Abstract
Urinary cotinine is one of the most commonly measured biomarkers reflecting recent exposure to nicotine. In some cases a simple qualitative dichotomization of smokers and non-smokers is all that is required. NicAlert® test strips have been evaluated for this purpose, but other recently introduced, inexpensive single-line test strips have not. In this study we evaluated two such strips with nominal cutoffs of 200 and 10 ng/mL. A total of 800 urine samples with known cotinine concentrations determined by an LC–MS-MS method were examined, including 400 urine samples ranging from 0.23 to more than 24,000 ng/mL by the 200 ng/mL strip, and 400 samples with concentrations <200 ng/mL by the 10 ng/mL cutoff strip. Both test strips performed well in these evaluations. Classification relative to LC–MS-MS by the 200 ng/mL strips had a sensitivity of 99.5% and specificity of 92%, with 95.8% accuracy. The 10 ng/mL strips had a sensitivity of 98.7% and specificity of 90.1%, with 93.3% accuracy. The positive predictive value for the 200 ng/mL strips was 92.6% and the negative predictive value was 99.5%. For the 10 ng/mL strips, the corresponding values were 85.4 and 99.2%, respectively. The prevalence of positive samples was 50% in the 200 ng/mL group, and 37% in the 10 ng/mL set. Each strip was read by two readers with an overall agreement of >98%. Our results suggest that these simple and inexpensive lateral flow immunoassay test strips can provide useful qualitative estimates of nicotine exposures for appropriate applications within the inherent limitations of sensitivity and precision of the immunoassay test strip format.
Introduction
Tobacco use and cessation studies often include measurements of cotinine, a primary nicotine metabolite, as a biomarker of exposure. These assays are typically conducted in readily accessible matrices such as urine or saliva (1–3). Sensitive liquid-chromatography tandem mass spectrometry assays for accurately measuring cotinine in blood, urine, saliva and other matrices are well-established and available in several academic, government and commercial laboratories (3). The reliable performance of these assays in both serum and urine has been documented by inter-laboratory evaluations (4, 5). However, while these assays are valuable and indeed essential for many studies, they also are relatively time-consuming and expensive, and laboratory capacity is often limited. Therefore, for certain less demanding purposes such as an initial separation of presumed tobacco users from non-users, or confirming compliance in smoking cessation programs, investigators may consider the use of accessible and relatively inexpensive non-laboratory based tests such as exhaled carbon monoxide measurements or the use of lateral flow immunoassay (LFIA) cotinine test strips (3). The latter are of particular interest since unlike CO measurements which reflect recent exposure to combustion by-products (e.g. from smoking), cotinine assays are specific for nicotine exposure.
To date, most literature reports concerning the use of cotinine test strips in urine or saliva have used Nymox NicAlert®, which is a multilevel device consisting of several “reland” bands with varying affinities for cotinine which is described as providing semi-quantitative results. NicAlert® strips are based on a standard competitive LFIA approach for each band based on the use of monoclonal cotinine antibodies and colloidal gold nanoparticles for visual detection. The performance of NicAlert® strips in estimating urine or saliva cotinine concentrations has been evaluated and reported previously by several authors (6–10).
More recently, newer LFIA strips for urine or in some cases saliva cotinine measurements have become available based on a traditional design with a single test band with a defined cutoff value. For urine samples, the nominal strip cutoff level for smokers vs non-smokers is usually set by the manufacturer to 200 ng/mL (3), although lower cutoff levels are also available. These newer cotinine LFIA devices are significantly less expensive than NicAlert® strips and are widely available online as over-the-counter (OTC) devices from many suppliers. However, unlike NicAlert®, there is presently no information available on the reliability of these newer strips other than the limited insert reports that are (usually) provided by the manufacturers. In this study we evaluated the performance of two LFIA cassette devices purchased from on-line suppliers, and compared them relative to the total cotinine concentrations as measured by LC tandem mass spectrometry in a total of 800 urine samples.
Materials and Methods
Test devices
We purchased urinary cotinine test strips in cassette format (DCT-102–200) with a reported cutoff of 200 ng/mL (COT200) from CLIAwaived (https://www.cliawaived.com, 1578 Sorrento Valley Road, San Diego, CA 92121), and another set of test strip cassettes (DCT-102) with a 10 ng/mL cutoff concentration (COT10) from All Test (http://www.custom-monoclonalantibody.com, 550 Yinhai Street, Hangzhou 310018, PR China). All strips were individually packaged in sealed pouches with desiccant, and were opened immediately prior to use. The CLIAwaived test strips were all lot number COT602004 and those from All Test were lot number COT17060012.
