvested for further analysis using immunohistochemistry.
2.11. Immunohistochemistry (IHC)
Tissues were immersed and allowed to fix in 10% buffered formalin for 24 hours, and subsequently transferred to 70% ethanol prior to processing and embedding. Tissue sections were stained with hematoxylin and eosin for histopathological evaluation. Tissue sections, 3–4 μm in thickness, were deparaffinized in CitriSolve (Decon Labs., King of Prussia, PA) and rehydrated by processing through graded alcohols. For F4/80 staining, antigen retrieval was performed by treating tissue sections with Proteinase K (0.1 mg/mL) for 5 minutes at room temperature. Antigen retrieval for cleaved caspase-3 was performed by heating the slides to 95°C in Rodent Decloaker, a citrate-based solution, (Biocare Medical; Pacheco, CA) for 40 minutes using a rice steamer. For both procedures, endogenous IgG and non-specific background were blocked with Rodent Block M (Biocare Medical, Pacheco, CA), followed by an alkaline phosphatase block (BLOXALL; Vector Laboratories, Burlingame, CA). For F4/80 staining, primary antibody (GTX26640; GeneTex, Irvine, CA) was incubated on tissue sections at a 1:100 dilution for 1 h at room temperature. Subsequently, sections were sequentially incubated with a rat-on-rodent tissue horseradish peroxidase-based polymer (Biocare Medical) and Vina Green chromogen (Biocare Medical). For caspase-3 staining, the primary antibody (GTX22302; Gene Tex, Irvine, CA) was incubated on the tissue sections at a dilution of 1:100 for 1 hour at room temperature. Subsequently, sections were sequentially incubated with a rabbit-on-rodent tissue alkaline phosphatase-based polymer (Biocare Medical) containing blocking reagent (XM Factor, Biocare Medical), and fast red (Warp Red; Biocare Medical). For both procedures, sections were counterstained with Tacha’s hematoxylin (Biocare Medical) and mounted using a permanent mounting medium (EcoMount; Biocare Medical).
2.12. Statistical analysis
Statistical analysis was carried out with GraphPad Prism 7 (GraphPad Software, San Diego, CA). Visualization was performed in GraphPad Prism 7 or R 3.5.2 [41] using the ggplot2 package [42] and ggthemes [43]. Specific details of each analysis can be found in the respective figure legends.
3. RESULTS
3.1. Enrichment of primary mouse KCs and LSECs
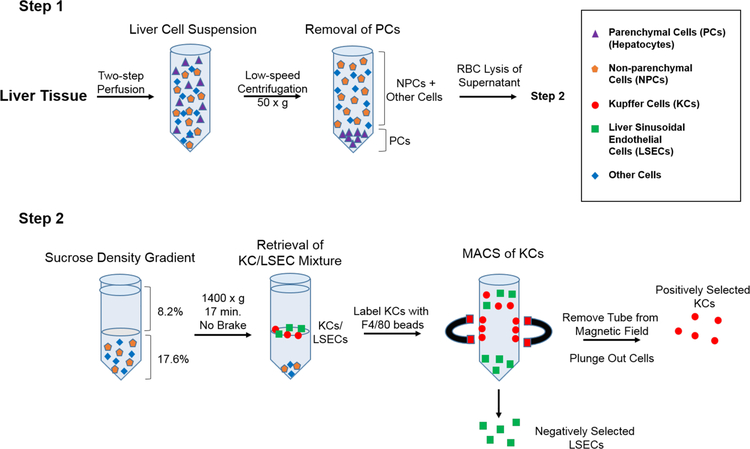
Studies in rats and mice have indicated that KCs and LSECs are extremely vulnerable to the effects of ricin toxin [20, 24, 44, 45]. To better understand the interaction of ricin toxin with these cell types and what role antibodies may play in protection, we sought to establish an ex vivo model with primary mouse KCs and LSECs. As depicted in Figure 1, primary KCs and LSECs were isolated from the livers of Swiss Webster mice using a two-step collagenase perfusion method. The two cell types were enriched using a combination of low-speed centrifugation, density gradient centrifugation, and antibody-conjugated magnetic microbeads [46–49]. For the magnetic separation, anti-F4/80 microbeads were used to positively select for mouse KCs. LSECs were collected in the flow-through (negative selection), as there are no known universal markers for these cell types to enable positive selection [50–52]. While numerous studies have reported using anti-CD146 magnetic microbeads for LSECs selection, this strategy does not capture the CD146− cell subset [48]. For that reason, we relied on negative selection to enrich for both the CD146− and CD146+ LSEC subsets.
Figure 1. Isolation and enrichment protocol for KCs and LSECs from mouse livers.
In Step 1, liver tissues were subjected to a retrograde two-step perfusion followed by low speed centrifugation to remove hepatocytes. The RBCs were then lysed with ammonium chloride and the remaining cell mixture was subjected to sucrose density gradient centrifugation. In Step 2, the KC/LSECs populations partition was collected and mixed with anti-F4/80 antibody-coated magnetic beads. KCs were positively selected by magnetic separation, while LSECs were enriched in the flow through. Relative enrichment and viability of primary KCs and LSECs was determined by flow cytometry and phase contrast microscopy.
To assess the relative enrichment of the two primary cell populations, the KC and LSEC pools were triple-stained with antibodies specific for F4/80, CD146, and CD45, and then subjected to flow cytometry [53, 54]. The isolation and purification protocol yielded positively-selected KCs at 95.8 ± 0.608% enrichment, based on F4/80 and CD45 expression (Table 1; Figure S1). The KCs were also positive for CD11b (data not shown). The average KC yield per mouse liver was ~2 × 106, and cell viability was > 90% (Table 1; Figure S1E). The LSEC pool consisted of two subpopulations that varied in expression levels of CD45: CD146−/CD45hi and CD146+/CD45+ (Table 1; Figure S1). These two LSEC populations have been described previously in rats [55]. The CD146− subset is not hepatic stellate cells (HSCs) or vascular endothelial cells because those two cell types do not express CD45 [56, 57]. The LSEC pool also contained a fraction (~8–10%) of contaminating KCs. The viability of LSECs was ~90% based on trypan blue (TB) and propidium iodide (PI) staining (Table 1; Figure S1). Both the KC and LSEC populations adhered to collagen-coated microtiter plates and assumed morphologies consistent with KCs and LSECs (Figure S1) [58, 59].
Table 1.
Enrichment and viability of mouse KCs and LSECs.
| KCsa | LSECsb | |
|---|---|---|
| Yield | 1.6–2.9 × 106 | 7.07 to 11.0 × 106 |
| % Purityc | 95.8 ± 0.608 | 85.16 ± 1.76 |
| % Viability (TB) | 92.8 ± 2.58 | 90.42 ± 3.45 |
| % Viability (PI)d | 94.68 ± 2.55 | 91.79 ± 1.53 |
per mouse, based on positive KC or negative LSEC selection
Refer to Figure S1A–D for flow cytometry purity plots
Refer to Figure S1E,F for cell viability plots; TB = Trypan Blue, PI = Propidium Iodide
We next examined the sensitivity of primary KCs and LSECs to ricin intoxication. J774E cells, a well-characterized mouse macrophage cell line reported to express the MR (CD206) were included as a control for these experiments [60–62]. Ricin cytotoxicity was initially evaluated across a range of KC and LSEC densities (1 × 104 to 1 × 105 cells total), as described in the Materials and Methods. We ultimately chose 5 × 104 total cells as a working concentration since there was little variation in toxin sensitivity across the range of cell densities we tested (data not shown). Depending on the experiments, we performed cytotoxicity profiles with either a 2 h pulse with ricin or a prolonged (18 h) incubation period, followed by a series of wash steps to remove residual extracellular toxin before a 24 h incubation.
3.2. Ex vivo sensitivity of KCs to ricin.
KCs were remarkably sensitive to the effects of ricin toxin, as evidenced by EC50 values of 1.3 and 0.02 ng/ml in the 2 h and 18 h exposure assays, respectively (Table 2; Figure 2). For J774E cells, EC50 values were 44 and 1.5 ng/ml for the 2 and 18 h exposures, respectively. Ricin toxin was reported to enter BDMCs by two distinct mechanisms: one mediated by RTB that is inhibited by lactose, and the other mediated by the MR that is inhibited by mannan {Kimura, 1988 #1688; Magnusson, 1993 #1983; Simmons, 1986 #1981. By flow cytometry, we confirmed that the isolated mouse KC populations were positive for the MR (Figure S2).
Table 2.
