Abstract
Methylprednisolone (MP) is used to treat a variety of neurological disorders involving white matter injury, including multiple sclerosis, acute disseminated encephalomyelitis, and spinal cord injury (SCI). While its mechanism of action has been attributed to anti-inflammatory or anti-oxidant properties, we examined the possibility that MP may have direct neuroprotective activities. Neurons and oligodendrocytes treated with AMPA (alpha-amino-3-hydroxy-5- methylisoxazole-4-propionate) or staurosporine died within 24 hrs after treatment. MP attenuated oligodendrocyte death in a dose-dependent manner; however, neurons were not rescued by the same doses of MP. This protective effect was reversed by the glucocorticoid receptor (GR) antagonist, RU486, and siRNA directed against GR, suggesting a receptor-dependent mechanism. MP reversed AMPA-induced decreases in the expression of antiapoptotic Bcl-xL, caspase-3 activation, and DNA laddering, suggesting anti-apoptotic activity in oligodendrocytes. To examine if MP demonstrated this selective protection in vivo, neuronal and oligodendrocyte survival was assessed in rats subjected to SCI; groups of rats were treated with or without MP in the presence or absence of RU486. Eight days after SCI, MP significantly increased oligodendrocytes (CC-1 immunoreactive cells) following SCI, but neuronal (NeuN immunoreactive cells) number remained unchanged; RU486 reversed this protective effect. MP also inhibited SCI-induced decreases in Bcl-xL and caspase-3 activation. Consistent with these findings, the volume of demyelination, assessed by Luxol Fast Blue staining, was attenuated by MP and reversed by RU486. These results suggest that MP selectively inhibits oligodendrocyte but not neuronal cell death via a receptor-mediated action, and may be a mechanism for its limited protective effect following SCI.
Keywords: Spinal cord injury, oligodendrocytes, neurons, glucocorticoids, methylprednisolone, apoptosis
Introduction
Methylprednisolone (MP), a synthetic glucocorticoid (GC) agonist with potent anti-inflammatory and anti-oxidant properties, is the mainstay of therapy for a variety of neurological disorders involving white matter injury. For example, MP is routinely used to treat multiple sclerosis (MS) exacerbations and acute disseminated encephalopmyelitis. It is widely used for acute spinal cord injury (SCI) where white matter injury plays a prominent but not exclusive role, though marginal efficacy and a poor side-effect profile have recently raised concerns (Nesathurai, 1998; Sayer et al., 2006). Despite its entrenched role in therapy for these disorders, MP's mechanisms of action are not well understood. Because of the high doses required for protection, it has been proposed that MP's action is through a receptor-independent inhibition of oxygen free radical-induced lipid peroxidation (Demopoulos et al., 1982; Hall and Braughler, 1982; Anderson and Means, 1985; Bracken, 1991). MP's potent anti-inflammatory activity (Barnes, 1998), suggest another mechanism which may be more prominent in the treatment of MS. In animal models of inflammatory disorders, GCs have been shown to reduce eicosanoid formation (Perkins and Kniss, 1997), inhibit the expression of cytokines (Arzt et al., 1994), adhesion molecules (van de Stolpe et al., 1994), iNOS (Brenner et al., 1994) and COX2 (Lukiw et al., 1998), and other effector actions of inflammatory mediators (Heyderman et al., 1995; Funk et al., 1995). In addition, GCs induce apoptosis in thymocytes, T and B lymphocytes, and peripheral blood monocytes (Sikora, 1966; Schmidt et al., 1999).
While GCs are best known for their pro-apoptotic activities in inflammatory blood cells, there are increasing examples of anti-apoptotic actions in other cell types. For example, the GR agonist, dexamethasone, attenuated TNF-α-induced apoptosis of the human mammary carcinoma cell line, MCF-7, by preventing the down-regulation of inhibitors of apoptosis (IAP) family of proteins (Messmer et al., 2001). Dexamethasone also suppressed pro-apoptotic Bcl-xS expression, and enhanced basal levels of anti-apoptotic Bcl-xL in gastric cancer TMK-1 cells (Chang et al., 1997) and C6 glioma cells (Ni Chonghaile et al., 2006), enhancing cell survival. Moreover, spontaneous apoptosis in primary cultures of human and rat hepatocytes was inhibited by dexamethasone, in parallel with an increase in Bcl-xL expression (Bailly-Maitre et al., 2001).
Several studies have reported that following SCI, some neural cells die with features of apoptosis (Crowe et al., 1997; Liu et al., 1997; Springer et al., 2001). Ultrastructural features, biochemical markers, and activated apoptotic mediators or end-products have been observed in contused cords (Hamada et al., 1996; Crowe et al., 1997; Liu et al., 1997; Emery et al., 1998; Springer et al., 1999; Li et al., 2000; Keane et al., 2001). Prominent among these dying cells were oligodendrocytes which were observed to die hours to weeks after injury near and distant to the epicenter (Crowe et al., 1997; Shuman et al., 1997; Yong et al., 1998; McTigue et al., 2001; McEwen and Springer, 2005).
In light of its contrasting apoptotic activities in different cell types, we investigated whether MP could have differential anti-apoptotic activities in neural cells after SCI.
Material and Methods
SCI model in rats
The SCI model in rats has been detailed elsewhere (Xu et al., 1990; Xu et al., 1991; Xu et al., 1992; Liu et al., 1997; Xu et al., 1998a). Briefly, female rats (Long Evans, body weight 300 ± 25 g) were subjected to chloral hydrate anesthesia (400 mg/kg, i.p.). Following a T9 - T11 laminectomy, SCI was induced using a New York University (NYU) Impactor by dropping a 10 g weight at a height of 12.5 mm. Animals subjected to identical surgical procedures, without impaction, served as sham-operated controls. In some situations, animals undergoing no surgery were used as controls. Perioperative care followed MASCIS guidelines, which has been described in previous publications (Basso et al., 1996; Liu et al., 1997; Xu et al., 1998a). In addition, all procedures were approved by the Washington University Animal Studies Committee.
Cell culture
Cortical neuronal cultures
Cortical neuronal cultures were prepared as described (Ross et al 1993). Briefly, cortices were isolated from 14-16 day rat embryos, dissociated and plated on poly-lysine coated dishes or 24-well plates in MEM (with glutamine-free Earle's salts) supplemented with 5% fetal bovine serum, 5% horse serum, 20 mM glucose and 2 mM glutamine. After 3 days in vitro, non-neuronal cells were eliminated with a 3 day exposure to10 μM cytosine-arabinoside, yielding >90% pure neuronal cultures. Cultures were maintained in a 37 °C humidified incubator in a 5% CO2 atmosphere.
