Abstract
Theoretical models for lexical access to visual objects have been based mainly on adult data. To investigate the developmental aspects of object recognition and lexical access in children, a large-scale functional MRI (fMRI) study was performed in 283 normal children ages 5−18 using a word–picture matching paradigm in which children would match an aurally presented noun to one of two pictures (line drawings). Using group Independent Component Analysis (ICA), six task-related components were detected, including (a) the posterior superior temporal gyrus bilaterally; (b) the fusiform, inferior temporal, and middle occipital gyri bilaterally; (c) the dorsal aspect of the inferior frontal gyrus bilaterally, the left precuneus, the left superior/middle temporal gyrus, and the anterior cingulate; (d) the right medial fusiform gyrus; (e) a left-lateralized component including the inferior/middle frontal, middle temporal, medial frontal, and angular gyri, as well as the thalamus and the posterior cingulate; and (f) the ventral/anterior aspect of the inferior frontal gyrus bilaterally. Increased activation associated with age was seen in the components (b) and (d) (ventral visual pathway) for object recognition, and (c) and (f) likely associated with semantic maintenance and response selection. Increased activation associated with task performance was seen in components (b) and (d) (ventral visual pathway) while decreased activation associated with task performance was seen in component (f) (ventral/anterior inferior frontal gyrus). The results corroborate the continued development of the ventral visual pathway throughout the developmental period.
Keywords: Independent Component Analysis, visual perception, auditory perception, semantics, age factors
INTRODUCTION
The ability to understand that verbal labels reference objects in the environment is a major building block for language acquisition in young children. In addition to physical objects, children are able to recognize pictorial representations of objects from an early age and will attend to pictures during storybook activities and other situations during which words refer to pictured objects and actions. Children as young as 3 years old understand that pictures have a stable meaning (Apperly et al., 2004), as opposed to written words, about which many young children incorrectly change their judgment according to context (Bialystok, 2000). Stability of meaning with pictures has been hypothesized to stem from children's experiences with producing pictures of their own and attaching meaning to them (Adi-Japha et al., 1998; Richert and Lillard, 2002). The distribution of perceptual and functional features in objects generated by young children appears to be similar to adults (Hughes et al., 2005; McRae and Cree, 2002).
There is a dependent relationship between ability of young children to represent object features and their ability to label (Jones and Smith, 2005). Likewise, children's ability to generalize labels to multiple exemplars of the same item, and even to over-extend the use of those labels to different objects, is influenced by the perceptual similarities among the objects (Landau and Leyton, 1999; Samuelson and Smith, 2005; Smith et al., 1996b). However, children's representation of object attributes can differ from adults, which is another potential influence on over-extension of both object categories and the labels used to represent the objects (Abecassis et al., 2001).
To investigate the developmental aspects of object encoding and association with verbal labels, we performed a large-scale functional MRI (fMRI) study in normal children aged 5−18 using a word–picture matching paradigm. Children matched an aurally presented noun to one of two line drawings; the control task consisted of meaningless stimuli in both the auditory and visual domains. This particular task is designed to tap word-level (lexical) skills that should be fully established by the age of 5 years, and is part of a battery of fMRI language development paradigms we have used to investigate age dependencies in the neural substrates at the lexical and sentential level in the developing brain.
While the ideal paradigm would be to incorporate trials involving only meaningful auditory and visual stimuli, in order to dissociate the neuronal circuitry involved with each, such a design would have been impracticable in this study population (with very young children) due to its length. To overcome this limitation we used group Independent Component Analysis (ICA) (Calhoun et al., 2001; Schmithorst and Holland, 2004) as a method for analyzing the data, with a recently developed method (Schmithorst et al., 2006) of investigating developmental changes in the components. ICA provides the advantage of being able to separate out cortical networks involved with different cognitive components without needing to know the hemodynamic response functions a priori. The resulting components detected through ICA can be compared with models, such as the one we will propose here, to understand their contribution to cognitive tasks, and investigated for any effects related to age or task performance (Schmithorst et al., 2006). Under a limited set of assumptions (Calhoun et al., 2004; Duann et al., 2002), ICA components can be associated with separate cognitive components and be held to represent distinct processing modules in the neural substrates supporting a given task. The limitation in this approach is that the specific cognitive function (e.g. visual association, motor function, etc.) associated with each ICA component must be inferred from prior knowledge about the functionality of the detected cortical regions.
Various models that link visual representations (e.g., words, pictures) to verbal codes exist in the literature. We propose a simple model of this process (displayed in Fig. 1), which incorporates aspects of broader models (e.g. Beeson and Hillis, 2001; Ramus, 2001) that have sought to explain visual–verbal associations. However, our model has been modified from these to more closely mirror the aspects of the specific task used in this study. In previous models, recognition of a visual object as a known object permits access to a semantic lexicon that associates meaning with the object. Current models consider the semantic system to be available to both verbal and nonverbal processing for meaning (Beeson and Hillis, 2001; Ramus, 2001). This general semantic system provides the bridge for comparing meaning from the visual domain with that coming from the parallel auditory–verbal stream. At this level, task specific demands are represented by the need to compare the semantic content of the verbal and visual representations. This process may also require both attention and memory as the verbal label is held in memory during the semantic comparison with the pictures. Once the child has established a match, a response is initiated.
Figure 1.
