Abstract
Radiation therapy is primarily a modality to kill cancer cells in the treatment field. It is becoming increasingly clear that radiation therapy can also be used to direct immune responses that have the potential to clear residual local or distant disease outside the treatment field. We believe that cancer cell death is the critical link between these processes. Understanding the handling of dying cancer cells by immune cells in the tumor environment is crucial to facilitate immune responses following radiation therapy. We review the role of the TAM (Tyro3 Axl Mertk) group of receptor tyrosine kinases and their role following radiation-induced cancer cell death in the tumor environment.
Cell-mediated immunity to tumors is thought to require cytotoxic effector CD8 T cells expanded from naive precursors that can traffic to the tumor and kill their targets. Cancer cells can accumulate an array of mutated proteins with the potential to act as neoantigen targets for effector T cells, but cancer cells lack the requisite costimulatory molecules to active naive T cells and naive T cells do not traffic efficiently outside of lymphoid organs. Thus, to initiate new immune responses from naive T cells, the dogma is that dendritic cells (DCs) must serve to transport antigen from peripheral sites to lymphoid organs and cross-present antigen to CD8 T cells. One of the critical populations of dendritic cells for tumor immunity is the CD8a+CD103+Batf3+ subpopulation, as these are uniquely capable of cross-presenting tumor-associated antigen on MHC class I [1,2]. Batf3+ cross-presenting dendritic cells are critical for T-cell-mediated rejection of immunogenic tumors [2], are required for T-cell-mediated rejection of tumors treated with immunotherapy [3], and are also required for tumor control by the combination of immunotherapy and radiation therapy [4]. However, the CD103+Batf3+ dendritic cell is one of the least common myeloid cells in tumors, representing a mere 1–3% of immune cells in the tumor environment [1,5]. Tumor-associated macrophages can be 10–20× more common, and the less phagocytic granulocyte and immature monocyte subtypes may be more common than that [1,5]. Multiple studies have shown that tumors with high levels of myeloid infiltration are associated with poor prognosis; however, often these studies are unable to distinguish the various myeloid populations within the tumor environment which may explain mixed results upon meta-analysis [6,7].
Thus, while targeted radiation leads to focused killing of cancer cells and release of cancer antigens and immune adjuvant within the irradiated tumor, the released antigen is most probably taken up by multiple myeloid populations other than CD103+Batf3+ dendritic cells, and therefore, to cells incapable of initiating naive T-cell responses. If tumor immunity is to be facilitated with targeted radiation, then efficient delivery of irradiated cancer cell antigen to dendritic cells must be attained. For these reasons, it would be valuable to characterize individual contributions of the various phagocytic myeloid populations in the tumor on cancer antigen processing in order to optimize the flow of antigen toward CD103+Batf3+ dendritic cells.
While the primary goal of radiation therapy is to kill cancer cells, apoptotic cancer cells can have negative impacts on the immune properties of the surrounding tumor immune environment. Apoptotic cancer cells have been shown to drive accelerated repopulation of residual viable cancer cells [8], and interaction with apoptotic cells results in macrophage secretion of suppressive cytokines including IL-10 and TGF-β [9–12]. In addition, resident immune populations within the treatment field are susceptible to ionizing radiation or indirectly suppressed by exposure to dying cancer cells. As discussed, tumors are typically heavily infiltrated with myeloid cells polarized to phenotypes that suppress immune responses at the time of radiation therapy; however, our prior studies have demonstrated that radiation therapy increases infiltration of macrophages into tumors and that over time these macrophages exhibit increased suppressive differentiation [13]. As the macrophages convert into these suppressive differentiation phenotypes following radiation therapy, they are capable of suppressing T-cell responses that were successfully initiated by radiation therapy [13–15]. As discussed in a prior review, this macrophage response to dying cells is a normal feature of wound repair, where inflammatory damage leads to inflammatory resolution [15]. Phagocytosis and clearance of apoptotic cells normally prevent immune activation in the absence of additional danger signals. Thus, in vivo administration of apoptotic cells is an efficient means to generate antigen-specific tolerance [16,17], and in patients, tumors progress despite readily detectable areas of cell destruction [18]. These data suggest that antigens and adjuvants released following cell death do not necessarily generate immune responses that control residual tumors, but instead that additional processes tightly regulate immune responses to dying cells.
