Abstract
Hemoglobin A1c (HbA1c), a long-term, integrated average of tissue exposure to hyperglycemia, is the best reflection of average glucose concentrations and the best proven predictor of microvascular complications of diabetes mellitus. However, HbA1c fails to capture glycemic variability and the risks associated with extremes of hypoglycemia and hyperglycemia.
These risks are the primary barrier to achieving the level of average glucose control that will minimize both the microvascular and the long-term macrovascular complications of type 1 diabetes. High blood glucose levels largely due to prandial excursions produce oxidative and inflammatory stress with potential acceleration of preexisting atherosclerosis and increased cardiovascular risk. Moreover, some temporal aspects of glycemic variation, including the rates of rise and fall of glucose, are associated with adverse cognitive and mood symptoms in those with diabetes.
Methods to quantify the risk of glycemic extremes, both high and low, and the variability including its temporal aspects are now more precise than ever. These important endpoints should be included for use in clinical trials as useful metrics and recognized by regulatory agencies, which has not been the case in the past. Precise evaluation of glycemic variability and its attendant risks are essential in the design of optimal therapies; for these reasons, inclusion of these metrics and the pulsatile hormone patterns in mathematical models may be essential. For the clinician, the incursion of mathematical models that simulate normal and pathophysiological mechanisms of glycemic control is a reality and should be also gradually incorporated into clinical practice.
Keywords: counterregulation, glucagon, hypoglycemia, mathematical model, variability
Introduction
The Gaussian or bell-shaped curve is used by mathematicians and researchers to describe the normal distribution of events and to predict and detect significant differences between groups. Comparisons are based on mean or median values and their distribution of variability around them; median values are abstraction, while variability is the day-to-day reality according a famous essay by Stephen Gould. This emphasis on variability aptly applies to the many patients with insulin-deficient diabetes mellitus and highlights their struggle to achieve good glycemic control safely and that of clinicians trying to help them.
The Importance of Glycemic Variability
The variability of glycemia in diabetes is typically at the root of the inability of clinicians working with diabetic patients to safely achieve near-normal average glycemia, reflected by the hemoglobin A1c (HbA1c). Primarily, this is because of hypoglycemia, which itself leads to both high and low blood glucose (BG) extremes. Target HbA1c values of 7% or less (American Diabetes Association recommendations) result in the decreased risk of severe micro- and macrovascular complications of diabetes mellitus. Hypoglycemia extremes are critical for safety, however, and represent the largest barrier to safe control of hyperglycemia.1,2 One critical question is how to best quantify the risk associated with hypoglycemia. In clinical studies, the rates of severe and moderate hypoglycemia are usually given. In theory, a cumulative distribution or probability distribution function curve would best quantify risk. For clinical applicability, however, it would help patients and physicians to have an analysis of glycemic patterns that will characterize their hypoglycemia risk in real time, possibly on their meter or continuous glucose monitoring (CGM) system. Ideally, it should be based on their glycemia patterns, their extent of variability, and its temporal aspects and would warn of hypoglycemia events. Such warning could thus help avoid moderate lows that precede the vicious cycle leading to severe hypoglycemia.
Difficulties with Predicting Hypoglycemia
Unfortunately, the distribution of clinical BG values in patients with diabetes mellitus is notoriously asymmetrical and commonly has a lognormal distribution. As a result, it is unwieldy to predict hypoglycemia. Moreover, the predictive values of many standard parameters of central tendency (mean, median) poorly reflect the risk of extremes, hyper- and hypoglycemia, and the variability of glycemia. Hemoglobin A1c, a 3-month integrated average of the tissue exposure to hyperglycemia, is nonetheless an excellent reflection of average glycemia and the best proven predictor of microvascular complications of diabetes mellitus. Despite this, HbA1c fails to capture glycemic variability and the risks associated with extremes of hypo- and hyperglycemia. The asymmetry of the glucose scale can, however, be mathematically rectified and thereby be used to permit its power of analysis and prediction to be restored.
Glycemia and the Prediction of Chronic Diabetes Complications
Many studies show mean BG values are the best predictors of the chronic microvascular complications of diabetes: neuropathy, retinopathy, and nephropathy.3-5 The mean BG and HbA1c are weaker predictors of macrovascular complications such as coronary artery disease and stroke, two of the most deadly and costly complications of diabetes. Hyperglycemia is associated with atherosclerosis with increased risk starting at minimally elevated average glucose or A1c values, even in the normal range and a shallow rise in risk.4-7 Some recent clinical trials suggest that hypoglycemia may also be an important predictor of increased mortality from vascular disease in type 2 diabetes. Some studies that link glycemic extremes to diabetes complications are reviewed here.