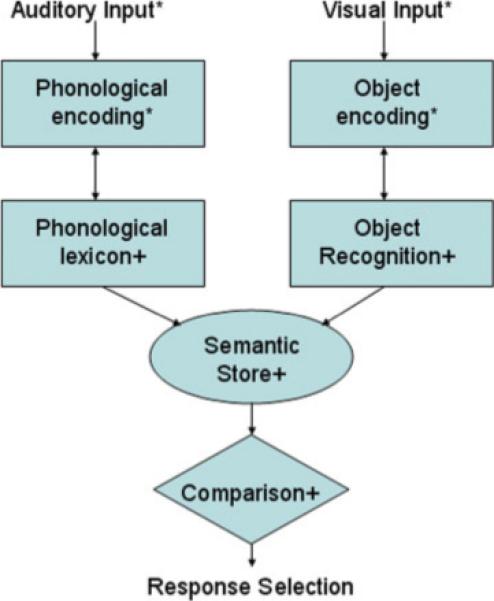
Model of task requiring semantic association between pictures and spoken words. * indicates processes requiring sustained attention to stimuli. + indicates processes requiring working memory. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We will compare the physiological components identified through ICA with the theoretical constructs of the model we have proposed, with specific attention to developmental aspects. ICA should identify independent components related to auditory and visual processing streams. Other components should reflect the cognitive processes of memory, attention, and semantic association. Since we also predict improved performance with age, due to the developing neuronal circuitry, task performance will be incorporated as a covariate in all developmental analyses.
We make several predictions related to development of maturing neuronal circuitry. Based on previous studies (Gathers et al., 2004; Kovacs, 2000; Kovacs et al., 1999), we predict significant developmental changes in the ventral visual areas associated with object recognition. The object recognition component of the model involves attention to and encoding of the visual representation (in the primary visual cortex), with subsequent object encoding (in the ventral visual pathway). Functional imaging studies (e.g., Gerlach et al., 1999; Kellenbach et al., 2005) make a distinction between object processing in general vs. recognition of an object as real. The latter may require comparison to stored representations of objects previously encountered (Gerlach et al., 1999). Neuroimaging evidence suggests developmental changes in the functional organization of the ventral visual processing stream (used for object recognition) in children aged 5−11 years (Gathers et al., 2004). While children of 9−11 years showed face-preferential activation near the classical fusiform face area, children of 5−8 years old showed face-preferential activation in the posterior ventral visual processing pathway. An anterior shift in the ventral processing stream locus with age was hypothesized for visual processing tasks, which require skilled perceptual differentiation of objects. Developmental changes have also been shown in visual object perception. Adults possess greater ability to recognize degraded objects than children, and also display less perceptual interference (Bernstein et al., 2005). Infants and young children often over-extend their use of object labels to include other objects that are similar in shape (Clark, 1973; Gershkoff-Stowe and Smith, 1997, 2004), such as calling a piece of irregularly hardened clay a “dinosaur” (Samuelson and Smith, 2005). This, along with the tendency of children to organize shape information differently than adults (Abecassis et al., 2001), suggests how children represent objects conceptually changes with age.
We do not predict a similar developmental change in the auditory to phonological transcoding branch of the model. The words selected for use in the study are typically in the receptive vocabulary of children at about 2 years of age and should be familiar in meaning to even our youngest subjects at the age of five. We predict, however, that activation associated with semantic processing will show a developmental change due to changes in both the conceptualization of visual objects and refinements in the understanding of word meanings. For objects, young children can show over-extension of object classifications, which can result in the generation of erroneous or tangential semantic information in association with those objects. Likewise, word meanings undergo both over- and under-extensions of meaning until the child converges on an adult-like conceptual representation. We therefore predict increased activation in areas used for semantic processing as the word meanings attached to phonological strings become increasingly familiar, semantically refined, and even overlearned with age. As such, children's processing of words should reflect an expansion of word processing from simply phonological to increasingly semantic with age. Such a shift from phonological to semantic representations has been seen previously in children aged 5−11 (Dewhurst and Robinson, 2004).
MATERIALS AND METHODS
Subjects were recruited by advertisement following Institutional Review Board approval for the study. Advertisements were broadcast on local television stations and newspapers, and posted in primary care clinics within the hospital and in the metropolitan area. Healthy siblings of patients at our hospital were invited to participate. All potential participants were pre-screened by questionnaire and structured telephone interview for any conditions (such as the presence of orthodontic braces), which would prevent an MRI scan from being acquired, prior to being scheduled for further examinations.
All children underwent a brief neurological examination administered by a pediatric neurologist and subjects who did not test within the normal range were excluded. At the neurological examination, our inclusion criteria were reviewed with the subject/family, and subjects who were failing to maintain a C-average in school or had a positive history for neurologic or psychiatric disease, or a previous clinically indicated MRI scan, were excluded. Subjects were likewise excluded if they were under treatment (including medication) for any neurological or psychiatric conditions. These criteria excluded any subjects being treated with psychoactive drugs such as atypical stimulants, anti-depressants, or serotonin reuptake inhibitors. Additional exclusion criteria included learning disability, head trauma with loss of consciousness, pregnancy, and birth at 37 weeks gestational age or earlier.
The structural MR images obtained for each subject during this study were read by a pediatric neuroradiologist. Abnormal findings were reported to the subjects’ primary physicians through an IRB-approved process. One boy had an abnormal anatomical MRI image (arteriovenous malformation) and was therefore subsequently excluded from the study. Other subjects were excluded for the following reasons: 2 were greater than the 95th percentile for weight; and 4 were greater than the 95th percentile for height. Two children had previously had an MRI scan of the brain for clinical indications (one left facial droop and one back pain and tingling).