Oligodendrocyte cultures
Neurospheres were cultured as previously described (Zhang et al., 1999) with modifications. Briefly, embryonic rat cortices (E14-16) were dissected, homogenized gently in DMEM/F-12 medium, and centrifuged at 350x g for 5 min. The pellet was digested with 1.5 ml of 0.025% trypsin/0.27 mM EDTA at 37°C for 30 min followed by the addition of 1.5 ml DMEM/F-12 with 20% FBS and then filtered through 10 μm nylon mesh. The filtrate was centrifuged at 350x g for 5 min, and the pellet was washed twice with DMEM/F-12. Dissociated cells were layered on a pre-equilibrated Percoll gradient (formed by centrifuging 50% Percoll and 50% DMEM/F-12 at 23,500x g for 1 h at 4°C) and centrifuged at 23,500x g for 15 min. The fraction containing glial progenitors, banding between myelin and red blood cell layers, were recovered and washed twice with DMEM/F-12 followed by a wash with neurosphere culture medium (DMEM/F-12; N1 supplement; 25 μg/ml insulin; 20 ng/ml basic fibroblast growth factor, bFGF and 20 ng/ml epidermal growth factor, EGF). The cell pellet was resuspended in 20 ml of neurosphere culture medium and seeded in 75 mm culture flasks. After 24 h, when neurospheres formed, 5 ml of fresh medium was added to each culture every other day for 7 d; the neurosphere cultures were split (1:2): dissociated gently 10 times with a syringe with needle (25 gauge) and centrifuged at 350x g. The resulting cell pellets were treated with 0.05% trypsin/0.53 mM EDTA and centrifuged at 350x g for 10 min. The cells were resuspended in progenitor medium (69% DMEM/F-12 containing N1 supplement, 10 μg/ml insulin, 20 nM progesterone, 100 units penicillin/streptomycin, 30% conditioned medium from B104 cells and 1% FBS) and plated on 100-mm culture dishes pre-coated with poly-L-ornithine. Disaggregated oligosphere cells displayed bipolar or tripolar morphology.
For differentiated oligodendrocyte cultures, progenitor cells were detached with trypsin and cultured on poly-L-ornithine-coated dishes in differentiating oligodendrocyte medium (DMEM/F-12; N1 supplement, 20 ng/ml biotin; 20 ng/ml triiodo-L-thyronine, T3, 20 ng/ml retinoic acid, 0.1 μg/ml heregulin 1-β and 1% FBS). We have previously shown that oligoprogenitor cells differentiated from rat neurospheres demonstrate morphologic and antigenic characteristics of oligodendrocytes; immunohistochemistry revealed that virtually all cells immunostained for oligodendrocyte-specific antigens, CNP, Rip, GalC, and PLP (Lee et al., 2004).
Cell death assessment
Cell viability was quantitated by the LDH assay (Xu et al., 1998b).
DNA fragmentation
DNA laddering (Xu et al., 1998a)
Briefly, at various intervals post injury, a 10 mm spinal cord segment (5 mm rostral and 5 mm caudal from the epicenter) was dissected following intracardiac perfusion with 100 ml saline under anesthesia. In cultures, oligodencrocytes were collected after drug treatments. The samples were homogenized in lysis buffer (50 mMTris-HCl, 10 mM EDTA and 0.5% SDS, pH 8.0), incubated with RNase (0.5 mg/ml) at 55 °C for 1h, following by proteinase K (0.5 mg/ml) at 37 °C overnight. The solution was extracted with the same volume of phenol and chloroform (1:1, v/v) and centrifuged at 25,000x g at room temperature for 10 min. Equal volumes of isopropanol was added to the supernatant and centrifuged at 13,000x g for 15 min. Pellets were washed with 70% ethanol and rehydrated with TE buffer and electrophoresed on a 1.5% agarose gel and visualized by UV for quantification.
Elisa
For cultures, oligodendrocytes were collected after drug treatment. The cytosolic levels of histone-associated DNA fragments were determined using a Cell Death Detection ELISA kit (Boehringer Mannheim, Indianapolis, IN) (Xu et al., 1998a).
siRNA
Two siRNAs were acquired to knockdown GR (Qiagen). SiGR1: sense—r(GAU GUU AGG UGG GCG UCA A)dTdT; antisense—r(UUG ACG CCC ACC UAA CAU G)dTdT; target sequence—AAC ATG TTA GGT GGG CGT CAA; and siGR2: sense—r(GGG ACA AAU AUA AUU GGU A)dTdT; antisense—r(UAC CAA UUA UAU UUG UCC C)dAdG; target sequence—CTG GGA CAA ATA TAA TTG GTA. A nonspecific duplex was used as a control siRNA (5'-r(CAG UGG AGA UCA ACG UGC AAG UU)-3', which did not affect GR mRNA levels relative to the untransfected controls. SiRNAs in Opti-MEM medium was mixed with lipofectamine 2000 in Opti-MEM medium (Invitrogen) for 20 min, then added to oligodendrocyte cultures (without antibiotics) for 48 hours (final concentration 50-200 nM). After transfection, medium was changed to DMEM/F12 and MP (1μM) was added for 4 hours followed by staurosporine (100 nM) for 24 hours. LDH from the medium was measured for cytotoxicity.
Western blot analysis
This method has been reported previously (Xu et al., 1991; Xu et al., 1997; Xu et al., 1998a). Briefly, at various intervals post injury, a 10 mm spinal cord segment (5 mm rostral and 5 mm caudal from the epicenter) was dissected following intracardiac perfusion with 100 ml saline. The cord segment was homogenized in Western blot buffer (10 mM Tris-HCl containing 10 mM HEPES, 1.5 mM MgCl, 10 mM KCl, 0.5 mM DTT, 1 mM PMSF, 0.1 unit/ml protinin, pH 7.9) and centrifuged at 600x g. The pellets were collected for nuclear protein extraction (see below). The supernatant (cytoplasm) was subjected to high-speed (25,000x g) centrifugation. Twenty-sixty μg protein from the supernatant was loaded onto 10-12% polyacrylamide gel (depending on protein molecular weight), separated by SDS/PAGE, and transferred to nitrocellulose membranes by electrophoresis. The membranes were blocked in TBST buffer containing 20 mM Tris-HCl, 5% non-fat milk, 150 mM NaCl, and 0.05% Tween-20 (pH 7.5) for 1 h at room temperature. Primary antibodies, anti-activated caspase-3 (1:500, Cell Signaling), anti-Bcl-xL (1:1000, Sigma), anti-NeuN (1:500, Chemicon), and anti-MBP (1:1000, Chemicon), were added to the membrane and incubated at 4°C overnight. The membrane was washed with TBST, incubated with alkaline phosphatase or horseradish peroxidase conjugated anti-rabbit, anti-mouse or anti-rat secondary antibody (1:5,000) at room temperature for 1 h, and detected using the Blot AP System (Promega) or SuperSignal West Pico Chemiluminescent Substrate System (Pierce) as described in the technical manual provided by the manufacturers.