In type 1 diabetes mellitus, the Diabetes Control and Complications Trial (DCCT)3 study showed a nearly two point difference in HbA1c and predicted a 39-76% reduction in chronic microvascular complications. Severe hypoglycemia was increased by approximately threefold. The Epidemiology of Diabetes Interventions and Complications study8 found that HbA1c retained its predictive power for microvascular complications and revealed a new reduction (57% in major cardiovascular events) to macrovascular complications despite HbA1c values in the low 8% range.8 Whether glycemic variability is predictive of chronic complications in type 1 diabetes remains a disputed concept.6,7
In the United Kingdom Prospective Diabetes Study (UKPDS) in type 2 diabetes, median HbA1c over more than 10 years of an ∼1% difference between subjects on intensive glucose and standard policy9 reduced microvascular complications by approximately half that in the DCCT. A 10-year follow-up of UKPDS has shown that microvascular complications remain10 reduced despite HbA1c above 8%. Follow-up of UKPDS also found that cardio-vascular and mortality benefits were clearly significantly benefited by tight glycemia control.10 Much epidemiological evidence and some clinical trial data in type 2 diabetes suggest that hyperglycemic excursions, after meals or after glucose challenge, predict macrovascular complications in prediabetes and type 2 diabetes11-17 to a greater degree than fasting glycemia or HbA1c. The contribution of prandial hyperglycemia to cardiovascular risk has been thought plausible because of a biological relationship to oxidant stress or endothelial dysfunction.
New Measures to Help Predict Glycemic Extremes
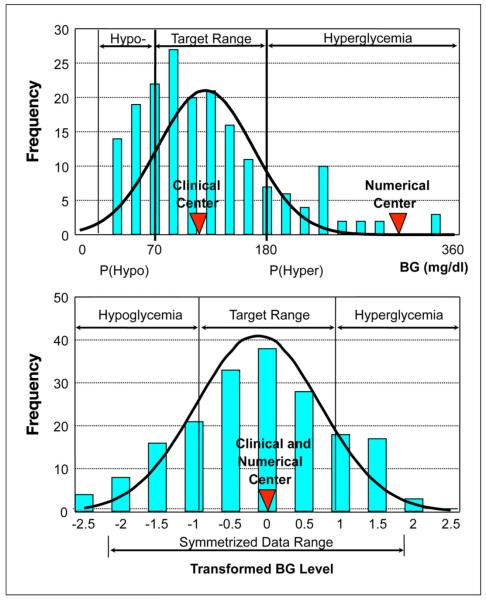
Despite excellent prediction of many chronic diabetes complications by HbA1c, the predictive value of HbA1c for acute hypoglycemia risk is weak. Approximately 8% of the risk of severe hypoglycemia is accounted for by the HbA1c.18 An important predictor of hypoglycemia is derived from use of a nonlinear transformation of the BG scale to restore symmetry to its distribution of glucose values.19-21 The concept is illustrated in Figure 1. The plots show how the asymmetrical non-Gaussian distribution of clinical values within the range typically observed in diabetes (upper panel) is transformed by a non-linear transformation of the BG scale to a Gaussian distribution (lower panel), which is more suitable for risk analysis.
Figure 1.
A typical asymmetrical non-Gaussian distribution of BG values within the range commonly observed in diabetes (upper panel) transformed into a symmetric Gaussian distribution using the nonlinear transformation of the BG scale (lower panel).
The benefit of reshaping the distribution of BG concentrations is that predicting the risk of both hyper- and hypoglycemia can be dramatically improved as compared to HbA1c. Several important parameters capture the risk of glycemia extremes and variability. The low BG index (LBGI) is a measure of the risk of hypoglycemia, and the high BG index (HBGI) is a measure of the risk of hyperglycemia.19-21 The BG rate of change (ROC) captures the speed at which BG values rise and fall. The average daily risk range (ADRR) is a composite of risk of high and low extremes and is the single best predictor of glucose extremes. These measures are derived from self-monitored BG (SMBG) concentrations. The LBGI, HGBI, and ROC have been adapted for use with CGM systems,22 while ADRR22 is used only with SMBG.
