Summary
Here we critically review two recent hypotheses about the mechanism of strong alkalinization by the anterior midgut of mosquito larvae and our tests of these hypotheses. We present experimental evidence against the major components of transport models proposed in these hypotheses. Measurements of the transapical and transbasal proton electrochemical gradients provide an indication of driving forces faced by and generated by the transport mechanisms of the tissue. These measurements confirmed that basal V-ATPase energizes alkalinization. Serotonin stimulates the V-ATPase, as indicated by the ensuing increase in proton-motive force across the basal membrane. Moreover, the neurohormone resulted in a surprisingly large increase in the intracellular pH. The results of inhibitor studies indicate that, contrary to previous proposals, carbonic anhydrase is apparently not involved in supplying acid–base equivalents to the respective transporters. Furthermore, any apical processes proposed to be involved in alkali secretion or acid absorption must be Cl− independent and insensitive to DIDS, amiloride, Zn2+ and ouabain. These results argue against the involvement of putative apical Cl−/HCO3− exchangers, apical H+ channels, apical cation/proton exchangers and the importance of the apical Na+/K+ pump. The studies analyzed here thus provide both a limitation and direction for further studies of the mechanism of strong alkalinization in this system.
Keywords: larval mosquito, midgut alkalinization, H+ V-ATPase, proton electrochemical gradient, anion exchanger, Na+/H+ exchanger, H+ channel, DIDS, amiloride, ouabain, zinc
Introduction
The V-type H+ ATPase, expressed at the plasma membrane, energizes a wide variety of epithelial transport processes in insects and other animals, leading, depending on the system, to secretion or absorption of fluid, mineral ions and amino acids (c.f. Harvey and Wieczorek, 1997; Harvey et al., 1999; Beyenbach, 2001). The implication of H+ transport by the V-ATPase for intracellular and extracellular pH is determined by the nature of the secondary transport processes to which the ATPase is coupled. So, for example, in the midgut of lepidopteran insect larvae, an apical V-ATPase is coupled to processes that result in luminal alkalinization (Azuma et al., 1995); in dipteran insect larvae, it is a basal V-ATPase that, coupled to apical processes, drives luminal alkalinization (Shanbhag and Tripathi, 2005), whereas, in insect Malpighian tubules, an apical V-ATPase is coupled to secondary processes that do not result in strong alkalinization or acidification of the tubule lumen (Maddrell and O’Donnell, 1992; Petzel et al., 1999). In the anterior midgut of larval mosquitoes, the V-ATPase provides much or all of the energy for absorption of dietary amino acids and for raising the pH of the lumen to values as high as 10.5 (Dadd, 1975). The latter process has been the focus of study in our laboratory for almost a decade. We will review our tests of two major hypotheses about the mechanism of strong alkalinization in the anterior midgut of the yellow fever mosquito Aedes aegypti, including as yet unpublished results, and suggest directions that new hypotheses might take.
Methodological approaches
The perfused gut preparation
Studies in our laboratory center on an isolated, perfused preparation of the anterior midgut developed initially by Clark and colleagues (Clark et al., 1999) and modified by Onken and colleagues (Onken et al., 2004). Briefly, for the experiments reported here, the excised gut is tied to a glass perfusion pipette at one end. The other end is left open, except that a blunt rod of appropriate dimensions is inserted partway into the lumen to support the tissue in the focal plane of a dissection microscope. This preparation offers the possibility of studying the function of a tissue in a setting in which the composition of solutions on both sides of the tissue can be controlled and in which complicating structures such as the peritrophic membrane and adjacent gut regions are absent. This preparation becomes nonfunctional within minutes after mounting, with the transepithelial electrical potential (Vte) falling to a value near zero and alkali secretion occurring at immeasurable rates. However, administration of submicromolar serotonin restores and sustains transepithelial potential values and alkali secretion for up to several hours. It is noteworthy that this is the only insect tissue shown so far to engage in extreme alkalinization in vitro.
For the experiments described herein, the hemolymph-side superfusate was Aedes saline (Clark et al., 1999) and the luminal perfusate was 100mmoll−1 NaCl, unless otherwise noted. In most experiments, the luminal perfusate was not buffered and contained the pH-sensitive dye m-cresol purple (0.04%). A visual indication of alkali secretion can be obtained at any point in the experiment by stopping perfusion and watching for the orange-to-purple color transition of the m-cresol purple that occurs at a pH of approximately 8.3. This method was verified with luminal pH-sensitive microelectrodes. Typically, the rate of alkali secretion is sufficiently vigorous that only a few minutes are required for this transition.
Three types of experiments using the perfused preparation are presented here: inhibitor studies, in which the inhibitor is applied in luminal or hemolymph-side perfusate and changes in the transepithelial potential and the capability to secrete alkali are measured; optical measurements of intracellular pH (pHi) using the H+-sensitive dye BCECF; and microelectrode experiments in which the tissue is penetrated with intracellular glass microelectrodes for measurement of the transbasal electrical potential (Vbl).
Optical measurements of pHi with BCECF were performed using a method modified from that of Parks and colleagues (Parks et al., 2007). In brief, the BCECF trapped in the tissue was excited at its absorption peak of 495nm; 440nm was used as an isobestic wavelength. Images at these wavelengths were captured digitally. The 495-to-440 nm ratios were compiled as an indication of the pHi. At the beginning of each experiment, areas of interest were established on the CCD image of the gut. At the end of each experiment, fluorescence ratios recorded during the experiment were calibrated by superfusing the tissue successively with high-K+ solutions containing 5µmoll−1 nigericin, adjusted to cover the range of pH values 6.6–8.4.
