Abstract
Chemotherapy and checkpoint inhibitor immunotherapies are increasingly used in combinations. We determined associations between the presence of anti-PD-1/PD-L1 therapeutic biomarkers and protein markers of potential chemotherapy response. Data was extracted from a clinical-grade testing database (Caris Life Sciences; February 2015 through November 2017): immunotherapy response markers (microsatellite instability high (MSI-H), tumor mutation burden-high (TMB-H), and PD-L1 protein expression; and protein chemotherapy response markers (ERCC1, TOPO1, TOP2A, TS, TUBB3, RRM1, and MGMT. Relationships were determined by the Mantel-Haenszel chi-squared test or Fischer’s exact tests. Overall, 28,034 patients representing a total of 40 tumor types were assessed. MSI-H was found in 3.3% of patients (73% were also TMB-H); TMB-H, 8.4% (28.3% were also MSI-H); and PD-L1 expression in 11.0% of patients (5.1% were also MSI-H; 16.4% were also TMB-H). Based on concurrent biomarker expression, combinations of immunotherapy with platinum (ERCC1 negativity) or with doxorubicin, epirubicin, or etoposide (TOP2A positivity), have a higher probability of response while combinations with irinotecan or topotecan (TOPO1 positivity), with gemcitabine (RRM1 negativity), and fluorouracil, pemetrexed, or capecitabine (TS negativity) may be of less benefit. The potential for immunotherapy and taxane (TUBB3 negativity) combinations is present for MSI-H but not TMB-H or PD-L1-expressing tumors; for temozolomide and dacarbazine (MGMT negative), PD-L1 is frequently co-expressed, but MSI-H and TMB-H are not associated. Protein markers of potential chemotherapy response along with NGS for immunotherapy response markers can help support rational combinations as part of an individualized, precision oncology approach.
Keywords: immunotherapy, cytotoxic chemotherapy, PD-L1, MSI, TMB
INTRODUCTION
Combinations of immunotherapy and cytotoxic chemotherapy are increasingly being used and tested in clinical trials 1–3. Chemotherapy has the potential to enhance antitumor immune responses 4 by several mechanisms including activation of immune effectors such as monocytic-derived dendritic cells 5, and sensitizing tumor cells to lysis 6,7 However, preclinical studies have shown that chemotherapy can also deplete immunosuppressive cells, including myeloid-derived suppressor cells8, and T-regulatory cells 9,10. It is unclear which cytotoxic chemotherapeutic agents will synergize best with immunotherapy. However, several biomarkers have been associated with responses to anti-PD-1/PD-L1 checkpoint inhibitors: microsatellite instability high status (MSI-H) 11, high tumor mutational burden (TMB-H) 12, 13, programmed death-ligand 1 (PD-L1) amplification, and increased expression of PD-L1 on immunohisochemistry 14–18.
Protein markers may aid in predicting response or resistance to specific cytotoxic chemotherapeutic agents (Supplemental Table 1). Elevated topoisomerase 2 (TOP2A) expression has been linked to doxorubicin response in soft tissue sarcomas 19 while increased topoisomerase 1 (TOPO1) expression has been associated with response to irinotecan in colorectal cancer 20. Expression of TOP2A can also predict responses to etoposide and other anthracyclines 21. High thymidylate synthase (TS) was associated with decreased response to capecitabine in metastatic breast cancer 22 while low TS was associated with better response to 5-fluorouracil in colorectal cancer 23, and longer progression-free survival with pemetrexed in nonsmall cell lung cancer 24, 25. Tubulin beta 3 (TUBB3) expression has been linked to resistance to taxanes in ovarian cancer and lower survival in prostate cancer 26–28. Expression of excision repair complementation group 1 (ERCC1) negativity predicts improved response in bladder cancer and longer survival in ovarian and gastric cancers in with the use of platinum agents 29, 30 O-6-methyl guanine DNA methyltransferase (MGMT) deficiency may predict response to dacarbazine in melanoma31 and temozolomide (glioblastoma, neuroendocrine tumors) 32,33. Ribonucleotide reductase regulatory subunit M1 (RRM1) negativity may predict response to gemcitabine in non-small cell lung cancer 34.
The aim of the current study was to determine associations between protein expression markers of response to chemotherapy and immunotherapy response markers (MSI-H, TMB-H, and PD-L1 expression) in order to determine which immunotherapy and chemotherapy combinations could be more likely benefit various patient populations.
MATERIALS AND METHODS
Patient population:
Cases submitted to Caris Life Sciences (www.carislifesciences.com), a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory, between February 2015 to November 2017 that had results for MSI status, TMB, and IHC analysis (PD-L1, ERCC1, TOPO1, TOP2A, TS, TUBB3, RRM1, and/or MGMT) were analyzed. Tissue diagnoses were based on pathology reports from requesting physicians and were verified by a Caris laboratory-based pathologist. Formalin-fixed paraffin-embedded tissues were processed as previously described 35. Patient identity protection was maintained throughout the study and the information reflected a de-identified database, so the study was considered exempt and institutional review board approval was waived.
Techniques for evaluating markers
A variety of technologies were used to evaluate markers and are summarized in Supplemental Table 2.
Next-Generation Sequencing (NGS):
MSI status and TMB were determined using NGS analysis. NGS was performed on genomic DNA isolated from formalin-fixed paraffin embedded tissue using a NextSeq platform (Illumina Inc., San Diego, CA, USA). An Agilent custom-designed SureSelectXT assay (Agilent Technologies, Santa Clara, CA, USA) then was utilized to enrich the 592 whole-gene targets that comprised the NGS panel (592 genes). All reported variants were detected with greater than 99% confidence, based on the frequency of the mutation present and the amplicon coverage. The average depth of coverage for this assay is 500x with an analytic sensitivity of 5% variant frequency. To calculate TMB, the number of somatic non-silent protein-coding mutations with exclusion of copy number gene alterations and structural rearrangements were determined 36. TMB-H was defined as greater than or equal to 17 mutations per megabase (Muts/Mb), TMB- intermediate was 6–16 Muts/Mb, and TMB-low <6 Muts/Mb.
MSI instability by NGS microsatellite loci in the targeted genes of the panel were first identified using the multi-objective immune system algorithm (MISA) (8,921 locations identified). Subsequent analyses excluding sex chromosome loci, microsatellite loci in regions that typically have lower coverage depth relative to other genomic regions, and microsatellites with repeat unit lengths greater than five nucleotides, led to 7,317 target microsatellite loci. After DNA was sequenced by NGS, the 7,317 target microsatellite loci were examined and compared to the reference genome hg19 from the University of California Santa Cruz Genome Browser database. The number of microsatellite loci that were altered by somatic insertion or deletion was counted for each patient sample and only insertions or deletions that increased or decreased the number of repeats were considered. A locus was not counted more than once even in the setting of multiple lengths of insertions or deletions. Thresholds were calibrated based on a comparison of total number of altered loci per patient to MSI-fragment analysis (MSI-FA) results 37.