Sensitivity of LSECs, KCs, and J774E cells to ricin toxin and RTAa.
| Ricin | RTA | |||
|---|---|---|---|---|
| EC50 | EC90 | EC50 | EC90 | |
| LSEC | 0.87 | 2.63 | 50.0 | >400 |
| KC | 0.02 | 0.23 | 5.30 | 177 |
| J774E | 1.5 | 2.97 | >400 | >400 |
EC50 and EC90 values in ng/mL
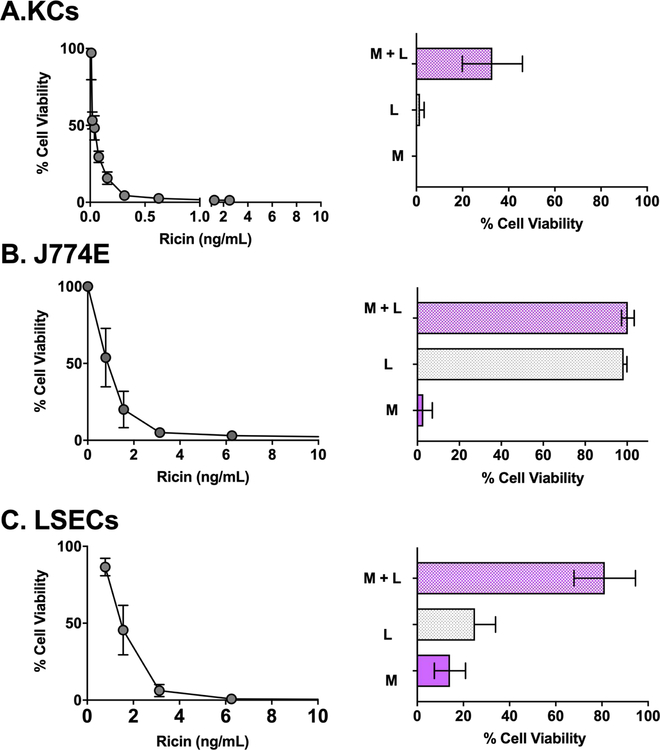
Figure 2. Sensitivity of KCs, J774E cells and LSECs to ricin toxin.
Left panels: Average cell viability of KCs (panel A), J774E cells (panel B), and LSECs (panel C) after an 18 h exposure to indicated concentrations of ricin toxin from two independent experiments. Right panels: Effects of co-incubation of ricin with α-mannan (M; 1 mg/mL), lactose (L; 0.1 M) and the combination of α-mannan and lactose (M+L) on viability of the three different cell types. Each bar represents the average (with corresponding standard deviations) of six independent experiments.
To examine the contribution of these two pathways in ricin uptake, KCs were pulsed for 2 h with ricin in the presence of saturating amounts of exogenous lactose (0.1 M), α-mannan (1 mg/mL) or both lactose and α-mannan {Magnusson, 1993 #1983; Simmons, 1986 #1981}. KC viability was assessed 24 h later. Neither lactose nor mannan alone abrogated the effect of ricin (Figure 2). However, the combination of lactose and mannan afforded partial protection (~40% viability) against ricin-induced cell death, consistent with ricin internalization into KCs occurring via RTB and the MR. To examine the factors influencing ricin attachment to KCs, freshly isolated cells were incubated at 4°C and pulsed with FITC-labeled ricin in the absence or presence of a large excess of lactose, mannan, or the combination of lactose and mannan. After which the cells were analyzed by flow cytometry. Lactose inhibited ricin binding to KCs by ~90%, whereas mannan alone affected attachment by just ~10% (Figure S2). The combination of lactose and mannan did not significantly inhibit binding of ricin to KCs beyond lactose alone. These results indicate that the vast majority of ricin associates with KCs by virtue of RTB’s Gal/GalNAc-specific lectin activity, while the MR accounts for just a fraction of ricin binding. However, despite the differences in binding, the two pathways apparently contribute equally to ricin uptake, since neither lactose nor mannan alone impacted ricin cytotoxicity on KCs. This apparent disconnect is explained by the fact that RTB-mediated uptake is inefficient, whereas the MR uptake is extremely efficient [6, 63]. Thus, even small amounts of ricin bound to the MR can result in productive intoxication and cell death.
It should be pointed out that the mannan and lactose mixture only partially protected KCs from ricin, indicating the existence of a third uptake pathway in these cells. While not investigated here, RTA-derived peptides containing the LDV sequence have been shown to trigger apoptosis of HUVECs via a process that is inhibited by fibronectin and likely integrin mediated [64–66].
In the case of J774E cells, lactose alone completely inhibited ricin binding to cell surfaces and also protected cells from ricin intoxication (Figure 2; Figure S2). Mannan did not affect ricin attachment to J774E cells nor did it impact ricin’s cytotoxic activity. Thus, ricin intoxication of J77E cells is solely dependent on RTB’s lectin activity, even though the cells express the MR on their surface (Figure S2). MR expression and half-life has been reported to vary widely among different J774E clones, possibly explaining the failure of MR to promote ricin uptake here [60].
3.3. Capacity of anti-RTA and anti-RTB MAbs to protect KCs from ricin intoxication ex vivo.
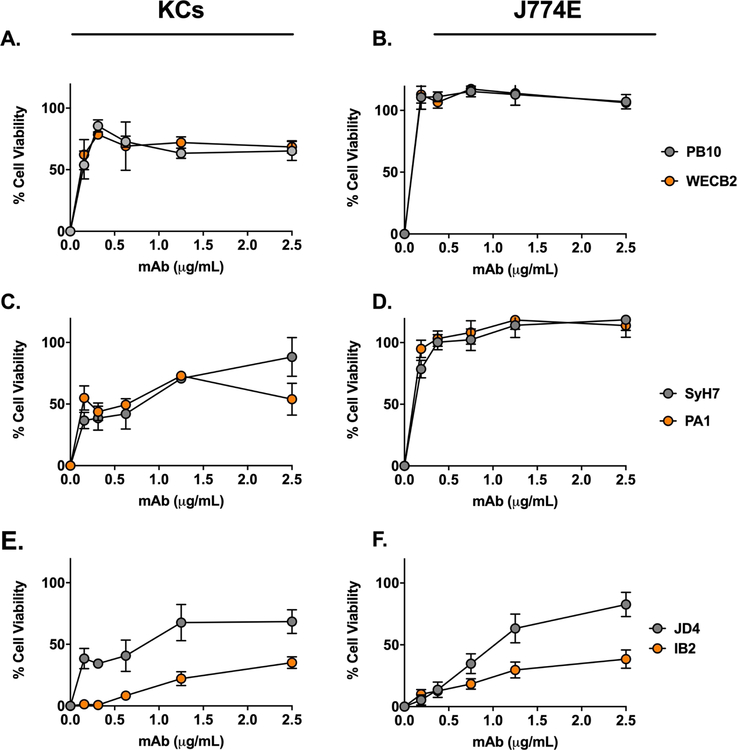
We next examined the capacity of ricin-specific MAbs to protect KCs against ricin toxicity ex vivo. We have previously described a collection anti-RTA and anti-RTB MAbs with a range of neutralizing activities (IC50) on Vero cells [29, 31–35]. The anti-RTA MAbs are directed against four spatially distinct epitope clusters (I-IV). PB10 and WECB2 recognize cluster I, SyH7 and PA1 recognize cluster II, and MAbs IB2 and JD4 bind clusters III and IV, respectively (Table S1). The mechanisms of toxin-neutralization may differ depending on epitope specificity [67].
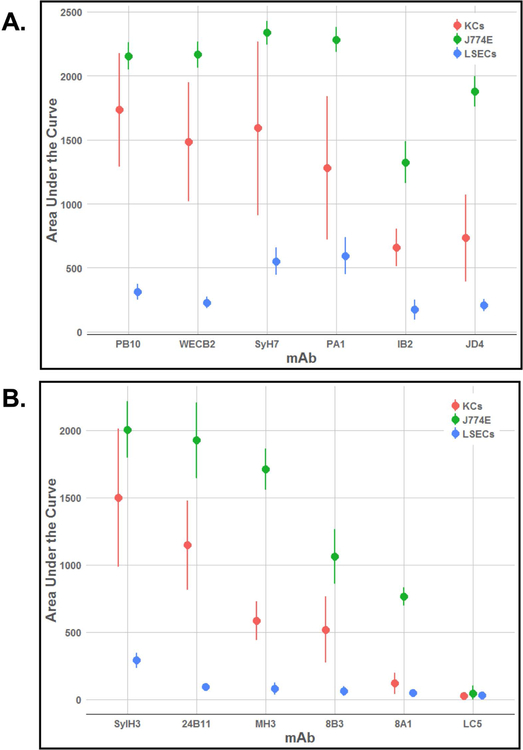
The four RTA-specific MAbs against epitope clusters I (PB10, WECB2) and II (SyH7, PA1) readily neutralized ricin on the J774E cells (IC50 <0.2 µg/mL) (Figure 3). The MAbs also protected KCs from ricin toxin (40–80%), although much less effectively than in the J774E cell assay (Figure 3). Moreover, PB10, WECB2, SyH7, and PA1 toxin-neutralizing activities plateaued at ~80% in the KCs, even at high MAb concentrations. IB2 and JD4 also afforded some degree of toxin-neutralizing activity on both J774E and KCs (Figure 3). In addition, individual anti-RTB antibodies were able to partially protect KCs from ricin, but less effectively than in the J774E cytotoxicity assay (Figure S3). For comparison purposes, the overall toxin-neutralizing activity for each MAb tested in the KC and J774E assay was plotted as area under the curve (AUC) (Figure 4). The plot illustrates the superior neutralizing activity of the MAbs in the J774E assay, as compared to the KC assay.
Figure 3. Protection of KCs and J774E cells from ricin toxicity by individual anti-RTA MAbs.
Freshly isolated KCs (panels A, C, E) or J774E cells (panels B, D, F) were seeded into 96-well microtiter plates and then treated with ricin plus indicated amount of anti-RTA MAbs for 18 h. The cells were washed, and cell viability was assessed 24 h later. The MAbs are grouped based on their epitopes clusters on RTA: cluster I (A, B), cluster II (C, D) and Clusters III and IV (E, F). Each data point represents the average (with corresponding standard deviations) of three independent experiments.