In the microelectrode experiments, the tissue was penetrated across the hemolymph-side surface with KCl-filled microelectrodes, as in previous studies (Clark et al., 2000). From simultaneous measurements of Vbl and the transepithelial potential Vte, the transapical electrical potential (Vapi) can be calculated. These measurements were combined with measurements of pHi under similar experimental conditions to yield estimations of the electrochemical forces acting on H+ as it passes through the cells.
Results
The proton electrochemical gradients
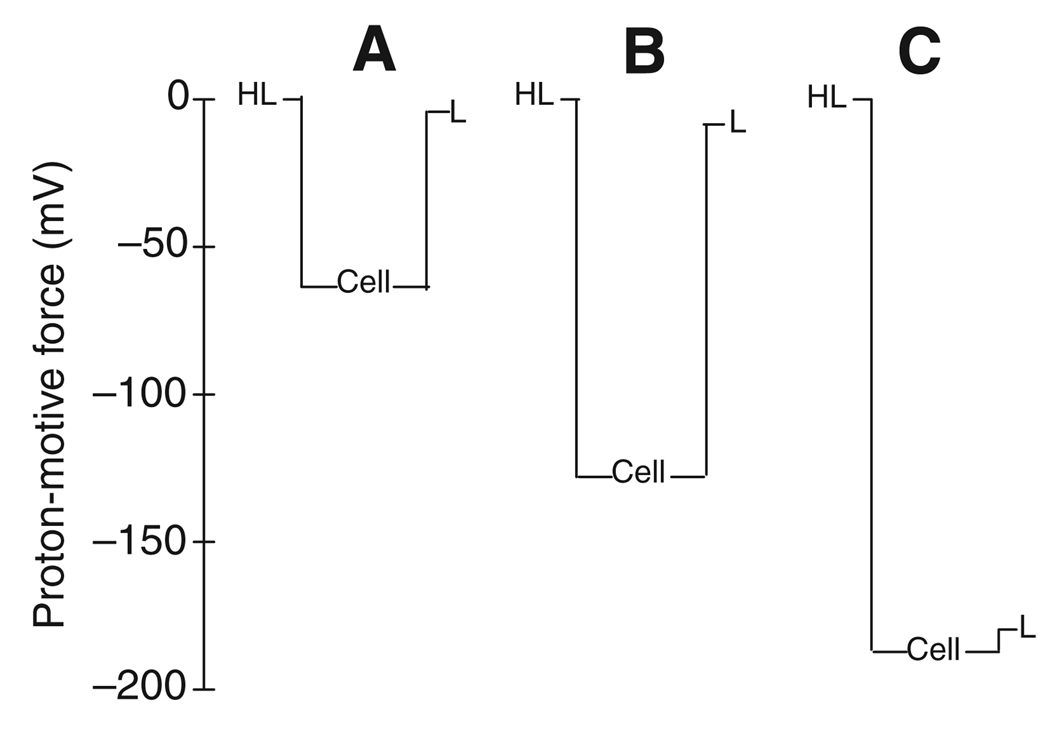
Fig.1 shows the proton electrochemical gradients for three conditions: before serotonin stimulation, after serotonin with both luminal side and hemolymph-side buffered to pH 7.0, and after increasing the luminal pH to an in vivo-like pH of 10. The unstimulated anterior midgut tissue presented pHi values near or slightly below neutrality (H.O., S. K. Parks, G. G. Goss and D.F.M., unpublished observations). In the unstimulated gut (Fig. 1A), with a luminal pH of 7.0, the combination of the large, inside-negative Vapi (Clark et al., 2000) with the negligible transapical H+ activity gradient results in a substantial electrochemical gradient favoring H+ entry across the apical membrane. There is a similar large electrical gradient that opposes H+ movement from the cell to hemolymph (Clark et al., 2000).
Fig. 1.
Transepithelial profiles of the proton-motive force (in mV) calculated from the average membrane voltages and transmembrane pH gradients for (A) a control condition with mosquito saline of pH 7 on both sides of the tissue, (B) after stimulation with serotonin (pH=7 on both sides; lumen 100mmoll−1 NaCl) and (C) after increasing the pH of the luminal perfusate to 10. HL, hemolymph; L, lumen.
Addition of serotonin resulted in hyperpolarization of both Vbl and Vapi (Clark et al., 2000); generally, the effect on Vbl is the larger of the two, so that Vte increased to lumen-negative values of up to several tens of millivolts. Serotonin also had a dramatic effect on pHi, increasing it to a mean of 7.7 (H.O., S. K. Parks, G. G. Goss and D.F.M., unpublished observations). When the solutions on both sides of the tissue were buffered to pH 7.0, the combination of these effects increased both the gradient favoring proton entry from the lumen and that opposing proton exit across the basal membrane (Fig. 1B).
When the pH of the luminal perfusate of a serotonin-stimulated tissue was raised from 7 to 10, pHi rose substantially to ~8.6 (H.O., S. K. Parks, G. G. Goss and D.F.M., unpublished observations). This change dramatically increased the transbasal proton electrochemical gradient to approximately −190 mV. This value approximates the maximal pump electromotive force predicted for the V-ATPase (Moffett, 1980; Grabe et al., 2000; Luo et al., 2004). At the same time, the H+ electrochemical gradient across the apical membrane was essentially abolished, so that, at a luminal pH of 10, cytoplasmic H+ is close to electrochemical equilibrium with luminal H+ (Fig. 1C).
When micromolar Zn2+, a blocker of H+ channels (DeCoursey, 2003), was included in the luminal perfusate, neither the magnitude nor the rate of the change in pHi in response to alkaline luminal perfusate was affected (H.O., S. K. Parks, G. G. Goss and D.F.M., unpublished observations). This result argues against participation of apical H+ channels in the mechanism of luminal alkalinization.