Immunohistochemical Analysis (IHC):
IHC was performed on the tumor samples using commercially available detection kits and autostainers (BenchmarkXT, Ventana Medical Systems Inc, and Autostainer Link 48, Dako). Primary antibodies used for protein detection were: excision repair complementation group 1 (ERCC1; 8F1) from Abcam; TOPO1 (1D6) and TOP2A (3F6) from Leica Microsystems; MGMT (MT21.2) from Invitrogen; RRM1 (polyclonal) from Proteintech Group, TS (TS106, Dako); TUBB3 (PRB-435P, BioLegend); and PD-L1 (SP142; Ventana). The laboratory used staining protocols by Ventana Medical Systems, Inc. or the Dako automated staining systems. Appropriate positive and negative control specimens were included for all the antibodies tested. Scoring for all slides was performed manually by board-certified pathologists with results reported as a percentage of tumor cells that stained positive and intensity of staining (0, 1+, 2+, and 3+).
Statistics:
All statistical analysis was verified by our biostatistician (DAB). Associations between MSI status, PD-L1 expression, or TMB status and protein markers (ERCC1, TOPO1, TOP2A, TS, TUBB3, RRM1, MGMT) were analyzed with the Mantel-Haenszel chi-square test using tumor type as stratification. The association between the protein markers and the presence of any marker predicting response to immunotherapy (MSI-H, TMB-H, or PD-L1 expression) was also determined. The Breslow-Day test was used to determine if the odds ratios for different tumor types were similar such that they could be combined in the analysis. If the Breslow-Day test was not significant (p≥0.05), then the Mantel-Haenszel statistic and adjusted odds ratio were used to describe the data. If the Breslow-Day test was significant (p<0.05) then Fisher’s exact test for each tumor type were used to determine significant relationships and the relationships were described by odds ratios in each tumor type separately. P-values less than or equal to 0.05 were considered significant.
Data Availability:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
RESULTS
Data were available for 28,034 patients with MSI status and classified by 40 cancer types (Table 1, Supplemental Tables 3–4). Overall, MSI-H was found in 3.3% of patients; TMB-H, in 8.4%; and PD-L1 expression in 11.0% of patients. TMB-H was found in 2,340 patients; of these, 662 (28.3%) were also MSI-H and 24.9% of those tested expressed PD-L1. TMB-intermediate was found in 7,990 patients; of these, 1.6% (125) were MSI-H. MSI-H was found in 911 patients with a TMB result; of these 73% (662) were TMB-H (125 patients had TMB-intermediate and 124 patients, TMB-low) and 15.4% of tested patients expressed PD-L1. Of the PD-L1 expressing tumors that were tested for MSI, 5.1% were MSI-H; of the PD-L1 expressing tumors that were tested for TMB, 16.4% were TMB-H.
Table 1:
Microsatellite status, tumor mutational burden, and protein expression in 28,034 patients. (Bolded numbers indicated percentage of patients that may be responsive)a
|
Positive (%) |
Low or Negative (%) |
Number of patients tested |
Comment | |
|---|---|---|---|---|
| MSI-H | 3.3% | 96.7% | 28,034 | MSI-H is one marker for checkpoint inhibitor immunotherapy response. Therefore 3.3% of patients tested for MSI-H status may benefit from checkpoint inhibitors 11. |
| TMB-H | 8.4% | 91.6% | 27,847 | TMB-H is one marker of immunotherapy response 12. |
| PD-L1 | 11.0% | 89.0% | 22,114 | PD-L1 expression is a marker for immunotherapy response 14. |
| ERCC1 | 20.9% | 79.1% | 21,802 | ERCC1 negative correlates with platinum response 29, 30; 79% of patients tested are ERCC negative/low. |
| MGMT | 55.4% | 44.6% | 5,200 | MGMT negative correlates with dacarbazine/temozolomide response 31–33; 45% of patients tested have MGMT negative/low |
| RRM1 | 19.9% | 80.1% | 17,205 | RRM1 negative correlates with gemcitabine response 34; 80% of patients tested have RRM1 negative/low. |
| TOP2A | 75.8% | 24.2% | 12,907 | TOP2A positive correlates with doxorubicin 19, etoposide, epirubicin21 response; 76% of patients have TOPO2A high |
| TOPO1 | 58.7% | 41.3% | 22,211 | TOPO1 positive correlates with irinotecan and topotecan response 20; 59% of patients have TOPO1 positive disease |
| TS | 34.0% | 66.0% | 20,491 | TS negative correlates with fluorouracil/pemetrexed/capecitabine response 22–25; 66% of patients tested have TS negative/low. |
| TUBB3 | 56.8% | 43.2% | 19,863 | TUBB3 positive correlates with taxane resistance 26–28; 43% of patients tested have TUBB3 negative/low |
No patients had all markers. See also Figure 1 for graphical presentation. See Methods and Supplemental Table 2 for the methods used in each case to determine positive or negative/low and Supplemental Table 1 for the implication of positivity and negativity Abbreviations: ERCC1=excision repair complementation group 1, MGMT= O-6-methyl guanine DNA methyltransferase, MSI =microsatellite instability, RRM1=ribonucleotide reductase regulatory subunit M1, TMB = tumor mutational burden; TOP2A=topoisom erase 2, TOPO1=topoisomerase 1, TS=thymidylate synthetase, TUBB3=tubulin beta 3
Positivity of protein marker expression for all cancers combined was: ERCC1 20.9%, MGMT 55.4%, RRM1 19.9%, TOPO1 58.7%, TOP2A 75.8%, TS 34.0%, and TUBB3 56.8% (Table 1). The percentage of protein expression positivity varied between cancer types (Supplemental Table 4). For some of these proteins, e.g., ERCC1, RRM1, MGMT, TS, and TUBB3, it is loss of expression that correlates with either sensitivity or less resistance to chemotherapy23–34. Decreased expression for these proteins was found in the following patients by percent: ERCC1 79.1%, RRM1 80.1%, MGMT 44.6%, TS 66%, and TUBB3 43.2%.
MSI-H and Chemotherapy Protein Marker Relationships:
The relationship between the percentage of patients with protein expression indicating sensitivity to specific drugs was compared between MSI-H and MSI-Stable patients (Figure 1A, Table 2). The Mantel-Haenszel (M-H) test was used to compare the likelihood of MSI-H status with ERCC1, MGMT, RRM1, TOP2A, TOPO1, TS, and TUBB3 expression indicating drug sensitivity (Table 2). Decreased ERCC1 expression, a marker of potential benefit from platinum chemotherapy 29, 30, was associated with MSI-H status (Mantel-Haenszel Odds Ratio (OR) (95% confidence interval (CI)): 0.68 (0.55–0.85); p<0.001). Similarly, low TUBB3 expression (high TUBB3 is a marker of taxane resistance 26–28 was found more commonly in MSI-H patients (MH OR 0.71 (0.60–0.83); p<0.001). On the other hand, decreased TOPO1 expression (positivity is a marker for likely irinotecan or topotecan response 20), was associated with MSI-H; similarly, RRM1 over-expression (under-expression is a marker of gemcitabine response 34), was more commonly found in with MSI-H patients. No significant relationship was found between MSI status and MGMT expression (p=0.59).
