Abstract
Extended access to cocaine produces an increase in cocaine self-administration in rats that mimics aspects of compulsive drug intake in human addicts. While emerging evidence implicates the endogenous cannabinoid system in aspects of opioid and ethanol addiction, a role of the endocannabinoid system in cocaine addiction remains largely inconclusive. Here, we investigate the effects of systemic and intra-accumbal administration of the CB1 antagonist SR141716A (Rimonabant) on cocaine self-administration (0.5 mg/kg/infusion) under a progressive ratio (PR) schedule in rats with extended (long access, LgA; 6 h/day) or limited (short access, ShA; 1 h/day) access to cocaine. LgA rats, but not ShA rats showed an increase in cocaine intake as previously reported, and responding for cocaine by LgA rats was higher than in ShA rats under a PR schedule. Systemic SR141716A induced a dramatic dose-dependent decrease in the break-point for cocaine by LgA rats, whereas only the highest dose of the antagonist had a significant effect in the ShA group. Anandamide levels in the nucleus accumbens (NAc) shell were decreased in ShA rats but unchanged in LgA rats during cocaine self-administration. Both phosphorylated and total CB1 receptor protein expression were upregulated in LgA rats in the NAc and the amygdala compared to ShA and drug-naïve rats, 24 h after last cocaine session. Finally, intra-NAc infusions of SR141716A reduced cocaine break-points selectively in LgA animals. These results suggest that neuroadaptations in the endogenous cannabinoid system may be part of the neuroplasticity associated with the development of cocaine addiction.
Keywords: Cocaine, self-administration, CB1 receptors, endocannabinoids, nucleus accumbens, addiction
INTRODUCTION
Cocaine addiction constitutes a major health problem and an estimated 1.6 million Americans meet the criteria for cocaine abuse or dependence (NSDUH, 2006). Animal models for components of psychostimulant addiction have been well characterized (Ahmed and Koob, 1998; Kitamura et al., 2006), and mimic valid aspects of the loss of control over drug intake observed in humans (American Psychiatric Association, 2000; Koob, 2008). Specifically, cocaine self-administration with extended access (LgA) gradually increases over days, whereas the intake remains stable in rats with limited access (ShA) (Ahmed and Koob, 1998; Wee et al., 2007, 2008; Specio et al., 2008). This escalated drug intake is associated with increased break-points or responding for cocaine under a progressive-ratio (PR) schedule, suggesting an enhanced motivation to seek cocaine and/or an enhanced efficacy of cocaine reward (Paterson and Markou, 2003; Wee et al., 2008), and is also characterized by a withdrawal syndrome as measured by increased brain reward thresholds (Ahmed et al., 2002). Thus, investigations into the neuroadaptations that occur in LgA rats are invaluable for understanding the enhanced motivation to seek cocaine associated with the development of addiction.
The endogenous cannabinoid system is associated with aspects of drug addiction, as endocannabinoids (eCB) modulate the rewarding effects of ethanol, nicotine, opioids and marijuana (for reviews: Tanda and Goldberg, 2003, Lupica et al., 2004, Cohen et al., 2005; Gardner, 2005). Moreover, CB1 cannabinoid receptors are expressed in regions of the brain reward circuitry (Mailleux and Vanderhaeghen, 1992; Herkenham et al., 1990, 1991). However, the specific role of eCB in cocaine addiction remains largely inconclusive (Wiskerke et al., 2008). Whereas several studies suggest no role for CB1 receptors in cocaine self-administration per se (Fattore et al., 1999; Martin et al., 2000; Cossu et al., 2001; Lesscher et al., 2005; Caille & Parsons, 2006; Filip et al., 2006; Caille et al., 2007), other reports demonstrate a CB1 receptor involvement in both the acquisition and consolidation of cocaine reward (Soria et al., 2005; Xi et al. 2008) and in relapse to cocaine-seeking behavior (De Vries et al., 2001; Xi et al., 2006). However, the above studies evaluated the effects of CB1 receptor deletion/antagonism in animals given limited access to cocaine. It is unknown if long term extended access cocaine exposure could alter eCB-CB1 function.
We tested the hypothesis here that alterations in eCB transmission contribute to the increased motivation for cocaine associated with stimulant use. Thus, we examined the effects of systemic and intra-accumbal injections of the CB1 receptor antagonist SR141716A (Rimonabant) on cocaine self-administration under a PR schedule of reinforcement and showed a selective enhanced sensitivity in LgA rats compared to ShA rats. Additionally, LgA rats maintained an increased nucleus accumbens (NAc) eCB tone during cocaine self-administration compared to ShA rats, and showed an upregulation of both phosphorylated (pCB1) and total CB1 receptor protein expression in the NAc and amygdala 24 h after last cocaine session. Results together suggest a dramatic increased activity of the eCB system in rats with extended access to cocaine self-administration.
MATERIAL AND METHODS
Animals
Sixty-eight adult male Wistar rats (Charles River, Hollister, CA) weighting 225-250 g at the start of the experiment were used. Rats were housed 2 or 3 per cage with food and water available ad libitum, and maintained in a reverse 12 h light/dark cycle, with lights on at 8 am. Twenty-three rats did not complete the experiments due to catheter failure or health complications. In three animals the i.v. catheter was replaced for a new one which was implanted in the left jugular vein, and two animals were excluded of analysis due to misplaced cannula locations. All procedures were approved by The Scripps Research Institute Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines (NIH Publication no. 85-23, revised 1996).
Drugs and reagents
Cocaine hydrochloride and SR141716A (Rimonabant) [N-piperidino-5-(4-chorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide] were generously provided by the National Institute on Drug Abuse. Cocaine HCL was dissolved in sterile 0.9% saline and filtered. SR141716A (Rimonabant) was dissolved in a vehicle of ethanol:emulphor:saline (1:1:18) by sonication for 30 seconds and was prepared fresh for every test session. AEA, 2-AG, 1(3)-arachidonoylglycerol (1-AG) and (S)-(+)-arachidonyl-2′-hydroxy-1′-propylamide (S-2 methanandamide) were used as chromatographic standards for microdialysis and were purchased from Cayman Chemical (Ann Arbor, MI). Rabbit anti-CB1 receptor (C1108) was purchased from Sigma (St. Louis, MO). Rabbit polyclonal anti-pCB1 receptor (sc-17555) and mouse anti-β-tubulin (sc-53140) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), goat anti-rabbit and goat anti-mouse IgG secondary antibodies were from BioRad (Hercules, CA).
Intravenous surgery
Rats were anesthetized with 2-3% of isofluorane mixed in oxygen and implanted with a sterilized silastic catheter (0.64 ID × 1.19 OD mm; Dow Corning Co. Midland, MI) into the right jugular vein under aseptic conditions. The distal end of the catheter was threaded under the skin to the back of the rat and exited the skin via a metal guide cannula (22G, Plastic One, Inc., Roanoke, VA). Immediately after surgery, Flunixin® (2.5 mg/kg, s.c.) was given as analgesic. The rats were subjected to antibiotic therapy (Timentin®, 20 mg, i.v) for at least ten days. Catheters were flushed daily with heparinized saline (30 USP units/ml). The patency of catheters was tested using methohexital sodium (Brevital®, 10 mg/ml, 2 mg/rat).
Cocaine self-administration procedure
After at least five days of post-operative recovery, rats were trained to self-administer cocaine during daily 1h sessions under a fixed ratio 1 (FR1) schedule of reinforcement in standard operant boxes (MedAssociates, St. Albans, VT). The dose of cocaine (0.5 mg/kg/infusion) was prepared individually for each rat based on the body weight and updated every two or three days. Cocaine at 0.5 mg/kg/infusion accounts for a highly stable self-administration behavior in rats, and previous findings showed as well that the dose of 0.5 mg/kg/infusion of cocaine is within the range of the descending limb of the cocaine dose-response self-administration curve in rats (Wee et al., 2007; Wee et al., 2008; Xi et al., 2005), and thus this dose of cocaine is appropriate to measure reliable and stable dose-dependent effects. In addition, when LgA and ShA rats were compared using a dose/response test, there was an upwards shift at all doses bracketing this dose (see Ahmed and Koob, 1998, 1999, 2005; Wee et al., 2007; Deroche et al.,1999). The dose of cocaine was carefully chosen based on on our and other’s previous studies to reflect a dose where both increases and decreases in PR responding could be observed (Wee et al., 2007, Wee et al., 2008; Xi et al., 2005; Xi et al., 2008).