Figure 4. Comparative analysis of anti-RTA and anti-RTB MAbs in protecting KCs, J774E and LSECs from ricin toxin.
Area under the curve (AUC) analysis of ricin-toxin neutralizing activities of a collection of MAbs targeting (A) RTA and (B) RTB. Cytotoxicity assays were performed across a range of MAb concentrations (starting at 20 μg/ml), as described in the Materials and Methods. AUC values and corresponding 95% confidence intervals were determined for each antibody from the results of ricin neutralization assays, with 4–13 biological replicates per concentration.
3.4. Protecting KCs from ricin-induced killing in vivo.
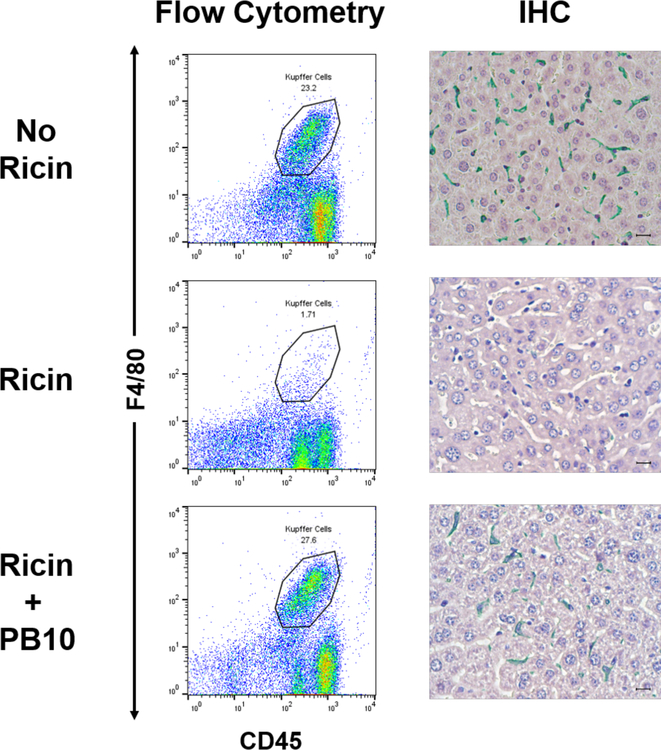
The results of the ex vivo studies prompted us to examine the degree to which individual anti-ricin MAbs are able to protect KCs in vivo. We chose PB10 (anti-RTA) and SylH3 (anti-RTB) for these studies since they represent two of our most potent classes of toxin-neutralizing MAbs [18, 33, 34, 68]. Ricin was injected into mice by the intraperitoneal (IP) or intravenous (IV) routes in absence or presence of PB10 or SylH3. It is known that injection of ricin into mice by the IV or IP routes results in toxin accumulation in liver [69]. The mice were euthanized 18 h later and KCs were isolated from livers as described above. In the absence of either PB10 or SylH3, the KC populations, defined by F4/80+ staining, declined from ~23% to < 2%, consistent with ricin’s capacity to essentially ablate these cells from the liver (Figure 5; Figure S4). The loss of KCs in situ was confirmed by IHC analysis with F4/80 antibodies (Figure 5). By comparison, co-administration of ricin with PB10 resulted in complete protection of KCs from toxin-induced death, as determined by flow cytometry and IHC. Identical results were obtained when ricin was co-administered by SylH3 and delivered to mice by IV injection (Figure S4). Thus, individual MAbs against RTA or RTB are able to protect KCs in vivo from the effects of ricin exposure, even though they afforded less than maximal activity ex vivo. From an intervention standpoint, a single MAb like PB10 or SylH3 would be considered appropriate to protect KCs against ricin.
Figure 5. PB10 protects KCs from the effects of ricin in vivo.
Groups of mice were challenged by IP injection with saline (top row), ricin toxin (middle row) or ricin toxin plus PB10 (bottom row). Eighteen hours later, the mice were euthanized, and liver tissues collected for KC isolation (left panels) or IHC (right panel). For flow cytometric analysis, KCs were immunostained for F4/80 (y-axis) and CD45 (x-axis), as described in the Materials and Methods. The CD45+ F4/80+ KC populations are encircled, and the percentage of the total cell numbers are noted. For IHC (right panels), formalin-fixed tissue sections were stained with anti-F4/80 followed by HRP-based polymer and Vina Green chromogen, also as described in the Materials and Methods. Note the complete absence of F4/80+ cells in ricin treated mice, while near normal numbers of F4/80+ cells in ricin plus PB10-treated animal.
3.5. Sensitivity and neutralizing activity of MAbs in LSEC assay.
We next examined the sensitivity of LSECs to ricin. As shown in Table 2 and Figure 2, ricin induced LSEC death in a dose-dependent manner, with an estimated EC50 of 4 ng/ml. Co-administration of ricin with either lactose (0.1 M) or α-mannan (1 mg/mL) afforded limited protection (20–30%) against killing, whereas the combination of lactose and α-mannan resulted in ~80% viability. These results are consistent with ricin uptake into LSECs occurring via RTB- and MR-dependent pathways [9]. By flow cytometry, MR expression was detected on the CD146+ LSEC population, but not the CD146− population (Figure S2).
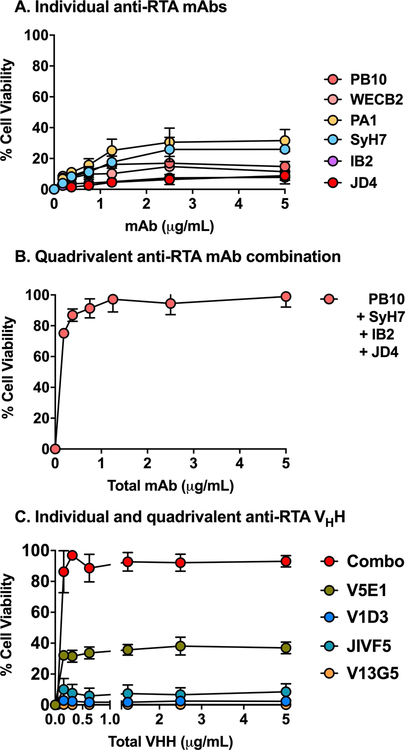
Our collection of RTA-specific MAbs were evaluated for the ability to protect LSECs from the effects of ricin. Surprisingly, individual anti-RTA MAbs were largely, if not completely, ineffective at neutralizing ricin in the LSEC cytotoxicity assay (Figures 4, 6). For example, even molar excess amounts of PB10 afforded only 10% protection against ricin killing (Figure 6). Two other MAbs, SyH7 and PA1, fared slightly better (~40% viability), but never achieved complete protection.
Figure 6. Protection of LSECs by anti-RTA MAb cocktails.
LSECs were seeded in 96 well microtiter plates and then treated with ricin plus indicated concentrations of (panel A) individual anti-RTA MAbs, (panel B) an anti-RTA quadrivalent MAb cocktail, or (panel C) individual and combinations of anti-RTA VHHs. Cell viability was normalized to LSECs treated with vehicle (saline), as described in the Materials and Methods. Each data point represents the average (with corresponding standard deviations) of three independent experiments.
The failure of the individual MAbs to neutralize ricin in the LSEC cytotoxicity assay prompted us to test a combination of all four anti-RTA MAbs, effectively creating a quadrivalent cocktail targeting the four known neutralizing hotspots on RTA. The quadrivalent antibody mixture demonstrated extremely potent toxin-neutralizing activity in the LSEC cytotoxicity assay, with an IC50 of <0.2 µg/mL (Figure 6). In an effort to ascribe the neutralizing activity to particular MAb combination, the four different MAbs were tested in all possible combinations (Figure S5). Certain pairs, namely PB10 and SyH7, afforded between 80–90% protection at high concentrations (>1 µg/mL), but no two or three MAb combination achieved the potency observed with the quadrivalent cocktail.
To further examine the combinatorial effects of antibodies on ricin toxin neutralizing activity in the LSEC cytotoxicity assay, we turned to a collection of alpaca-derived single domain antibodies (VHHs) [39, 70]. VHHs differ from conventional IgG MAbs in that they are monovalent and are devoid of Fc elements, thereby eliminating any contribution of aggregation and FcR involvement in the cytotoxicity assay. VHHs targeting RTA’s four neutralizing hotspots were tested individually and in combination. While only one of the four individual VHHs had notable toxin-neutralizing activity on its own, the quadrivalent mixture proved nearly as potent at inactivating ricin as the four MAb cocktail (Figure 6). Thus, co-occupancy of key neutralizing epitopes on RTA rather than agglutination or FcR-mediated uptake is likely responsible for the protection of LSECs from the effects of ricin.