Figure 1:
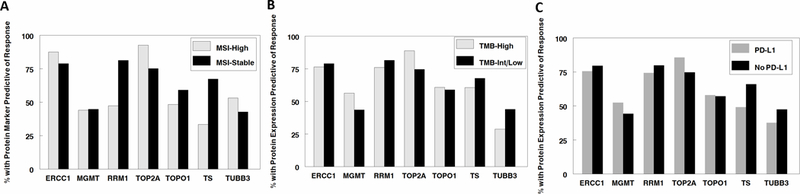
Protein markers predictive of response to chemotherapy compared to immunotherapy response makers. A. MSI-H (predictive of checkpoint inhibitor response) vs. MSI-stable. 88% of MSI-H also have ERCC1 negativity (predictive of platinum response); 44% of MSI-H also have MGMT negativity (predictive of dacarbazine and temozolomide response); 47% of MSI-H also have RRM1 negative (predictive of gemcitabine response); 93% of MSI-H patients have TOP2A positivity (predictive of with doxorubicin, epirubicin, etoposide response), 48% of MSI-H patients have TOPO1 positivity (predictive of irinotecan or topotecan response), 33% of MSI-H patients have TS negativity (predictive of fluorouracil/pemetrexed/capecitabine response), and 53% of MSI-H patients have TUBB3 negativity (predictive of taxane response).
B. TMB-H (predictive of checkpoint inhibitor response) vs. TMB-intermediate/low. 76% of TMB-H also have ERCC1 negativity (predictive of platinum response); 56% of TMB-H also have MGMT negativity (predictive of dacarbazine and temozolomide response); 76% of TMB-H also have RRM1 negative (predictive of gemcitabine response); 89% of TMB-H patients have TOP2A positivity (predictive of with doxorubicin, epirubicin, etoposide response), 61% of TMB-H patients have TOPO1 positivity (predictive of or irinotecan or topotecan response), 61% of TMB-H patients have TS negativity (predictive of fluorouracil/pemetrexed/capecitabine response), and 29% of TMB-H patients have TUBB3 negativity (predictive of better response to taxanes).
C. PD-L1 positive (predictive of checkpoint inhibitor response) vs. PD-L1 negative. 75% of PD- L1 positive also have ERCC1 negativity (predictive of platinum response); 52% of PD-L1 positive also have MGMT negativity (predictive of dacarbazine and temozolomide response); 74% of PD-L1 positive also have RRM1 negative (predictive of gemcitabine response); 86% of PD-L1 positive patients have TOP2A positivity (predictive of with doxorubicin, epirubicin, etoposide response), 58% of PD-L1 positive patients have TOPO1 positivity (predictive of irinotecan or topotecan response), 49% of PD-L1 positive patients have TS negativity (predictive of fluorouracil/pemetrexed/capecitabine response), and 38% of PD-L1 positive patients have TUBB3 negativity (predictive of better response to taxanes).
See Table 2 for additional data.
Table 2:
Relationship between protein biomarkers and MSI-H, TMB-H, or PD-L1 expression status a
| Biomarker | Mantel-Haenszel Odds Ratio (95% CI)b |
p-value | Number of patients |
Potential benefit in combination therapy [Yes/No] |
|---|---|---|---|---|
| Protein markers and MSI-H status | ||||
| ERCC1 | 0.68(0.55 – 0.85) | 0.001 | 21,772 | Yes for benefit of combination of immunotherapy and platinum (ERCC1 negativity, which is associated with platinum response 29, 30, was correlated with MSI-H.) |
| MGMT | 0.91 (0.62 – 1.30) | 0.59 | 5,175 | No significant correlation between MGMT status and MSI-H |
| RRM1 | 3.49 (2.91 – 4.17) | <0.001 | 17,190 | No for benefit of combination of immunotherapy and gemcitabine (RRM1 negativity is associated with gemcitabine response 34. However, data shows that it is RRM1 positivity that was correlated with MSI-H.) |
| TOPO1 | 0.77 (0.67 – 0.89) | 0.001 | 22,186 | No for benefit of combination of immunotherapy and irinotecan or topotecan. (TOPO1 positivity is associated with irinotecan response 20. However, data shows that TOPO1 negativity was correlated with MSI-H.) |
| TUBB3 | 0.71 (0.60 – 0.83) | <0.001 | 19,839 | Yes for benefit of combination of immunotherapy and taxanes (TUBB3 positivity is associated with taxane resistance 26–28. Data shows that TUBB3 negativity was correlated with MSI-H.) |
| Protein markers and TMB-H status | ||||
| ERCC1 | 0.83(0.72 – 0.96) | 0.013 | 21,665 | Yes for benefit of combination of immunotherapy and platinum (ERCC1 negativity, which is associated with platinum response 29,30, was correlated with TMB-H.) |
| MGMT | 0.98(0.80 – 1.20) | 0.86 | 5,160 | No significant correlation between MGMT status and TMB-H |
| TOP2A | 2.80 (2.15 – 3.66) | <0.001 | 12,828 | Yes for benefit of combination of immunotherapy and doxorubicin (TOPO2A positivity, which is associated with doxorubicin, etoposide, epirubicin response 19, 21, was correlated with TMB-H.) |
| TOPO1 | 0.83 (0.75 – 0.93) | 0.001 | 22,090 | No for benefit of combination of immunotherapy and irinotecan or topotecan (TOPO1 positivity is associated with irinotecan or topotecan response 20. However, data shows that it TOPO1 negativity was correlated with TMB-H.) |
| Protein markers and PD-L1 positivity | ||||
| MGMT | 0.78(0.65 – 0.95) | 0.011 | 4,919 | Yes for benefit of combination of immunotherapy and temozolomide, dacarbazine. (MGMT negativity, which is associated with response to dacarbazine 31 and temozolomide 32, 33, was correlated with PD-L1 positivity.) |
| TOPO1 | 1.01 (0.91 – 1.12) | 0.87 | 18,931 | No significant correlation between TOPO1 status and PD-L1 positivity. |
If odds ratio of biomarker is less than 1 and p-value is significant, then biomarker negativity is associated with MSI-H or TMB-H. See summary of these results in Table 3.
Tumor types were pooled and described in this table by the Mantel-Haenszel odds ratio if the Breslow-Day test was not significant; if the Breslow Day test is significant, you cannot pool the tumor types because there are significant differences between histologies. Protein markers with significant Breslow-Day results were not included in the table and relationships are summarized in Supplemental Tables 5–7
Abbreviations: CI=confidence interval, ERCC1=excision repair complementation group 1, H = high, MGMT= O-6-methyl guanine DNA methyltransferase, MSI=microsatellite instability, TMB=tumor mutational burden, TS=thymidylate synthase, TOPO1=topoisomerase 1, TUBB3=tubulin beta 3
TS and TOP2A could not be evaluated by the Mantel-Haenszel test, which looks at pooled data for all histologies providing that the individual histologies do not differ significantly from each other. If the individual histologies differed significantly, we examined them with the Fisher’s exact test. For TS, the Fisher’s exact test was significant in 9/40 tumor types in the direction of drug resistance (Supplemental Table 5). Specifically TS positivity (reflects 5-fluoruracil resistance 22), was associated with MSI-H status in all nine tumor types (colorectal (p<0.001), cholangiocarcinoma (p=0.022), epithelial ovarian cancer (p=0.031), female genital tract malignancy (p<0.0001), gastric cancer (p<0.001), neuroendocrine tumor (p=0.025), cancer with unknown primary (p<0.001), pancreatic (p<0.001), and small intestinal cancers (p=0.003)). For TOP2A, Fisher’s exact tests were significant in 4/40 tumor types for drug sensitivity (Supplemental Table 5). TOP2A positivity, a marker of potential doxorubicin, epirubicin, and etoposide benefit19, 21, was significantly associated with MSI-H status in all four cancers (epithelial ovarian (p=0.0018), female genital tract malignancy (p=0.0011), gastric cancer (p=0.043), and neuroendocrine tumors (p=0.0038)).