The transbasal processes
Transbasal processes parallel to the V-ATPase are potentially important in alkali secretion, particularly if the hemolymph is a source for bicarbonate. The presence of the V-ATPase alone cannot result in mass absorption of protons without the support of an anion transport pathway. Boudko and colleagues (Boudko et al., 2001) detected a DIDS-sensitive transbasal Cl− efflux in a semi-intact preparation. We reported effects of hemolymph-side DIDS (0.1mmoll−1) as well as DPC (0.5mmoll−1) on Vte (Onken et al., 2004). The effects of hemolymph-side application of these two relatively nonspecific inhibitors of anion exchangers and anion channels on alkalinization has not yet been evaluated. If, in addition to Cl− channels, there were a basal anion exchanger, it might provide HCO3− for alkali secretion. Addition of Ba2+ (5mmoll−1), an inhibitor of K+ channels (Nagel, 1979), to the hemolymph-side superfusate, reduces Vte by ~26% (Onken et al., 2004). In the presence of basal K+ channels, the V-ATPase could serve a housekeeping role by driving the accumulation of K+ in the cells, thus substituting for the conventional role of the basal Na+/K+ pump, which is absent in these cells (Patrick et al., 2006). For values of Vbl of the order of those reported here, intracellular [K+] would be approximately 70–90mmoll−1, a value not unusual for insect cells.
The transapical processes
Boudko–Onken hypothesis
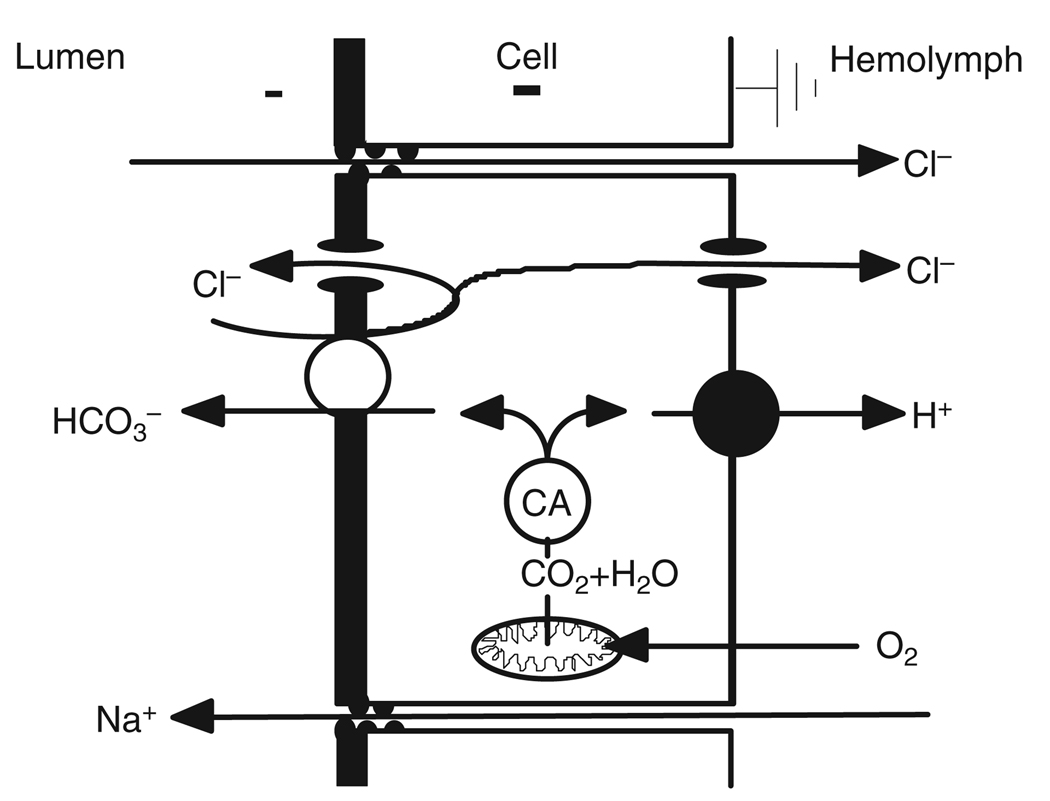
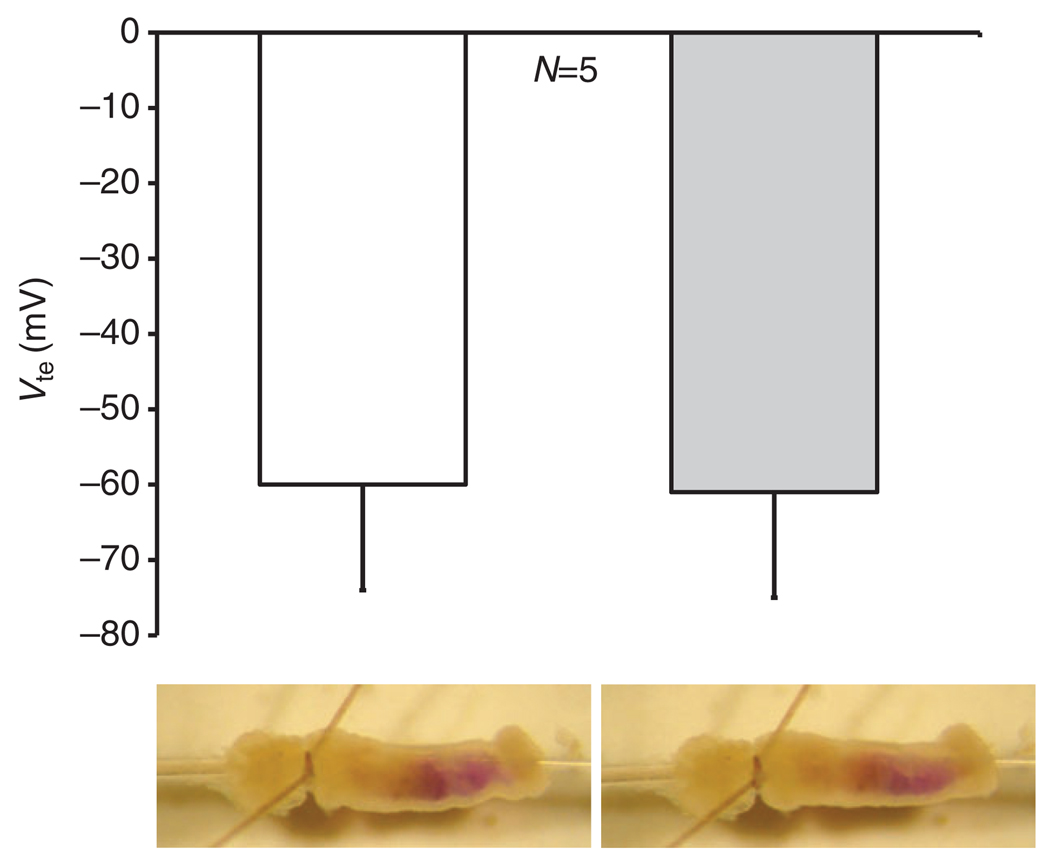