The consequence of radiation therapy on the various myeloid populations in the tumor will depend on the balance between recruitment, differentiation and trafficking/death of these cells [15]. As discussed, radiation results in increased recruitment and suppressive differentiation, but can also result in death of myeloid cells. Broadly speaking, fully differentiated myeloid cells are relatively radio-resistant; however, radiation therapy can suppress the ability of ex vivo cultured dendritic cells to stimulate T-cell responses [19,20]. Moreover, regulation of DNA damage response pathways in irradiated DC can alter the pattern of cytokine secretion of stimulated DC [21], potentially resulting in altered T-cell response profiles stimulated by these DC. Little is known about the relative effects of radiation on the distinct DC subtypes in vivo, but recent studies suggest that multiple DC subtypes are negatively affected. Price et al. [22] demonstrated that in the skin of tumor-free mice, both epidermal Langerhans cells and dermal DC are decreased in number following radiation and these cells repopulate ~10 days following treatment. While Langerhans cells exhibit DNA damage following radiation, they do not undergo cell death, but instead repair DNA damage and migrate to draining lymph nodes where they are more effective at driving T regulatory cell expansion than unirradiated Langerhans cells [22]. These data suggest that radiation therapy effectively causes DC and Langerhans cell migration to draining lymph nodes, but that these drive expansion of suppressive T-cell populations. While there may be differential effects of radiation therapy at lower doses (reviewed in ref. [23]), in the absence of additional therapeutic interventions, the data seem to suggest that high-dose radiation therapy drives immunosuppressive macrophage differentiation and suboptimal DC function in the treatment field. This is consistent with long-term data demonstrating that in the absence of additional interventions, radiation therapy is poorly able to generate systemic immune responses that can result in abscopal tumor control [24]. There are now many papers describing the recruitment of macrophages to tumors following radiation therapy and that these cells limit tumor control [13,15,25–27]. One strategy is to apply therapies that remove these cells, which can therefore remove the potential for suppressive differentiation and also increase the likelihood that antigen is made available to dendritic cells. One such approach has been to target CSF1R, which is a critical growth factor receptor pathway that drives macrophage differentiation and supports them in peripheral tissues. CSF1R inhibition with blocking antibodies or small molecules has synergized with both chemotherapy and radiation therapy to control tumors [25,26,28–30]; however, this approach has not been shown to result in tumor cures.
An alternative to decreasing the overall number of macrophages is to intervene to prevent suppressive polarization of these cells, or to prevent their ability to respond to dying cancer cells. We recently discussed a range of innate adjuvants as options to repolarize the tumor immune environment [31], so here we will focus on the response to dying cells. One of the most significant signals exposed on dying cells and indirectly recognized by phagocytes is phosphatidylserine (PS). PS is normally restricted to the inner leaflet of cells and classically it is exposed on the exterior of cells beginning in the early phases of apoptosis, though it can also become exposed in response to stress within the tumor. Exposed PS causes macrophages to secrete IL-10 and TGF-β [11], which are immunosuppressive cytokines in the tumor environment. Antibodies that interfere with the availability of PS significantly enhance the efficacy of radiation therapy [32] and chemotherapy [33] in preclinical models. Milk fat globulin-E8 (MFG-E8) can directly bind exposed PS on apoptotic cells and serve as a bridge to integrins on the phagocyte [34]. Mice lacking MFG-E8 exhibit autoimmune disease [35] as a result of enhanced cross-presentation of cell-associated antigens [36]. Further experiments demonstrated that dendritic cell clearance of apoptotic cells was defective in the absence of MFG-E8, resulting in uptake of cellular debris rather than apoptotic cells and improved cross-presentation to CD8 T cells [36]. Importantly, blockade of MFG-E8 has been shown to improve responses to radiation therapy and chemotherapy in preclinical models [37]. Mertk has been shown to play a critical role in MFG-E8-mediated opsonization of apoptotic cells [38], and the dominant ligand for Mer is Gas6, a protein S-related gene that has been shown to bind exposed PS on apoptotic cells. Gas6 bridging of apoptotic cells to macrophages results in apoptotic cell phagocytosis [39], and like mice lacking MFG-E8, mice lacking Mertk show defective handling of apoptotic cells and autoimmunity [40,41]. Our studies demonstrated that ligation of Mertk resulted in the accumulation of NF-κB p50 in macrophages, which repolarized the macrophage response to innate adjuvants [13,42]. This is consistent with prior data demonstrating that tumor-associated macrophages and M2 macrophages accumulate NF-κB p50, which defines their polarized response to stimulation [43,44]. Thus, despite the availability of potential endogenous adjuvants such as HMGB1 released from dying cancer cells, the interaction of dying cancer cells with Mertk on macrophages limits their inflammatory potential and therefore the ability of radiation therapy to initiate immunity as a single agent [42,45,46].
