Introduction
The United States has the highest antiretroviral treatment (ART) prices yet the lowest rate of HIV viral suppression (54%) compared to all other well-resourced countries, including Britain, Australia and Canada.1 High ART costs are among many structural barriers that lead to poor treatment access and adherence, contributing to suboptimal HIV outcomes in the US.2 We sought to characterize costs of recommended initial ART regimens in the US from 2012–2018 and the magnitude of cost changes over time.
Methods
The US Department of Health and Human Services updates the Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents annually; since 2012, these Guidelines include the Average Wholesale Price (AWP) of available regimen components.3 Recommended initial ART regimens for “most people with HIV (PWH)” demonstrate durable virologic efficacy, favorable tolerability/toxicity, and ease of use. Alternative initial ART regimens are recommended in “certain clinical situations,” such as presence of co-morbidities. We used data from the Guidelines to determine the annual per person cost of each of the recommended initial regimens from 2012–2018. We calculated the trend in average regimen costs, annually, and the percent change over time for all regimens recommended for most PWH, as well as in certain clinical situations. Then, for each regimen that was recommended for most PWH and subsequently recommended only in certain clinical situations, we compared the changes in costs over time as those recommendations evolved. All cost trends were compared to the consumer price index (CPI, U.S city average, all urban consumers). We used the CPI-U, rather than the medical care component of the CPI, because of its standard use in discounting and rebating formulas for Federal and Medicaid Ceiling Pricing.4
Results
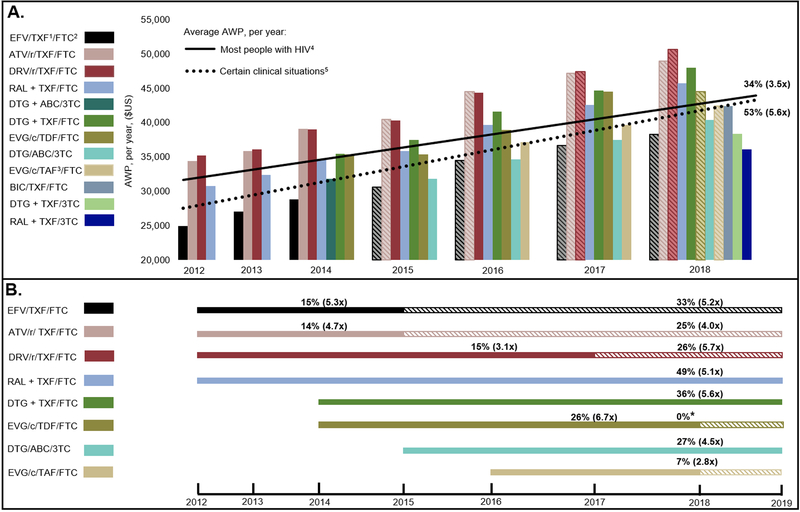
In 2012, the annual AWP of initial ART regimens recommended for most PWH ranged $24,970-$35,160 and increased to $36,080-$48,000 in 2018. The average annual cost of ART recommended for most PWH has increased 34% since 2012, 3.5 times faster than inflation (Figure, Panel A). The average annual cost of initial ART regimens recommended for PWH in certain clinical situations was lower ($25,930-$39,670) from 2012–2018, but has increased 53%, 5.6 times faster than inflation. Regimens recommended for most PWH increased 2.8–6.7 times faster than inflation during their recommended horizon (Figure, Panel B, solid bars); after falling out of recommendation for most PWH to certain clinical situations, the AWP of these regimens still increased 4.0–5.7 times faster than inflation (Figure, Panel B, hatched bars).
Figure. Average Wholesale Price of Initial ART, 2012–2018.
A) Change in ART Average Wholesale Prices (AWPs) among initial ART regimens over time: Time (2012–2018) is given on the horizontal axis. The vertical axis shows the annual AWP ($US). The solid black line represents the average AWP of all initial ART recommended for most PWH from 2012–2018. The dotted black line represents the average AWP of all initial ART regimens recommended in certain clinical situations from 2012–2018, which includes some regimens not depicted in the figure. For example, because darunavir/cobicistat- and doravirine-based regimens were never recommended for most PWH, they are not shown as bars, but their costs are calculated into the average cost of initial regimens for people with HIV in certain clinical situations (dotted line). The percent change in initial ART regimen costs from 2012–2018 is shown. In parentheses, the change compared to inflation is shown (CPI-U: U.S. city average, all urban consumers, not seasonally adjusted). Each colored solid bar represents a different initial ART regimen recommended for most PWH. Each hatched bar represents the regimen after it was moved from recommended for most PWH to recommended only in certain clinical situations. DTG + ABC/3TC alone fell completely out of recommendations for initial ART regimens during this time horizon.