At the beginning of each session rats were presented with two levers into the chamber. Responding on the right lever resulted in the 4 sec delivery of 0.1 ml of cocaine and the illumination of a light above the lever that lasted throughout a time-out period of 20 sec. Responding on the left lever was recorded but had no programmed consequences. Rats were trained to self-administer a higher cocaine dose (1 mg/kg/infusion) for two days before the dose of cocaine was reduced to 0.5 mg/kg/infusion over 14 subsequent baseline sessions. After this baseline period the rats were divided into two groups, balanced by the average number of cocaine infusions per session during the last three baseline sessions. One group of rats were allowed to self-administer cocaine (0.5 mg/kg/infusion) during daily 6 h sessions (long access, LgA), whereas the other group of rats was maintained on daily 1 h sessions (short access, ShA) for the next 22 sessions. Cocaine self-administration sessions were conducted 6 days per week.
SR141716A (Rimonabant) pretreatment on cocaine self-administration
After 22 escalation sessions, the effect of the CB1 receptor antagonist SR141716A was tested in LgA and ShA rats under a PR schedule of reinforcement. In the PR schedule, the response requirement began at one lever press/infusion and increased exponentially according to the following equation: lever press/infusion=[5× e(infusion number × 0.2)]-5 (Richardson and Roberts, 1996). The session length was limited to 12 h or ended when a rat failed to achieve the response requirement within 1 h. Test sessions (PR) were conducted 2 days per week and were preceded by two FR1 cocaine self-administration session days (LgA, 6 h session; ShA, 1 h session) in order to assure stable cocaine intake levels between the previous and the subsequent test session. Each rat received systemic vehicle pretreatments prior to four PR test sessions to habituate them to the injection procedure. Three doses of the CB1 antagonist SR141716A (0.3 mg/kg, 1.0 mg/kg and 3.0 mg/kg) and vehicle (see reagents) were tested in a within-subject, Latin square design. The CB1 receptor antagonist was injected intraperitoneally 30 min before a test session. The SR141716A doses were chosen based on previous literature (Rodríguez de Fonseca et al., 1999; Navarro et al., 2001). Finally, each rat was again tested with vehicle injections in one last test session at the end of the study. No changes in responding to vehicle treatment were found on PR cocaine self-administration during and after the Latin square design.
Brain microdissection procedure
Animals from the behavioral study were allowed to self-administer cocaine (LgA, 6 h session n=6; ShA, 1 h session, n=6; FR1) during a SR141716A wash-out period of at least seven days after the last dose tested. For the purpose of comparison, age- and weight-matched drug-naïve rats (n=4) were included as a control group for the biochemical analyses. Twenty-four hours after the last escalation session rats were decapitated in random order under light isofluorane anesthesia. The brains were rapidly removed, snap frozen in 2-methylbutane, and stored at -80°C until dissection. On the day of dissection, brains were mounted and sliced in a cryostat (Cryocut 1800; Reichert-Jung, Leica). Brain tissue from the prefrontal cortex (PFC, +3.20 to +2.20 mm from bregma), nucleus accumbens core (NAc core, +2.20 to +0.70), nucleus accumbens shell (NAc shell, +1.70 to +0.70), dorsal striatum (DS, +1.70 to +2.20), bed nucleus of the stria terminalis (BNST, -0.30 to -0.80), amygdaloid nucleus (Amy, -1.80 to -2.80), ventral hippocampus (VHp, -2.80 to -4.30), and dorsal hippocampus (DHp, -4.30 to -5.8) were punched out bilaterally in successive 500 μm-sections with the assistance of a rat brain atlas (Paxinos G. and Watson C., 1998). Punch needles were kept on dry ice during the procedure to prevent tissue from thawing within the needle, and brain punches were subsequently kept at -80°C until assay.
Western blot analysis of phosphorylated or total CB1 receptor and β-tubulin
Brain punches were sonicated in a homogenization buffer containing 50 mM Tris-HCl (pH =7.4), 10% w/v sucrose, 5 mM EDTA, 1 mM dithiotreitol, 1% w/v SDS, with Protease Inhibitor Cocktail and Phosphatase Inhibitor Cocktails I and II diluted 1:100 each (Sigma, St. Louis, MO). Protein content was determined in homogenized samples using a detergent-compatible protein assay (Bio-Rad, Hercules, CA). Equal amounts of protein (15 μg) were loaded per lane including 3-5 animals per group in each gel. In a separate experiment, equal amounts of protein (15 μg) from various brain structures were loaded to compare basal brain wide CB1 receptor and phosphorylated CB1 receptor (pCB1) immunoreactivies in naïve animals. Proteins were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) using Tris-Glycine-SDS buffer system (Bio-Rad), and transferred to polyvinylidene membranes (GE Healthcare, Piscataway). Membranes were then blocked for 2 h in PBS containing 5% non-fat milk to reduce nonspecific binding of antibodies, followed by an overnight incubation at 4°C with polyclonal rabbit anti-CB1 (1:500 dilution) or anti-pCB1 (Ser 317) (1:250 dilution), and mouse anti-β-tubulin (1:2500 dilution). Membranes were washed and then incubated for 1 h with species-specific peroxidase-conjugated secondary antibody (1:10000 dilution; Bio-Rad). Immunoreactivity was detected with an enhanced chemiluminescence (ECL) Western blot detection system (GE Healthcare), following by exposure to Amersham Hyperfilm ECL for 1-5 min. Different film exposure times were used to ensure that bands were not saturated, and immunoreactivity was quantified by ImageJ software (NIH). The anti-pCB1 antibody used in this study was raised against the CB1 receptor, which is phosphorylated by PKC at Ser 316 (humans)/Ser 317 (rodents) (Garcia et al., 1998; Matsuda et al., 1990; Gerard et al., 1991). The antibody recognized two bands corresponding to the glycosylated (63 kDa) and the non-glycosylated (53 kDa) protein, both reflecting functional pCB1 receptor protein (Porcella et al., 2000; Howlett et al. 1998). Both glycosylated and non-glycosylated phosphorylated forms were quantified and expressed as total pCB1 protein. The antibody for total CB1 receptor density recognized one band of 64 KDa corresponding to the monomeric form of the fully glycosylated receptor (Fusco et al., 2004) that was quantified and expressed as total CB1 receptor protein (the antibody recognized also one non-specific band of 33 KDa which is due probably to the secondary antibody used, see Fusco et al., 2004 for details). β-tubulin was used as a loading control and so the immunoreactivity of CB1 and pCB1 receptors was corrected for β-tubulin levels.
Intracerebral microdialysis cannula implantations
To investigate cocaine’s regulation of eCB levels in LgA and ShA rats during cocaine self-administration, another group of rats was trained as described above. After the escalation period (22 sessions), rats were anesthetized (2-3% isofluorane) and implanted with a guide cannula (SciPro, Sanborn, NY) aimed at the NAc shell using a stereotaxic frame with the tooth bar at -3.3 mm below the interaural zero. Coordinates for the shell cannula implantation were: +1.6 mm from bregma, ± 0.8 mediolateral and -5.7 mm below dura (Paxinos and Watson, 1998). Cannulae were implanted in the right or left NAc shell of the rats in random order. To avoid disruption in animal behavior resulting from surgery, rats were allowed to self-administer cocaine during at least six more sessions prior to the microdialysis experiment.
Probe implantation and in vivo microdialysis
For probe implantation, each animal was lightly anesthetized (1-2 % isofluorane) and the dialysis probes (2 mm polyethyl sulfone membrane, 15 kDa MW cutoff; SciPro) were inserted in the guide cannulae and secured with a light layer of cement (Double/Bubble® Epoxy, Hardman®) . The probes were perfused overnight with artificial CSF (0.1 μl/min) composed of the following: 149 NaCl nM, 2.8 KCl, 1.2 CaCl2, 1.2 MgCl2, 0.25 ascorbic acid and 5.4 D-glucose. The morning of the microdialysis experiment, the artificial CSF was replaced by an artificial CSF solution containing 30% (w/v) hydroxypropyl-β-cyclodextrin and probes were perfused for 90 min at 0.6 μl/min flow. Subsequently, dialysis samples were collected at 15 min intervals during the baseline period (90 min) and subsequent operant cocaine self-administration (90 min). Animals remained in the same cage and room during the microdialysis perfusion and were transferred to the operant chambers in the same room exclusively for cocaine self-administration.
Liquid chromatography/mass spectrometry analysis of dialysate eCB content
Dialysate levels of arachidonoylethanolamide (anandamide, AEA), 2-arachidonoylglycerol (2-AG) and 1-arachidonoylglycerol (1-AG) were determined using high performance liquid chromatography coupled with electrospray ionization mass spectrometry. 2-AG levels were calculated by summing 1-AG and 2-AG peak areas to control for 2-AG degradation over time. The procedure used has been described in detail elsewhere (Caillé S. et al., 2007).
