Abstract
Female mice treated neonatally with the phytoestrogen genistein (50 mg/kg/day) have multioocyte follicles, lack regular estrous cyclicity, and are infertile even after superovulation. To determine the cause of their infertility, we examined both oocyte developmental competence and timing of embryo loss. Eggs obtained by superovulation of genistein-treated or control females were equally capable of being fertilized in vitro and cultured to the blastocyst stage. However, if eggs were fertilized in vivo, retrieved at the pronucleus stage and cultured, there was a significant reduction in the percentage of embryos from genistein-treated females reaching the blastocyst stage. When these blastocysts were transferred to pseudopregnant recipients, the number of live pups produced was similar to controls. Preimplantation embryo development in vivo was examined by flushing embryos from the oviduct and/or uterus. Similar numbers of 1-cell and 2-cell embryos were obtained from genistein-treated and control females. However, significantly fewer embryos (<50%) were obtained from genistein-treated females on postcoital days 3 and 4. To determine if neonatal genistein treatment altered the ability of the uterus to support implantation, blastocysts from control donors were transferred to control and genistein-treated pseudopregnant recipients. These experiments demonstrated that genistein-treated females are not capable of supporting normal implantation of control embryos. Taken together, these results suggest that oocytes from mice treated neonatally with genistein are developmentally competent; however, both the oviductal environment and the uterus have abnormalities that contribute to the observed reproductive failure.
Keywords: endocrine disruptor, oviduct, ovary, development, estrogens, soy, phytoestrogens
Introduction
Naturally occurring phytoestrogens (estrogenic chemicals found in plants) are readily available in the diet, particularly in soy products [1–3]. Isoflavones comprise the major class of phytoestrogens in soy and the isoflavone that has received the most attention is genistein (Gen). The glycosylated form of genistein, genistin, is found in soy products including soy-based infant formulas and makes up greater than 65% of the isoflavone content [4]. Infants fed soy-based formulas have high circulating levels of Gen (1–5 µM), indicating that genistin is readily absorbed and converted to Gen by the infant [4]. This finding has been confirmed in a recent study showing Gen metabolites in the urine of infants fed soy-based infant formulas [5].
Limited information is available regarding long-term effects of infant exposure to soy-based infant formulas. An epidemiological study of women (20–34 years of age) who had been fed either soy-based or cow milk-based formulas as infants demonstrated significant differences in menstrual cycle length and a significant increase in pain associated with the menstrual cycle [6]. A more recent study of infants fed soy formula, cow milk formula, or breast milk showed that female infants fed soy formula have re-estrogenization of vaginal cells at 6 months of age whereas this effect is not observed in the other two groups [7]. Together, these studies suggest that the level of phytoestrogens found in soy infant formulas exhibits estrogenic activity in the developing human reproductive tract.
In rodents, developmental exposure to Gen causes adverse effects on the female reproductive system including altered estrous cyclicity, subfertility/infertility and cancer [8–14]. Our laboratory has characterized the effects of neonatal (postpartum days 1–5) exposure to Gen on the developing female reproductive system [9]. At the highest dose used in these studies (50 mg/kg/day), serum circulating levels of Gen are ~6.8 µM [15], similar to the levels measured in infants fed soy formula [4]. Female mice treated neonatally with this dose of Gen lack regular estrous cyclicity and do not ovulate spontaneously. If ovulation is induced, some of the mice become pregnant buft do not deliver pups because fewer embryos implant and there is failure of post-implantation development [9]. Lower doses of Gen (5 mg/kg/day) result in subfertility, with some mice in persistent estrus, fewer mice becoming pregnant, and fewer pups delivered per litter.
Neonatal Gen treatment also results in an increased incidence of multioocyte follicles caused by disruption of oocyte nest breakdown that normally occurs in the neonatal period [8, 16]. This is a direct effect of Gen on the ovary because control neonatal ovaries cultured in the presence of Gen also exhibit this phenotype [17]. The Gen-induced multioocyte phenotype appears to be mediated by estrogen receptor (ER) β because multioocyte follicles are not observed in Gen-treated mice lacking ERβ [8]. A similar multioocyte phenotype is observed following developmental exposure to diethylstilbestrol (DES) and bisphenol A [18]. Further, there is evidence that oocytes from DES-induced multioocyte follicles have reduced developmental competence when compared to oocytes from single oocyte follicles [19]. These findings suggest that the infertility of Gen-treated mice could be explained by diminished oocyte quality resulting in lower developmental competence.
In this study, the underlying cause of infertility in Gen-treated mice was examined. We report here that most eggs ovulated from Gen-treated mice were developmentally competent; therefore the infertility cannot be attributed to poor oocyte quality. However, the reproductive tract of these mice was limited in its ability to support both preimplantation embryo development and implantation, suggesting that alterations in reproductive tract development induced by Gen in the neonatal period contribute substantially to their infertility.
