Abstract
Rationale: Acute exposure to chlorine gas results in respiratory impairment, but few data are available on the pathobiology of the underlying lung damage. Objectives: To assess lung function and potential lung damage pathways in the acute phase and longitudinally over a 15-month follow-up after chlorine exposure. Methods: Ten previously-healthy children were accidentally exposed to chlorine gas at a swimming pool due an erroneous servicing procedure. Exhaled nitric oxide (FENO), exhaled breath condensate (EBC) compounds and serum Clara cell protein (CC16) were repeatedly measured. Main results: In the acute phase, all patients had respiratory distress (one child required mechanical ventilation) and reduced lung function (median and IQR: FVC 51% pred. [43-60], FEV1 51% pred. [46-60]). This was accompanied by low FENO (4.7 [3.9-7.9] ppb), high EBC leukotriene B4 (LTB-4) levels (24.4 [22.5-24.9] pg/mL) and increased serum CC16 levels (mean ± SE 23.4 ± 2.5 μg/L). Lung function returned to normal in 15 days (FVC 97% pred. [82-108] and FEV1 92% pred. [77-102]). FENO reached normal values after 2 months (12.6 [11.4-15] ppb), while LTB-4 levels were still increased (12 [9.3-17.1] pg/mL). Conclusion: Children acutely exposed to chlorine in a swimming pool presented a substantial lung function impairment associated with biochemical exhaled breath alterations, mainly represented by an increase in LTB-4 and a reduction in FENO. While lung function and FENO improved within a few weeks, the increased levels of exhaled LTB-4 persisted for several months.
Keywords: Chlorine inhalation, Pulmonary function, Exhaled nitric oxide, Exhaled breath condensate, Pneumoproteinemia
Introduction
Acute chlorine inhalation results in a variety of dose-related lung effects ranging from respiratory mucous membrane irritation to pulmonary edema with acute respiratory failure, but few data are available on the pathobiology of lung damage underlying this intoxication (1-4). Though a recovery is the most likely outcome, there is still concern as to the possibility of long-term sequelae (5-9).
In the last decade, there has been an increasing interest in the application of noninvasive methods to assess the pathobiological mechanisms underlying respiratory disorders. In particular, analyzing biomarkers in exhaled breath, considering exhaled gases and exhaled condensate, has been widely used in pulmonology research settings (10). Being completely non-invasive, the analysis of exhaled breath has the potential for addressing unmet medical needs because the respiratory tract can be repeatedly sampled, enabling longitudinal studies in a wide range of settings.
Among the broad spectrum of gaseous compounds detectable in exhaled air, exhaled NO (FENO) is the most extensively studied marker. In the lung, NO plays a key role in the physiological regulation of vessel and airway tone, and it can be altered in several heart-lung diseases (11).
Exhaled breath condensate (EBC) is a fluid obtained by freezing exhaled air under spontaneous breathing conditions and provides a non-invasive means for exploring several aspects of lung biology and pathobiology. A great variety of molecules originating from the surface of the airways can be measured in EBC, including pro-inflammatory cytokines, oxidative stress indicators and other compounds involved in airway inflammation, such as arachidonic acid metabolites (12).
Another recently-developed non-invasive diagnostic approach to assess lung injury is the so-called pneumoproteinemia concept, i.e. assaying lung-specific proteins (e.g. Clara cell-specific protein CC-16) in serum (13). In fact, because CC-16 is mainly secreted within the respiratory tract, its occurrence in the vascular compartment suggests leakage from the lung into the bloodstream and it is thought to reflect both the rate of synthesis and the permeability of the lung epithelium.
Although non-invasive methods are being used for research purposes and are gradually being introduced in clinical settings (14), there have been no reports on their application in a real clinical scenario following acute exposure and poisoning to pneumotoxic substances.
This paper reports on the application of non-invasive methods to assess the possible pathways of lung injury, in the acute phase and during a 15-month follow-up, in a group of children accidentally exposed to chlorine gases in a swimming pool.
Some of the results of this study have been previously reported in the form of an abstract (15).