Intracerebral infusion cannulas
Animals were trained as described in the cocaine self-administration procedure. After 24 escalation sessions, rats were anesthetized (2-3% isofluorane) and implanted with bilateral microinfusion guide cannula (22 gauge, 12 mm length, stainless steel) that terminated 2 mm above the surface of the NAc (from bregma: AP + 1.7, ML ± 0.9, DV - 5.4 from dura) (Paxinos and Watson, 1998). After post-operative recovery, rats were allowed to self-administer cocaine until similar self-administration rates to those before surgery were established prior to the SR141716A testing.
Intra-NAc SR141716A (Rimonabant) testing on PR cocaine self-administration
The effects of local SR141716A administration on cocaine self-administration under a PR schedule were evaluated in a separate group of animals. Animals were trained for responding in a PR schedule during a total of four sessions (two sessions before i.c. surgery and two sessions post-surgery) which were always preceeded by cocaine FR1 sessions. After establishment of stable self-administration behavior, animals received an initial sham microinjection (insertion but no liquid infusion) immediately before a PR cocaine session in order to acclimate them to the procedure and to produce the initial tissue damage from injection insertion. Subsequently, vehicle and SR141716A (1.0 and 3.0 μg per side) were injected via bilateral 33 gauge microinjectiors that extended 2 mm beyond the tip of the guide cannulas. Infusions of 0.5 μl per side were made over a 2 min period, followed by an additional 1 min to allow drug diffusion before injector removal. The doses of SR141716A for intracerebral microinjections were chosen on the basis of a ratio of ∼ 1000:1 for systemic/intracerebral microinjection dosing (Xi et al., 2006) and following our previous work with SR141716A (Caille et al. 2007). Intra-NAc administration of SR141716A was tested immediately before cocaine PR sessions in a counter balance order across the rats and each dose was tested only once in each animal.
Histology
Microdialysis probe locations were examined after completion of the experiments in all the animals. Rats were sacrificed by decapitation and brains removed and rapidly frozen on dry ice. Brains were coronally sliced in a cryostat and the probe placements were verified with the help of a rat atlas (Paxinos and Watson, 1998).
Cannula placements for intra-NAc infusions were examined as follows: frozen coronal brain section (60 μm) were dehydrated and rehydrated in ethanol, stained in 0.1% cresyl violate acetate for 10 minute and cover slipped for histological verification of the implant location under a light microscope.
Statistical analyses
Data were analyzed using GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA). Cocaine intake was expressed as the mean number of infusions in both the first hour of the session and in the total session for each group of rats. Cocaine self-administration was compared across daily sessions over the initial 22 sessions using repeated-measures two-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test (access x daily session). The effect of pretreatment of SR141716A on cocaine PR self-administration was compared between LgA and ShA rats using repeated-measures two-way ANOVA with cocaine access as the between-subjects factor and SR141716A dose as the within-subjects factor followed by the Bonferroni post test. Additionally, when a significant access x dose interaction was found, one-way ANOVA followed by Newman-Keuls post hoc test was conducted on simple main effects, and Student’s t-test was performed on the appropriate pairing (vehicle-injected rats). To identify possible rate-dependent effects, a median split analysis was performed on the LgA animal’s response under a PR schedule (cocaine injections/session) that yielded high and low subgroups, which were compared using an independent samples t-test. Subsequently, the percent change from baseline following SR141716A administration was compared for the high and low responding groups using an independent samples t-test. Differences in baseline dialysate AEA and 2-AG concentrations between LgA and ShA rats were analyzed by two-tailed Student’s t-test. Subsequently, dialysate AEA and 2-AG levels were expressed as a percentage of the average six dialysate baseline concentrations for each group. Changes in dialysate sample content between groups were investigated measuring the area under the curve (AUC) for AEA and 2-AG of the transformed data. The AUC was calculated for each animal by subtracting each percentage of baseline data points from 100 and summing all data points collected during the experimental period. Calculated cumulative AUC was then compared between groups by Student’s t-test for AEA and 2-AG. Additionally, repeated-measures two-way ANOVA analysis of the calculated data was used to investigate interaction between groups. Percentage of changes from baseline levels in each group were reported by using repeated measures one-way ANOVA following Newman-Keuls multiple comparison test when a significant interaction or main effect was found.
Data from Western blot analyses were expressed as a percentage of the drug-naïve control group and were analyzed using one-way ANOVA followed by Newman-Keuls multiple comparison test when a significant F value was obtained.
RESULTS
Cocaine self-administration under a fixed ratio-schedule in ShA and LgA rats
CB1 receptor antagonist group
A two-way ANOVA found a significant interaction between group and daily session in escalation of cocaine self-administration (0.5 mg/kg/infusion) within a whole session [F (21, 374) = 7.27, p<0.0001] as well as during the first hour of each session [F (21, 374) = 3.08, p<0.0001]. Additionally, there were main effects of group [F (1, 374) = 2561, p<0.0001; first hour F (21, 374) = 169.7, p<0.0001] and daily session [F (21, 374) = 7.58, p<0.0001; first hour F (21, 374) = 3.85, p<0.0001] on cocaine self-administration levels. Subsequent analyses revealed that cocaine self-administration in ShA rats (n=10) remained stable over 22 sessions (Fig. 1a,b; open circles). However, LgA rats (n=9) self-administered more cocaine over time, with the increase becoming evident in session five (within the whole session), and in session six (during the first hour), compared with respective cocaine intake in session one (Fig, 1a,b; filled circles). Escalated cocaine self-administration by LgA rats was maintained over the course of the study.
Figure 1. Cocaine self-administration under a FR1 schedule during the escalation period.
Left panels (a, b) are data from animals in the CB1 receptor antagonist study, and right panels (c, d) are data from animals in the microdialysis study. Top panels (a, c) are data from the entire 6 h sessions, and bottom panels (b, d) are data from the first hour of the sessions. Filled circles correspond to cocaine (0.5 mg/kg/infusion) extended access rats (6 h session, LgA) and open circles correspond to cocaine limited access rats (1 h session, ShA). Data are expressed as the mean ± SEM of the number of cocaine infusions on the left axis and mg/kg on the right axis. *p<0.05, **p<0.01, ***p<0.001 compared to session 1.
Endocannabinoid microdialysis group
Similarly, in the microdialysis study, LgA rats (n=6) showed an escalation in the cocaine intake over 22 sessions, compared with session one, within the 6 h session (Fig.1c, filled circles) and during the first hour of each session (Fig 1d), which started in session number six. A two-way ANOVA found a significant interaction between group and daily session in cocaine self-administration within session [F (21,220) = 3.61, p<0.0001] and during the first hour session [F (21, 220) = 2.446, p<0.001]. Additionally, there were main effects of group [F (1, 220) = 1406, p<0.0001; first hour F (1, 220) = 400.6, p<0.0001], and daily session [F(21, 220)= 3.61, p<0.0001; first hour F (1, 220) = 2.44, p<0.0001] in the levels of cocaine self-administration. Self-administration in the ShA group (n=6) remained stable over the 22 sessions (Fig. 1b, d, open circles).
Effect of CB1 receptor antagonist SR141716A (Rimonabant) on cocaine self-administration under a PR schedule in LgA and ShA rats
To determine whether CB1 activation drives the increased motivation for cocaine observed in dependent animals, we tested a CB1 antagonist on cocaine self-administration under a PR schedule of reinforcement. LgA rats responded ∼ 228 times at the active lever (cumulative response) to achieve break-point of 52.79 ratio/infusion (12.67 reinforcements) (Fig. 2, left panel). ShA rats responded ∼ 97 times to achieve an average break-point of 25.83 (9.3 reinforcements) (Fig. 2, right panel). The break-point for cocaine (0.5 mg/kg/infusion) in vehicle-injected rats was significantly higher in LgA rats versus ShA rats (Student’s t-test, #p<0.01).
Figure 2. Effects of systemic CB1 receptor antagonist SR141716A on the break-point for cocaine in LgA and ShA rats.