Immunohistochemistry
Rats were sacrificed at various times post-injury under anesthesia and perfused transcardialy with 100 ml of 0.9% saline followed by 500 ml of 4% paraformaldehyde (Holets et al., 1987; Xu et al., 1995). After perfusion, the spinal cords were carefully dissected and 10 mm segments centered at the epicenter were blocked and post-fixed for an additional 2 h in the same fixative. The specimens were transferred to a solution containing 30% sucrose in 0.1M phosphate buffer (PB, pH7.4) overnight.
Immunofluorescence double labeling for confocal microscopy
Spinal cord segments from control, sham-operated, or injured animals were embedded in tissue freezing medium, and longitudinally sectioned (16 μm thick sections) on a cryostat, and mounted on gelatin-coated slides. The sections were permeabilized and blocked with 0.3% Triton X-100/ 10% normal goat serum in 0.01 M PBS for 30 min. Primary antibodies, activated caspase-3 (1:100, polyclonal antibody, Sigma) and CC1 (1:100, monoclonal antibody, Oncogene), were applied to the sections overnight at 4°C. On the following day, the sections were incubated with fluorescein-conjugated goat anti-rabbit (FITC) and rhodamine-conjugated rabbit anti-mouse (RITC) antibodies. Slides were mounted and examined with a Zeiss Fluorescence microscope (Axiovert 200). In control sections, the primary antibody was substituted by 1% normal mouse serum.
Stereological analysis
Total oligodendrocyte or neuron number in the dorsal funiculus or gray matter was assessed respectively using a computer-based stereology system (StereoInvestigator, MicroBrightField, Williston, VT, USA). Neuron and oligodendrocyte numbers were estimated with the optical fractionator (West et al., 1991) 8 d after SCI. CC1- and NeuN-positive cells in 6 equally-spaced 30 μm-sections in a 1-mm block 3.5 to 4.5 mm rostral and 3.5 to 4.5 mm caudal to the epicenter were counted in the dorsal column and the gray matter respectively. CC1 positive cells were only counted when the cell body and proximal processes were darkly labeled and were within the inclusive zone of each dissector frame. NeuN positive cells whose nucleus top came into focus within the inclusive zone of each dissector frame were counted. Results were reported in units of density (cells per mm3).
Statistical Analyses
Differences among groups were analyzed by one-way ANOVA followed by post-hoc Tukey test.
Results
Methylprednisolone attenuates oligodendrocyte but not neuronal cell death via glucocorticoid receptors
In order to determine if MP has direct neuroprotective activities, we treated cultures of neurons or oligodendrocytes with 200 μM AMPA and 100 μM cyclothiazide (for sustained activation of AMPA receptors), or 0.2 μM staurosporine (STP). The cells were assessed for death 24 hrs after treatment, by LDH release. Both AMPA and STP induced significant cell death within this time period. MP treatment (1 μM) attenuated oligodendrocyte (Fig. 1A) but not neuronal (Fig. 1C) cell death, while the caspase inhibitor zVAD (50 μM) attenuated cell death for both cell types (Fig. 1A, C). MP attenuated STP-induced oligodendrocyte cell death over a wide range of concentrations; significant reductions in LDH release were detected at 30 nM with maximal effect at 1 μM (Fig. 1B). However, at these same doses, MP had no effect on STP-induced neuronal death (Fig. 1D), suggesting a selective protective effect in oligodendrocytes.
Fig. 1.
MP selectively attenuates oligodendrocyte cell death. Oligodendrocytes (A,B) or neurons (C,D) grown in the presence or absence of AMPA (200 uM) + cyclothiazide (100 uM) or staurosporine (STP, 0.2 uM) were pre-treated for 2 h with vehicle, methylprednisolone (1 μM) or the caspase inhibitor, zVAD (50 μM). Oligodendrocyte cell death (assessed by LDH release) induced by treatment with AMPA or STP was attenuated by MP (A) in a dose-dependent manner (B). AMPA- and STP-induced neuronal cell death was not inhibited by MP (C), even at doses that protected oligodendrocytes (D). zVAD inhibited AMPA- and STP-induced cell death for both cell types (A,C). * p<0.01, compared to control; ** p<0.05, compared to STP or AMPA treatment alone (ANOVA with post-hoc Tukey's test). Error bars represent SD. Results were replicated in two independent experiments, using triplicate cultures in each group.
To determine if MP's protective actions in oligodendrocytes were receptor-mediated, we added the glucocorticoid receptor antagonist, RU486, to STP-treated oligodendrocytes to examine its effect on cell death. In a dose-dependent manner, RU486 antagonized MP's protective effect with maximal effect at 1-3 μM (Fig. 2A). RU486 alone had no effect on cell death. To confirm the specific pharmacological effect of RU486 on GR, we also utilized two different siRNAs to knockdown GR expression. Both GR siRNAs resulted in an attenuation of MP's protective activity (Fig. 2B), concomitant with knockdown of GR mRNA (Fig 2C). These results suggest that MP selectively protects oligodendrocytes (but not neurons) from injury via a glucocorticoid receptor-dependent mechanism.
Fig. 2.
MP's protective effect is mediated by the glucocorticoid receptor. (A) Oligodendrocytes grown in the presence or absence of STP (0.2 μM) and/or MP, were treated with escalating doses of the glucocorticoid receptor antagonist, RU486, and assessed for cell death by LDH release. RU486 inhibited the protective activity of MP in a dose-dependent manner. * p<0.001 compared to control; ** p<0.001 compared to STP treatment; † p<0.001 compared to STP+MP treatment (ANOVA with post-hoc Tukey's test). (B) Oligodendrocytes grown in the presence or absence of vehicle, scrambled siRNA, or two different siRNAs directed against GR, were treated with STP or MP as indicated, and assessed for cell death. Tranfection efficiencies were 85-90%. *p<0.001 control vs. STP; ** p<0.001 STP vs. MP+STP (ANOVA with post-hoc Tukey's test). Error bars represent SD. Results were replicated in two independent experiments, using triplicate cultures in each group. Both GR siRNAs (50 nM) attenuated the protective effect of MP (B), concomitant with GR mRNA knockdown (C).