Urine samples
The urine samples used in this study were residual frozen aliquots from samples that had been analyzed as part of the NIH-FDA Population Assessment of Tobacco and Health (PATH) study (11, 12). Westat’s Institutional Review Board approved the study design and data collection protocol including cotinine measurements for PATH. All samples had been analyzed for total cotinine, trans-3′-hydroxycotinine and usually several other analytes by one of two fully validated LC tandem mass spectrometry methods depending on the concentration levels (7, 13, 14). The total dataset from which these samples were drawn consisted of 10,263 samples, ranging in total cotinine concentration from 0.23 to 24,900 ng/mL. For the COT200 test strips we divided the dataset into two subsets with samples either above or below 200 ng/mL for total cotinine. The two sets were then sorted by increasing concentration and every nth sample from that set was selected for analysis. A total of 400 samples were selected overall with 200 from each of the two subsets. For the COT10 strips we selected 400 samples from the low subset (200 ng/mL or less of total cotinine), again by selecting every nth sample from the ranked set for a distributed range of values. Of the total 800 samples, 22 (2.8%) were included in both data sets.
Analysis
LFIA assays were conducted according to the manufacturer’s directions. Urine vials were completely thawed and well-mixed prior to assay. Packs were opened immediately before use and 80 μL of well-mixed urine was pipetted into the sample well. The strips were placed on the bench and left to develop for 5 min at which point the results (presence or absence of colored bands) were independently read and recorded by two analysts who were blind to the identity of the samples. LFIA strips were coded as negative (i.e. below the defined cutoff concentration) if any red color was visible in the test band region; otherwise they were coded as positive. All control bands were visible in each case, confirming normal development. Data were collected over time by entry into an Excel file, and the final results were processed using SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Table I summarizes the results obtained for both test devices in this study. The agreement between the two independent readers was excellent at 98–99% in both cases. There was seldom any ambiguity in interpreting the bands. The results given in Table I are based on those of the primary reader (H.A.) only, since test strips would normally be evaluated by a single individual.
Table I.
Classification using (a) 200 ng/mL strips (COT200) and (b) 10 ng/mL strips (COT10)
| (a) LC tandem mass spectrometry | (b) LC tandem mass spectrometry | |||
|---|---|---|---|---|
| Total cotinine >200 ng/mL | Total cotinine <200 ng/mL | Total cotinine >10 ng/mL | Total cotinine <10 ng/mL | |
| Test strip positive (%) | 199 (49.8) | 16 (4) | 146 (36.5) | 25 (6.3) |
| Test strip negative (%) | 1 (0.25) | 184 (46) | 2 (0.5) | 227 (56.8) |
| Sensitivity = 99.5% | Sensitivity = 98.7% | |||
| Specificity = 92.0% | Specificity = 90.1% | |||
| Positive predictive value = 92.6% | Positive predictive value = 85.4% | |||
| Negative predictive value = 99.5% | Negative predictive value = 99.2% | |||
| Accuracy = 95.8% | Accuracy = 93.3% | |||
| n = 400 | n = 400 | |||
| Agreement between both readers: 99.4% | Agreement between both readers: 97.9% | |||
All tabulated results are from the Primary reader.
Figure 1A and C shows the log distribution of the selected samples for the COT200 and COT10 test strips, respectively. For a classification based on 200 ng/mL of total cotinine, the a priori prevalence of positive samples in the COT200 group was 50%. For the COT10 classification group the prevalence of positive samples was 37% (148/400). Figure 1B and D shows the range of individual classification results for the corresponding dataset (COT200 or COT10) as a function of concentration.
Figure 1.