Mertk is a member of the TAM (Tyro3 Axl Mertk) group of receptor tyrosine kinases, and each bind Gas6 or protein S with varying affinity [47]. Ligation of these receptors can result in up-regulation of anti-inflammatory regulators such as SOCS1 and suppress the ability of cells to produce inflammatory cytokines in response to stimuli [48]. These receptors have a varying expression pattern, but there is a great deal of interest in TAM inhibitors as cancer therapies [47]. Analysis of the gene expression profile of a range of immune cell populations demonstrated that Mertk was selectively expressed on macrophages when compared with dendritic cells [49]. Similarly, Broz et al. [5] recently characterized the myeloid populations of the tumor and developed a flow cytometry panel to identify the unique cross-presenting CD103+ dendritic cell subpopulation, and used this information to extract a gene signature that characterized the cross-presenting CD103+ DC and another gene signature for the other DC and tumor-associated macrophage populations. As anticipated, the CD103+ cells were enriched for expression of Batf3, Clec9a and Irf8, among other genes. However, it is notable that the other myeloid populations were enriched for genes such as C1qa, b and c, and Mertk [5]. As discussed for MFG-E8, Mertk is also the signaling component of apoptotic cell phagocytic complexes involving C1q [50], placing this molecule at the center of a complex of genes involved in clearance of dying cancer cells that does not lead to cross-presentation.
These data suggest that Mertk could be targeted to specifically redirect antigen uptake away from macrophages, which can engender suppressive responses, and toward dendritic cells, which can cross-present antigen. However, Mertk expression has also been detected on cancer cells, suggesting that therapies targeting Mertk have potential efficacy on both cancer cells and tumor-infiltrating myeloid cells. Yi et al. [51] discovered aberrant Mertk expression in a small subset of gastric cancers associated with worse clinical outcomes. In vitro, gastric cancer cell lines that overexpress Mertk saw growth reduction with shRNA-mediated knockdown of Mertk, suggesting a role for Mertk in early tumor development of some gastric cancers [51]. Melanoma cell lines harboring both BRAF and NRAS mutations demonstrated reduced cell motility and transient reductions in cell proliferation after knockdown of Mertk [52]. Glioblastoma (GBM) cells have been shown to express Mertk, which is associated with In vitro GBM invasiveness [53]. However, non-cancer cell expression of Mertk can influence tumor growth. In Mertk−/− hosts, MMTV-PyMT orthotopic grafts show delayed tumor growth, decreased pulmonary metastases, increased expression of proinflammatory cytokines IL-6 and IL-12 and increased infiltration of CD8+ T cells, suggesting a role for Mertk in early tumor development [54]. This is not true in all models, as CT26 colorectal carcinoma and 4T1 mammary carcinoma in BALB/c mice, and Panc02 pancreatic adenocarcinoma, 3LL lung adenocarcinoma and B16 melanoma in C57BL/6 mice backgrounds were not different between wild-type and Mertk−/− hosts [42]. In addition, spontaneous pulmonary metastases derived from orthotopic 4T1 mammary carcinoma were not different between wild-type and Mertk−/− hosts [42]. Thus, the role of Mertk in tumor growth and progression may be dependent on the experimental model and also influenced by expression of Mertk in cancer cells and the host.
A family of small molecule inhibitors with specificity for Mertk have been developed and described in many preclinical studies. Within this family, UNC1062 has shown In vitro efficacy in reducing growth and motility of Mertk expressing melanoma and gastric cancer cells [51,55]. The related agent UNC569 induced apoptosis in acute lymphocytic leukemia (ALL) cell lines Jurkat and 697, and reduced colony formation in ALL and pediatric atypical teratoid rhabdoid tumor cell lines compared with vehicle-treated and non-Mertk-targeted small molecule inhibitor controls [56]. Another related agent UNC2025 reduced cell proliferation and colony formation of U251, A172 and SF188 GBM cell lines In vitro [57], and in syngeneic orthotopic tumor studies was found to have significant penetration into tumors in the central nervous system resulting in delayed growth and increased response rate to radiation therapy [58]. UNC2025 inhibited Mertk phosphorylation in human non-small cell lung carcinoma (NSCLC) cell lines (H2228, A549, Colo699 and H1299), induced apoptosis and inhibited colony formation In vitro and inhibited growth of subcutaneous tumor xenografts with H2228 and A549 cells in Nude mice [59]. Similar effects on reduced Mertk phosphorylation were seen In vitro with a blocking monoclonal antibody to the Mertk extracellular domain on A549, H2009, HCC15 and Colo699 NSCLC cell lines [60]. These data using Mertk-targeting antibodies are important evidence for the potential of Mertk-specific inhibition, since UNC2025, UNC569 and UNC1062 also suppress Axl and Tyro3 in the nano-molar range. However, since Axl may play an important role in cancer cell resistance to treatment (reviewed in ref. [61]), inhibitors that suppress both Axl and Mertk have the potential to act as drugs working on both sides of the tumor–host divide.