SR141716A (SR, Rimonabant) was injected (i.p.) 30 min before the test session. Sessions under a PR schedule ended when rats did not achieve cocaine (0.5 mg/kg/infusion) reinforcement within 1 h. Data are expressed as the mean ± SEM of the number of infusions per session on the left axis and the break-point (maximal ratio per infusion) on the right axis. Bars on the left side are data from cocaine extended access (LgA) rats and bars on the right side are data from limited access (ShA) rats. Responding for cocaine was different under a PR schedule in vehicle-injected rats between the access conditions: #p<0.01 compared to ShA. SR141716A (0.3 and 1.0 mg/kg) decreased the break-point for cocaine selectively in LgA rats compared with vehicle-injected animals; the highest dose of SR141716A (3.0 mg/kg) had a significant effect in both LgA and ShA rats compared to their respective controls: *p<0.05, ***p<0.001 compared to vehicle-injected rats in the same access condition. Different from ShA vehicle-injected rats: #p<0.01.
Figure 2 shows the effects of pretreatment with SR141716A (0.3, 1.0 and 3.0 mg/kg, i.p.) on the break-point for cocaine in both long and short access conditions. A two-way ANOVA between SR141716A pretreatment and cocaine access showed a significant interaction between both factors [F (3, 51) = 5.80, p=0.0017] and a main effect of SR141716A on cocaine break-point [F (3, 51) = 23.49, p<0.0001]. Additional post test revealed a significant reduction in the break-point for cocaine in LgA rats at all doses of SR141716A tested versus vehicle in the same access condition (Fig. 2; 0.3 mg/kg *p<0.05; 1.0 and 3.0 mg/kg ***p<0.001). Similar analysis in ShA rats showed that pretreatment with 0.3 and 1.0 mg/kg doses of SR141716A did not modify the break-point for cocaine compared with vehicle-injected rats in the same access condition (p>0.05, n.s.), and only the highest dose (3.0 mg/kg) had a significant effect in the limited access animals (*p<0.05). Furthermore, even with the lowest dose of SR141716A there was no longer a significant difference between LgA and ShA rats in responding for cocaine (p>0.05, n.s.). One-way ANOVA analyses identified a main effect of SR141716A dose in LgA and ShA rats (F(3,24)=18.42, p<0.0001 and F(3,27)=5.24, p<0.01, respectively) and post hoc tests confirmed a reduction in cocaine break-point in LgA rats at 1.0 and 3.0 mg/kg SR141716A dose (p<0.001) and in ShA rats at 3.0 mg/kg dose (p<0.05), compared with the respective vehicle-injected animals. Finally, SR141716A at 3.0 mg/kg decreased cocaine break-points in the LgA group to even lower ratios than ShA vehicle-treated rats (t-test p<0.01). This fact suggests that high doses of SR141716A may interact with neuroadaptative changes in the LgA group that are not present in the ShA group and/or that CB1 receptor signaling provides a general influence on the motivational effects in LgA animals beyond simply conferring the increased cocaine intake and/or break-points observed in LgA vs. ShA animals.
To address the possibility that higher response rates would be more susceptible to pharmacological modulation by the CB1 antagonist, we conducted a median split analysis on the LgA animals’s response rates. A median split analysis of the response of the LgA animals in a PR schedule (cocaine injections/ session) yielded high and low responder subgroups with mean lever-presses (± S.E.M.) 14.6 ± 0.25 and 11.83 ± 0.79, respectively. SR141716A induced equivalent decreases in percent change from baseline at all doses tested regardless of whether the animals had high or low response rates initially. For example, SR141716A at dose of 1 mg/kg produced 41.1% ± 9.98% and 43.63% ± 11.41% decrease in responding in the high and low responders, respectively. This further supports the hypothesis that SR141716A acted independently of response rate and was more potent in LgA animals.
The amount of cocaine in the rat’s system during testing was calculated based on data in Figure 2 and is represented in Table 1. Analysis of the cocaine intake data presented in the table shows that on the test days there were no robust differences in the amount of cocaine ingested between the two groups. For example, there are no differences in the amount of cocaine in the rat’s system on the test days following SR141716A 0.3 and 1.0 mg/kg pretreatment (5.06 mg/kg vs. 4.85 mg/kg and 3.45 mg/kg vs. 3.75 mg/kg, respectively). However, during those test days, SR141716A decreased responding selectively in the LgA group and not in the ShA group (Fig.2). This supports the hypothesis that SR141716A acted independently of the amount of cocaine currently in the rat’s system during testing. Rather the data suggest that SR141716A altered the motivation for obtaining cocaine in the LgA group.
Table 1. Cocaine intake (mg/kg) by LgA and ShA rats during systemic SR141716A testing (PR sessions).
The amount of cocaine ingested by LgA and ShA rats on the test days was calculated based on data in Figure 2. Data on table are represented as mean ± S.E.M. Analysis of the cocaine intake paired-data presented in the table (LgA versus ShA, t-test) shows differences in the amount of cocaine between vehicle-injected LgA and ShA animals and no differences in the amount of cocaine ingested between the two groups on the other test days. This data support the hypothesis that SR141716A acted independently of the amount of cocaine currently in the rat’s system during testing. SR: SR141716A; n.s.: non significant.
| Cocaine (mg/kg)-LgA | Cocaine (mg/kg)-ShA | P value | |
|---|---|---|---|
| SR vehicle | 6.33 ± 0.34 | 4.65 ± 0.40* | < 0.01 |
| SR 0.3 mg/kg | 5.06 ± 0.58 | 4.85 ± 0.62 | n.s. |
| SR 1.0 mg/kg | 3.45 ± 0.70 | 3.75 ± 0.55 | n.s. |
| SR 3.0 mg/kg | 1.95 ± 0.69 | 3.30 ± 0.53 | n.s. |
Endocannabinoid levels during cocaine self-administration in the NAc shell of rats with a history of LgA and ShA to cocaine
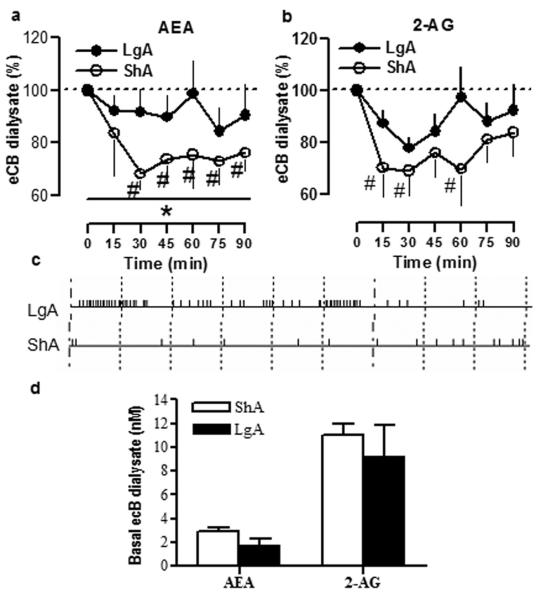
To test whether eCB transmission is differentially affected by extended versus limited access to cocaine, we measured eCB levels during cocaine self-administration in LgA and ShA rats using in vivo microdialysis. Anandaminde (AEA) and 2-arachidonoylglycerol (2-AG) levels (see methods) were measured in the NAc shell, where changes in eCB transmission were reported previously following heroin and ethanol self-administration (Caillé et al., 2007).
Baseline AEA and 2-AG levels are depicted in Fig. 3d. No differences in the baseline levels of either AEA or 2-AG were found between ShA and LgA rats (Student’s t-test, p>0.05, n.s.), although AEA baseline levels in the LgA group were relatively lower (∼37 %) compared to the ShA group (2.89 ± 0.33 versus 1.80 ± 0.51 nM, p>0.05, n.s.). As expected, LgA rats self-administered more cocaine than ShA rats during the 90 min microdialysis session (Student’s t-test, p<0.01). Total cocaine intake during the test session was 21.63 ± 5.67 mg/kg for LgA rats and 5.33 ± 1.28 mg/kg for ShA rats. Fig. 3c shows the cocaine response record for a representative animal in each group. Area-under-the curve (AUC) analysis revealed a significant difference in AEA levels between LgA and ShA rats during the entire session (Fig. 3a; Student’s t-test (AUC), *p<0.05). Two-way ANOVA revealed a significant interaction between group and cumulative AUC over time for AEA levels [F (6,48) = 4.30, p≤0.01] and main effects of group [F(1,48)= 6.30, p<0.05] and time [F (6,48) = 17.79, p<0.0001] in the AEA dialysate. Further analyses revealed that cocaine self-administration in ShA rats produced rapid decreases in AEA (Fig. 3a; repeated measures one-way ANOVA, #p<0.05), whereas AEA levels in the LgA group remained similar to baseline during the 90 min session (Fig. 6a; repeated measures one-way ANOVA, p>0.05, n.s.). Regarding 2-AG levels, no statistical difference in the AUC analysis was found between groups (Fig. 3b; Student’s t-test (AUC), p>0.05, n.s.). Two-way ANOVA analyses of 2-AG dialysate failed to find an interaction between group and the cumulative AUC over time [F (6,48)= 1.02, p>0.05, n.s.), although there was a main effect of time in the 2-AG levels [F (6,48)= 10.27, p<0.0001). Further analyses revealed decreases in 2-AG levels over time in the ShA group (Fig. 3b; repeated measures one-way ANOVA, #p<0.05) but no significant effect in the LgA group (Fig. 3b; repeated measures one-way ANOVA, p>0.05, n.s.).