TMB-H and Chemotherapy Protein Marker Relationships:
The relationship between the percentage of patients with positive protein expression indicating sensitivity to specific drugs was compared between TMB-H and TMB- Intermediate/Low (Figure 1B). The Mantel-Haenszel test was used to compare the likelihood of TMB-H status with ERCC1, MGMT, RRM1, TOP2A, TOPO1, TS, and TUBB3 expression indicating drug sensitivity (Table 2). Decreased ERCC1 expression, a marker of potential benefit from platinum chemotherapy 29, 30, was associated with TMB-H status (Mantel-Haenszel Odds Ratio (OR) (95% confidence interval (CI)): 0.83 (0.72–0.96); p=0.013). TOP2A over-expression, a marker of doxorubicin, etoposide, and epirubicin response 19, 21, was found more commonly in TMB-H (M-H OR 2.80 (2.15–3.66); p<0.001). No significant relationship was found between TMB status and MGMT expression (p=0.86).
RRM1, TS, and TUBB3 could not be evaluated by the Mantel-Haenszel test. For RRM1, the Fisher’s exact test was significant in 9/40 tumor types (Supplemental Table 6). RRM1 negativity, a marker of gemcitabine response 34, was associated with TMB-H status in non-melanoma skin cancer (p=0.031). RRM1 positivity (negativity has been related to gemcitabine response 34) was associated with TMB-H status in eight tumor types: small intestinal cancer (p=0.005), pancreatic cancer (p=0.004), cancer with unknown primary (p=0.010), non-small cell lung cancer (p=0.012), female genital tract malignancy (p<0.001), epithelial ovarian cancer (p<0.001), breast cancer (p=0.040), and bladder cancer (p=0.019).
For TS, the Fisher’s exact test was significant in 11/40 tumor types (Supplemental Table 6). TS negativity, a marker for fluorouracil, pemetrexed, and capecitabine response 23–25, was associated with TMB-H status in two tumor types: breast cancer (p=0.029) and Merkel cell cancer (p=0.048). TS positivity (negativity is associate with improved responses to 5-flurouracil, pemetrexed, capecitabine 23–25) was associated with TMB-H status in nine tumor types: small intestinal cancer (p=0.001), pancreatic cancer (p=0.002), cancer with unknown primary tumors (p=0.001), non-small cell lung cancer (p<0.001), gastric cancer (p<0.001), female genital tract malignancy (p<0.001), epithelial ovarian cancer (p=0.031), cholangiocarcinoma (p=0.029), and colorectal cancer (p<0.001).
For TUBB3, the Fisher’s exact test was significant in 4/40 tumor types (Supplemental Table 6). TUBB3 positivity, a marker for taxane resistance26–28 was associated with TMB-H status in two tumor types: non-small cell lung cancer (p=0.035) and melanoma (p<0.001). TUBB3 negativity (positivity is associated with taxane resistance 26–28) was associated with TMB-H status in two tumor types: female genital tract malignancy (p=0.039) and colorectal cancer (p=0.046).
PD-L1 expression and Chemotherapy Protein Marker Relationships:
The relationship between the percentage of patients with positive protein expression indicating drug sensitivity was compared between PD-L1 expressing tumors and PD-L1 non-expressing tumors (Figure 1C). The Mantel-Haenszel test was used to compare the likelihood of PD-L1 expression with ERCC1, MGMT, RRM1, TOP2A, TOPO1, TS, and TUBB3 positivity (Table 2). Decreased MGMT expression, a marker for temozolomide and dacarbazine response 31–33, was found more commonly with PD-L1 expression (Mantel-Haenszel Odds Ratio (95% confidence interval): 0.78 (0.65–0.95); p=0.011). No relationship was found between TOPO1 and PD-L1 expression (p=0.87).
ERCC1, RRM1, TOP2A, TS, and TUBB3 could not be evaluated by the Mantel- Haenszel test. For ERCC1, the Fisher’s exact test was significant in 4/40 tumor types (Supplemental Table 7). ERCC1 negativity, a marker of platinum response 29, 30, was associated with PD-L1 expression in GIST tumors (p=0.032), while ERCC1 positivity was associated with PD-L1 expression in glioblastoma (p=0.030), female genital tract malignancies (p<0.001), and esophageal tumors (p=0.010).
For RRM1, the Fisher’s exact test was significant in 5/40 tumor types (Supplemental Table 7). RRM1 positivity (RRM1 negativity has been associated with gemcitabine response 34), was associated with PD-L1 expression in non-epithelial ovarian cancer (p=0.025), soft tissue sarcoma (p=0.010), pancreatic cancer (p=0.032), female genital tract malignancy (p=0.016), and cholangiocarcinoma (p=0.002).
For TOP2A, the Fisher’s exact test was significant in 10/40 tumor types (Supplemental Table 7). TOP2A positivity, a marker of doxorubicin, etoposide, and epirubicin response 19, 21, was associated with PD-L1 expression in non-epithelial ovarian cancer (p=0.029), soft tissue sarcoma (p<0.001), cancer with unknown primary (p<0.001), non-melanoma skin cancer (p=0.045), non-small cell lung cancer (p<0.001), neuroendocrine tumors (p=0.003), mesothelioma (p=0.013), kidney cancer (p<0.001), head and neck cancer (p=0.001), and female genital tract malignancy (p<0.001).
For TS, the Fisher’s exact test was significant in 15/40 tumor types (Supplemental Table 7). TS positivity (TS negativity has been associated with fluorouracil, pemetrexed, and capecitabine response 23–25), was associated with PD-L1 expression in small intestinal cancer (p=0.007), pancreatic cancer (p<0.001), cancer with unknown primary (p<0.001), non-small cell lung cancer (p=0.036), neuroendocrine tumors (p=0.035), melanoma (p=0.006), kidney cancer (p=0.015), head and neck cancers (p<0.001), gastric cancer (p<0.001), female genital tract malignancy (p=0.031), epithelial ovarian cancer (p=0.008), cholangiocarcinoma (p=0.006), colorectal cancer (p<0.001), breast cancer (p<0.001), and bladder cancer (p=0.001).
For TUBB3, the Fisher’s exact test was significant in 8/40 tumor types (Supplemental Table 7). TUBB3 positivity, a marker for taxane resistance 26–28, was associated with PD-L1 expression in soft tissue sarcoma (p=0.027), cancer with unknown primary tumors (p=0.019), non-small cell lung cancer (p<0.001), kidney cancer (p=0.006), head and neck cancer (p=0.035), gastric cancer (p=0.001), esophageal cancer (p=0.004), and bladder cancer (p=0.001).