Materials and Methods
Animals and genistein treatment
Timed pregnant CD-1 (Crl: CD-1 [ICR] BR) mice were obtained from the in-house breeding colony at NIEHS (Research Triangle Park, NC). Mice were housed in a temperature controlled environment (21–22° C) under a 12 h light:12 h dark cycle and fed NIH-31 mouse diet and deionized water ad libitum. The diet used in this study contains phytoestrogens at levels below the dose used in the current study [20, 21]. All animal procedures complied with NIH/NIEHS animal care guidelines. Dams delivered their young at 19 days of gestation; pups were pooled together, separated into two groups by sex, and then randomly standardized to 8 female pups per dam (male pups were used in another experiment). All female pups were treated by subcutaneous injections on neonatal days 1 through 5 with either Gen 50 mg/kg per day (98% pure; Sigma, St. Louis, MO) suspended in corn oil or with corn oil alone (controls), previously referred to as Gen-50 [8, 9, 16]. Mice were weaned at 22 days of age and housed 5 per cage.
Oocyte and embryo collection and culture
To stimulate ovulation, 6–9 wk old Gen-treated and control female mice received a single subcutaneous injection of 5.0 IU eCG (Calbiochem, Gibbstown, NJ) in 0.1 ml of saline followed 48 h later with 5.0 IU hCG (Calbiochem) in 0.1 ml saline. Metaphase II-arrested eggs were collected from the oviducts into FHM medium (Catalog # MR-024-D, Invitrogen, Carlsbad, CA) 14 h after hCG administration. For experiments requiring embryos, females were bred singly to proven fertile CD-1 male mice immediately after the hCG injection and checked for a vaginal plug the following morning. Only plug-positive females were used in subsequent experiments. Embryos were flushed from the oviducts and/or uterus using FHM at the following hours after hCG administration: 18, 21, or 24 h (1-cell); 48 h (2-cell); 72 h (4-cell to morula) and 92 h (blastocyst). All embryos were cultured in microdrops of KSOM/AA (Catalog # MR-106-D, Millipore, Phillipsburg, NJ) under mineral oil in a humidified environment of 5% CO2, 5% O2, and 90% N2. For all culture experiments, the number of embryos in each microdrop of medium was consistently kept at approximately one embryo/µl [22].
Immunofluorescence Analysis of Meiotic Spindles
Cumulus cells were removed from the eggs by brief incubation in 1% hyaluronidase, and the zona pellucidae were removed using acid Tyrodes medium, pH 1.6 [22]. The eggs were fixed for 45 min at room temperature in 2% formaldehyde (Ted Pella, Redding, CA) in PBS containing 1 µM Taxol (Sigma) to maintain spindle stability. Eggs were washed in blocking buffer (PBS containing 0.3% BSA, 0.01% Tween-20, and 0.01% NaN3) and then permeabilized 15 min in PBS containing 0.3% BSA, 0.1% Triton X-100, and 0.01% NaN3. The eggs were again washed in blocking buffer, then incubated for 1 h with FITC-conjugated anti-mouse α-tubulin (final concentration 12 µg/ml; Abcam, Cambridge, MA; cat # ab11303). The eggs were mounted in Vectashield containing 1.5 µg/ml DAPI (Vector). The immunofluorescence signal was observed and captured using a Zeiss LSM 510 multiphoton confocal microscope and the images were processed using Photoshop software (Adobe, San Jose, CA).
In Vitro Fertilization
Sperm were released from the cauda epididymides of a single 3–4 month old B6SJLF1/J male mouse (Jackson Laboratory, Bar Harbor, ME) into HTF medium (Catalog # MR-070-D, Millipore) containing 3% BSA (Albumax I, Invitrogen) and immediately covered with mineral oil. Sperm were allowed to capacitate for 1.5 h at 37° C in a humidified environment of 5% CO2, 5% O2, and 90% N2. Two egg/cumulus cell masses from each of 8 individual superovulated mice (4 control and 4 Gen-treated) were placed into separate 250 µl drops of HTF medium and then inseminated with approximately 106 capacitated sperm/ml. The eggs were washed free of unbound sperm 4.5 h following insemination and cultured in KSOM/AA media. Fertilization was determined by the presence of two pronuclei 6–8 h after insemination. Embryos were observed for progression to the 2-cell and blastocyst stages of development.
Blastocyst Transfer
Genistein Blastocysts Transferred into Control Recipients
At 8 weeks of age, untreated CD-1 female mice were bred with vasectomized CD-1 males to stimulate pseudopregnancy. Vaginal plug-positive females were removed and housed singly for use as recipients. Blastocysts cultured from 1-cell embryos collected from the oviduct of control and Gen-treated mice were transferred to recipients on day 3 of pseudopregnancy according to standard procedures [22]. Seven or eight blastocysts were transferred into each uterine horn. Recipients were allowed to deliver their pups to determine blastocyst developmental competence. All pups from these litters were weaned at 21 days of age. Ten female pups from each group were bred for 3 days at 8 wks of age to CD-1 males. The females were checked daily for vaginal plugs, and the pregnant females were allowed to deliver pups.
