Abstract
The use of DNA-based nanomaterials in biomedical applications is continuing to grow, yet more emphasis is being put on the need for guaranteed structural stability of DNA nanostructures in physiological conditions. Various methods have been developed to stabilize DNA origami against low concentrations of divalent cations and presence of nucleases. However, most strategies require a complete encapsulation of nanostructures, which makes accessing the encased DNA strands difficult, or chemical modification such as covalent crosslinking of DNA strands. We present a novel stabilization method by synthesizing DNA brick nanostructures with dendritic oligonucleotides attached on the outer surface. We find that nanostructures assembled from DNA brick motifs remain stable against denaturation without any chemical modifications. Additionally, densely coating the outer surface of DNA brick nanostructures with dendritic oligonucleotides prevents nuclease digestion.
Keywords: DNA nanotechnology, DNA bricks, Self-assembly, Nanostructures, Dendrimers
Graphical Abstract
DNA brick nanostructures of certain design parameters remain structurally stable in physiological conditions without any chemical modifications.
DNA nanotechnology enables synthesis of rationally designed DNA nanostructures of arbitrary geometric configuration,1 and such molecular-scale nanodevices can be used in biomedical applications, driving the field towards programmably customizable nanomedicine.2,3 Examples of DNA nano- cages,4,5 capsules,6 and carriers7 have been studied as delivery vehicles or diagnostic devices for drug delivery, cancer treatment, and immunotherapy.2,7 Nevertheless, more recent literature highlights the importance on the structural stability of DNA-based materials under physiological conditions when using them in vitro and/or in vivo.8–11 This is due to two main factors involved in degradation of DNA nanostructures when exposed to biological conditions: (i) denaturation caused by low divalent cation concentration (physiological salt concentration ranges ~ 0.04 – 0.8 mM MgCl2), and (ii) digestion caused by the presence of nucleases.8
Multiple strategies have been developed to chemically or physically stabilize the DNA nanostructures from falling apart in cellular media (e.g. 10% FBS),12–20 and in general, encapsulation of DNA nanostructures with different coating moieties prolongs the survival time the longest yet to date.12,14,17–19 For example, while most bare DNA origami falls apart easily in physiological condition, PEG-oligolysines,12 lipid molecules,17 or cationic polymers18,19 can become applied as coating material and extend the half-life of DNA origami up to the order of ~100.12 Such encasing strategy, however, often covers the entire outer surface of DNA origami and therefore in theory, makes it difficult to access the DNA strands post-coating. Ideally, one should be able to access the DNA strands and nanostructures for complementary lock-and-key mechanism without having to penetrate through a thick layer of overlaid material.
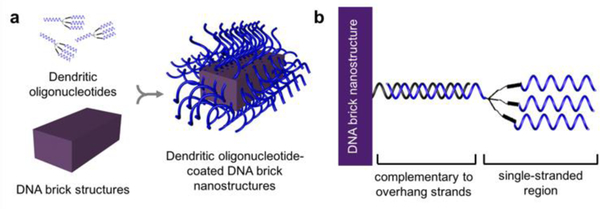
In this work, we aim to structurally stabilize DNA nanostructures with only oligonucleotide strands such that both biocompatible stability and DNA accessibility remain viable. And in doing so, we present two significant findings: (i) nanostructures consisted of certain DNA brick motifs21,22 remain structurally stable at low divalent salt concentrations (e.g. 1× PBS), and (ii) functionalizing the outer surface of DNA brick nanostructures with dendritic oligonucleotides prevents digestion of nanostructures from nucleases due to putative steric hindrance (Figure 1). As a result, our strategy suggests that neither chemical protectants or covalent base-pair interlocking is necessary to enhance the stability of DNA brick nanostructures.
Figure 1.
DNA nanostructures are constructed by (a) attaching dendritic oligonucleotides to the outer surface of DNA brick nanostructures via (b) hybridization to the complementary, protruding overhang strands.