Glucocorticoid agonists attenuate oligodendrocyte apoptosis
Previous studies have demonstrated that MP exerts protective effects in other cell types by modulating apoptotic regulators (Chang et al., 1997; Bailly-Maitre et al., 2001; Messmer et al., 2001). We sought to determine if MP's protective activity in oligodendrocytes were mediated through a similar mechanism. Oligodendrocytes treated with AMPA demonstrated classical features of apoptosis including down-regulation of the anti-apoptotic regulator, Bcl-xL (Fig 3A), caspase-3 activation (Fig. 3A, B), and DNA fragmentation (Fig. 3C), consistent with previous reports (Liu et al., 2002; Sanchez-Gomez et al., 2003). Furthermore, as demonstrated above, oligodendrocytes died after exposure to STP (Fig. 1A,B), a non-selective inhibitor of protein kinase often used to induce apoptosis. Treatment with 1 μM MP increased Bcl-xL expression, decreased caspase-3 activation and DNA fragmentation, and attenuated cell death (Fig 3A-D). RU486 (1 μM) antagonized MP's effects on the apoptotic mediators and reversed MP's protective effects (Figs. 3A-D). Furthermore, dexamethasone (DEX, 1 μM) also demonstrated similar protective effects on oligodendrocytes, attenuating AMPA-induced apoptosis. MP did not rescue STP-treated neurons (Figs 1C,D), nor did it increase Bcl-xL levels in these cells (Fig. 3E). Collectively, these results suggest that glucocorticoid agonists inhibit oligodendrocyte apoptosis via glucocorticoid receptor-mediated inhibition of apoptotic mediators.
Fig. 3.
Glucocorticoid agonists inhibit oligodendrocyte but not neuronal apoptosis. Oligodendrocytes were cultured in the presence or absence of AMPA, MP, dexamethasone (DEX) or RU486 (RU) as indicated. RNA and protein were extracted for Bcl-xL RT-PCR and activated caspase-3 Western Blotting, respectively (A). In addition, caspase-3 activity (B) and DNA fragmentation (C) was measured. Cell death was assessed using an LDH release assay (D). Cortical neurons were grown in the presence or absence of stuarosporine (STP) and/or MP as indicated. Protein was extracted for Bcl-xL Western Blotting (E). * p<0.001 compared to control; ** p<0.001 compared to AMPA treatment; † p<0.01 compared to AMPA+MP (ANOVA with post-hoc Tukey's test). Error bars represent SD. Results were replicated in two independent experiments, using triplicate cultures in each group.
Methylprednisolone inhibits SCI-induced apoptosis
We sought to examine if MP could have similar anti-apoptotic activity following SCI in a rat model. Groups of rats were subjected to SCI using the NYU impactor (see Methods), and the molecular signatures of apoptosis were examined 24 hrs after contusion. In agreement with previous reports (Qiu et al., 2001; Nesic-Taylor et al., 2005), we found that SCI decreased Bcl-xL mRNA (Fig 4A) and protein (Fig 4B), increased caspase-3 activation (Fig 4C), and induced DNA laddering (Fig 4D), similar to our in vitro findings above. All of these changes in the apoptotic indicators were reversed when rats were treated with MP (30 mg/kg, iv) 15 min after SCI. Moreover, MP's effects were abrogated by RU-486 (15 mg/kg, ip), 30 min prior to SCI (Fig 4A-D), suggesting that the anti-apoptotic effects were GR-mediated. Prominent among cells which demonstrated activated caspase-3 immunoreactivity (Act casp-3) were oligodendrocytes which co-labeled with CC-1 (Fig 4E). Activated caspase-3 immunostaining was absent in sham-operated animals (data not shown).
Fig. 4.
MP inhibits apoptosis following rat SCI. Groups of rats were subjected to no surgery, sham surgery, SCI, SCI + MP (30 mg/kg, iv, 15 min prior to SCI), or SCI + MP + RU486 (15 mg/kg, ip, 30 min prior to SCI) as indicated. 24 hrs after surgery, spinal cords were removed, RT-PCR was performed to measure Bcl-xL mRNA (A); Western Blot was performed to measure Bcl-xL (B) and activated caspase-3 (C). Spinal cord extracts were also run on a DNA gel to assess DNA laddering (D). Sections from contused spinal cords were immunostained for activated caspase-3 (green) and CC-1 (red) 8 d after surgery, demonstrating colabelling (left panel, low power; right panel, high power; bars = 10 μm) (E). Blots and gels are representative of at least 3 independent replicates.
Methylprednisolone selectively attenuates oligodendrocyte cell death and demyelination following SCI
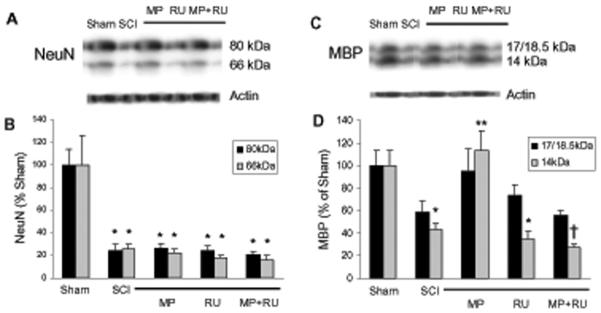
To determine if MP demonstrated selective protection of oligodendrocytes after SCI (as found in our in vitro models), we used unbiased stereology to quantify surviving oligodendrocytes and neurons 8d after SCI, to allow time for degenerating cells to disappear. Groups of rats were subjected to sham surgery, SCI (using NYU impactor—see Materials and Methods), treatment with MP (30 mg/kg, iv) 15 min after SCI, or treatment with MP (15 min before) and RU-486 (15 mg/kg, ip) 30 min prior to SCI as indicated (Fig 5). Because of the gross morphological alterations at the epicenter of the contusion, we counted surviving cells 3.5-4.5 mm from the epicenter (both rostral and caudal), specifically counting CC1-immunostained oligodendrocytes in the dorsal columns, or NeuN-immunostained neurons in the gray matter (Fig. 5A). 8d after SCI, the number of surviving oligodendrocytes in the dorsal columns was significantly decreased compared to sham-operated controls. MP treatment (30 mg/kg, iv) attenuated this SCI-induced oligodendrocyte death, and RU486 (15 mg/kg, ip) reversed MP's protective effect (Fig. 5B). RU486 alone had no effect on cell number. SCI also significantly decreased neuronal number in the gray matter; however, MP had no effect on neuronal cell survival (Fig 5C). These results suggest that MP selectively attenuates oligodendrocyte but not neuronal cell death following SCI. Furthermore, this protective effect appears to be mediated via the glucocorticoid receptor. A similar trend was observed in spinal cord homogenates (8 d after contusion) subjected to Western blotting, using anti-NeuN and anti-myelin basic protein (MBP) antibodies. SCI reduced expression of both antigens, MP rescued MBP (14 kDa isform) but not NeuN expression, and RU486 antagonized this effect (Fig 6A-D).
Fig. 5.
