Abstract
In chick development, ciliary ganglion (CG) neurons go through a period of axon extension from approximately embryonic day (E)4 to E8, followed by a period of synaptogenesis and neuronal cell death. By examining the immunohistochemical localization of laminin, in conjunction with Dil labeling of the ciliary nerve projection, we have determined that the pathway taken by these neurons is rich in laminin expression. Therefore, laminins are good candidate molecules for mediating outgrowth of these neurons in vivo. In vitro, the ability of CG neurons to extend neurites on laminin-1 (EHS laminin, α1β1γ1) is maximal up to E8, then declines dramatically. CG neuron outgrowth on laminin-1 requires the activity of β1-class integrins. We have used subunit-specific antibodies to determine which of the five β1-containing heterodimers known to be laminin receptors (α1β1, α2β1, α3β1, α6β1, α7β1) are expressed, and which mediate neurite outgrowth. While we could not detect expression of α2 or α7, we have found that α1, α3β1, and α6β1 are expressed on the surface of ciliary ganglion neuron cell bodies and axons, both in vitro and in vivo. Furthermore, antibodies against α3 and α6, but not α1, interfered with CG neurite outgrowth on laminin-1 in vitro. Taken together, these data suggest that interactions of cell surface α3β1 and α6β1 integrins with laminin-1 are likely to mediate growth of CG neurons during pathfinding in vivo.
Keywords: ciliary ganglion, development, axon outgrowth, integrin, laminin, merosin
In nervous system development, axons must traverse formidable distances to reach and synapse upon their specific targets. En route, their growth cones negotiate complex environments which provide cellular and extracellular guidance cues. Secreted matrices and basal laminae contain specific glycoproteins which are likely promoters of axon outgrowth (reviewed in Reichardt and Tomaselli, 1991). Notable among these are members of the laminin family. In vitro, laminin isoforms have been shown to promote neurite outgrowth of peripheral neurons (cf. Manthorpe et al., 1983; Engvall et al., 1986). Neuronal interactions with laminin-l (EHS laminin, α1β1γl, for laminin nomenclature see Burgeson et al., 1994) and merosin (laminin-2, α2β1γ1) are mediated primarily by members of the integrin family. Integrins are heterodimeric receptors, each composed of an α-subunit non-covalently associated with a β-subunit, which bind a variety of cellular and extracellular matrix molecules (Hynes, 1992). Regulated expression of integrins and their ligands correlates with, and may mediate periods of axon outgrowth and regeneration in vivo (de Curtis et al., 1991; Lefcort et al., 1992; de Curtis and Reichardt, 1993).
The avian ciliary ganglion (CG) has been a model system for studying neuronal development and synaptogenesis. CG neuron axons grow to their targets within the eye from approximately E4 until E8, whereupon a period of synaptogenesis and cell death begins (Landmesser and Pilar, 1978). CG neurons cultured from E8 and younger embryos extend profuse neurites on a heart cell conditioned medium substrate, and this response is gradually lost from E8 to E14 (Collins and Lee, 1982). The active component of this substrate probably consists of one or more isoforms of laminin (Lander et al., 1985; Coughlin et al., 1986) and similar results have been obtained using purified laminin-1 substrates (cf. Tomaselli et al., 1986). Loss of responsiveness to laminin-1 in vitro is thus temporally correlated with a switch from neurite outgrowth to synaptogenesis “mode” in vivo. However, older neurons retain the ability to extend neurites on cellular surfaces (Tomaselli et al., 1986; Bixby et al., 1988), suggesting a specific loss of laminin receptor function.
As a first step toward understanding how the ability of these neurons to interact with laminin-1 is developmentally regulated, we have attempted to identify the laminin-1 receptors involved. Since CG neuron outgrowth on laminin-1 requires β1-class integrin function, we used subunit-specific antibodies to determine which of the α-subunits known to form laminin receptors with β1 (α1β1, α2β1, α3β1, α6β1, α7β1) are expressed and mediate neurite outgrowth on laminin. We found that CG neurons express α1, α3β1, and α6β1 on their cell bodies and axons, and that polyclonal antibodies against α6 and α3, but not α1, perturb neurite outgrowth of these neurons on laminin-1. Furthermore, the pathway taken by CG axons within the eye is rich in laminin expression. These data suggest that α6β1 and α3β1 may mediate interactions with laminin-1 which are important for CG neuron growth in vivo.
Materials and Methods
Reagents
White Leghorn chicken eggs were purchased from Feather Hill Farm (Petaluma, CA) and incubated at 38°C and 95% humidity until use. Laminin-1 was purified from Engelbreth-Holm-Swarm sarcomas as described (Timpl et al., 1979). Nerve growth factor (NGF) was purified from male mouse submaxillary glands (Mobley et al., 1976). Collagenase, trypsin and protease-dispase were from Worthington (Freehold, NJ). Thiopropyl Sepharose 6B, protein A–Sepharose and Sepharose CL-4B were from Pharmacia (Piscataway, NJ). 125I-Goat anti-rabbit IgG and ECL (enhanced chemiluminescence) kits were from Amersham (Arlington Heights, IL). Dulbecco’s Minimal Essential Medium (DMEM), Ham’s F-12, Eagle’s MEM with Earle’s balanced salts, RPMI, Dulbecco’s phosohate-buffered saline (PBS), horse serum, fetal bovine serum (FBS), glutamine, and penicillin-streptomycin (pen/strep) were all from the UCSF cell culture facility. All other chemicals were from Sigma Chemical Co. (St. Louis, MO) unless otherwise noted in the text.
Antibodies
Polyclonal antibodies to the extracellular domains of α6 (anti-α6Ex; de Curtis and Reichardt, 1993), α8 (anti-α8Ex; Müller et al., 1995) and αv (anti-αvL; Bossy and Reichardt, 1990), and a monoclonal antibody to integrin β1 subunit, TASC (Neugebauer and Reichardt, 1991) have been described previously. A polyclonal antibody to the cytoplasmic domain of the integrin subunit α3A (de Curtis et al., 1991) was affinity purified using a peptide-Thiopropyl Sepharose 6B column. The human α2 cytoplasmic domain peptide CGFFKRKYEKMTKBPDEIDETTGLSS was synthesized by Dr. C. Turck of the Howard Hughes Medical Institute, coupled to keyhole limpet hemocyanin (Harlow and Lane, 1988) and used to generate the polyclonal antiserum named anti-α2 cyto. To prepare the polyclonal antibodies anti-α3Exl and 2, a chicken α3 cDNA encoding 17 amino acids of the putative signal sequence and the N-terminal 558 amino acids was cloned into pAR3039 at the BamHI site (Studier and Moffatt, 1986; Hoey and Levine, 1988). The final fusion protein, containing an N-terminal 15 amino acids and 24 C-terminal amino acids derived from the plasmid, was used for immunization of rabbits after purification by SDS-PAGE and electroelution. IgG was purified from these polyclonal sera using protein-G Mac Discs according to the manufacturer’s directions (Amicon, Beverly, MA). Fab fragments were generated according to Harlow and Lane (1988), except that papain-agarose (Sigma, P-4406) was substituted for papain and then removed by centrifugation. In anti-β1 immunoprecipitates of chick fibroblast extract, anti-α3Exl and anti-α3Ex2 each recognize a band characteristic of chick α3, also recognized by anti-α3 cyto (A-form specific, de Curtis et al., 1991). Anti-α3Exl, used in neurite outgrowth assays (see Fig. 6, Table 1), immunoprecipitates this band directly from the same extract (data not shown). It is possible, however, that this antibody cross-reacts with a β1-associated protein which co-migrates with α3.
Figure 6.