While much of the research on Axl inhibition has focused on the cancer cell, it is becoming clear that Axl expressed on cancer cells can influence the tumor immune environment. Recent studies demonstrated that expression of Axl by cancer cells was a critical factor in determining a suppressive tumor immune environment, which in turn resulted in poor responses to radiation therapy and immunotherapy [62]. Interestingly, activation of Axl in the cancer cells resulted in suppression of antigen-processing and presentation machinery [62]. Even between subclones of cancer cell lines, MHC expression can vary, and the level of MHC expression by subclones directly correlates with their ability to be killed by cytotoxic T lymphocytes [63]. Loss of antigen-presenting machinery in cancer cells, along with local immune suppression, is highly predictive of poor outcome in cancer patients [64]. MHC I expression in cancer cells is limited by activation of the MEK pathway, and small molecule MEK inhibitors restored MHC expression and increased sensitivity to T-cell killing [65]. Importantly, Tap1 and Tap2, as well as other critical components involved in antigen presentation, were also up-regulated following MEK inhibition in cancer cells [65]. Since Tyro3, Axl and Mertk are strong activators of the MEK pathway following ligation, it makes sense that in an environment where these family members are chronically activated due to constant environmental stress and treatment-induced cell death, the consequence will be a poor environment for immune control of cancer.
One concern in the use of small molecule TAM inhibitors is that where tested, they also block Flt3 at similar affinity to Mertk blockade [47,61,66]. Flt3 is a critical growth factor receptor needed to generate cross-presenting DC [67,68]. These data suggest that the currently available small molecules blocking Mertk may not be useful if our goal is to redirect antigen from macrophages and toward cross-presenting dendritic cells. This may play a role in the differential effects of treatment in Mertk−/− mice versus in vivo Mertk small molecule inhibition. Inhibition of Mertk with the small molecule inhibitor, UNC2025, did not affect tumor growth in murine orthotopic GBM tumor graft models [58], and combined treatment with UNC2025 and radiation therapy did not significantly increase overall survival compared with radiation alone. However, combined treatment did result in some tumor cures that were not seen in mice treated with radiation therapy alone [58]. In contrast, Mertk−/− mice bearing CT26 colorectal carcinoma showed significantly increased overall survival following radiation compared with wild-type mice [42]. Similar studies with Panc02 pancreatic adenocarcinoma cell lines did not have increased responsiveness to radiation therapy in Mertk−/− hosts and required the addition of a TGF-β inhibition to achieve high rates of tumor control following radiation [42]. In addition to direct targeting of Mertk or Axl, an alternative approach is targeting of its endogenous ligands protein S and Gas6 [69]. Silencing of the gene for Protein S with siRNA reduced migration of the GBM cell line LN18 and induced apoptosis In vitro [70]. Pharmaceutical inhibition of Gas6 and Protein S can be attained with warfarin, which blocks the vitamin K-dependent γ-carboxylation of Gas6 and Protein S needed for binding these ligands to exposed phosphatidylserine on apoptotic cells [71]. Warfarin administration had been shown to reduce melanoma metastases in murine subcutaneous tumor grafts with B16F10 melanoma cells [72], and reduced meta-static burden was also demonstrated in many pancreatic adenocarcinoma tumor models subjected to low-dose warfarin administration [73]. Thus, TAM signaling inhibition may be a contributor to the long-standing but poorly understood connection between anticoagulation and cancer [74].
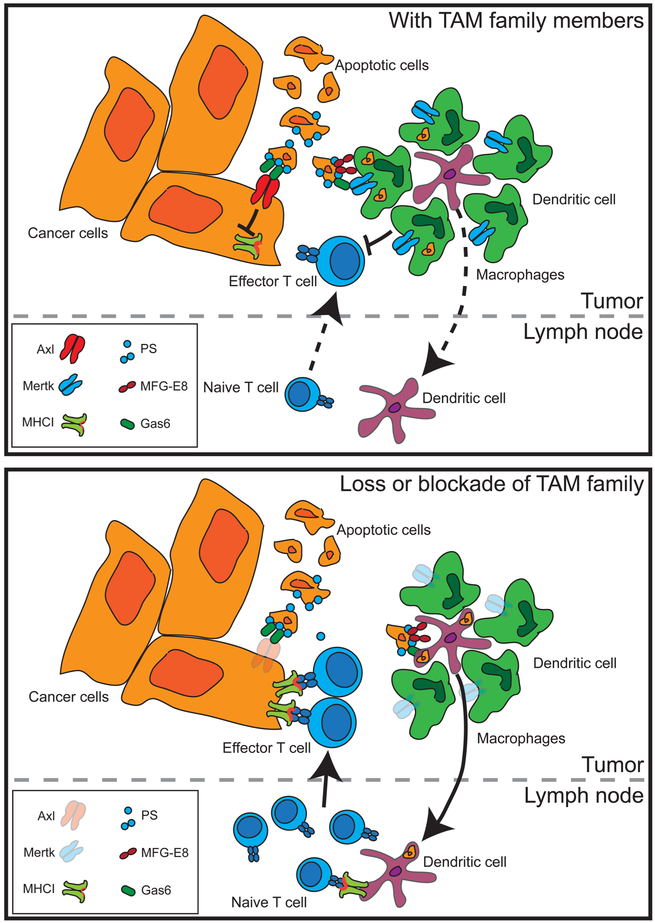
We believe that if radiation therapy is to be improved as an immunotherapy — due to its ability to kill cancer cells to permit cross-presentation of tumor-associated antigens to T cells — then it is critical to understand the handling of dying cells following radiation therapy. That radiation therapy as a single agent is rarely capable of causing immune-mediated regression of distant tumors suggests that without intervention, the normal handling of dying cells does not permit immune responses. We believe that the TAM family members, and Mertk in particular, are an excellent target to manipulate the handling of dying cells and improve immune responses to cell-associated antigens (Figure 1).