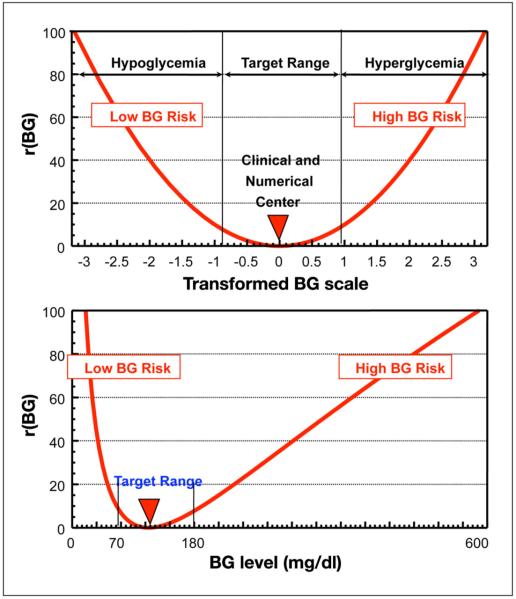
The risk function for the LBGI and HBGI is shown in Figure 2 (upper panel) and is derived from the distribution of the transformed BG levels shown in Figure 1 (bottom panel). When this risk function is brought back into the original glucose scale, the risk values associated with progressive hypoglycemia increase much faster than the risk values associated with progressive hyperglycemia (Figure 2, lower panel). This corresponds to the clinical notion of acutely increasing risk related to numerically minor (compared to hyperglycemia) glucose excursions in the low BG range.
Figure 2.
The upper panel presents the risk function used to define the LBGI and the HBGI as derived from the distribution of the transformed BG levels shown in Figure 1 (lower panel). The lower panel shows this risk function brought back into the original glucose scale. It is evident that the risk values associated with progressive hypoglycemia increase much faster than the risk values associated with progressive hyperglycemia.
The formulas for calculating the LBGI and HBGI have been described.19-21,23,24 Empirically derived risk categories, derived from large numbers of subjects with type 1 and type 2 diabetes who provided self-monitored glucose data, are as follows for the risk of hypoglycemia and hyperglycemia, respectively: LBGI minimal (LBGI ≤ 1.1), low (1.1 < LBGI ≤ 2.5), moderate (2.5 < LBGI ≤ 5), and high (LBGI > 5.0); and HBGI low (HBGI ≤ 4.5), moderate (4.5 < HBGI ≤ 9.0), and high (HBGI > 9.0).
Low HbA1c is associated with the increased risk of hypoglycemia, and the DCCT investigators looked at its predictive value. Fewer than 10% of serious events were predicted over the next 6 months by HbA1c. By comparison, the LBGI can predict up to 40-60% of severe hypoglycemic risk within the next few days. Its predictive accuracy is dependent on frequency of BG self-monitoring. Through monitoring four or more times daily, the LBGI can predict more than half of the severe hypoglycemia events within the next 48 hours.
Hypoglycemic Extremes Associated with Unstable and Rapid Blood Glucose Changes
It has also been observed that severe hypoglycemia is associated with instability in BG levels. Blood glucose levels are more likely to have high and low extreme values in diabetes patients about to experience or who have recently had a severe hypoglycemic reaction (requiring help to recover). A classic example of this is a patient seeking islet-cell transplantation who has frequent and severe lows and rapid BG ROC,22 which islet transplantation reverses. Thus the risks of both hyper- and hypoglycemic extremes are increased when severe hypoglycemia is recent or impending. Moreover, the speed of transitions between high and low values is often very rapid. This “roller-coaster effect,” the rapid occurrence of frequent extremes of both hyperglycemia and hypoglycemia around the time of a severe hypoglycemic episode, makes therapy decisions very difficult. Patients and physicians may be uncertain about which to address first. The instability may be made worse by overcorrection of highs and overtreatment of extreme lows due to fear of severe loss of control or severe hypoglycemia.