Two hundred eighty-three children (143 boys, 140 girls) were successfully scanned using the picture–word matching task as part of this study following informed consent by the child's parent or guardian (assent was also obtained from subjects 8 years and older). All subjects were native monolingual English speakers. A complete age and gender breakdown of the subjects is detailed in Table I. Two hundred and sixty-five of the subjects were right-handed, 16 were left-handed, and 2 were ambidextrous according to the Edinburgh (Oldfield, 1971) test for handedness. The racial/ethnic background of the subjects was 248 Caucasian, 23 African-American, 2 Asian, 3 Hispanic, 1 Native American, 6 Multi-Ethnic. Neurocognitive assessment and testing was done under the supervision of a board certified pediatric neuropsychologist. All subjects received the Wechsler Preschool and Primary Scale of Intelligence, Revised (WPPSI-R); Wechsler Intelligence Scale for Children, Third Edition (WISC-III) or the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III); and the Oral and Written Language Scales (OWLS) (Carrow-Woolfolk, 1996). Mean age = 12.2 ± 3.67 years (range = 5.2−18.9 years.); Mean Wechsler Full-Scale IQ = 111.0 ± 13.62 (range = 74−146); Mean OWLS = 107.3 ± 14.14 (range = 72−149). Three subjects had a Full-Scale IQ < 80 and five subjects had an OWLS score < 80; they were not excluded from the study population as there was no documented history of learning disability, and the frequency of such findings was not greater than expected given the total sample size.
TABLE I.
The study population, task performance (mean % correct ± std. dev.), and reaction time in seconds (mean ± std. dev.), broken down by age and gender
| Age | No. of M | No. of F | Performance (M) | Performance (F) | Reaction time (M) | Reaction time (F) |
|---|---|---|---|---|---|---|
| 5 | 6 | 4 | 74 ± 6 | 81 ± 10 | 2.32 ± 0.18 | 2.36 ± 0.18 |
| 6 | 7 | 9 | 76 ± 9 | 83 ± 8 | 2.37 ± 0.19 | 2.38 ± 0.08 |
| 7 | 9 | 9 | 81 ± 8 | 84 ± 3 | 2.36 ± 0.15 | 2.38 ± 0.17 |
| 8 | 12 | 10 | 82 ± 7 | 82 ± 9 | 2.34 ± 0.12 | 2.38 ± 0.16 |
| 9 | 12 | 11 | 89 ± 4 | 86 ± 7 | 2.35 ± 0.16 | 2.40 ± 0.09 |
| 10 | 12 | 10 | 86 ± 5 | 87 ± 9 | 2.42 ± 0.84 | 2.45 ± 0.07 |
| 11 | 15 | 7 | 90 ± 4 | 87 ± 8 | 2.44 ± 0.08 | 2.43 ± 0.05 |
| 12 | 15 | 14 | 89 ± 3 | 90 ± 5 | 2.42 ± 0.12 | 2.33 ± 0.18 |
| 13 | 14 | 17 | 88 ± 6 | 88 ± 7 | 2.34 ± 0.14 | 2.40 ± 0.13 |
| 14 | 10 | 10 | 86 ± 8 | 85 ± 6 | 2.27 ± 0.13 | 2.19 ± 0.24 |
| 15 | 9 | 7 | 88 ± 7 | 91 ± 3 | 2.35 ± 0.14 | 2.43 ± 0.07 |
| 16 | 9 | 9 | 90 ± 3 | 90 ± 6 | 2.31 ± 0.16 | 2.40 ± 0.11 |
| 17 | 10 | 12 | 90 ± 5 | 92 ± 2 | 2.38 ± 0.11 | 2.40 ± 0.10 |
| 18 | 3 | 11 | 89 ± 4 | 89 ± 7 | 2.31 ± 0.17 | 2.43 ± 0.11 |
MRI scans were obtained using a Bruker 3T Medspec (Bruker Medizintechnik, Karlsruhe, Germany) imaging system. An MRI-compatible audiovisual system was used for presentation of the stimuli as well as a movie during the preparation (e.g. shimming) and acquisition of the whole-brain anatomical scans. Details of the techniques used to obtain fMRI data from younger children, as well as the success rates, are given in Byars et al. (2002). EPI-fMRI scan parameters were the following: TR/TE = 3,000/38 ms; BW = 125 kHz; FOV = 25.6 × 25.6 cm2; matrix = 64 × 64; slice thickness = 5 mm. Twenty-four slices were acquired, covering the entire cerebrum. One hundred ten scans were acquired (the first 10 were discarded to allow the spins to reach relaxation equilibrium) for a total scan time of 5 min 30 s. Techniques detailed elsewhere (Byars et al., 2002) were used to acclimatize the subjects to the MRI procedure and render them comfortable inside the scanner. An elastic strap was attached to either side of the head coil apparatus by means of Velcro strips and stretched over the subjects’ foreheads in order to minimize head motion. In addition to the fMRI scans, whole-brain T1-weighted MP-RAGE scans were acquired for anatomical coregistration.
The fMRI scan paradigm consisted of a 30 s on–off block design, with five active and five control epochs. All stimuli were presented using MacStim (White Ant Software, Melbourne, Australia). Stimuli were presented at a rate of one every 3 s for 10 stimuli during each epoch. During the “active” epochs, the subjects matched visually-presented, line-drawn (without color), pictures to aurally-presented nouns. Subjects were shown two pictures, one on the left side, and one on the right side of the viewing field. Simultaneously to the video presentation, subjects heard a noun corresponding to one of the pictures. Subjects pressed a button in the left or right hand, corresponding to the side of the video screen on which the picture was presented that matched the aurally presented noun.