The key features of this anion-dominated model (Fig. 2) (Boudko et al., 2001; Onken et al., 2004) are the presence of the basal V-ATPase, the absence of basal Na+/K+-ATPase ordinarily present in animal epithelia, and the presence of an apical Cl−/HCO3− exchanger supported by cytoplasmic carbonic anhydrase (CA). A basal Cl− channel provides an avenue for transepithelial Cl− absorption. A putative apical anion channel could provide for transapical Cl− recycling and/or even for HCO3− secretion. We have found that hemolymph-side Na+ is required for a normal Vte (in Na+-free solution, Vte falls from a mean of −45mV to <−10mV) and for alkalinization. Amiloride applied to the hemolymph-side saline has a similar effect (Onken et al., 2004; Onken et al., 2008). The latter observations were interpreted as reflecting a transbasal Na+-dependent process and a transapical Na+-dependent component of HCO3− secretion. Note that Boudko and colleagues (Boudko et al., 2001) assume a cytoplasmic pH of 7.2, giving a transapical H+ gradient of 3–4 orders of magnitude. A major piece of evidence against this hypothesis was provided by our recent finding that neither methazolamide, an inhibitor of CA (Fig. 3), nor DIDS, an inhibitor of anion exchangers, nor bilateral Cl−-free saline, affect alkalinization in the isolated and perfused anterior midgut (Onken et al., 2008).
Fig. 2.
Model of hypothetical transport mechanisms involved in strong alkalinization based on earlier proposals (Boudko et al., 2001; Onken et al., 2004), focusing on anionic pathways in the apical membrane. CA, carbonic anhydrase.
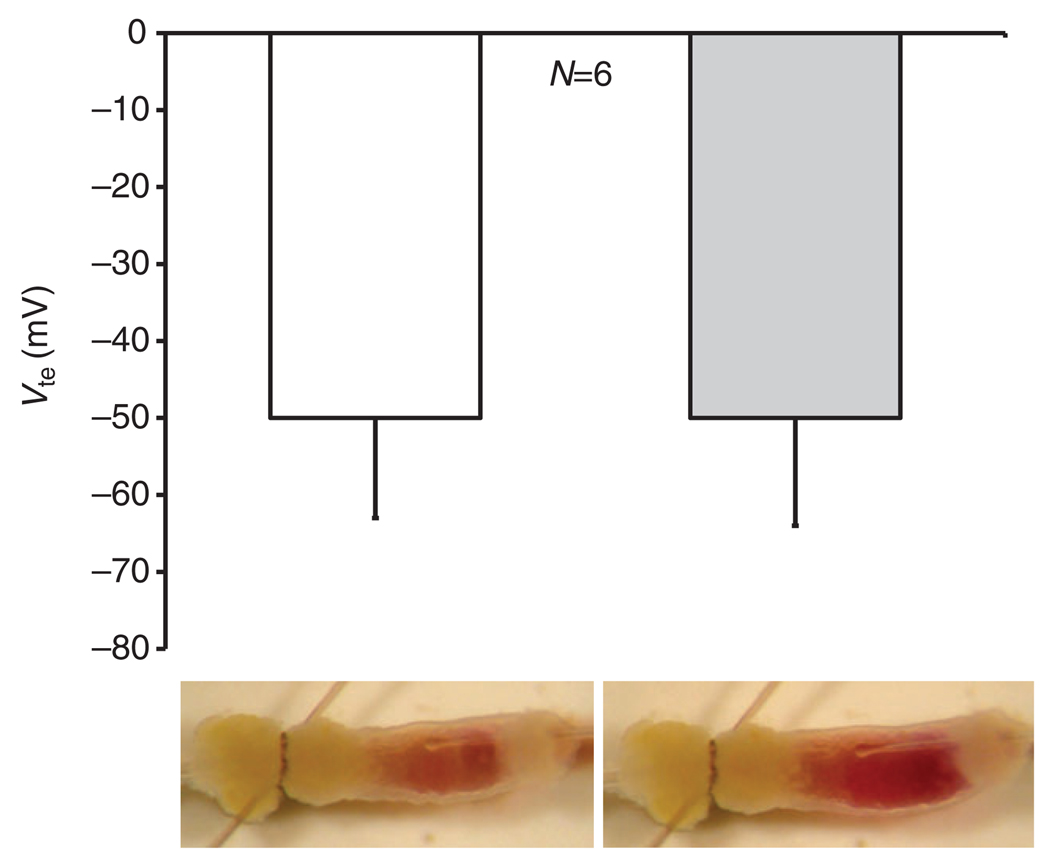
Fig. 3.
