Abstract
Objective:
Fibromyalgia (FM) is characterized by pain and fatigue, particularly during physical activity. Transcutaneous electrical nerve stimulation (TENS) activates endogenous pain inhibitory mechanisms. We evaluated if using TENS during activity would improve movement-evoked pain and other patient-reported outcomes in women with FM.
Methods:
Participants were randomly assigned to receive active-TENS (n=103), placebo-TENS (n=99) or no-TENS (n=99) and instructed to use it at home 2h/day during activity for 4-weeks. TENS was applied to the lumbar and cervicothoracic regions using a modulated frequency (2–125Hz) at the highest tolerable intensity. Participants rated movement-evoked pain (primary outcome) and fatigue on an 11-point scale before and during application of TENS. Primary and secondary patient-reported outcomes were assessed at randomization and 4weeks.
Results:
After 4-weeks, the active-TENS group reported a greater reduction in movement-evoked pain and fatigue than placebo-TENS (Pain, Group mean difference(95% CI): −1.0(−1.8, −0.2), p=0.008; Fatigue: −1.4(−2.4, −0.4), p=0.001) and no-TENS groups (Pain: −1.8(−2.6. −1.0), p<0.0001; Fatigue: −1.9(−2.9, −0.9), p=<0.0001). A greater percentage of the active-TENS group reported improvement on the global impression of change when compared to placebo-TENS (70% vs. 31%, p<0.0001) and no-TENS (9%, p<0.0001). There were no TENS-related serious adverse events and less than 5% of participants experienced minor adverse events from TENS.
Conclusion:
Among women with FM and stable medication, 4-weeks of active-TENS use compared with placebo-TENS or no-TENS resulted in a significant improvement in movement-evoked pain and other clinical outcomes. Further research is needed to examine effectiveness in a real world, pragmatic setting to establish clinical importance of these findings.
Clinical Trial Registration Number:
Keywords: Movement Pain, TENS, Fibromyalgia
Introduction
Fibromyalgia (FM) is a complex condition characterized by widespread pain and fatigue. Pharmacological interventions are modestly effective for FM with most individuals experiencing activity-limiting pain despite use of multiple drugs (1,2). It has become increasingly recognized that non-pharmacological interventions should be considered first-line treatments for chronic pain (3–5) and as safe, low cost treatments that can be added to pharmacological approaches. While there is strong evidence that exercise is an effective treatment for FM (6,7), individuals report movement-evoked pain limits activity-participation (8,9). Use of non-pharmacological approaches that reduce movement-evoked pain would theoretically increase activity-participation resulting in a perceived global improvement.
Transcutaneous electrical nerve stimulation (TENS) is a non-pharmacological intervention that delivers electrical current through the skin for pain control. Animal studies show that TENS activates endogenous inhibitory mechanisms to reduce central excitability (10–14). In contrast, individuals with FM show reduced endogenous inhibition and enhanced central excitability (15,16). Thus, based on the mechanism of action of TENS, it may be useful in individuals with FM.
Although TENS is effective for several pain conditions, recent systematic reviews show mixed results (17–20). Johnson and colleagues note limitations of the existing literature for TENS including inadequate sample size, limited outcome data, and moderate risk of bias (17, 21). We further suggest variables not considered in TENS clinical trials also lead to equivocal results (22). Strong, but comfortable stimulation intensity or greater is critical for TENS effectiveness (14,23,24). TENS works best for movement-evoked pain (24,25) and provides the greatest effects when the unit is turned on (22,26), yet prior studies routinely measure resting pain or assess pain after treatment when physiological effects of TENS are no longer optimal (25,27–30). Furthermore, few studies examine effects on other domains such as fatigue, quality of life, or function.
We designed a double-blinded, randomized, controlled trial to examine effects of TENS in women with FM addressing weaknesses of prior studies. The primary aim tested effectiveness of repeated TENS on movement-evoked pain in women with FM after random assignment to three groups: active-TENS, placebo-TENS, or no-TENS. Secondary aims tested effects of TENS on fatigue, function and other patient-reported outcomes. We hypothesized that TENS would reduce movement-evoked pain resulting in perceived global improvement in women with FM.
Methods
The Fibromyalgia Activity Study with TENS (FAST) is a phase II randomized, double-blind, placebo controlled dual-site clinical trial conducted at The University of Iowa and Vanderbilt University Medical Center that was approved by institutional review boards of both universities; a study protocol is published (31) and registered ( NCT01888640). The research was carried out in compliance with the Helsinki Declaration.
Design and Study Participants
We examined effects of home use of TENS in women with FM with 4-weeks of a randomized, placebo-controlled period followed by 4-weeks where all subjects received active-TENS (see Figure 1A). Participants were recruited from two sites using a variety of strategies (for details see Supplementary Methods). Verification of eligibility was completed at both Visit-1 and Visit-2. Inclusion criteria: women between 18–70 years; met 1990 American College of Rheumatology FM criteria; stable medication for last 4-weeks and projected stable treatment for next 2-months. Exclusion criteria: pain <4/10 on a numerical rating scale (NRS) at both Visit-1 and Visit-2; inability to walk 6 minutes without assistance; TENS use in last 5 years; pacemaker; neuropathic or autoimmune disorder; spinal fusion; metal implants in spine; allergy to adhesive or nickel; pregnancy; epilepsy; serious or unstable medical or psychiatric condition that would preclude participation (31). Prior to enrolling, participants provided written informed consent. All participants continued current treatments prescribed by their healthcare provider and were asked not to change medications during the study. Current medications were recorded at each visit. Analgesic use before Visit 2 was not different between groups (Supplementary Table 1).
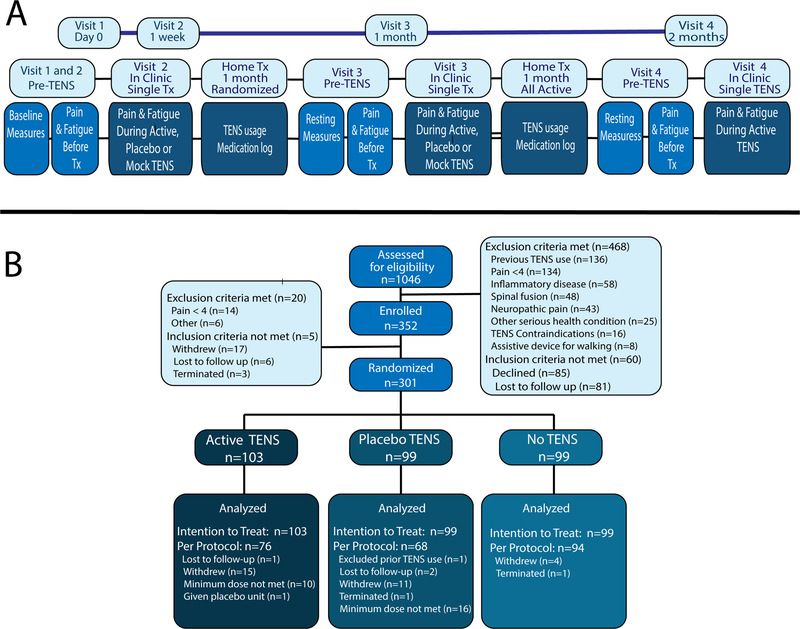
Figure 1. Study Design and CONSORT.
A. Diagram of study design for all four Visits. On Visit-1 participants were screened for pain and the 1990 FM American College of Rheumatology criteria. On Visit-2, subjects were re-screened for pain and randomized. Baseline questionnaires were assigned, and subjects were assessed for pain and fatigue at rest and during functional tasks. TENS was applied during Visit-2 and remained on for 30-minutes prior to re-assessment of pain, fatigue and function. Participants were given the TENS unit for home and returned 4-weeks later for Visit-3. Visit-3 followed the same protocol as Visit-2. After Visit-3, all subjects received Active-TENS for 4-weeks and were reassessed with the same protocol as Visits-2 and 3. All assessments were the same across treatment arms. B. CONSORT diagram. We assessed 1046 participants for eligibility with 468 excluded prior to enrollment. The main reasons for exclusion were previous TENS use and pain <4. After enrollment on Visit-1 5 subjects did not meet the FM criteria (n=5) and on Visit-2 1-week later 14 subjects were excluded for pain <4. After enrollment on Visit-1, 17 subjects withdrew due to personal reasons. Thus, 301 participants were randomized to active-TENS (n=103), placebo-TENS (n=99), or no-TENS (n=99).