Mathematical Methods and Clinical Studies
Translation of clinical phenomena into mathematical parameters has more power and potential importance than many clinicians or those in regulatory agencies may realize. For example, a rapid downward BG ROC nicely captures what happens in defective insulin counterregulation. The power of prediction of hypoglycemia remains a clinical and research challenge that is incompletely addressed. Many newer pharmaceuticals, such as basal and prandial analog insulins, appear in a variety of studies to have a reduced risk of hypoglycemia compared to older insulins, such as neutral protamine Hagedorn and regular insulin,27,28 although their overall efficacy gauged by HbA1c lowering is equal. Given the importance of hypoglycemia avoidance, particularly in type 1 diabetes and arguably also in long-duration insulin-treated type 2 diabetes, it is surprising that no therapeutics have an indication for avoidance of hypoglycemia. Cryer and colleagues1,2 underscore the importance of hypoglycemia avoidance for safe control of diabetes. It is arguable that precise and validated risk category assignment or its lack using conventional measures of hypoglycemia is one factor that has limited authorities from indicating significant benefit through hypoglycemia avoidance. Moreover, even therapies intrinsically better for avoiding hypoglycemia risk (such as basal insulin) may be nonetheless misused, creating severe hypoglycemia risk when prescribers have insufficient experience or expertise.
In most clinical trials the risk of severe hypoglycemia is low—those designing the trials would be unwise to make it otherwise. Nonetheless, the risks of hypoglycemia are on a continuum, and this continuum can be given precise risk estimation by using tools like the LBGI or ADRR. Calculation of the risk for hypoglycemia and the amount of time in which study participants are in a high-, moderate-, or low-risk state can be estimated using such a tool. Indeed, risk-space analysis22-24 can further refine the temporal variation in risk to a more precise determination of both timing and severity more than simply that of mild, moderate, or the rare risk found in short-terms studies.
Recent Clinical Studies and Glycemic Extremes
The recent premature termination of the intensive glycemic arm of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial29 brings up this issue of hypoglycemic risk as it applies to clinical trials. More than 10,000 subjects with longstanding type 2 diabetes and with relatively high cardiovascular risk were assigned to a control target HbA1c of <6% (achieving approximately 6.4%) versus less intensive control aiming for 7-7.9% (and achieving 7.5%). An increase in mortality of 22% led to the study being stopped by the data safety monitoring committee. The increased mortality occurred despite a decrease by 24% in risk of nonfatal myocardial infarction, which was significant and a trend toward benefit in the primary composite cardiovascular endpoint. Two hundred fifty-seven patients in the intensive therapy group died, and 203 patients in the standard therapy group died, resulting in a hazard ratio of 1.22 (95% confidence interval; 1.01 to 1.46; p = .04). Severe hypoglycemia and weight gain of more than 10 kg were more frequent in the intensive treatment group (p < .001). Although there was not a clear association between severe hypoglycemia and the increased death rates primarily from myocardial infarction, there is speculation that there may be mechanisms in which hypoglycemia could have made a significant role in increased mortality risk in the intensive treatment group, perhaps with hypoglycemia even converting nonfatal myocardial infarctions to cardiovascular deaths in some subjects.
It should be noted that the ADVANCE Collaborative Group30 and the Veterans AffairsDiabetes Trial (VADT)31 also did not show a benefit in type 2 diabetes of tight glycemia in protecting against cardiovascular disease, although these studies did not indicate any aggregate increased cardiovascular risk of tight control. Of particular interest perhaps is the finding in post hoc analysis in the VADT that, in patients with longstanding diabetes, hypoglycemia was a powerful predictor of cardiovascular mortality. The VADT investigators looked at diabetes duration and hazard ratio for cardiovascular events in the study subjects and found a nearly linear relationship—the shorter the duration, the lower the hazard ratio with intensive therapy. Conversely, the longer the duration, the higher the hazard ratio with the crossover point to an increased risk occurring between a 12- and 15-year duration. Hypoglycemia that was severe anytime within the last 3 months was found to be an important predictor of cardiovascular risk and mortality.
It might be instructive to use the highly predictive risk analysis approach with these types of trials. A risk analysis using known indicators of higher risk of intensive glycemic intervention, such as the duration of type 2 diabetes mellitus, might allow patient selection for clinical intensive control to be refined and at the same time avoid the unexpected increased vascular disease risk observed in the ACCORD trial and some in VADT. It should be possible, for example, to use the risk calculation for LBGI or even HBGI and build it into a sophisticated BG meter that would then be a risk meter as well.