Figure 3. In vivo microdialysis for AEA and 2-AG in the NAc shell of LgA and ShA rats during cocaine self-administration.
NAc shell eCB dialysates were collected every 15 min in cocaine long access (LgA, filled circles) and short access (ShA, open circles) rats. Time zero represents basal levels for AEA (a) and 2-AG (b) which were calculated as mean of the six dialysated samples collected prior to the self-administration session. Values are expressed as percentage of the baseline samples ± SEM. Differences in the AUC between LgA and ShA: *p<0.05. Different from baseline levels in the same condition: #p<0.05 (one-way ANOVA, Newman-Keuls test). Central panel (c) represents the response records for representative dialysated LgA and ShA animals during the cocaine self-administration test session. Small marks represent an i.v. infusion of cocaine, and larger marks represent 10 min-periods within the session. Basal concentrations for AEA and 2-AG are depicted in d).
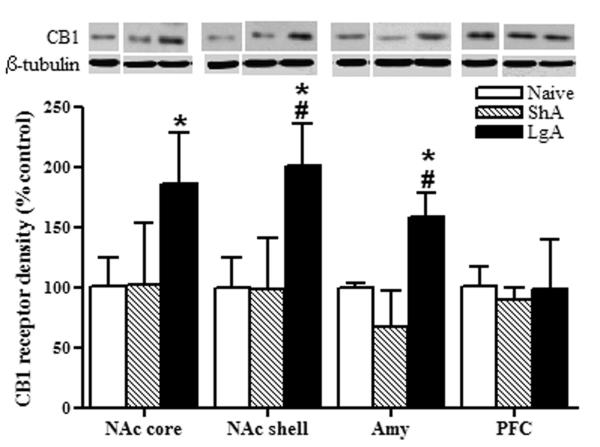
Figure 6. Western blot analysis of pCB1 receptor immunoreactivities in NAc core, NAc shell, Amy and PFC of drug-naïve, and cocaine ShA and LgA rats.
Top panels show representative immunoblots for pCB1 and the loading control β-tubulin in nucleus accumbens core (a, NAc core), nucleus accumbens shell (b, NAc shell), amygdala (c, Amy) and prefrontal cortex (d, PFC). Bar graphs show the quantification of pCB1 receptors immunoreactivity (double band quantification, see methods). Data on graphs are represented as mean ± SEM (n=4-5) and expressed as percentage values of the drug-naïve rats for each group in every brain structure after normalization by β-tubulin. Animals were sacrificed 24 h after the last cocaine session. LgA: long access to cocaine (filled bars); ShA: short access to cocaine (striped bars); Naïve: drug-naïve rats (open bars). Different from drug-naïve: *p<0.05; different from ShA rats: #p<0.05. β-tubulin expression was constant across the groups (p>0.05, n.s.).
Histology
The active portion of the dialysis membrane was located in the NAc shell between 1.6 and 1.7 mm anterior to bregma in all cases (n=10) (Fig. 4).
Figure 4. Schematic representation of the microdialysis probe placements.

The depicted section represents a coronal brain slice 1.6 mm anterior to bregma (Paxinos and Watson, 1998). Vertical bars correspond to the location of the active microdialysis membrane (2 mm) within the NAc shell. Cannulae for probe insertion were implanted in the right or left NAc shell of the rats in random order (n=10).
Cannula placements for intra-accumbal infusions were located in the NAc between 2.28 and 2.76 mm anterior to bregma (Fig. 9). Two animals (one LgA and one ShA rats) had misplaced cannulas, and therefore, only rats with cannulas implanted in the NAc shell were included in the analysis (n=10).
Figure 9. Schematic representation of cannula implants for intra-NAc infusions.
Placements of each drug microinjector in the NAc are depicted in the coronal sections. Each brain section represents a coronal slice 2.76 mm, 2.52 mm and 2.28 mm anterior to bregma (Paxinos and Watson, 1998), and each circle corresponds to the location of the microinjector tip within the NAc (n=10). Animals with cannula implants outside of the NAc were excluded of statistical analysis.
CB1 and pCB1 receptor distribution in the brain
Figure 5 shows brain wide total CB1 and phosphorylated CB1 receptor distribution in a representative drug-naïve animal. We observed that functional CB1 receptors are widely distributed in the brain and concentrated in addiction-related brain areas such as the NAc core and shell, amygdala, bed nucleus of the stria terminalis, and prefrontal cortex, in agreement with other reports (Herkenham et al., 1990, 1991; Mailleux and Vanderhaeghen, 1992; Tsou et al., 1998; Hermann & Lutz, 2005). The pattern of CB1 receptor expression slightly differs from the pCB1 levels, with high density of both proteins observed in prefrontal cortex, dorsal striatum and hippocampus, in accordance with the well-known regulatory effect of CB1 receptors on many other processes in the brain, including locomotion and memory. The bar graph in Fig. 5 represents the quantification of total CB1 receptor and pCB1 receptor density (see methods) normalized by β-tubulin levels (51 kDa) in each brain area and expressed as a ratio (Fig. 5).
Figure 5. Regional differences in total CB1 receptor and pCB1 protein expression in the brain.
Top: representative immunoblots for total CB1, pCB1 receptors and the loading control β-tubulin show the pattern of functional CB1 receptor distribution throughout the brain. Bottom: the bar graph shows the quantification of the single band corresponding to CB1 receptor protein expression and the double band (see methods) corresponding to pCB1 receptor immunoreactivity, both normalized by β-tubulin immunoreactivity. Data are expressed as receptor / β-tubulin immunoreactivity ratio (AU: arbitrary units). PFC: prefrontal cortex; NAc core: nucleus accumbens core; NAc shell: nucleus accumbens shell; DS: dorsal striatum; BNST: bed nucleus of the stria terminalis; Amy: amygdala; VHp: ventral hippocampus; DHp: dorsal hippocampus.
Increased phosphorylated and total CB1 receptor protein expression in the amygdala and NAc of cocaine LgA rats
High densities of phosphorylated CB1 receptor protein were observed in LgA rats compared to ShA and drug-naive rats specifically in the NAc core [F(2, 10)= 6.25, p<0.05], NAc shell [F(2, 11)= 6.45, p<0.05] and the amygdala [F(2, 11)= 4.50, p<0.05] twenty-four hours after the last cocaine escalation session (Fig. 6; Newman-Keuls multiple comparison test: #p<0.05 compared to ShA; *p<0.05 compared to drug-naïve rats). In contrast, no changes in pCB1 immunoreactivity were found in other brain structures such as the prefrontal cortex (Fig. 6), bed nucleus of the stria terminalis, dorsal striatum and hippocampus (data not shown). There were no differences in pCB1 immunoreactivity between ShA and drug-naïve rats (p>0.05, n.s.) in any of the brain structures tested (Fig. 6). Moreover, cocaine self-administration did not change β-tubulin levels in any region tested (Fig. 6).
To elucidate whether pCB1 receptor increases reflected changes in total CB1 receptor densities, we analyzed total CB1 receptor immunoreactivity across the groups in the brain structures where we found an increase in pCB1 receptor (Fig. 7). Total CB1 receptor protein was increased in LgA rats in the NAc core [F(2, 12)= 4.851, p<0.05], the NAc shell [F(2, 12)= 4.225, p<0.05] and the amygdala [F(2, 16)= 5.483, p<0.05] twenty-four hours after the last cocaine escalation session (Fig. 7; Newman-Keuls multiple comparison test: #p<0.05 compared to ShA; *p<0.05 compared to drug-naïve rats). No changes in CB1 receptor were observed between ShA and drug-naïve rats (p>0.05, n.s.). Whereas most drugs of abuse alter CB1 receptor density in different directions (Basavarajappa et al., 1998; Gonzalez et al., 2002a), no changes were observed after non-contingent cocaine administration in limbic forebrain (Gonzalez et al., 2002a), which is in agreement with the lack of receptor changes observed in our ShA rats. However, we observed increased CB1 receptor density specifically in cocaine dependent animals and, similarly, opioid and ethanol dependent animals showed a permanent CB1 receptor upregulation in reward-related areas and enhanced sensitivity to reward disruption induced by cannabinoid receptor antagonists (Rodriguez de Fonseca et al., 1999; Navarro et al., 2001; Rimondini et al., 2002; Navarro et al., 2004).
