Abstract
There has been no comparison of excessive daytime sleepiness (EDS) in patients with Alzheimer’s disease dementia (ADem), dementia with Lewy bodies (DLB) and behavioral variant frontotemporal dementia (bvFTD). We identified patients with mild dementia who met criteria for these disorders who also had the Epworth Sleepiness Scale (ESS) completed. The sample included 17 bvFTD, 111 AD and 31 DLB. An ESS score ≥10 was considered abnormal and consistent with EDS. Analyses with age and sex as covariates revealed higher mean ESS scores for DLB compared to the other groups (DLB 13.9 ± 5, bvFTD 9.6 ± 8, AD 8.8 ± 5, p<0.05). An ESS score ≥10 was significantly more likely to occur in DLB compared to bvFTD or AD (DLB 81% vs. bvFTD 47% vs. AD 45%, p<0.01). In patients with mild dementia, EDS is greatest in DLB and comparably lower in bvFTD and AD.
Keywords: excessive daytime somnolence, Epworth Sleepiness Scale, Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia
Introduction
Excessive daytime sleepiness (EDS) is a common feature among aging individuals, especially in those with dementia. EDS has been described in dementia with Lewy bodies (DLB),1–4 but has not been well characterized in terms of its frequency and severity. Less is known regarding EDS in patients with Alzheimer’s disease dementia (ADem) and behavioral variant frontotemporal dementia (bvFTD).1–8 The Epworth Sleepiness Scale (ESS) is a measure that assesses subjective sleepiness over the prior two weeks.9 In most settings, the patient completes the measure. In dementia populations, this measure is typically completed by informants.1 We sought to compare ESS scores between patients with mild DLB, AD and bvFTD as rated by their informants, and hypothesized that mild DLB patients would have greater degrees of severity and frequency of EDS compared to those with mild bvFTD and AD.
Material and Methods
The Mayo Clinic Alzheimer’s Disease Research Center (ADRC) database was queried to identify patients evaluated over a ten year period who a) met published clinical criteria for bvFTD, AD, or DLB,10–12 b) had a Clinical Dementia Rating (CDR)13 score of 1, and c) had the Epworth Sleepiness Scale (ESS) completed by an informant. The ADRC program enrolls patients who are followed annually with a comprehensive battery of clinical measures; for those patients who had multiple ESS scores, only the initial score was used for this analysis. The ESS is a scale that queries the likelihood of dozing (i.e., 0= no chance of dozing, 1= slight chance of dozing, 2= moderate chance of dozing or sleeping, 3= high chance of dozing) in eight different circumstances: 1. Sitting and reading, 2. Watching TV, 3. Sitting inactive in a public place, 4. As a passenger in a motor vehicle for an hour or more, 5. Lying down to rest in the afternoon when circumstances permits, 6. Sitting and talking to someone, 7. Sitting quietly after lunch without alcohol, 8. In a car, while stopped for a few minutes in traffic. A higher score reflects a higher likelihood of dozing, with a maximum score of 24. An ESS score ≥10 is considered abnormal and consistent with excessive daytime sleepiness.
The Mayo Sleep Questionnaire (MSQ)14 is always completed concurrently with the ESS at our center. An affirmative response to question 1 on the MSQ [Have you ever seen the patient appear to “act out his/her dreams” while sleeping? (punched or flailed arms in the air, shouted or screamed)], was considered consistent with probable REM sleep behavior disorder (RBD). The MSQ has been validated, and is 98–100% sensitive and 74–95% specific for polysomnography-confirmed RBD.14, 15
Descriptive statistics were used to compare demographic data, total ESS scores and the frequency of ESS score ≥10 among the three dementia syndrome groups. ANCOVA was used to compare ESS scores in AD versus DLB versus bvFTD. A p<0.05 was considered statistically significant.