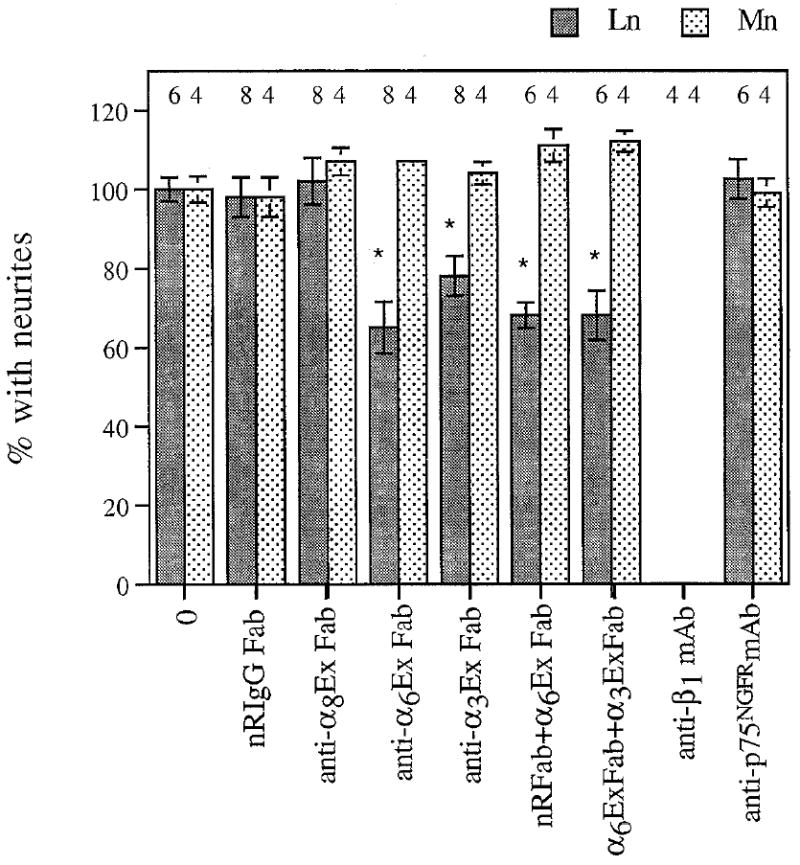
Antibodies to α6 and α3 inhibit CG outgrowth on laminin-1, but not on merosin. Dissociated E7.5 CG neurons were plated on laminin-1 (Ln) or merosin (Mn) and cultured for 4–5 hr (Mn) or 9–10 hr (Ln) in the presence of no antibody (0), normal rabbit IgG Fab (NRIgG Fab, 500 μglml), anti-α8EX Fab (500 μg/ml), anti-α6Ex Fab (500 μg/ml), anti-α3Exl Fab (500 μg/ml), Fab combinations, anti-β1 mAb (W1B10, 25 μg/ml), or control mAb (anti-P75NGFR, 25 μg/ml). Values were normalized to the percentage of neurons with neurites in the absence of antibody (mean ± SD). The number of cultures examined per condition is indicated above each bar. Dunnett’s test for multiple comparisons against a single control was used to determine significance (Glanz, 1992). *, p < 0.01, compared to no antibody (0) control.
Table 1.
Neurite lengths in the presence of anti-α6Ex and anti-α3Ex1
| Condition | Laminin (% neurite length) | Merosin (% neurite length) |
|---|---|---|
| nRIgG Fab | 100 ± 5 (79) | 100 ± 9 (38) |
| anti-α6Ex Fab | 71 ± 7 (80)** | 106 ± 9 (40) |
| anti-α3Ex1 Fab | 85 ± 15.8 (80)† | 105 ± 8 (40) |
| α3Ex + α6Ex Fabs | 81 ± 6 (79)* | 105 ± 5 (40) |
| anti-β1 mAb | 0 | 0 |
| anti-p75NGFR mAb | 99 ± 10 (80) | 106 ± 7 (40) |
Representative cultures from the experiments in Figure 6 were examined for effects of integrin-specific antibodies on neurite length. Processes longer than 1 cell body diameter were measured as described in Materials and Methods. Values represent mean neurite length ± SD, normalized to mean length under control conditions. (n) indicates the number of processes measured for each condition. Statistical significance was determined using Dunnett’s test for multiple comparisons against a single control.
p < 0.05.
p < 0.01.
p > 0.05 (not significantly different from control).
Several antibodies and/or hybridomas were kind gifts: polyclonal anti-chick α1 (Syfrig et al., 1991; University of Bern, Switzerland), anti-α1 mAb 3A3 (Turner et al., 1989; McGill University), anti-β1 mAb W1B10 (Hayashi et al., 1990), and anti-chick α7 mAb H1 (Bao et al., 1993; University of Illinois at Urbana-Champaign), anti-merosin antiserum (Leivo and Engvall, 1988; La Jolla Cancer Research Foundation), anti-laminin-heparin sulfate proteoglycan mAb INO (Chiu et al., 1986, from Dr. W. Matthew, Duke University), anti-neuron-specific βIII tubulin mAb TuJ1 (from Dr. A. Frankfurter, University of Virginia), and anti-rat p75NGFR mAb mc192 (Chandler et al., 1984; Stanford University). Polyclonal anti-mouse laminin-1 antibody JW2 was prepared by Dr. J. Winter and affinity purified as described (Lander et al., 1985). Anti-chick laminin mAb 31 (Bayne et al., 1984) was purchased from the Developmental Studies Hybridoma bank (University of Iowa, Iowa City, IA). Anti-150 kDa neurofilament subunit antibody was purchased from Calbiochem (cat #480722, San Diego, CA).
DiI labeling
E8 eyes, each with its ciliary ganglion attached, were fixed in 4% paraformaldehyde overnight at 4°C. Small crystals of DiI (Molecular Probes D282, Eugene, OR) were placed in the CG with a glass capillary micropipette. The tissue was reimmersed in 4% paraformaldehyde and incubated at 37°C for 20–25 d. The eyes were then hemisected, the vitreous was removed, and the eye “filet” containing the CG and ciliary nerve were embedded in 3% agarose. Vibratome sections were taken at 100 μm intervals and viewed under fluorescence optics. Photos were taken with TMAX 400 film (Kodak).
Immunohistochemistry
E7-8 ciliary ganglia were collected, fixed for 1 hr in 4% paraformaldehyde, and incubated overnight in 30% sucrose at 4°C. E6.5 eyes, E7-8 heads (fixed 2 hr), and E11 pectoral muscles were treated similarly. Tissues were embedded in Tissue-Tek OCT (Miles, Inc., Elkhart, IN), frozen in liquid N2, and cryostat sectioned at 10 μm intervals. Sections were incubated with 5–10% normal goat serum in Tris-buffered saline (TBS, 50 mm Tris-Cl, 150 mm NaCI, pH 7.4) with or without 0.1% Triton X-100 for 30 min. Primary antibodies diluted in the same solution were added to the sections and allowed to incubate overnight at 4°C. After extensive washing, secondary antibodies were incubated with the sections for 45 min at room temperature. Secondary antibodies were fluorescein-goat anti-rabbit (1:200; Kirkegaard and Perry, Gaithersburg, MD) or Texas red–goat anti-mouse IgG (1:200; Jackson, West Grove, PA) or IgM (1:400, Jackson), each of which was preadsorbed using E10 chick acetone powder (Harlow and Lane, 1988). Sections were rewashed, mounted in gelvatol plus 3% n-propyl gallate, pH 9.0, allowed to dry, and viewed under fluorescent optics. Photos of control and experimental samples/conditions were taken for equal exposure times and processed in parallel.
Tissue culture
E7.5 (stage 31–33, Hamburger and Hamilton, 1951) chick ciliary ganglia were prepared as described (Nishi and Berg, 1977), except that chick eye extract was substituted for chick embryo extract (Nishi and Berg, 1981). For neurite outgrowth assays (see also below), the base medium was 50% Eagle’s MEM with Earle’s balanced salts and 50% Ham’s F-12. E7 dorsal root ganglia (DRG) were prepared as described (Venstrom and Reichardt, 1995). For myotube and fibroblast cultures, pectoral muscles from E11 chick embryos were minced and digested with 0.05% protease-dispase for 5 min at 37°C with constant swirling. After gentle trituration, the cells were centrifuged and resuspended in DMEM with 10% horse serum, 5% chick embryo extract (GIBCO, Grand Island, NY), and 100 U/ml pen/strep. This cell suspension was passed through three disks of lens paper mounted in a Swinney filter and preplated on tissue culture plastic for l–3 hr. Plates were then rinsed and detached myoblast cells were collected and replated on plates coated with 10 μg/ml collagen I (Collaborative Research, Bedford, MA). These myoblasts were allowed to proliferate and fuse into myotubes. The fibroblast cells which remained attached were resubmerged in DMEM plus 10% FBS and 100 U/ml pen/strep, and cultured as necessary. PC12 cells were maintained as described previously (Greene et al., 1987).
Neurite outgrowth assays
Ninety-six well dish wells (Costar 3595, Pleasanton, CA) were coated with 10 μg/ml laminin-1 or 5 μg/ml merosin (Telios Pharmaceuticals, La Jolla, CA) in CMF-PBS for at least 3 hr at 37°C or overnight at 4°C. Only plate lots able to support neurite outgrowth under these conditions were used for these experiments. “Heat-treated laminin-1” was prepared as described previously (Goodman et al., 1991). Nonspecific binding was blocked with 1% BSA (Elisa grade, fraction V) in CMF-PBS for 1–3 hr at 37°C. The wells were washed five times with sterile CMF-PBS before cells (approximately 200–300 per well) were plated, in the presence of antibodies as indicated. Cells were immediately centrifuged to the bottom of the well, then incubated for 8–10 hr (CGs on laminin-1) or 4–5 hr (CGs on merosin, DRGs on laminin-1) at 37°C in 5% CO2 atmosphere. Experiments were terminated when approximately 50% of the cells bore neurites, defined as processes longer than two cell body diameters. Incubation periods were “titered” to comparable degrees of neurite outgrowth. All experiments were performed in duplicate. Cultures were fixed in 3% paraformaldehyde and scored for neurite outgrowth. For measuring neurite lengths, fixed cultures were examined on an inverted microscope (Olympus IMT2) and images were collected with a cooled CCD camera (Photometrics). Processes longer than 1 cell body diameter were measured using the PRISM modeling program (Chen et al., 1989). Statistical analyses were performed on data before they were normalized for graphing (see Fig. 6, Table 1).