Outcome Measures
The primary outcome was movement-evoked pain and secondary outcomes were resting pain, fatigue, function, disease impact, quality of life, fear of movement, and other psychological factors. These measures are described briefly below with more detail in the published protocol (31). Effects of TENS on pain and fatigue were examined before and during TENS treatment on Visits-2, −3 and −4. Patient-reported outcomes were examined before TENS treatment at these same visits.
Pain intensity at rest and during movement was measured with an 11-point NRS before and during TENS. Movement-evoked pain was measured during the Six-Minute-Walk-Test (6MWT) and Five-Time-Sit-to-Stand test (5TSTS). Pain intensity and interference was measured using the Brief Pain Inventory (BPI). Fatigue at rest and during movement were measured with an 11-point NRS before and during the 6MWT and 5TSTS, and with the Multidimensional Assessment of Fatigue (MAF). Physical Function was assessed using the 6MWT which measures the distance a person can walk in 6 min, 5TSTS which measures the time it takes to move from sitting to standing 5-times, physical activity for 1-week with accelerometry (Supplementary Methods), and the International Physical Activity Questionnaire short-form (IPAQ-sf).
Patient-reported outcomes.
We examined fear of movement with the Tampa Scale of Kinesiophobia (TSK), pain catastrophizing with the Pain Catastrophizing Scale (PCS), self-efficacy with the Pain Self-Efficacy Questionnaire (PSEQ), depression and anxiety with PROMIS short-forms. Disease impact was measured with the Fibromyalgia Impact Questionnaire-Revised (FIQR) and quality of life was assessed with the Short-Form-36 (SF-36). Rescue pain medication was examined from home logs for opioid and non-opioid analgesic use 1-week before Visit-2, −3, and −4. For of the 301 subjects enrolled, we had completed logs for 227 with opioid and non-opioid pain medications: 76 active-TENS group, 70 placebo-TENS group, 81 no-TENS group. Perceived improvement was examined with the Global Impression of Change (GIC) using a 7-point scale.
Randomization, Allocation, and Blinding
Participants were randomly assigned to active-TENS, placebo-TENS, or no-TENS using permuted blocks of size 6 and 9, stratified by site and opioid status (PROC PLAN, SAS/STAT v13.1). Participants were classified as opioid users if they had taken an opioid at least 5 of 7 days per week for the last 30 days. The randomization schedule was password protected with access granted only to those unblinded to the intervention, the statistician who generated the randomization schedule and the TENS-Allocator who provided the TENS intervention. Neither the statistician nor the TENS-Allocator had any role in patient recruitment, scheduling, or assessment of outcomes.
Assessments were performed by a separate person (Outcome-Assessor) than the TENS-Allocator. Participants were blinded to treatment group (active-TENS, placebo-TENS), and Outcome-Assessors were blinded to all three groups. TENS-Allocators were responsible for accessing the randomization schedule to assign subjects to groups, were not blinded to treatment, and were responsible for contact with subjects between Visit-2 and Visit-3 (blinded phase). The no-TENS group used a mock-TENS during Visit-2 and Visit-3 to blind Outcome-Assessors with electrodes that were attached to a TENS unit that provided no current intensity. For all groups a concealment pouch was used to maintain blinding of the Outcome-Assessor from viewing the TENS unit, and they were asked not to discuss treatment with Outcome-Assessors. A standardized script for each treatment group and specific to each visit was utilized so that all participants received the same instructions. The standardized script was kept identical, except for 1 line in the script, between the active-TENS and placebo-TENS group, to reduce bias (Supplementary Methods). Blinding of Outcome-Assessors was assessed after Visit-3 by asking if the participant received active-TENS, placebo-TENS, no-TENS, or didn’t know; and blinding of participants were determined by asking if they received active-TENS, placebo-TENS, or didn’t know. See supplemental methods for additional details on integrity of blinding.
TENS Intervention
The EMPI-Select TENS (DJO Global, Vista, CA) delivered both active-TENS and placebo-TENS interventions through butterfly electrodes placed at the cervicothoracic junction and lower back (Supplementary Figure 1). Active-TENS parameters were asymmetrical, biphasic waveform with a modulating frequency (2–125 Hz), pulse duration 200μsec, and highest tolerable stimulation intensity. During Visit-2, Visit-3 and Visit-4, TENS was applied by the TENS-Allocator in the clinic for 30 min prior to the Outcome-Assessor measuring effects on pain, fatigue, and function. The placebo-TENS unit delivered current for 45s ramping down to 0 in the last 15s and the appearance was identical to the active unit (32).
Following completion of Visit-2, active-TENS or placebo-TENS was sent home with participants with an instruction manual developed by study personnel. TENS-Allocators used a standardized script to instruct participants in home use and for weekly contact. Participants were instructed to use TENS at least 2h per day during activity. Both active-TENS and placebo-TENS units monitored number of sessions, number of minutes used, and average intensity per channel. All participants received active TENS between Visit-3 and Visit-4, the unblinded phase, with identical instructions.
Statistical Analysis
Sample size was determined using data from our pilot study using a single active-TENS treatment compared to placebo-TENS and no-TENS where the maximum SD (standard deviation) for movement-evoked pain was 1.96 (25). Therefore, assuming a SD of 2.0, a p<0.05 with 0.80 power, a correlation of r=0.5 between pain measurements from the same subject, and a sample size of 88/group, linear mixed model analysis for repeated measures with 3 time points (Visit-2 pre-TENS, Visit-3 pre-TENS, and Visit-3 post-TENS) would be able to detect a clinically meaningful mean difference of at least 1.5 (equivalent to a 30% improvement in pain for this sample that had an average baseline pain of 5 on a 0–10 NRS), which corresponds to an 0.75 effect size. A 30% improvement in pain is considered clinically significant (33).
Both intention-to-treat (ITT) and per-protocol (PP) analysis assessed treatment effect. For PP analysis, minimal effective treatment was defined as an average of 30 min/day and a minimum of 8 sessions over 4-weeks. Primary and secondary outcome variables, except for rescue medication, were compared among groups using linear mixed models for repeated measures controlling for site, as there were significant differences between sites at baseline (Supplementary Table 2)(33). For the outcome variables of movement evoked pain, resting pain, fatigue, and function during the randomized portion of the trial, the time variable comprised of 4 time points, Visit-2 pre-TENS, Visit-2 during-TENS; Visit-3 pre-TENS, Visit-3 during-TENS. In fitting the linear mixed model, the Akaike Information Criteria (AIC) and Schwarz’s Bayesian Information Criteria (BIC) was used to select the covariance structure that best fit these longitudinal measures within subject. The covariance types that were considered included compound symmetry (CS), heterogeneous CS, first order autoregressive (AR1), and unstructured. From these model parameters estimates and the fitted covariance structure, tests of mean contrast were performed to assess the effect of TENS, compared to placebo, and control on the primary outcome measures. These tests include: (1) test for immediate effect of TENS, during-TENS vs. Pre-TENS at Visit-2, and at Visit-3, within each treatment group; (2) test for long-term effect of TENS, Visit-3 during TENS vs. Visit-2 pre TENS and Visit-3 pre-TENS vs. Visit-2 pre-TENS, within each treatment group; and (3) comparison of long-term effect of TENS (i.e. Visit-3 during-TENS – Visit-2 pre-TENS; and Visit-3 pre-TENS – Visit-2 pre-TENS compared between treatment groups). TENS produces its analgesic effect by releasing inhibitory neurotransmitters – endogenous opioids, serotonin, GABA – and thus, has it maximal effects when the unit is on (14). We thus, tested the primary outcome when the TENS unit was active after 4-weeks of home use (Visit-3 during TENS) and compared to Visit-2 before TENS use (Visit-2 pre-TENS). To account for the number of tests performed within each of the 3 sets of tests, p-values have been adjusted using Bonferroni’s method (i.e. each set adjusted for 6 tests). Similar analysis was done, using linear mixed model for repeated measures, for the other secondary variables that were measured at 2 time points (Visit-2 and Visit-3), with the following tests performed (1) test for change (Visit-3 vs. Visit-2), within each treatment group (Bonferroni adjusted for 3 tests); and (2) comparison of visit 3-visit 2 change between treatment groups (Bonferroni adjusted for 3 tests). Since FIQR and marital status differed at baseline between groups, treatment effect on outcome measures was also tested with FIQR and marital status as a covariate in the model. Estimates of mean change or difference between groups with 95% were computed. Rescue pain medication, opioid and non-opioid analgesics, were analyzed using Cochran-Mantel-Haenszel static controlling for study site and opioid use. Rescue pain medication (opioid and non-opioid analgesics) was calculated as morphine equivalents for opioids, and as the number of pills for non-opioid analgesics. The groups were subdivided into those who reported rescue pain medication use at Visit-2 and those who did not use pain medication. We then examined the change in rescue medication between Visit-2 and Visit-3 to classify study participants into either: 1) those who decreased the amount of recue medication or remained non-users, or 2) those who increased or kept the same amount of usage. The percentage of subjects in these two categories were compared among the treatment groups using Cochran-Mantel-Haenszel statistic controlling for site and rescue pain medication use at Visit-2.