DISCUSSION
Activating the immune system to fight metastatic malignancies has been a major breakthrough in cancer therapy particularly for melanoma and lung cancer. Given the heterogeneity and complexity of metastatic solid tumors 38–41, it is important to give cancer therapy in combinations. Cytotoxic chemotherapy has the potential to augment the immune response and improve response rates and outcomes. However, cytotoxic chemotherapy can also have negative effects, including toxicities and immune cell depletion. It is unclear which chemotherapeutic agents would be most frequently effective when combined with checkpoint inhibitor immunotherapy.
The current study explored relationships between markers of chemotherapy response and of response to anti-PD1/PD-L1 immunotherapy, such as MSI-H, TMB-H, and PD-L1 expression. 11, 12, 14 The overall findings are summarized in Table 3. ERCC1 negativity, a marker of platinum response 29, 30, was frequently correlated with both MSI-H and TMB-H status in the pooled analysis of tumors, but was not correlated with PD-L1 IHC-positive status across tumor types. Overall, this would predict a potential benefit for immunotherapy and platinum agent combinations in patients with MSI-H or TMB-H. MGMT negativity correlated with PD-L1 expression, but was not significantly correlated with MSI-H and TMB-H evaluations. This would predict a potential benefit for dacarbazine or temozolomide 31–33 combined with checkpoint inhibitors in patients whose tumors expressed PD-L1 by IHC, but not necessarily in those with MSI-H or TMB-H. RRM1 positivity (negativity is a biomarker for gemcitabine response 34) was associated with MSI-H. This relationship was also found for many tumor types with TMB-H and PD-L1, suggesting that gemcitabine would not benefit most of these patients in combination with checkpoint blockade immunotherapy. TOP2A positivity, which predicts response to doxorubicin, epirubicin, and etoposide 19, 21, was associated with TMB-H. This relationship was also found for many tumor types with MSI-H and PD-L1. TOPO1 negativity correlated with MSI-H and TMB-H, but TOPO1 levels were not significantly associated with PD-L1 expression. Since TOPO1 negativity suggests lack of response to irinotecan and topotecan (positivity is predictive of response 20), these data indicate infrequent benefit from combining anti-PD-1/PD-L1 agents with irinotecan or topotecan. TS positivity, a marker of attenuated response to fluorouracil, pemetrexed, or capecitabine 22–25, correlated with MSI-H, TMB-H, and PD-L1 in many tumor types; thus combinations involving fluorouracil, pemetrexed, or capecitabine and immunotherapy are less likely to be of benefit. TUBB3 negativity, a marker of taxane response 26–28 was associated with MSI-H, while negativity (taxane resistance) was related to PD-L1 in many tumor types; thus the benefit for combining taxanes with immunotherapy is likely to be more frequent in patients with MSI-H and less frequent in those with PD-L1 expression (Table 3).
Table 3:
Summary of Benefit for Immunotherapy and Chemotherapy Combinations (see also Supplemental Table 1 for biomarker-chemotherapy relationships)a
| Markerb | MSI-H | TMB-H | PD-L1 expression |
|---|---|---|---|
|
ERCC1 (platinum) |
MSI-H and ERCC1 negative are associated; therefore immunotherapy and platinum combinations are likely beneficial | TMB-H and ERCC1 negative are associated; therefore immunotherapy and platinum combination are likely beneficial | PD-L1 expression and ERCC1 positive were associated in 3 of 39 tumor types; hence combinations of platinum and immunotherapy are not beneficial In 1 of 39 tumor types, PD-L1 expression and ERCC1 negative were associated; hence combinations of platinum and immunotherapy are likely beneficial in this tumor type. In the other 35 tumor types there was no relationship between PDL1 expression and ERCC1. |
|
MGMT (dacarbazine/temozolomide) |
No significant associations between MGMT negative and MSI-H | No significant associations between MGMT negative and TMB-H | MGMT decreased was associated with PD-L1 expression. Hence the combination of dacarbazine or temozolomide with immunotherapy is likely of benefit |
|
RRM1 (gemcitabine) |
MSI-H was associated with RRM1 positivity. Hence gemcitabine and immunotherapy is unlikely to be of benefit. | TMB-H was associated with RRM1 positivity in 8 of 40 tumor types and hence the combination of immunotherapy and gemcitabine is unlikely to be of benefit in these tumor types. TMB-H was associated with RRM1 negativity in 1 of 40 tumor types and hence the combination of immunotherapy and gemcitabine is likely to be of benefit in this tumor types. In the other 31 tumor types there was no relationship between TMB-H and RRM1. |
PD-L1 expression was associated with RRM1 positivity in 5 out of 40 tumor types; hence gemcitabine and immunotherapy is unlikely to be of benefit. In the other 35 tumor types there was no relationship between PD-L1 expression and RRM1. |
|
TOP2A (doxorubicin/etoposide/epirubicin) |
MSI-H was associated with TOP2A positivity in 4 of 40 tumor types; hence doxorubicin/etoposide/epirubicin and immunotherapy is likely to be of benefit. In the other 36 tumor types there was no relationship between MSI-H and TOP2A. |
TMB-H and TOP2A positivity are associated; therefore immunotherapy and doxorubicin/etoposide/epirubicin combination are likely beneficial |
PD-L1 expression was associated with TOP2A positivity in 10 of 40 tumor types; hence doxorubicin/etoposide/epirubicin and immunotherapy is likely to be of benefit. In the other 30 tumor types there was no relationship between PD-L1 expression and TOP2A. |
|
TOPO1 (irinotecan/topotecan |
MSI-H was associated with TOPO1 negativity. Hence irinotecan/topotecan and immunotherapy is unlikely to be of benefit. | TMB-H was associated with TOPO1 negativity. Hence irinotecan and immunotherapy is unlikely to be of benefit. | No significant associations between TOPO1 positive and PD-L1 expression |
|
TS (fluorouracil/pemetrexed/capecitabine) |
MSI-H was associated with TS positivity in 9 of 40 tumor types; hence fluorouracil/pemextrexed/capecitabine and immunotherapy is unlikely to be of benefit. In the other 31 tumor types there was no relationship between PDL1 expression and TS. |
TMB-H expression and TS positive were associated in 9 of 40 tumor types; hence combinations of fluoruracil/pemetrexed/capecitabine and immunotherapy are not beneficial In 2 of 40 tumor types, TMB-H and TS positive were associated; hence combinations of fluorouacil/pemetrexed/capecitabine and immunotherapy are likely beneficial. In the other 29 tumor types there was no relationship between TMB-H expression and TS. |
PD-L1 expression was associated with TS positivity in 15 of 40 tumor types; hence fluorouracil/pemextrexed/capecitabine and immunotherapy is unlikely to be of benefit. In the other 25 tumor types there was no relationship between PDL1 expression and TS. |
|
TUBB3 (taxane) |
MSI-H and TUBB3 negative are associated; therefore immunotherapy and doxorubicin/etoposide/epirubicin combination are likely beneficial | TMB-H and TUBB3 positive were associated in 2 of 40 tumor types; hence combinations of taxanes and immunotherapy are not beneficial In 2 of 40 tumor types, TMB-H and TUBB3 negative were associated; hence combinations of taxanes and immunotherapy are likely beneficial in this tumor type. In the other 36 tumor types there was no relationship between TMB-H and TUBB3. |
PD-L1 expression was associated with TUBB3 positivity in 8 of 40 tumor types; hence taxanes and immunotherapy is unlikely to be of benefit. In the other 32 tumor types there was no relationship between PDL1 expression and TUBB3. |
Bold “benefit” signifies the strongest evidence to for benefit of immunotherapy and chemotherapy combination
Summary from Mantel-Haenszel tests that pools data from the 40 histologies tested (Table 2). For patients where Mantel-Haenszel could not be used (i.e. different histologies showed different results), number of tumor types with significant results are listed—in that case, the Fisher exact test was used to calculate significant associations; See Supplemental Tables 5, 6, 7 for the exact tumor types in each case.
