Abstract
This report presents a case of spontaneous fungal peritonitis (SFP) in a patient with cardiogenic ascites. Physicians need to be aware of this life-threatening condition because early suspicion of SFP can improve survival.
Keywords: chronic heart failure, obesity, systolic heart failure
HISTORY OF PRESENTATION
This is the case of a 36-year-old man who presented with abdominal pain of 1-day evolution. He described the pain as 8/10 intensity, crampy, nonradiating, diffuse, exacerbated by movement, but without relieving factors. Associated symptoms included abdominal distension, unquantified fever, shortness of breath, and lower extremity edema. Physical examination was significant for hypotension of 80/43 mm Hg, fever of 38.9°C, and tachycardia of 110 beats/min. There was a positive ascitic fluid wave with shifting dullness and diffuse tenderness to palpation of the abdomen. Lung auscultation revealed bibasilar crackles and grade 3/4 pitting edema of the lower extremities.
MEDICAL HISTORY
He had a history of Class III morbid obesity and nonischemic dilated cardiomyopathy with an ejection fraction of 35%. Medications included carvedilol, lisinopril, spironolactone, and furosemide for the treatment of heart failure. He denied toxic habits, family history was noncontributory, and review of systems was unremarkable.
DIFFERENTIAL DIAGNOSIS
The differential diagnoses included spontaneous bacterial peritonitis (SBP), spontaneous fungal peritonitis (SFP), or gastrointestinal perforation.
INVESTIGATIONS
Laboratory values were significant for white blood cells of 6,780 cells/mm3 with 51% neutrophils and 31% bands; hemoglobin 11 g/dl, platelet count of 221,000 cells/mm3, blood urea nitrogen 46 mg/dl, serum creatinine 2.6 mg/dl, bicarbonate 27.9 mEq/l, and total bilirubin of 8.6 mg/dl with direct bilirubin of 7.5 mg/dl. Liver enzymes were normal with alanine aminotransferase of 5 IU/l and aspartate aminotransferase of 13 IU/l, lipase 17 IU/l, pro-B-type natriuretic peptide 9,318 pg/ml, serum lactate 18 IU/l, and erythrocyte sedimentation rate of 77 mm/h. An abdominal ultrasound demonstrated ascites and hepatomegaly; there was no evidence of cirrhosis or obstruction of the hepatic vein.
MANAGEMENT
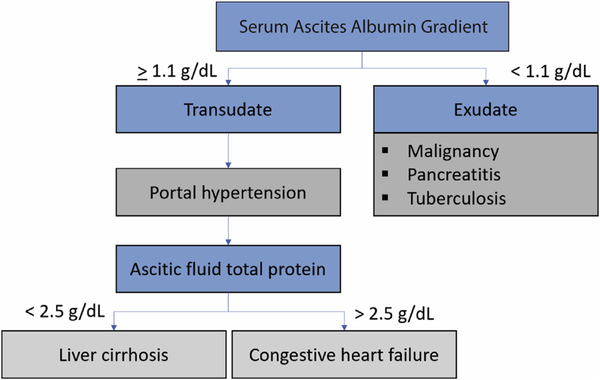
Initial paracentesis revealed >2,000 polymorphonuclear cells, serum-ascites albumin gradient of 1.3 mg/dl (Figure 1), and total ascitic fluid protein of 4.4 g/dl. The ascitic fluid culture was positive for Escherichia coli and Streptococcus sanguinis. These findings were consistent with SBP in the presence of cardiogenic ascites. Because of the presence of >1 organism in ascitic fluid, elevated lactate dehydrogenase, and low glucose levels, an abdominal computed tomography scan with oral contrast was obtained. This study showed no evidence of contrast leakage into the peritoneal cavity, which suggested a preserved intestinal mucosal lining.
FIGURE 1. Ascitic Fluid Diagnostic Algorithm.
This algorithm shows the differential diagnosis of ascites using serum ascites albumin gradient and total proteins.