Methods
On 17 February 2004, 18 children were accidentally exposed to chlorine gas during a swimming lesson. After an erroneous servicing procedure an excessive quantity of chlorine was added to the pool; the water turned yellow and the children began to feel ill, with coughing, vomiting, dyspnea, burning eyes and throat.
Ten children (5 males, age range 6 to 12 years) were taken to Padova hospital; 6 were admitted to the pediatric ward, 4 to the pediatric intensive care unit.
After admission, the children were evaluated using standard medical procedures. In addition FENO, spirometry and EBC analysis were performed and serum was stored. FENO measurement, spirometry and EBC collection were done in the first 24 hours after exposure in 9 children, and on day 4 (after extubation) in one child needing mechanical ventilation.
The children were then reassessed periodically (at days 1,3,8,15 and after 2,4,8 and 15 months). At month 8, the children also took an exercise challenge test.
The follow-up protocol was approved by the local ethical committee and both the parents and the children gave their informed consent to take part in the study. Fractional exhaled nitric oxide (FENO) was measured at a flow rate of 50 mL/sec with the NIOX system (Aerocrine, Stockholm, Sweden), using a single breath online method according to ATS/ERS recommendations (16). Lung function was analyzed by flow-volume spirometry according to international recommendations. In addition, a bronchodilation test was performed within 3 days of the intoxication. Exercise challenge was done on a treadmill as previously described (17).
EBC was collected with a condenser formed by five components: a mouthpiece set up to work also as a saliva trap, a non re-breathing polypropylene valve, a 10 cm Tygon® tube, a 50 ml polypropylene vial and a Dewar flask refrigerated with ice. Children breathed tidally through the mouth for 15 minutes, without using a nose-clip. They maintained a dry mouth during collection by periodically swallowing saliva. EBC samples were stored at -80 °C in polypropylene tubes until analytical determinations.
EBC-leukotriene B4 (LTB-4) and EBC-cysteinyl leukotrienes (Cys-LTs) were quantified using ELISA methods as previously described (17,18).
Serum Clara cell-specific protein CC16 (collected 3-5 hours after intoxication) was determined by latex immunoassay (19).
As a control group, we enrolled 10 healthy Caucasian children, relatives of doctors and nurses of our hospital, with no history of respiratory diseases or atopy, matched for age and gender with the intoxicated patients (5 males age range 6 to 12 years).
Statistical analysis
Results are expressed as median and interquartile range (IQR), except for CC16 values which are expressed as mean and SEM being normally distributed. Data in intoxicated children were compared over time using repeated measures analysis of variance, followed by Student-Newman-Keuls post hoc test. Correlations were tested with Spearman’s rank test. The Mann-Whitney U test was used to compare biomarker levels in exposed and control children. Statistical significance was assumed for p values of less than 0.05. Statistical analysis was performed using SigmaStat version 3.0.
Results
The past medical history of the poisoned children was essentially negative and none of them had ever had respiratory problems, in particular a diagnosis of asthma. Five children were atopic but only one was suffering from mild recurrent allergic rhinitis. At the time of the accident they were all healthy. Results of the standard medical procedures at admission are shown in table 1. In the first hours after the poisoning all of them were oxygen-dependent, whereas 4 children needed a more prolonged oxygen therapy (2-8 days after intoxication). A child needed mechanical ventilation for 4 days, during which time a bronchoscopy with bronchoalveolar lavage (BAL) was performed. Bronchoscopy revealed wide areas of disepithelization, with yellow membranes along the trachea and main bronchi. BAL analysis showed 90% of neutrophils.
Table 1.
Individual clinical presentation
| Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1* | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Gender, age | M,12 | F,6 | F,8 | M,9 | F,7 | F,10 | M,9 | M,9 | F,8 | M,8 |
| Chest wall indrawing | Severe | Mild | Mild | Moderate | Mild | Mild | Absent | Moderate | Absent | Severe |
| Nasal flaring | Present | Absent | Absent | Present | Absent | Absent | Absent | Absent | Absent | Present |
| Sat.O2 † at admission | <92% | 92-95% | 92-95% | <92% | 92-95% | 92-95% | 92-95% | <92% | 92-95% | <92% |
| Oxygen-dependence (days) | 8 days | <1 day | 1 day | 3 days | <1 day | <1 day | <1 day | 2 days | <1 day | 4 days |
| Chest x-ray at admission | Diffuse Interstitial and alveolar infiltrates | Normal | Normal | Interstitial and alveolar infiltrates | Normal | Normal | Normal | Normal | Mild interstitial infiltrates | Interstitial infiltrate |
Patient who needed mechanical ventilation.