Figure 7. Upregulation of total CB1 receptor density in NAc and Amy of LgA rats.
Quantification of total CB1 receptor density in the NAc core, NAc shell, amygdala (Amy) and prefrontal cortex (PFC) of LgA (long access to cocaine, filled bars) and ShA (short access to cocaine; striped bars) animals. Data on graphs are corrected for β-tubulin, expressed as percentage values of the drug-naïve rats and represented as mean ± SEM (n=3-6). Animals were sacrificed 24 h after the last cocaine session. Different from ShA animals: *p<0.05.
Intra-NAc infusion of the CB1 receptor antagonist SR141716A reduces cocaine PR responding selectively in LgA rats
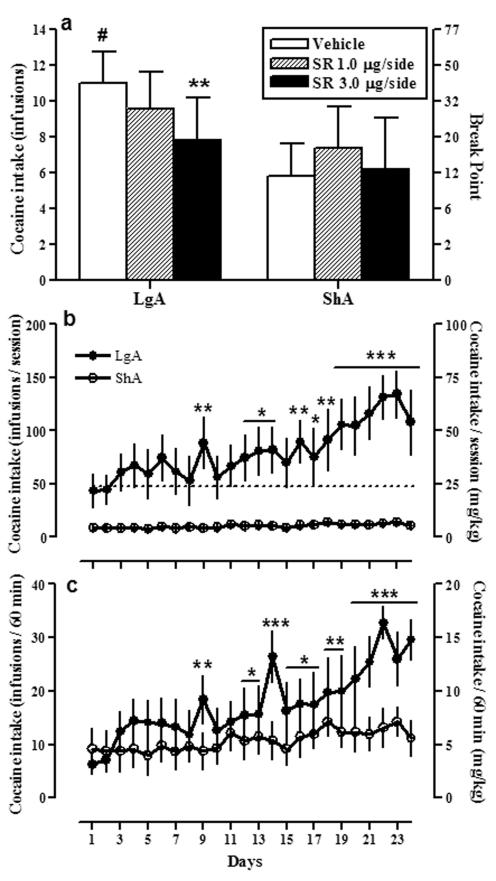
In order to confirm the hypothesized mechanism for the systemic effects of SR141716A, we performed an additional experiment where we infused bilaterally the CB1 antagonist SR141716A into the NAc immediately before a cocaine PR session. Rats were previously trained under a FR1 schedule in conditions of limited or extended access (see methods) and cocaine self-administration data (Fig. 8b, c) were analyzed by a mixed factor two-way ANOVA. The analysis identified a significant interaction between group and daily session within a whole session (Fig. 8b) [F (23,230) = 3.08, p<0.0001] and during the first hour of each session (Fig. 9c) [F (23,230) = 1.78, p=0.02]. Additionally, there were main effects of group [F (1, 230) = 14.12, p=0.004], and daily session [F (23, 230) = 3.79, p<0.0001; first hour F (23, 230) = 4.74, p<0.0001] on cocaine self- administration levels. Subsequent analyses revealed that cocaine self-administration in ShA rats (n=6) remained stable over 24 sessions (Fig. 8b, c; open circles). However, LgA rats (n=6) self-administered more cocaine over time, with the increase becoming evident in session nine, compared with respective cocaine intake in session one (Fig, 9a, b; filled circles).
Figure 8. Intra-NAc infusions of SR141716A reduce cocaine break-points in LgA but not ShA rats.
SR141716A (SR, Rimonabant) was infused locally into the NAc shell immediately before the test session (a). Sessions under a PR schedule ended when rats did not achieve cocaine (0.5 mg/kg/infusion) reinforcement within 1 h. Data in (a) are expressed as the mean ± SEM of the number of cocaine infusions per session on the left axis and the break-point (maximal ratio per infusion) on the right axis. Bars on the left side (a) are data from cocaine extended access (LgA) rats and bars on the right side are data from limited access (ShA) rats. Bilateral infusions of SR141716A (3.0 μg/side) decreased the break-point for cocaine selectively in LgA rats compared with vehicle-injected animals. Different to vehicle-injected rats in the same access condition: **p<0.01. Different from ShA vehicle-injected rats: #p<0.05. Cocaine self-administration data (FR1) during the entire (b) and first hour (c) of the session during the escalation period are represented in the bottom graphs. Filled circles represent cocaine (0.5 mg/kg/infusion) extended access rats (6 h session, LgA) and open circles correspond to cocaine limited access rats (1 h session, ShA). Data are expressed as the mean ± SEM of the number of cocaine infusions on the left axis and mg/kg on the right axis. *p<0.05, **p<0.01, ***p<0.001 compared to session 1.
Under a PR schedule LgA rats achieve an average break-point of ∼ 48 ratio/infusion (11.0 reinforcements) (Fig. 8a, left side), whereas ShA rats responded achieve an average break-point of ∼ 12 ratio/infusion (5.8 reinforcements) (Fig. 8a, right side). The break-point for cocaine in vehicle-injected rats was significantly higher in LgA rats versus ShA rats (Student’s t-test, #p<0.05). Fig. 8a shows the effects of bilateral infusions of SR141716A (0.0, 1.0 and 3.0 μg/ side; 0.5 μl per side in 2 min) on the break-point for cocaine in both long and short access conditions. A two-way ANOVA between SR141716A pretreatment and cocaine access showed a significant interaction between both factors [F (2, 16) = 4.56, p=0.027]. Additional analysis revealed that bilateral SR141716A accumbal infusions reduced break-points for cocaine selectively in LgA rats (Fig. 8a, left panel; F(2, 8) = 12.87; **p<0.01) and no effect was observed in the ShA group (Fig. 8a, right panel; p>0.05, n.s.).
A potential confound is that the site-specific injections may diffuse to other surrounding brain regions. Thus, the degree of spread of the antagonist was determined by an injection of 0.5 μl of cresyl violet using the same procedure as the microinjections. The dye was localized within the NAc in all animals included in the statistics. Two animals (one ShA and one LgA) were removed from the study because of misplaced cannula. Those animals presented no reduction in responding for cocaine after the administration of the antagonist (p>0.05). Within the NAc, the majority of cannulas were placed in the shell subregion (see histology in Fig.9). In two LgA animals one of the two cannulas had an angle or was shifted in medio lateral plane so that it touched the core subregion, and the other cannula was located in the medial shell. Those animals did present reductions in break-points for cocaine.
DISCUSSION
The following findings provide evidence that eCB system neuroplasticity contributes to the increased motivation for cocaine intake under extended-access conditions: 1) blockade of CB1 receptors by SR141716A systemic administration decreases the break-point for cocaine dose-dependently in LgA rats, whereas only the highest dose of the antagonist has an effect in the ShA group; 2) NAc shell eCB tone is maintained by LgA rats during cocaine self-administration, while decreases in eCB levels are observed in ShA rats; 3) pCB1 and CB1 receptors are upregulated in the NAc and amygdala of LgA rats; 4) intra-NAc SR141716A infusions reduce cocaine break-points by LgA rats but not ShA rats.
In agreement with previous reports, our LgA rats showed escalated cocaine intake and higher cocaine break-points versus the ShA rats, as an index of compulsivity and high motivation to seek cocaine associated with cocaine addiction (Koob, 2008; Wee et al., 2008). We observed enhanced sensitivity to CB1 receptor blockade in LgA rats, suggesting that eCB-CB1 signaling is increased in extended-access animals. Furthermore, SR141716A suppressed responding comparably in LgA animals regardless of whether they were high or low responders for cocaine and reduced cocaine intake by LgA rats but not ShA rats that have similar amount of cocaine in the system during testing, which supports the finding that SR141716A was acting independently of response rate and amount of cocaine in the animals’ system and was more potent in LgA rats.