Clinical Translation
The translation of mathematical phenomena into the clinical arena could become important if clinicians understand how a quantitative approach may help patients improve glycemia safely. The LBGI is simply the best predictor of hypoglycemic risk and can give a rapid risk assessment number (especially for values in excess of 5) to a patient and potentially contribute to averting a severe hypoglycemic episode. The HBGI, in contrast, reflects the relatively acute risk of hyperglycemia. Not only clinically important for diabetes patients, the HBGI also has clinical correlation with the risk of oxidative stress32 that characteristically presents in the postprandial period and that a considerable body of literature suggests may be correlated with complications.
Glycemic variability may be more important for prediction of macrovascular complications and perhaps complications in type 2 diabetes. One analysis suggested that glycemic variability may be important in the pathogenesis of glycemia-related microvascular complications as well,6 but this has been recently been disputed based on a reanalysis of the data from the DCCT.7
Temporal Variability, Symptoms, and Risk
The BG ROC is the rate in mg/dl (or mmols/liter) per minute of either rise or fall of BG; thus it measures change in either direction. Rate-of-change data from Cox and colleagues25,33,34 suggest that rapid BG ROC is associated with altered mood and dysphoria, whereas absolute hyperglycemia exceeding approximately 250 mg/dl is associated with physical symptoms and cognitive dysfunction. Rapid rise in BG often occurs after meals with a high glycemic load, and these are sometimes referred to a glucose spikes. Pramlintide and incretin agonists are newer types of diabetic pharmaceuticals that help to blunt these rapid rises, decreasing HBGI and ROC.
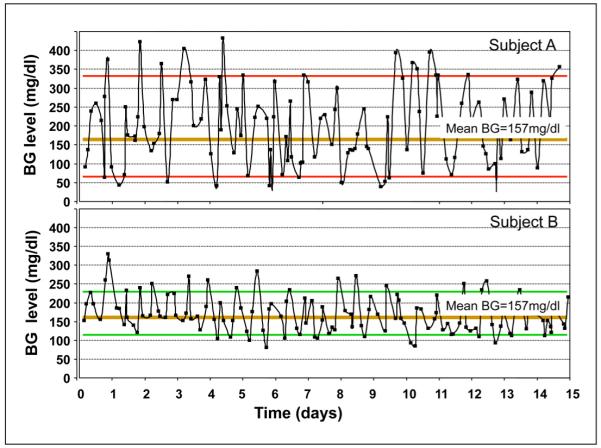
Rapid fall in glucose concentrations is found in the patient with undiagnosed hypoglycemia and defective glucose counterregulation resulting from decreased release of glucagon and epinephrine, the two hormones primarily responsible for early defense against hypoglycemia. The failure of this counterregulation may increase the ROC of BG to between 2 and 4 mg/dl/min, which is rapid22,26. Patients sometimes refer to this phenomenon as the elevator going down too fast. The combination of instability in BG patterns with rapid wide swings in glucose between high and low is sometimes called the roller-coaster effect, and in practice, it looks a bit like an oscilloscope that has gone haywire. Figure 3 illustrates two patients with type 1 diabetes. The bottom panel presents Patient B who has more stable control than Patient A who shows more severe extremes with rapid transitions between them with increased LBGI, HBGI, and a rapid ROC. Both have similar HbA1c values of around 8%.
Figure 3.
Blood glucose variability in two patients with type 1 diabetes. The top panel (Patient A) has more unstable control whereas the bottom panel (Patient B) is very stable. Both have similar HbA1c values around 8%, but Patient A is at higher risk for both hypoglycemia and hyperglycemia.
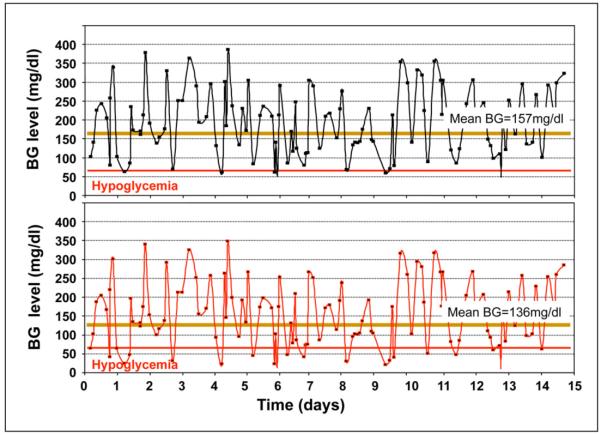
Perhaps the most important message is that reducing average glycemia without first reducing variability can potentially be dangerous. One may speculate that this adverse sequence could have occurred in some patients in ACCORD with highly variable glycemia initially, as it may do so in clinical practice. Figure 4 illustrates how a reduction in average glycemia in the same patient without reducing variability first may lead to a serious increase in hypoglycemia.