MP attenuates oligodendrocyte but not neuronal cell death following rat SCI. Groups of rats were subjected to: sham surgery (n=7), SCI (n=9), treatment with MP (30 mg/kg, iv, n=6) 15 min after SCI, RU486 (15mg/kg, ip, n=6) 30 min prior to SCI, or MP and RU486 (n=12). Unbiased stereology was used to count cells in equally-spaced transverse sections located 3.5-4.5 mm rostral and caudal to the epicenter (A), as described in Materials and Methods. Oligodendrocytes (CC1-positive cells) (B) or neurons (NeuN-positive cells) (C) were counted 8 d after SCI, and expressed as mean ± SD. Statistical tests were performed on total cell counts (rostral + caudal), and are significantly different as indicated: * p<0.001 for sham vs. SCI; ** p<0.001 for SCI vs. SCI+MP; † p<0.05 for SCI+MP vs. SCI+MP+RU (ANOVA with post-hoc Tukey's test).
Fig. 6.
MP rescues MBP but not NeuN expression following SCI. Spinal cord homogenates from rats subjected to sham surgery, SCI, SCI+MP, SCI+RU486, or SCI+MP+RU486 (n=4 per group) were immunoblotted using anti-NeuN (A) or anti-MBP antibodies (C), 8 days after surgery. Bands from blots were quantified by densitometry, normalized to actin, and expressed as mean ± SEM (% sham). NeuN expression was decreased after SCI, but neither MP, RU486, nor MP+RU486 had any effect on expression (B). MBP expression (14 kDa isoform) was decreased after SCI; but rescued by MP and antagonized by RU486 (D). * p<0.001 for sham vs. SCI; ** p<0.005 for SCI vs. SCI+MP; † p<0.001 for SCI+MP vs. SCI+MP+RU486 (ANOVA with post-hoc Tukey's test).
One consequence of SCI and oligodendrocyte death (which can occur at sites distant from the epicenter) is demyelination in and around the contusion cavity. To determine if MP altered the extent of demyelination after SCI, we used Luxol fast blue staining in groups of rats treated with vehicle, MP, or MP+RU486 (Fig. 7A). After SCI, a relatively large volume of demyelination was observed (~7mm3). Treatment with MP significantly reduced this volume of demyelination—an effect that was reversed by RU486 (Fig. 7B). These data suggest that MP reduces SCI-induced demyelination by attenuating oligodendrocyte cell death via the glucocorticoid receptor.
Fig. 7.

MP reduces SCI-induced demyelination via a receptor-mediated mechanism. Spinal cords from groups of 6 rats subjected to sham surgery, SCI, SCI+MP, or SCI+MP+RU486 were extracted, longitudinally sectioned, and stained with Luxol fast blue (A). The volume of demyelination was quantified and expressed as mean ± SD (B). * p<0.001 for SCI vs. SCI+MP; ** p<0.001 for SCI+MP vs. SCI+MP+RU486 (ANOVA with post-hoc Tukey's test).
Discussion
This study provides evidence that MP selectively attenuates oligodendrocyte apoptosis after injury, via a glucocorticoid receptor-mediated mechanism. In vitro, we have demonstrated that MP attenuates oligodendrocyte apoptosis induced by AMPA and staurosporine, while neuronal apoptosis was unaffected at identical doses. Moreover, this protective effect on oligodendrocytes was inhibited by RU486 and GR-targeted siRNA, suggesting a receptor-mediated mechanism. Oligodendrocyte-selective protection was also observed following rat SCI in vivo, where MP treatment selectively attenuated oligodendrocyte cell death without affecting neuronal survival via a glucocorticoid receptor-mediated mechanism. Furthermore, MP reversed apoptotic molecular signatures in oligodendrocytes in vitro, and following SCI in vivo: downregulation of Bcl-xL, activation of caspase-3, and DNA fragmentation was attenuated by MP.
Though better known for its pro-apoptotic activity in a variety of inflammatory cells, MP has also been reported to exert anti-apoptotic activity in a few cell types, including TMK-1 cells (Chang et al., 1997), bovine glomerular endothelial cells (Messmer et al., 1999), MCF-7 cells (Messmer et al., 2001), C6 glioma cells (Ni Chonghaile et al., 2006), and primary hepatocytes (Bailly-Maitre et al., 2001). Therefore, our novel finding that MP exerts anti-apoptotic activity in oligodendrocytes is consistent with these previous reports. Our findings are also consistent with preliminary evidence which suggested that MP may exert anti-apoptotic activity following SCI in rats (Vaquero et al., 2006), though this study did not report the selective anti-apopototic activity in oligodendrocytes.
In our studies, we have found that MP inhibits Bcl-xL down-regulation following injury stimuli along with downstream events (caspase-3 activation and DNA laddering). Bcl-xL, which resides on the mitochondrial outer membrane, is an important anti-apoptotic regulator involved in the cell-intrinsic apoptotic pathway. It is believed to exert its anti-apoptotic action by contributing to the maintenance of membrane integrity (thereby preventing the release of proapoptotic signaling molecules from the inner mitochondrial membrane) (for review see Lindsten et al., 2005). Endogenous Bcl-xL is highly expressed throughout the postnatal central nervous system (Gonzalez-Garcia et al., 1994; Krajewska et al., 2002), suggesting an important role in maintaining survival of adult neural cells, under physiological and pathological conditions. Indeed, the infusion of a Bcl-xL fusion protein following SCI in rats promoted neuronal survival (Nesic-Taylor et al., 2005).
The glucocorticoid receptor is ubiquitously expressed in cells throughout the nervous system, including neurons, astrocytes, and oligodendrocytes in the brain (Fuxe et al., 1985b) and spinal cord (Fuxe et al., 1985a). Following SCI, glucocorticoid receptor expression is upregulated in all of these cell types, with peak expression occurring 8 hrs after contusion (Yan et al., 1999). In the inactive state, glucocorticoid receptors (intracellular receptors) reside predominantly in the cytoplasm, associated with other proteins (Vedeckis, 1983; Mendel et al., 1986; Sanchez et al., 1987). Upon activation via steroid binding, these proteins dissociate, and the steroid-receptor complex dimerizes and translocates to the nucleus, where it binds to specific DNA consensus sequences, termed glucocorticoid response elements (GREs) (Tsai et al., 1988) to participate directly in gene regulation (Yamamoto, 1985). The bcl-x gene has several GRE consensus sequences in the promoter region (Gascoyne et al., 2003), suggesting a potential mechanism for its induction by MP in oligodendrocytes. Despite the expression of glucocorticoid receptors in neurons (Fuxe et al., 1985a; Fuxe et al., 1985b), MP did not exert protective effects on cortical neurons in vitro, or spinal cord neurons following SCI in vivo. Moreover, in our in vitro studies, MP did not induce Bcl-xL expression in neurons, while it was induced in oligodendrocytes. These findings suggest that MP's cell-type-specific activities may be mediated by downstream signaling events, which are likely to be different in neurons vs. oligodendrocytes.