Our present in vivo microdialysis study shows that accumbal AEA levels were decreased in ShA rats during cocaine self-administration, whereas this effect was absent in LgA rats. We also did not observe cocaine-related changes in eCB levels after high doses of cocaine in previous studies (Caille et al., 2007), where animals self-administered cocaine during 90 min dialysis session at high doses (∼15 mg/kg) similar to LgA animals of this study (∼20 mg/kg versus ∼5 mg/kg in ShA rats). Although the relative effect of the amount of cocaine intake on dialysate eCB levels requires further characterization, our present results highlight a potentially important difference in cocaine-induced alterations in NAc eCB levels between LgA and ShA animals. Even though baseline AEA levels appear to be lower in the LgA group, the lack of cocaine-induced deficits in interstitial AEA levels in LgA rats suggests maintenance of eCB tone in these animals during cocaine intake that contrasts with a likely reduction in CB1 signaling during cocaine intake by ShA rats. Interestingly, resistance to cocaine-induced decreases in eCB levels apparently is insufficient to observe an effect of intra-NAc SR141716A, according to our previous studies (Caille et al., 2007). Clearly, some differences between our previous and present studies, such as session duration history and ratio schedules, likely contribute to the differential effects of SR141716A. Of equal or perhaps greater importance are the observations of upregulated pCB1 and CB1 receptor levels in the NAc of LgA rats. The combined observation of relatively greater accumbal AEA levels during cocaine self-administration with increased CB1 receptor protein in this region of LgA versus ShA rats is consistent with enhanced eCB signaling in LgA animals and the relatively greater sensitivity of these animals to CB1 antagonist-induced reductions in the motivational effects of cocaine.
The influence of CB1 receptor phosphorylation on eCB signaling is not well characterized. Recent evidence suggests that phosphorylation of the CB1 receptor distal carboxy-terminus promotes internalization of agonist-activated full-length receptors (Daigle et al., 2008) similar to trafficking of delta opioid receptors (Whistler et al., 2001). Thus it is conceivable that increased pCB1 reflects a compensatory response aimed at reducing CB1-mediated signaling as a consequence of a relative overactivation of the system (Garcia et al., 1998), though it is presently not possible to make strong conclusions in this regard. It is interesting however that others noted a correlation between increased CB1 receptor expression and increased pCB1 levels within distinct brain regions (Diaz-Asensio C. et al., 2008). Finally, demonstration that intra-NAc shell SR141716A infusions reduce cocaine break-points selectively in LgA rats supports the hypothesis that the NAc shell is a brain structure where eCB-CB1 signaling may participate in the compulsive and motivational aspects of cocaine addiction (Pontieri FE, et al., 1995). However, we do not exclude an implication of the core subregion in this effect since we found similar changes in CB1 and pCB1 proteins in both subregions. It is possible that several brain regions (i.e. shell, core and/or amygdala) act together in the effect observed by SR141716A since the systemic administration produced stronger reductions in cocaine break-points than the intra-NAc shell CB1 antagonist infusions.
CB1 receptors appear to play a role in the determination of the hedonic value and sensitivity to the rewarding properties of natural (Sanchís-Segura et al., 2004; Maccioni et al., 2008) and cocaine reinforcement (Deroche-Gamonet et al., 2001; Xi et al., 2008). Thus, it is possible that the increased motivation to obtain drug (as reflected by the increased effort under PR schedules) is particularly sensitive to SR141716A. This may explain why our LgA rats —which have enhanced motivation to seek cocaine hypothesized to be due to changes in hedonic set point (Ahmed et al., 2002)- were much more sensitive to the effects of the CB1 antagonist. This hypothesis is consistent with previous reports, where CB1 antagonism was ineffective modulating limited effort-cocaine self-administration under FR schedules (Fattore et al., 1999; De Vries et al., 2001; Lesscher et al., 2005; Tanda et al., 2000; Soria et al., 2005; Caillé & Parsons, 2006; Caillé et al., 2007; Xi et al., 2008) but effective under PR schedules (Soria et al., 2005; Xi et al., 2008). Recently, Xi et al. (2008) showed that high doses of systemic SR141716A were ineffective modulating PR cocaine self-administration in limited-access rats, but 3.0 mg/kg of the potent CB1 antagonist AM251 reduced cocaine responding. We found that SR141716A clearly reduces cocaine break-points in LgA rats even at low doses such as 0.3 mg/kg, which make SR effects more selective for cocaine extended-access rats. However, we also observed a decrease in PR responding in the ShA group with the highest dose of SR, in contrast to Xi and colleagues. The reasons for the discrepancy between studies are not clear. The fact that intra-accumbal injections of SR141716A in the present study decreased cocaine break-points only in LgA rats and not in ShA rats at the doses tested strengthens the conclusion that SR141716A acts selectively for cocaine extended-access animals. However, one cannot exclude the possibility that relatively high doses of SR141716A (this study) or high selective CB1-antagonist (Xi et al., 2008) may also decrease cocaine-maintained behavior by ShA rats.
The neuropharmacological mechanism by which CB1 receptors could modulate motivational aspects of increased cocaine intake is unknown. Studies suggest potential interactions of CB1 receptor activation with the dopamine and/or glutamate systems. Cocaine addiction is associated with decreases in NAc basal dopamine levels (Weiss et al., 1992) and striatal D2 receptors (Volkow et al., 1999; Nader et al., 2006; Dalley et al., 2007). A compromised postsynaptic D2-like receptor function in LgA animals may contribute to an upregulated cAMP-PKA signaling in the NAc which is known to underlie tolerance to cocaine reinforcement (Self et al. 1998; but see Lynch and Taylor, 2005), and is also a requirement for AEA formation in neurons (Cadas et al., 1996). This hypothesis may explain why the LgA rats, which have compromised D1/D2 function (Ahmed & Koob, 2004), also present a maintained AEA tone compared with ShA animals (this study). Nevertheless, other reports indicate that, behaviorally, cocaine addiction increased D2 receptor function or sensitization (De Vries et al., 2002; Edwards et al., 2007) and, interestingly, striatal D2 activation may be also responsible for AEA release (Giuffrida et al., 1999; Beltramo et al., 2000; Centonze et al., 2004). Alternatively, a maintained AEA tone in LgA rats could be explained by an alteration in the mechanisms of eCB degradation, and not in the biosynthesis, as reported for ethanol preference (Hansson et al., 2007).
The CB1 signaling upregulation proposed in this study and the reduced glutamatergic activity observed in escalated animals (Allen et al., 2007) suggest that a retrograde eCB signaling in LgA animals may inhibit glutamate transmission via activation of presynaptic CB1 receptors located in NAc glutamate terminals (Robbe et al., 2002; Gerdeman et al., 2002). Thus, CB1 antagonists could neutralize a CB1-mediated inhibition of glutamate input (Xi et al., 2006) onto AMPA receptors in medium spiny neurons, normalizing the cocaine-evoked NAc synaptic plasticity that underlies the addictive behavior (Conrad et al., 2008; Bachtell et al., 2008). Alternatively, since dopaminergic and eCB systems exert mutual control on each other (van der Stelt & Di Marzo, 2003), it is also conceivable that the dopamine function impairment in LgA rats (Ahmed & Koob, 2004) may be the result of enhanced chronic CB1 neurotransmission, due to an eCB inhibitory feedback mechanism countering dopamine function (Giuffrida et al., 1999; Beltramo et al., 2000).
Taken together, our results suggest that eCB system neuroplasticity in the NAc may contribute to the motivational drive for cocaine associated with uncontrolled psychostimulant use and, therefore, may be a target for cocaine addiction pharmacotherapies.
Aknowledgements
This work was supported by N.I.D.A. grant DA004398 (G.F.K.), N.I.H. grant AA014619 (L.H.P.) and the Pearson Center for Alcoholism and Addiction Research. L.O. thanks Spanish MICINN, F.E.C.Y.T. and Fulbright Program for Fulbright Award (FU-2006-0200). The authors gratefully thank Dr. Sunmee Wee for helpful comments on the behavioral study and Mike Arends for editorial assistance. We also appreciate the excellent technical assistance of Ilham Polis, David G. Stouffer, Dr. Lily Alvarez-Jaimes and Lindsey Porterfield. We are grateful to N.I.D.A. for providing SR141716A. This is publication number 19783 from The Scripps Research Institute.