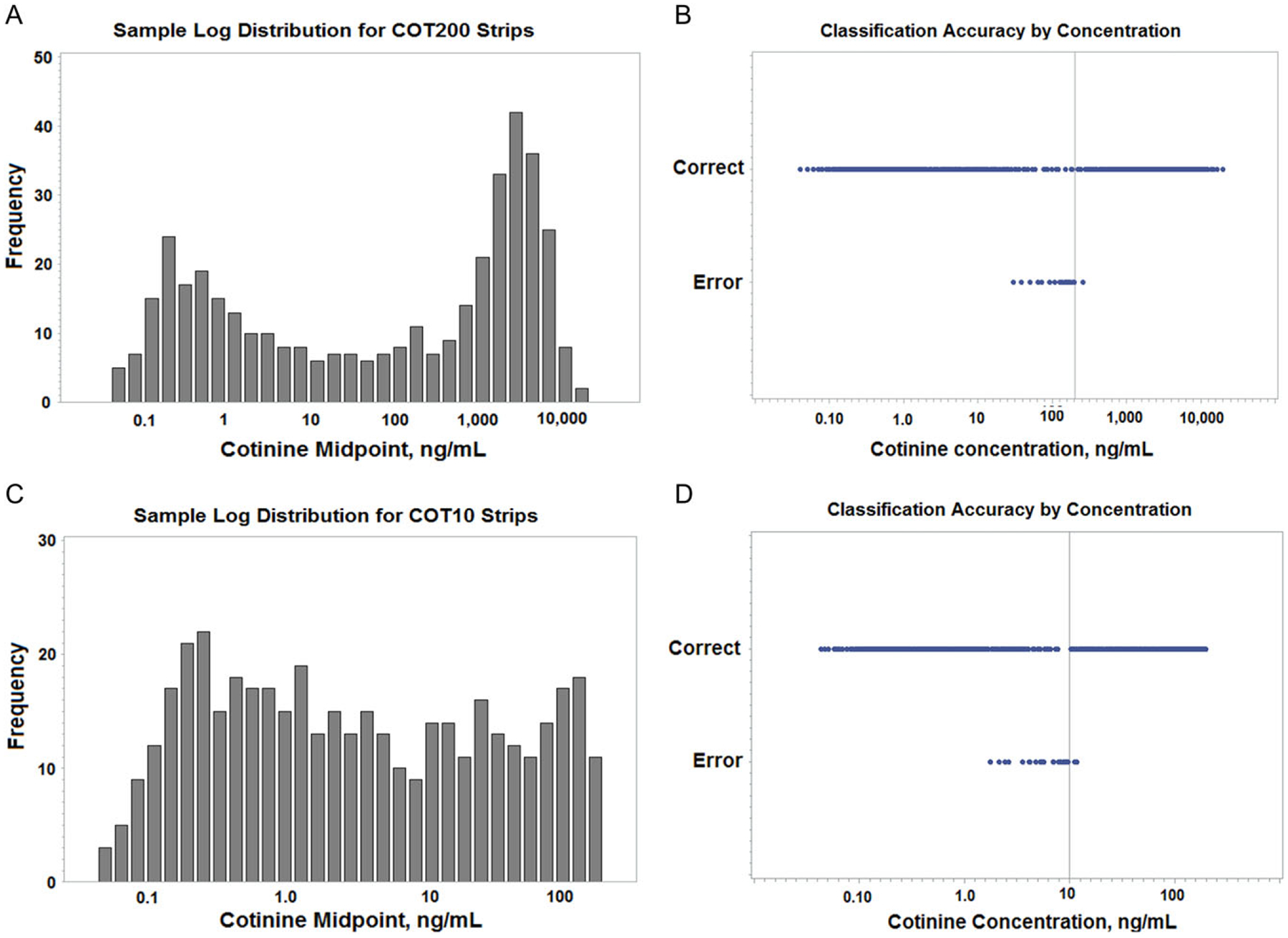
Sample distributions and classification accuracies by concentration. Panels (A) and (C) are the log distributions of the samples selected for use with the COT200 and COT10 test strips, respectively. Panels (B) and (D) are the classification results relative to concentration for COT200 and COT10 samples, respectively. Results labeled “Correct” were properly classified based on the stated cutoffs for the test strips. The results labeled “Error” are incorrect classifications, including both false positive and false negative results. The vertical lines represent the nominal cutoff concentration for the test strip. The classifications found for the samples analyzed with the COT200 strips were: true positive = 199, false positive = 16, true negative = 184, false negative = 1. For the COT10 strips the corresponding values were: true positive = 146, false positive = 25, true negative = 227, false negative = 2.
When constrained to samples with narrower limits around the nominal cutoff concentration, the strips retained good sensitivity although the specificities were noticeably lower. COT200 strips used to classify a subset of 63 samples with total cotinine concentrations between 10 and 500 ng/mL had a sensitivity of 92.8% and specificity of 67.3%. COT10 strips applied to 147 urine samples with total cotinine concentrations within the range 1–20 ng/mL had a sensitivity and specificity of 93.9 and 72.8%, respectively.