Although RU486 was first developed as a specific glucocorticoid antagonist (Teutsch et al., 1981), subsequent characterization revealed several other activities. RU486 binds avidly to but weakly activates GR, giving it agonist/antagonist properties (Bourgeois et al., 1984). In addition, RU486 antagonizes the progesterone receptor (Teutsch et al., 1981; Philibert, 1984), which is the basis for its contraceptive activity. In our studies, we found that RU486 antagonized MP's protective activities in oligodendrocytes treated with STP in vitro, and following spinal cord injury in vivo, suggesting that the protective effects of MP were mediated via GR. To rule out the possibility that RU486 antagonized progesterone-mediated protective effects, we examined the effect of RU486 administered alone after STP-induced cell death or after SCI. RU486 alone did not have any effect on cell survival either in vitro or in vivo.
Although MP is not FDA-approved for use in acute SCI, it is commonly used in this setting based on the results of several randomized controlled trials (Bracken et al., 1990; Bracken et al., 1997). However, many have questioned the efficacy of MP because of its marginal effects and criticisms of study design and interpretation (Nesathurai, 1998; Coleman et al., 2000; Hurlbert, 2000; Sayer et al., 2006). Moreover, there is concern that MP may have detrimental effects that may outweigh its marginal clinical benefits (Nesathurai, 1998; Sayer et al., 2006). There may still be utility in understanding mechanisms of MP's protective effects with hopes of developing interventions that promote protective effects, while minimizing adverse effects.
That MP is selectively protective for oligodendrocytes is intuitively appealing, as its clinical indications involve several CNS disorders involving WM injury. Despite its marginal efficacy in SCI, MP is the mainstay of therapy for a variety of demyelinating disorders, including multiple sclerosis and acute disseminated encephalomyelitis. Demyelination following SCI is thought to play an important role in disability, and has been proposed to be an important target for therapeutic intervention (for review, see McDonald and Belegu, 2006). Though current opinion favors an anti-oxidant or anti-inflammatory mechanism, based on our study we raise the possibility that MP's mechanisms of action may also involve direct anti-apoptotic activity in oligodendrocytes. This novel mechanism may have implications for MP's use in other disorders involving white matter injury.
Acknowledgements
This work was supported by NIH NINDS R01 NS40525, NS48283, P01 NS32636 (JML), and R01 NS40162 (JX). Technical assistance was provided by the In Vivo Animal Model Core from the Hope Center for Neurological Disorders.
References
- Anderson DK, Means ED. Iron-induced lipid peroxidation in spinal cord: protection with mannitol and methylprednisolone. J Free Radic Biol Med. 1985;1:59–64. doi: 10.1016/0748-5514(85)90030-3. [DOI] [PubMed] [Google Scholar]
- Arzt E, Sauer J, Pollmacher T, Labeur M, Holsboer F, Reul JM, Stalla GK. Glucocorticoids suppress interleukin-1 receptor antagonist synthesis following induction by endotoxin. Endocrinology. 1994;134:672–677. doi: 10.1210/endo.134.2.8299563. [DOI] [PubMed] [Google Scholar]
- Bailly-Maitre B, de Sousa G, Boulukos K, Gugenheim J, Rahmani R. Dexamethasone inhibits spontaneous apoptosis in primary cultures of human and rat hepatocytes via Bcl-2 and Bcl-xL induction. Cell Death Differ. 2001;8:279–288. doi: 10.1038/sj.cdd.4400815. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK, Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- Bourgeois S, Pfahl M, Baulieu EE. DNA binding properties of glucocorticosteroid receptors bound to the steroid antagonist RU-486. Embo J. 1984;3:751–755. doi: 10.1002/j.1460-2075.1984.tb01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken MB. Treatment of acute spinal cord injury with methylprednisolone: results of a multicenter, randomized clinical trial. J Neurotrauma. 1991;8(Suppl 1):S47–50. discussion S51-42. [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon J, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL, Jr., Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. Jama. 1997;277:1597–1604. [PubMed] [Google Scholar]
- Brenner T, Yamin A, Gallily R. Mycoplasma triggering of nitric oxide production by central nervous system glial cells and its inhibition by glucocorticoids. Brain Res. 1994;641:51–56. doi: 10.1016/0006-8993(94)91814-7. [DOI] [PubMed] [Google Scholar]
- Chang TC, Hung MW, Jiang SY, Chu JT, Chu LL, Tsai LC. Dexamethasone suppresses apoptosis in a human gastric cancer cell line through modulation of bcl-x gene expression. FEBS Lett. 1997;415:11–15. doi: 10.1016/s0014-5793(97)01083-1. [DOI] [PubMed] [Google Scholar]
- Coleman WP, Benzel D, Cahill DW, Ducker T, Geisler F, Green B, Gropper MR, Goffin J, Madsen PW, 3rd, Maiman DJ, Ondra SL, Rosner M, Sasso RC, Trost GR, Zeidman S. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord. 2000;13:185–199. doi: 10.1097/00002517-200006000-00001. [DOI] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- Demopoulos HB, Flamm ES, Seligman ML, Pietronigro DD, Tomasula J, DeCrescito V. Further studies on free-radical pathology in the major central nervous system disorders: effect of very high doses of methylprednisolone on the functional outcome, morphology, and chemistry of experimental spinal cord impact injury. Can J Physiol Pharmacol. 1982;60:1415–1424. doi: 10.1139/y82-210. [DOI] [PubMed] [Google Scholar]
- Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD. Apoptosis after traumatic human spinal cord injury. J Neurosurg. 1998;89:911–920. doi: 10.3171/jns.1998.89.6.0911. [DOI] [PubMed] [Google Scholar]
- Funk JO, Ernst M, Schonharting MM, Zabel P. Pentoxifylline exerts synergistic immunomodulatory effects in combination with dexamethasone or cyclosporin A. Int J Immunopharmacol. 1995;17:1007–1016. doi: 10.1016/0192-0561(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Harfstrand A, Agnati LF, Yu ZY, Cintra A, Wikstrom AC, Okret S, Cantoni E, Gustafsson JA. Immunocytochemical studies on the localization of glucocorticoid receptor immunoreactive nerve cells in the lower brain stem and spinal cord of the male rat using a monoclonal antibody against rat liver glucocorticoid receptor. Neurosci Lett. 1985a;60:1–6. doi: 10.1016/0304-3940(85)90372-6. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Wikstrom AC, Okret S, Agnati LF, Harfstrand A, Yu ZY, Granholm L, Zoli M, Vale W, Gustafsson JA. Mapping of glucocorticoid receptor immunoreactive neurons in the rat tel- and diencephalon using a monoclonal antibody against rat liver glucocorticoid receptor. Endocrinology. 1985b;117:1803–1812. doi: 10.1210/endo-117-5-1803. [DOI] [PubMed] [Google Scholar]