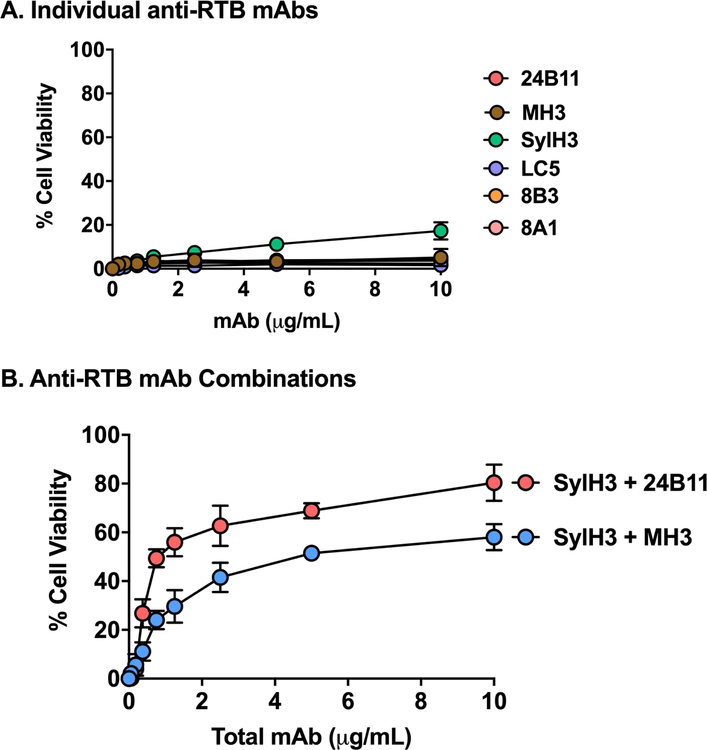
A similar pattern emerged when we tested a collection of anti-RTB mouse IgG MAbs and alpaca VHHs in the LSEC cytotoxicity assay (Table S1; Figure 7). Neither the individual anti-RTB MAbs (Figure 7A) nor the individual VHHs (data not shown) demonstrated a capacity to neutralize ricin in the LSEC assay. Mixtures of SylH3 combined with MH3 or 24B11 had only moderate toxin-neutralizing activity (50–75%) (Figure 7B).
Figure 7. Protection of LSECs by individual and pairs of anti-RTB MAbs.
LSECs were seeded into 96 well microtiter plates and then treated with ricin with individual MAbs (Panel A) or pairs of RTB-specific MAbs (Panel B) at indicated total concentrations, as described in the Materials and Methods. Each data point represents the average (with corresponding standard deviations) of three independent experiments.
3.6. Pairs of anti-RTA/anti-RTB MAbs protect LSECs from ricin intoxication.
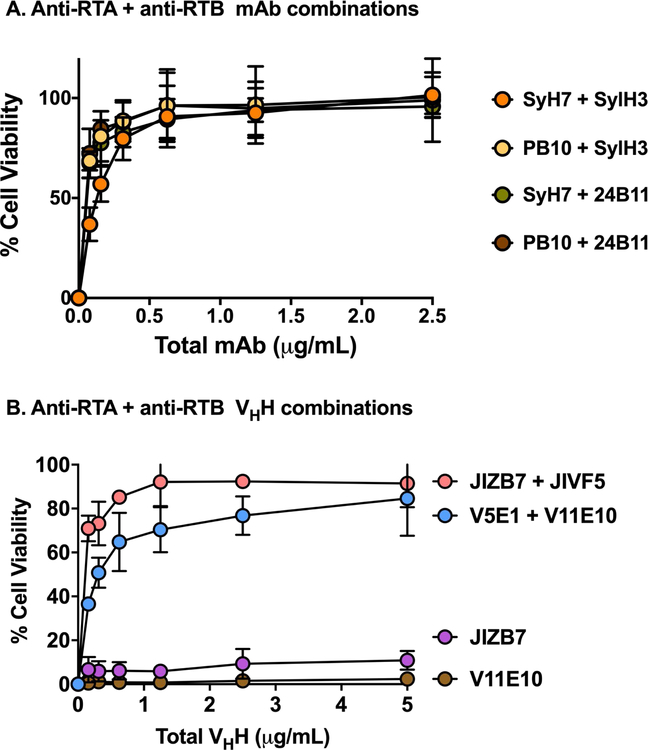
The next obvious line of investigation was to examine toxin-neutralizing activity of pairs of anti-RTA and anti-RTB MAbs. We generated four different anti-RTA and anti-RTB MAb combinations using PB10, SyH7, SylH3 and 24B11. All four anti-RTA/anti-RTB cocktails demonstrated remarkably potent toxin-neutralizing activity, as evidenced by IC50 values of <0.2 µg/mL (Figure 8A). In fact, the toxin-neutralizing profiles were essentially superimposable, indicating that equal potency was achieved irrespective of the different epitopes being targeted on RTA (i.e., cluster I or II) and RTB (i.e., domain 1 or 2).
Figure 8. Protection of LSECs by MAb and VHH cocktails.
LSECs were seeded into 96-well microtiter plates and then challenged ricin toxin incubated with pairs of anti-RTA (PB10, SyH7) and anti-RTB (SylH3, 24B11) MAbs (panel A) and VHHs (panel B). V11E10 is against RTA, and JIZB7 against RTB. Each data point represents the average (with corresponding standard deviations) of three independent experiments.
We also tested the potency of two different pairs of anti-RTA/anti-RTB VHHs single chain antibodies. Two different VHH pairs demonstrated potent toxin-neutralizing activities, although not to the extent observed with the MAbs (Figure 8B). Nonetheless, the activity associated with the VHH pairs was much greater than additive effects, indicating that targeting both of ricin’s subunits simultaneously results in synergistic activity and therefore an effective means to protect LSECs from ricin.
3.7. Anti-RTA and anti-RTB MAbs neutralize ricin via the mannose-dependent uptake pathway in LSECs.
We hypothesized that the observed synergy between anti-RTA and anti-RTB MAbs in the LSECs cytotoxicity assay might be explained by the MAbs acting on different ricin uptake pathways. Based on the literature, we postulated that anti-RTB MAbs block the lactose-dependent uptake pathway, while anti-RTA MAbs interfere with the mannan-dependent pathway [29, 67, 71]. To test this hypothesis, individual MAbs were mixed with lactose (to block RTB-mediated uptake) or mannan (to block the MR and other CTL uptake) and then tested them for the ability to protect LSECs from ricin challenge.
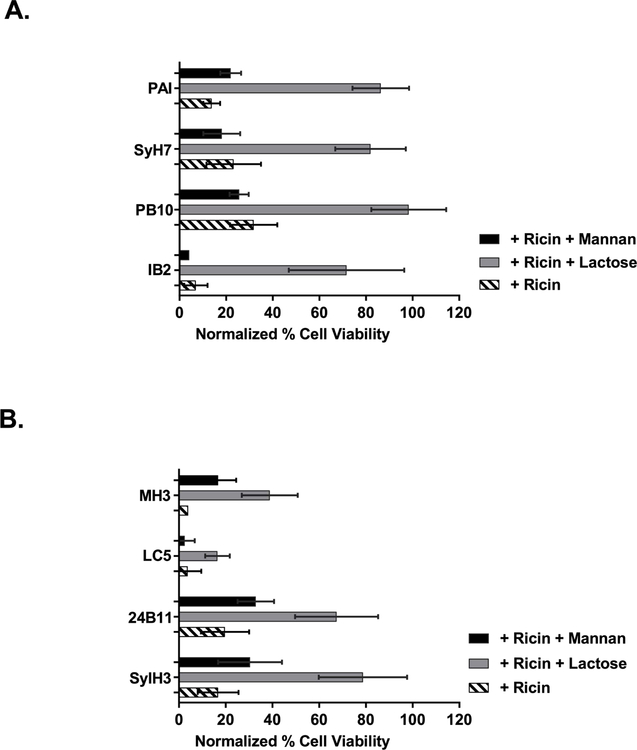
We found that the neutralizing activity of the anti-RTA MAbs was significantly enhanced by the addition of lactose, but not mannan (Figure 9). For example, LSEC viability in the presence of ricin and PB10 was ~30%, while the further addition of lactose resulted in >90% viability. The same was observed for MAbs SyH7 and PA1 (directed against epitope cluster II) and IB2 (against epitope cluster III). Overall, these results are consistent with anti-RTA MAbs blocking ricin uptake via the MR (or another mannose-dependent C-type lectin) in the LSEC cytotoxicity assay, not the lactose uptake pathway.
Figure 9. Anti-ricin MAbs function via the mannan-dependent uptake pathway in LSECs.
Freshly isolated LSECs were seeded in 96 well microtiter plates and pulsed for 2 h with ricin plus indicated anti-RTA (panel A) or anti-RTB (panel B) MAbs, in the presence of α-mannan (1 mg/mL) or lactose (0.1 M). Cell viability was normalized to LSECs that had not been treated with ricin toxin. In all cases, the addition of lactose resulted in a significant increase in cell viability, as compared to as compared to control (ricin only). Mannan addition impacted cell viability in only one instance (MH3). Kruskal-Wallis with Tukey’s multiple comparisons test (GraphPad 7) was applied to account for non-normal distribution of data.
Unexpectedly, the toxin-neutralizing activity of the anti-RTB MAbs was also enhanced by lactose. For example, the addition of lactose enhanced 24B11 and SylH3’s activities from ~20% viability to ~80% viability. The addition of mannan enhanced neutralizing activity to just 30% viability (Figure 9). MH3’s toxin-neutralizing activity was also enhanced by lactose and mannan, although total LSEC viability never exceeded 50%. These results suggest that anti-RTB MAbs may also interfere with ricin uptake via the MR pathway, not the lactose-dependent uptake pathway as we had postulated.