During the “control” epochs, subjects were shown a pair of “abstract” pictures side-by-side on the video screen. By pressing the left or right button they selected the target picture which they learned prior to the scanning session. Each abstract picture pair was accompanied by a tone, presented to parallel the auditory presentation of words during the active epochs. Examples of active and control stimuli are shown in Figures 2 and 3, respectively. All responses were recorded and stored for further analysis. All subjects met a criterion of P < 0.05 for responding correctly at a greater than chance level.
Figure 2.
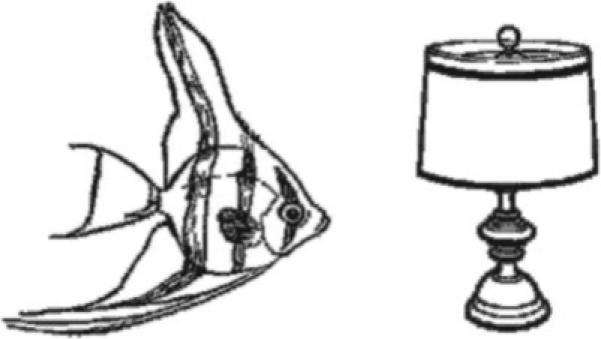
Example of a picture pair from the active phase of the picture-word matching task (subjects hear “lamp”). Figure reprinted with permission of the authors.
Figure 3.
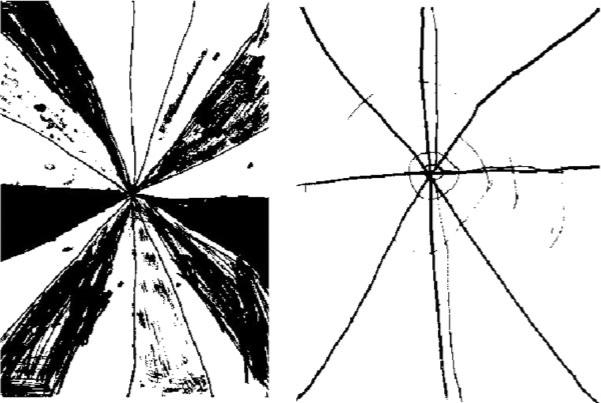
Example of a picture pair from the control phase of the picture-word matching task (the left image is the correct response, learned by the subjects prior to the scanning session; subjects hear constant frequency tone of 1 s duration). Figure reprinted with permission of the authors.
Data was processed using in-house software written in IDL (Research Systems Inc., Boulder, CO). Nyquist ghosts and geometric distortion due to B0 field inhomogeneity were corrected during reconstruction using a multi-echo reference scan (Schmithorst et al., 2001). Data was corrected for subject motion using a pyramid iterative algorithm (Thevenaz and Unser, 1998). Datasets were deemed to have an acceptable amount of subject motion if the median voxel displacement at the corner of the image space (corresponding to the maximum voxel displacement in each frame based on the parameters from the coregistration algorithm) was <3 mm (3/4 of a voxel), which roughly corresponds to <2 mm or half of a voxel (or less) in the center of the brain. The fMRI data was subsequently transformed into stereotaxic space (Talairach and Tournoux, 1988) using a linear affine transformation, previously validated for the age range in our study (Muzik et al., 2000; Wilke et al., 2002).
The subject-wise concatenation approach (Calhoun et al., 2001), shown to provide superior performance to other proposed methods (Schmithorst and Holland, 2004) was used for the group ICA analysis. The ICA time courses were subsequently analyzed via the data-driven analysis techniques outlined in Schmithorst et al. (2006). We refer the reader to the above-cited papers (Calhoun et al., 2001; Schmithorst et al., 2006) for a detailed description and only provide a summary here.
Hierarchical agglomerative clustering (Himberg et al., 2004) was used to validate the found components (due to the stochastic nature of the ICA algorithm). Task-related components were determined by testing the average (complex) Fourier component at the on–off task frequency from the time courses associated with each component for a significant difference (i.e. two-dimensional distance) from zero using a one-sample t-test using a threshold of P = 0.01 (Bonferroni-corrected for the 52 found components). There were 13 components deemed to be “task-related” using the aforementioned criteria; however, 7 components were rejected as related to motion artifacts via visual determination by the investigators (data not shown). For the 6 remaining components, a one-sample t-test was performed on the individual IC maps on a voxelwise basis; a stringent criterion of t > 12 was used, corresponding to P < 1e–10, Bonferroni-corrected for multiple voxel comparisons. For reference, a standard GLM analysis (Worsley and Friston, 1995) was also performed using the on–off task reference function as the regressor of interest; a random-effects analysis was performed using the same threshold as used for the IC maps.
The ICA associated time courses were tested for effects of age (covarying for performance) and performance (covarying for age) using the methods detailed in Schmithorst et al. (2006). Both the hypothesis-driven method (using a GLM-like analysis on the time courses) and the data-driven method (finding an optimized reference time course (Schmithorst et al., 2006)) were used. The data-driven optimized time course is then tested for task-relatedness by correlating with the on–off task reference function. The difference between the two approaches is that the first method tests for significant associations between subject age or performance and shape of the time courses, as determined by an a priori criterion (correlation with the task reference), while the second method finds a maximum-likelihood descriptor of any age or performance effect present, and then analyzes it a posteriori.