Recently, the Yin lab developed a strategy to assemble DNA nanostructures of different sizes and shapes using short, synthetic oligonucleotide strands.21,22 Such assembly of DNA bricks, which consist of four short binding domains arranged so that the bricks can interlock, does not require a scaffold. And while the first generation of bricks were 32 nucleotides (nt) long, consisting of four 8 nt binding domains,21 more recent developments investigated brick strands with longer binding domains (52 nt bricks with four 13 nt domains; 74 nt bricks with two 18 nt and two 19 nt domains).22
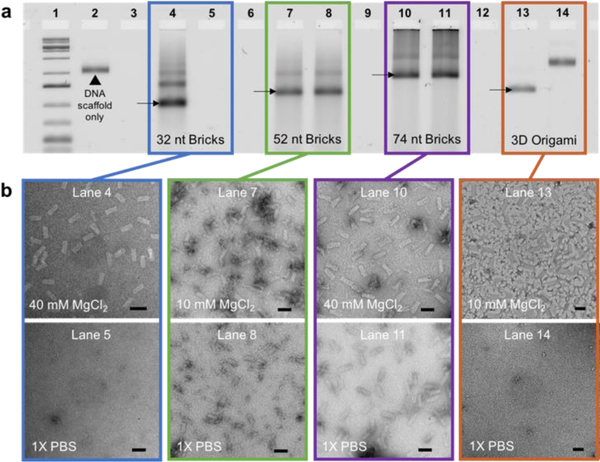
We discovered that DNA brick nanostructures consisted of strands with 13 or longer binding domains remain stable even when they are placed in 1× PBS with no divalent salt. First, the stability of DNA brick nanostructures was tested by removing cations from solution. Three separate sets, each consisted of approximately two hundred strands of 32-, 52-, or 74-nt bricks, were assembled at 10 mM or 40 mM MgCl2 (note that assembly salt conditions were chosen based on previous literature22; see SI for a detailed protocol). All sets of samples were divided into two sub-sets for comparison: (i) maintaining the overall MgCl2 concentration at 10 mM or 40 mM as control, and (ii) replacing the buffer with 1× PBS via incubating the assembled structures in 1× PBS at 37 °C for 1 hour, then using filtration to remove any excess divalent salt in solution. To note, 3D DNA origami with binding domain length of 8 nt was included for comparison (see SI for design and assembly conditions). Gel electrophoresis results show dissociation of structures in 1× PBS for 32-nt brick nanostructures and 3D DNA origami (lanes 5 and 14, respectively, in Figure 2a), while 52- and 74-nt brick nanostructures remained stable, implying the significance in binding domain length upon designing DNA nanostructures (lanes 8 and 11, respectively, in Figure 2a). This experiment was repeated with a longer incubation time (24 hours; Figure S2) and differently sized brick nanostructures (Figure S3), and results indicate that all 52 nt brick nanostructures remain stable regardless of their overall size. TEM data also verify the structural stability of DNA brick nanostructures in 1× PBS (Figure 1b), which remain structurally stable even after 4 days (Figure S4). Though it is expected that longer complementary binding lengths lead to stronger interlocking between base-pairs, all results emphasize the importance of binding domain length as a contributing factor in stabilizing DNA nanostructures. This finding also helps explain why most 3-D DNA origami structures with average binding domain lengths of 7–10 base-pairs degrade when the global divalent salt concentration is low (0 – 0.8 mM MgCl2).12–20
Figure 2.
Structural stability test against low salt denaturation. (a) 32-, 52-, and 74-nt brick nanostructures and 3D origami were assembled at either 10 or 40 mM MgCl2 (lanes 4, 7, 10, 13). Assembled samples were incubated at 37 °C in 1× PBS for 1 hour, then spin-filtered to completely remove any remaining salt in solution (lanes 5, 8, 11, 14). All samples were characterized via agarose gel electrophoresis. (b) TEM images were taken to confirm the preservation of structures. Scale bars indicate 50 nm in length.
DNA brick nanostructures that remain stable even in 1× PBS were still susceptible to nucleases. After confirming that DNA brick nanostructures were stable against low salt denaturation without any additional stabilization techniques, the same 52 nt brick nanostructures were tested against nuclease digestion (Figure S5). We followed a DNase I titration assay used in previous literature,8 and brick nanostructures were incubated with varying concentrations of DNase I at 37 °C for 1 hour, then characterized via gel electrophoresis. A decrease in band intensity as well as a slight shift in band position were found (Figures 3 and S5), suggesting that 52-nt bricks fall apart at a DNase I concentration above ~ 5 U/mL. Such nuclease resistance of DNA brick nanostructures is not high enough when compared to that of chemically stabilized or encapsulated DNA origami known in literature,12,15 and therefore additional method is required for stabilizing brick nanostructures against nuclease digestion.
