Abstract
Monocyte infection by HIV-1 is an important component of chronic HIV pathogenesis. Following infection by HIV-1, monocytes are able to cross the blood brain barrier and set up a viral reservoir in the central nervous system. Additionally, in the setting of chronic HIV-1 infection, monocytes can become activated either directly through HIV-1 infection or indirectly via HIV-1-mediated systemic immune activation. Currently, there are few studies looking at HIV-1 infection of primary human monocytes in vitro. Furthermore, detection of successful HIV-1 infection of monocytes can be laborious requiring an ELISA for p24 or assessing levels of HIV-1 mRNA or DNA. This protocol utilizes an HIV-1 strain expressing GFP to allow for easy quantification of HIV-1 infection by fluorescence-assisted cell sorting (FACS). By determining HIV-1 infection by FACS one can take advantage of its multiparametric nature allowing for the use of less cells and the ability to assess the expression of other markers on HIV-1+ and HIV-1− cells in the same experiment. Additionally, this protocol could be modified to study HIV-1 infection of other cells including CD4+ T cells.
Keywords: Monocytes, HIV, Infection, SLAMF7, Immunomodulation, Neuroinflammation, FACS, Flow cytometry
[Background]
This protocol will be of use to those looking to perform studies assessing the role that myeloid cells play in HIV-1 infection. Myeloid cells, in particular, monocytes, have been recognized as crucial mediators of chronic and acute inflammation in HIV-1 infection (Burdo et al., 2013; Anzinger et al., 2014; Merino et al., 2017). Gaining a deeper understanding of the interactions between the HIV-1 virus and monocytes will be fundamental in advancing our knowledge on how HIV-1 infection produces a chronic state of immune activation, even in the presence of combined anti-retroviral therapy (Anzinger et al., 2014). With persons infected by HIV-1 now exhibiting lower death rates, we are just now seeing the long-term effects of HIV-1-induced chronic immune activation which can present as: HIV-associated neurocognitive disorder (HAND), hepatic steatosis, renal failure, hepatitis, atherosclerosis, insulin resistance, osteoporosis, and more (Cardoso et al., 2013; Katlama et al., 2013).
The method presented here will be useful to both study how HIV-1 infection of monocytes alters cellular function, and to facilitate the development of novel methods to modulate or prevent HIV-1 infection of monocytes. In particular, this protocol is advantageous in that it allows for quick and easy analysis of monocyte HIV-1 infection using FACS without the need to intracellular stain for p24.
Materials and Reagents
LS columns (Miltenyi Biotec, catalog number: 130-042-401)
Glass Pasteur pipettes, autoclaved (VWR, catalog number: 14672-200)
Aspirating bulb for use with Pasteur pipet (CELLTREAT Scientific Products, catalog number: 229279)
50 ml conical tubes (Corning, catalog number: 430290)
Serological pipettes (Costar)
1 ml micropipette
96-well high-binding plates (Corning, catalog number: 3361)
Pipette tips
-
pNL-BaL-GFP recombinant HIV-1 virus
Construction of pNL-BaL-GFP HIV-1 vector. The pNL4-3env(−)GFP, which encodes full-length NL4-3 HIV-1 proviral DNA with a frameshift in env and expresses GFP in place of nef, was described previously (He et al., 1997). The CCR5-tropic HIV-1 proviral clone, R9BaL, was generated from R9 by replacing an EcoRI-XhoI fragment from the CCR5-tropic HIV-1 strain, BaL, which contains the entire env coding region (Gallay et al., 1995). pNL-BaL-GFP was constructed by replacing a SalI-BamHI fragment in pNL4-3env(−)GFP with that from R9BaL.