Mean lumen negative transepithelial voltages (Vte; −s.e.m.) of six anterior midguts stimulated with serotonin (0.2 µmol l−1) in the presence (gray bar) and absence (white bar) of luminal methazolamide (200 µmol l−1), and photographs of a representative preparation of the anterior midgut of larval (fourth instar) Aedes aegypti at identical times after perfusion stop in the presence (right) and absence (left) of luminal methazolamide (200 µmol l−1). [Figure reproduced from Onken and colleagues (Onken et al., 2008).]
Smith and colleagues (Smith et al., 2007) found CA in the ectoperitrophic space of the whole larval midgut of Anopheles gambiae. Although not an exclusive feature of the alkalinizing region of the midgut, the presence of CA in the midgut lumen supports the hypothesis that carbon dioxide, diffusing from the anterior midgut cells into the lumen, could be converted there to HCO3− and H+. Alkalinization could then depend solely on acid absorption (see also below). This hypothesis is indeed consistent with the findings by Boudko and colleagues (Boudko et al., 2001) and Corena and colleagues (Corena et al., 2002) that blockers of carbonic anhydrase inhibit alkalinization in semi-intact larvae or in excised midguts of Aedes aegypti. By contrast, strong alkalinization seems not to depend on ectoperitrophic carbonic anhydrase, as is demonstrated by the presence of strong alkalinization in our experiments with isolated and perfused midgut preparations where ectoperitrophic CA is removed and/or inhibited. Moreover, in our hands, CA inhibitors did not affect alkalinization in vivo. When the drugs were added together with m-cresol purple to the medium in which the larvae were maintained, all larvae still showed alkalinized anterior midguts (H.O., S. K. Parks, G. G. Goss and D.F.M., unpublished observations).
Okech–Patrick hypothesis
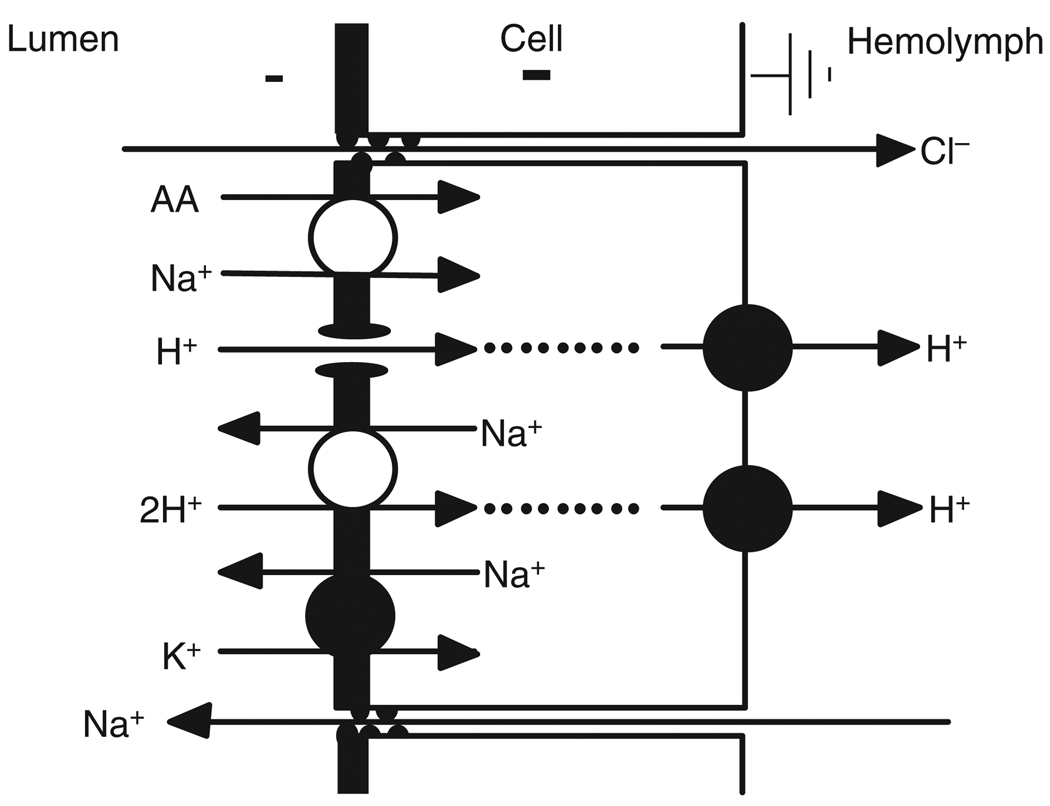
The key features of this cation-dominated model (Fig. 4) are the presence of apical Na+/2H+ exchanger, Na+-coupled amino acid transporter and apical Na+/K+-ATPase (Okech et al., 2008; Patrick et al., 2006). The postulated existence of an apical Na+/2H+ exchanger is logical as such an exchanger could exploit the large transapical proton motive force (Fig. 1B). If the luminal pH is 7, a transapical H+ gradient favorable for H+ absorption exists under the conditions of our experiments (see above); this could drive Na+/2H+ exchange, as long as the cytoplasmic [Na+] is low. If the gut becomes alkaline, this gradient ultimately disappears as the luminal pH approaches 10 (Fig.1C). However, luminal amiloride (200µmoll−1), a general inhibitor of such exchangers, did not affect alkalinization (Fig. 5) (Onken et al., 2008). A K+/2H+ exchanger is postulated in the lepidopteran midgut, another insect tissue that also develops strong alkalinization (Azuma et al., 1995). If such an exchanger were present in the mosquito, the gradient would be even more favorable. However, increased luminal K+ did not affect luminal alkalinization in the perfused preparations (Onken et al., 2008).