The handling of missing data in Linear Mixed Model analysis assumes that data is missing at random (MAR). However, a subsequent analysis examined sensitivity of the findings if data were missing not at random (MNAR) using a multiple imputation approach was performed using a control-based pattern imputation and delta-adjustment imputation (see Supplementary Methods and Supplementary Table 2).
Results
Participants
Between September 2013 through February 2018, 352 participants were enrolled, and 301 were randomly assigned to one of three groups: active-TENS (n=103); placebo-TENS (n=99), or no-TENS (n=99) and comprised ITT analysis (Figure 1B). The majority of participants enrolled but not randomized failed to meet the pain severity threshold required for inclusion at Visit-2. Of these, 238 participants were included in the PP analysis (active-TENS, n=77; placebo-TENS, n=68; no-TENS, n=94). Participant demographics and baseline characteristics prior to randomization at Visit-2 were similar between all three groups except for marital status and FIQR (Table 1, Supplementary Results). Subjects used active-TENS for a median time of 77.1 (IQR: 51.4–109.7) min/day and placebo-TENS for 72 (IQR: 39.4–104.6) min/day. The active group average intensity was 38.8 (SD: 7.98) for lumbar and 38.7 (SD: 7.2) for cervical locations.
Table 1:
Demographic and Baseline Characteristics of Study Participants.
| Active -TENS n=103 |
Placebo-TENS n=99 |
No-TENS n=99 |
p-value | |
|---|---|---|---|---|
| Demographic Variables* | ||||
| Age, mean (SD) | 44.7 (14.3) | 47.2 (12.6) | 48.6 (11.8) | 0.10 |
| Race, White | 92% | 92% | 92% | 0.99 |
| Ethnicity, Not Hispanic | 95% | 95% | 95% | 0.99 |
| Married / Living with partner | 33% | 51% | 52% | 0.010 |
| Less than college graduate | 61% | 61% | 64% | 0.48 |
| Working | 55% | 45% | 58% | 0.42 |
| Health Variables | ||||
| Never smoked | 82% | 80% | 70% | 0.16 |
| Body mass index (kg/m2) | 34.8 (8.7) | 33.7 (8.8) | 34.0 (8.9) | 0.65 |
| Duration of fibromyalgia, years | 7 (3–12) | 7 (2–14) | 7 (4–15) | 0.466 |
| Opioids for pain^ | 27 (26%) | 26 (26%) | 26 (26%) | -- |
| Baseline Measures | ||||
| Pain with movement (6MWT, 0–10)-primary outcome | 6.5 (1.9) | 6.2 (1.9) | 6.4 (1.9) | 0.50 |
| Pain with movement (5STS, 0–10) – primary outcome | 5.8 (2.4) | 5.5 (2.2) | 5.6 (2.2) | 0.63 |
| Pain at rest (NRS) (0–10) | 6.2 (1.5) | 5.9 (1.4) | 6.1 (1.6) | 0.33 |
| Fatigue at rest (NRS) (0–10) | 6.8 (2.0) | 6.1 (1.8) | 6.4 (2.0) | 0.075 |
| FIQR-pain (0–10) | 6.7 (1.8) | 6.0 (1.6) | 6.15 (1.8) | 0.020 |
| Disease Impact (FIQR, 0–100) | 59.2 (16.8) | 53.7 (15.9) | 55.6 (16.0) | 0.049 |
| Mental Quality of Life (SF-36 MCS, T-score) | 38.7 (10.0) | 40.2 (10.2) | 39.5 (10.6) | 0.57 |
| Physical Quality of Life (SF-36 PCS, T-score) | 32.7 (6.4) | 33.3 (6.2) | 32.7 (6.6) | 0.72 |
| Pain Catastrophizing (PCS, 0–52) | 23.1 (13.0) | 20.4 (12.5) | 20.8 (12.1) | 0.26 |
| Self-efficacy (PSEQ, 0–60) | 28.2 (13.3) | 29.9 (13.1) | 29.0 (13.2) | 0.67 |
| Fear of Movement (TSK, 17–68) | 36.5 (7.7) | 37.1 (8.0) | 37.4 (8.3) | 0.68 |
| PROMIS-Anxiety (T-score) | 58.8 (8.7) | 58.1 (8.0) | 58.3 (7.8) | 0.82 |
| PROMIS-Depression (T-score) | 58.1 (8.1) | 55.7 (8.5) | 56.6 (8.1) | 0.12 |
| Function (6MWT, ft) | 1386 (323) | 1358 (305) | 1316 (318) | 0.29 |
| Function (5TSTS, #TSTS/10sec) | 4.1 (1.5) | 4.0 (1.4) | 3.9 (1.5) | 0.75 |
| Physical Activity (MVPA, avg min/day) | 17.7 (7.4–29.0) | 16.5 (6.3–29.1) | 15.0 (7.3–36.0) | 0.67 |
| Physical Activity (IPAQ-sf METs/wk) | 1290 (504–3276) | 1108 (198–2839) | 1386 (297–2970) | |
| TENS Use | (n=94) | |||
| Intensity Lumbar (mA) | 38.67 (7.98) | -- | -- | -- |
| Intensity Cervical (mA) | 38.70 (7.24) | -- | -- | -- |
| Minutes/day | 77.1 (51.4–109.7) | -- | -- | -- |
percentages are for those choosing to respond
groups stratified for opioid use during randomization
NRS=numerical rating scale; FIQR= Fibromyalgia Impact Questionnaire-Revised; SF-36=short form 36; MCS=mental composite score; PCS=physical composite score; PSQI=Pittsburgh Sleep Quality Index; PCS=Pain Catastrophizing Scale; PSEQ= Pain Self-Efficacy Questionnaire; TSK=Tampa Scale of Kinesiophobia; MVPA= moderate to vigorous activity; IPAQ=International Physical Activity Questionnaire; 6MWT=six minute walk test; 5TSTS=5 times sit to stand test
Outcome Measures
Pain
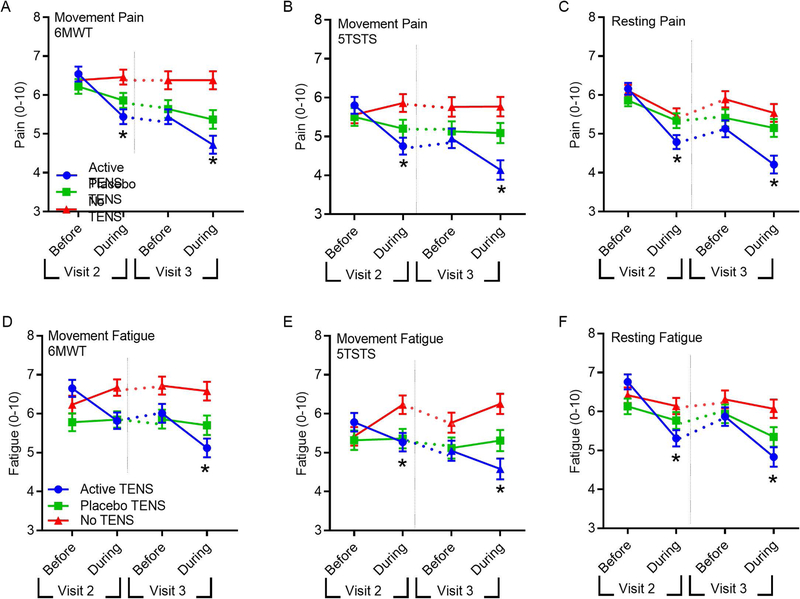
After 4-weeks of active-TENS, within group movement-evoked pain during 6MWT significantly reduced by −1.8 points (95%-CI: −2.3, −1.2) compared to pre-TENS treatment on Visit-2, and was greater than placebo-TENS (p=0.008; mean −0.8; 95%-CI: −1.4, −0.2) and no-TENS (p<0.0001; mean −0.006; 95%-CI: −0.5, 0.6) for intention-to-treat analysis (Figure 2, Table 2). Similar results were obtained after adjusting for baseline FIQR and marital status. There were also significant reductions with active-TENS for resting pain (NRS), pain intensity and interference (BPI), and FIQR-pain when compared to placebo-TENS or no-TENS after 4-weeks of home use (p<0.05)(Figure 2, Table 2). Per protocol analysis demonstrated similar results for pain (Supplementary Figure 2; Supplementary Table 4). There was no significant change at Visit-3 in rescue pain medication use after 1 month of active-TENS compared to placebo-TENS or no-TENS (Supplementary Table 6).