- Antibodies:
- CD14, PE-Cy7 (clone: 61D3) (Thermo Fisher, catalog number: 25-0149-42)
- CD16, BV510 (Clone: 3G8) (BD BioSciences, catalog number: 563830)
- SLAMF7, PE (Clone: 162) (BioLegend, catalog number: 331806)
- CCR5, unconjugated (Clone: 2D7) (BD BioSciences, catalog number: 555990)
Viability dye: Live/Dead Violet (Thermo Fisher, catalog number: L34964)
CD14 microbeads, human (Miltenyi Biotec, catalog number: 130-050-201)
Fresh human buffy coat (Gulf Coast Regional Blood Center, Texas, USA)
Lympholyte-H (Cedarlane Labs, catalog number: CL5020)
DPBS without calcium or magnesium (Gibco, catalog number: 14190250)
RPMI 1640 (Gibco, catalog number: 11875093)
ACK Lysis buffer (Gibco, catalog number: A1049201)
Fetal Bovine Serum (FBS) (Atlanta Biologicals, catalog number: S11550)
Antibiotic/Antimycotic (Penicillin, Streptomycin, Fungizone) (PSF) (Gibco, catalog number: 15240062)
Ethylenediaminetetraacetic acid (EDTA) (Millipore Sigma, catalog number: E6758)
BSA (Millipore Sigma, catalog number: A9056)
Sodium azide (Millipore Sigma, catalog number: S2002)
Fixation buffer from BD Fix/Perm kit (BD Biosciences, catalog number: 554714)
Cross-linking antibody of choice (for stimulation of monocytes during HIV-1 infection)
FACS solution (see Recipes)
MACS solution (see Recipes)
Complete RPMI solution (see Recipes)
Equipment
Biosafety cabinet
Gun pipette
QuadroMACS magnetic cell separator (Miltenyi Biotec, catalog number: 130-090-976)
LSRII flow cytometer (BD Biosciences)
Tabletop centrifuge with swinging bucket rotor
Automated cell counter (Countess) (Invitrogen)
Software
FlowJo version 10.5 (https://www.flowjo.com)
GraphPad Prism V7 (https://www.graphpad.com/scientific-software/prism/)
Procedure
-
Isolate PBMCs from a fresh human buffy coat using Lympholyte-H under sterile conditions.
Note: Perform all the following steps in a sterile biosafety cabinet.
Allow Lympholyte-H to equilibrate to room temperature. This is important to ensure efficient isolation of peripheral blood mononuclear cells (PBMCs).
Split approximately 50 ml buffy coat evenly into four 50 ml conical tubes (12.5 ml per tube).
Add 22.5 ml DPBS to each tube containing pure buffy coat to bring the volume up to 35 ml. (The ratio of DPBS to buffy coat should be greater than 1:1.)
Shake vigorously (or vortex) the 50 ml conical tubes for 10 s. This is important to prevent clumping of cells which will affect isolation and can cause RBC contamination.
Shake lympholyte-H vigorously just before use.
-
Work in a biosafety cabinet with lights off.
Note: Lympholyte-H is light sensitive.
Place a sterile glass Pasteur pipette into the bottom of a 50 ml conical tube containing the blood/DPBS mixture. Add Lympholyte-H to the glass Pasture pipette using a gun pipette. This is best accomplished using a 10 ml stripette with the power setting on the gun pipette set to “gravity”. A total of 10 ml of Lympholyte-H should be added per 50 ml conical tube.
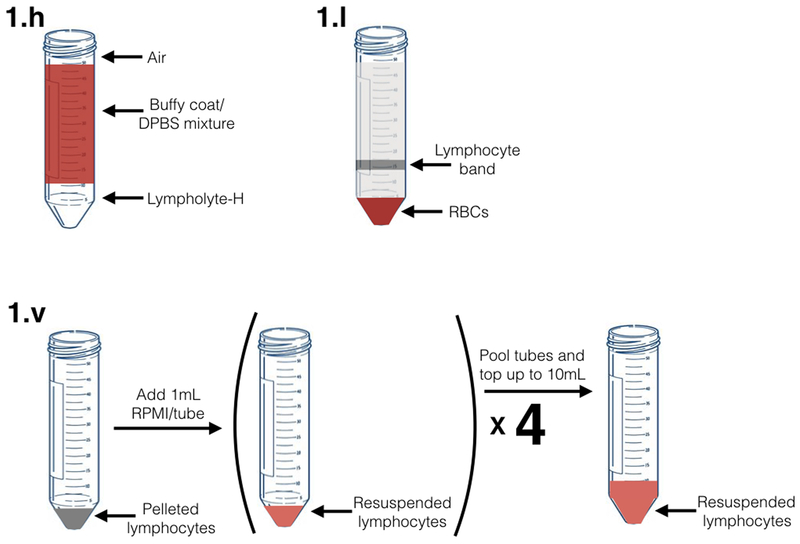
Lympholyte-H should immediately begin to form a layer on the bottom of the conical tube, clearly separated from the buffy coat (Figure 1.h).