Results
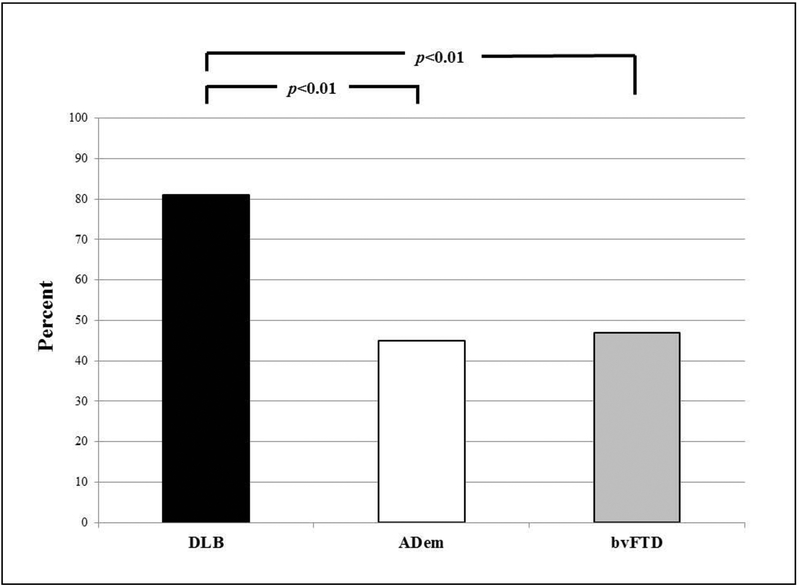
There were 1044 subjects with the qualifying diagnoses with the following breakdown: 641 AD, 250 DLB, and 153 FTD. Among these, 159 had the ESS completed and had a CDR=1, resulting in the sample (total=159) being 111 AD, 31 DLB, and 17 bvFTD. All subjects were Caucasian. There were no group differences in education (mean 14.9 ±3). Male representation was lowest in AD (52%) compared to bvFTD (77%) and DLB (88%) (p<0.01). The mean (SD) of the bvFTD group was significantly younger than either the AD or DLB groups (bvFTD 65.9 ±12 yrs vs. AD 75.6 ±11 yrs vs. DLB 71.1±9 yrs, p<0.05). Separate ANCOVA analyses with age and sex as covariates revealed higher mean ESS scores for DLB compared to bvFTD or AD (DLB 13.9 ±5, bvFTD 9.6±8, AD 8.8±5, p<0.05). Moreover, the frequency of an ESS score ≥10 was significantly higher in DLB compared to either the bvFTD or AD groups (DLB 81% vs. bvFTD 47% vs. AD 45%, p<0.01) (Figure). The frequency of probable RBD based on the MSQ was AD 21% vs DLB 87% vs bvFTD 35% (p<0.001).
Frequency of excessive daytime sleepiness (EDS) in patients with dementia with Lewy bodies (DLB) versus Alzheimer’s disease dementia (AD) versus behavioral variant frontotemporal dementia (bvFTD).
Discussion
Patients with mild DLB had a higher frequency and greater severity of EDS compared to patients with AD and bvFTD who also had a mild dementia. Excessive daytime sleepiness has been described in patients with AD and bvFTD, but the degree and frequency was similar for those two groups and is not as prominent as in patients with DLB.8, 16
Our data is similar to that reported by others on DLB, in which hypersomnia has been shown based on subjective (i.e., the ESS) and objective (i.e., the Multiple Sleep Latency Test [MSLT] and Maintenance of Wakefulness Test [MWT]) measures in DLB.1–4, 8, 17
The underlying cause(s) for EDS being more frequent and severe in DLB compared to AD and bvFTD is likely multifactorial. EDS can be due to obstructive or central sleep apnea, sleep fragmentation for many reasons, restless legs syndrome, effect of medications, depression, etc., as well as being intrinsic aspects of the underlying disease processes themselves.18 Our analyses focused on those with mild dementia, and our data underscores the potential discriminative value of EDS in differentiating DLB vs AD vs bvFTD early in the dementia course. EDS is also now considered a supportive clinical feature for the diagnosis of DLB.12 Whether EDS has any prognostic implications in terms of rate of progression for any of the dementia subtypes will require further study.