Control Blastocysts Transferred into Genistein-Treated Recipients
At 8 wks of age, control (n=21) and Gen-treated female mice (n=25) were superovulated and then bred with vasectomized CD-1 males to stimulate pseudopregnancy. Vaginal plug-positive females were removed and housed singly for use as recipients. Blastocysts were flushed from the uteri of superovulated females mated to control mice 4 days after hCG administration and pooled together. Pseudopregnant control (n=2) and Gen-treated (n=6) recipient mice underwent the embryo transfer procedure as above, except that either 7 or 8 control blastocysts were transferred into only one uterine horn for each recipient. Despite uneventful blastocyst transfer procedures, 3 of the Gen-treated recipients died during the first two postoperative days. This was apparently because of significant blood loss that we later speculated could be a side effect of the non-steroidal anti-inflammatory analgesic (flunixin meglumine 2.5 mg/kg IP) used postoperatively. This occurrence is very rare following embryo transfer procedures, and suggests that neonatal exposure to Gen may alter vascularity or blood clotting efficiency. The experiment was therefore repeated with an additional 3 control and 6 Gen-treated females; a narcotic analgesic (buprenorphine 0.1 mg/kg SC) was used preoperatively and all animals remained healthy. The recipients were euthanized by CO2 asphyxiation eight days after surgery to determine the presence of implantation sites.
Statistical Analysis
The data were analyzed using JMP software (SAS, Cary, NC). Statistical significance was determined by using the nonparametric Wilcoxon or Mann-Whitney tests, or Fisher’s Exact test, as appropriate, with p<0.05 reported as significant.
Results
Egg Developmental Competence Assessment
To test the hypothesis that female mice treated neonatally with Gen were infertile because of poor oocyte quality, we induced ovulation and then examined the ovulated eggs for their general morphology and spindle structure. Gen-treated females (n=8) ovulated 29.6 ± 5.5 eggs and control females (n=6) ovulated 28.8 ± 5.1 eggs. The appearance of the ovulated eggs in both groups was similar. When spindles of eggs obtained from superovulated Gen-treated or control females were stained for α-tubulin and DNA, the spindle morphology was normal in almost all cases [controls, 81/83 (98%); Gen, 130/131 (99%)] (Fig. 1, A and B), with only a few spindle abnormalities observed in both groups (Fig. 1C). In vitro fertilization of cumulus-enclosed eggs was performed and resulted in efficient fertilization in both groups as indicated by formation of two pronuclei [control, 86/89 (96.6%); Gen, 112/114 (98.2%)]. To further assess developmental competence, the fertilized eggs were cultured to the blastocyst stage of development. Of the eggs that were fertilized, there was no difference between treatment groups in the timing of development or the percentage of embryos that reached the blastocyst stage [control, 82/86 (95.3%); Gen, 112/112 (100%)]. These findings suggested that egg quality as indicated by capacity to undergo fertilization and preimplantation development in vitro was not adversely affected by neonatal Gen treatment.
Figure 1.
Confocal microscopy of metaphase II-arrested eggs immunostained for alpha tubulin and DNA. Examples of normal-appearing meiotic spindles in eggs from control mice (panel A) and Gen-treated mice (panel B). Example of abnormal spindle from Gen-treated mouse (panel C).
To confirm full developmental competence, 1-cell embryos (two-pronuclei stage) were collected from the oviduct of Gen-treated and control females and cultured to the blastocyst stage. The resulting blastocysts were transferred to untreated control pseudopregnant recipients. Recipients received 16 blastocysts (8 per uterine horn) from one treatment group and were allowed to deliver their pups. All recipients delivered pups, and there were similar numbers of live pups per litter in both groups (Table 1). Furthermore, all pups were apparently healthy and survived to weaning. Ten female pups from both groups were bred at 8 weeks of age to determine their fertility. All vaginal plug-positive females in each group (N=9 in Gen group and N=8 in control group) became pregnant and delivered offspring 19 days after the plug was documented. The average litter size was 14.4 ± 1.09 in the females derived from embryos of Gen-treated donors, and 15.0 ± 1.07 in the females derived from embryos of control donors (not significantly different, Mann-Whitney test, p=0.88). These data demonstrate that eggs from Gen-treated mice are fully developmentally competent to the point of generating fertile female offspring if removed from the maternal environment at the two-pronuclei stage.
Table 1.
Live pups produced following embryo transfer of blastocysts derived from control and Gen-treated mice.
| Neonatal Treatment |
# Embryos Transferred |
# Recipients | # Pregnant | Total # Live Pups |
Avg. # Live Pups/Litter |
Live Pups per Transferred Embryos |
|---|---|---|---|---|---|---|
| Control | 124 | 8 | 8 | 49 | 6.1 ± 1.3 | 40% |
| Genistein | 140 | 9 | 9 | 48 | 5.3 ± 1.2 | 34% |
Reproductive Tract Environment Assessment
Fertilization in vivo
To determine if there was an effect of the reproductive tract environment on fertilization, eggs were collected from superovulated control and Gen-treated vaginal plug-positive mice at either 17–18 or 21–22 h after hCG administration and placed in culture. The appearance of two pronuclei was used to indicate that fertilization had occurred. At 17–18 h post-hCG, there were fewer eggs fertilized in the Gen treatment group [143/395 (36%)] when compared to controls [194/219 (89%)]. However, by 21–22 h post-hCG, most eggs were fertilized in both treatment groups [control, 231/252 (92%); Gen, 373/381 (98%)]. These findings indicated that there was a small delay in the fertilization process in vivo but given sufficient time, most eggs were fertilized.