Add a total of 10 ml lympholyte-H to each 50 ml conical tube.
Repeat for each 50 ml conical tube. The final volume in each tube will now be 45 ml.
Spin 50 ml conical tubes at 1,800 x g, 24 °C, no break, maximum acceleration, and minimum deceleration, for 30 min in any swinging bucket benchtop centrifuge.
Attach a rubber suction bulb to a fresh Pasteur pipette. Carefully remove the middle lymphocyte band (approximately 5 ml) from each 50 ml conical tube. This band will be cloudy, may contain some RBCs, and will be located above the pelleted RBCs (at the bottom of the tube) (Figure 1.I).
Transfer these cells to a fresh 50 ml conical tube.
Repeat this for each of the four 50 ml conical tubes.
Add DPBS to a final volume of 50 ml into each new 50 ml conical tube containing pure lymphocytes.
Spin the tube at 1,500 x g, 24 °C, full break for 5 min (“full break” means the centerfuge break is on).
Discard the supernatant.
Resuspend each pellet in 2 ml ACK Lysis buffer using a 1 ml micropipette. Ensure pellet is fully resuspended.
Incubate cells in ACK lysis buffer for exactly 6 min at room temperature.
Top with DPBS, spin the tube at 900 x g, 24 °C, full break for 5 min.
Discard the supernatant.
Resuspend in complete RPMI pre-warmed to 37 °C using a 1 ml micropipette. Ensure cells are in a single cell suspension. Add a total of 10 ml of media per buffy coat. At this stage, you can combine cells from the 4 separate fractions (Figure 1.v).
Prepare 1:100 dilution of cells into complete RPMI and count on a Countess (or your preferred method) per manufacturer’s instructions.
-
Isolate CD14+ monocytes by positive selection (CD14 microbeads, Miltenyi Biotec) per manufacturer recommendation using MACS buffer.
Note: Incubate cells with anti-CD14 microbeads for 25 min to increase the purity of isolated monocytes.
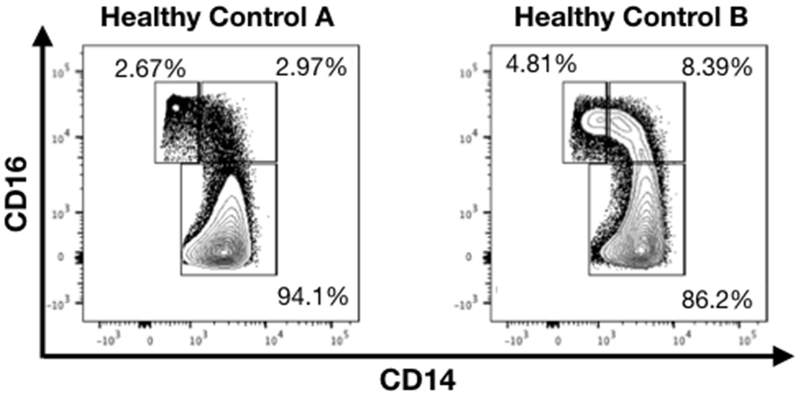
Check the purity of monocytes before proceeding. Stain cells (pre and post-isolation) with CD14 PE-Cy7 mAb and Live/Dead Violet viability dye per manufacturer’s instructions and as previously described (O’Connell et al., 2018). Run cells on LSRII flow cytometer (or other flow cytometers) and determine % of cells CD14+ that are viable (Figure 2). If isolated monocyte samples are less then 90% CD14+ it is recommended not to proceed. Should monocyte purity be < 90% you may return the monocytes to MACS buffer (if you have already resuspended them in complete RPMI) and pass them through a fresh MACS LS column exactly as performed in Step 2.
Resuspend monocytes in complete RPMI and plate in 96-well high-binding cell culture plates at 2 x 105 cells/well.