Fig. 4.
Model of hypothetical transport mechanisms involved in strong alkalinization and amino acid absorption based on earlier proposals (Okech et al., 2008; Patrick et al., 2006), focusing on cationic pathways in the apical membrane.
Fig. 5.
Mean lumen negative transepithelial voltages (Vte; −s.e.m.) of five anterior midguts stimulated with serotonin (0.2 µmol l−1) in the presence (gray bar) and absence (white bar) of luminal amiloride (200 µmol l−1), and photographs of a representative preparation of the anterior midgut of larval (fourth instar) Aedes aegypti at identical times after perfusion stop in the presence (right) and absence (left) of luminal amiloride (200 µmol l−1). [Figure reproduced from Onken and colleagues (Onken et al., 2008).]
The location of the Na+/K+-ATPase on the apical membrane is an unusual expression pattern for this pump, which is generally expected to have a basal location. As freshwater mosquito larvae such as Aedes and Anopheles consume a low-Na+ diet, it might serve in vivo to deliver Na+ to the gut lumen at the proximal end of the gut to support the Na+-dependent nutrient-absorption processes. Even more striking is that the Na+/K+-ATPase is located in the apical membrane in exactly the region of high luminal alkalinity and the hypothesis arises that it could serve as an ATP-driven Na+/H+ exchanger. It has been known that the ATPase can accept H+ instead of Na+ and/or K+ (Polvani and Blostein, 1988). However, when we tested this hypothesis by addition of ouabain (5mmoll−1), a specific inhibitor of the Na+/K+ ATPase, to the luminal perfusate, there was no significant effect on Vte, and alkalinization was not impaired (Onken et al., 2009).
Okech and colleagues (Okech et al., 2008) found amino acid transporters in the anterior midgut by using immunohistochemistry. The presence of such transporters is indeed confirmed by electrophysiological measurements in which a significant increase in Vte is observed when individual amino acids are added to the luminal perfusate (S. Izeirovski, S. B. Moffett, D.F.M. and H.O., personal communication). The effect of glutamine, which gives the largest response of the amino acids present in the Aedes saline, is shown in Fig. 6. Interestingly, amino acid transport, as indicated by changes of Vte, is stimulated by serotonin. In our recent experiments, the luminal perfusate did not include amino acids, but alkalinization was nevertheless observed. Therefore, the presence of luminal nutrients might facilitate alkali secretion but is not a requirement for it. By contrast, if amino acids are eliminated from the hemolymph-side superfusate, the Vte drops significantly and alkali secretion is retarded (S. Izeirovski, S. B. Moffett, D.F.M. and H.O., personal communication).
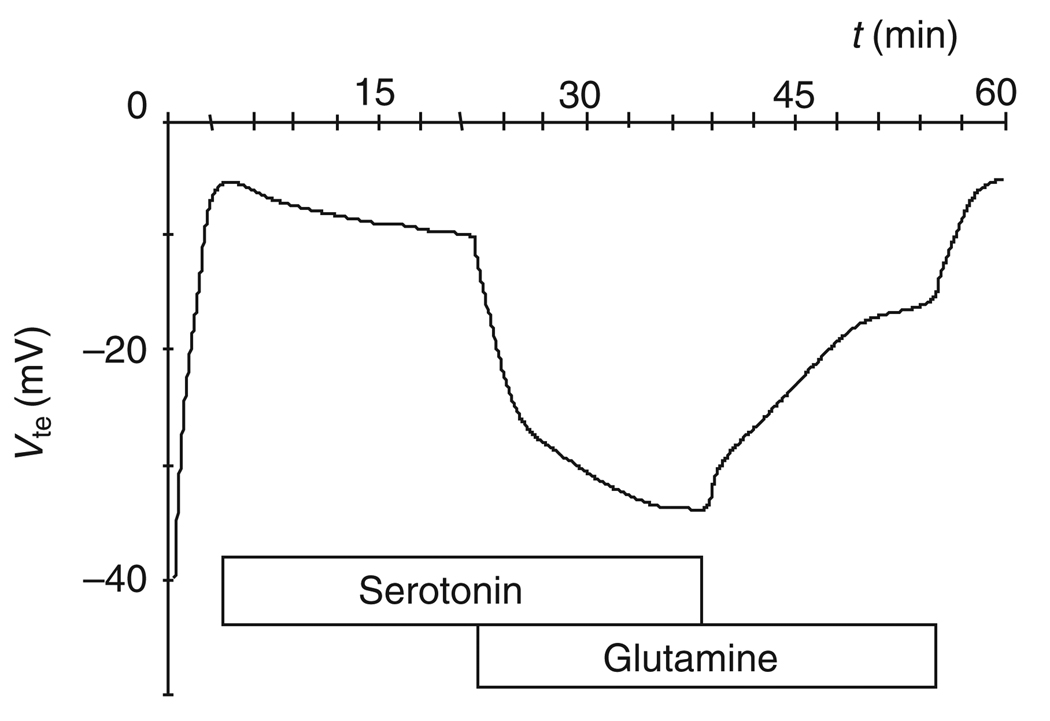
Fig. 6.