The fact that SylH3 and PB10 plus lactose are much more effective than saturating amounts of mannan plus lactose at protecting LSECs from ricin intoxication indicates that the MAbs have “effector functions” not mimicked by mannan. We postulate that the most plausible explanation is simply binding affinity. For example, PB10 and SylH3 each bind to ricin with sub-nanomolar affinities and would be expected to stay associated with ricin following endocytosis, whereas mannan has only micromolar affinity for the MR and would be expected to serve as a relatively weak competitive inhibitor, even at saturating doses. However, the story could be more complicated if the ricin-MAb complexes are delivered into the cytosol where actors like TRIM-21 are able to promote the clearance of IgG-based immune complexes [72].
3.8. Interaction of ricin with the MR and LSECtin.
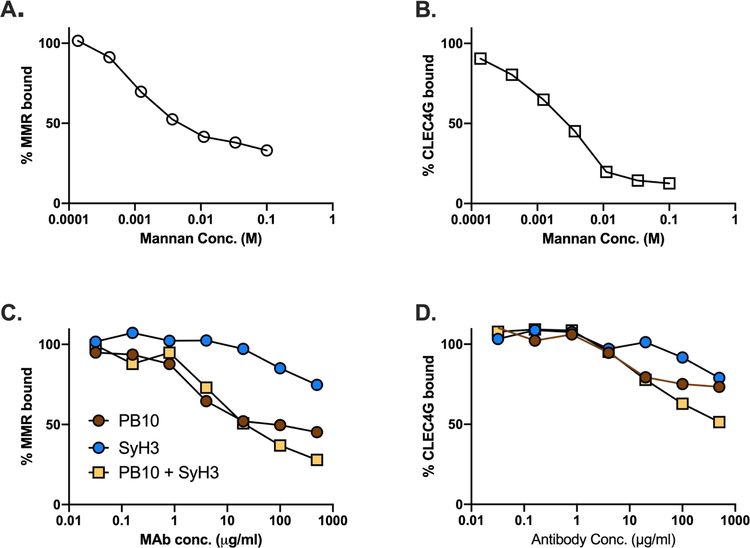
To examine the possibility that anti-RTA and anti-RTB MAbs interfere with toxin-MR interactions, we established a solid phase binding assay in which immobilized ricin toxin was probed with recombinant mouse MR. The interaction of the MR with immobilized ricin was inhibited by mannan in a dose-dependent manner (Figure 10). At high concentrations, PB10 and the PB10/SylH3 combination partially inhibited (50–60% reduction) the binding of the MR to ricin. SylH3 also affected the MR-ricin interaction but to a lesser extent, although it is unclear whether these reductions in binding account for the observed effects of the MAbs on toxin-neutralizing activity.
Figure 10. Effect of MAbs on the interaction between ricin and the MR and CLECtin.
Immulon microtiter plates were coated with ricin (1 µg/ml) and then probed with recombinant mouse MR or CLECtin, as described in the Materials and Methods, in the presence of increasing amounts of mannan (panels A, B) or five-fold serial dilutions of MAbs (panels C, D). Shown are the averages of representative experiments with three technical replicates.
We also established a binding assay between ricin and LSECtin (CLEC4G), another C-type lectin enriched in the liver that recognizes GlcNAcMan residues like those on RTA and RTB [73]. LSECtin recognized immobilized ricin in a mannose-dependent manner, although neither PB10 nor SyH3 were particularly effective at blocking this interaction (Figure 10). Collectively, these results suggest that PB10 and SylH3 protect LSECs from ricin toxin by a mechanism other than interfering with the initial recognition between ricin and the MR or another C-type lectin receptor such as LSECtin.
3.9. A candidate ricin toxin subunit vaccine elicits antibodies that protect KCs and LSECs from ricin.
RiVax is a recombinant RTA subunit vaccine that has been shown to elicit immunity to ricin in mice and non-human primates [74, 75]. It is also safe in humans when administered intramuscularly [76]. We therefore wished to examine whether anti-RiVax antisera is able to protect KCs and LSECs ex vivo. Ricin was incubated with serial dilutions of anti-sera from RiVax vaccinated mice and then applied to KCs and LSECs. We found that the anti-serum had similar IC50 titers (e.g., 1:2500) for both KCs and LSECs, consistent with roughly equal degrees of protection (Figure S6). This result may explain in part the relative efficacy of RiVax as a vaccine against ricin intoxication.
4. DISCUSSION
The liver plays an essential role in detoxification and clearance of xenobiotics from circulation [25, 77]. Non-parenchymal cells also function in immune surveillance and uptake of SIC [28, 78, 79]. In this report, we investigated the capacity of anti-RTA and anti-RTB MAbs, individually and in combination, to protect primary mouse KCs and LSECs from ricin toxin-induced death. This study was motivated by the fact that KCs and LSECs are notoriously sensitive to the effects of ricin, a point recognized decades ago when RTA was first being explored as an immunotoxin [7, 45, 80–83]. Indeed, we found that freshly isolated mouse KCs were 10–40 times more sensitive to ricin than standard mouse cell lines like J774E cells. Nonetheless, individual MAbs against RTA or RTB were able to protect KCs ex vivo with efficiencies similar to J774E cells. In addition, two MAbs, PB10 and SyH3, were each able to protect KCs from ricin-induced apoptosis in vivo. Thus, despite their remarkable sensitivity to ricin toxin, KCs are readily protected from toxin-induced death by anti-RTA and anti-RTB MAbs.
LSECs were another story. We found that individual anti-RTA and anti-RTB MAbs had virtually no capacity to protect LSECs from ricin intoxication. For example, even PB10, which has been shown to be able to rescue Rhesus macaques from lethal dose ricin exposure by aerosol, afforded less than 20% protection in the LSEC killing assay. This shortcoming was overcome when PB10 was combined with other MAbs. The most effective combination was a two-part cocktail targeting RTA and RTB (i.e., PB10 + SyH3), although other pairs and oligoclonal mixtures were equally functional. Combinations of VHHs, which are monovalent and lack Fc elements, also had neutralizing activity, demonstrating that neither ricin crosslinking (aggregation) nor FcR engagement is necessary to protect LSECs from ricin intoxication.
When considering possible mechanisms by which MAbs protect KCs and LSECs, it is important to keep in mind that ricin uptake by these two cell types occurs by at least two distinct pathways: one inhibited by exogenous lactose (“lactose-sensitive”) and the other by exogenous mannose or mannan (“mannose-sensitive”). The lactose-dependent endocytic pathway, which has been studied in great detail, is mediated by RTB’s affinity for terminal Gal/GalNAc residues on cell surface glycoproteins and glycolipids [63]. Following endocytosis, ricin holotoxin is trafficked retrograde to the TGN and the ER, where RTA is liberated from RTB and translocated into the cytoplasm [84]. This uptake pathway is inefficient, however, as only a small fraction (1–10%) of the total amount of ricin bound to any given cell surface actually results in delivery of RTA into the cytoplasm [84, 85]. Moreover, ricin is easily derailed during retrograde transport, particularly if the toxin is aggregated or complexed with another protein [86]. All things considered, perhaps it is not surprising that ricin-MAb complexes are recycled or inefficiently routed to the TGN [67, 71, 87].
The mannose-sensitive uptake pathway exploited by ricin to intoxicate liver cells is much less well understood. While uptake has historically been attributed to the MR, this assumption is inconsistent with demonstration that MR knock-out mice are significantly more susceptible (not more resistant) to toxin-induced death than age-matched controls [6, 8–10, 88]. Moreover, LSECs are sensitive to ricin toxin even though the MR is only expressed on the CD146+/CD45+ LSEC subset (~18% of total population) and not on the CD146-/CD45+ subset. Therefore, it is plausible that mannose-sensitive uptake of ricin is mediated by a second C-type lectin. One candidate is LSECtin, a single domain C-type lectin with homology to DC-SIGN and DC-SIGNR expressed exclusively in the liver [89]. LSECtin serves as a receptor for Ebola virus, SARS coronavirus, and Japanese encephalitis virus [73, 90]. We found that recombinant LSECtin recognized ricin toxin in a solid phase binding assay and that the interaction was inhibited by mannan. Assessing the contribution of LSECtin in promoting ricin intoxication of LSECs in vivo awaits testing in LSECtin knock-out mice [91].
Nevertheless, it remains unclear how ricin and ricin-IC evade degradation following uptake by LSECs. A cardinal feature of LSECs is their extraordinary capacity to degrade endocytosed materials, including SIC [26, 27, 50, 92, 93]. Scavenging receptors like the MR, for example, release cargo within acidified endosomes before being recycled back to the cell surface [94, 95]. That cargo is then destined for degradation via the lysosome or proteolytically processed and loaded onto major histocompatibility complexes. How ricin evades these fates following uptake by the MR or another C-type lectin is unknown. There is evidence that ricin passes through an acidic compartment following uptake via the mannose-sensitive pathway, but ricin has no known mechanism to broach the endocytic membrane [6].
Similarly, ricin-MAb complexes such as those generated in this study would be expected to be taken up by LSECs by way of FcγRIIb2 [28, 79, 96, 97]. FcγRIIb2 is an inhibitory Fc-receptor primarily expressed on LSECs and secondarily on KCs and is the largest contributor to clearance of SICs from circulation. While FcγRIIB would not be able to internalize one-to-one toxin-antibody complexes (e.g., PB10-ricin), it would be predicted to engage with ricin decorated by two or more MAbs (e.g., PB10 + SyH3) and route them for degradation. Whether or not FcγRIIB plays a role in clearance of ricin immune complexes in vivo remains to be determined.