Figure 1. Effect of TAM family members on the immune response to cancer cell death.
Activation of Axl or Mertk on cancer cell results in suppression of antigen processing and presentation to T cells, while Mertk on tumor macrophages results in phagocytosis of dying cells to the exclusion of dendritic cell uptake. Cell uptake drives suppressive differentiation of macrophages, which can, in turn, suppress T cells in the tumor environment. Where TAM family members are absent, antigen is more available for uptake by dendritic cells that can expand naive T cells that can traffic to the tumor. Tumor-infiltrating T cells find decreased macrophage suppression and increased antigen presentation, permitting increased control of tumors.
Summary.
Dying cancer cells activate a series of suppressive mechanisms in the tumor environment that limits immune responses to cell-associated antigens.
TAM family members are central to handling of dying cancer cells, and suppression of TAM family members is a potent therapeutic strategy to initiate immune responses following radiation-induced cancer cell death.
Abbreviations
- ALL
acute lymphocytic leukemia
- DCs
dendritic cells
- GBM
glioblastoma
- MFG-E8
milk fat globulin-E8
- NSCLC
non-small cell lung carcinoma
- PS
phosphatidylserine
- TAM
Tyro3 Axl Mertk
Footnotes
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S et al. (2016) Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44, 924–938 10.1016/j.immuni.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M et al. (2008) Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 10.1126/science.1164206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez-Paulete AR, Cueto FJ, Martinez-Lopez M, Labiano S, Morales-Kastresana A, Rodriguez-Ruiz ME et al. (2016) Cancer immunotherapy with immunomodulatory anti-CD137 and anti-PD-1 monoclonal antibodies requires BATF3-dependent dendritic cells. Cancer Discov. 6, 71–79 10.1158/2159-8290.CD-15-0510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Ruiz ME, Rodriguez I, Garasa S, Barbes B, Solorzano JL, Perez-Gracia JL et al. (2016) Abscopal effects of radiotherapy are enhanced by combined immunostimulatory mAbs and are dependent on CD8 T cells and crosspriming. Cancer Res. 76, 5994–6005 10.1158/0008-5472.CAN-16-0549 [DOI] [PubMed] [Google Scholar]
- 5.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ et al. (2014) Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 638–652 10.1016/j.ccell.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C et al. (2016) Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: a systemic review and meta-analysis. Oncotarget 7, 34217–34228 10.18632/oncotarget.9079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q. w., Liu L, Gong C. y., Shi H. s., Zeng Y. h., Wang X. z. et al. (2012) Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE 7, e50946 10.1371/journal.pone.0050946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Q, Li F, Liu X, Li W, Shi W, Liu F-F et al. (2011) Caspase 3–mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med 17, 860–866 10.1038/nm.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY and Henson PM (1998) Macrophages that have ingested apoptotic cells In vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest 101, 890–898 10.1172/JCI1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gough MJ, Melcher AA, Ahmed A, Crittenden MR, Riddle DS, Linardakis E et al. (2001) Macrophages orchestrate the immune response to tumor cell death. Cancer Res. 61, 7240–7247 PMID:11585761 [PubMed] [Google Scholar]
- 11.Huynh M-LN, Fadok VA and Henson PM (2002) Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J. Clin. Invest 109, 41–50 10.1172/JCI0211638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP and Henson PM (2006) Apoptotic cells, through transforming growth factor-β, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J. Biol. Chem 281, 38376–38384 10.1074/jbc.M605146200 [DOI] [PubMed] [Google Scholar]
- 13.Crittenden MR, Cottam B, Savage T, Nguyen C, Newell P and Gough MJ (2012) Expression of NF-κB p50 in tumor stroma limits the control of tumors by radiation therapy. PLoS ONE 7, e39295 10.1371/journal.pone.0039295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crittenden MR, Savage T, Cottam B, Baird J, Rodriguez PC, Newell P et al. (2014) Expression of arginase I in myeloid cells limits control of residual disease after radiation therapy of tumors in mice. Radiat. Res 182, 182–190 10.1667/RR13493.1 [DOI] [PubMed] [Google Scholar]