Sat.O2:oxygen saturation in room air determined using a pulse oximeter.
All children were treated with oxygen therapy and inhaled steroids, while 6 were also given antibiotics and systemic steroids. The chest X-ray was pathological in 4 children with a picture of interstitial involvement accompanied in two cases by patchy and irregular areas of density.
FENO
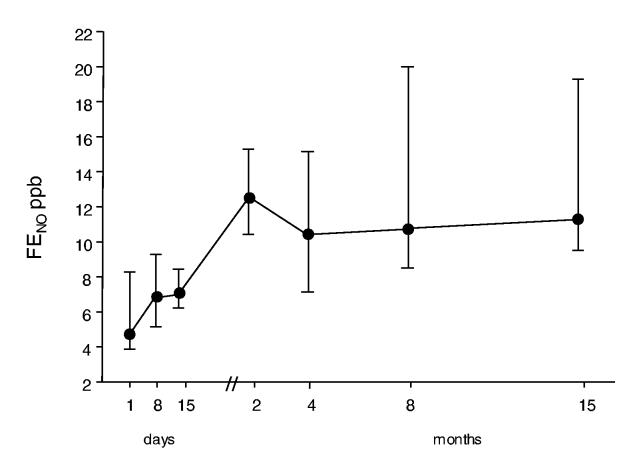
In the exposed children, the median FENO levels in the acute phase (4.7 [3.9-7.9] ppb) were significantly lower than in healthy matched controls (10.8 [8.9-12.2] ppb) and gradually increased during the follow-up reaching normal values at 2 months (12.6 [11.4-15] ppb) (figure 1).
Figure 1.
Time course of FENO levels (median and IQR). Exhaled NO (FENO) was measured on the day of admission in 9 children and at several time points in all the patients (10) after acute chlorine inhalation. (Control values 10.8 [8.9-12.2] ppb)
Pulmonary function test
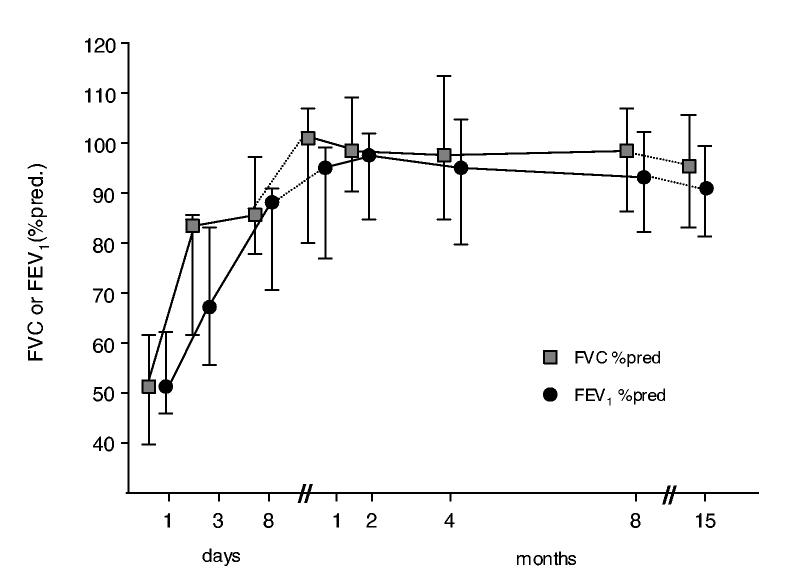
The spirometric findings are shown in figure 2. In the acute phase, there was evidence of an important reduction in FVC and FEV1 (median value 51% pred., both), with a normal ratio. There was a significant improvement in both parameters after 3 and 8 days; normal spirometric values were reached 15 days after the exposure to chlorine (median FVC 97%, FEV1 92% pred.). In the acute phase, 4 patients presented reversibility to β2-agonists, defined as a more than 12% increase in FEV1 after salbutamol inhalation.