Embryo development in culture after fertilization in vivo
One-cell embryos collected 21–22 h post-hCG were monitored daily to determine developmental progression. The average percentage of embryos reaching each stage was determined for each individual mouse and was expressed as a percentage of number of pronuclear stage embryos placed in culture (Fig. 2A). There was no difference in the ability to progress to the 2-cell stage in embryos from Gen-treated mice compared to controls, suggesting once the egg is fertilized, it is capable of the first cleavage division. However, with additional time in culture, slightly fewer embryos from Gen-treated mice progressed to the subsequent preimplantation embryo stages; this finding reached statistical significance by the blastocyst stage (Fig. 2A). These results, taken together with our observation that embryos derived from IVF of eggs from Gen-treated mice survive just as well as controls, suggests that the slightly reduced embryo developmental competence can be attributed to the reproductive tract environment, even though the exposure time was minimal (only a few hours).
Figure 2.
Graph of embryo progression over four days in vitro. Fertilized eggs from mice treated neonatally with Gen (n=15 mice; 373 embryos) or corn oil alone (n=11 mice; 231 embyros) were followed for four days in culture to determine progression of development. Panel A: The percentage of embryos that progressed to each stage was determined for each mouse and the mean ± S.E. is plotted. The asterisk denotes statistical significance at p<0.05 (Wilcoxon test). Panel B: Percentage of embryos at the 4-cell or 5–8 cell stage of development 48 h post-hCG. A total of 211 embryos from control mice and 356 embryos from Gen-treated mice were evaluated. There were significantly fewer 5–8 cell embryos in the Gen group than the control group (Fisher’s Exact test, p<0.0001).
Consistent with our observations regarding delayed timing of fertilization in vivo, embryos from Gen-treated females reached cleavage stages beyond the 4-cell stage more slowly than controls. At 72 h post-hCG, control embryos were split evenly between 4-cell and 5–8 cell stages of development while Gen-treated embryos were predominantly at the 4-cell stage with only a few at the 5–8 stage of development (Fig. 2B). Differences in timing of development between groups were not apparent in later stage embryos in which subtle differences in developmental progression are difficult to distinguish.
Embryo Development In Vivo
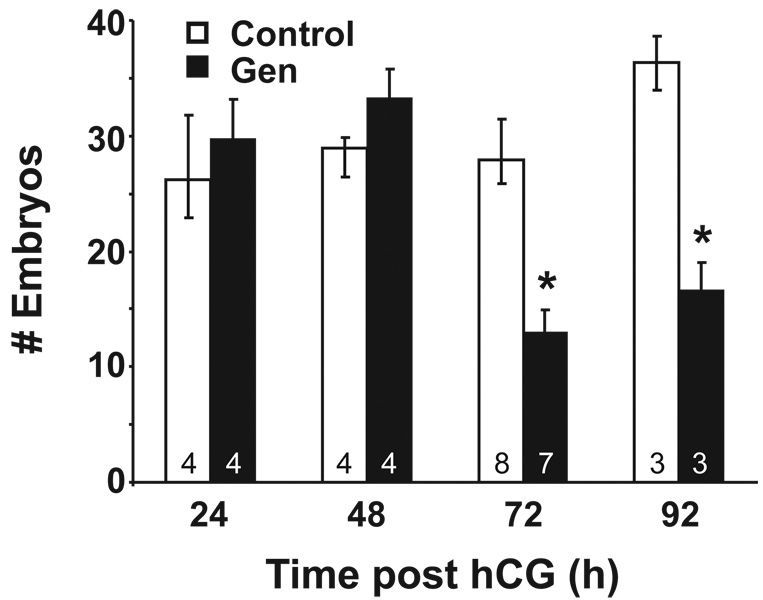
To determine effects of the reproductive tract environment on preimplantation embryo development in vivo, embryos were flushed from the oviduct and/or uterus of vaginal plug-positive females at 24, 48, 72 or 92 h post-hCG and mating. The average number of embryos per mouse collected at 24 and 48 h was not different between treatment groups (Fig. 3). However, at 72 and 92 hrs post-hCG, there was a significant reduction in the number of embryos retrieved, with about 50% fewer embryos retrieved from Gen-treated mice than controls at both time points. Because both the oviduct and uterus were flushed at these time points and the embryos were found in the expected locations (oviduct at 72 h and uterus at 92 h), the reduction in embryo number could not be explained by disruption of oviductal transport. These findings indicate that there was a loss of embryos from Gen-treated females between the 2-cell and 4–8 cell stages of development. Embryos flushed from the oviducts were subsequently cultured in vitro to determine progression to the blastocyst stage. Embryos collected at 24 h post hCG showed a slight reduction in blastocyst development while the majority of embryos retrieved at 48, 72 and 92 h post hCG progressed to the blastocyst stage (Table 2). These data support the idea that the reproductive tract environment is not favorable for embryo development, with longer exposure to the reproductive tract environment resulting in lower embryo survival, and that the critical time point for this effect is during the transition from the 2-cell to 4-cell stages. However, embryos that survive to at least the 4-cell stage continue to develop either in vivo or in vitro.