If activation of a monocyte cell surface receptor is desired (e.g., SLAMF7 [O’Connell et al., 2018]), coat high-binding plate with an antibody against the desired receptor for 24 h at 4 °C beforehand. Dilute cross-linking antibody in DPBS before adding it to wells for cross-linking. Wash wells 3 times with DPBS before addition of cells and make sure wells do not dry out at any point.
As a positive control, if using an R5-tropic HIV-1 virus, add 10 μg/ml of the CCR5-blocking mAb (2D7) per appropriate well. Wells containing this soluble mAb should retain appropriate concentrations of this mAb at all media changes.
After 24 h of cell culture at 37 °C and 4% CO2, remove media and add fresh media containing 190 pg/ml pNL-BaL-GFP.
After 4 h, wash pNL-BaL-GFP infected cells 2 times with complete RPMI.
Resuspend cells in 200 μl complete RPMI/well and culture at 37 °C and 4% CO2.
Every 24-36 h remove an aliquot of cells (i.e., one well) per condition and wash them with FACS.
Stain cells with CD14, CD16, Live/Dead Violet viability dye, and any other antibodies (i.e., SLAMF7) desired as previously described (O’Connell et al., 2018).
Fix cells for 20 min at room temperature, protected from light, by adding 200 μl of BD Fixation buffer per well and thoroughly resuspending cells.
Wash cells twice with FACS.
-
Analyze stained cells on an LSRII (or similar flow cytometer) to determine (%) CD14+ Live/Dead Violet GFP+ cells.
Note: Cells should be analyzed within 24 h of picking them up to ensure GFP signal is not lost. Fixation will not affect GFP signal over short term (less than 24 h), but prolonged fixation will degrade GFP
GFP+ cells are considered to be HIV-1+ as all infected cells using this virus will express GFP. The addition of CD14 and viability markers ensure that cells analyzed are monocytes (i.e., CD14+) and living (i.e., Live/Dead Violet−).
Figure 1. Graphical diagram for selected steps in part 1 of procedure (PBMC) isolation.
Figure labels correspond to indicated steps in PBMC isolation procedure.
Figure 2. FACS plots showing subtypes of monocytes between 2 healthy blood donors.
Healthy control B has considerably more CD16+ monocytes. Cells shown are gated on singlets, myeloid cell population (based on FSC and SSC), living, and expressing CD14.
Data analysis
Analyze all data using FlowJo v10.5 or greater (or other FACS analysis software per your preference).
Analyses should be conducted by assessing the (%) CD14+ Live/Dead Violet GFP+ cells per condition. Inclusion of other markers can be included to allow for additional analyses.
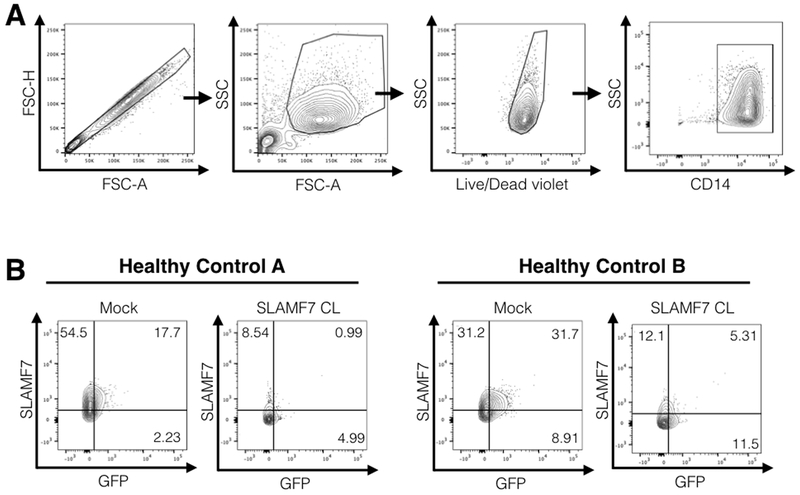
Example analyses are presented in Figure 3.
When comparing infectivity between groups, use a student’s t-test or one-way ANOVA as appropriate.
Data can be displayed as an X-Y graph with connecting lines (O’Connell et al., 2018, Figure 6d) and also as a FACS dot plot to provide additional support for your results (O’Connell et al., 2018, Figure 6d).
Figure 3. Example FACS analysis.