Figure 2.
Time course of FVC% pred., FEV1 % pred. (median and IQR) after acute chlorine inhalation. Pulmonary function tests were performed on the day of admission and at several time points during the 15 months follow-up (FEV1/FVC ratio values range: 84-87). (Spirometry performed in 9 children at day 1st and 3rd and then in all the 10 patients during the follow -up).
Exhaled breath biomarkers
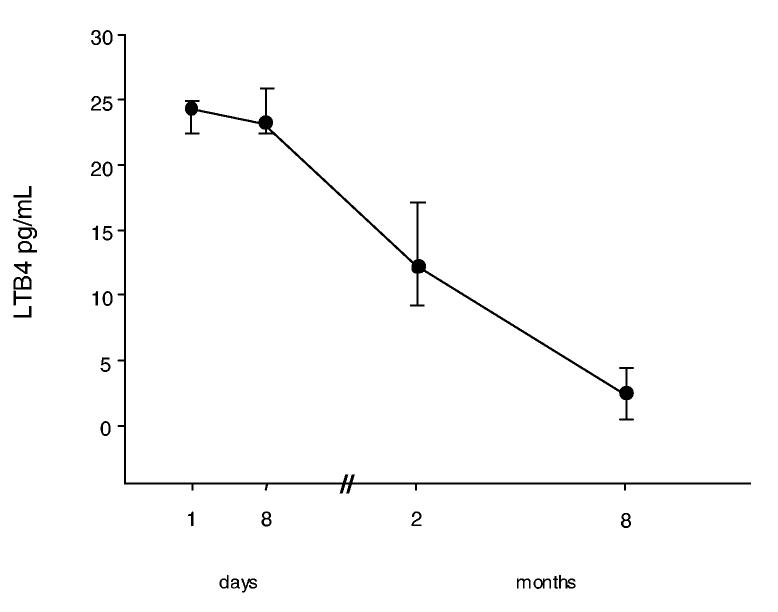
At admission, LTB4 levels were significantly higher in the EBC of the exposed children than in those of the healthy controls (24.4 [22.5-24.9] vs 4.7 [3-10.9] pg/mL); their concentration remained high after the first 8 days (23.3 [22.4-25.8] pg/mL), then progressively declined over 8 months when they were not statistically different from controls (2.5 [0.5-4.4] pg/mL) (figure 3).
Figure 3.
Time course of LTB-4 levels (median and IQR). Leukotriene B4 (LTB-4) was measured on exhaled breath condensates (EBC) collected at admission in 9 patients, and then in all the 10 patients after 1 week, 2 and 8 months after acute chlorine inhalation. (Control values 4.7 [3-10.9] pg/mL)
Cysteinyl leukotrienes (Cys-LTs) levels were also higher in exposed children at admission than in controls (25.6 [13.1-38.3] vs 7.2 [4-15.8] pg/mL), than gradually dropped during the follow-up; at 8 months they didn’t differ from controls (4.3 [2-5.9] pg/mL).
Serum CC16
Only one sampling time was available for CC16 (within 3-5 hours from the exposure); serum CC16 levels were significantly higher in the exposed children than in healthy children (mean 23.4 ± 2.5 μg/L vs 9.5 ± 0.5μg/L).
Exercise challenge test
The exercise challenge, performed 8 months after the accident, revealed no exercise-induced bronchoconstriction; none of the children had a drop in FEV1 > than 12% and the mean FEV1 reduction was 5.5 ± 0.6%.
Correlations
There was a significant negative correlation between EBC LTB-4 levels and FEV1% pred. (r=-0.4, p=0.01) and between EBC-LTB-4 levels and FENO values (r=-0.4, p=0.03). FENO and FVC% were not correlated at any time point (p >0.2 at all time points).