Figure 2. Active-TENS significantly decreased pain and fatigue during activity and at rest compared to placebo-TENS or no-TENS (intention-to-treat analysis).
Line graphs show movement and resting pain and fatigue before and during treatment at Visit-2, and before and during treatment at Visit-3. Between Visit-2 and Visit-3 participants used TENS at home for 4 weeks (dotted lines). A. Movement-pain during the 6MWT. B. Movement pain during 5TSTS. C. Resting Pain. D Movement-fatigue during 6MWT, E. Movement-fatigue during 5TSTS, F. Resting fatigue. Data are mean ± SEM *, significantly different from placebo-TENS and no-TENS.
Table 2.
Primary and secondary outcomes for intention-to-treat analysis corrected for site differences. Mean change (from baseline, Visit 2) at Visit 3 (after 4 weeks of home TENS use, or placebo TENS, or no TENS) and at Visit 4 (after 4 weeks of home TENS all groups) with 95% adjusted confidence intervals. Change scores are presented for the randomized phase between Visit-2 and Visit-3, and for the difference from baseline at Visit 4 when all subjects received active-TENS.
| Visit #, change from Visit2 | Active-TENS n=103 |
Placebo-TENS n=99 |
No-TENS n=99 |
Group Mean Difference (95% CI) P-value |
||
|---|---|---|---|---|---|---|
| Active-TENS vs Placebo-TENS | Active-TENS vs No-TENS | |||||
| Pain 6MWT
(0–10) |
Visit 3 (randomized) | −1.8 (−2.3, −1.2)^^ | −0.8 (−1.4, −0.2)^ | 0.0 (−0.5, 0.6) | −1.0 (−1.8, −0.2) 0.008 | −1.8 (−2.6, −1.0) <0.0001 |
| Visit 4 (all with TENS) | −2.0 (−2.8, −1.3)^^ | −1.9 (−2.7, −1.2)^^ | −1.9 (−2.6, −1.2)^^ | |||
| Pain 5xSTS (0–10) |
Visit 3 (randomized) | −1.6 (−2.3, −1.0)^^ | −0.3 (−1.0, 0.3) | 0.2 (−0.4, 0.9) | −1.3 (−2.2, −0.4) 0.002 | −1.8 (−2.8, −1.0) <0.0001 |
| Visit 4 (all with TENS) | −1.9 (−2.6, −1.1)^^ | −1.4 (−2.2, −0.7)^^ | −1.3 (−2.1, −0.6)^^ | |||
| Resting Pain (0–10) |
Visit 3 (randomized) | −1.9 (−2.5, −1.4)^^ | −0.7 (−1.3, −0.1)* | −0.5 (−1.1, 0.0) | −1.2 (−2.1, −0.4) 0.0006 | −1.4 (−2.2, −0.6) <0.0001 |
| Visit 4 (all with TENS) | −2.2 (−2.9, −1.6)^^ | −1.9 (−2.6, −1.2)^^ | −2.2 (−2.8, −1.5)^^ | |||
| Fatigue 6MWT (0–10) |
Visit 3 (randomized) | −1.5 (−2.2, −0.8)^^ | −0.1 (−0.9, 0.7) | 0.4 (−0.3, 1.1) | −1.4 (−2.4, −0.4) 0.001 | −1.9 (−2.9, −0.9) <0.0001 |
| Visit 4 (all with TENS) | −1.3 (−2.0, −0.6)^^ | −0.9 (−1.7, −0.2)^ | −0.9 (−1.7, −0.2)^ | |||
| Fatigue 5xSTS (0–10) |
Visit 3 (randomized) | −1.2 (−1.9,−0.5)^^ | 0.0 (−0.8, 0.7) | 0.8 (0.1, 1.5)* | −1.2 (−2.2, −0.2) 0.011 | −2.0 (−3.0, −1.0) <0.0001 |
| Visit 4 (all with TENS) | −1.1 (−1.9, −0.4)^^ | −0.8 (−1.6, −0.1)* | −0.6 (−1.4, 0.1) | |||
| Resting Fatigue (0–10) |
Visit 3 (randomized) | −1.9 (−2.6, −1.2)^^ | −0.8 (−1.5, −0.04)* | −0.4 (−1.0, 0.4) | −1.2 (−2.2, −0.1) 0.016 | −1.57 (−2.6, −0.6) 0.0002 |
| Visit 4 (all with TENS) | −2.1 (−2.9, −1.4)^^ | −1.6 (−2.4, −0.8)^^ | −1.8 (−2.6, −1.1)^^ | |||
| Disease Impact FIQR (0–100) |
Visit 3 (randomized) | −8.5 (−12.9, −4.0)^^ | −3.4 (−6.5, −0.3)* | −1.39 (−4.4, 1.6) | −5.0 (−10.4, 0.3) 0.074 | −7.1 (−12.4, −1.8) 0.005 |
| Visit 4 (all with TENS) | −9.6 (−13.8, −5.4)^^ | −11.1 (−15.2, 7.0)^^ | −10.7 (−14.8, −6.6)^^ | |||
| Pain
FIQR-pain (0–10) |
Visit 3 (randomized) | −1.3 (−1.8, −0.7)^^ | −0.4 (−0.9, 0.2) | −0.1 (−0.6, 0.4) | −0.9 (−1.7, −0.1) 0.018 | −1.2 (−1.9, −0.4) 0.0006 |
| Visit 4 (all with TENS) | −1.4 (−2.0, −0.8)^^ | −1.2 (−1.7, −0.6)^^ | −1.4 (−1.9, −0.8)^^ | |||
| Pain
BPI-Interference (0–10) |
Visit 3 (randomized) | −0.9 (−1.4, −0.5)^^ | −0.3 (−0.7, 0.2) | −0.3 (−0.7, 0.2) | −0.7 (−1.3, −0.01) 0.043 | −0.6 (−1.3, −0.0) 0.048 |
| Visit 4 (all with TENS) | −1.1 (−1.6, −0.6)^^ | −0.9 (−1.4, −0.3)^^ | −1.2 (−1.7, −0.7)^^ | |||
| Pain
BPI-Intensity (0–10) |
Visit 3 (randomized) | −0.8 (−1.1, −0.4)^^ | −0.3 (−0.6, 0.1) | 0.15 (−0.2, 0.5) | −0.5 (−1.0, −0.0) 0.036 | −0.9 (−1.4, −0.4) <0.0001 |
| Visit 4 (all with TENS) | −1.0 (−1.4, −0.6)^^ | −0.9 (−1.3, −0.5)^^ | −0.9 (−1.2, −0.5)^^ | |||
| Fatigue MAF GFI (1–50) |
Visit 3 (randomized) | −4.6 (−6.4, −2.8)^^ | −1.5 (−3.3, 0.4) | −0.3 (−2.0, 1.5) | −3.2 (−5.7, −0.6) 0.009 | −4.4 (−6.8, −1.9) <0.0001 |