In the setting of dementia, EDS as well as RBD can be viewed as risk factors, prodromal markers, or parts of the symptom complex. Our data on EDS pertains to the symptom complex issue. Recent findings suggest an association between beta-amyloid deposition (as reflected by amyloid PET imaging) and EDS in the cognitively normal elderly.19 Future analyses are necessary to determine if EDS is a risk factor or prodromal marker of one or more of the major dementia subtypes.
There is compelling evidence that RBD is a prodromal feature of the synucleinopathies, including DLB.20, 21 While RBD is far more common in DLB than in AD and bvFTD,20, 21 which is also supported by our data, EDS is not usually associated with RBD.22, 23
We acknowledge the limitations of this study. This is a retrospective study using a measure of subjective sleepiness as reported by an informant. We did not control for other sleep comorbidities such as obstructive sleep apnea since most patients had not undergone a polysomnogram. However, in contrast to the other studies described above which included patients with mild to severe dementia, we purposefully focused on those with mild dementia (as reflected by the global CDR score being 1) and adjusted comparisons based on age and sex, which strengthen the finding of EDS being more significant in DLB compared to AD and bvFTD. The large differences in age and sex led us to control for these in our analyses. The small number of females (N=4 DLB and N=4 bvFTD) did not allow us to stratify our analyses by sex.
Future directions should include determining if EDS in the prodromal phase of each dementia syndrome (i.e., mild cognitive impairment) may be predictive of eventual phenoconversion from normal to MCI or MCI to dementia. In other words, one could hypothesize that EDS in the setting of MCI may predict phenoconversion to DLB versus AD or bvFTD. Another key point is that EDS is a problematic symptom/feature in DLB which is worthy of treatment trials; while one open label pilot study in DLB has suggested improvement in EDS with armodafinil,17 double-blind placebo-controlled studies with wake-promoting agents are worthy of further investigation.
Acknowledgements
This study was supported by P50 AG016574, R01 AG015866, Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program, Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program. We extend our appreciation to the patients and their caregivers for participating in aging/dementia research.
Footnotes
Disclosures/Potential Conflicts of Interest
Angelica Boeve, BA—nothing to disclose
Tanis J. Ferman, PhD—nothing to disclose
Jeremiah Aakre, MPH—nothing to disclose
Erik St. Louis, MD– nothing to disclose
Michael Silber, MBChB– nothing to disclose
Mary Machulda, PhD– nothing to disclose
Julie Fields, PhD– nothing to disclose
Neill Graff-Radford, MD– receives research support from NIH, has received publishing royalties from UpToDate, Inc.; and receives research support from Biogen, Lilly and Axovant and Novartis. He has consulted for Cytox.
Michelle Mielke, PhD– served as a consultant to Eli Lilly and Lysosomal Therapeutics, Inc. She receives research support from the NIH and unrestricted research grants from Biogen, Lundbeck, and Roche.
Yonas Geda, MD– nothing to disclose
David Jones, MD– nothing to disclose
Jonathon Graff-Radford, MD– nothing to disclose
David Knopman, MD—serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by TauRX Pharmaceuticals, Lilly Pharmaceuticals, Biogen and the Alzheimer’s Disease Cooperative Study; and receives research support from the NIH.
Ronald Petersen, MD, PhD—serves on data monitoring committees for Pfizer, Inc., Janssen Alzheimer Immunotherapy, and is a consultant for Biogen, Roche, Inc., Merck, Inc. and Genentech, Inc.; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003), and receives research support from the NIH.
Brad Boeve, MD—investigator for clinical trials sponsored by Axovant and Biogen; receives royalties from the publication of a book entitled Behavioral Neurology Of Dementia (Cambridge Medicine, 2009, 2017); serves on the Scientific Advisory Board of the Tau Consortium; receives research support from the NIH, Mangurian Foundation and the Little Family Foundation.