- 15.Gough MJ, Young K and Crittenden M (2013) The impact of the myeloid response to radiation therapy. Clin. Dev. Immunol 2013, 1–14 10.1155/2013/281958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N et al. (2009) Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31, 245–258 10.1016/j.immuni.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffith TS and Ferguson TA (2011) Cell death in the maintenance and abrogation of tolerance: the five Ws of dying cells. Immunity 35, 456–466 10.1016/j.immuni.2011.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leek RD, Landers RJ, Harris AL and Lewis CE (1999) Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br. J. Cancer 79, 991–995 10.1038/sj.bjc.6690158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merrick A, Errington F, Milward K, O’Donnell D, Harrington K, Bateman A et al. (2005) Immunosuppressive effects of radiation on human dendritic cells: reduced IL-12 production on activation and impairment of naïve T-cell priming. Br. J. Cancer 92, 1450–1458 10.1038/sj.bjc.6602518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anton D, Dabadghao S, Palucka K, Holm G and Yi Q (1998) Generation of dendritic cells from peripheral blood adherent cells in medium with human serum. Scand. J. Immunol 47, 116–121 10.1046/j.1365-3083.1998.00284.x [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Franks HA, Lax SJ, El Refaee M, Malecka A, Shah S et al. (2013) The ataxia telangiectasia mutated kinase pathway regulates IL-23 expression by human dendritic cells. J. Immunol 190, 3246–3255 10.4049/jimmunol.1201484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price JG, Idoyaga J, Salmon H, Hogstad B, Bigarella CL, Ghaffari S et al. (2015) CDKN1A regulates Langerhans cell survival and promotes Treg cell generation upon exposure to ionizing irradiation. Nat. Immunol 16, 1060–1068 10.1038/ni.3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manda K, Glasow A, Paape D and Hildebrandt G (2012) Effects of ionizing radiation on the immune system with special emphasis on the interaction of dendritic and T cells. Front. Oncol 2, 102 10.3389/fonc.2012.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abuodeh Y, Venkat P and Kim S (2016) Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer 40, 25–37 10.1016/j.currproblcancer.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Escamilla J, Mok S, David J, Priceman S, West B et al. (2013) CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 73, 2782–2794 10.1158/0008-5472.CAN-12-3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC and Coussens LM (2015) TH2-polarized CD4+ T cells and macrophages limit efficacy of radiotherapy. Cancer Immunol. Res 3, 518–525 10.1158/2326-6066.CIR-14-0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn G-O, Tseng D, Liao C-H, Dorie MJ, Czechowicz A and Brown JM (2010) Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc. Natl Acad. Sci. U.S.A 107, 8363–8368 10.1073/pnas.0911378107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF et al. (2011) Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 1, 54–67 10.1158/2159-8274.CD-10-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J et al. (2014) CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 74, 5057–5069 10.1158/0008-5472.CAN-13-3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE et al. (2013) Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 73, 1128–1141 10.1158/0008-5472.CAN-12-2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baird JR, Monjazeb AM, Shah O, McGee H, Murphy WJ, Crittenden MR et al. (2017) Stimulating innate immunity to enhance radiation therapy–induced tumor control. Int. J. Radiat. Oncol. Biol. Phys 99, 362–373 10.1016/j.ijrobp.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He J, Yin Y, Luster TA, Watkins L and Thorpe PE (2009) Antiphosphatidylserine antibody combined with irradiation damages tumor blood vessels and induces tumor immunity in a rat model of glioblastoma. Clin. Cancer Res 15, 6871–6880 10.1158/1078-0432.CCR-09-1499 [DOI] [PubMed] [Google Scholar]
- 33.Huang X, Bennett M and Thorpe PE (2005) A monoclonal antibody that binds anionic phospholipids on tumor blood vessels enhances the antitumor effect of docetaxel on human breast tumors in mice. Cancer Res. 65, 4408–4416 10.1158/0008-5472.CAN-05-0031 [DOI] [PubMed] [Google Scholar]
- 34.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A and Nagata S (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417, 182–187 10.1038/417182a [DOI] [PubMed] [Google Scholar]
- 35.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y et al. (2004) Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304, 1147–1150 10.1126/science.1094359 [DOI] [PubMed] [Google Scholar]