FENO values at admission were correlated with initial clinical severity (p= 0.03, r=-0.6) and with the length of oxygen-dependence (p=0.01, r=-0.7).
Atopic and non-atopic children didn’t differ in any of the inflammatory markers studied, nor in pulmonary function.
Discussion
To our knowledge this is the first study in humans to have applied non-invasive techniques (exhaled breath analysis) to assess lung injury in a real clinical scenario following acute chlorine exposure and poisoning.
Case reports of lung injuries following acute chlorine inhalation in occupational environments and also after community accidents have already been published (1,3,4) but they describe lung injuries in terms of lung function and imaging techniques, whereas little information is available on the underlying lung pathobiology after chlorine exposure in humans (9,20).
Biological events in the lung can be evaluated using invasive methods, such as bronchoscopy and BAL, which have provided important insight on the biological processes occurring in lung diseases, and still represent the gold standard. However these methods have a limited applicability, mainly due to the invasiveness of the sampling procedures, which makes them unsuitable for repeated measurements, particularly in children. As a recent alternative, several lung disease biomarkers can be analyzed from exhaled air and blood (10,15,21).
Previous studies reported respiratory symptoms in the acute phase after chlorine exposure, accompanied by restrictive or mixed deficits at pulmonary function tests, with symptoms fading over few days and pulmonary function test findings returning to normal over a few months in most cases (5,6). Nevertheless some authors reported a persistent airway hyper-responsiveness and obstruction in association with exposure to respiratory irritants and physical exertion even years after intoxication (7,9).
In this longitudinal study, we found a severe pulmonary function derangement in the first week after chlorine inhalation, with a progressive improvement leading to a normalization after 15 days (figure 2). At the exercise challenge performed 8 months after the accident, none of the children presented a significant drop in FEV1 suggesting a normal bronchial hyperresponsiveness to an indirect stimulus. However because direct and indirect challenges are weakly correlated and measure different mechanisms of bronchial hyperresponsiveness, we recognize that the choice of an exercise challenge instead of a methacholine test can be a limit of our study.
FENO values were lower at admission and in the first weeks by comparison with age-matched healthy controls and progressively increased to normal levels after two months. The low FENO levels observed after chlorine inhalation may be the consequence of massive epithelial destruction with subsequent damage of NO-producing cells of the airway wall, i.e epithelial, endothelial and nervous cells (11,22). This hypothesis is supported by the bronchoscopic findings of large areas of airway epithelial loss with proteinaceous exudates in the child who underwent endoscopy. Another possible cause of low FENO values could be the reduction in lung volumes. However this hypothesis seems unlikely because a non significant relationship was found between vital capacity and FENO values. Also steroid therapy could have affected FENO values, however the lowest FENO levels were registered at admission when administration of steroids had been initiated a few hours previously. In addition FENO levels progressively increased in the first week when all children were receiving inhaled steroids.
The hypothesis of a diffuse epithelial damage is also supported by the increased values of serum CC-16 we observed in the intoxicated children, which may be interpreted as a sign of injury to the lung epithelial permeability barrier. CC-16 is secreted by polarized cells in the lumen of respiratory tract, so its occurrence in the vascular compartment is suggestive of its passage from the lung into the bloodstream via the bronchoalveolar-blood barrier (13). Moreover, increased CC-16 levels, together with the alveolar infiltrates on chest x-rays in two patients, suggest a peripheral lung involvement after chlorine inhalation, beside the bronchial damage shown by functional tests.
EBC LTB-4 levels were clearly higher in our patients at admission than in control children. Leukotrienes are potent lipid mediators derived from arachidonic acid metabolism; LTB-4 is involved in a number of events, including stimulation of leukocyte migration from the bloodstream, neutrophil recruitment and activation, increased interleukins production (23). The high EBC LTB-4 levels that we observed in the intoxicated children may indicate an active neutrophilic inflammation in the airways of these patients, with the subsequent release of proteolytic enzymes, O2 radicals and lipid mediators, resulting in tissue damage. This is consistent with the differential cell count we observed in the child needing mechanical ventilation, whose BAL contained 90% of neutrophils. The possible role of LTB-4 in lung damage is further supported by its negative correlation with the lung function test results. Published data on in vitro tests also indicate a role of neutrophils in the lung’s response to acute chlorine exposure in mice and rats (24,25).