For ERCC1, MGMT, RRM1, TS and TUBB3, it is low or negative expression of the marker that is associated with chemotherapy benefit; for TOP2A and TOPO1, it is positive expression that is associated with chemotherapy benefit. Therefore, as examples, in this Table, ERCC1 negativity (potential benefit from platinums) is associated with MSI-H (potential immunotherapy benefit); TOP2A positivity (potential benefit from doxorubicin, etoposide and epirubicin) is associated with MSI-H (potential benefit from immunotherapy) in 4 of 40 tumor types)
Abbreviations: ERCC1=excision repair complementation group 1, MGMT= O-6-methyl guanine DNA methyltransferase, MSI =microsatellite instability, PD-L1=programmed death-ligand 1, RRM1=ribonucleotide reductase regulatory subunit M1, TMB = tumor mutational burden; TOP2A=topoisomerase 2, TOPO1=topoisomerase 1, TS=thymidylate synthetase, TUBB3=tubulin beta 3
Prior studies of the relationships between protein markers and response to cytotoxic chemotherapy were performed in a disease-specific manner. These studies evaluated commonly used chemotherapeutic agents for each cancer type. However, these relationships may hold for other cancer types 21, 42. In our study, while many of the statistical relationships determined were valid across tumor types, most of the PD-L1 assessments and all TS relationships were evaluated by individual tumor type as the significant differences between tumor types did not allow for pooling. Thus, any relationships found only held for a subset of the tumor types evaluated. In some cases, the lack of significance for other tumor types may be due to lack of power for the individual tumor types.
Prior oncology therapeutics and regimens were approved by the Food and Drug Administration (FDA) and administered based on tissue of origin of the tumor. However, recent advances in NGS and molecular profiling have demonstrated that each tumor has a unique molecular profile, which mandates a more personalized approach 39, 40, 43. Recent studies have explored dosing of novel combinations of targeted agents, cytotoxics, and immunotherapies 3,44–46. The FDA recently approved pembrolizumab in a tissue-agnostic manner for use in all patients with MSI-H status or mismatch gene alterations 47, which signifies a major shift in drug approval practice. Additional studies have suggested that patients with high TMB 12 or PD-L1 expression 14 will have superior responses to checkpoint blockade immunotherapy. While cytotoxic chemotherapy has also traditionally been administered based on tumor of origin, more recent data 19–34 supports the use of protein markers to provide insight into how best to match these agents to an individual patient.
All patients would ideally have molecular profiling including MSI, TMB, PD-L1, and protein marker information prior to the start of therapy, but due to delays in acquiring tissue from pathology and conducting NGS, patients may not have a full genomic and protein marker profile at the start of treatment. This is especially true for a patient who needs urgent initiation of therapy due to organ failure from malignancy. Immunotherapy and chemotherapy combinations are increasingly being studied in clinical trials; thus a better understanding of the relationships between markers of response to chemotherapy and immunotherapy—in particular, which combinations will give a higher probability of response—as evaluated in the current study, is essential.
Other findings of interest also emerged from out interrogation of tissue markers. MSI-H status was only observed in 3% while TMB-H was seen in 8.4% of the patients reviewed and PD-L1 expression was present in 11.0% patients in the current study. Other studies have shown corresponding percentages of 7.1% (TMB-H) 48 and 3.5% (MSI-H) 49. In the current study, only 28% of patients with TMB-H status had concurrent MSI-H; however, the majority of patients with MSI-H status were TMB-H (73%). A prior study of TMB across tumor types showed that 83% of patients with MSI-H status had TMB-H; however only 16% of TMB-H patients had MSI-H status 48. Given that MSI-H, TMB-H, and PD-L1 expression often do not co-occur, it is not surprising that the current study often found distinct relationships between each of these three immunotherapy markers and the protein markers.
This study had several important limitations. First, the database was de-identified; hence future studies will need to determine if these relationships correlate with better outcomes for the cognate combinations. Some markers can be evaluated by more than one methodology, and precise-points for important markers such as TMB-H are still a matter of debate. Third, the markers of response to chemotherapy were assumed to hold for all tumor types, however they may not have been validated in all tumor types. Of note, ERCC1 has not been found to be predictive of non-small cell lung cancer responses to platinum agents 50; thus we did not evaluate ERCC1 relationships with MSI, TMB, and PD-L1 in non-small cell lung cancer. Further, a significant Mantel-Haenszel test indicates that a relationship exists between immunotherapy and chemotherapy response markers when taking into account possible confounding from the different tumor types, but does not mean there is a relationship present for each individual tumor type. The study aimed to make overall conclusions regarding immunotherapy and chemotherapy response marker associations to provide clinically useful information to help guide precision medicine treatments and clinical trials. Fourth, the mechanisms underlying the associations described in this report are not clear. Fifth, specific treatments might result in a change in expression of PD-L1 or other markers as the tumor evolves. Finally, the ability of protein markers to predict chemotherapy response is still not considered as robust as the predictive power of NGS for immunotherapy or gene-targeted agents.
In conclusion, MSI-H, TMB-H, and PD-L1 expression were found to correlate with specific protein markers of response to chemotherapy. Based on the co-occurrence of these biomarkers, combinations of PD1/PD-L1 checkpoint inhibitor immunotherapy with temozolomide, dacarbazine, doxorubicin, epirubicin, etoposide, and platinum will have a higher probability of a response while combinations of immunotherapy with irinotecan, topotecan, gemcitabine, fluorouracil, pemetrexed, and capecitabine may less frequently have salutary effects. Taxanes may be of more frequent benefit to patients with MSI-H, but not those with TMB-H or PD-L1 expression. Protein markers of chemotherapy response along with NGS for immunotherapy response markers should be evaluated in prospective trials to determine if these markers can help support the rational use of chemotherapy as part of an individualized, precision medicine approach to oncology therapy.
Supplementary Material
Novelty and Impact:
Combinations of anti-PD-1 checkpoint inhibitors and chemotherapy are increasingly being tested in clinical trials. Understanding the protein markers that are associated with immunotherapy markers may aid in determining which immunotherapy-chemotherapy combinations will provide the highest frequencies of responses. Based on concurrent biomarker expression, combining platinum, doxorubicin, epirubicin, or etoposide with checkpoint (PD-1/PD-L1) blockade immunotherapy would have a higher probability of response.