PC12 cells were primed with 50 ng/ml NGF for 7 d (Greene et al., 1987), then replated as described above in RPMI with 100 ng/ml NGF, 1% horse serum, and 100 U/ml pen/strep.
Surface labeling and immunoprecipitation
E7-8 ciliary ganglia were collected in PBS with 0.6% glucose and dissociated by addition of 300–400 U of collagenase for 40 min at 37°C. Digested ganglia were then centrifuged and pellets were resuspended in CG medium and triturated with a fire-polished pasteur pipette. The cell suspension was pelleted, washed once in PBS, resuspended in PBS with 0.1 m Na-HEPES, pH 8.0, and passed through a 25-gauge needle. 0.8 mg of sulfo-NHS-biotin (#21217, Pierce, Rockford, IL) was added and rocked with the cells at 4°C for 1 hr. Cells were then washed three times in TBS and extracted in RIPA (0.01 m Tris-Cl, pH 7.2, 0.15 m NaCl, 1% Na-deoxycholate, 1% Triton X-100, 0.1% SDS, 1% aprotinin, 4 mm PMSF, and 10 μg/ml each antipain, leupeptin, pepstatin, and chymostatin) for 1 hr at 4°C. The extracts were centrifuged at 9170 × g for 15 min and DNase was added to the soluble fraction at a final concentration of 100 μl/ml.
For immunoprecipitations, all steps were carried out at 4°C unless otherwise noted. Extracts were precleared once with Sepharose CL-4B followed by protein A–Sepharose (each 50 μl/lane, 45 min). then incubated overnight at 4°C with anti-β1 mAb W1B10 coupled directly to protein A–Sepharose (Harlow and Lane, 1988). Beads from all precipinitation steps were collected, washed three times each in RIPA, two times in TBS with 0.5% Tween-20, and once in TBS with 0.5% Tween-20 and 0.1% ovalbumin. Precipitates were boiled in nonreducing sample buffer, subjected to SDS-PAGE, and transferred to nitrocellulose (Schleicher and Schuell; Keene, NH). Transfer membranes were blocked for 1 hr at room temperature in PBS with 10% BSA, 0.05% Tween-20, then incubated in PBS with 1% BSA, 0.05% Tween-20 (reaction buffer) with streptavidin–HRP (1:4000; Zymed, South San Francisco CA) for 1 hr at room temperature. After a brief rinse in TBS, the transfers were washed twice in 1% Triton X-100, 0.1% SDS, 0.5% Nadeoxycholate in TBS for 5 min each, then twice more in TBS. The transfers were then processed for chemiluminescent detection of HRP reaction product according to the manufacturer’s specifications.
In order to identify specific bands in the β1-immunoprecipitates, transfer membranes were washed in TBS and reblocked in TBS with 10% BSA, 0.05% Tween-20 for 1 hr at room temperature. After overnight incubation at 4°C with anti-α6Ex IgG (50 μg/ml in reaction buffer), they were washed five times in reaction buffer and incubated with 15 μCi of 125I goat anti-rabbit IgG for 45 min at room temperature. Blots were washed again and exposed to Kodak XAR-5 film. For anti-α3 immunoblotting of β1-immunoprecipitates, the procedure was the same except the cells were not biotinylated so that immunoblotted bands could be detected by the more sensitive ECL method.
Immunoblots
E7-8 ciliary ganglia were dissected out and either used directly, dissociated as described above and cultured for 24 hr on laminin-1, or collected and snap-frozen in dry-ice ethanol and stored at −70°C. Whole ganglia and cultured neurons were extracted either in sample buffer, 1% Triton X-100, or used to make membrane preps (see below). E6-8 chick retinae were collected, dissociated in 0.5% trypsin with swirling for 2–5 min, and then washed in PBS and extracted in sample buffer. E11 breast myotubes were prepared as described above and extracted in 1% Triton X-100 or used to prepare membranes. Triton X-100 extracts were prepared by resuspending tissue in PBS with 1% Triton X-100, 1 mm PMSF plus 10 μg/ml each antipain, leupeptin, aprotinin, pepstatin, and chymostatin and incubating for 30 min at 4°C. Extracts were centrifuged at 9170 × g for 15 min, and the soluble supernatant fraction was saved and subjected to further analysis. To prepare membranes, cell pellets or whole ciliary ganglia were homogenized in 10 mm Na-HEPES, pH 7.4, 0.32 m sucrose, 2 mm EDTA, 2 mm N-ethyl maleimide, 1 mm PMSF, plus 10 μg/ml each antipain, leupeptin, pepstatin, and chymostatin, then centrifuged at 14300 × g for 35 min at 4°C. The resulting pellet was considered the membrane fraction.
For antigen blots, approximately equal amounts of protein were loaded for SDS-PAGE under nonreducing conditions. After transfer to nitrocellulose, the blots were blocked in Blotto (5% Carnation nonfat dry milk in 50 mm Tris-Cl, pH 7.5, 150 mm NaCl, 1 mm CaCl2) for at least 1 hr at room temperature. Blots were then incubated in primary antibody overnight at 4°C or at room temperature for 1–2 hr. After washing with Blotto, the blots were incubated in alkaline phosphatase-conjugated goat anti-rabbit or goat anti-mouse IgG (Promega, 1:7500 in Blotto) for 45 min at room temperature. After another round of washing, immuno-reactivity was detected by developing with 0.33 mg/ml nitro blue tetrazolium and 0.16 mg/ml 5-bromo-3-indolyl-phosphate in 0.1 m Tris-Cl, pH 9.0, 0.1 m NaCl, 5 mm MgCl2.
Results
CG axons project through the laminin-rich choroid layer of E8 eye
Efferent fibers from the chick ciliary ganglion consist of two large ciliary nerves and three to five smaller choroid nerves. Piercing the sclera, these branches run within the optic cup toward their targets (Landmesser and Pilar, 1974). The choroid branches innervate the smooth muscle cells of the choroidal coat, while the ciliary axons innervate the striated iris and ciliary body muscles (Marwitt et al., 1971). To verify the presence of laminin-1 in the path that ciliary ganglion axons take in vivo, we labeled ciliary ganglia with the lipophilic dye DiI in order to visualize the exact nerve projections within the eye (Fig. 1). When the dye was visible in a target (the iris), indicating that it had diffused the entire length of the nerve, the eye was hemisected and the resulting “filet” was sectioned with a vibratome at 100–150 μm intervals for examination. An example of such a section (Fig. 1B) is schematized in Figure 1A. The box in Figure 1A corresponds to the photomicrograph in Figure 1B. In this section, the entire ganglion (cg) appears to be labeled, and a main ciliary nerve branch exits the ganglion and runs within the choroid layer of the eye (ch).
Figure 1.
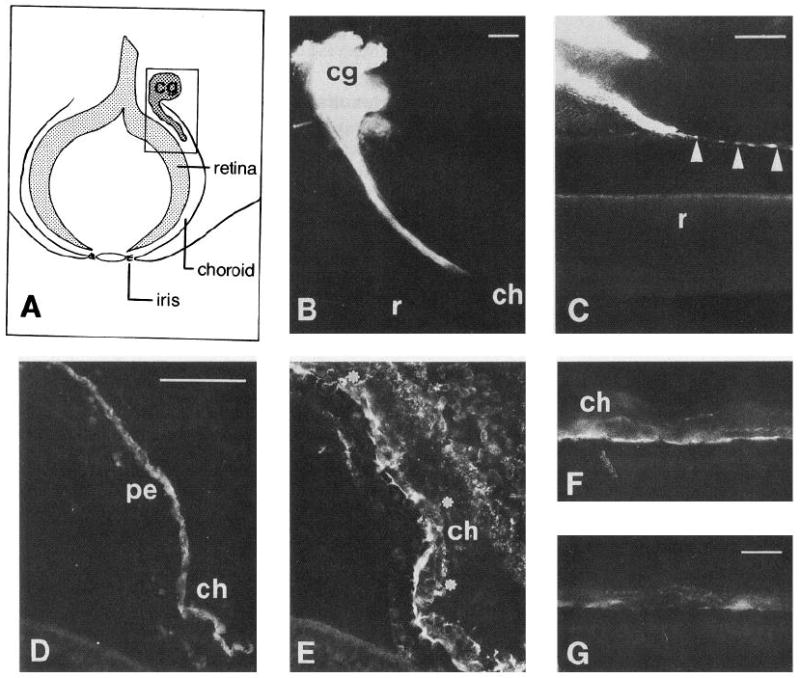
Ciliary ganglion neuron axons project through laminin-rich choroid layer of E7.5-8 eye. A, Schematic of eye and its relationship to ciliary ganglion in approximate plane of vibratome section (not to scale). Box encloses representation of B. B, Section of DiI labeled ciliary ganglion. A main ciliary nerve branch penetrates the choroid layer of the eye and runs within it towards target tissues. C, Section containing minor ciliary or choroid nerve branch which appears to run on the edge of Bruch’s membrane (arrowheads), the basal lamina between the pigment epithelium and choroid layers. The pigment epithelium has separated from the retina in this section. This is a common artifact of preparation, which has also occurred in D/E. D and E, Section of E7.5 eye, double labeled with anti-β III tubulin (TuJ1), a neuronal marker (D), and an affinity-purified polyclonal antibody to laminin-1 (E). Dots indicate position of axons; note that the axon bundle lies within the laminin-1-rich choroid layer. F and G, Sections of E7.5 eye, stained with anti-laminin mAb 31 (F) and anti-laminin heparin sulfate proteoglycan mAb INO (G). Both are immunoreactive with material in the choroid layer of the eye. cg, Ciliary ganglion; ch, choroid; r, retina; pe, pigment epithelium. Scale bars: B and C, 100 μm; D and E, 50 μm; F and G, 10 μm.