Figure 4.
Reduction in average glycemia (HbA1c) without reducing glucose variability is indicated by downward shift of the glucose profile from the upper panel to the lower panel, which results in increased occurrence and severity of hypoglycemic episodes.
As a single number, melding the power of risk prediction for both high and low BG extremes, the ADRR is another method for characterizing the instability of glycemic control.23 It is the most powerful single statistic for capturing glucose variability and is a far superior indicator than other methods such as standard deviation or mean amplitude of glycemic excursion. It captures highs and lows with roughly equal precision and estimates risk and is therefore the best available tool for predicting patient outcomes.
Data obtained from CGM are now able to be analyzed using some of the variability methods described earlier, although not the ADRR because of technical analytical reasons. It is hoped that the interpretation of the data obtained by CGM will help to guide algorithms for patient care.
Mathematical Modeling
A few comments about variability and modeling are worth noting. The tendency is still prevalent in physiological research to exam single hormone control mechanisms in the study of glycemic control counterregulation. Farhy and colleagues35 published an interdisciplinary work studying the control of pulsatile glucagon release in response to hypoglycemia using both experimental and modeling methods. Pulsatile secretion of hormones can now be precisely characterized, and its presence is indicative of feedback regulation, which can be detected by mathematical methods (such as deconvolution) and is yet another approach to analyze variability, although in this case, it is hormonal variability not substrate (glucose) variability as discussed earlier.
Feedback control is typically not the result of a single influence, but instead it is the result of a series of controlling mechanisms, e.g., as in defective counter-regulation in type 1 diabetes mellitus. Because the hormonal control is very complex, the mechanisms of control are manifold. Therefore, it is unlikely that they can be understood if its separate elements are studied in isolation. This means that most likely the only correct way to study such phenomena is by using a network model or system analysis. Examples of this approach include the already classical minimal model36 suggested as an alternative to hyperinsulinemic euglycemic clamping for measuring insulin sensitivity in vivo and its numerous extensions and applications (e.g., Reference 37).
In the study by Farhy et al.,35 a network model was used to explore the unknown mechanisms of glucagon counterregulation in rats and its failure related to β-cell loss. These studies were extended by Farhy and McCall,38 where model-based analyses predicted that different signals may improve defective glucagon counterregulation in β-cell deficiency through different but complementary mechanisms. Most importantly, these studies suggest strategies to enhance the glucagon response to hypoglycemia in type 1 diabetes by manipulation of the glucagon control axis and are clinically relevant as they could have application to design of an artificial pancreas by providing ways to augment glucagon counterregulation that would not require glucagon infusion. Such modeling is theoretical and must have evidence of a direct relevance to experimental data.
On the other hand, no single physiological experiment can successfully control or even study the multiple confounding variables. Use of a hybrid experimental approach, a combination of classic physiological mechanisms studied either in vivo or in vitro or both, are married with a modeling analysis using a series of differential equations to approximate the known and putative control mechanisms. By using such an approach, one can study these complex control mechanisms in silico as well as in vivo and in vitro. This hybrid approach is a more sophisticated method for probing these complex systems of control. Elucidation of these control mechanisms may eventually allow extrapolation and incorporation into a control mechanism for artificial pancreas.
Advantages and Limits of Modeling
Modeling in this kind of research is used as a tool and is never an end unto itself. Modeling, when done with precise mathematical constructs that have been experimentally validated, does permit physiological simulation on computer, which is an advantage of this approach. Most physiologists do use models in their work, although they are often not explicitly delineated. Even the use of standard statistical methods in analyzing the influence of one or several aspects of physiological control of hormone systems and hormone variability does imply some degree of inchoate modeling.
Modeling may be particularly an advantage when it is explicitly incorporated into experimental work and precisely mathematically defined. Under these conditions, modeling may become a more robust hypothesis-generating tool.26 The importance of modeling as a tool is that when it is combined with the physiological experimental assessment, the model itself becomes refined and extended, adding terms that delineate the importance of additional mathematical constructs. This can be further used in an iterative manner to refine both the experimental work and the model itself for subsequent use as a tool to further the systematic analysis of complex biological control mechanisms.