RESULTS
All subjects performed at a better than chance level with P < 0.05 (corresponding to 32 correct responses out of 50). A slightly better performance was seen in girls compared to boys, only significant at the level of a trend (U = 1.37, P = 0.085, Mann-Whitney -test). A significant effect was seen for subject age (Spearman's R = 0.43, P < 0.001) on performance. However, no significant effect was seen for Full-Scale IQ (Spearman's R = 0.037, P > 0.5), and the effect for OWLS scores just reached significance (Spearman's R = 0.12, P < 0.05). For mean reaction times, no significant effect was seen due to gender ( = 1.21, P > 0.1), age (Spearman's R = −0.007, P > 0.9), or OWLS (Spearman's R = 0.09, P > 0.1); a barely significant effect was seen due to IQ (Spearman's R = 0.12, P < 0.05). There was a highly significant correlation (Spearman's R = 0.27, P < 1e–5) between reaction time and performance, with greater mean reaction time correlating with improved performance. This effect retained significance when subject age was included as a covariate (partial R = 0.35, P < 1e–5); and was significant both for younger (ages < 12 years) children (Spearman's R = 0.35, P < 1e–4) and older (ages ≥ 12 years) children (Spearman's R = 0.27, P < 1e–3). A breakdown of reaction times and performance by age and gender are given in Table I.
In addition to the 283 children with acceptable data, datasets from 17 children were rejected due to excessive motion, while datasets from 50 children were rejected due to failure to perform at a better than chance level. In the accepted data, there was a significant correlation of motion with subject age (Spearman's R = −0.22, P < 0.001) and task performance (Spearman's R = 0.21, P < 0.001), as would be expected. In the children 7 years and younger, however, the mean amount of motion (computed according to the method described in the Materials and Methods section) was only ∼1 mm at the corner of the image space; thus corresponding to less than 1 mm in the brain. Thus motion artifacts are unlikely to contribute significantly to our results. In addition, ICA is able to separate out spurious “activation” related to small amounts of motion in separate components, as was the case here.
The six task-related ICA components were ordered (leading to lagging) according to the phase of the average Fourier component relative to the reference on–off time course and are displayed in Figure 4. The average time courses associated with each component are plotted in Figure 5. All components were found in 24 or 25 of the ICA runs (e.g. the cluster size was 24 or 25), indicating a high degree of reliability. The GLM analysis (see Fig. 6) resulted in the detection of the same general regions as the ICA analysis, including the fusiform gyrus and middle occipital gyrus bilaterally, and Broca's area and the middle temporal gyrus in the left hemisphere; the lack of complete agreement between ICA and GLM results for group analyses has been discussed previously (Schmithorst and Brown, 2004).
Figure 4.
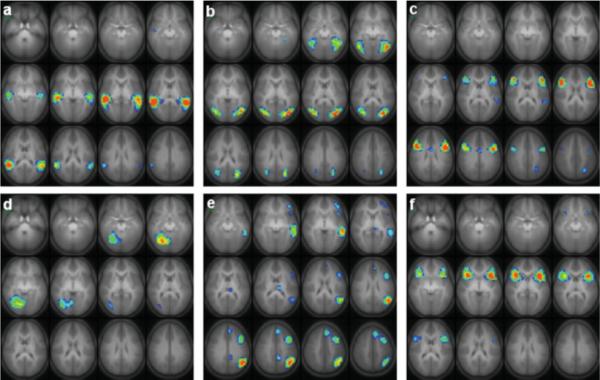
Six task-related independent components found from group analysis of 283 children aged 5−18 performing the task of word-picture matching (matching the correct choice of two pictures to an aurally presented noun). Slice ranges (Talairach coordinates): (a) Z = −25 to +30 mm; (b) Z = −20 to +35 mm; (c) Z = −15 to +40 mm; (d) Z = −25 to +30 mm; (e) Z = −10 to +45 mm; (f) Z = −25 to +30 mm. All images in radiologic orientation. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 5.
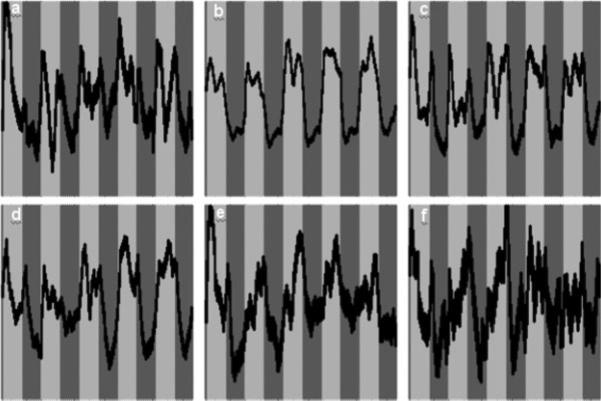
Time courses (bands ± 1σ) associated with the independent components shown in Figure 4.
Figure 6.