Figure 3.
Graph of embryo development over four days in vivo. The number of embryos retrieved (mean ± S.E.) from control or Gen-treated pregnant mice at 24, 48, 72 and 92 hrs post-hCG is shown. The number of mice per group is indicated at the base of the column. The asterisk denotes statistical significance at p<0.05 (Wilcoxon test).
Table 2.
Blastocyst development in vitro of embryos flushed from the oviduct and/or uterus of control and Gen-treated mice.
| Neonatal Treatment |
Embryo Collection Time (hours post hCG) |
|||
|---|---|---|---|---|
| 24 | 48 | 72 | 92 | |
| Control | 98/101 (97.0%) | 115/116 (99.1%) | 218/221 (98.6%) | 109/109 (100%) |
| Gen | 92/102 (90.2%) | 127/133 (95.5%) | 90/93 (96.8%) | 50/50 (100%) |
Reproductive Tract Competence to Support Implantation
To determine if the uterine environment, in addition to the oviductal environment, contributed to the infertility phenotype of Gen-treated mice, blastocysts were collected from untreated control mice and transferred into pseudopregnant control or Gen-treated recipients. Control blastocysts (7 or 8) were transferred into a single uterine horn of each recipient, and the uterus was examined 8 days following transfer for the presence of implantation sites. Three of five control recipients had normal size implantation sites; the other two were not pregnant (Table 3). In contrast, only two of nine genistein-treated recipients had any implantation sites, and these few sites were obviously smaller (less than half the size) than implantation sites in controls. This implantation site appearance was similar to that observed previously in Gen-treated mice in vivo [9]. These data are consistent with the idea that the uterus is unable to support implantation even after transfer of normal control blastocysts. Together with the finding that Gen-treated females have occasional implantation sites but no full term development [9], these findings suggest that the uterine environment contributes to the infertility of Gen-treated mice.
Table 3.
Implantation sites following transfer of control blastocysts into control and Gen-treated mice.
| Neonatal Treatment |
# Embryos Transferred |
# Recipients | # Pregnant (%) |
Total # implantation sites |
# implantation sites per pregnant recipient |
# embryos transferred per implantation site |
|---|---|---|---|---|---|---|
| Control | 37 | 5 | 3 (60) | 15 | 5.0 | 2.5b |
| Genistein | 66 | 9 | 2 (22) | 3a | 1.5 | 22.0b |
All implantation sites in Gen-treated females were significantly smaller (<50% size) than those in controls.
Significantly different, Fisher’s Exact test, p<0.0001
Discussion
This study is a comprehensive analysis of the factors contributing to infertility in Gen-treated female mice; a schematic summarizing the experiments done is shown in Figure 4. The data presented herein demonstrate that eggs from neonatal Gen-treated females are morphologically indistinguishable from controls and are capable of being fertilized in vitro, developing to the blastocyst stage, and developing to term after transfer to control recipients. We conclude that the complete infertility of neonatal Gen-treated and superovulated females cannot be explained on the basis of poor egg quality. Although other studies have demonstrated that environmentally relevant chemicals such as bisphenol A can cause disruptions in oocyte meiosis [18], this does not appear to be the case with neonatal exposure to Gen. Several estrogenic compounds induce multioocyte follicles in the mouse, with a proposed mechanism being disruption of the gonadotropin-estrogen-inhibin/activin system that regulates folliculogenesis [23]. Disruption of endocrine/paracrine mechanisms of folliculogenesis could explain suboptimal egg quality. In one multioocyte model, prenatal exposure to high dose DES, eggs from multioocyte follicles have reduced fertilization competence when compared to eggs from single oocyte follicles [19]. However, in the DES-exposed mice the multioocyte follicles have many more oocytes/follicle (up to 28) than is observed in our neonatal Gen model (up to 6), likely due to the much higher dose and estrogenic activity of the DES administered. Our results indicate that the existence of the multioocyte phenotype does not necessarily indicate that all eggs from such ovaries are of lower quality. Of note, our experiments were performed when the Gen-treated females were 6–9 wks of age. Because oocyte quality can decrease with age [24, 25], and higher quality oocytes may be recruited to develop earlier in reproductive life, it is possible that Gen treatment affects oocyte quality but that this would not be observed in young females.
Figure 4.
Schematic diagram of experimental design. All female mice were superovulated and egg/cumulus masses were collected at 14 h post-hCG for IVF and IHC experiments. For embryo development, superovulated female mice were bred to males and embryos were collected from the oviduct and/or uterus at the times indicated above each developmental stage. Embryos were then placed in culture to continue development to the blastocyst stage. Embryo transfers were done using blastocysts derived from 1-cell embryos. Developmental stages inside of the oviduct represent in vivo progression and developmental stages outside of the oviduct represent in vitro progression.