Acknowledgments
Funding: This study was funded in part by the Joan and Irwin Jacobs philanthropic fund and by National Cancer Institute grant P30 CA023100.
Disclosures: Dr. Kurzrock has stock and equity interests in IDbyDNA, CureMatch Inc, and Soluventis; has consulting or advisory roles in Gaido, LOXO, X-Biotech, Actuate Therapeutics, Roche, NeoMed, Soluventis, and Pfizer; speaker’s fee from Roche; research funding from Incyte, Genetech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, Guardant Health, Grifols, Konica Minolta, DeBiopharm, Boerhinger Ingelheim, and Omniseq; and is a board member at CureMatch Inc. David Arguello, Zoran Gatalica, and Jeff Swenson are employed by Caris Life Sciences, Inc.
With the emerging success of immunotherapy of cancers, combinations with conventional chemotherapies are increasingly being tested in clinical trials. Here the authors examined concurrent biomarker expression of checkpoint (PD-1/PD-L1) blockade immunotherapy and various cytotoxic chemotherapies to determine which chemotherapeutic agents will best synergize with immunotherapy. They predict that combining platinum or doxorubicin, epirubicin, or etoposide treatments with PD-1/PD-L1 inhibitors would have a higher probability of response than other treatments, supporting a rational combination strategy in a possibly individualized treatment approach.
Abbreviations:
- TMB-H
tumor mutational burden high
- MSI-H
microsatellite instability high
- ERCC1
excision repair complementation group 1
- TOPO1
topoisomerase 1
- TOP2A
topoisomerase 2
- TS
thymidylate synthetase
- TUBB3
tubulin beta 3
- RRM1
ribonucleotide reductase regulatory subunit M1
- MGMT
O-6-methyl guanine DNA methyltransferase
- CLIA
Clinical Laboratory Improvement Amendments
- NGS
next generation sequencing
References:
- 1.Morrissey KM, Yuraszeck TM, Li CC, Zhang Y, Kasichayanula S. Immunotherapy and Novel Combinations in Oncology: Current Landscape, Challenges, and Opportunities. Clinical and translational science 2016;9: 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris SJ, Brown J, Lopez J, Yap TA. Immuno-oncology combinations: raising the tail of the survival curve. Cancer biology & medicine 2016; 13: 171–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikanjam M, Patel H, Kurzrock R. Dosing immunotherapy combinations: Analysis of 3,526 patients for toxicity and response patterns. Oncoimmunology 2017;6: e1338997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD. Combination immunotherapy: a road map. Journal for immunotherapy of cancer 2017; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesterhuis WJ, Punt CJ, Hato SV, Eleveld-Trancikova D, Jansen BJ, Nierkens S, Schreibelt G, de Boer A, Van Herpen CM, Kaanders JH, van Krieken JH, Adema GJ, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. The Journal of clinical investigation 2011;121: 3100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Biasi AR, Villena-Vargas J, Adusumilli PS. Cisplatin-induced antitumor immunomodulation: a review of preclinical and clinical evidence. Clinical cancer research : an official journal of the American Association for Cancer Research 2014;20: 5384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nature reviews Clinical oncology 2011;8: 151–60. [DOI] [PubMed] [Google Scholar]
- 8.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer research 2010;70: 3052–61. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Liu N, Xiong SD, Zheng YJ, Chu YW. CD4+Foxp3+ regulatory T-cell impairment by paclitaxel is independent of toll-like receptor 4. Scandinavian journal of immunology 2011;73: 301–8. [DOI] [PubMed] [Google Scholar]
- 10.Fridlender ZG, Sun J, Singhal S, Kapoor V, Cheng G, Suzuki E, Albelda SM. Chemotherapy delivered after viral immunogene therapy augments antitumor efficacy via multiple immune-mediated mechanisms. Molecular therapy : the journal of the American Society of Gene Therapy 2010;18: 1947–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England journal of medicine 2015;372: 2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, Stephens PJ, Daniels GA, Kurzrock R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Molecular cancer therapeutics 2017;16: 2598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voong KR, Feliciano J, Becker D, Levy B. Beyond PD-L1 testing-emerging biomarkers for immunotherapy in non-small cell lung cancer. Annals of translational medicine 2017;5: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Molecular cancer therapeutics 2015;14: 847–56. [DOI] [PubMed] [Google Scholar]
- 15.Khagi Y, Kurzrock R, Patel SP. Next generation predictive biomarkers for immune checkpoint inhibition. Cancer Metastasis Rev 2017;36: 179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol 2017; 14: 203–20. [DOI] [PubMed] [Google Scholar]
- 17.Jardim DL, de Melo Gagliato D, Giles FJ, Kurzrock R. Analysis of Drug Development Paradigms for Immune Checkpoint Inhibitors. Clin Cancer Res 2018;24: 1785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman AM, Piccioni D, Kato S, Boichard A, Wang H-Y, Frampton G, Lippman SM, Connelly C, Fabrizio D, Miller V, Sicklick JK, Kurzrock R. PD-L1 (CD274) Amplification: Analysis of Prevalence in 118,187 Patients and Preliminary Response to Immune Checkpoint Blockade. JAMA Oncology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigo RS, Nathalie A, Elodie T, Gonzalo GA, Philippe T, Francoise D, Julien D, Angela C, Berenice B, Jean-Yves B, Jean-Michel C, Jean B, et al. Topoisomerase II-alpha protein expression and histological response following doxorubicin-based induction chemotherapy predict survival of locally advanced soft tissues sarcomas. European journal of cancer 2011;47: 1319–27. [DOI] [PubMed] [Google Scholar]
- 20.Braun MS, Richman SD, Quirke P, Daly C, Adlard JW, Elliott F, Barrett JH, Selby P, Meade AM, Stephens RJ, Parmar MK, Seymour MT. Predictive biomarkers of chemotherapy efficacy in colorectal cancer: results from the UK MRC FOCUS trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008;26: 2690–8. [DOI] [PubMed] [Google Scholar]
- 21.Heestand GM, Schwaederle M, Gatalica Z, Arguello D, Kurzrock R. Topoisomerase expression and amplification in solid tumours: Analysis of 24,262 patients. Eur J Cancer 2017;83: 80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SJ, Choi YL, Park YH, Kim ST, Cho EY, Ahn JS, Im YH. Thymidylate synthase and thymidine phosphorylase as predictive markers of capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Cancer chemotherapy and pharmacology 2011; 68: 743–51. [DOI] [PubMed] [Google Scholar]
- 23.Qiu LX, Tang QY, Bai JL, Qian XP, Li RT, Liu BR, Zheng MH. Predictive value of thymidylate synthase expression in advanced colorectal cancer patients receiving fluoropyrimidine-based chemotherapy: evidence from 24 studies. International journal of cancer 2008;123: 2384–9. [DOI] [PubMed] [Google Scholar]