Figure 3.
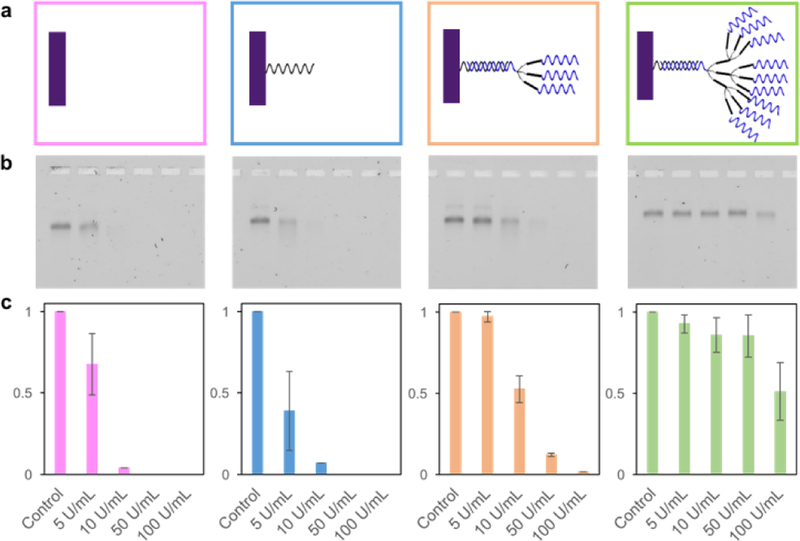
Structural stability test against nuclease digestion. (a) DNA brick nanostructures with four different surface densities were studied – bare, single, triple, and nonuple. All assembled DNA brick nanostructures were incubated at 37 °C for 1 hour with varying DNase I concentrations (0 to 100 U/mL). (b) Incubated samples were characterized via gel electrophoresis. (c) Analysis was performed on three separate gel results, calculating the average, normalized, band intensities and standard deviations.
We thenceforth envisioned that densely functionalizing the outer surface of DNA brick nanostructures with additional oligonucleotide strands would stabilize them against enzymes. This was mainly inspired by spherical nucleic acids (SNAs)23 which are formed by organizing nucleic acids radially around a nanoparticle core. SNAs can enter cells without transfection reagents, and once inside the cell, the nucleic acid components of SNAs resist nuclease degradation, leading to longer intracellular lifetimes.23 Because bare DNA brick nanostructures alone were not stable against nucleases, we hypothesized that introducing surrounding oligonucleotides in high density would prevent enzyme accessibility and therefore stabilize against digestion.
Because only a limited number of overhang strands can be designed into a given DNA brick nanostructure, the surface density could be increased by attaching dendritic oligonucleotides. To do this, DNA brick nanostructures were carefully designed to have ~ 100 protruding overhang strands at which dendritic oligonucleotides can become attached on 52 nt DNA brick nanostructures (Figure 1b). Dendritic oligonucleotides were synthesized by incorporating a trebler phosphoramidite, a branching reagent that can be incorporated in regular DNA synthesis protocol. Integrating one trebler modifier enables each single stranded DNA to branch into three separate single stranded arms (3×), while including two repeated trebler moieties enables the dendritic oligonucleotide to have nine single stranded arms (9×). 3× or 9× dendrimers can hybridize to the overhang strands to systematically increase the total number of available single-stranded oligonucleotides on the outermost surface.
DNA brick nanostructures were tested against nuclease digestion at four different surface oligonucleotide densities – (i) bare, with no protruding strands from the brick nanostructures, (ii) single, with approximately 100 protruding DNA, (iii) triple, via attachment of 3× dendrimers, and (iv) nonuple, via attaching 9× dendrimers (Figure 3a). All samples were exposed to different concentrations of DNase I, ranging from 0 to 100 U/mL. These concentrations were chosen based on previous literature,12 at which a DNase I titration assay was used to characterize nuclease resistance. Gel analysis shows that DNA brick nanostructures have an increase in tolerance against DNase I at higher oligonucleotide density on the outer surface. Some samples show formation of multimeric (e.g. dimer) structures, however, this phenomenon is found in most synthesized brick structures (including bare ones), and therefore we do not believe the multimeric structures themselves dramatically increase the overall structural stability. Quantitative analysis was performed to calculate the number of oligonucleotides available on the outer surface via fluorescence measurements (Figures S7, S8). And the estimated numbers of available DNA strands, relatively close to the expected numbers with some error, indirectly validates the accessibility of DNA sequences at the outer surface of brick nanostructures.