Attempts to immunostain the vibratome sections containing ciliary nerve were unsuccessful, probably due to the extended fixation of the tissue (typically 20–25 d) which may have reduced antigenicity significantly. To determine whether laminin immunoreactivity is also present in the choroid, cryostat sections were double labeled with antibodies to the axonal marker anti-β III tubulin (Fig. 1D) and a polyclonal anti-laminin-1 antibody (Fig. 1E). Anti-laminin immunoreactivity is concentrated in the choroid layer, surrounding a nerve bundle. A monoclonal antibody to chicken laminin (Fig. 1F) and INO, a function-blocking monoclonal antibody which recognizes an epitope present on laminin-heparin sulfate proteoglycan complexes (Fig. 1G), also label antigens in the choroid layer. Thus, a laminin isoform appears to be present in the immediate environment of CG axons in vivo.
CG neurite outgrowth on laminin-1 requires activity of the E8–E3 region
Dissociated E7.5 (stage 31–33, Hamburger and Hamilton, 1951) CG neurons plated on laminin-1 or merosin extend neurites in a manner characteristic of each substrate. On merosin, at least 50% of the neurons plated typically extend neurites within 4 hr. This outgrowth was inhibited in the presence of an antiserum to merosin, but was unchanged by control serum or anti-laminin-1 antibodies (Fig. 2A). In contrast, outgrowth on laminin-1 is considerably slower; 50% of neurons typically bear neurites 8–10 hr after plating. Outgrowth on laminin-1 was virtually abolished in the presence of affinity-purified anti-laminin-1 antibody, but was not reduced by the anti-merosin antiserum. Thus, both laminin-1 and merosin can specifically promote CG neurite outgrowth.
Figure 2.

A, In vitro, CG neurite outgrowth on laminin-1 and merosin are sensitive to anti-laminin-1 and anti-merosin antibodies, respectively. Dissociated E7.5 CG neurons were plated on laminin-1 (Ln) and merosin (Mn) and allowed to extend neurites for 4–5 hr (Mn) or 9–10 hr (Ln) in the presence of no antibody (0), anti-Mn serum (1:25), normal rabbit serum (NRS, 1:25), or affinity-purified anti-laminin-1 (JW2, 20 μg/ml). Values represent the mean ± SD of four determinations from two experiments, each performed in duplicate. B, CG neurite outgrowth on laminin-1 requires activity of heat-sensitive E8–E3 region. Dissociated E7.5 CG neurons and primed PC12 cells were plated on laminin-1 (Ln) or laminin-1 which had been heated to 80°C for 10 min (80° Ln). This heat treatment inactivates the E8–E3 region of laminin-1, while the E1-4 region remains intact. In 16–18 hr, PC12 cells, but not CG neurons, are able to extend neurites on this substrate. An anti-α1 mAb, 3A3, blocks this outgrowth. Values represent the percentage of neurons with neurites (average ± range of determinations made on duplicate cultures from a single representative experiment).
In order to determine which region(s) of laminin-1 was neurite-promoting for CG neurons, we heat-treated laminin-1 at 80°C for 10 min to inactivate the E8–E3 region, leaving activity present in the E1–4 domain intact (Goodman et al., 1987, 1991). CG neurons and primed PC12 cells were allowed to interact with this substrate for 16–18 hr (Fig. 2B). The PC12 cells extended neurites on heat-treated laminin-1, demonstrating that the El-4 domain was still active. As previously reported (Turner et al., 1989), PC 12 cell neurite outgrowth on this substrate was blocked completely by 3A3, a monoclonal antibody to the rat α1 subunit. In contrast, CG neurite outgrowth was not supported by heat-treated laminin-1, indicating that neurite extension by these neurons is mediated via interactions with the E8–E3 portion of laminin-1. This finding confirms and extends those of Engvall et al. (1986), who found that monoclonal antibodies recognizing sites at the end of the long arm of laminin-1 significantly reduced the extent of E8 CG neurite outgrowth on laminin substrates.
Characterization of CG integrin expression
Extracts of whole E7-8 ciliary ganglia, CG neurons plated on laminin-1, and positive: control tissues were prepared and subjected to immunoblot analysis to determine whether CG neurons express integrin subunits that form laminin receptors, that is, β1, α1,α2, α3, α6, and/or α7. The results of this analysis are shown in Figure 3A. Whole CG and cultured CG neurons express β1, α1, α3, and α6. Although present in extracts of control tissues (thrombocytes and myotubes, respectively), α2 and α7 were not detected in CG extracts.
Figure 3.
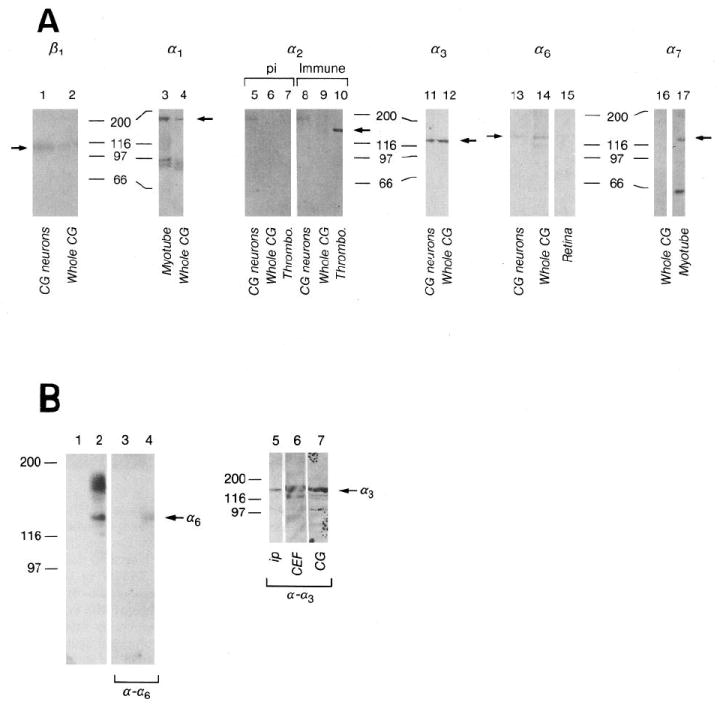
A, Integrin expression by E7-8 CG and purified CG neurons cultured on laminin-1. Whole CG (lanes 2, 4, 6, 9, 12, 14), CG membranes (lane 16), CG neurons plated on laminin-1 (lanes 1, 5, 8, 11, 13), E11 myotubes (lane 3), E11 myotube membranes (lane 17), thrombocytes (lanes 7, 10) and E6–8 retinae (lane 15) were extracted in sample buffer (lanes 1, 2, 5–17) or 1% Triton X-100 (lanes 3, 4) as described in Materials and Methods. Approximately equal amounts of protein were loaded per lane for each blot. Blots were incubated with polyclonal antibodies against α1 (lanes 3, 4), α2 cyto (preimmune, lanes 5, 6, 7; immune, lanes 8, 9, 10), α3 (Ex2, lanes 11, 12), α6 (Ex, lanes 13, 14, 15), or monoclonal antibodies against β1 (TASC, lanes 1, 2) or α7 (H1, lanes 16, 17). Immunoreactivity was visualized with alkaline phosphatase-conjugated anti-rabbit or anti-mouse antibodies. Arrows indicate bands corresponding to each subunit. The doublets around 97 kDa in lanes 3 and 4 are probably breakdown products of α1 (Duband et al., 1992). The band at 190 kDa in lanes 5 (preimmune) and 8 (immune) is nonspecific. The lower Mr band in lane 17 is a common breakdown product of α7 (Bao et al., 1993). Numbers denote positions of Mr marker proteins in kilodaltons. pi, Preimmune. B, α6 and α3 heterodimerize with β1 in CG neurons. β1-containing integrin heterodimers were immunoprecipitated from surface-biotinylated CG cells with anti-β1 (W1B10) mAb coupled to protein A–Sepharose. Immunoprecipitates were visualized directly by chemiluminescent detection of HRP–streptavidin (lane 1, protein A–Sepharose control; lane 2, antibody-coupled Sepharose), then probed with anti-α6Ex (lanes 3, 4). Similar immunoprecipitates were probed with anti-α3Ex2 (ip, lane 5). Triton X-100 extracts of chick breast fibroblasts (CEF, lane 6) and whole ciliary ganglia (CG, lane 7) were also blotted as positive controls. Arrows indicate bands corresponding to α6 or α3. Numbers indicate Mr in kilodaltons.