- 36.Peng Y and Elkon KB (2011) Autoimmunity in MFG-E8–deficient mice is associated with altered trafficking and enhanced cross-presentation of apoptotic cell antigens. J. Clin. Invest 121, 2221–2241 10.1172/JCI43254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jinushi M, Sato M, Kanamoto A, Itoh A, Nagai S, Koyasu S et al. (2009) Milk fat globule epidermal growth factor–8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J. Exp. Med 206, 1317–1326 10.1084/jem.20082614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Y, Singh S, Georgescu M-M and Birge RB (2005) A role for Mer tyrosine kinase in αvβ5 integrin-mediated phagocytosis of apoptotic cells. J. Cell Sci 118, 539–553 10.1242/jcs.01632 [DOI] [PubMed] [Google Scholar]
- 39.Shao W-H, Zhen Y, Eisenberg RA and Cohen PL (2009) The Mer receptor tyrosine kinase is expressed on discrete macrophage subpopulations and mainly uses Gas6 as its ligand for uptake of apoptotic cells. Clin. Immunol 133, 138–144 10.1016/j.clim.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RAS et al. (2002) Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J. Exp. Med 196, 135–140 10.1084/jem.20012094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL et al. (2001) Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411, 207–211 10.1038/35075603 [DOI] [PubMed] [Google Scholar]
- 42.Crittenden MR, Baird J, Friedman D, Savage T, Uhde L, Alice A et al. (2016) Mertk on tumor macrophages is a therapeutic target to prevent tumor recurrence following radiation therapy. Oncotarget 7, 78653–78666 10.18632/oncotarget.11823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D et al. (2009) Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor κB. Proc. Natl Acad. Sci. U.S.A 106, 14978–14983 10.1073/pnas.0809784106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L et al. (2006) P50 nuclear factor-κB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 66, 11432–11440 10.1158/0008-5472.CAN-06-1867 [DOI] [PubMed] [Google Scholar]
- 45.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y et al. (2009) Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114, 589–595 10.1182/blood-2009-02-206870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gough MJ, Crittenden MR, Sarff M, Pang P, Seung SK, Vetto JT et al. (2010) Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J. Immunother 33, 798–809 10.1097/CJI.0b013e3181ee7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graham DK, DeRyckere D, Davies KD and Earp HS (2014) The TAM family: phosphatidylserine-sensing receptor tyrosine kinases gone awry in cancer. Nat. Rev. Cancer 14, 769–785 10.1038/nrc3847 [DOI] [PubMed] [Google Scholar]
- 48.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MBA and Lemke G (2007) TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131, 1124–1136 10.1016/j.cell.2007.10.034 [DOI] [PubMed] [Google Scholar]
- 49.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S et al. (2012) Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol 13, 1118–1128 10.1038/ni.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galvan MD, Foreman DB, Zeng E, Tan JC and Bohlson SS (2012) Complement component C1q regulates macrophage expression of Mer tyrosine kinase to promote clearance of apoptotic cells. J. Immunol 188, 3716–3723 10.4049/jimmunol.1102920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yi JH, Jang J, Cho J, Do I-G, Hong M, Kim ST et al. (2015) MerTK is a novel therapeutic target in gastric cancer. Oncotarget 8, 96656–96667 10.18632/oncotarget.3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tworkoski KA, Platt JT, Bacchiocchi A, Bosenberg M, Boggon TJ and Stern DF (2013) MERTK controls melanoma cell migration and survival and differentially regulates cell behavior relative to AXL. Pigment Cell Melanoma Res. 26, 527–541 10.1111/pcmr.12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Moncayo G, Morin P Jr, Xue G, Grzmil M, Lino MM et al. (2013) Mer receptor tyrosine kinase promotes invasion and survival in glioblastoma multiforme. Oncogene 32, 872–882 10.1038/onc.2012.104 [DOI] [PubMed] [Google Scholar]