ROC analyses of the COT200 and COT10 results (not shown) resulted in AUC values of 0.9970 and 0.9914, respectively. The optimum cutoff concentration point estimated from a logistic model for COT200 was 126.2 ng/mL based on the maximum Youden’s J index. The corresponding value for the COT10 model was 5.3 ng/mL. In both cases the effective cutoff value of the strips was lower (~53–63%) than the stated cutoff concentration.
Reclassification of the COT200 test strip data based on a cotinine cutoff of 126.2 ng/mL improved the specificity with values for sensitivity and specificity of 97.6 and 95.7%, respectively. Similarly, reclassification of the COT10 results using a total cotinine value of 5.3 ng/mL also improved the results with sensitivity and specificity of 95.3 and 95.7%, respectively. However, any increase in specificity necessarily reduces the sensitivity, and the optimum classification efficiency of nicotine exposures typically will encompass a concentration range rather than a single point estimate.
Discussion
Our results indicate that simple, inexpensive LFIA devices are capable of providing good results in the qualitative screening of urine samples for their cotinine content. It should be noted that our analysis was specifically of the prediction of total urinary cotinine cutoffs by the test strips relative to LC–MS-MS concentrations, and did not address reported tobacco use status of the individuals. The cotinine concentration of spot urine samples is generally regarded as a good estimate of recent nicotine intake, but it is known to be subject to substantial variability both within and between individuals (15), and urinary cotinine concentrations among similarly active tobacco users may differ. Hydration and relative concentration differences of samples is another established issue that may be partially addressed by creatinine adjustments, although the value of such corrections remains controversial (16, 17). Consequently, classification of an individual as a smoker or non-smoker from a single urine analysis is not definitive even when sophisticated and reliable assays such as LC–MS-MS are used. However, in most cases the difference in urinary cotinine concentration between regular smokers and non-smokers is several orders of magnitude.
As can be seen in Figure 1B and D, all misclassified values using either strip were in the vicinity of the nominal cutoff concentration and were predominately false positives. For COT200 strips the false positive concentrations ranged from 196 down to as low as 30 ng/mL. The false negative result sample had a concentration of 260 ng/mL. With COT10 strips the false positive samples had total cotinine concentrations of 1.8–9.6 ng/mL; the two false negatives were 11.2 and 11.8 ng/mL. Outside of those limits, all samples were correctly classified.
Trans-3′-hydroxycotinine is another important metabolite in urine that often is more abundant than cotinine. Furthermore, cotinine and hydroxycotinine may exist in both free and glucuronide forms. Our LC–MS-MS assays were of total cotinine following hydrolysis of the glucuronides, and included the sum of both forms in the sample. Conversely, our evaluation of the LFIA cassettes with pure standards indicated that they were not sensitive to cotinine-N-glucuronide and thus the test strips likely measured only the free cotinine form. However, some cross-reactivity with standards of both free hydroxycotinine and its O-glucuronide was observed and this might have contributed to at least some misclassifications since with both strips, the misclassified groups had hydroxycotinine/cotinine ratios that averaged twice as high (4.4 vs 2.2) as the correctly classified samples.
Although our results indicate that good classification efficiency can be obtained with these LFIA cotinine test strips, there are a number of limitations. One of the major problems of LFIA continues to be reproducibility between strips which at least partly reflects the complexity of the preparation, handling and storage of these devices. In addition, the number of different companies providing OEM (Original Equipment Manufacturer) sourcing and the difficulty in determining the provenance of a local suppliers products is a concern. Of the two cassettes that we evaluated in this study, the COT10 devices were manufactured by All Test, but we could not confirm the source of the COT200 product, although it may also have been All Test. Until greater transparency is available concerning the manufacture and sourcing of these devices, care in selecting cotinine LFIA suppliers is important. Nevertheless, our results indicate that these relatively new, low cost LFIA test strips for cotinine are capable of providing useful initial qualitative screening information on the urinary cotinine concentrations of individuals when appropriately applied to the types of studies for which they are suited.
Acknowledgments
This project was supported in part by an appointment to the Research Participation Program at the US Centers for Disease Control and Prevention, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and CDC.
Funding
This work was supported by funds from the US Centers for Disease Control and Prevention, and with Federal funds from the National Institute on Drug Abuse, National Institutes of Health, and the Center for Tobacco Products, Food and Drug Administration, Department of Health and Human Services, under a contract to Westat (Contract Nos. HHSN271201100027C and HHSN271201600001C).
Footnotes
Supplementary data
Supplementary material is available at Journal of Analytical Toxicology online.