| Visit 4 (all with TENS) | −4.0 (−6.0, −1.9)^^ | −4.3 (−6.4, −2.2)^^ | −3.2 (−5.1, 1.2)^^ | |||
| Self-efficacy PSEQ# (0–60) |
Visit 3 (randomized) | 3.2 (0.8, 5.6)^^ | 1.5 (−0.9, 4.0) | 0.8 (−1.5, 3.1) | 1.6 (−1.8, 5.1) 0.75 | 2.3 (−1.0, 5.7) 0.28 |
| Visit 4 (all with TENS) | 5.3 (2.6, 8.0)^^ | 4.4 (1.7, 7.1)^^ | 4.2 (1.6, 6.8)^^ | |||
| Pain Catastrophizing PCS (0–52) |
Visit 3 (randomized) | −3.4 (−5.3, −1.4)^^ | −3.1 (−5.1, −1.2)^^ | −1.4 (−3.3, 0.5) | −0.3 (−3.0, 2.5) >0.99 | −2.0 (−4.7, 0.7) 0.23 |
| Visit 4 (all with TENS) | −6.1 (−8.2, −3.9)^^ | −4.5 (−6.8, −2.3)^^ | −4.9 (−7.0, −2.8)^^ | |||
| Fear of Movement TSK (17–68) |
Visit 3 (randomized) | −0.7 (−2.0, 0.6) | −0.3 (−1.7, 1.0) | −0.2 (−1.4, 1.1) | −0.4 (−2.3, 1.5) >0.99 | −0.6 (−2.4, 1.3) >0.99 |
| Visit 4 (all with TENS) | −0.3 (−1.6, 1.1) | −2.3 (−3.7, −0.9)^^ | −3.3 (−4.6, −2.0)^^ | |||
| Mental Quality of Life SF-36 MCS# (T-score) |
Visit 3 (randomized) | 2.3 (0.2, 4.4)* | 1.2 (−0.9, 3.4) | −0.04 (−2.1, 2.0) | 1.1 (−1.9, 4.1) >0.99 | 2.4 (−0.6, 5.3) 0.17 |
| Visit 4 (all with TENS) | 2.1 (−0.2, 4.4) | 3.6 (1.3, 6.0)^^ | 2.8 (0.6, 5.0)^ | |||
| Physical Quality of Life SF-36 PCS# (T-score) |
Visit 3 (randomized) | 2.4 (1.0, 3.7)^^ | 1.2 (−0.2, 2.5) | 1.4 (0.1, 2.6) | 1.2 (−0.7, 3.1) 0.36 | 1.00 (−0.8, 2.8) 0.58 |
| Visit 4 (all with TENS) | 3.5 (2.0, 5.1)^^ | 3.2 (1.6, 4.8)^^ | 4.4 (2.9, 5.9)^^ | |||
| Anxiety PROMIS (T-score) |
Visit 3 (randomized) | −1.1 (−2.6, 0.5) | −0.6 (−2.1, 1.0) | −0.7 (−2.1, 0.8) | −0.5 (−2.7, 1.7) >0.99 | −0.4 (−2.5, 1.7) >0.99 |
| Visit 4 (all with TENS) | −0.5 (−2.1, 1.2) | −1.6 (−3.3, 0.1) | −2.2 (−3.8, −0.6)^ | |||
| Depression PROMIS (T-score) |
Visit 3 (randomized) | −2.8 (−4.2, −1.5)^^ | −0.1 (−1.5, 1.3) | 0.4 (−0.9, 1.7) | −2.7 (−4.7, −0.8) 0.002 | −3.2 (−5.1, −1.3) 0.0001 |
| Visit 4 (all with TENS) | −2.0 (−3.4, −0.6)^ | −1.3 (−2.7, 0.1) | −1.2 (−2.6, 0.2) | |||
| Self-report function | ||||||
| FIQR Function (0–30) |
Visit 3 (randomized) | −2.7 (−4.0, −1.4)^^ | −1.4 (−2.7, −0.1)* | −0.6 (−1.8, 0.7) | −1.3 (−3.2, 0.5) 0.25 | −2.1 (−3.9, −0.4) 0.013 |
| Visit 4 (all with TENS) | −2.8 (−4.2, −1.4)^^ | −3.7 (−5.1, −2.3)^^ | −3.6 (−4.9, −2.3)^^ | |||
| SF-36
Physical Function# (T-score) |
Visit 3 (randomized) | 1.4 (0.1, 2.7)* | 0.5 (−0.8, 1.8) | 0.8 (−0.5, 2.0) | 0.9 (−1.0, 2.7) 0.79 | 0.6 (−1.2, 2.4) >0.99 |
| Visit 4 (all with TENS) | 2.9 (1.5, 4.4)^^ | 2.3 (0.9, 3.8)^^ | 3.0 (1.6, 4.4)^^ | |||
| Physical Activity IPAQ MET/wk % change |
Visit 3 (randomized) | 8.1 (−22.0, 49.9) | 52.1 (9.0, 112.2)^ | −14.2 (−37.5, 17.8) | 0.24 | 0.67 |
| Visit 4 (all with TENS) | 38.1 (−1.2, 93.0) | 46.3 (4.1, 105.5)* | 29.9 (−6.3, 80.1) | |||
| Performance-based function | ||||||
| 6MWT (Feet) |
Visit 3 (randomized) | −1 (−55, 54) | −20 (−75, 36) | −42.1 (−95, 11) | 19 (−58, 96) >0.99 | 42 (−34, 117) >0.99 |
| Visit 4 (all with TENS) | 15 (−42, 71) | 16 (−41, 74) | −2 (−57, 53.0) | |||
| 5TSTS (#STS/10sec) |
Visit 3 (randomized) | 0.6 (0.3, 1.0)^^ | 0.4 (0.0, 0.7)* | 0.1 (−0.3, 0.4) | 0.2 (−0.2, 0.7) 0.96 | 0.6 (0.1, 1.0) 0.008 |
| Visit 4 (all with TENS) | 0.6 (0.2, 1.0)^^ | 0.8 (0.4, 1.3)^^ | 0.6 (0.1, 1.0)^ | |||
| Physical Activity MVPA avg min/day % change |
Visit 3 (randomized) | −9.4 (−27.8, 13.5) | 2.5 (−18.6, 29.1) | −14.1 (−29.5, 4.7) | >0.99 | >0.99 |
| Visit 4 (all with TENS) | −6.8 (−27.2, 19.3) | −0.3 (−22.3, 27.9) | −17.0 (−33.9, 4.1) | |||
Significant change within group
p<0.05
p<0.01
p<0.001
Higher score represents improvement; p-values represent post hoc comparisons between groups
As part of our recruitment and retention strategy, we provided active-TENS to all participants after Visit-3 for 4-weeks of home use and tested effects on Visit-4. The active-TENS group (n=75) continued to show a reduction in resting and movement-evoked pain after an additional 4-weeks of home use (Table 2, Supplementary Table 5). After 4-weeks of active-TENS, those in the placebo-TENS (n=68) and no-TENS (n=94) groups had a significant decrease in resting and movement-evoked pain (Table 2, Supplementary Table 5).