- Gascoyne DM, Kypta RM, Vivanco MM. Glucocorticoids inhibit apoptosis during fibrosarcoma development by transcriptionally activating Bcl-xL. J Biol Chem. 2003;278:18022–18029. doi: 10.1074/jbc.M301812200. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia M, Perez-Ballestero R, Ding L, Duan L, Boise LH, Thompson CB, Nunez G. bcl-XL is the major bcl-x mRNA form expressed during murine development and its product localizes to mitochondria. Development. 1994;120:3033–3042. doi: 10.1242/dev.120.10.3033. [DOI] [PubMed] [Google Scholar]
- Hall ED, Braughler JM. Effects of intravenous methylprednisolone on spinal cord lipid peroxidation and Na+ + K+)-ATPase activity. Dose-response analysis during 1st hour after contusion injury in the cat. J Neurosurg. 1982;57:247–253. doi: 10.3171/jns.1982.57.2.0247. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Ikata T, Katoh S, Nakauchi K, Niwa M, Kawai Y, Fukuzawa K. Involvement of an intercellular adhesion molecule 1-dependent pathway in the pathogenesis of secondary changes after spinal cord injury in rats. J Neurochem. 1996;66:1525–1531. doi: 10.1046/j.1471-4159.1996.66041525.x. [DOI] [PubMed] [Google Scholar]
- Heyderman RS, Klein NJ, Daramola OA, Levin M. Modulation of the endothelial procoagulant response to lipopolysaccharide and tumour necrosis factor-alpha in-vitro: the effects of dexamethasone, pentoxifylline, iloprost and a polyclonal anti-human IL-1 alpha antibody. Inflamm Res. 1995;44:275–280. doi: 10.1007/BF02032568. [DOI] [PubMed] [Google Scholar]
- Holets VR, Hokfelt T, Ude J, Eckert M, Penzlin H, Verhofstad AA, Visser TJ. A comparative study of the immunohistochemical localization of a presumptive proctolinlike peptide, thyrotropin-releasing hormone and 5-hydroxytryptamine in the rat central nervous system. Brain Res. 1987;408:141–153. doi: 10.1016/0006-8993(87)90366-0. [DOI] [PubMed] [Google Scholar]
- Hurlbert RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93:1–7. doi: 10.3171/spi.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- Keane RW, Kraydieh S, Lotocki G, Bethea JR, Krajewski S, Reed JC, Dietrich WD. Apoptotic and anti-apoptotic mechanisms following spinal cord injury. J Neuropathol Exp Neurol. 2001;60:422–429. doi: 10.1093/jnen/60.5.422. [DOI] [PubMed] [Google Scholar]
- Krajewska M, Mai JK, Zapata JM, Ashwell KW, Schendel SL, Reed JC, Krajewski S. Dynamics of expression of apoptosis-regulatory proteins Bid, Bcl-2, Bcl-X, Bax and Bak during development of murine nervous system. Cell Death Differ. 2002;9:145–157. doi: 10.1038/sj.cdd.4400934. [DOI] [PubMed] [Google Scholar]
- Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI, Chen S, Hsu CY. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol. 2004;164:123–131. doi: 10.1083/jcb.200307017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ona VO, Chen M, Kaul M, Tenneti L, Zhang X, Stieg PE, Lipton SA, Friedlander RM. Functional role and therapeutic implications of neuronal caspase-1 and -3 in a mouse model of traumatic spinal cord injury. Neuroscience. 2000;99:333–342. doi: 10.1016/s0306-4522(00)00173-1. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Zong WX, Thompson CB. Defining the role of the Bcl-2 family of proteins in the nervous system. Neuroscientist. 2005;11:10–15. doi: 10.1177/1073858404269267. [DOI] [PubMed] [Google Scholar]
- Liu HN, Giasson BI, Mushynski WE, Almazan G. AMPA receptor-mediated toxicity in oligodendrocyte progenitors involves free radical generation and activation of JNK, calpain and caspase 3. J Neurochem. 2002;82:398–409. doi: 10.1046/j.1471-4159.2002.00981.x. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Pelaez RP, Martinez J, Bazan NG. Budesonide epimer R or dexamethasone selectively inhibit platelet-activating factor-induced or interleukin 1beta-induced DNA binding activity of cis-acting transcription factors and cyclooxygenase-2 gene expression in human epidermal keratinocytes. Proc Natl Acad Sci U S A. 1998;95:3914–3919. doi: 10.1073/pnas.95.7.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Belegu V. Demyelination and remyelination after spinal cord injury. J Neurotrauma. 2006;23:345–359. doi: 10.1089/neu.2006.23.345. [DOI] [PubMed] [Google Scholar]
- McEwen ML, Springer JE. A mapping study of caspase-3 activation following acute spinal cord contusion in rats. J Histochem Cytochem. 2005;53:809–819. doi: 10.1369/jhc.4A6467.2005. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21:3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel DB, Bodwell JE, Gametchu B, Harrison RW, Munck A. Molybdate-stabilized nonactivated glucocorticoid-receptor complexes contain a 90-kDa non-steroid-binding phosphoprotein that is lost on activation. J Biol Chem. 1986;261:3758–3763. [PubMed] [Google Scholar]
- Messmer UK, Winkel G, Briner VA, Pfeilschifter J. Glucocorticoids potently block tumour necrosis factor-alpha- and lipopolysaccharide-induced apoptotic cell death in bovine glomerular endothelial cells upstream of caspase 3 activation. Br J Pharmacol. 1999;127:1633–1640. doi: 10.1038/sj.bjp.0702726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer UK, Pereda-Fernandez C, Manderscheid M, Pfeilschifter J. Dexamethasone inhibits TNF-alpha-induced apoptosis and IAP protein downregulation in MCF-7 cells. Br J Pharmacol. 2001;133:467–476. doi: 10.1038/sj.bjp.0704093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesathurai S. Steroids and spinal cord injury: revisiting the NASCIS 2 and NASCIS 3 trials. J Trauma. 1998;45:1088–1093. doi: 10.1097/00005373-199812000-00021. [DOI] [PubMed] [Google Scholar]
- Nesic-Taylor O, Cittelly D, Ye Z, Xu GY, Unabia G, Lee JC, Svrakic NM, Liu XH, Youle RJ, Wood TG, McAdoo D, Westlund KN, Hulsebosch CE, Perez-Polo JR. Exogenous Bcl-xL fusion protein spares neurons after spinal cord injury. J Neurosci Res. 2005;79:628–637. doi: 10.1002/jnr.20400. [DOI] [PubMed] [Google Scholar]