The patient was admitted with a diagnosis of septic shock secondary to SBP and acute decompensation of chronic systolic heart failure. He was started on intravenous ceftriaxone, furosemide drip, and norepinephrine to maintain a mean arterial pressure >65 mm Hg. A transthoracic echocardiogram revealed a severe biventricular dilation, global hypokinesis, and an ejection fraction of 10%. There was severe tricuspid regurgitation with elevated right ventricular pressure of 72 mm Hg. Right ventricular systolic function was severely decreased.
The patient showed no clinical improvement and continued to deteriorate 48 h later. Antibiotic therapy was changed to ertapenem and daptomycin to cover community-acquired infections.
After changing antibiotic therapy, the patient demonstrated an initial improvement. However, 2 weeks after admission and despite uninterrupted antibiotic therapy, the patient developed increased abdominal distension, pain, recurrence fever, and hypotension that required vasopressor therapy. A new paracentesis was done. Ascitic fluid was still consistent with cardiogenic ascites, but the fluid appearance was turbid, dark yellow; and polymorphonuclear cells were markedly elevated at 118,624 cells/mm3. The ascitic fluid culture was positive for fluconazole-susceptible Candida albicans, consistent with a diagnosis of SFP. He was started on fluconazole with a loading dose of 800 mg, and 400 mg for maintenance, but due to a lack of clinical response, antibiotic therapy was changed to ceftazidime-avibactam and caspofungin. Resolution of SFP was achieved after 30 days of caspofungin. The authors performed a follow-up paracentesis that demonstrated culture negative results for 5 days.
DISCUSSION
SFP is defined as a fungal infection of peritoneal fluid with no apparent intra-abdominal source, anatomical malformation, or malignancy. Although SBP is relatively common in patients with end-stage liver disease, occur ring in approximately 30% of patients as per initial fluid culture, SFP remains rare, with an incidence of 3.5% (1). The organisms most frequently associated with this condition, in descending order, are Candida albicans, Candida glabrata, Candida krusei, Cryptococcus spp., and Aspergillus spp. (2).
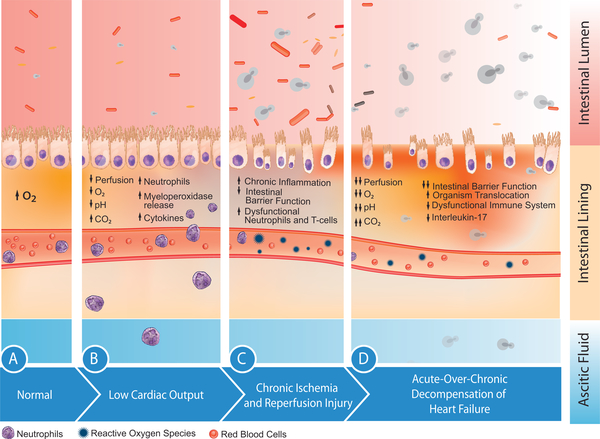
SFP in patients with cirrhosis is attributed to opsonin deficiency, as well as inadequate neutrophil and T-cell response. However, SFP can also happen in patients with cardiogenic ascites (although rarely), in whom impaired host defense seen in patients with end-stage liver disease is usually absent. Some studies have proposed the “gut hypothesis of heart failure” as the predisposing mechanism in this population. This hypothesis states that the gut experiences chronic ischemia–reperfusion damage, compromising the gut barrier morphology and functionality (Figure 2). In animal models, the intestinal mucosal permeability increased in the presence of low blood flow and hypoxemia. Underperfused tissue develops localized intestinal hypoxia and acidic pH, along with a cascade effect of decreased nitric oxide release, increased release of reactive oxygen species, and interleukins. Ischemia leads to tissue malfunction, which then leads to a constant state of inflammation that promotes intestinal flora proliferation.
FIGURE 2. Gut Hypothesis of Heart Failure.
Progressive effects of hypoperfusion on the mucosal barrier. (A) Normal mucosa is affected by (B) low cardiac output, which results in ischemia. (C) Chronic ischemia and reperfusion–injury cause a release in reactive oxygen species, erosion, and distortion of the mucosal barrier. (D) Acute decompensation of chronic heart failure increases the risk of microperforations that facilitates organism translocation into the ascitic fluid. With a weakened immune response and low interleukin-17, the host becomes predisposed to multiple infections, including fungi. Antibiotics change intestinal flora, which facilitates the growth of fungal species, which then may translocate into the ascitic fluid. CO2 = carbon dioxide; O2 = oxygen.