Our study adds to the growing list of examples in which MAb cocktails display synergistic neutralizing activity well beyond what is achieved with single MAbs alone [98–101]. In the case of botulinum neurotoxin (BoNT), for example, there is synergy with two- and three-component MAb cocktails that was attributed to either conformational changes induced by one antibody that enhanced secondary antibody recognition and/or toxin aggregation/multimerization [102–104]. In the case staphylococcus enterotoxin B (SEB), the combination of a MAb (20B1) that occludes the T-cell receptor binding site with MAbs of lesser (6D3,14G8) neutralizing activity enhanced Fc-mediated clearance of SEB in a mouse model. Similarly, combinations of non-neutralizing MAbs have been shown to neutralize Ebola and Marburg virus in animal models [100, 101]. In a companion study, we demonstrated that the two-part cocktail consisting of PB10 + SyH3 is far superior at protecting AMs and tissue resident macrophages from ricin-induced death in vivo, as compared to either of the MAbs individually (Rong et al., manuscript submitted). AMs are hypersensitive to the effects of ricin, possibly due to high levels of MR expression [105]. The success of PB10 + SylH3 in that study and the current study has prompted us to generate a humanized version of SyH3 with the goal of testing it in combination with humanized PB10 in the Rhesus macaque model [17].
One limitation of our study is that we restricted our examination of the liver to LSECs and KCs, essentially ignoring other effects that ricin and ricin-IC may have on hepatic cellularity, inflammation, and physiology. For example, Roche and colleagues demonstrated in mice that hepatic inflammation and pathology following ricin exposure includes an influx of neutrophils, chemokine expression (e.g., MCP-1), fibrin deposition, red blood cell congestion of the hepatic parenchyma and glycogen depletion [106]. These are obvious metrics that could be examined in the context of the MAbs and MAb cocktails like PB10 and SylH3 when administered concurrently or at timepoints after ricin challenge. Roche and colleagues did demonstrate a marked reduction in hepatic damage with the addition of anti-RTA MAbs and MAbs cocktails.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful Dr. Renjie Song (Wadsworth Center Immunology Core Facility) for his assistance with flow cytometry. We extend a special thanks to Helen Johnson of the Wadsworth Center’s Histopathology Core facility for preparation of tissue sections. We thank Dr. David Vance for providing VHHs. We are grateful to Dr. Yinghui Rong for her scientific input and technical advice throughout the course of the study. We acknowledge Beth Cavosie for administrative assistance.
1, Research reported in this work was supported by Contract No. HHSN272201400021C from the National Institutes of Allergy and Infectious Diseases, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
DISCLOSURE
The authors have no conflicts of interest to declare.
Supplementary information accompanies this paper
REFERENCES
- 1.Audi J, Belson M, Patel M, Schier J, Osterloh J (2005) Ricin poisoning: a comprehensive review. JAMA 294, 2342–51. [DOI] [PubMed] [Google Scholar]
- 2.Cieslak TJ, Kortepeter MG, Wojtyk RJ, Jansen HJ, Reyes RA, Smith JO, And the, N. B. M. A. P. (2018) Beyond the Dirty Dozen: A Proposed Methodology for Assessing Future Bioweapon Threats. Mil Med 183, e59–e65. [DOI] [PubMed] [Google Scholar]
- 3.Endo Y, Mitsui K, Motizuki M, Tsurugi K (1987) The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem 262, 5908–12. [PubMed] [Google Scholar]
- 4.Endo Y and Tsurugi K (1987) RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem 262, 8128–30. [PubMed] [Google Scholar]
- 5.Tesh VL (2012) The induction of apoptosis by Shiga toxins and ricin. Curr Top Microbiol Immunol 357, 137–78. [DOI] [PubMed] [Google Scholar]
- 6.Simmons BM, Stahl PD, Russell JH (1986) Mannose receptor-mediated uptake of ricin toxin and ricin A chain by macrophages. Multiple intracellular pathways for a chain translocation. J Biol Chem 261, 7912–20. [PubMed] [Google Scholar]
- 7.Bourrie BJ, Casellas P, Blythman HE, Jansen FK (1986) Study of the plasma clearance of antibody--ricin-A-chain immunotoxins. Evidence for specific recognition sites on the A chain that mediate rapid clearance of the immunotoxin. Eur J Biochem 155, 1–10. [DOI] [PubMed] [Google Scholar]
- 8.Magnusson S and Berg T (1993) Endocytosis of ricin by rat liver cells in vivo and in vitro is mainly mediated by mannose receptors on sinusoidal endothelial cells. Biochem J 291 (Pt 3), 749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnusson S, Berg T, Turpin E, Frenoy JP (1991) Interactions of ricin with sinusoidal endothelial rat liver cells. Different involvement of two distinct carbohydrate-specific mechanisms in surface binding and internalization. Biochem J 277 (Pt 3), 855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magnusson S, Kjeken R, Berg T (1993) Characterization of two distinct pathways of endocytosis of ricin by rat liver endothelial cells. Exp Cell Res 205, 118–25. [DOI] [PubMed] [Google Scholar]
- 11.Newton DL, Wales R, Richardson PT, Walbridge S, Saxena SK, Ackerman EJ, Roberts LM, Lord JM, Youle RJ (1992) Cell surface and intracellular functions for ricin galactose binding. J Biol Chem 267, 11917–22. [PubMed] [Google Scholar]
- 12.Rutenber E, Katzin BJ, Ernst S, Collins EJ, Mlsna D, Ready MP, Robertus JD (1991) Crystallographic refinement of ricin to 2.5 A. Proteins 10, 240–50. [DOI] [PubMed] [Google Scholar]
- 13.Youle RJ, Murray GJ, Neville DM Jr. (1981) Studies on the galactose-binding site of ricin and the hybrid toxin Man6P-ricin. Cell 23, 551–9. [DOI] [PubMed] [Google Scholar]
- 14.Hadebe S, Brombacher F, Brown GD (2018) C-Type Lectin Receptors in Asthma. Front Immunol 9, 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gal Y, Mazor O, Falach R, Sapoznikov A, Kronman C, Sabo T (2017) Treatments for Pulmonary Ricin Intoxication: Current Aspects and Future Prospects. Toxins (Basel) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenfeld R, Alcalay R, Mechaly A, Lapidoth G, Epstein E, Kronman C, S, J. F., Mazor O (2017) Improved antibody-based ricin neutralization by affinity maturation is correlated with slower off-rate values. Protein engineering, design & selection : PEDS, 1–7. [DOI] [PubMed]
- 17.Roy CJ, Ehrbar DJ, Bohorova N, Bohorov O, Kim D, Pauly M, Whaley K, Rong Y, Torres-Velez FJ, Vitetta ES, Didier PJ, Doyle-Meyers L, Zeitlin L, Mantis NJ (2019) Rescue of rhesus macaques from the lethality of aerosolized ricin toxin. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Slyke G, Sully EK, Bohorova N, Bohorov O, Kim D, Pauly MH, Whaley KJ, Zeitlin L, Mantis NJ (2016) Humanized Monoclonal Antibody That Passively Protects Mice against Systemic and Intranasal Ricin Toxin Challenge. Clin Vaccine Immunol 23, 795–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhaskaran M, Didier PJ, Sivasubramani SK, Doyle LA, Holley J, Roy CJ (2014) Pathology of lethal and sublethal doses of aerosolized ricin in rhesus macaques. Toxicol Pathol 42, 573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bingen A, Creppy EE, Gut JP, Dirheimer G, Kirn A (1987) The Kupffer cell is the first target in ricin-induced hepatitis. J Submicrosc Cytol 19, 247–56. [PubMed] [Google Scholar]
- 21.Frenoy JP, Turpin E, Janicot M, Gehin-Fouque F, Desbuquois B (1992) Uptake of injected 125I-ricin by rat liver in vivo. Subcellular distribution and characterization of the internalized ligand. Biochem J 284 (Pt 1), 249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skilleter DN and Foxwell BM (1986) Selective uptake of ricin A-chain by hepatic non-parenchymal cells in vitro. Importance of mannose oligosaccharides in the toxin. FEBS Lett 196, 344–8. [DOI] [PubMed] [Google Scholar]
- 23.Worrell NR, Skilleter DN, Cumber AJ, Price RJ (1986) Mannose receptor dependent uptake of a ricin A chain--antibody conjugate by rat liver non-parenchymal cells. Biochem Biophys Res Commun 137, 892–6. [DOI] [PubMed] [Google Scholar]
- 24.Zenilman ME, Fiani M, Stahl PD, Brunt EM, Flye MW (1989) Selective depletion of Kupffer cells in mice by intact ricin. Transplantation 47, 200–3. [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, Evers S, Roeder D, Parlow AF, Risteli J, Risteli L, Lee YC, Feizi T, Langen H, Nussenzweig MC (2002) Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 295, 1898–901. [DOI] [PubMed] [Google Scholar]
- 26.Maynard Y and Baenziger JU (1981) Oligosaccharide specific endocytosis by isolated rat hepatic reticuloendothelial cells. J Biol Chem 256, 8063–8. [PubMed] [Google Scholar]
- 27.Anderson CL (2015) The liver sinusoidal endothelium reappears after being eclipsed by the Kupffer cell: a 20th century biological delusion corrected. J Leukoc Biol 98, 875–6. [DOI] [PubMed] [Google Scholar]