Since antibodies recognizing the β1, α3, and α6 subunits interfere with neurite outgrowth (see Fig. 6, below), we wanted to verify that these subunits exist as heterodimers in CG cells. Dissociated CG cells were surface-labeled and β1 heterodimers were immunoprecipitated with the monoclonal anti-β1 antibody W1B10. In Figure 3B, protein A–Sepharose (lane 1) failed to precipitate any labeled material, but anti-β1 (lane 2) precipitated β1 (the prominent band between the Mr 97 and 116 × 103 markers) and several other bands which probably correspond to integrin α-subunits. These precipitates were blotted with polyclonal antibodies to individual α-subunits and visualized with 125I-conjugated secondary anti-rabbit antibodies. Material in lanes 1 and 2 was incubated with anti-α6Ex. The β1 immunoprecipitate contains an anti-α6 immunoreactive band (lane 4) at approximately Mr 140 × 103 corresponding to the Mr of the chicken α6 subunit (de Curtis et al., 1991). A similar immunoprecipitation was incubated with anti-α3Ex2, which reacted with a band at approximately Mr 130 × 103 (lane 5). Extracts of chick embryo fibroblasts (lane 6) and ciliary ganglia (lane 7) were immunoblotted in parallel as positive controls. Thus it appears that the heterodimers α6β1 and α3β1 are expressed on the surface of CG cells.
We immunostained cryostat sections of E7-8 ciliary ganglia with antibodies against specific integrin subunits to examine their expression at the cellular level in situ. The results are illustrated in Figure 4. In one such section, double-labeled with the monoclonal anti-β1 antibody TASC (Fig. 4A) and anti-neurofilament (Fig. 4B), anti-β1 immunoreactivity appears on the surfaces of cells which are also labeled with the neuronal marker. This colocalization is also apparent on strnctures which appear to be axons (see arrows). Polyclonal antibodies against α3 (Fig. 4C) and α6 (Fig. 4D) also label the surfaces of neurofilament-positive (not shown) CG neurons. A control polyclonal (anti-α6 pre-immune IgG; Fig. 4E) was not immunoreactive with material in these sections. CG neurons were not reactive with anti-α2 (Fig. 4F) or anti-α7 (Fig. 4G) antibodies, although both antibodies were immunoreactive with control tissues stained in parallel (data not shown).
Figure 4.
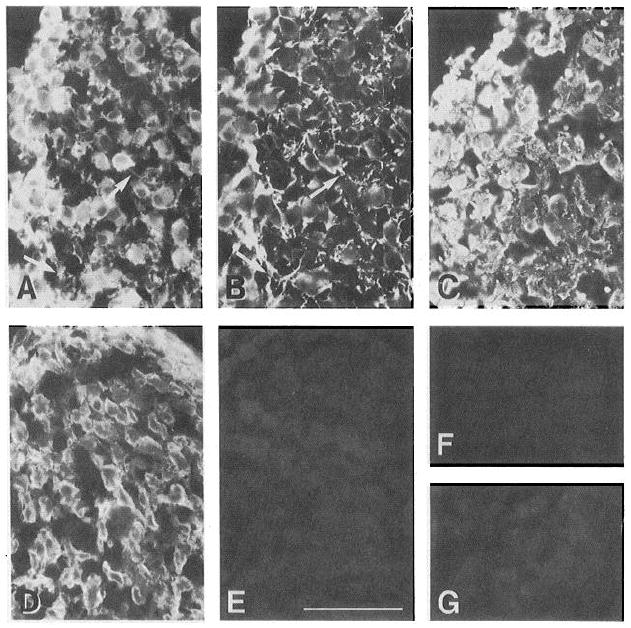
CG neurons ex press α6, α3, and β1, but not α2 or α7, in situ. Cryostat sections of E7-8 ciliary ganglia were stained with anti-integrin antibodies as described in Materials and Methods. A and B show a double-labeled section incubated with anti-β1, mAb (TASC, A) and a polyclonal antibody to neurofilament (B). Anti-β1 immunoreactivity appears to be highly coincident with that of the neuronal marker, notably in structures which appear to be axons (arrows). Other sections were incubated with antibodies to α3 (cytoplasmic domain A-form, C), α6 (D), α6 preimmune IgG (E), α2 (cytoplasmic domain, F), and α7, mAb (G). As for β1, α3 and α6 immunoreactivity often colocalizes with that of neuronal markers (data not shown). The α2 and α7 antibodies were immunoreactive with control tissues (epithelium and muscle, respectively) in the same experiments (data not shown). Scale bar, 100 μm.
In order to determine whether the distribution of integrin subunit expression is consistent with a role in CG neuron outgrowth in vivo, we immunostained cryostat sections of E6.5 eye containing ciliary nerve. At this stage, CG axons are still actively growing towards their targets. The ciliary nerve, labeled with the neuronal marker anti-β III tubulin monoclonal TuJl (Fig. 5A), was strongly immunoreactive with affinity purified anti-α6Ex IgG (Fig. 5B) in double-labeling experiments. In adjacent sections, anti-β1 (TASC, Fig. 5C) and anti-α3 (cytoplasmic domain A, Fig. 5E) immunoreactivity was found not only in the ciliary nerve, but throughout most of the layers of the eye. A polyclonal antibody against the cytoplasmic domain of α2 did not label the ciliary nerve (Fig. 5F). Anti-laminin-1 immunoreactivity is concentrated in Bruch’s membrane (b), and is also present in the choroid and sclera (Fig. 6D). Thus, it appears that the laminin receptor subunits α3, α6, and β1 are expressed on CG axons during their outgrowth period, and that a laminin isoform is present in their pathway at this time.
Figure 5.
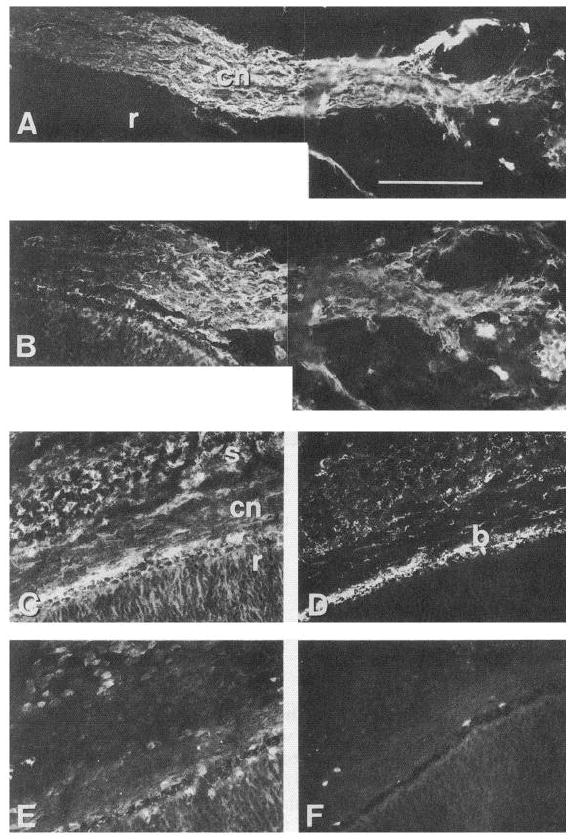
Ciliary ganglion axons express α6, α3 and β1. At this stage, CG axons are still en route to their target. Cryostat sections of E6.5 eye were double labeled with antibodies against β-III tubulin (A) and α6 (B). Most of the axons within the ciliary nerve (cn) appear to express α6. Adjacent sections were incubated with anti-β1 mAb (TASC, C), affinity purified anti-laminin-1 (D), anti-α3 cyto (A form specific, E), and anti-α2 (cytoplasmic domain, F). β1 and α3 immunoreactivities are found not only within the ciliary nerve, but throughout most of the layers of the eye. Anti-laminin-1 immunoreactivity is concentrated in Bruch’s membrane, and is also present in the choroid and sclera. cn, ciliary nerve; r, retina; s, sclera; b, Bruch’s membrane. Scale bar, 100 μm.