Publisher's Disclaimer: Disclaimer
The views and opinions expressed in this article are those of the authors only and do not necessarily represent the views, official policy or position of the US Department of Health and Human Services or any of its affiliated institutions or agencies. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the US Department of Health and Human Services, CDC or FDA.
Presentation
A preliminary account of this work was presented at the 24th Annual Meeting of the Society for Research on Nicotine and Tobacco, “Evaluation of the accuracy and reliability of commonly available cotinine immunochromatographic test kits.” Lanqing Wang, June Feng, John Bernert, Honest Achilihu.
References
- 1.Jarvis MJ, Russell MAH, Benowitz NL, Feyerabend C (1988) Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. American Journal of Public Health, 78, 696–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benowitz NL (1996) Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiologic Reviews, 18, 188–204. [DOI] [PubMed] [Google Scholar]
- 3.Schick SF, Blount BC, Jacob P III, Saliba NA, Bernert JT, El Hellani A, et al. (2017) Biomarkers of exposure to new and emerging tobacco delivery products. American Journal of Physiology—Lung Cellular and Molecular Physiology, 313, L425–L452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernert JT, Jacob P III, Holiday DB, Benowitz NL, et al. (2009) Interlaboratory comparability of serum cotinine measurements at smoker and nonsmoker concentration levels: a round-robin study. Nicotine & Tobacco Research, 11, 1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Bernert JT, Benowitz NL, Feng J, Jacob P, McGahee E, et al. (2018) Collaborative method performance study of the measurement of nicotine, its metabolites, and total nicotine equivalents in human urine. Cancer Epidemiology Biomarkers and Prevention. DOI: 10.1158/1055-9965.EPI-17-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooke F, Bullen C, Whittaker R, McRobbie H, Chen MH, Walker N (2008) Diagnostic accuracy of cotinine test strips in saliva for verifying smoking status. Nicotine & Tobacco Research, 10, 607–612. [DOI] [PubMed] [Google Scholar]
- 7.Bernert JT, Harmon TL, Sosnoff CS, McGuffey JE (2005) Use of cotinine immunoassay test strips for preclassifying urine samples from smokers and nonsmokers prior to analysis by LC-MS-MS. Journal of Analytical Toxicology, 29, 814–818. [DOI] [PubMed] [Google Scholar]
- 8.Marrone GF, Shakleya DM, Scheidweiler KB, Singleton EG, et al. (2011) Relative performance of common biochemical indicators in detecting cigarette smoking. Addiction (Abingdon, England), 106, 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Best D, Green EM, Smith JH, Perry DC (2010) Dipstick tests for secondhand smoke exposure. Nicotine & Tobacco Research, 10, 551–556. [DOI] [PubMed] [Google Scholar]
- 10.Scheidweiler KB, Marrone GF, Shakleya DM, Singleton EG, Heishman SJ, Huestis MA (2011) Oral fluid nicotine markers to assess smoking status and recency of use. Therapeutic Drug Monitoring, 33, 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Population assessment of tobacco and health. (2017). https://pathstudyinfo.nih.gov/UI/HomeMobile.aspx (accessed July 30, 2018). [Google Scholar]
- 12.PATH Study Team. (2017) Design and methods of the Population Assessment of Tobacco and Health (PATH) study. Tobacco Control, 26, 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGuffey JE, Wei B, Bernert JT, Morrow JC, Xia B, Wang L, et al. (2014) Validation of an LC/MS/MS method for quantifying urinary nicotine, six nicotine metabolites and the minor tobacco alkaloids—anatabine and anabasine—in smoker’s urine. PLoS One, 9, e101816 Doi: 10.1371/journal.pone.0101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei B, Feng J, Rehmani IJ, Miller S, McGuffey JE, Blount BC, et al. (2014) A high-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clinica Chimica Acta, 436, 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benowitz NL, Lessov-Schlaggar CN, Swan GE (2008) Genetic influences in the variation in renal clearance of nicotine and cotinine. Clinical Pharmacology and Therapeutics, 84, 243–247. [DOI] [PubMed] [Google Scholar]
- 16.Carrieri M, Trevisan A, Bartolucci GB (2001) Adjustment to concentration-dilution of spot urine samples: correlation between specific gravity and creatinine. International Archives of Occupational and Environmental Health, 74, 63–67. [DOI] [PubMed] [Google Scholar]
- 17.Waikar SS, Sabbisetti VS, Bonventre JV (2010) Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney International, 78, 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]