Fatigue
After 4-weeks of active-TENS, there was a significant reduction in movement-evoked fatigue when compared to placebo-TENS (p=0.001) and no-TENS (p<0.0001) for the intention-to-treat analysis (Figure 2, Table 2). Resting fatigue, and MAF global fatigue index showed significant differences between active-TENS and placebo-TENS or no-TENS (p<0.05) (Table 2). The per protocol analysis demonstrated similar results for fatigue (Supplementary Figure 2; Supplementary Table 4).
Function, Disease Impact, Quality of Life, and Pain-Related Psychological Factors
Active-TENS produced significant reductions in disease impact (FIQR) and self-reported function (FIQR function subscale) compared to no-TENS but not compared to placebo-TENS (Table 2) for the intention-to-treat analysis. No differences between groups were observed for performance-based function (6MWT, FTSTS), physical activity (accelerometry, IPAQ-sf), fear of movement (TSK), pain catastrophizing (PCS), self-efficacy (PSEQ), anxiety (PROMIS), or quality of life (SF-36), except for a small decrease in depression (PROMIS) with active-TENS (Table 2).
Global Impression of Change (GIC)
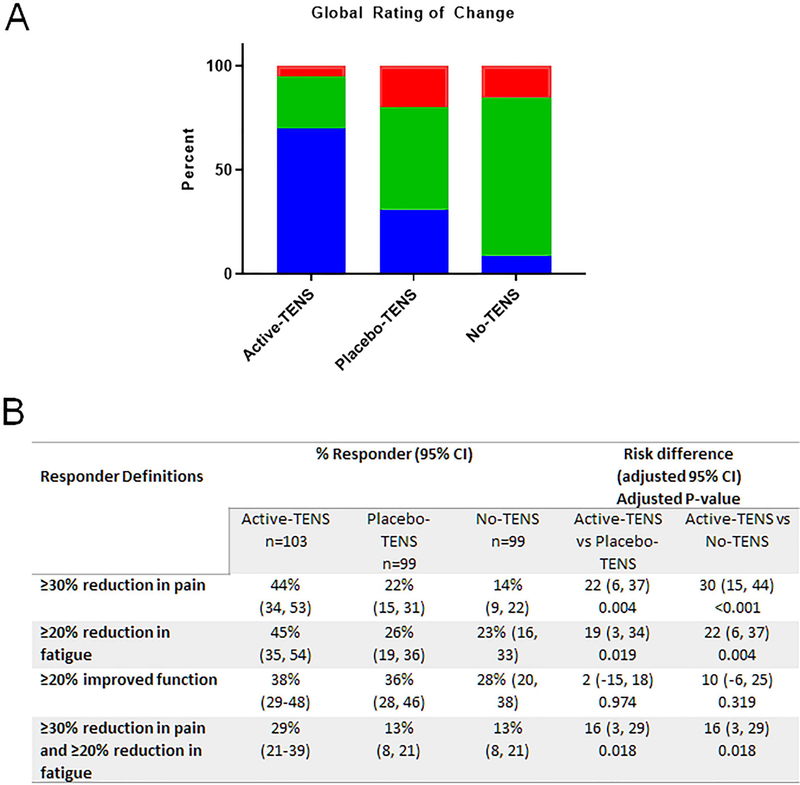
The GIC showed that 70% of those in the active-TENS group reported global improvement compared to 31% in the placebo-TENS group (p<0.0001) and 9% in the no-TENS group (p<0.0001) for the intention-to-treat analysis (Figure 3A). The GIC rating showed a moderate relationship to the change in movement-evoked pain (r=−0.39, p=0.0001, n=242, Spearman’s test)(Supplementary Figure 3).
Figure 3. Active-TENS resulted in improved perception of change and a greater number of responders for changes in pain or fatigue compared to placebo-TENS or no-TENS.
A. Stacked bar graph showing the percentage of people reporting better or much better (blue), no change (green), and worse or extremely worse (red) after 4 weeks of active-TENS, placebo-TENS or no-TENS. The majority of individuals reported a significant overall improvement after using active-TENS when compared to placebo-TENS (p<0.0001) or no-TENS groups (p<0.0001). There were no differences between placebo-TENS and no-TENS groups (p=0.175). B. Table showing the percentage of responders to active-TENS for pain (≥30% reduction), fatigue (≥20% reduction) and function (≥20% reduction), and pain and fatigue both improved. These percentages were based on percentages suggested by Arnold and colleagues as clinically meaningful (34). There were a significant greater number of responders for pain, fatigue, and both pain and fatigue for the active-TENS group compared to either the placebo-TENS or no-TENS group. Pain = movement-evoked pain during 6MWT; Fatigue = movement-evoked fatigue during 6MWT; Function = FIQR Function. Data are percentage with 95% confidence intervals.
Responders to TENS Intervention
We defined responders as follows: pain ≥30% reduction, fatigue ≥20% reduction, and function ≥20% improvement based on percentages suggested as clinically meaningful in prior studies (33, 34). For pain, active-TENS showed significantly more responders compared to placebo-TENS (p=0.004) and no-TENS (p<0.001)(Figure 3B). Similarly, for fatigue, active-TENS had significantly more responders compared to placebo-TENS (p=0.019) and no-TENS (p=0.004). For function, measured with FIQ-R-function, the number of responders did not differ among groups (Figure 3B).
Blinding
The Outcome-Assessor was adequately blinded to active-TENS (45% correct), placebo-TENS (13% correct) and no-TENS (20% correct). The participant was blinded to placebo-TENS (49% correct) but correctly identified active-TENS 70% of the time. The reduction in movement-evoked pain during 6MWT after 4-weeks of active-TENS were not significantly different in those that correctly identified active-TENS (−1.9; 95%-CI: −2.6, −1.3) compared to those that did not correctly identify active-TENS (−1.4; 95%-CI: −2.5, −0.3), with a mean difference of −0.56 (95%-CI: −1.7, 0.6; p=0.50).
Adverse Events
There were 30 adverse events in 30 participants related to TENS intervention on Visit-1, −2 or −3. The most common adverse events were pain with TENS (4.8% active-TENS, 4% placebo-TENS; 1% no-TENS), and skin irritation with electrodes (4.8% active TENS; 1% placebo-TENS; 0% no-TENS). Adverse events reported on Visit-2 were from the first treatment during this visit, and those adverse events reported on Visit-3 were from the treatment on Visit-3 and 4-weeks of home use. Supplementary Table 7 shows rates for TENS-related adverse events by visits. There were four serious adverse events, none related to TENS (Supplementary Results).
Discussion
This double-blind, randomized, controlled trial shows that active-TENS significantly reduced movement-evoked and resting pain and fatigue compared to placebo-TENS or no-TENS in women with FM. The current study used a classical design to examine for active-TENS-specific effects by comparing to placebo-TENS, but also added a unique design comparing to no-TENS as a more pragmatic application that includes both specific and non-specific treatment effects. While the active-TENS group correctly identified the intervention 70% of the time, there was no difference in pain reduction between participants who correctly guessed the treatment compared to participants who did not, arguing against a difference based on inadequate blinding. We also showed adequate blinding to the placebo-TENS using our novel transient-placebo TENS unit with repeated use, further validating the placebo-TENS from our prior study showing excellent blinding with a single use (32).
Our primary outcome of movement-evoked pain lacks formally validated minimally important difference in the published literature however we utilized the general thresholds of 30% reduction in pain, 20% reduction in fatigue, and 20% reduction in functional impairment recommended by OMERACT (34). For active-TENS, 44% showed a clinically important 30% reduction in pain, 45% a clinically reduction in fatigue, and 29% showed a reduction in both pain and fatigue suggesting that a sub-population of individuals with FM responds well to TENS. The responder rates for pain are similar to FDA-approved pharmaceutical agents for FM such as duloxetine or pregabalin which show responder rates of between 31–41% for a 30% reduction in pain (35,36). The comparative reductions in movement-evoked pain with active-TENS compared to placebo were small, averaging a 1-point difference on an 11-point scale. However, when compared to no-TENS comparative reduction was 1.8. A 1.8-point decrease equates to a ≥30% reduction for people with pain rating of 6 or less (33). It should be noted that some studies also suggest a 2-point reduction in pain is the clinically relevant threshold (37). Future studies should determine who will respond to TENS, which is an inexpensive, safe, and easy to disseminate pain management intervention.