Antibodies against the β1, α6, and α3 subunits inhibit CG outgrowth on laminin-1, but not on merosin
CG neurons were cultured on laminin-1 or on merosin in the absence or presence of function-blocking, subunit-specific antibodies against chick β1, α3, α6 (Fig. 6), and α1, (Fig. 7, described below). Neurite outgrowth on laminin-1 and merosin was completely abolished in the presence of an anti-β1 monoclonal antibody (Fig. 6), corroborating previous reports that the function of β1-class integrins is required for neurite outgrowth on each isoform of laminin (Tomaselli et al., 1986; Engvall et al., 1992). To examine the roles of α6β1, and α3β1, we cultured the neurons in the presence of monovalent Fab fragment preparations of anti-α6Ex and anti-α3Exl. The percentage of neurons bearing neurites on laminin-1 was reduced by an average of 35% (Fig. 6), and mean neurite length was reduced to 71% of control (p < 0.01, Table 1) in the presence of anti-α6Ex Fab. Affinity-purified anti-α6Ex IgG and Fab fragments reduced outgrowth on laminin-1 by similar extents (not shown). Anti-α3Ex1 Fab reduced CG outgrowth on laminin-1 by 22% (p < 0.01), but did not significantly reduce neurite lengths (p > 0.05). The effects of anti-α6EX plus anti-α3Ex1 were not additive. Clearly, the combination of these two antibodies fails to inhibit outgrowth as completely as anti-β1 antibodies, suggesting that one or more additional β1 integrin heterodimer(s) may contribute to CG neurite outgrowth on laminin-1. In contrast to their effects on laminin-1, neither anti-α6Ex Fab nor anti-α3Ex1 Fab interfered with CG outgrowth on merosin. Control mAb, and Fab fragments of normal rabbit IgG (nRIgG) or anti-α8Ex IgG, a function-blocking polyclonal raised in a similar manner, had no discernable effect on neurite outgrowth. Thus, α6 and α3, presumably as heterodimers with β1 (see Fig. 3B), mediate outgrowth on laminin-1. The identity of the β1-class receptor(s) involved in CG neuron outgrowth on merosin remains unclear.
Figure 7.
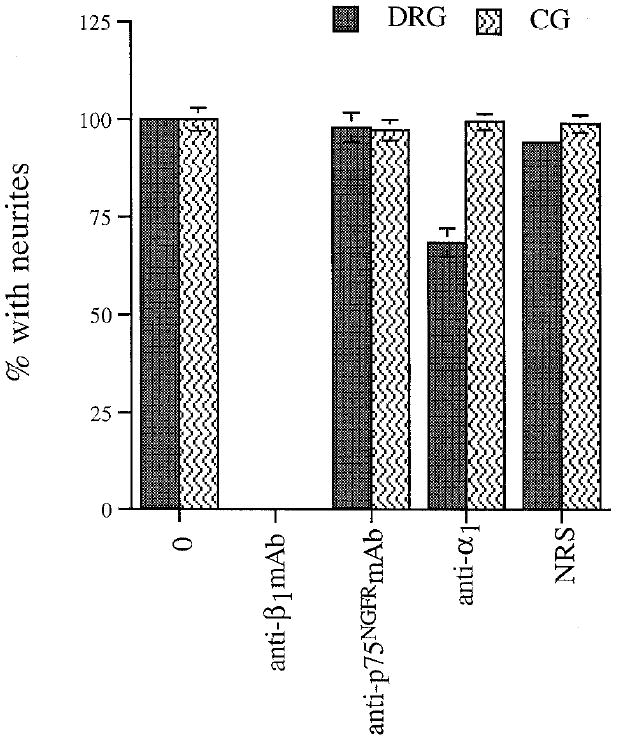
Anti-α1 antibodies fail to block outgrowth of CGs, but reduce DRG outgrowth on laminin-1. Dissociated E7 DRGs and CGs were plated on laminin-1 and allowed to extend neurites for 4–5 hr (DRGs) or 8–10 hr (CGs) in the presence of no antibodies (0), anti-β1 mAb (W1B10, 50 μg/ml), anti-p75NGFRmAb (mc 192, 50 μg/ml), anti-α1, serum (1:25), or control serum (NRS, 1:25). The degree of outgrowth reduction observed in the presence of the anti-α1 serum is very similar to that reported by Tomaselli et al. (1993) for human DRG cultured in the presence of a monoclonal anti-α1 antibody. Values represent mean ± SD of at least four determinations from at least two separate experiments, each performed in duplicate.
Antibodies to the α1 subunit do not inhibit CG neurite outgrowth on laminin-1
In order to determine whether the α1 integrin subunit is involved in CG neurite outgrowth, we performed outgrowth experiments in the presence of a polyclonal antibody raised to chicken α1. Parallel experiments comparing outgrowth of CG and dorsal root ganglion (DRG) neurons were performed, as DRG neurite outgrowth on laminin-1 is sensitive to anti-α1 antibodies (Tomaselli et al., 1993). As illustrated in Figure 7, control antibodies had no effect on the percentage of DRG or CG neurons bearing neurites, but anti-β1 abolished neurite outgrowth of both cell types. In the presence of the anti-α1 serum, 33% fewer DRG neurons extended neurites on laminin-1; this degree of reduction is very similar to that reported previously for embryonic mammalian DRG neurons (Tomaselli et al., 1993). In contrast, there was no effect of anti-α1 on the percentage of CG neurons which developed neurites. Therefore, we conclude that the function of α1, presumably in association with β1, is not required for CG neurite outgrowth on laminin-1.
Discussion
We draw three major conclusions from the work presented here. First, a laminin isoform is prominently expressed in the chick CG nerve pathway during the period of axon extension in vivo. Second, two β1-class integrins, α6β1 and α3β1, mediate neurite outgrowth of CG neurons on laminin-1 substrates in vitro. Third, α6β1 and α3β1 are expressed on the surface of CG cell bodies and axons, both in vivo and in vitro, and are therefore in an appropriate position to mediate interactions of these axons with laminin during axonogenesis.
Laminin is present in the ciliary nerve pathway
We have visualized E8 CG nerve projections by inserting DiI crystals into the CG and allowing the nerves to be labeled in an anterograde manner. Ciliary and choroid nerve branches were observed to exit the ganglion and immediately enter the choroid layer of the eye, through which they course until they contact their targets. These anatomical observations are consistent with schematic and written descriptions of the ganglion and its projections reported previously (Marwitt et al., 1971; Landmesser and Pilar, 1974).
In double-labeling experiments performed on cryostat sections, we have found that axon bundles in the choroid are surrounded by anti-laminin-1 immunoreactivity at E7-8, towards the end of CG neuron extension (Fig. 1D,E). While we cannot be certain that these particular fibers originate in the CG, anti-laminin-1 immunoreactivity is also present along the pathway of bona fide ciliary nerve, (based on tracing directly from the CG) at E6.5, when CG axons are actively growing (Fig. 5). The monoclonal antibody INO (for “inhibitor of neurite outgrowth”), which recognizes a laminin-heparin sulfate proteoglycan complex (Matthew and Patterson, 1983; Chiu, et al., 1986) was also immunoreactive with material in the choroid. Tissue-specific localization of the INO antigen has been correlated with a unique functional neurite outgrowth-promoting activity that can be inhibited by the antibody in vivo (Sandrock and Matthew, 1987a,b). Thus, the spatiotemporal expression patterns of laminin and the INO antigen suggest that a laminin may support CG outgrowth in vivo.
CG neurons extend neurites on two isoforms of laminin
We found that the neurite outgrowth responses of dissociated CG neurons to substrate-bound laminin-1 and merosin are similar but distinct, extending earlier work of Engvall et al. (1992). Antibodies to laminin-1 specifically inhibited neurite outgrowth on laminin-1 substrates but not on merosin, while the anti-merosin serum blocked outgrowth on merosin, but not on laminin-1. This is the first report that cellular interactions with a merosin preparation can be inhibited by an anti-merosin antibody. This control is important because merosin associates tightly with heparin, which in turn binds several ECM proteins and growth factors whose presence might influence neurite outgrowth (Rouslahti, 1989; Vaca et al., 1989).
Receptor expression in vitro and in vivo
Using integrin subunit-specific antibodies, we find that CG neurons express α1 (probably α1β1), α3β1 and α6β1 during the period of axonogenesis and pathfinding. We failed to detect expression of α2 or α7 on these cells by immunoblot or immunohistochemical analysis, but we cannot rule out the possibility that either of these subunits is expressed at low levels, or is present in a form not recognized by our antibodies. β1, α6 and α3-like immunoreactivities all appear to be associated with CG neuron cell bodies and axons, and these axons are found in close apposition to a laminin-rich matrix. Therefore these receptors appear to be in position to mediate interactions with a laminin isoform which may result in neurite extension. The relevance of the finding that α1 is expressed by these neurons (see also Duband et al., 1992) is unclear at the moment. Since anti-α1 antibodies failed to perturb ciliary ganglion neuron outgrowth on laminin-1, α1 (presumably dimerized with β1) may function as a receptor for collagen, but not laminin-1 on these neurons (Turner et al., 1989; Syfrig et al., 1991). Alternatively, it may associate with a different β-subunit to yield a yet unidentified receptor.