B) Change in individual initial ART regimen costs for most PWH over time. Each solid bar represents a regimen that was recommended for most PWH for more than one year, over the timeline below it. Each hatched bar represents a regimen that was recommended for most PWH (initially indicated as solid), and then became limited to recommendation only for certain clinical situations (indicated as hatched). The percent change in the regimen cost over the time it was recommended is shown and, in parentheses, the change compared to inflation is provided (CPI-U: U.S. city average, all urban consumers, not seasonally adjusted).
1TDF or TAF are denoted as “TXF”. In 2012–2015, tenofovir is tenofovir disoproxil fumarate (TDF). In 2016–2018, tenofovir may be TDF or tenofovir alafenamide (TAF). Unless otherwise specified, Average Wholesale Prices (AWPs) of TDF and TAF are the same.
2FTC may be substituted with cheaper 3TC, which is available generically; we did not include 3TC substitution costs here. We included TDF/3TC in 2018.
3Cost of TDF and TAF are only different in combination with EVG/c/FTC.
4,5DHHS guidelines distinguish recommended regimens for “most people with HIV” from those in “certain clinical situations”, who may not tolerate the most common initial ART regimens and are therefore eligible for alternative regimens.
*This may indicate a lack of updated cost data or no change in cost from 2017–2018.
Regimens recommended for only one year (DTG + ABC/3TC, BIC/TXF/FTC, DTG+TXF/3TC, RAL+TXF/3TC) are not shown in Panel B.
Discussion
Even with new generic options in 2018, initial regimens recommended for most PWH today are all priced over $36,000/patient/year with annual costs that increased 6%, on average, since 2012. Increases in ART costs far outpaced the overall inflation rate.
Although US HIV prevalence is low, ART is the nation’s fifth costliest therapeutic class, accounting for US$22.5 billion in spending in 2018.5 The federal “Ending the HIV Epidemic” initiative aims to achieve a 90% decrease in new HIV infections by 2030. To do so, the US needs to increase viral suppression by 33%,1 which will require a total of $35.6 billion in annual spending on ART alone.
Complex systems of discounts and rebates help insulate public and private insurers from the full brunt of high ART costs and federally funded safety nets may minimize costs for patients. However, in response to mounting ART costs and prolonged survival among PWH, insurers are increasingly seeking to manage ART access through formulary design, utilization management, and cost-sharing.6 We provide these details so that physicians can be sensitive to drug costs when recommending initial regimens of similar efficacy, as costs borne directly by patients can affect their adherence and engagement in care. Slowing the trend of rapidly increasing ART costs is essential to expand and sustain access to effective individualized care and treatment for PWH and to meet “End the HIV Epidemic” goals.
Funding Sources
This work was supported by the National Institute of Allergy and Infectious Diseases (R01 AI042006) and the National Heart Lung and Blood Institute (K01HL123349). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.HIV viral suppression rate in U.S. lowest among comparable high-income countries. The Henry J. Kaiser Family Foundation. https://www.kff.org/hivaids/slide/hiv-viral-suppression-rate-in-u-s-lowest-among-comparable-high-income-countries/. Published 2019. Accessed November 7, 2019.
- 2.Zamani-Hank Y. The Affordable Care Act and the Burden of High Cost Sharing and Utilization Management Restrictions on Access to HIV Medications for People Living with HIV/AIDS. Popul Health Manag. 2016;19(4):272–278. doi: 10.1089/pop.2015.0076 [DOI] [PubMed] [Google Scholar]
- 3.U.S Department of Health and Human Services. Archived Adult and Adolescent Guidelines. AIDSinfo. https://aidsinfo.nih.gov/guidelines/archive/adult-and-adolescent-guidelines. Accessed November 7, 2019.
- 4.CPI for All Urban Consumers (CPI-U). United States Department of Labor: Bureau of Labor Statistics. https://data.bls.gov/PDQWeb/cu. Accessed November 7, 2019. [Google Scholar]
- 5.Medicine use and spending in the U.S: a review of 2018 and outlook to 2023. The IQVIA Institute; https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published 2019 Accessed November 5, 2019. [Google Scholar]
- 6.Discriminatory Design: HIV Treatment in the Marketplace. NASTAD. https://www.nastad.org/sites/default/files/Discriminatory-Design-HIV-Treatment-in-the-Marketplace.pdf. Published 2016. Accessed November 5, 2019.