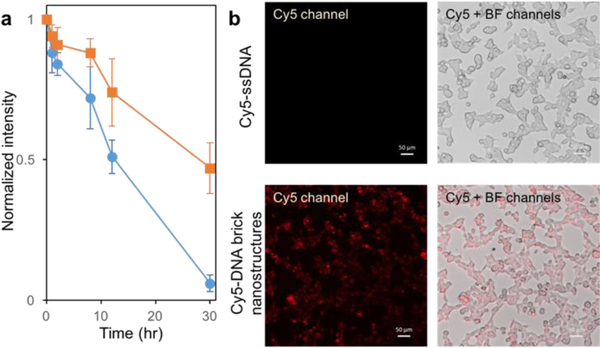
Lastly, in vitro studies were carried out on DNA brick nanostructures incubated in 10% FBS cellular media for different lengths of times. To compare the effect of dendritic brushes, bare and nonuple brick nanostructures were tested in 10% FBS (Figure S9). Results reveal a shorter survival time for bare brick nanostructures while nonuple brick nanostructures survived up to 30 hours without significant degradation (Figures 4a and S10). Cellular uptake studies were also performed by incubating nonuple DNA brick nanostructures in HEK293T cells. Nonuple DNA brick nanostructures were fluorescently labeled via hybridizing complementary Cy5-ssDNA to the single-stranded region of the dendritic oligonucleotides. In situ imaging results show a clear difference in fluorescence between Cy5-ssDNA only versus Cy5-DNA brick nanostructures (Figure 4b), as well as successful uptake of brick nanostructures inside the cells.
Figure 4.
Testing stability in cellular media (10% FBS) and conducting cellular uptake. (a) Bare (marked in blue circles) and nonuple (marked in orange squares) DNA brick nanostructures were assembled, then incubated in 10% FBS at 37 °C for varying lengths of time (0, 1, 2, 8, 12, 30 hours). Normalized band intensities, from three separately conducted gel electrophoresis experiments, were averaged and plotted against time. (b) Nonuple DNA brick nanostructures (200 nM) were incubated with complementary Cy5-ssDNA (2 μM) to fluorescently label the structures. HEK293T cells were incubated with 200 nM fluorescently labeled nonuple DNA brick nanostructures, and as control, a separate set of HEK293T cells were incubated with 2 μM Cy5-ssDNA. Both sets were characterized via an inverted fluorescence microscope using the Cy5 and bright-field channels.
In conclusion, (i) DNA brick nanostructures with binding domain lengths 13-nt or longer are stable against denaturation in low divalent salt concentration, and (ii) attaching dendritic oligonucleotides at the outer surface of DNA brick nanostructures stabilizes them against nuclease digestion. Such dendritic-oligonucleotide-coated DNA brick nanostructures do not require chemical base-pair interlocking techniques or encapsulation coating methods yet still display structural stability in cellular media as well as accessibility of DNA sequences at the surface. As a result, brick nanostructures offer as a promising alternative to DNA origami, especially while the fundamental question remains unanswered whether viral DNA scaffold is safe to be used in the context of drug delivery. Furthermore, unlike spherical nucleic acids in which DNA strands are coated in an isotropic manner, these brick nanostructures can become asymmetrically functionalized with precise location control, which will have important implications as research efforts shift to the use of multi-functionalized nanomaterials.24,25
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (1DP1GM133052), the Office of Naval Research (N00014-18-1-2549), the National Science Foundation (1540214), and the Wyss Institute’s Immune Materials Platform. Y. K. thanks Dr. Ayush Verma, Prof. William M. Shih, Dr. Zhao Zhao, Dr. Frances Anastassacos for helpful discussions, and Dr. Nikhil Gopalkrishnan for insight in DNA origami.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].Seeman NC. Annu. Rev. Biochem 2010, 79, 65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hu Q, Li H, Wang L, Gu H, Fan C, Chem. Rev 2019, 119, 10, 6459–6506. [DOI] [PubMed] [Google Scholar]