Summary
The use of glycemic averages has always been the best proven predictor of microvascular complications of diabetes mellitus. However, averages and HbA1c fail to capture glycemic variability and the attendant risks associated with extremes of hypo- and hyperglycemia. By employing the methods described here, data and analyses from clinical studies would be enhanced and would consequently result in more successful methods of assessing diabetes control and its risks in patient populations. Additionally, use of mathematical models in tandem with physiological experimentation is a hybrid approach, whereby hormonal variability and control mechanisms for feedback regulation and pulsatile hormone secretion can be analyzed on a network or system control level. Models are tools rather than ends. Pairing the two approaches, modeling and metabolism physiology experiments, permits more rapid approximation of complex control mechanisms analytically and further may allow the establishment of computer-based simulation to design repair or circumvention of defective physiological control such as defective insulin counterregulation.
Acknowledgment
The authors thank Teresa Olsen M.S. for editorial assistance.
Funding:
This work was supported by grants in aid from the National Institutes of Health, NIDDK P30 DK063609 and R21 DK072095.
Abbreviations
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- ADRR
average daily risk range
- BG
blood glucose
- CGM
continuous glucose monitoring
- DCCT
Diabetes Control and Complications Trial
- HbA1c
hemoglobin A1c
- HBGI
high blood glucose index
- LBGI
low blood glucose index
- ROC
rate of change
- SMBG
self-monitored blood glucose
- UKPDS
United Kingdom Prospective Diabetes Study
- VADT
Veterans Affairs Diabetes Trial
References
- 1.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902–12. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 2.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45(7):937–48. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial Research Group The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44(8):968–83. [PubMed] [Google Scholar]
- 4.Khaw KT, Wareham N, Luben R, Bingham S, Oakes S, Welch A, Day N. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European Prospective Investigation of Cancer and Nutrition (EPIC¬Norfolk) BMJ. 2001;322(7277):15–8. doi: 10.1136/bmj.322.7277.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19(3):178–81. doi: 10.1016/j.jdiacomp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN, DCCT/EDIC Research Group Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—revisited. Diabetes. 2008;57(4):995–1001. doi: 10.2337/db07-1618. [DOI] [PubMed] [Google Scholar]
- 8.Nathan DM, Cleary PA, backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B, Diabetes Control and Complications Trial. Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study Group (UKPDS) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 10.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 11.DECODE Study Group. the European Diabetes Epidemiology Group Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161(3):397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- 12.Esposito K, Giugliano D, Nappo F, Marfella R, Companion Postprandial Hyperglycemia Study Group Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110(2):214–19. doi: 10.1161/01.CIR.0000134501.57864.66. [DOI] [PubMed] [Google Scholar]
- 13.Muggeo M, Zoppini G, Bonora E, Brun E, Bonadonna RC, Moghetti P, Verlato G. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: the Verona Diabetes Study. Diabetes Care. 2000;23(1):45–50. doi: 10.2337/diacare.23.1.45. [DOI] [PubMed] [Google Scholar]
- 14.Meigs JB, Nathan DM, D’Agostino RB, Sr., Wilson PW, Framingham Offspring Study Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25(10):1845–50. doi: 10.2337/diacare.25.10.1845. [DOI] [PubMed] [Google Scholar]
- 15.Takei Y, Tomiyama H, Tanaka N, Yamashina A. Close relationship between sympathetic activation and coronary microvascular dysfunction during acute hyperglycemia in subjects with atherosclerotic risk factors. Circ J. 2007;71(2):202–6. doi: 10.1253/circj.71.202. [DOI] [PubMed] [Google Scholar]
- 16.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, STOP-NIDDM Trial Research Group Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290(4):486–94. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 17.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, STOP-NIDDM Trial Research Group Acarbose for the prevention of Type 2 diabetes, hypertension and cardiovascular disease in subjects with impaired glucose tolerance: facts and interpretations concerning the critical analysis of the STOP-NIDDM trial data. Diabetologia. 2004;47(6):969–75. doi: 10.1007/s00125-004-1409-4. [DOI] [PubMed] [Google Scholar]