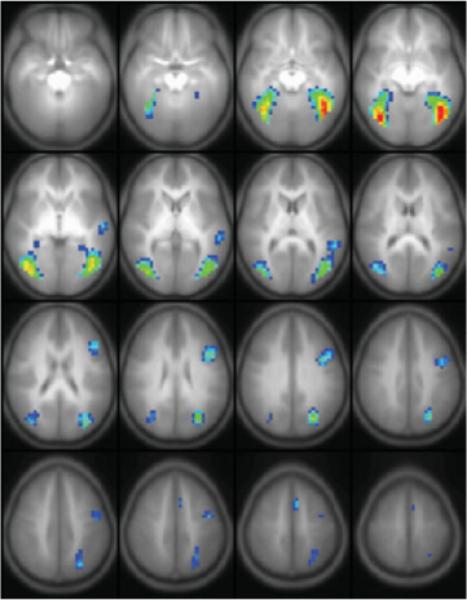
Results from a random-effects GLM analysis of 283 children aged 5−18 performing the task of word-picture matching. Slice range: Z = −20 to +55 mm (Talairach coordinates). All images in radiologic orientation. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Components were found with activation in the most posterior part of the superior temporal gyrus bilaterally (Fig. 4a); the fusiform gyrus, inferior temporal gyrus, and middle and superior occipital gyrus bilaterally (Fig. 4b); the inferior frontal gyrus bilaterally (BA 44/45), as well as the anterior cingulate and left superior temporal gyrus and precuneus (Fig. 4c); the right fusiform gyrus (Fig. 4d); a left-lateralized component including the middle frontal gyrus, middle temporal gyrus, medial frontal gyrus, inferior frontal gyrus, left angular gyrus, thalamus, and posterior cingulate (Fig. 4e); and the inferior frontal gyrus bilaterally (BA 47) (Fig. 4f). A summary of the activation foci (determined by finding the peak in the IC source map) for each component is listed in Table II. All averaged time courses were correlated with the task on–off reference function with a correlation coefficient of R > 0.3 or greater.
TABLE II.
Activation foci (Talairach coordinates) for each of the ICA components displayed in Figure 4
| Component | BA | Region | X, Y, Z |
|---|---|---|---|
| 1a | 22 | R. superior temporal gyrus | 46, −41, 10 |
| 22 | L. superior temporal gyrus | −54, −45, 10 | |
| 1b | 37 | R. fusiform/inferior temporal gyrus | 42, −61, −5 |
| 37 | L. fusiform/inferior temporal gyrus | −42, −65, −5 | |
| 19 | R. middle/superior occipital gyrus | 34, −69, 10 | |
| 19 | L. middle/superior occipital gyrus | −34, −73, 10 | |
| 1c | 21/22 | L. middle/superior temporal gyrus | −54, −41, 15 |
| 44/45 | R. inferior frontal gyrus | 42, 19, 20 | |
| 44/45 | L. inferior frontal gyrus | − 42, 7, 25 | |
| 24 | Anterior cingulate | −6, −1, 30 | |
| 7 | L. precuneus | −26, −57, 40 | |
| 1d | 19/37 | R. fusiform/lingual | 18, −65, −10 |
| 1e | 10/46 | L. inferior/middle frontal gyrus | −38, 43, 0 |
| 21 | L. middle temporal gyrus | −54, −33, 0 | |
| L. thalamus | −6, −17, 15 | ||
| 39 | L. angular gyrus | −46, −57, 30 | |
| 31 | Posterior cingulate | −10, −45, 35 | |
| 44/9 | L. inferior/middle frontal gyrus | −38, 11, 40 | |
| 8 | L. medial frontal gyrus | −6, 31, 45 | |
| 1f | 47 | R. inferior frontal gyrus | 30, 15, 0 |
| 47 | L. inferior frontal gyrus | −34, 15, 0 |
For the analysis of developmental trends using the a priori criterion of fit to the on–off task reference function, the components in Figure 4d (R = 0.17, corrected P < 0.05) and Figure 4f (R = 0.17, corrected P < 0.05) showed a significant increase (Bonferroni-correcting for the multiple comparisons across the six components) of task-relatedness with age (covarying for performance). The components in Figure 4b (R = 0.12, uncorrected P < 0.05) and Figure 4c (R = 0.13, uncorrected P < 0.03) showed only nominally significant age-related effects, which would not retain significance after correcting for the multiple comparisons across the six components. Using the data-driven approach of finding a maximum-likelihood reference time course, the null distribution was found via Monte Carlo simulation as described in Schmithorst et al. (2006) (e.g. substituting random numbers for the independent variables). The components in Figure 4b,d (corrected P < 0.01) and Figure 4c (corrected P < 0.025) displayed a significant effect due to subject age when compared with the null distribution; the component in Figure 4f was significant at the level of a trend (corrected P < 0.075). Correlating the reference time course with the on–off task reference function for those components verified that the age-related components were also task-related (R > 0.45 for all components, P < 1e–5). The results indicate increasing use of the regions shown in Figure 4b (lateral fusiform, inferior temporal, and middle/superior occipital), Figure 4c (inferior frontal; BA 44/45), Figure 4d (right medial fusiform), and Figure 4f (bilateral inferior frontal gyrus; BA 47) with subject age. The ICA methodology used is not able to detect changes in lateralization within a single component, which is generated from all subjects. However, ICA can be used to detail the source of changes in laterality via isolating the specific lateralized components where they occur. The slightly left-lateralized component in Figure 4c increases in activation with age, as does the right-lateralized component in Figure 4d.
For analysis of performance effects, using the a priori criterion of fit to the on–off task reference function, the components in Figure 4b (partial R = 0.18, corrected P < 0.02) and Figure 4f (partial R = −0.19, corrected P < 0.01) displayed a significant effect related to performance (covarying for age); the component in Figure 4d (partial R = 0.14, uncorrected P < 0.02) displayed a nominally significant effect. Using the data-driven approach, the components in Figure 4b (corrected P < 0.001), Figure 4d (corrected P < 0.05), and Figure 4f (corrected P = 0.03) showed significant performance-related effects (covarying for age). Correlating the optimal data-driven reference function with the on–off task reference function, the components in Figure 4b (R = 0.86, corrected P < 1e–6), Figure 4d (R = 0.53, corrected P < 1e–6), and Figure 4f (R = −0.54, corrected P < 1e–6) also showed significant task-related effects. The results indicate a positive association of activation in the regions shown in Figures 4b,d (visual association areas), and a negative association of activation in the regions shown in Figure 4f (bilateral inferior frontal gyrus; BA 47) with task performance.