The reduction in pain and fatigue, two common symptoms in FM, likely contributed to improvements in GIC reported by individuals receiving active-TENS. While pain is a defining characteristic of FM, fatigue is a common symptom occurring in the majority of individuals with FM (38). Both pain and fatigue contribute significantly to perceived disability and function (39), and global rating of change is associated with improvements in pain and fatigue in individuals with FM (40, 41). We show for the first time, the relationship between global improvement and movement-evoked pain, with similar results to prior studies on pain and GIC (40,41). The magnitude of reduction in both pain and fatigue observed in the current study is likely to have a significant impact on the lived experience of FM (42,43).
A recent Cochrane review examined efficacy of TENS for individuals with FM and concluded there was insufficient high-quality evidence (17). The main concerns were inadequately powered studies (n=5–43 participants/group) with incomplete and limited outcome reporting. Additional concerns for clinical TENS studies include use of an adequate placebo with blinding of participant and assessor, timing of outcome measurements, adequate dosing of TENS, and monitoring concurrent analgesia (21,22). The current study was designed to address these concerns, as well as the multiple dimensions of FM recommended by professional societies as clinically important domains (44). While we showed significant effects on pain, fatigue and global improvement, we failed to detect a change in several FM domains including function, psychological factors, and quality of life after 4-weeks of TENS use. The lack of effect on function could be related to the short-duration of TENS use, as functional changes may take a longer period of time to change or are harder to achieve, particularly in long-standing conditions such as FM. As an example, most exercise studies are require 8–24 weeks for improved function with improvements varying between 10 and 20% (45). It is also possible that TENS may improve adherence to an exercise task, while not directly improving function. Future studies are needed to examine more long-term effects in a pragmatic setting to examine the interactions between function and adherence with physical activity interventions.
Uniquely, the current study, compared with prior TENS studies (22), examined outcomes during TENS treatment. TENS activates endogenous inhibitory pathways in the central nervous system releasing the inhibitory neurotransmitters serotonin, opioids, and GABA to reduce sensitization of central neurons (14,46), and greatest effects occur during stimulation when endogenous inhibitory neurotransmitters are released. We also show active-TENS has a cumulative effect because there was a greater reduction after 4-weeks of home use when compared to the first single treatment. Further, active-TENS remained effective after 8-weeks of repeated home use in the active-TENS group. This is important because we previously reported development of analgesic tolerance to repeated use of high-frequency or low-frequency TENS in animals and human participants, a phenomenon mediated by endogenous opioids (47, 48). The lack of tolerance to repeated use is likely a result of the mixed frequency used in the current study as prior studies in animals show decreased tolerance with mixed frequencies (49). Thus, understanding mechanisms underlying the analgesia produced by non-pharmacological interventions is critical to design an adequate clinical trial to detect clinical effectiveness.
Importantly, the current study collected adverse events to active-TENS and placebo-TENS and shows minimal adverse events. Less than 5% of individuals receiving active-TENS or placebo-TENS reported pain with TENS or irritation with electrodes. This demonstrates that TENS is safe and could be a useful treatment for home use.
There are several limitations to the study. The inability to fully blind the active intervention, as noted above, may lead to reporting bias by the subject. However, if TENS is given at an adequate intensity to produce analgesic effects, i.e. strong but comfortable or greater (23,32), this limitation may be unavoidable in a clinical trial of TENS. Medication usage was collected by self-report and thus subject recall bias and willingness for the subject to fill out the log can influence results. In the current study, 75% of subjects filled out enough logs for us to examine change in medication use as an outcome. A greater number of subjects withdrew from the active-TENS (n=15) and the placebo-TENS (n=11) groups than the no-TENS group (n=4) which might suggest an unwillingness for some individuals to use the units on a regular basis. Additionally, this study was only performed in women with fibromyalgia and thus may not translate to men with fibromyalgia. Lastly, the trial was designed as a 4-week intervention and while we saw significant effects on pain and fatigue, we did not see significant effects on function, rescue medication usage, or psychological outcomes. It is possible that longer treatment is necessary to see effects on these other outcomes and thus future experiments will be needed to examine more long-term impact of TENS use.
In conclusion, among women with FM and stable medication, the use of active-TENS compared with placebo-TENS for 4-weeks resulted in significantly reduced movement-evoked pain. The use of active-TENS compared with no-TENS, as would be done clinically, resulted in a statistically and clinically meaningful improvement in movement-evoked pain. Further research is needed for replication, to assess duration of effect, and to establish clinical importance of the findings.
Supplementary Material
Acknowledgements:
Funding: NIH UM1 AR063381 and NIH UM1 AR063381-S1. Also, supported by the National Center for Advancing Translational Sciences Award Number U54TR001356 to University of Iowa and the National Center for Advancing Translational Sciences Award Number UL1TR000445 to Vanderbilt University Medical Center. Active and placebo TENS units and electrodes were provided by DJO, Inc. The National Institute for Arthritis and Musculoskeletal and Skin Diseases at the National Institute of Health provided financial support and an external Data Safety and Monitoring Board, but was not involved in the design and conduct of the study in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. DJO, Inc. provided active and placebo TENS units but was not involved in the design and conduct of the study in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest/Disclosures:
Kathleen A. Sluka, PT, PhD, FAPTA serves as a consultant for Novartis Consumer Healthcare/GSK Consumer Healthcare, has an active grant from the American Pain Society/Pfizer, and receives royalties from IASP Press. The remaining authors of this manuscript have no conflicts of interest to report.