- 24.Nicolson MC, Fennell DA, Ferry D, OByrne K, Shah R, Potter V, Skailes G, Upadhyay S, Taylor P, Andre V, Nguyen TS, Myrand SP, et al. Thymidylate synthase expression and outcome of patients receiving pemetrexed for advanced nonsquamous non-small-cell lung cancer in a prospective blinded assessment phase II clinical trial. Journal of thoracic oncology : official publication of the International Association for the Study of Lang Cancer 2013;8: 930–9. [DOI] [PubMed] [Google Scholar]
- 25.Sun JM, Ahn JS, Jung SH, Sun J, Ha SY, Han J, Park K, Ahn MJ. Pemetrexed Plus Cisplatin Versus Gemcitabine Plus Cisplatin According to Thymidylate Synthase Expression in Nonsquamous Non-Small-Cell Lung Cancer: A Biomarker-Stratified Randomized Phase II Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33: 2450–6. [DOI] [PubMed] [Google Scholar]
- 26.Hetland TE, Hellesylt E, Florenes VA, Trope C, Davidson B, Kaern J. Class III beta-tubulin expression in advanced-stage serous ovarian carcinoma effusions is associated with poor survival and primary chemoresistance. Human pathology 2011;42: 1019–26. [DOI] [PubMed] [Google Scholar]
- 27.Mozzetti S, Ferlini C, Concolino P, Filippetti F, Raspaglio G, Prislei S, Gallo D, Martinelli E, Ranelletti FO, Ferrandina G, Scambia G. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research 2005;11: 298–305 [PubMed] [Google Scholar]
- 28.Ploussard G, Terry S, Maille P, Allory Y, Sirab N, Kheuang L, Soyeux P, Nicolaiew N, Coppolani E, Paule B, Salomon L, Culine S, et al. Class III beta-tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxel-based chemotherapy. Cancer research 2010;70: 9253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Dosso S, Zanellato E, Nucifora M, Boldorini R, Sonzogni A, Biffi R, Fazio N, Bucci E, Beretta O, Crippa S, Saletti P, Frattini M. ERCC1 predicts outcome in patients with gastric cancer treated with adjuvant cisplatin-based chemotherapy. Cancer chemotherapy and pharmacology 2013;72: 159–65. [DOI] [PubMed] [Google Scholar]
- 30.Scheil-Bertram S, Tylus-Schaaf P, du Bois A, Harter P, Oppitz M, Ewald-Riegler N, Fisseler-Eckhoff A. Excision repair cross-complementation group 1 protein overexpression as a predictor of poor survival for high-grade serous ovarian adenocarcinoma. Gynecologic oncology 2010;119: 325–31. [DOI] [PubMed] [Google Scholar]
- 31.Busch C, Geisler J, Lillehaug JR, Lonning PE. MGMT expression levels predict disease stabilisation, progression-free and overall survival in patients with advanced melanomas treated with DTIC. European journal of cancer 2010;46: 2127–33. [DOI] [PubMed] [Google Scholar]
- 32.Chinot OL, Barrie M, Fuentes S, Eudes N, Lancelot S, Metellus P, Muracciole X, Braguer D, Ouafik L, Martin PM, Dufour H, Figarella-Branger D. Correlation between O6-methylguanine-DNA methyltransferase and survival in inoperable newly diagnosed glioblastoma patients treated with neoadjuvant temozolomide. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2007;25: 1470–5. [DOI] [PubMed] [Google Scholar]
- 33.Kulke MH, Hornick JL, Frauenhoffer C, Hooshmand S, Ryan DP, Enzinger PC, Meyerhardt JA, Clark JW, Stuart K, Fuchs CS, Redston MS. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 2009;15: 338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong W, Zhang X, Wu J, Chen L, Li L, Sun J, Lv Y, Wei X, Du Y, Jin H, Dong J. RRM1 expression and clinical outcome of gemcitabine-containing chemotherapy for advanced non-small-cell lung cancer: a meta-analysis. Lang cancer 2012;75: 374–80. [DOI] [PubMed] [Google Scholar]
- 35.Millis SZ, Bryant D, Basu G, Bender R, Vranic S, Gatalica Z, Vogelzang NJ. Molecular profiling of infiltrating urothelial carcinoma of bladder and nonbladder origin. Clinical genitourinary cancer 2015;13: e37–49. [DOI] [PubMed] [Google Scholar]
- 36.Stadler ZK, Battaglin F, Middha S, Hechtman JF, Tran C, Cercek A, Yaeger R, Segal NH, Varghese AM, Reidy-Lagunes DL, Kemeny NE, Salo-Mullen EE, et al. Reliable Detection of Mismatch Repair Deficiency in Colorectal Cancers Using Mutational Load in Next- Generation Sequencing Panels. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34: 2141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanderwalde A, Spetzler D, Xiao N, Gatalica Z, Marshall J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer medicine 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, Leiserson MD, Miller CA, et al. Mutational landscape and significance across 12 major cancer types. Nature 2013;502: 333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wheler JJ, Parker BA, Lee JJ, Atkins JT, Janku F, Tsimberidou AM, Zinner R, Subbiah V, Fu S, Schwab R, Moulder S, Valero V, et al. Unique molecular signatures as a hallmark of patients with metastatic breast cancer: implications for current treatment paradigms. Oncotarget 2014;5: 2349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wheler J, Lee JJ, Kurzrock R. Unique molecular landscapes in cancer: implications for individualized, curated drug combinations. Cancer Res 2014;74: 7181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsimberidou AM, Iskander NG, Hong DS, Wheler JJ, Falchook GS, Fu S, Piha-Paul S, Naing A, Janku F, Luthra R, Ye Y, Wen S, et al. Personalized medicine in a phase I clinical trials program: the MD Anderson Cancer Center initiative. Clinical cancer research : an official journal of the American Association for Cancer Research 2012;18: 6373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gatalica Z, Millis SZ, Vranic S, Bender R, Basu GD, Voss A, Von Hoff DD. Comprehensive tumor profiling identifies numerous biomarkers of drug response in cancers of unknown primary site: analysis of 1806 cases. Oncotarget 2014;5: 12440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurzrock R, Giles FJ. Precision oncology for patients with advanced cancer: the challenges of malignant snowflakes. Cell cycle 2015;14: 2219–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu S, Nikanjam M, Kurzrock R. Dosing de novo combinations of two targeted drugs: Towards a customized precision medicine approach to advanced cancers. Oncotarget 2016;7: 11310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nikanjam M, Liu S, Kurzrock R. Dosing targeted and cytotoxic two-drug combinations: Lessons learned from analysis of 24,326 patients reported 2010 through 2013. Int J Cancer 2016; 139: 2135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nikanjam M, Liu S, Yang J, Kurzrock R. Dosing Three-Drug Combinations That Include Targeted Anti-Cancer Agents: Analysis of 37,763 Patients. Oncologist 2017;22: 576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm Accessed: March 31, 2018. .
- 48.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, Huang F, He Y, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cortes-Ciriano I, Lee S, Park WY, Kim TM, Park PJ. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun 2017;8: 15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friboulet L, Olaussen KA, Pignon JP, Shepherd FA, Tsao MS, Graziano S, Kratzke R, Douillard JY, Seymour L, Pirker R, Filipits M, Andre F, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med 2013;368: 1101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