Because the infertility phenotype could not be explained on the basis of poor egg quality, we examined fertilization in vivo and progressive development of preimplantation embryos to determine their developmental competence within the reproductive tract. Although almost all of the eggs were eventually fertilized, there was a delay in fertilization of Gen-treated eggs compared to controls. This delay could be explained by differences in the timing of mating, progression of sperm through the reproductive tract, timing of capacitation, or alterations in the cumulus cell matrix or zona pellucida properties caused by the oviductal environment that delay successful sperm-egg interactions. Further studies to determine the precise timing of fertilization in vivo and the factors that delay this event may provide insights into effects of the female reproductive tract on sperm function in vivo.
Once placed in culture, pronuclear stage embryos flushed from Gen-treated mice progressed efficiently to the 2-cell stage; however, they did not progress as well as controls thereafter, with a small but significant reduction in development to the blastocyst stage (Fig. 2). This difference was not observed when eggs from Gen-treated mice were fertilized in vitro, suggesting that even a very short exposure to the oviductal environment during the time of fertilization was enough to adversely impact early embryo development. However, blastocysts generated from these pronuclear stage embryos and then transferred into control recipients generated pups equally as well as controls, and these pups were apparently healthy with all live pups surviving to weaning. These findings suggest that although there is a very small difference in development from pronuclear stage to blastocyst stage, the embryos that do survive are fully competent to develop.
Detailed examination of preimplantation embryo development in vivo demonstrated a loss of about 50% of the embryos between the 2-cell and 4-cell stages. This reduction in embryo number was attributed to exposure to the oviductal environment because it did not occur if the eggs were fertilized and cultured in vitro. It is clear that the environment in which preimplantation embryos develop has a strong impact on embryo development and survival. Numerous growth factors that act in both autocrine and paracrine fashions promote preimplantation embryo development [26]. In addition, other components including ions, steroids, prostaglandins, energy substrates, and amino acids comprising culture media or oviductal fluids strongly influence preimplantation development and can even influence postnatal development and adult health [27, 28].
It is notable that the failure of development in vivo occurred during the 2-cell to 4-cell transition. This is reminiscent of the “two-cell block” phenomenon in which embryos arrest at the 2-cell stage when cultured in suboptimal media in vitro but can complete development if transferred back to the oviduct [29]. The two-cell block can be caused by environmental toxicity, deficiency, or imbalance [30]; any or all of these could explain the poor development in vivo observed in our study. Deficiencies in the oviduct of the Gen-treated mouse could include lack of required energy substrates, osmolytes, or amino acids that are provided in vitro by the KSOM/AA culture medium. Tumor necrosis factor, a proinflammatory cytokine normally produced by the oviduct, causes decreased proliferation and increased apoptosis in preimplantation embryos unless these effects are countered by the presence of certain growth factors [31, 32]. An imbalance in this or a similar system could cause poor development in vivo.
The 2-cell to 4-cell stage transition is also the timing of the maternal to zygotic transition in the mouse, and the time when a failure of zygotic genome activation becomes manifest by an arrest in development. Several events critical for continued embryogenesis occur during this time frame, including degradation of maternal mRNAs, chromatin remodeling, development of a transcriptionally repressive state, and expression of zygotic mRNAs [33]. Whether specific pathways involved in zygotic genome activation or chromatin remodeling are disrupted because of factors within (or lacking from) the oviductal environment of Gen-treated females will be addressed in future experiments.
The reduction in embryo number after the 2-cell stage could account for the lower numbers of implantation sites observed previously in neonatal Gen-treated mice [9]. However, the presence of morphologically normal-appearing blastocyst stage embryos, albeit a reduced number, in the uterus of Gen-treated mice suggests that an additional mechanism besides loss of cleavage stage embryos contributes to the infertility of Gen-treated females. Indeed, we found that the uterus of pseudopregnant Gen-treated females did not support normal implantation of control blastocysts. One explanation for this finding could be that the Gen-treated mice had suboptimal estrogen or progesterone levels that would cause decreased uterine receptivity. However, it was shown previously that there is no difference between control and Gen-treated mice in serum levels of estrogen, progesterone, or testosterone on days 6, 8, and 10 of pregnancy [9]. Instead, the poor uterine receptivity is likely due to diminished endometrial responsiveness to normal levels of circulating steroid hormones. This would not be surprising because the uterus of Gen-treated mice does not respond fully to estrogen stimulation at puberty [34], and implantation is dependent on the presence of functional estrogen receptor alpha that mediates estrogen responses in the uterus [35]. Taken together, these data suggest that the uterus of Gen-treated females has compromised responsiveness to hormonal cues, leading to abnormal uterine receptivity and failed implantation and/or fetal resorption.