Anti-integrin antibodies reduce CG neurite outgrowth in vitro
In our neurite outgrowth experiments, anti-α6 and anti-α3 antibodies each reduced the percentage of CG neurons extending neurites on laminin-1. These results add to the growing body of evidence implicating α6β1 and α3β1 in neuronal interactions with laminins (de Curtis and Reichardt, 1993; Tomaselli et al., 1993). However, these receptors seem to mediate distinct ligand interactions in different cellular and functional contexts. For example, although α3Aβ1 and α6Aβ1 are capable of mediating cell adhesion to placental laminins (containing primarily merosin; Delwel et al., 1994) and anti-α3 antibodies inhibit sensory neuron outgrowth on merosin (Tomaselli et al., 1993), we have been unable to interfere with CG neurite extension on merosin with anti-α3 or anti-α6 antibodies. Moreover, there are conflicting reports concerning the ability of α3β1 to mediate interactions with laminin-1 (cf. Gehlsen et al., 1992; Weitzman et al., 1993). Our findings may provide additional examples of cell type-specific receptor function such as that identified for α2β1, a laminin-1 receptor in some cells, but not in others (Elices and Hemler, 1989). Complexity in receptor function may arise from alternative splicing of the cytoplasmic (to generate A and B forms) or extracellular domains (Xl and X2) of α6 and α3, or from cellular activation (e.g., Tamura et al., 1991; Ziober et al., 1993; Dewel et al., 1994). We have not determined which splice forms of α3 or α6 are present in CG neurons, except that the α3 population includes α3A.
Furthermore, reports on receptor binding specificity are largely based on the results of cell adhesion assays, but a receptor which mediates adhesion may not engage in cell-substratum interactions which favor neurite outgrowth. This point is illustrated in a study of retinal neurons (Neugebauer et al., 1991), whose adhesion and neurite outgrowth responses to thrombospondin appear to be mediated by different receptors, demonstrating that these processes are distinct, even on the same substrate. Apparent differences in the role of α6β1 and α3β1 in attachment and neurite outgrowth on laminin-1 or merosin may reflect a similar type of functional distinction. Indeed, it has often been suggested that dynamic interactions, such as those observed during periods of growth cone motility, require that the cell adhere less strongly (cf. Gundersen, 1987). In light of the apparently low avidity of α3β1-ligand interactions, perhaps it is not surprising that we find a role for α3β1 in neurite outgrowth by CG neurons and DRG neurons (Tomaselli et al., 1993) on laminin-1, even though its ability to mediate attachment to laminin-1 is debatable.
Clearly, we have been unable to account for all of the outgrowth response to laminin-1 by blocking the activities of α6β1 and α3β1. We have considered three explanations for this finding. First, these experiments were performed in the presence of crude eye extract, added to support neuron survival (Nishi and Berg, 1981). This extract contains integrin subunits (C. D. Weaver, unpublished observation) which could compete with surface receptors for antibody binding. This explanation seems unlikely, however, since neurons cultured in the presence of β1-integrin-depleted eye extract were no more sensitive to the presence of anti-integrin antibodies than those cultured with crude extract (not shown). Second, the titer of the anti-α6Ex and anti-α3Ex polyclonals may be too low to bind all the surface receptors, leaving some available to mediate outgrowth. To address this possibility, we affinity purified anti-α6Ex and used it in the neurite outgrowth assay. In dose–response assays, its maximal effect on outgrowth was not significantly greater than that observed using IgG preparations (not shown). Thus it appears that sufficient levels of α6-specific antibody are present to block all surface receptors in these assays. However, we cannot exclude the possibility that the affinity of either polyclonal antibody may be too low to compete efficiently with ligand interactions. Third, it is possible that a heterodimer whose expression we did not detect is actually expressed and mediates outgrowth. In the case of α7, for example, the monoclonal antibody we used may only recognize a subset of the identified isoforms (Ziober et al., 1993). Alternatively, yet another, previously unidentified β1 heterodimer may be involved. Consistent with this idea is the recent report that a new integrin, α9β1, has been purified and binds the E8 fragment of laminin-1 in a solid phase receptor assay (Palmer et al., 1993; Forsberg et al., 1994).
Ultimately, it is important to understand how the ability of CG neurons to extend neurites on laminin-1 is developmentally regulated. Given the apparently complex set of receptors required for outgrowth on laminin-1 substrates in vitro, a simple model might be that β1 subunit function may be regulated at the time of synapse formation (Neugebauer and Reichardt, 1991). The expression and/or function of individual receptor subunits, or their splice forms may also be regulated. Models such as these can now be tested.
Acknowledgments
We thank Eva Engvall, Josef Syfrig, Mats Paulsson, Z. Z. Bao, Alan Horwitz, William Matthew, Anthony Frankfurter, and Salvatore Carbonetto for generous gifts of antibodies, Richard Hynes for providing chick α3 cDNA, Karla Neugebauer for providing thrombocyte extract, and Kristine Venstrom for generating the anti-α2 antibody. We also thank Barbara Varnum-Finney for instruction on the use of the CCD and for helpful discussions and Michael DeFreitas, Kristine Venstrom, Frances Lefcort, and Ulrich Müller for thoughtful suggestions and/or helpful comments on the manuscript. This work was supported by USPHS Grant NS 19090. C.D.W. was supported by NIH Vision Training Grant EY07120. L.F.R. is an investigator of the Howard Hughes Medical Institute.
References
- Bao ZZ, Lakonishok M, Kaufman S, Horwitz AF. Alpha 7 beta 1 integrin is a component of the myotendinous junction on skeletal muscle. J Cell Sci. 1993:579–589. doi: 10.1242/jcs.106.2.579. [DOI] [PubMed] [Google Scholar]
- Bayne EK, Anderson MJ, Fambrough DM. Extracellular matrix organization in developing muscle: correlation with acetylcholine receptor aggregates. J Cell Biol. 1984:1486–1501. doi: 10.1083/jcb.99.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby JL, Lilien J, Reichardt LF. Identification of the major proteins that promote neuronal process outgrowth on Schwann cells in vitro. J Cell Biol. 1988;107:353–361. doi: 10.1083/jcb.107.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy B, Reichardt LF. Chick integrin alpha V subunit molecular analysis reveals high conservation of structural domains and association with multiple beta subunits in embryo fibroblasts. Biochemistry. 1990;29:10191–10198. doi: 10.1021/bi00496a006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Klienman H, Martin GR, Meneguzzi G, Paulsson M, Sanes J, Timpl R, Tryggvason K, Yamada Y, Yurchenco P. A new nomenclature for the laminins. Matrix Biol. 1994:209–211. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Chandler CE, Parsons LM, Hosang M, Shooter EM. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J Biol Chem. 1984;259:6882–6889. [PubMed] [Google Scholar]
- Chen HJ, Sedat JW, Agard DA. Manipulation, display and analysis of three dimensional biological images. In: Pawley J, editor. The handbook of biological confocal microscopy. Madison, WI: IMR; 1989. [Google Scholar]
- Chiu AY, Matthew WD, Patterson PH. A monoclonal antibody that blocks the activity of a neurite regeneration-promoting factor: studies on the binding site and its localization in vivo. J Cell Biol. 1986;103:1383–1398. doi: 10.1083/jcb.103.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F, Lee MR. Reversible developmental change in the ability of ciliary ganglion neurons to extend neurites in culture. J Neurosci. 1982;2:424–430. doi: 10.1523/JNEUROSCI.02-04-00424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin MD, Grover AK, Jung CY. Determination of the molecular weight of neuronectin, a conditioned medium-derived, substrate-binding neurite-extension factor: comparison with laminin using radiation-inactivation analysis. J Neurosci. 1986;6:1553–1559. doi: 10.1523/JNEUROSCI.06-06-01553.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis I, Reichardt LF. Function and spatial distribution in developing chick retina of the laminin receptor alpha 6 beta 1 and its isoforms. Development. 1993;118:377–388. doi: 10.1242/dev.118.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis I, Quaranta V, Tamura RN, Reichardt LF. Laminin receptors in the retina: sequence analysis of the chick integrin alpha 6 subunit. Evidence for transcriptional and posttranslational regulation. J Cell Biol. 1991;113:405–416. doi: 10.1083/jcb.113.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwel GO, de MA, Hogervorst F, Jaspars LH, Fles DL, Kuikman I, Lindblom A, Paulsson M, Timpl R, Sonnenberg A. Distinct and overlapping ligand specificities of the alpha 3A beta 1 and alpha 6A beta 1 integrins: recognition of laminin isoforms. Mol Biol Cell. 1994;5:203–215. doi: 10.1091/mbc.5.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband JL, Belkin AM, Syfrig J, Thiery JP, Koteliansky VE. Expression of alpha 1 integrin, a laminin-collagen receptor, during myogenesis and neurogenesis in the avian embryo. Development. 1992;116:585–600. doi: 10.1242/dev.116.3.585. [DOI] [PubMed] [Google Scholar]