- Ni Chonghaile T, Concannon CG, Szegezdi E, Gorman AM, Samali A. Dexamethasone inhibits apoptosis in C6 glioma cells through increased expression of Bcl-XL. Apoptosis. 2006;11:1247–1255. doi: 10.1007/s10495-006-7233-1. [DOI] [PubMed] [Google Scholar]
- Perkins DJ, Kniss DA. Tumor necrosis factor-alpha promotes sustained cyclooxygenase-2 expression: attenuation by dexamethasone and NSAIDs. Prostaglandins. 1997;54:727–743. doi: 10.1016/s0090-6980(97)00144-5. [DOI] [PubMed] [Google Scholar]
- Philibert D. An original multifaceted antihormone in In. 1984 [Google Scholar]
- Qiu J, Nesic O, Ye Z, Rea H, Westlund KN, Xu GY, McAdoo D, Hulsebosch CE, Perez-Polo JR. Bcl-xL expression after contusion to the rat spinal cord. J Neurotrauma. 2001;18:1267–1278. doi: 10.1089/089771501317095304. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gomez MV, Alberdi E, Ibarretxe G, Torre I, Matute C. Caspase-dependent and caspase-independent oligodendrocyte death mediated by AMPA and kainate receptors. J Neurosci. 2003;23:9519–9528. doi: 10.1523/JNEUROSCI.23-29-09519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez ER, Meshinchi S, Schlesinger MJ, Pratt WB. Demonstration that the 90-kilodalton heat shock protein is bound to the glucocorticoid receptor in its 9S nondeoxynucleic acid binding form. Mol Endocrinol. 1987;1:908–912. doi: 10.1210/mend-1-12-908. [DOI] [PubMed] [Google Scholar]
- Sayer FT, Kronvall E, Nilsson OG. Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J. 2006;6:335–343. doi: 10.1016/j.spinee.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Pauels HG, Lugering N, Lugering A, Domschke W, Kucharzik T. Glucocorticoids induce apoptosis in human monocytes: potential role of IL-1 beta. J Immunol. 1999;163:3484–3490. [PubMed] [Google Scholar]
- Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res. 1997;50:798–808. doi: 10.1002/(SICI)1097-4547(19971201)50:5<798::AID-JNR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Sikora U. [On cutaneous emphysemas in the maxillo-facial region] Dtsch Stomatol. 1966;16:648–652. [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med. 1999;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- Springer JE, Nottingham SA, McEwen ML, Azbill RD, Jin Y. Caspase-3 apoptotic signaling following injury to the central nervous system. Clin Chem Lab Med. 2001;39:299–307. doi: 10.1515/CCLM.2001.046. [DOI] [PubMed] [Google Scholar]
- Teutsch G, Costerousse G, Deraedt R, Benzoni J, Fortin M, Philibert D. 17 alpha-alkynyl- 11 beta, 17-dihydroxyandrostane derivatives: a new class of potent glucocorticoids. Steroids. 1981;38:651–665. doi: 10.1016/0039-128x(81)90084-2. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Carlstedt-Duke J, Weigel NL, Dahlman K, Gustafsson JA, Tsai MJ, O'Malley BW. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988;55:361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- van de Stolpe A, Caldenhoven E, Stade BG, Koenderman L, Raaijmakers JA, Johnson JP, van der Saag PT. 12-O-tetradecanoylphorbol-13-acetate- and tumor necrosis factor alpha-mediated induction of intercellular adhesion molecule-1 is inhibited by dexamethasone. Functional analysis of the human intercellular adhesion molecular-1 promoter. J Biol Chem. 1994;269:6185–6192. [PubMed] [Google Scholar]
- Vaquero J, Zurita M, Oya S, Aguayo C, Bonilla C. Early administration of methylprednisolone decreases apoptotic cell death after spinal cord injury. Histol Histopathol. 2006;21:1091–1102. doi: 10.14670/HH-21.1091. [DOI] [PubMed] [Google Scholar]
- Vedeckis WV. Subunit dissociation as a possible mechanism of glucocorticoid receptor activation. Biochemistry. 1983;22:1983–1989. doi: 10.1021/bi00277a038. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Xu J, Qu ZX, Moore SA, Hsu CY, Hogan EL. Receptor-linked hydrolysis of phosphoinositides and production of prostacyclin in cerebral endothelial cells. J Neurochem. 1992;58:1930–1935. doi: 10.1111/j.1471-4159.1992.tb10071.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Hsu CY, Junker H, Chao S, Hogan EL, Chao J. Kininogen and kinin in experimental spinal cord injury. J Neurochem. 1991;57:975–980. doi: 10.1111/j.1471-4159.1991.tb08246.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Wu Y, He L, Yang Y, Moore SA, Hsu CY. Regulation of cytokine-induced iNOS expression by a hairpin oligonucleotide in murine cerebral endothelial cells. Biochem Biophys Res Commun. 1997;235:394–397. doi: 10.1006/bbrc.1997.6800. [DOI] [PubMed] [Google Scholar]
- Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY. Methylprednisolone inhibition of TNF-alpha expression and NF-kB activation after spinal cord injury in rats. Brain Res Mol Brain Res. 1998a;59:135–142. doi: 10.1016/s0169-328x(98)00142-9. [DOI] [PubMed] [Google Scholar]
- Xu J, Yeh CH, Chen S, He L, Sensi SL, Canzoniero LM, Choi DW, Hsu CY. Involvement of de novo ceramide biosynthesis in tumor necrosis factor-alpha/cycloheximide-induced cerebral endothelial cell death. J Biol Chem. 1998b;273:16521–16526. doi: 10.1074/jbc.273.26.16521. [DOI] [PubMed] [Google Scholar]
- Xu JA, Hsu CY, Liu TH, Hogan EL, Perot PL, Jr., Tai HH. Leukotriene B4 release and polymorphonuclear cell infiltration in spinal cord injury. J Neurochem. 1990;55:907–912. doi: 10.1111/j.1471-4159.1990.tb04577.x. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol. 1995;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- Yamamoto KR. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Yan P, Xu J, Li Q, Chen S, Kim GM, Hsu CY, Xu XM. Glucocorticoid receptor expression in the spinal cord after traumatic injury in adult rats. J Neurosci. 1999;19:9355–9363. doi: 10.1523/JNEUROSCI.19-21-09355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong C, Arnold PM, Zoubine MN, Citron BA, Watanabe I, Berman NE, Festoff BW. Apoptosis in cellular compartments of rat spinal cord after severe contusion injury. J Neurotrauma. 1998;15:459–472. doi: 10.1089/neu.1998.15.459. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Ge B, Duncan ID. Adult brain retains the potential to generate oligodendroglial progenitors with extensive myelination capacity. Proc Natl Acad Sci U S A. 1999;96:4089–4094. doi: 10.1073/pnas.96.7.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]