It is also possible that factors from the oviductal fluid influence the ability of the endometrium to support implantation. In women undergoing in vitro fertilization procedures, fluid from hydrosalpinges (fluid-filled Fallopian tubes) has a negative impact on pregnancy establishment after embryo transfer to the uterine cavity and may be associated with early miscarriage [36, 37]. It is unknown if this is because of detrimental effects on uterine receptivity or direct effects on the transferred embryos. The negative effects are abrogated if the hydrosalpinx is removed, and are not observed if there is no tubal patency between the tube and uterus, suggesting that luminal transit of the fluid into the endometrial cavity is required [36].
In summary, eggs from Gen-treated females are developmentally competent despite the multioocyte follicle phenotype observed in their ovaries. However, cumulative defects in the reproductive tract result in infertility. First, fertilization appears to be delayed in Gen-treated mice and although the mechanism for this is currently unknown, this could lead to altered developmental timing. Second, the oviductal environment contributes to infertility seen in Gen-treated mice because over half of the embryos were lost during this time in early embryo development. Finally, even if the embryos survive the oviductal environment, the reproductive tract is not capable of sustaining pregnancy as demonstrated by embryo transfer studies. Taken together, these data show that neonatal Gen exposure adversely impacts the function of the female reproductive tract such that the cumulative effect is complete infertility.
Acknowledgements
The authors thank Dr. Grace Kissling for assistance with statistical analysis, Dr. Jeff Tucker for assistance with confocal microscopy, and Linwood Koonce for assistance with animal care.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institutes of Environmental Health Sciences, Z01-ES-102405-01.
Footnotes
Summary Sentence: Neonatal genistein treatment does not affect oocyte developmental competence but disrupts the ability of the adult female reproductive tract to support both preimplantation embryo development and implantation.
References
- 1.Adlercreutz H, Yamada T, Wahala K, Watanabe S. Maternal and neonatal phytoestrogens in Japanese women during birth. Am J Obstet Gynecol. 1999;180:737–743. doi: 10.1016/s0002-9378(99)70281-4. [DOI] [PubMed] [Google Scholar]
- 2.Lapcik O, Hill M, Hampl R, Wahala K, Adlercreutz H. Identification of isoflavonoids in beer. Steroids. 1998;63:14–20. doi: 10.1016/s0039-128x(97)00104-9. [DOI] [PubMed] [Google Scholar]
- 3.Whitten PL, Lewis C, Russell E, Naftolin F. Potential adverse effects of phytoestrogens. J Nutr. 1995;125:771S–776S. doi: 10.1093/jn/125.3_Suppl.771S. [DOI] [PubMed] [Google Scholar]
- 4.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phytooestrogens from soy-based infant formula. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 5.Hoey L, Rowland IR, Lloyd AS, Clarke DB, Wiseman H. Influence of soya-based infant formula consumption on isoflavone and gut microflora metabolite concentrations in urine and on faecal microflora composition and metabolic activity in infants and children. Br J Nutr. 2004;91:607–616. doi: 10.1079/BJN20031083. [DOI] [PubMed] [Google Scholar]
- 6.Strom BL, Schinnar R, Ziegler EE, Barnhart KT, Sammel MD, Macones GA, Stallings VA, Drulis JM, Nelson SE, Hanson SA. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. Jama. 2001;286:807–814. doi: 10.1001/jama.286.7.807. [DOI] [PubMed] [Google Scholar]
- 7.Bernbaum JC, Umbach DM, Ragan NB, Ballard JL, Archer JI, Schmidt-Davis H, Rogan WJ. Pilot studies of estrogen-related physical findings in infants. Environ Health Perspect. 2008;116:416–420. doi: 10.1289/ehp.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta-mediated and nonestrogenic actions. Biol Reprod. 2002;67:1285–1296. doi: 10.1095/biolreprod67.4.1285. [DOI] [PubMed] [Google Scholar]
- 9.Jefferson WN, Padilla-Banks E, Newbold RR. Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biol Reprod. 2005;73:798–806. doi: 10.1095/biolreprod.105.041277. [DOI] [PubMed] [Google Scholar]
- 10.Kouki T, Kishitake M, Okamoto M, Oosuka I, Takebe M, Yamanouchi K. Effects of neonatal treatment with phytoestrogens, genistein and daidzein, on sex difference in female rat brain function: estrous cycle and lordosis. Horm Behav. 2003;44:140–145. doi: 10.1016/s0018-506x(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 11.Lewis RW, Brooks N, Milburn GM, Soames A, Stone S, Hall M, Ashby J. The effects of the phytoestrogen genistein on the postnatal development of the rat. Toxicol Sci. 2003;71:74–83. doi: 10.1093/toxsci/71.1.74. [DOI] [PubMed] [Google Scholar]