References
- 1.Ferman T, Smith G, Dickson D, et al. Abnormal daytime sleepiness in dementia with Lewy bodies compared to Alzheimer’s disease using the Multiple Sleep Latency Test. Alzheimer Res Ther 2014;16:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cagnin A, Fragiacomo F, Camporese G, et al. Sleep-Wake Profile in Dementia with Lewy Bodies, Alzheimer’s Disease, and Normal Aging. J Alzheimers Dis 2017;55:1529–1536. [DOI] [PubMed] [Google Scholar]
- 3.Chwiszczuk L, Breitve M, Hynninen M, Gjerstad MD, Aarsland D, Rongve A. Higher Frequency and Complexity of Sleep Disturbances in Dementia with Lewy Bodies as Compared to Alzheimer’s Disease. Neurodegener Dis 2016;16:152–160. [DOI] [PubMed] [Google Scholar]
- 4.Raggi A, Neri W, Ferri R. Sleep-related behaviors in Alzheimer’s disease and dementia with Lewy bodies. Rev Neurosci 2015;26:31–38. [DOI] [PubMed] [Google Scholar]
- 5.Anderson KN, Hatfield C, Kipps C, Hastings M, Hodges JR. Disrupted sleep and circadian patterns in frontotemporal dementia. Eur J Neurol 2009;16:317–323. [DOI] [PubMed] [Google Scholar]
- 6.Bonakis A, Economou NT, Paparrigopoulos T, et al. Sleep in frontotemporal dementia is equally or possibly more disrupted, and at an earlier stage, when compared to sleep in Alzheimer’s disease. J Alzheimers Dis 2014;38:85–91. [DOI] [PubMed] [Google Scholar]
- 7.Merrilees J, Hubbard E, Mastick J, Miller BL, Dowling GA. Sleep in persons with frontotemporal dementia and their family caregivers. Nurs Res 2014;63:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guarnieri B, Adorni F, Musicco M, et al. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross-sectional study on 431 patients. Dem Geriatr Cog Disord 2012;33:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johns M A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 10.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKhann G, Knopman D, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 14.Boeve B, Molano J, Ferman T, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med 2011;12:445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boeve B, Molano J, Ferman T, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. J Clin Sleep Med 2013;9:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liguori C, Romigi A, Mercuri NB, et al. Cerebrospinal-fluid orexin levels and daytime somnolence in frontotemporal dementia. J Neurol 2014;261:1832–1836. [DOI] [PubMed] [Google Scholar]
- 17.Lapid MI, Kuntz KM, Mason SS, et al. Efficacy, Safety, and Tolerability of Armodafinil Therapy for Hypersomnia Associated with Dementia with Lewy Bodies: A Pilot Study. Dem Geriatr Cog Disord 2017;43:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasanuki K, Ferman TJ, Murray ME, et al. Daytime sleepiness in dementia with Lewy bodies is associated with neuronal depletion of the nucleus basalis of Meynert. Park Rel Disord 2018;50:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carvalho DZ, St Louis EK, Knopman DS, et al. Association of Excessive Daytime Sleepiness With Longitudinal beta-Amyloid Accumulation in Elderly Persons Without Dementia. JAMA Neurol 2018;75:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boeve B, Silber M, Ferman T, Lucas J, Parisi J. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord 2001;16:622–630. [DOI] [PubMed] [Google Scholar]
- 21.Boeve B, Silber M, Ferman T, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med 2013;14:754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schenck C, Mahowald M. REM sleep behavior disorder: Clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 2002;25:120–138. [DOI] [PubMed] [Google Scholar]
- 23.Iranzo A, Serradell M, Santamaria J. Excessive Daytime Sleepiness Does Not Predict Neurodegeneration in Idiopathic REM Sleep Behavior Disorder. SLEEP 2017;40. [DOI] [PubMed] [Google Scholar]