Furthermore, acute decompensation of systolic heart failure worsens tissue edema, and splanchnic hypoperfusion because the hepatosplanchnic circulation receives approximately 30% of the total cardiac output. In a decreased cardiac output state, such as in this patient, it is understood that perfusion to organs is proportionately decreased. Our patient had septic shock that required vasopressors, on top of systolic heart failure, which worsened splanchnic circulation.
Fungi are much larger than bacteria, which makes it more difficult for this infection to occur. Some have theorized that antibiotic exposure leads to fungal overgrowth within the alimentary tract, increasing their virulence and making translocation conceivable. Our patient received antibacterial therapy for the management of the initial SBP. This might explain the progression to SFP.
Other investigators classify SFP as a nosocomial infection, arguing that peritoneal fungal infections can be explained by direct percutaneous inoculation in patients with refractory ascites, which leads to repeated paracentesis. However, this patient had undergone only 1 paracentesis 2 weeks before the diagnosis of SFP.
SFP is a life-threatening complication, with an estimated mortality of 56% to 90%. Tardy suspicion, current diagnostic (culture) methods, the difficulty of early differentiation from SBP, and delay in therapy may account for its high mortality (3). Some studies support the use of polymerase chain reaction (PCR) for (1→3)-β-D-glucan and galactomannan testing for early diagnosis of fungal peritonitis. Wherever available, PCR is encouraged because it is a cost-effective alternative that delivers rapid and reliable results, leading to early antifungal therapy (4).
The authors found only 1 other reported case of SFP in a patient with cardiogenic ascites. Unlike this patient, this one was infected by C. glabrata and was successfully treated with intravenous caspofungin (5). Similar to this case, this patient was treated with antibiotics for >2 weeks before presenting with SFP. Our patient had an unsatisfactory response to azole therapy but responded well to caspofungin. Although this condition is uncommon, azole therapy is generally considered first-line therapy when SFP is suspected, until the organism and sensitivity are identified.
FOLLOW-UP
After 5 months of hospitalization and multiple complications, the patient lost 178 lbs of weight, and there was a complete resolution of ascites; left ventricular ejection fraction improved to 35%. Subsequently, serum creatinine decreased to 1.6 mg/dl.
CONCLUSIONS
SFP of cardiogenic ascites is extremely rare and can be easily underdiagnosed. Nonculture-based fungal diagnostic tests, such as PCR, should be considered in patients with a history of repeated paracentesis or who fail to respond to antibacterial therapy for SBP. Fluconazole is an acceptable first-line therapy whenever invasive candidiasis is suspected because it can improve overall prognosis.
LEARNING OBJECTIVES.
To suspect SFP in patients with cardiogenic ascites.
To become familiar with the gut hypothesis of heart failure.
ABBREVIATIONS AND ACRONYMS
- SBP
spontaneous bacterial peritonitis
- SFP
spontaneous fungal peritonitis
- PCR
polymerase chain reaction
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Hwang SY, Yu SJ, Lee JH, et al. Spontaneous fungal peritonitis: a severe complication in patients with advanced liver cirrhosis. Eur J Clin Microbiol Infect Dis 2014;33: 259–64. [DOI] [PubMed] [Google Scholar]
- 2.Shi L, Wu D, Wei L, et al. Nosocomial and community-acquired spontaneous bacterial peritonitis in patients with liver cirrhosis in China: comparative microbiology and therapeutic implications. Sci Rep 2017;7: 46025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shizuma T Spontaneous bacterial and fungal peritonitis in patients with liver cirrhosis: a literature review. World J Hepatol 2018;10:254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagès A, Iriart X, Molinier L, et al. Cost-effectiveness of candida polymerase chain reaction detection and empirical antifungal treatment among patients with suspected fungal peritonitis in the intensive care unit. Value Heal 2017;20: 1319–28. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Gandhi S, Attar BM. Spontaneous fungal peritonitis in ascites of cardiac origin. ACG Case Rep J 2017;4:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]