- 12.Nagao T, Yoshimura S, Saito Y, Nakagomi M, Usumi K, Ono H. Reproductive effects in male and female rats of neonatal exposure to genistein. Reprod Toxicol. 2001;15:399–411. doi: 10.1016/s0890-6238(01)00141-1. [DOI] [PubMed] [Google Scholar]
- 13.Newbold RR, Banks EP, Bullock B, Jefferson WN. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res. 2001;61:4325–4328. [PubMed] [Google Scholar]
- 14.Nikaido Y, Yoshizawa K, Danbara N, Tsujita-Kyutoku M, Yuri T, Uehara N, Tsubura A. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod Toxicol. 2004;18:803–811. doi: 10.1016/j.reprotox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Doerge DR, Twaddle NC, Banks EP, Jefferson WN, Newbold RR. Pharmacokinetic analysis in serum of genistein administered subcutaneously to neonatal mice. Cancer Lett. 2002;184:21–27. doi: 10.1016/s0304-3835(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 16.Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod. 2006;74:161–168. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- 18.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iguchi T, Kamiya K, Uesugi Y, Sayama K, Takasugi N. In vitro fertilization of oocytes from polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol. In Vivo. 1991;5:359–363. [PubMed] [Google Scholar]
- 20.Jefferson WN, Padilla-Banks E, Newbold RR. Disruption of the developing female reproductive system by phytoestrogens: genistein as an example. Mol Nutr Food Res. 2007;51:832–844. doi: 10.1002/mnfr.200600258. [DOI] [PubMed] [Google Scholar]
- 21.Thigpen JE, Setchell KD, Ahlmark KB, Locklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab Anim Sci. 1999;49:530–536. [PubMed] [Google Scholar]
- 22.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo. Cold Spring Harbor, NY: John Inglis; 2003. [Google Scholar]
- 23.Guillette LJ, Jr, Moore BC. Environmental contaminants, fertility, and multioocytic follicles: a lesson from wildlife? Semin Reprod Med. 2006;24:134–141. doi: 10.1055/s-2006-944419. [DOI] [PubMed] [Google Scholar]
- 24.Eichenlaub-Ritter U. Genetics of oocyte ageing. Maturitas. 1998;30:143–169. doi: 10.1016/s0378-5122(98)00070-x. [DOI] [PubMed] [Google Scholar]
- 25.Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Hardy K, Spanos S. Growth factor expression and function in the human and mouse preimplantation embryo. J Endocrinol. 2002;172:221–236. doi: 10.1677/joe.0.1720221. [DOI] [PubMed] [Google Scholar]
- 27.Aguilar J, Reyley M. The uterine tubal fluid: secretion, composition and biological effects. Anim. Reprod. 2005;2:91–105. [Google Scholar]
- 28.Leese HJ, Hugentobler SA, Gray SM, Morris DG, Sturmey RG, Whitear SL, Sreenan JM. Female reproductive tract fluids: composition, mechanism of formation and potential role in the developmental origins of health and disease. Reprod Fertil Dev. 2008;20:1–8. doi: 10.1071/rd07153. [DOI] [PubMed] [Google Scholar]
- 29.Whittingham DG, Biggers JD. Fallopian tube and early cleavage in the mouse. Nature. 1967;213:942–943. doi: 10.1038/213942a0. [DOI] [PubMed] [Google Scholar]
- 30.Biggers JD. Reflections on the culture of the preimplantation embryo. Int J Dev Biol. 1998;42:879–884. [PubMed] [Google Scholar]
- 31.Glabowski W, Kurzawa R, Wiszniewska B, Baczkowski T, Marchlewicz M, Brelik P. Growth factors effects on preimplantation development of mouse embryos exposed to tumor necrosis factor alpha. Reprod Biol. 2005;5:83–99. [PubMed] [Google Scholar]
- 32.Kawamura K, Kawamura N, Kumagai J, Fukuda J, Tanaka T. Tumor necrosis factor regulation of apoptosis in mouse preimplantation embryos and its antagonism by transforming growth factor alpha/phosphatidylionsitol 3-kinase signaling system. Biol Reprod. 2007;76:611–618. doi: 10.1095/biolreprod.106.058008. [DOI] [PubMed] [Google Scholar]
- 33.Schultz RM. The molecular foundations of the maternal to zygotic transition in the preimplantation embryo. Hum Reprod Update. 2002;8:323–331. doi: 10.1093/humupd/8.4.323. [DOI] [PubMed] [Google Scholar]
- 34.Jefferson WN, Padilla-Banks E, Newbold RR. Disruption of the female reproductive system by the phytoestrogen genistein. Reprod Toxicol. 2007;23:308–316. doi: 10.1016/j.reprotox.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Curtis Hewitt S, Goulding EH, Eddy EM, Korach KS. Studies using the estrogen receptor alpha knockout uterus demonstrate that implantation but not decidualization-associated signaling is estrogen dependent. Biol Reprod. 2002;67:1268–1277. doi: 10.1095/biolreprod67.4.1268. [DOI] [PubMed] [Google Scholar]
- 36.Johnson NP, Mak W, Sowter MC. Surgical treatment for tubal disease in women due to undergo in vitro fertilisation. Cochrane Database Syst Rev. 2004:CD002125. doi: 10.1002/14651858.CD002125.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strandell A, Waldenstrom U, Nilsson L, Hamberger L. Hydrosalpinx reduces in-vitro fertilization/embryo transfer pregnancy rates. Hum Reprod. 1994;9:861–863. doi: 10.1093/oxfordjournals.humrep.a138606. [DOI] [PubMed] [Google Scholar]