- [3].Seeman NC, Slieman HF, Nat. Rev. Matter 2018, 3, 17068. [Google Scholar]
- [4].Douglas SM, Bachelet I, Church GM, Science 2012, 335, 831–834. [DOI] [PubMed] [Google Scholar]
- [5].Li S, Jiang Q, Liu S, Zhang Y, Tian Y, Song C, Wang J, Zou Y, Anderson GJ, Han J-Y, ang, Liu Y, Zhang C, Chen L, Zhou G, Nie G, Yan H, Ding B, Zhao Y, Nat. Biotechnol 2018, 36, 258–264. [DOI] [PubMed] [Google Scholar]
- [6].Ijas H, Hakaste I, Shen B, Kostiainen MA, Linko V, ACS Nano 2019, 13, 5, 5959–5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Madhanagopal BR, Zhang S, Demirel E, Wady H, Chandrasekaran AR, Trends Biochem. Sci 2018, 43, 12, 997–1013. [DOI] [PubMed] [Google Scholar]
- [8].Hahn J, Wickham SFJ, Shih WM, Perrault SD, ACS Nano 2014, 8, 8765–8775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ramakrishnan S, Ijas H, Linko V, Keller A, Comput. Struct. Biotechnol. J 2018, 16, 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kielar C, Xin Y, Shen B, Kostiainen MA, Grundmeier G, Linko V, Keller A, Angew. Chem. Int. Ed 2018, 57, 9470–9474. [DOI] [PubMed] [Google Scholar]
- [11].Bila H, Kurisinkal EE, Bastings MMC, Biomat. Sci 2019, 7, 532–541. [DOI] [PubMed] [Google Scholar]
- [12].Ponnuswamy N, Bastings MMC, Nathwani B, Ryu J, Chou LYT, Vinther M, Li WA, Anastassacos FM, Mooney DJ, Shih WM, Nat. Comm 2017, 8, 15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ahmadi Y, Llano ED, Barisic I, Nanoscale 2018, 10, 7494–7504. [DOI] [PubMed] [Google Scholar]
- [14].Perrault SD, Shih WM, ACS Nano 2014, 8, 5132–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bastings MMC, Anastassacos FM, Ponnuswamy N, Leifer FG, Cuneo G, Lin C, Shih WM, Nano Lett 2018, 18, 3557–3564. [DOI] [PubMed] [Google Scholar]
- [16].Gerling T, Kube M, Kick B, Dietz H, Sci. Adv 2018, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mikkila J, Eskelinen A-P, Niemela EH, Linko V, Frilander MJ, Torma P, Nano Lett 2014, 14, 2196–2200. [DOI] [PubMed] [Google Scholar]
- [18].Auvinen H, Zhang H, Kopilow A, Niemela EH, Nummelin S, Adv. Healthcare Mater 2017, 6. [DOI] [PubMed] [Google Scholar]
- [19].Agarwal NP, Matthies M, Gur FN, Osada K, Schmidt TL, Angew. Chem. Int. Ed 2017, 56, 5460–5464. [DOI] [PubMed] [Google Scholar]
- [20].Cassinelli V, Oberleitner B, Sobotta J, Nickels P, Grossi G, Kempter S, Angew. Chem. Int. Ed 2015, 54, 7795–7798. [DOI] [PubMed] [Google Scholar]
- [21].Ke Y, Ong LL, Shih WM, Yin P, Science 2012, 338, 1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ong LL, Hanikel N, Yaghi OK, Grun C, Strauss MT, Bron P, Lai-Kee-Him J, Schueder F, Wang B, Wang P, Kishi JY, Myhryold CA, Zhu A, Jungmann R, Bellot G, Ke Y, Yin P, Nature 2017, 552, 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cutler JI, Auyeung E, Mirkin CA, J. Am. Chem. Soc 2012, 134, 3, 1376–1391. [DOI] [PubMed] [Google Scholar]
- [24].Chang M, Yang C-S, Huang D-M, ACS Nano 2011, 5, 6156–61163. [DOI] [PubMed] [Google Scholar]
- [25].Wang S, Qin L, Yamankurt G, Skakuj K, Huang Z, Chen P, Dominguez D, Lee A, Zhang B, Mirkin CA, Proc. Natl. Acad. Sci 2019, 116, 21, 10473–10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.