Representative time-course of the lumen negative transepithelial voltage (Vte) of the anterior midgut of larval (fourth instar) Aedes aegypti in the presence of hemolymph-side mosquito saline and luminal 100mmoll−1 NaCl. After mounting of the tissue, Vte declines but successively recovers after addition of serotonin (0.2 µmol l−1) to the hemolymph-side bath and glutamine (10mmoll−1) to the luminal perfusate. Washout of serotonin in the presence of luminal glutamine indicates that the effect of luminal glutamine is stimulated by serotonin.
Conclusions
The larval mosquito anterior midgut has a number of elements that seem unusual to one familiar with the well-studied vertebrate epithelia. In this system, the activity of the V-ATPase has the most profound impact on intracellular H+ yet reported, with changes in intracellular H+ concentration of more than an order of magnitude. The absence of the Na+/K+-ATPase on the basal membrane and its presence on the apical membrane (Patrick et al., 2006; Okech et al., 2008) certainly violates a time-honored paradigm of epithelial physiologists; only one other exception is known – the pigment epithelium of the vertebrate eye (Okami et al., 1990). Another very uncommon observation for an epithelium so much involved in acid–base transport is the absence of high intracellular carbonic anhydrase activity. Abundant intracellular carbonic anhydrase is present in the anterior midgut of lepidopteran larvae, the other well-studied model for strong alkalinization in insects (Ridgway and Moffett, 1986), and is almost universal in epithelial cells involved in acid–base transport (Maren, 1967).
It would be facile to say that the studies presented here add to our understanding of how the V-ATPase functions in a system in which it energizes absorption of acid equivalents and secretion of alkali equivalents. Unfortunately, much of the impact of these studies was simply to reveal the extent of what we don’t know. We are now in a position to rule out, or at least cast in serious doubt, most elements of the current reasonable hypotheses about the apical membrane transport processes in this system. We cannot rule out uptake of H+ by a charge-carrying process (i.e. either through proton channels or by an electrogenic exchanger), but we can say that any such process is insensitive to both Zn2+ and amiloride at a concentration that could be expected to bring about at least substantial inhibition of most of the known exchangers of this type. We cannot entirely rule out secretion of bicarbonate or carbonate, but we can stipulate that it occurs by a process that is Cl− independent and DIDS insensitive. Although there is a very substantial interaction of amino acid transport with acid–base transport, we have also shown that amino acids do not need to be present in the lumen in order for luminal alkalinization to occur. There is a potential interaction of amino acid metabolism with alkali secretion, in that amino acids are a source of both HCO3− and NH4+. Both of these become strong bases if H+ is removed. The process of strong luminal alkalinization almost certainly rests on as-yet-unknown mechanisms that deliver one or both of these to the lumen, either in their protonated or nonprotonated forms, combined with the known ability of the V-ATPase to remove protons from the cytoplasm and some unknown mechanism that transports protons from the gut lumen to the cytoplasm.
Finally, it is important to note that the studies reviewed here mingle results from both Anopheles gambiae and Aedes aegypti. Major differences between the two species could well exist, presenting complications that need to be addressed with parallel studies in both species. Moreover, we compare results obtained with very different experimental approaches (in vitro, in situ, in vivo), and our interpretations rely to some degree on the effectiveness of drugs known to impact certain transporters in certain tissues. Nevertheless, we believe that the work with isolated and perfused midgut segments is very productive in relation to uncovering the mechanisms of strong alkalinization in larval mosquitoes, especially because the tissue actually maintains its alkalinizing activity in vitro. Based on the present findings, future work with isolated anterior midguts should be directed to more in vivo-like conditions.
Acknowledgments
The work of the authors cited here was supported by NIH RO1 AI 063463 and a Wagner College faculty research grant to H.O. Deposited in PMC for release after 12 months.
List of abbreviations
- pHi
cytoplasmic pH
- Vapi
transapical electrical potential
- Vbl
transbasal electrical potential
- Vte
transepithelial electrical potential
References
- Azuma M, Harvey WR, Wieczorek H. Stoichiometry of K+/H+ antiport helps to explain extracellular pH 11 in a model epithelium. FEBS Lett. 1995;361:153–156. doi: 10.1016/0014-5793(95)00146-z. [DOI] [PubMed] [Google Scholar]
- Beyenbach KW. Energizing epithelial transport with the vacuolar H+-ATPase. News Physiol. Sci. 2001;16:145–151. doi: 10.1152/physiologyonline.2001.16.4.145. [DOI] [PubMed] [Google Scholar]
- Boudko DY, Moroz LL, Harvey WR, Linser PJ. Alkalinization by chloride/bicarbonate pathway in larval mosquito midgut. Proc. Natl. Acad. Sci. USA. 2001;98:15354–15359. doi: 10.1073/pnas.261253998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TM, Koch A, Moffett DF. The anterior and posterior ‘stomach’ regions of larval Aedes aegypti midgut: regional specialization of ion transport and stimulation by 5-hydroxytryptamine. J. Exp. Biol. 1999;202:247–252. doi: 10.1242/jeb.202.3.247. [DOI] [PubMed] [Google Scholar]