Both analyses of age and performance effects display an overall greater sensitivity of the data-driven approach over the hypothesis-driven approach; however, the hypothesis-driven approach may display greater sensitivity for an individual component, indicating the complementarity of the two techniques. The hypothesis-driven approach, incorporating an a priori time course, will yield greater sensitivity when the time course can be specified with sufficient accuracy; however, the data-driven approach is useful when there is insufficient prior knowledge, yielding information otherwise inaccessible via a hypothesis-driven approach. Scatterplots of the fit of the individual time courses to the optimized reference time course found from the data-driven analyses for the four components displaying an age effect are displayed in Figure 7. Performance effects were removed via stepwise regression and the data was separated out for boys and girls; a two-way ANCOVA with age and sex as the independent variables revealed neither a significant main effect for sex nor an age-by-sex interaction in any of the four components.
Figure 7.
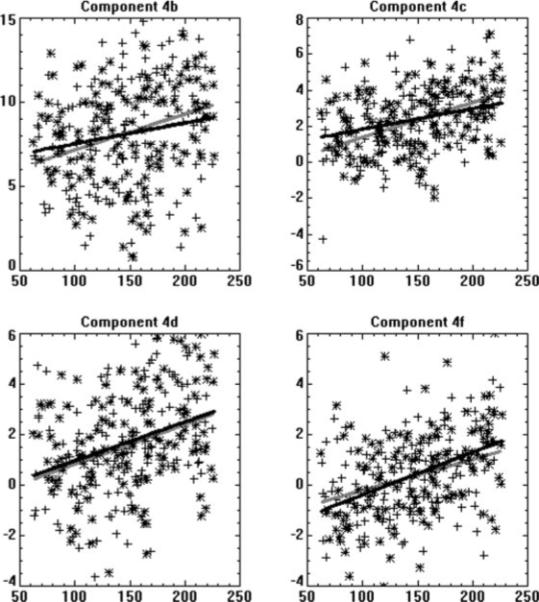
Scatterplots of the goodness-of-fit parameters (T-scores) of regression of the associated IC time courses from each subject to the reference time course found from the data-driven developmental ICA analyses components for the components displayed in Figures 4b,c,d, and f (Y axis) as a function of subject age in months (X axis); effects of task performance were removed via stepwise regression. Legend: Boys = plus signs, gray line; Girls = asterisks; black line.
DISCUSSION
Interpretation of ICA Results
Spatial ICA reveals “chronoarchitectonically identified areas” (Bartels and Zeki, 2004) or functionally connected regions. The presence of different cortical regions in the same ICA component implies that they are active at the same time (subject to the limitations of the temporal resolution of the fMRI data acquisition); otherwise, the ICA algorithm would have separated them out into different components. ICA provides different information than other techniques such as diffusion tensor imaging (DTI), or functional connectivity maps based on resting-state fMRI data (Lowe et al., 1998). The existence of strong anatomical connections between two cortical regions is a necessary but not sufficient condition for them to appear in the same task-related ICA component; if a given cognitive task recruits only one of those regions then there will be a component separated by ICA containing only that region.
In addition, under certain minimal assumptions (e.g. Calhoun et al., 2004; Duann et al., 2002) the spatial independence of the ICA components may be equated with their modularity, linking each ICA component found to a specific cognitive task. This is subject to the caveat that, due to the finite number of voxels, spatial independence of the components found cannot be assumed with absolute certainty. This limitation is likely not significant in the current study, however, due to the excellent signal-to-noise ratio provided by the very large number of subjects.
Neural Correlates of the Task Components
As ICA is a data-driven analysis, the precise roles of the found components cannot be determined directly. However, the components can be compared with the cognitive model to determine areas of convergence, based on previous neuroanatomical and neuroimaging findings. The cognitive model for the task employed posits initial independent processing of visual and auditory information, both of which are linked to a general semantic store. Shared access to semantic information allows the participant to compare the visual and auditory information to match a target pictures with a spoken word. Although our model of the task begins with sensory input in the auditory and linguistic modalities, our task design controlled for these processes by including both auditory and visual processes in the control task. Therefore, it is not surprising that primary visual and primary auditory cortex were not seen as ICA components. Note also that a motor response was also executed in both the experimental and control conditions, so that the motor response component was not task-related. The remaining elements of the cognitive model were expected to generate task-related ICA components.
Visual Processing
Our model posits a transcoding of visual input to object perception and object recognition. These cognitive processes should draw heavily on the ventral visual stream and should be independent of functions related to auditory processing. We also expected these processes to show age-related changes, based on findings on the development of object recognition in children (Gershkoff-Stowe and Smith, 2004; Samuelson and Smith, 1999). Two components (Fig. 4b,d) identified through the ICA analysis fit this description.
These components in Figure 4b,d (ventral visual pathway) were the most strongly correlated with age, and had the strongest associations with task performance. Human visual development has been thought previously to be completed at an early age (e.g. Bornstein et al., 1976; Dobson and Teller, 1978; Kellman and Spelke, 1983; Nelson and Horowitz, 1987). However, more recent research indicates maturation of neuronal circuitry used for visual processing may extend well into later childhood (Burkhalter, 1993; Burkhalter et al., 1993). Integration of contours against a dense noise field was shown to exhibit significant development in children between 5 and 14 years of age (Kovacs et al., 1999). Our data supports the interpretation of a longer developmental time course for the simple visual processing that is a prerequisite for object recognition.