- 28.Ganesan LP, Kim J, Wu Y, Mohanty S, Phillips GS, Birmingham DJ, Robinson JM, Anderson CL (2012) FcgammaRIIb on liver sinusoidal endothelium clears small immune complexes. J Immunol 189, 4981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yermakova A and Mantis NJ (2011) Protective immunity to ricin toxin conferred by antibodies against the toxin’s binding subunit (RTB). Vaccine 29, 7925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leshin J, Danielsen M, Credle JJ, Weeks A, O’Connell KP, Dretchen K (2010) Characterization of ricin toxin family members from Ricinus communis. Toxicon 55, 658–61. [DOI] [PubMed] [Google Scholar]
- 31.Toth RTI, Angalakurthi SK, Van Slyke G, Vance DJ, Hickey JM, Joshi SB, Middaugh CR, Volkin DB, Weis DD, Mantis NJ (2017) High-Definition Mapping of Four Spatially Distinct Neutralizing Epitope Clusters on RiVax, a Candidate Ricin Toxin Subunit Vaccine. Clin Vaccine Immunol 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Hara JM, Kasten-Jolly JC, Reynolds CE, Mantis NJ (2014) Localization of non-linear neutralizing B cell epitopes on ricin toxin’s enzymatic subunit (RTA). Immunol Lett 158, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Hara JM, Neal LM, McCarthy EA, Kasten-Jolly JA, Brey RN 3rd, Mantis NJ (2010) Folding domains within the ricin toxin A subunit as targets of protective antibodies. Vaccine 28, 7035–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rong Y, Van Slyke G, Vance DJ, Westfall J, Ehrbar D, Mantis NJ (2017) Spatial location of neutralizing and non-neutralizing B cell epitopes on domain 1 of ricin toxin’s binding subunit. PLoS One 12, e0180999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yermakova A, Vance DJ, Mantis NJ (2012) Sub-Domains of Ricin’s B Subunit as Targets of Toxin Neutralizing and Non-Neutralizing Monoclonal Antibodies. PLoS One 7, e44317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudolph MJ, Vance DJ, Cassidy MS, Rong Y, Mantis NJ (2017) Structural Analysis of Single Domain Antibodies Bound to a Second Neutralizing Hot Spot on Ricin Toxin’s Enzymatic Subunit. J Biol Chem 292, 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudolph MJ, Vance DJ, Cassidy MS, Rong Y, Shoemaker CB, Mantis NJ (2016) Structural analysis of nested neutralizing and non-neutralizing B cell epitopes on ricin toxin’s enzymatic subunit. Proteins 84, 1162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudolph MJ, Vance DJ, Cheung J, Franklin MC, Burshteyn F, Cassidy MS, Gary EN, Herrera C, Shoemaker CB, Mantis NJ (2014) Crystal structures of ricin toxin’s enzymatic subunit (RTA) in complex with neutralizing and non-neutralizing single-chain antibodies. J Mol Biol 426, 3057–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vance DJ, Tremblay JM, Rong Y, Angalakurthi SK, Volkin DB, Middaugh CR, Weis DD, Shoemaker CB, Mantis NJ (2017) High-Resolution Epitope Positioning of a Large Collection of Neutralizing and Nonneutralizing Single-Domain Antibodies on the Enzymatic and Binding Subunits of Ricin Toxin. Clin Vaccine Immunol 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vance DJ, Greene CJ, Rong Y, Mandell LM, Connell TD, Mantis NJ (2015) Comparative Adjuvant Effects of Type II Heat-Labile Enterotoxins in Combination with Two Different Candidate Ricin Toxin Vaccine Antigens. Clin Vaccine Immunol 22, 1285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.R_Core_Team (2014) R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 42.Wickham H (2009) ggplot2: Elegant Graphics for Data Analysis Springer-Verlag New York, New York. [Google Scholar]
- 43.Arnold JB (2019) ggthemes: Extra Themes, Scales and Geoms for ‘ggplot2’
- 44.Derenzini M, Bonetti E, Marionozzi V, Stirpe F (1976) Toxic effects of ricin: studies on the pathogenesis of liver lesions. Virchows Arch B Cell Pathol 20, 15–28. [DOI] [PubMed] [Google Scholar]
- 45.Zenilman ME, Fiani M, Stahl P, Brunt E, Flye MW (1988) Use of ricin A-chain to selectively deplete Kupffer cells. J Surg Res 45, 82–9. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Huang X, Werner M, Broering R, Yang D, Lu M (2017) Advanced Method for Isolation of Mouse Hepatocytes, Liver Sinusoidal Endothelial Cells, and Kupffer Cells. In Hepatitis B Virus: Methods and Protocols (Guo H and Cuconati A, eds), Springer New York, New York, NY: %@ 978-1-4939-6700-1 249–258. [DOI] [PubMed] [Google Scholar]
- 47.Liu W, Hou Y, Chen H, Wei H, Lin W, Li J, Zhang M, He F, Jiang Y (2011) Sample preparation method for isolation of single-cell types from mouse liver for proteomic studies. Proteomics 11, 3556–64. [DOI] [PubMed] [Google Scholar]
- 48.Meyer J, Gonelle-Gispert C, Morel P, Buhler L (2016) Methods for Isolation and Purification of Murine Liver Sinusoidal Endothelial Cells: A Systematic Review. PLoS One 11, e0151945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C, Lu Y, Zhou H, Lu H, Qian X, Liu X, Wang X, Ding Z, Zhang F, Lu L (2015) Acquiring Kupffer cells in mice using a MACS-based method. Transplantation proceedings 47, 553–7. [DOI] [PubMed] [Google Scholar]
- 50.Poisson J, Plessier A, Kiladjian JJ, Turon F, Cassinat B, Andreoli A, De Raucourt E, Goria O, Zekrini K, Bureau C, Lorre F, Cervantes F, Colomer D, Durand F, Garcia-Pagan JC, Casadevall N, Valla DC, Rautou PE, Marzac C, French national network for vascular liver, d. (2017) Selective testing for calreticulin gene mutations in patients with splanchnic vein thrombosis: A prospective cohort study. J Hepatol 67, 501–507. [DOI] [PubMed] [Google Scholar]
- 51.Sørensen KK, Simon-Santamaria J, McCuskey RS, Smedsrød B (2011) Liver Sinusoidal Endothelial Cells. In Comprehensive Physiology John Wiley & Sons, Inc. [DOI] [PubMed] [Google Scholar]
- 52.Strauss O, Phillips A, Ruggiero K, Bartlett A, Dunbar PR (2017) Immunofluorescence identifies distinct subsets of endothelial cells in the human liver. Sci Rep 7, 44356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holt MP, Cheng L, Ju C (2008) Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol 84, 1410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krenkel O and Tacke F (2017) Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol 17, 306–321. [DOI] [PubMed] [Google Scholar]
- 55.Xie G, Wang L, Wang X, Wang L, DeLeve LD (2010) Isolation of periportal, midlobular, and centrilobular rat liver sinusoidal endothelial cells enables study of zonated drug toxicity. Am J Physiol Gastrointest Liver Physiol 299, G1204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeLeve LD (2013) Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest 123, 1861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ichikawa S, Mucida D, Tyznik AJ, Kronenberg M, Cheroutre H (2011) Hepatic stellate cells function as regulatory bystanders. J Immunol 186, 5549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bourgognon M, Klippstein R, Al-Jamal KT (2015) Kupffer Cell Isolation for Nanoparticle Toxicity Testing. J Vis Exp, e52989. [DOI] [PMC free article] [PubMed]
- 59.Tatsumi K, Ohashi K, Mukobata S, Kubo A, Koyama F, Nakajima Y, Shima M, Okano T (2012) Hepatocyte Is a Sole Cell Type Responsible for the Production of Coagulation Factor IX In Vivo. Cell Med 3, 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fiani ML, Beitz J, Turvy D, Blum JS, Stahl PD (1998) Regulation of mannose receptor synthesis and turnover in mouse J774 macrophages. J Leukoc Biol 64, 85–91. [DOI] [PubMed] [Google Scholar]
- 61.Frankel AE, Fu T, Burbage C, Tagge E, Harris B, Vesely J, Willingham MC (1997) Lectin-deficient ricin toxin intoxicates cells bearing the D-mannose receptor. Carbohydrate research 300, 251–8. [DOI] [PubMed] [Google Scholar]
- 62.Wahome PG, Ahlawat S, Mantis NJ (2012) Identification of small molecules that suppress ricin-induced stress-activated signaling pathways. PLoS One 7, e49075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sandvig K, Olsnes S, Pihl A (1976) Kinetics of binding of the toxic lectins abrin and ricin to surface receptors of human cells. J Biol Chem 251, 3977–84. [PubMed] [Google Scholar]
- 64.Baluna R, Ghetie V, Oppenheimer-Marks N, Vitetta ES (1996) Fibronectin inhibits the cytotoxic effect of ricin A chain on endothelial cells. Int J Immunopharmacol 18, 355–61. [DOI] [PubMed] [Google Scholar]
- 65.Baluna R, Rizo J, Gordon BE, Ghetie V, Vitetta ES (1999) Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome. Proc Natl Acad Sci U S A 96, 3957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baluna R, Coleman E, Jones C, Ghetie V, Vitetta ES (2000) The effect of a monoclonal antibody coupled to ricin A chain-derived peptides on endothelial cells in vitro: insights into toxin-mediated vascular damage. Exp Cell Res 258, 417–24. [DOI] [PubMed] [Google Scholar]