- 54.Cook RS, Jacobsen KM, Wofford AM, DeRyckere D, Stanford J, Prieto AL et al. (2013) MerTK inhibition in tumor leukocytes decreases tumor growth and metastasis. J. Clin. Invest 123, 3231–3242 10.1172/JCI67655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlegel J, Sambade MJ, Sather S, Moschos SJ, Tan A-C, Winges A et al. (2013) MERTK receptor tyrosine kinase is a therapeutic target in melanoma. J. Clin. Invest 123, 2257–2267 10.1172/JCI67816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Christoph S, Deryckere D, Schlegel J, Frazer JK, Batchelor LA, Trakhimets AY et al. (2013) UNC569, a novel small-molecule mer inhibitor with efficacy against acute lymphoblastic leukemia In vitro and in vivo. Mol. Cancer Ther 12, 2367–2377 10.1158/1535-7163.MCT-13-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sufit A, Lee-Sherick AB, DeRyckere D, Rupji M, Dwivedi B, Varella-Garcia M et al. (2016) MERTK inhibition induces polyploidy and promotes cell death and cellular senescence in glioblastoma multiforme. PLoS ONE 11, e0165107 10.1371/journal.pone.0165107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu J, Frady LN, Bash RE, Cohen SM, Schorzman AN, Su Y-T et al. (2017) MerTK as a therapeutic target in glioblastoma. Neuro-oncology 10.1093/neuonc/nox111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cummings CT, Zhang W, Davies KD, Kirkpatrick GD, Zhang D, DeRyckere D et al. (2015) Small molecule inhibition of MERTK is efficacious in non-small cell lung cancer models independent of driver oncogene status. Mol. Cancer Ther 14, 2014–2022 10.1158/1535-7163.MCT-15-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cummings CT, Linger RMA, Cohen RA, Sather S, Kirkpatrick GD, Davies KD et al. (2014) Mer590, a novel monoclonal antibody targeting MER receptor tyrosine kinase, decreases colony formation and increases chemosensitivity in non-small cell lung cancer. Oncotarget 5, 10434–10445 10.18632/oncotarget.2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vouri M and Hafizi S (2017) TAM receptor tyrosine kinases in cancer drug resistance. Cancer Res. 77, 2775–2778 10.1158/0008-5472.CAN-16-2675 [DOI] [PubMed] [Google Scholar]
- 62.Aguilera TA, Rafat M, Castellini L, Shehade H, Kariolis MS, Hui AB-Y et al. (2016) Reprogramming the immunological microenvironment through radiation and targeting Axl. Nat. Commun 7, 13898 10.1038/ncomms13898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rivoltini L, Barracchini KC, Viggiano V, Kawakami Y, Smith A, Mixon A et al. (1995) Quantitative correlation between HLA class I allele expression and recognition of melanoma cells by antigen-specific cytotoxic T lymphocytes. Cancer Res. 55, 3149–3157 PMC:2248458 [PMC free article] [PubMed] [Google Scholar]
- 64.Feng Z, Bethmann D, Kappler M, Ballesteros-Merino C, Eckert A, Bell RB et al. (2017) Multiparametric immune profiling in HPV–oral squamous cell cancer. JCI Insight 2, e93652 10.1172/jci.insight.93652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brea EJ, Oh CY, Manchado E, Budhu S, Gejman RS, Mo G et al. (2016) Kinase regulation of human MHC class I molecule expression on cancer cells. Cancer Immunol. Res 4, 936–947 10.1158/2326-6066.CIR-16-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang W, DeRyckere D, Hunter D, Liu J, Stashko MA, Minson KA et al. (2014) UNC2025, a potent and orally bioavailable MER/FLT3 dual inhibitor. J. Med. Chem 57, 7031–7041 10.1021/jm500749d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E et al. (2000) Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 95, 3489–3497 PMID:10828034 [PubMed] [Google Scholar]
- 68.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D et al. (2009) The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med 206, 3115–3130 10.1084/jem.20091756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hafizi S and Dahlbäck B (2006) Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 273, 5231–5244 10.1111/j.1742-4658.2006.05529.x [DOI] [PubMed] [Google Scholar]
- 70.Mat MFC, Murad NAA, Ibrahim K, Mohd NM, Ngah WZW, Harun R et al. (2016) Silencing of PROS1 induces apoptosis and inhibits migration and invasion of glioblastoma multiforme cells. Int. J. Oncol 49, 2359–2366 10.3892/ijo.2016.3755 [DOI] [PubMed] [Google Scholar]
- 71.Hasanbasic I, Rajotte I and Blostein M (2005) The role of γ-carboxylation in the anti-apoptotic function of gas6. J. Thromb. Haemost 3, 2790–2797 10.1111/j.1538-7836.2005.01662.x [DOI] [PubMed] [Google Scholar]
- 72.Paolino M, Choidas A, Wallner S, Pranjic B, Uribesalgo I, Loeser S et al. (2014) The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature 507, 508–512 10.1038/nature12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kirane A, Ludwig KF, Sorrelle N, Haaland G, Sandal T, Ranaweera R et al. (2015) Warfarin blocks Gas6-mediated Axl activation required for pancreatic cancer epithelial plasticity and metastasis. Cancer Res. 75, 3699–3705 10.1158/0008-5472.CAN-14-2887-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tieken C and Versteeg HH (2016) Anticoagulants versus cancer. Thromb. Res 140, S148–S153 10.1016/S0049-3848(16)30114-1 [DOI] [PubMed] [Google Scholar]