- 18.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46(2):271–86. [PubMed] [Google Scholar]
- 19.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21(11):1870–5. doi: 10.2337/diacare.21.11.1870. [DOI] [PubMed] [Google Scholar]
- 20.Kovatchev BP, Cox DJ, Gonder-Frederick L, Clarke WL. Methods for quantifying self-monitoring blood glucose profiles exemplified by an examination of blood glucose patterns in patients with type 1 and type 2 diabetes. Diabetes Technol Ther. 2002;4(3):295–303. doi: 10.1089/152091502760098438. [DOI] [PubMed] [Google Scholar]
- 21.Kovatchev BP, Cox DJ, Kumar A, Gonder-Frederick L, Clarke WL. Algorithmic evaluation of metabolic control and risk of severe hypoglycemia in type 1 and type 2 diabetes using self-monitoring blood glucose data. Diabetes Technol Ther. 2003;5(5):817–28. doi: 10.1089/152091503322527021. [DOI] [PubMed] [Google Scholar]
- 22.Kovatchev BP, Clarke WL, Breton M, Brayman K, McCall A. Quantifying temporal glucose variability in diabetes via continuous glucose monitoring: mathematical methods and clinical application. Diabetes Technol. Ther. 2005;7(6):849–62. doi: 10.1089/dia.2005.7.849. [DOI] [PubMed] [Google Scholar]
- 23.Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29(11):2433–8. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 24.Kovatchev BP, Straume M, Cox DJ, Farhy LS. Risk analysis of blood glucose data: A quantitative approach to optimizing the control of insulin dependent diabetes. J Theor Med. 2000;3(1):1–10. [Google Scholar]
- 25.Kovatchev BP, Cox DJ, Summers KH, Gonder-Frederick L, Clarke WL. Postprandial glucose dynamics and associated symptoms in type 2 diabetes mellitus. J Appl Res. 2003;3(4):449–58. [Google Scholar]
- 26.Kovatchev BP, Cox DJ, Kumar A, Gonder-Frederick L, Clarke WL. Algorithmic evaluation of metabolic control and risk of severe hypoglycemia in type 1 and type 2 diabetes using self-monitoring blood glucose data. Diabetes Technol Ther. 2003;5(5):817–28. doi: 10.1089/152091503322527021. [DOI] [PubMed] [Google Scholar]
- 27.Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080–6. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 28.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29(6):1269–74. doi: 10.2337/dc05-1365. [DOI] [PubMed] [Google Scholar]
- 29.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr., Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr., Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 31.Abraira C, Duckworth W, McCarren M, Emanuele N, Arca D, Reda D, Henderson W, VA Cooperative Study of Glycemic Control and Complications in Diabetes Mellitus Type 2 Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications. 2003;17(6):314–22. doi: 10.1016/s1056-8727(02)00277-5. [DOI] [PubMed] [Google Scholar]
- 32.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–7. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 33.Cox D, Gonder-Frederick L, McCall A, Kovatchev B, Clarke W. The effects of glucose fluctuation on cognitive function and QOL: the functional costs of hypoglycaemia and hyperglycaemia among adults with type 1 or type 2 diabetes. Int J Clin Pract Suppl. 2002;129:20–6. [PubMed] [Google Scholar]
- 34.Cox DJ, McCall A, Kovatchev B, Sarwat S, Ilag LL, Tan MH. Effects of blood glucose rate of changes on perceived mood and cognitive symptoms in insulin-treated type 2 diabetes. Diabetes Care. 2007;30(8):2001–2. doi: 10.2337/dc06-2480. [DOI] [PubMed] [Google Scholar]
- 35.Farhy LS, Du Z, Zeng Q, Veldhuis PP, Johnson ML, Brayman KL, McCall AL. Amplification of pulsatile glucagon secretion by switch-off of α-cell suppressing signals in Streptozotocin (STZ)-treated rats. Am J Physiol Endocrinol Metab. 2008;295:E575–85. doi: 10.1152/ajpendo.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236(6):E667–77. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 37.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Measurement of selective effect of insulin on glucose disposal from labeled glucose oral test minimal model. Am J Physiol Endocrinol Metab. 2005;289(5):E909–14. doi: 10.1152/ajpendo.00299.2004. [DOI] [PubMed] [Google Scholar]
- 38.Farhy LS, McCall AL. System-level control to optimize glucagon counterregulation by switch-off of alpha-cell suppressing signals in beta-cell deficiency. J Diabetes Sci Technol. 2009;3(1):21–33. doi: 10.1177/193229680900300104. [DOI] [PMC free article] [PubMed] [Google Scholar]