- Clark TM, Koch A, Moffett DF. The electrical properties of the anterior stomach of the larval mosquito (Aedes aegypti) J. Exp. Biol. 2000;203:1093–1101. doi: 10.1242/jeb.203.6.1093. [DOI] [PubMed] [Google Scholar]
- Corena MDP, Seron TJ, Lehman HK, Ochrietor JD, Kohn A, Tu C, Linser PJ. Carbonic anhydrase in the midgut of larval Aedes aegypti: cloning, localization and inhibition. J. Exp. Biol. 2002;205:591–602. doi: 10.1242/jeb.205.5.591. [DOI] [PubMed] [Google Scholar]
- Dadd RH. Alkalinity within the midgut of mosquito larvae with alkaline-active digestive enzymes. J. Insect Physiol. 1975;21:1847–1853. doi: 10.1016/0022-1910(75)90252-8. [DOI] [PubMed] [Google Scholar]
- DeCoursey TH. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- Grabe M, Wang H, Oster G. The mechanochemistry of V-ATPase proton pumps. Biophys. J. 2000;78:2798–2813. doi: 10.1016/S0006-3495(00)76823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey WR, Wieczorek H. Animal plasma membrane energization by chemiosmotic H+ V-ATPases. J. Exp. Biol. 1997;200:203–216. doi: 10.1242/jeb.200.2.203. [DOI] [PubMed] [Google Scholar]
- Harvey WR, Maddrell SHP, Telfer WH, Wieczorek H. H+ V-ATPases energize animal plasma membranes for secretion and absorption of ions and fluids. Am. Zool. 1999;38:426–441. [Google Scholar]
- Luo C, Clark JW, Jr, Heming TA, Bidani A. A simplified model for V-ATPase H+ extrusion. IEEE Trans. Nanobioscience. 2004;3:257–264. doi: 10.1109/tnb.2004.837905. [DOI] [PubMed] [Google Scholar]
- Maddrell SHP, O’Donnell MJ. Insect Malpighian tubules: V-ATPase action in ion and fluid transport. J. Exp. Biol. 1992;172:417–429. doi: 10.1242/jeb.172.1.417. [DOI] [PubMed] [Google Scholar]
- Maren TH. Carbonic anhydrase: chemistry, physiology and inhibition. Physiol. Rev. 1967;47:595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Moffett DF. Voltage-current relation and K+ transport in tobacco hornworm (Manduca sexta) midgut. J. Membr. Biol. 1980;54:213–219. doi: 10.1007/BF01870237. [DOI] [PubMed] [Google Scholar]
- Nagel W. Inhibition of potassium conductance by barium in frog skin epithelium. Biochim. Biophys. Acta. 1979;522:346–357. doi: 10.1016/0005-2736(79)90289-x. [DOI] [PubMed] [Google Scholar]
- Okami T, Yamamoto A, Omori K, Takada T, Uyama M, Tashiro Y. Immunocytochemical localization of Na+, K+ ATPase in rat retinal pigment epithelial cells. J. Histochem. Cytochem. 1990;38:1267–1275. doi: 10.1177/38.9.2167328. [DOI] [PubMed] [Google Scholar]
- Okech BA, Boudko DY, Linser PJ, Harvey WR. Cationic pathway of pH regulation in larvae of Anopheles gambiae. J. Exp. Biol. 2008;211:957–968. doi: 10.1242/jeb.012021. [DOI] [PubMed] [Google Scholar]
- Onken H, Moffett SB, Moffett DF. The transepithelial voltage of the isolated anterior stomach of mosquito larvae (Aedes aegypti): pharmacological characterization of the serotonin-stimulated cells. J. Exp. Biol. 2004;207:1779–1787. doi: 10.1242/jeb.00964. [DOI] [PubMed] [Google Scholar]
- Onken H, Moffett SB, Moffett DF. Alkalinization in the isolated and perfused anterior midgut of the larval mosquito, Aedes aegypti. J. Insect Sci. 2008;8:43. doi: 10.1673/031.008.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken H, Patel M, Javoroncov M, Izeirovski S, Moffett SB, Moffett DF. Strong alkalinization in the anterior midgut of larval yellow fever mosquitoes (Aedes aegypti): Involvement of luminal Na+/K+-ATPase. J. Exp. Zool. 2009 doi: 10.1002/jez.512. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks SK, Tresguerres M, Goss GG. Interactions between Na+ channels and Na+-HCO3− cotransporters in the freshwater fish gill MR cell: a model for transepithelial Na+ uptake. Am. J. Physiol. 2007;292:C935–C944. doi: 10.1152/ajpcell.00604.2005. [DOI] [PubMed] [Google Scholar]
- Patrick ML, Karlygash A, Sanders HR, Gill SS. P-type Na+/K+-ATPase and V-type H+-ATPase expression patterns in the osmoregulatory organs of larval and adult mosquito Aedes aegypti. J. Exp. Biol. 2006;209:4638–4651. doi: 10.1242/jeb.02551. [DOI] [PubMed] [Google Scholar]
- Petzel DH, Pirotte PT, Van Kerkhove E. Intracellular and luminal pH measurements of Malpighian tubules of the mosquito Aedes aegypti: the effects of cAMP. J. Insect Physiol. 1999;45:937–982. doi: 10.1016/s0022-1910(99)00076-1. [DOI] [PubMed] [Google Scholar]
- Polvani P, Blostein R. Protons as substitutes for sodium and potassium in the sodium pump reaction. J. Biol. Chem. 1988;260:16757–16763. [PubMed] [Google Scholar]
- Ridgway RL, Moffett DF. Regional differences in the histochemical localization of carbonic anhydrase in the midgut of tobacco hornworm (Manduca sexta) J. Exp. Zool. 1986;237:407–412. [Google Scholar]
- Shanbhag S, Tripathi S. Electrogenic H+ transport and pH gradients generated by a V-H+-ATPase in the isolated perfused larval Drosophila midgut. J. Membr. Biol. 2005;206:61–72. doi: 10.1007/s00232-005-0774-1. [DOI] [PubMed] [Google Scholar]
- Smith KE, VanEkeris LA, Linser PJ. Cloning and characterization of AgCA9, a novel α-carbonic anhydrase from Anopheles gambiae Giles sensu stricto (Diptera: Culicidae) larvae. J. Exp. Biol. 2007;210:3919–3930. doi: 10.1242/jeb.008342. [DOI] [PubMed] [Google Scholar]