- 67.Yermakova A, Klokk TI, O’Hara JM, Cole R, Sandvig K, Mantis NJ (2016) Neutralizing Monoclonal Antibodies against Disparate Epitopes on Ricin Toxin’s Enzymatic Subunit Interfere with Intracellular Toxin Transport. Sci Rep 6, 22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sully EK, Whaley KJ, Bohorova N, Bohorov O, Goodman C, Kim DH, Pauly MH, Velasco J, Hiatt E, Morton J, Swope K, Roy CJ, Zeitlin L, Mantis NJ (2014) Chimeric plantibody passively protects mice against aerosolized ricin challenge. Clin Vaccine Immunol 21, 777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fodstad O, Olsnes S, Pihl A (1976) Toxicity, distribution and elimination of the cancerostatic lectins abrin and ricin after parenteral injection into mice. Br J Cancer 34, 418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vance DJ, Tremblay JM, Mantis NJ, Shoemaker CB (2013) Stepwise engineering of heterodimeric single domain camelid VHH antibodies that passively protect mice from ricin toxin. J Biol Chem 288, 36538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yermakova A, Klokk TI, Cole R, Sandvig K, Mantis NJ (2014) Antibody-mediated inhibition of ricin toxin retrograde transport. MBio 5, e00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rhodes DA and Isenberg DA (2017) TRIM21 and the Function of Antibodies inside Cells. Trends Immunol 38, 916–926. [DOI] [PubMed] [Google Scholar]
- 73.Pipirou Z, Powlesland AS, Steffen I, Pohlmann S, Taylor ME, Drickamer K (2011) Mouse LSECtin as a model for a human Ebola virus receptor. Glycobiology 21, 806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roy CJ, Brey RN, Mantis NJ, Mapes K, Pop IV, Pop LM, Ruback S, Killeen SZ, Doyle-Meyers L, Vinet-Oliphant HS, Didier PJ, Vitetta ES (2015) Thermostable ricin vaccine protects rhesus macaques against aerosolized ricin: Epitope-specific neutralizing antibodies correlate with protection. Proc Natl Acad Sci U S A 112, 3782–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smallshaw JE, Firan A, Fulmer JR, Ruback SL, Ghetie V, Vitetta ES (2002) A novel recombinant vaccine which protects mice against ricin intoxication. Vaccine 20, 3422–7. [DOI] [PubMed] [Google Scholar]
- 76.Vitetta ES, Smallshaw JE, Schindler J (2012) Pilot phase IB clinical trial of an alhydrogel-adsorbed recombinant ricin vaccine. Clin Vaccine Immunol 19, 1697–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trefts E, Gannon M, Wasserman DH (2017) The liver. Curr Biol 27, R1147–R1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kasturirangan S, Rainey GJ, Xu L, Wang X, Portnoff A, Chen T, Fazenbaker C, Zhong H, Bee J, Zeng Z, Jenne C, Wu H, Gao C (2017) Targeted Fcgamma Receptor (FcgammaR)-mediated Clearance by a Biparatopic Bispecific Antibody. J Biol Chem 292, 4361–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wohlleber D and Knolle PA (2016) The role of liver sinusoidal cells in local hepatic immune surveillance. Clin Transl Immunology 5, e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fodstad O, Kvalheim G, Godal A, Lotsberg J, Aamdal S, Host H, Pihl A (1984) Phase I study of the plant protein ricin. Cancer Res 44, 862–5. [PubMed] [Google Scholar]
- 81.Skilleter DN, Paine AJ, Stirpe F (1981) A comparison of the accumulation of ricin by hepatic parenchymal and non-parenchymal cells and its inhibition of protein synthesis. Biochim Biophys Acta 677, 495–500. [DOI] [PubMed] [Google Scholar]
- 82.Houston LL (1982) Transport of ricin A chain after prior treatment of mouse leukemia cells with ricin B chain. J Biol Chem 257, 1532–9. [PubMed] [Google Scholar]
- 83.Vitetta ES and Thorpe PE (1985) Immunotoxins containing ricin A or B chains with modified carbohydrate residues act synergistically in killing neoplastic B cells in vitro. Cancer Drug Deliv 2, 191–8. [DOI] [PubMed] [Google Scholar]
- 84.Rapak A, Falnes PO, Olsnes S (1997) Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc Natl Acad Sci U S A 94, 3783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Deurs B, Sandvig K, Petersen OW, Olsnes S, Simons K, Griffiths G (1988) Estimation of the amount of internalized ricin that reaches the trans-Golgi network. J Cell Biol 106, 253–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Deurs B, Tonnessen TI, Petersen OW, Sandvig K, Olsnes S (1986) Routing of internalized ricin and ricin conjugates to the Golgi complex. J Cell Biol 102, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song K, Mize RR, Marrero L, Corti M, Kirk JM, Pincus SH (2013) Antibody to ricin a chain hinders intracellular routing of toxin and protects cells even after toxin has been internalized. PLoS One 8, e62417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gage E, Hernandez MO, O’Hara JM, McCarthy EA, Mantis NJ (2011) Role of the mannose receptor (CD206) in innate immunity to ricin toxin. Toxins (Basel) 3, 1131–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu W, Tang L, Zhang G, Wei H, Cui Y, Guo L, Gou Z, Chen X, Jiang D, Zhu Y, Kang G, He F (2004) Characterization of a novel C-type lectin-like gene, LSECtin: demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J Biol Chem 279, 18748–58. [DOI] [PubMed] [Google Scholar]
- 90.Shimojima M, Takenouchi A, Shimoda H, Kimura N, Maeda K (2014) Distinct usage of three C-type lectins by Japanese encephalitis virus: DC-SIGN, DC-SIGNR, and LSECtin. Arch Virol 159, 2023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang L, Yang J, Liu W, Tang X, Chen J, Zhao D, Wang M, Xu F, Lu Y, Liu B, Sun Q, Zhang L, He F (2009) Liver sinusoidal endothelial cell lectin, LSECtin, negatively regulates hepatic T-cell immune response. Gastroenterology 137, 1498–508 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elvevold K, Smedsrod B, Martinez I (2008) The liver sinusoidal endothelial cell: a cell type of controversial and confusing identity. Am J Physiol Gastrointest Liver Physiol 294, G391–400. [DOI] [PubMed] [Google Scholar]
- 93.Sorensen KK, McCourt P, Berg T, Crossley C, Le Couteur D, Wake K, Smedsrod B (2012) The scavenger endothelial cell: a new player in homeostasis and immunity. Am J Physiol Regul Integr Comp Physiol 303, R1217–30. [DOI] [PubMed] [Google Scholar]
- 94.Hu Z, Shi X, Yu B, Li N, Huang Y, He Y (2018) Structural Insights into the pH-Dependent Conformational Change and Collagen Recognition of the Human Mannose Receptor. Structure 26, 60–71 e3. [DOI] [PubMed] [Google Scholar]
- 95.Martinez-Pomares L (2012) The mannose receptor. J Leukoc Biol 92, 1177–86. [DOI] [PubMed] [Google Scholar]
- 96.Anderson CL, Ganesan LP, Robinson JM (2015) The biology of the classical Fcgamma receptors in non-hematopoietic cells. Immunol Rev 268, 236–40. [DOI] [PubMed] [Google Scholar]
- 97.Lovdal T, Andersen E, Brech A, Berg T (2000) Fc receptor mediated endocytosis of small soluble immunoglobulin G immune complexes in Kupffer and endothelial cells from rat liver. J Cell Sci 113 (Pt 18), 3255–66. [DOI] [PubMed] [Google Scholar]
- 98.Chow SK and Casadevall A (2012) Monoclonal antibodies and toxins--a perspective on function and isotype. Toxins (Basel) 4, 430–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chow SK, Smith C, MacCarthy T, Pohl MA, Bergman A, Casadevall A (2013) Disease-enhancing antibodies improve the efficacy of bacterial toxin-neutralizing antibodies. Cell Host Microbe 13, 417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hiatt A, Pauly M, Whaley K, Qiu X, Kobinger G, Zeitlin L (2015) The emergence of antibody therapies for Ebola. Hum Antibodies 23, 49–56. [DOI] [PubMed] [Google Scholar]
- 101.Marzi A, Haddock E, Kajihara M, Feldmann H, Takada A (2018) Monoclonal Antibody Cocktail Protects Hamsters From Lethal Marburg Virus Infection. J Infect Dis 218, S662–S665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng LW, Stanker LH, Henderson TD 2nd, Lou J, Marks JD (2009) Antibody protection against botulinum neurotoxin intoxication in mice. Infect Immun 77, 4305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nowakowski A, Wang C, Powers DB, Amersdorfer P, Smith TJ, Montgomery VA, Sheridan R, Blake R, Smith LA, Marks JD (2002) Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc Natl Acad Sci U S A 99, 11346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sepulveda J, Mukherjee J, Tzipori S, Simpson LL, Shoemaker CB (2010) Efficient serum clearance of botulinum neurotoxin achieved using a pool of small antitoxin binding agents. Infect Immun 78, 756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H (2013) Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol 49, 503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roche JK, Stone MK, Gross LK, Lindner M, Seaner R, Pincus SH, Obrig TG (2008) Post-exposure targeting of specific epitopes on ricin toxin abrogates toxin-induced hypoglycemia, hepatic injury, and lethality in a mouse model. Lab Invest 88, 1178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.