References
- 1.Okifuji A, Hare BD. Management of fibromyalgia syndrome: review of evidence. Pain Ther. 2013;2(2):87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent A, Whipple MO, McAllister SJ, Aleman KM, St Sauver JL. A cross-sectional assessment of the prevalence of multiple chronic conditions and medication use in a sample of community-dwelling adults with fibromyalgia in Olmsted County, Minnesota. BMJ Open. 2015;5(3):e006681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qaseem A, Wilt TJ, McLean RM, Forciea MA, Clinical Guidelines Committee of the American College of P. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166(7):514–30. [DOI] [PubMed] [Google Scholar]
- 4.Macfarlane GJ, Kronisch C, Atzeni F, Hauser W, Choy EH, Amris K, et al. EULAR recommendations for management of fibromyalgia. Ann Rheum Dis. 2017;76(12):e54. [DOI] [PubMed] [Google Scholar]
- 5.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain-United States. MMWR Recomm Rep; 2016. p. 1–49. [DOI] [PubMed] [Google Scholar]
- 6.Hoeger Bement MK, Sluka KA. Exercise-induced hypoalgesia: An Evidence-based review In: Sluka KA, editor. Pain Mechanisms and Management for the Physical Therapist. 2nd edition ed. Philadelphia: Wolters Kluwer; 2016. p. 177–202. [Google Scholar]
- 7.Bidonde J, Busch AJ, Bath B, Milosavljevic S. Exercise for adults with fibromyalgia: an umbrella systematic review with synthesis of best evidence. Curr Rheumatol Rev. 2014;10(1):45–79. [DOI] [PubMed] [Google Scholar]
- 8.Dailey DL, Keffala VJ, Sluka KA. Do cognitive and physical fatigue tasks enhance pain, cognitive fatigue, and physical fatigue in people with fibromyalgia? Arthritis Care Res. 2015;67(2):288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bair MJ, Matthias MS, Nyland KA, Huffman MA, Stubbs DL, Kroenke K, et al. Barriers and facilitators to chronic pain self-management: a qualitative study of primary care patients with comorbid musculoskeletal pain and depression. Pain Med. 2009;10(7):1280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma YT, Sluka KA. Reduction in inflammation-induced sensitization of dorsal horn neurons by transcutaneous electrical nerve stimulation in anesthetized rats. Exp Brain Res. 2001;137(1):94–102. [DOI] [PubMed] [Google Scholar]
- 11.Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289(2):840–6. [PubMed] [Google Scholar]
- 12.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS). J Pharmacol Exp Ther. 2001;298(1):257–63. [PubMed] [Google Scholar]
- 13.Radhakrishnan R, King EW, Dickman J, Richtsmeier C, Schardt N, Spurgin M, et al. Blockade of spinal 5-HT receptor subtypes prevents low, but not high, frequency TENS-induced antihyperalgesia in rats. Pain. 2003;105:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vance CG, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: the state of the evidence. Pain Manag. 2014;4(3):197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clauw DJ. Fibromyalgia: a clinical review. J American Med Assn. 2014;311(15):1547–55. [DOI] [PubMed] [Google Scholar]
- 16.Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neurosci. 2016;338:114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson MI, Claydon LS, Herbison GP, Jones G, Paley CA. Transcutaneous electrical nerve stimulation (TENS) for fibromyalgia in adults. Cochrane Database Syst Rev. 2017;10:CD012172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson MI, Paley CA, Howe TE, Sluka KA. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database Syst Rev. 2015;6:CD006142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen LX, Zhou ZR, Li YL, Ning GZ, Li Y, Wang XB, et al. Transcutaneous Electrical Nerve Stimulation in Patients With Knee Osteoarthritis: Evidence From Randomized-controlled Trials. Clin J Pain. 2016;32(2):146–54. [DOI] [PubMed] [Google Scholar]
- 20.Resende L, Merriwether E, Rampazo EP, Dailey D, Embree J, Deberg J, et al. Meta-analysis of transcutaneous electrical nerve stimulation for relief of spinal pain. Eur J Pain. 2018;22(4):663–78. [DOI] [PubMed] [Google Scholar]
- 21.Bennett MI, Hughes N, Johnson MI. Methodological quality in randomised controlled trials of transcutaneous electric nerve stimulation for pain: low fidelity may explain negative findings. Pain. 2011;152(6):1226–32. [DOI] [PubMed] [Google Scholar]
- 22.Sluka KA, Marchand S, Bjordal JM, Rakel BA. What makes TENS work? Making sense of the clinical literature. Phys Ther. 2013;93(10):1397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moran F, Leonard T, Hawthorne S, Hughes CM, Crum-Gardner E, Johnson MI, et al. Hypoalgesia in Response to Transcutaneous Electrical Nerve Stimulation (TENS) Depends on Stimulation Intensity. J Pain. 2011;12(8):929–35. [DOI] [PubMed] [Google Scholar]
- 24.Rakel B, Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. J Pain. 2003;4(8):455–64. [DOI] [PubMed] [Google Scholar]
- 25.Dailey DL, Rakel BA, Vance CG, Liebano RE, Amrit AS, Bush HM, et al. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. 2013;154(11):2554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowan S, McKenna J, Crum-Gardner E, Johnson MI, Sluka KA, Walsh DM. An investigation of the hypoalgesic effects of TENS delivered by a glove electrode. J Pain. 2009;10(7):694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunshine W, Field TM, Quintino O, Fierro K, Kuhn C, Burman I, et al. Fibromyalgia benefits from massage therapy and transcutaneous electrical stimulation. J Clin Rheumatol. 1996;2(1):18–22. [DOI] [PubMed] [Google Scholar]
- 28.Lofgren M, Norrbrink C. Pain relief in women with fibromyalgia: a cross-over study of superficial warmth stimulation and transcutaneous electrical nerve stimulation. J Rehabil Med. 2009;41(7):557–62. [DOI] [PubMed] [Google Scholar]
- 29.Lauretti GR, Chubaci EF, Mattos AL. Efficacy of the use of two simultaneously TENS devices for fibromyalgia pain. Rheumatol Int. 2013;33(8):2117–22. [DOI] [PubMed] [Google Scholar]
- 30.Carbonario F, Matsutani LA, Yuan SL, Marques AP. Effectiveness of high-frequency transcutaneous electrical nerve stimulation at tender points as adjuvant therapy for patients with fibromyalgia. Eur J Phys Rehabil Med. 2013;49(2):197–204. [PubMed] [Google Scholar]
- 31.Noehren B, Dailey DL, Rakel BA, Vance CG, Zimmerman MB, Crofford LJ, et al. Effect of transcutaneous electrical nerve stimulation on pain, function, and quality of life in fibromyalgia: a double-blind randomized clinical trial. Phys Ther. 2015;95(1):129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rakel B, Cooper N, Adams HJ, Messer BR, Frey Law LA, Dannen DR, et al. A New Transient Sham TENS Device Allows for Investigator Blinding While Delivering a True Placebo Treatment. J Pain. 2010;11:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrar JT, Young JP Jr., LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–58. [DOI] [PubMed] [Google Scholar]
- 34.Arnold LM, Williams DA, Hudson JI, Martin SA, Clauw DJ, Crofford LJ, et al. Development of responder definitions for fibromyalgia clinical trials. Arthritis Rheum. 2012;64(3):885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straube S, Derry S, Moore RA, Cole P. Cervico-thoracic or lumbar sympathectomy for neuropathic pain and complex regional pain syndrome. Cochrane Database Syst Rev. 2013;9:CD002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;4:CD007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(4):283–91. [DOI] [PubMed] [Google Scholar]
- 38.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RL, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–29. [DOI] [PubMed] [Google Scholar]
- 39.Dailey DL, Frey Law LA, Vance CG, Rakel BA, Merriwether EN, Darghosian L, et al. Perceived function and physical performance are associated with pain and fatigue in women with fibromyalgia. Arthritis Res Ther. 2016;18:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnold LM, Zlateva G, Sadosky A, Emir B, Whalen E. Correlations between fibromyalgia symptom and function domains and patient global impression of change: a pooled analysis of three randomized, placebo-controlled trials of pregabalin. Pain Med. 2011;12(2):260–7. [DOI] [PubMed] [Google Scholar]
- 41.Rampakakis E, Ste-Marie PA, Sampalis JS, Karellis A, Shir Y, Fitzcharles MA. Real-life assessment of the validity of patient global impression of change in fibromyalgia. RMD Open. 2015;1(1):e000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnold LM, Crofford LJ, Mease PJ, Burgess SM, Palmer SC, Abetz L, et al. Patient perspectives on the impact of fibromyalgia. Patient Educ Couns. 2008;73(1):114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okifuji A, Bradshaw DH, Donaldson GW, Turk DC. Sequential analyses of daily symptoms in women with fibromyalgia syndrome. J Pain. 2011;12(1):84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mease PJ, Clauw DJ, Christensen R, Crofford LJ, Gendreau RM, Martin SA, et al. Toward development of a fibromyalgia responder index and disease activity score: OMERACT module update. J Rheumatol. 2011;38(7):1487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bidonde J, Busch AJ, Schachter CL, Overend TJ, Kim SY, Goes SM, et al. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev. 2017;6:CD012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sluka KA, Walsh DM. Transcutaneous Electrical Nerve Stimulation and Interferential Therapy In: Sluka KA, editor. Pain Mechanisms and Management for the Physical Therapist. 2nd Edition ed. Philadelphia: Wolters Kluwer; 2016. p. 203–23. [Google Scholar]
- 47.Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102(1–2):195–201. [DOI] [PubMed] [Google Scholar]
- 48.Liebano R, Rakel B, Vance C, Walsh DM, Sluka KA. An investigation of the development of analgesic tolerance to transcutaneous electrical nerve stimulation (TENS) in humans. Pain. 2011;152:335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeSantana JM, Santana-Filho VJ, Sluka KA. Modulation between high- and low-frequency transcutaneous electric nerve stimulation delays the development of analgesic tolerance in arthritic rats. Arch Phys Med Rehabil. 2008;89:754–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.