A. Representative gating scheme used for analysis of monocyte infectivity. B. Representative FACS plots showing examples of positive and negative GFP monocytes from the CD14+ gate above. Mock conditions are monocytes infected with pNL-BaL-GFP and no immune cell receptor cross-linking. Note how HIV-1+ monocytes tend to be positive for the SLAMF7 receptor and activation of the SLAMF7 receptor via cross-linking prevents monocyte infection. Co-expression analyses like this can be performed with any other marker of interest. CL; cross-linking.
Notes
This protocol has been reproducible in our hands, and we have only noticed variability in the degree of infectivity between individual blood donors.
Refresh media on cultured monocytes every 48 h.
Culture cells for a maximum of 7 days. Primary human monocytes will not typically survive more than 7 days post-isolation.
Monocytes should show a time-dependent increase in the number of HIV-1+ cells until approximately 6-7 days, at which point the infectivity should level out as monocytes reach their terminal ex-vivo lifespan.
The efficiency of HIV-1 infection of monocytes will vary between subjects and is dependent on the percent of CD16+ monocytes. Individuals with higher levels of CD16+ monocytes will show a greater percent of HIV-1+ monocytes (Figures 2 and 3) (Tuttle et al., 1998; Ellery et al., 2007).
Recipes
-
FACS solution
500 ml DPBS (1 bottle)
1% (W/V) BSA
0.1% (W/V) sodium azide
-
MACS solution
500 ml DPBS (1 bottle)
0.5% BSA
2 mM EDTA (used for CD14 isolation)
-
Complete RPMI solution
500 ml RPMI 1640
10% FBS
1% PSF
Acknowledgments
This work was supported by the NIH in the form of an R21 grant to YAA (1R21AI122808-01). We also thank Sarah Godbehere for her critical review of this manuscript. This method is adapted from Wang et al., 2018.
Footnotes
Competing interests
PO and YAA have a patent pending regarding modulation of SLAMF7 signaling for the treatment of inflammation. The authors declare no additional conflicts.
References
- 1.Anzinger JJ, Butterfield TR, Angelovich TA, Crowe SM and Palmer CS (2014). Monocytes as regulators of inflammation and HIV-related comorbidities during cART. J Immunol Res 569819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burdo TH, Lackner A and Williams KC (2013). Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev 254(1): 102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso SW, Torres TS, Santini-Oliveira M, Marins LM, Veloso VG and Grinsztejn B (2013). Aging with HIV: a practical review. Braz J Infect Dis 17(4): 464–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S and Crowe SM (2007). The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol 178(10): 6581–6589. [DOI] [PubMed] [Google Scholar]
- 5.Gallay P, Swingler S, Aiken C and Trono D (1995). HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell 80(3): 379–388. [DOI] [PubMed] [Google Scholar]
- 6.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay CR, Sodroski J and Gabuzda D (1997). CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature 385(6617): 645–649. [DOI] [PubMed] [Google Scholar]
- 7.Katlama C, Deeks SG, Autran B, Martinez-Picado J, van Lunzen J, Rouzioux C, Miller M, Vella S, Schmitz JE, Ahlers J, Richman DD and Sekaly RP (2013). Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet 381 (9883): 2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merino KM, Allers C, Didier ES and Kuroda MJ (2017). Role of monocyte/macrophages during HIV/SIV infection in adult and pediatric acquired immune deficiency syndrome. Front Immunol 8: 1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connell P, Pepelyayeva Y, Blake MK, Hyslop S, Crawford RB, Rizzo MD, Pereira-Hicks C, Godbehere S, Dale L, Gulick P, Kaminski NE, Amalfitano A and Aldhamen YA (2019). SLAMF7 is a critical negative regulator of IFN-α-Mediated CXCL10 production in chronic HIV infection. J Immunol 202(1): 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuttle DL, Harrison JK, Anders C, Sleasman JW and Goodenow MM (1998). Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J Virol 72(6): 4962–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B, Zuo J, Kang W, Wei Q, Li J, Wang C, Liu Z, Lu Y, Zhuang Y, Dang B, Liu Q, Kang W and Sun Y (2018). Generation of Hutat2:Fc knockin primary human monocytes using CRISPR/Cas9. Mol Ther Nucleic Acids 11: 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]