The high LTB-4 levels observed after 2 months from the exposure suggests a persistent neutrophilic inflammation despite the lack of respiratory symptoms and the normalization of routine lung function test findings. Neutrophil recruitment is probably not the only factor involved in the pathogenesis of lung injury due to chlorine inhalation, in fact, chlorine exposure also induced an increase in EBC Cys-LTs levels. These eicosanoids are produced by several cell types in the lung, including mast cells, basophils, eosinophils and macrophages. It is known that Cys-LT production induces the contraction of the airways and vascular smooth muscle, stimulates mucus secretion and increases microvascular permeability (26).
Taken together, these latter findings led us to hypothesize a role of arachidonic acid pathway in the lung damage seen in our children, and we speculate that in addition to steroids, medication inhibiting the 5-lipoxygenase pathway and thus the production of LTB-4 and Cys-LT may be useful in such cases of acute lung injury.
In conclusion, children acutely exposed to chlorine in a swimming pool had a substantial lung function impairment associated with biochemical exhaled breath alterations, mainly represented by an increase in leukotrienes and a reduction in FENO. While lung function and exhaled NO improved within a few weeks, the increased levels of exhaled LTB-4 persisted for several months. These findings shed new light on the pathobiology of chlorine-induced lung damage and may suggest new therapeutic implications for these patients.
Supplementary Material
Acknowledgements
We are grateful to Prof. A. Bernard and his staff (University of Louvain) for CC16 analysis of serum samples. We thank Dr. FLM Ricciardolo (University of Genova) for his useful suggestions.
Footnotes
This study was supported in part by grant 1R01 HL72323-01 from the National Heart, Blood and Lung Institute (NHLBI; Bethesda, USA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI or National Institute of Health.
References
- 1.Sexton JD, Pronchik DJ. Chlorine Inhalation: the big picture. Clin Toxicol. 1998;36:87–93. doi: 10.3109/15563659809162593. [DOI] [PubMed] [Google Scholar]
- 2.Mrvos R, Dean BS, Krenzelok EP. Home exposures to chlorine/chloramine gas: Review of 216 cases. South Med J. 1993;86:654–657. doi: 10.1097/00007611-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Agabiti N, Ancona C, Forastiere F, Di Napoli A, Lo Presti E, Corbo GM, D’Orsi F, Perucci CA. Short term respiratory effects of acute exposure to chlorine due to a swimming pool accident. Occup Environ Med. 2001 Jun;58:399–404. doi: 10.1136/oem.58.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parimon T, Kanne JP, Pierson DJ. Acute inhalation injury with evidence of diffuse bronchiolitis following chlorine gas exposure at a swimming pool. Respir Care. 2004;49:291–294. [PubMed] [Google Scholar]
- 5.Jones RN, Hughes JM, Glindmeyer H. Lung function after acute chlorine exposure. Am Rev Respir Dis. 1986;134:1190–1195. doi: 10.1164/arrd.1986.134.6.1190. [DOI] [PubMed] [Google Scholar]
- 6.Abhyankar A, Bhambure N, Kamath NN, Pajankar SP, Nabar ST, Shrenivas A, Shah AC, Deshmukh SN. Six month follow-up of fourteen victims with short-term exposure to chlorine gas. J Soc Occup Med. 1989;39:131–132. doi: 10.1093/occmed/39.4.131. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz DA, Smith DD, Lakshminarayan S. The pulmonary sequelae associated with accidental inhalation of chlorine gas. Chest. 1990;97:820–825. doi: 10.1378/chest.97.4.820. [DOI] [PubMed] [Google Scholar]
- 8.Brooks SM, Weiss MA, Bernstein I. Reactive airway dysfunction syndrome (RADS): persistent asthma syndrome after high level irritant exposures. Chest. 1985;88:376–384. doi: 10.1378/chest.88.3.376. [DOI] [PubMed] [Google Scholar]