REFERENCES
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Changes in response to a dopamine receptor antagonist in rats with escalating cocaine intake. Psychopharmacology (Berl) 2004;172:450–454. doi: 10.1007/s00213-003-1682-9. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- Allen RM, Dykstra LA, Carelli RM. Continuous exposure to the competitive N-methyl-D: -aspartate receptor antagonist, LY235959, facilitates escalation of cocaine consumption in Sprague-Dawley rats. Psychopharmacology (Berl) 2007;191:341–351. doi: 10.1007/s00213-006-0661-3. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. IV-TR edn American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- Bachtell RK, Choi KH, Simmons DL, Falcon E, Monteggia LM, Neve RL, Self DW. Role of GluR1 expression in nucleus accumbens neurons in cocaine sensitization and cocaine-seeking behavior. Eur J Neurosci. 2008;27:2229–2240. doi: 10.1111/j.1460-9568.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212–218. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- Beltramo M, de Fonseca FR, Navarro M, Calignano A, Gorriti MA, Grammatikopoulos G, Sadile AG, Giuffrida A, Piomelli D. Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J Neurosci. 2000;20:3401–3407. doi: 10.1523/JNEUROSCI.20-09-03401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadas H, Gaillet S, Beltramo M, Venance L, Piomelli D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J Neurosci. 1996;16:3934–3942. doi: 10.1523/JNEUROSCI.16-12-03934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille S, Parsons LH. Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology. 2006;31:804–813. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692:47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Batulis DK, Landry KL, Morgan AD. Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacology (Berl) 2005;180:414–426. doi: 10.1007/s00213-005-2182-x. [DOI] [PubMed] [Google Scholar]
- Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agro A, Bernardi G, Calabresi P, Maccarrone M. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal gabaergic Transmission. Neuropsychopharmacology. 2004;29:1488–1497. doi: 10.1038/sj.npp.1300458. [DOI] [PubMed] [Google Scholar]
- Cohen C, Kodas E, Griebel G. CB1 receptor antagonists for the treatment of nicotine addiction. Pharmacol Biochem Behav. 2005;81:387–395. doi: 10.1016/j.pbb.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M, Fratta W. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65. doi: 10.1016/s0166-4328(00)00311-9. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V, Le MM, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci. 1999;11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Le MM, Piazza PV, Soubrie P. SR141716, a CB1 receptor antagonist, decreases the sensitivity to the reinforcing effects of electrical brain stimulation in rats. Psychopharmacology (Berl) 2001;157:254–259. doi: 10.1007/s002130100804. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Raaso H, Vanderschuren LJ. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology. 2002;26:18– 26. [Google Scholar]
- Daigle TL, Kwok ML, Mackie K. Regulation of CB1 cannabinoid receptor internalization by a promiscuous phosphorylation-dependent mechanism. J Neurochem. 2008;106:70–82. doi: 10.1111/j.1471-4159.2008.05336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Asensio C, Setien R, Echevarria E, Casis L, Casis E, Garrido A, Casis O. Type 1 diabetes alters brain cannabinoid receptor expression and phosphorylation status in rats. Horm Metab Res. 2008;40:454–458. doi: 10.1055/s-2008-1065323. [DOI] [PubMed] [Google Scholar]
- Edwards S, Whisler KN, Fuller DC, Orsulak PJ, Self DW. Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacology. 2007;32:354–366. doi: 10.1038/sj.npp.1301062. [DOI] [PubMed] [Google Scholar]
- Fattore L, Martellotta MC, Cossu G, Mascia MS, Fratta W. CB1 cannabinoid receptor agonist WIN 55,212-2 decreases intravenous cocaine self-administration in rats. Behav Brain Res. 1999;104:141–146. doi: 10.1016/s0166-4328(99)00059-5. [DOI] [PubMed] [Google Scholar]
- Filip M, Golda A, Zaniewska M, McCreary AC, Nowak E, Kolasiewicz W, Przegalinski E. Involvement of cannabinoid CB1 receptors in drug addiction: effects of rimonabant on behavioral responses induced by cocaine. Pharmacol Rep. 2006;58:806–819. [PubMed] [Google Scholar]
- Fusco FR, Martorana A, Giampa C, De MZ, Farini D, D’Angelo V, Sancesario G, Bernardi G. Immunolocalization of CB1 receptor in rat striatal neurons: a confocal microscopy study. Synapse. 2004;53:159–167. doi: 10.1002/syn.20047. [DOI] [PubMed] [Google Scholar]
- Garcia DE, Brown S, Hille B, Mackie K. Protein kinase C disrupts cannabinoid actions by phosphorylation of the CB1 cannabinoid receptor. J Neurosci. 1998;18:2834–2841. doi: 10.1523/JNEUROSCI.18-08-02834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de FF, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Fernandez-Ruiz J, Sparpaglione V, Parolaro D, Ramos JA. Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB(1) receptor binding and mRNA levels. Drug Alcohol Depend. 2002;66:77–84. doi: 10.1016/s0376-8716(01)00186-7. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Bermudez-Silva FJ, Malinen H, Hyytia P, Sanchez-Vera I, Rimondini R, Rodriguez de FF, Kunos G, Sommer WH, Heilig M. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2007;32:117–126. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann H, Lutz B. Coexpression of the cannabinoid receptor type 1 with the corticotropin-releasing hormone receptor type 1 in distinct regions of the adult mouse forebrain. Neurosci Lett. 2005;375:13–18. doi: 10.1016/j.neulet.2004.10.080. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Song C, Berglund BA, Wilken GH, Pigg JJ. Characterization of CB1 cannabinoid receptors using receptor peptide fragments and site-directed antibodies. Mol Pharmacol. 1998;53:504–510. doi: 10.1124/mol.53.3.504. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, Hoogveld E, Burbach JP, van Ree JM, Gerrits MA. Endogenous cannabinoids are not involved in cocaine reinforcement and development of cocaine-induced behavioural sensitization. Eur Neuropsychopharmacol. 2005;15:31–37. doi: 10.1016/j.euroneuro.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol. 2004;143:227–234. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Persistent changes in motivation to self-administer cocaine following modulation of cyclic AMP-dependent protein kinase A (PKA) activity in the nucleus accumbens. Eur J Neurosci. 2005;22:1214–1220. doi: 10.1111/j.1460-9568.2005.04305.x. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Pes D, Carai MA, Gessa GL, Colombo G. Suppression by the cannabinoid CB1 receptor antagonist, rimonabant, of the reinforcing and motivational properties of a chocolate-flavoured beverage in rats. Behav Pharmacol. 2008;19:197–209. doi: 10.1097/FBP.0b013e3282fe8888. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12:4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- National Surveys on Drug Use and Health, NSDUH . Office of Applied Studies. Substance Abuse and Mental Health Services Administration (SAMHSA); Rockville, MD: 2006. [Google Scholar]
- Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, Chowen JA, Gomez R, del A,I, Villanua MA, Maldonado R, Koob GF, Rodriguez de FF. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Carrera MR, del A,I, Trigo JM, Koob GF, Rodriguez de FF. Cannabinoid receptor antagonist reduces heroin self-administration only in dependent rats. Eur J Pharmacol. 2004;501:235–237. doi: 10.1016/j.ejphar.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di CG. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Porcella A, Maxia C, Gessa GL, Pani L. The human eye expresses high levels of CB1 cannabinoid receptor mRNA and protein. Eur J Neurosci. 2000;12:1123–1127. doi: 10.1046/j.1460-9568.2000.01027.x. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de FF, Roberts AJ, Bilbao A, Koob GF, Navarro M. Cannabinoid receptor antagonist SR141716A decreases operant ethanol self administration in rats exposed to ethanol-vapor chambers. Zhongguo Yao Li Xue Bao. 1999;20:1109–1114. [PubMed] [Google Scholar]
- Sanchis-Segura C, Cline BH, Marsicano G, Lutz B, Spanagel R. Reduced sensitivity to reward in CB1 knockout mice. Psychopharmacology (Berl) 2004;176:223–232. doi: 10.1007/s00213-004-1877-8. [DOI] [PubMed] [Google Scholar]
- Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Mendizabal V, Tourino C, Robledo P, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–1680. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms--a review of recent preclinical data. Psychopharmacology (Berl) 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- van der SM, Di M,V. The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: implications for neurological and psychiatric disorders. Eur J Pharmacol. 2003;480:133–150. doi: 10.1016/j.ejphar.2003.08.101. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther. 2007;320:1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- Wee S, Mandyam CD, Lekic DM, Koob GF. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992;593:314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- Whistler JL, Tsao P, von ZM. A phosphorylation-regulated brake mechanism controls the initial endocytosis of opioid receptors but is not required for post-endocytic sorting to lysosomes. J Biol Chem. 2001;276:34331–34338. doi: 10.1074/jbc.M104627200. [DOI] [PubMed] [Google Scholar]
- Wiskerke J, Pattij T, Schoffelmeer AN, De Vries TJ. The role of CB1 receptors in psychostimulant addiction. Addict Biol. 2008;13:225–238. doi: 10.1111/j.1369-1600.2008.00109.x. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr., Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, Peng XQ, Gardner EL. Cannabinoid CB1 Receptor Antagonists Attenuate Cocaine’s Rewarding Effects: Experiments with Self-Administration and Brain-Stimulation Reward in Rats. Neuropsychopharmacology. 2008;33:1735–45. doi: 10.1038/sj.npp.1301552. [DOI] [PubMed] [Google Scholar]