- Elices MJ, Hemler ME. The human integrin VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc Natl Acad Sci USA. 1989;86:9906–9910. doi: 10.1073/pnas.86.24.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E, Davis GE, Dickerson K, Ruoslahti E, Varon S, Manthorpe M. Mapping of domains in human laminin using monoclonal antibodies: localization of the neurite-promoting site. J Cell Biol. 1986:2457–2465. doi: 10.1083/jcb.103.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E, Earwicker D, Day A, Muir D, Manthorpe M, Paulsson M. Merosin promotes cell attachment and neurite outgrowth and is a component of the neurite-promoting factor of RN22 schwannoma cells. Exp Cell Res. 1992;198:115–123. doi: 10.1016/0014-4827(92)90156-3. [DOI] [PubMed] [Google Scholar]
- Forsberg E, Ek B, Engstrom A, Johansson S. Purification and characterization of integrin alpha 9 beta 1. Exp Cell Res. 1994;213:183–190. doi: 10.1006/excr.1994.1189. [DOI] [PubMed] [Google Scholar]
- Gehlsen KR, Sriramarao P, Furcht LT, Skubitz AP. A synthetic peptide derived from the carboxy terminus of the laminin A chain represents a binding site for the alpha 3 beta 1 integrin. J Cell Biol. 1992;117:449–459. doi: 10.1083/jcb.117.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanz S. Primer of biostatistics. New York: McGraw-Hill; 1992. [Google Scholar]
- Goodman SL, Deutzmann R, von der Mark K. Two distinct cell-binding domains in laminin can independently promote nonneuronal cell adhesion and spreading. J Cell Biol. 1987;105:589–598. doi: 10.1083/jcb.105.1.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SL, Aumailley M, von der Mark H. Multiple cell surface receptors for the short arms of laminin: alpha 1 beta 1 integrin and RGD-dependent proteins mediate cell attachment only to domains III in murine tumor laminin. J Cell Biol. 1991;113:931–941. doi: 10.1083/jcb.113.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Aletta JM, Rukenstein A, Green SH. PC12 pheochromocytoma cells: culture, nerve growth factor treatment, and experimental exploitation. Methods Enzymol. 1987;147:207–216. doi: 10.1016/0076-6879(87)47111-5. [DOI] [PubMed] [Google Scholar]
- Gundersen RW. Response of sensory neurites and growth cones to patterned substrata of laminin and fibronectin in vitro. Dev Biol. 1987;121:423–431. doi: 10.1016/0012-1606(87)90179-5. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- Hayashi Y, Haimovich B, Reszka A, Boettiger D, Horwitz A. Expression and function of chicken integrin beta 1 subunit and its cytoplasmic domain mutants in mouse NIH 3T3 cells. J Cell Biol. 1990;110:175–184. doi: 10.1083/jcb.110.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey T, Levine M. Divergent homeo box proteins recognize similar DNA sequences in Drosophila. Nature. 1988;332:858–861. doi: 10.1038/332858a0. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Lander AD, Fujii DK, Reichardt LF. Laminin is associated with the “neurite outgrowth-promoting factors” found in conditioned media. Proc Natl Acad Sci USA. 1985;82:2183–2187. doi: 10.1073/pnas.82.7.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L, Pilar G. Synapse formation during embryogenesis on ganglion cells lacking a periphery. J Physiol (Lond) 1974;241:715–736. doi: 10.1113/jphysiol.1974.sp010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L, Pilar G. Interactions between neurons and their targets during in vivo synaptogenesis. Fed Proc. 1978;37:2016–2022. [PubMed] [Google Scholar]
- Lefcort F, Venstrom K, McDonald JA, Reichardt LF. Regulation of expression of fibronectin and its receptor, alpha 5 beta 1, during development and regeneration of peripheral nerve. Development. 1992;116:767–782. doi: 10.1242/dev.116.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivo I, Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc Natl Acad Sci USA. 1988;85:1544–1548. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M, Engvall E, Ruoslahti E, Longo FM, Davis GE, Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983;97:1882–1890. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwitt R, Pilar G, Weakly JN. Characterization of two ganglion cell populations in avian ciliary ganglia. Brain Res. 1971;25:317–334. doi: 10.1016/0006-8993(71)90441-0. [DOI] [PubMed] [Google Scholar]
- Matthew WD, Patterson PH. The production of a monoclonal antibody that blocks the action of a neurite outgrowth-promoting factor. Cold Spring Harbor Symp Quant Biol. 1983;2:625–631. doi: 10.1101/sqb.1983.048.01.066. [DOI] [PubMed] [Google Scholar]
- Mobley WC, Schenker A, Shooter EM. Characterization and isolation of proteolytically modified nerve growth factor. Biochemistry. 1976;15:5543–5552. doi: 10.1021/bi00670a019. [DOI] [PubMed] [Google Scholar]
- Müller U, Bossy B, Venstrom K, Reichardt LF. Integrin alpha 8 beta 1 promotes attachment, cell spreading and neurite outgrowth on fibronectin. Mol Biol Cell. 1995;6:433–448. doi: 10.1091/mbc.6.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer KM, Reichardt LF. Cell-surface regulation of beta 1-integrin activity on developing retinal neurons. Nature. 1991;350:68–71. doi: 10.1038/350068a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer KM, Emmett CJ, Venstrom KA, Reichardt LF. Vitronectin and thrombospondin promote retinal neurite outgrowth: developmental regulation and role of integrins. Neuron. 1991;6:345–358. doi: 10.1016/0896-6273(91)90244-t. [DOI] [PubMed] [Google Scholar]
- Nishi R, Berg DK. Dissociated ciliary ganglion neurons in vitro: survival and synapse formation. Proc Natl Acad Sci USA. 1977;74:5171–5175. doi: 10.1073/pnas.74.11.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi R, Berg DK. Two components from eye tissue that differentially stimulate the growth and development of ciliary ganglion neurons in cell culture. J Neurosci. 1981;1:505–513. doi: 10.1523/JNEUROSCI.01-05-00505.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer EL, Ruegg C, Ferrando R, Pytela R, Sheppard D. Sequence and tissue distribution of the integrin alpha 9 subunit, a novel partner of beta 1 that is widely distributed in epithelia and muscle. J Cell Biol. 1993;123:1289–1297. doi: 10.1083/jcb.123.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF, Tomaselli KJ. Extracellular matrix molecules and their receptors: functions in neural development. Annu Rev Neurosci. 1991;14:531–570. doi: 10.1146/annurev.ne.14.030191.002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989;264:13369–13372. [PubMed] [Google Scholar]
- Sandrock AJ, Matthew WD. Identification of a peripheral nerve neurite growth-promoting activity by development and use of an in vitro bioassay. Proc Natl Acad Sci USA. 1987a;84:6934–6938. doi: 10.1073/pnas.84.19.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrock AJ, Matthew WD. An in vitro neurite-promoting antigen functions in axonal regeneration in vivo. Science. 1987b;237:1605–1608. doi: 10.1126/science.3306923. [DOI] [PubMed] [Google Scholar]
- Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Syfrig J, Mann K, Paulsson M. An abundant chick gizzard integrin is the avian alpha 1 beta 1 integrin heterodimer and functions as a divalent cation-dependent collagen IV receptor. Exp Cell Res. 1991;194:165–173. doi: 10.1016/0014-4827(91)90349-y. [DOI] [PubMed] [Google Scholar]
- Tamura RN, Cooper HM, Collo G, Quaranta V. Cell type-specific integrin variants with alternative alpha chain cytoplasmic domains. Proc Nat1 Acad Sci USA. 1991;88:10183–10187. doi: 10.1073/pnas.88.22.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin—a glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- Tomaselli KJ, Reichardt LF, Bixby JL. Distinct molecular interactions mediate neuronal process outgrowth on non-neuronal cell surfaces and extracellular matrices. J Cell Biol. 1986:2659–2672. doi: 10.1083/jcb.103.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli KJ, Doherty P, Emmett CJ, Damsky CH, Walsh FS, Reichardt LF. Expression of beta 1 integrins in sensory neurons of the dorsal root ganglion and their functions in neurite outgrowth on two laminin isoforms. J Neurosci. 1993;13:4880–4888. doi: 10.1523/JNEUROSCI.13-11-04880.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DC, Flier LA, Carbonetto S. Identification of a cell-surface protein involved in PC12 cell-substratum adhesion and neurite outgrowth on laminin and collagen. J Neurosci. 1989;9:3287–3296. doi: 10.1523/JNEUROSCI.09-09-03287.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca K, Stewart SS, Appel SH. Identification of basic fibroblast growth factor as a cholinergic growth factor from human muscle. J Neurosci Res. 1989;23:55–63. doi: 10.1002/jnr.490230108. [DOI] [PubMed] [Google Scholar]
- Venstrom K, Reichardt L. Beta 8 integrins mediate interactions of chick sensory neurons with laminin-1, collagen IV, and fibronectin. Mol Biol Cell. 1995;6:419–431. doi: 10.1091/mbc.6.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman JB, Pasqualini R, Takada Y, Hemler ME. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading, and homotypic cell aggregation. J Biol Chem. 1993;268:8651–8657. [PubMed] [Google Scholar]
- Ziober BL, Vu MP, Waleh N, Crawford J, Lin CS, Kramer RH. Alternative extracellular and cytoplasmic domains of the integrin alpha 7 subunit are differentially expressed during development. J Biol Chem. 1993;268:26773–26783. [PubMed] [Google Scholar]