- 9.Schonhofer B, Voshaar T, Kohler D. Long-term lung sequelae following accidental chlorine gas exposure. Respiration. 1996;63:155–9. doi: 10.1159/000196536. [DOI] [PubMed] [Google Scholar]
- 10.Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163:1693–722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 11.Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84:731–65. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 12.Hunt J. Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease. J Allergy Clin Immunol. 2002;110:28–34. doi: 10.1067/mai.2002.124966. [DOI] [PubMed] [Google Scholar]
- 13.Hermans C, Bernard A. Lung epithelium-specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med. 1999;159:646–78. doi: 10.1164/ajrccm.159.2.9806064. [DOI] [PubMed] [Google Scholar]
- 14.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352:2163–73. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 15.Bonetto G, Corradi M, Carraro S, Zanconato S, Bastardo C, Mutti A, Bernard A, Baraldi E. Swimming pool acute chlorine inhalation in children: pulmonary function and markers of lung damage over a 8 month follow-up. Eur Respir J. 2005;26(S49):395. [Google Scholar]
- 16.Baraldi E, de Jongste JC. ERS/ATS statement. Measurement of exhaled nitric oxide in children, 2001. Eur Respir J. 2002;20:223–237. doi: 10.1183/09031936.02.00293102. [DOI] [PubMed] [Google Scholar]
- 17.Carraro S, Corradi M, Zanconato S, Alinovi R, Pasquale MF, Zacchello F, Baraldi E. Exhaled breath condensate cysteinyl leukotrienes are increased in children with exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2005;115:764–70. doi: 10.1016/j.jaci.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 18.Bodini A, Peroni D, Vicentini L, Loiacono A, Baraldi E, Ghiro L, Corradi M, Alinovi R, Boner AL, Piacentini GL. Exhaled breath condensate eicosanoids and sputum eosinophils in asthmatic children: a pilot study. Pediatr Allergy Immunol. 2004;15:26–31. doi: 10.1046/j.0905-6157.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 19.Lagerkvist BJ, Bernard A, Blomberg A, Bergstrom E, Forsberg B, Holmstrom K, Karp K, Lundstrom NG, Segerstedt B, Svensson M, Nordberg G. Pulmonary epithelial integrity in children: relationship to ambient ozone exposure and swimming pool attendance. Environ Health Perspect. 2004;112:1768–71. doi: 10.1289/ehp.7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leroyer C, Malo JL, Infante-Rivard C, Dufour JG, Gautrin D. Changes in airway function and bronchial responsiveness after acute occupational exposure to chlorine leading to treatment in a first aid unit. Occup Environ Med. 1998;55:356–9. doi: 10.1136/oem.55.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson WP, Mutti A. Role of biomarkers in monitoring exposures to chemicals: present position, future prospects. Biomarkers. 2004;9:211–42. doi: 10.1080/13547500400015642. [DOI] [PubMed] [Google Scholar]
- 22.de Gouw HW, Grunberg K, Schot R, Kroes AC, Dick EC, Sterk PJ. Relationship between exhaled nitric oxide and airway hyperresponsiveness following experimental rhinovirus infection in asthmatic subjects. Eur Respir J. 1998;11:126–132. doi: 10.1183/09031936.98.11010126. [DOI] [PubMed] [Google Scholar]
- 23.Freeland HS, Schleimer RP, Schulman ES, Lichtenstein LM. Peters SP.l Generation of leukotriene B4 by human lung fragments and purified human lung mast cells. Am Rev Respir Dis. 1988;138:389–394. doi: 10.1164/ajrccm/138.2.389. [DOI] [PubMed] [Google Scholar]
- 24.Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med. 2003;168:568–74. doi: 10.1164/rccm.200201-021OC. [DOI] [PubMed] [Google Scholar]
- 25.Demnati R, Fraser R, Ghezzo A, Martin JG, Plaa G, Malo JL. Time-course of functional and pathological changes after a single high acute inhalation of chlorine in rats. Eur Respir J. 1998;11:922–928. doi: 10.1183/09031936.98.11040922. [DOI] [PubMed] [Google Scholar]
- 26.Drazen JM, Israel E, O’Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.