Previous studies that have involved various forms of object recognition from line drawings have reported bilateral activation of posterior inferior structures, including lateral occipital cortex and fusiform gyrus (Gerlach et al., 1999; Kellenbach et al., 2005; Shen et al., 1999; Simons et al., 2003). The activation described in these studies corresponds most closely with that seen in Figure 4b. The ventral visual pathway (the “what” pathway), includes the lateral fusiform and inferior temporal gyrus (BA 37), and extends into the middle occipital gyrus (BA 19). In our study, the component shown in Figure 4b is the most task-related (e.g. most highly correlated with the task reference function). This component is somewhat left-lateralized, corresponding to previous studies showing left-dominant activation in visual form recognition of unnamable pictures (object encoding in our model) (Shen et al., 1999). A recent fMRI study (Simons et al., 2003) has shown differential activation in the right and left fusiform gyrus to the presentation of novel or repeated items. However, when the repeated item is represented from a different view, activation is reduced only for the left fusiform gyrus, suggesting recognition of the picture as the same object as previously seen. This further suggests that this area represents the object as a unique item regardless of the specific perceptual features viewed (object recognition in our model). Additionally, activity in the left fusiform modulated according to whether a real word or nonword was presented. This manipulation varied the semantic content associated with a phonological signal, suggesting the left fusiform in particular supports linkages between the perceptual representation of recognized objects and the semantic store. The left fusiform gyrus has also been recruited when subjects performed a context verification task of the semantic fit of target words within preceding sentential contexts (Hoenig and Scheef, 2005). The component in Figure 4b, however, is left-lateralized rather than left localized, as the information regarding visual form available from the right fusiform also needs to be used.
The ICA revealed a second, separate component involving the fusiform gyrus, which is almost completely right-lateralized (Fig. 4d). The existence of this independent component may have been masked in other studies because of its close spatial proximity with areas active in Figure 4b, which has been previously described. However, a similar region in the right fusiform was found in a study of children who viewed faces and pictured objects (Gathers et al., 2004), although this same area was not seen in the adult subjects also studied. This suggests a developmental effect that is consistent with the age correlation for Figure 4d in the present study. The Gathers et al. study did not require semantic mapping in order to complete the task; children merely indicated when they saw an image. However, the children may not have been able to suppress semantic associations for items either. Other studies have suggested that the right anterior fusiform may contribute to semantic tasks such as naming (Etard et al., 2000), and bilateral fusiform activation was seen for writing words as compared to writing letters of the alphabet (Beeson et al., 2003). Intracranial recordings of the human brain also differentiate two areas within the basal temporal lobe, with the more anterior regions showing preferential responses to the semantic context of words whereas the posterior portion responded equally to printed words and nonwords (Nobre et al., 1994). The nature of the associated time course for component 4d also suggests some involvement in semantic processing. This component indicates greater activation intensity during the last three active epochs as compared to the first two. This may be due to the subjects’ recognition over the sequential blocks that the pairs of pictures were quite distinct in terms of their semantic content (e.g., “cat” vs. “door”), resulting in the possibly more “efficient” strategy of using the right fusiform gyrus for naming (Etard et al., 2000), although further research will be necessary to verify this hypothesis.
Phonological Processes
Several of the ICA components involve cortical regions classically associated with language functions (Fig. 4a,c,e,f). Our model makes a distinction between processes related to the phonological encoding of incoming acoustic stimuli and the recognition of the string of phonological units as a real word, prior to accessing the meaning of that word. Processing of speech sounds has been associated with regions in the posterior superior temporal lobe (e.g. Belin et al., 2002; Binder et al., 2004; Jancke et al., 2002; Specht and Reul, 2003). Several of our ICA components showed activation in this general region (Fig. 4a,c,e). However, they differed notably in their lateralization and extent. Previous research has described bilateral regions of activation similar to that shown in Figure 4a (posterior superior temporal gyrus). However, this component shows a right lateralization, which is not consistent with the well established pattern of left lateralization for speech sounds (Jancke et al., 2002; Specht and Reul, 2003). Instead, the smaller regions of superior temporal activation seen in Figure 4c show that left lateralization is more consistent with the role of phonetic processing. The modest negative association with task performance of this component supports this interpretation. We would expect less effortful processing of speech sound sequences, and therefore a weaker BOLD response, to be associated with highly recognizable speech sound sequences that comprise familiar words.
The anterior activation in Figure 4c (inferior frontal) may relate to cognitive processes associated with phonetic processing. The inferior frontal region, including the insula and inferior frontal gyrus, activates when subjects are asked to remember and make decisions about both words and nonmeaningful phonological stimuli (Chee et al., 2004; Dehaene-Lambertz et al., 2005; Gelfand and Bookheimer, 2003; Jacquemot et al., 2003; LoCasto et al., 2004; Seghier et al., 2004; Shivde and Thompson-Schill, 2004). Similar to activation in the left temporal region, we might also expect less effort devoted to phonological maintenance would be associated with familiar words, resulting in a negative correlation with the task performance. The idea that this activation corresponds to the maintenance demands of these studies is further supported by a TMS study showing impairment only when subjects were instructed to remember stimuli for later use vs. when they made judgments concerning those stimuli (Nixon et al., 2004). However, we know from previous studies that these areas are differentially active to phonological vs. semantic (LoCasto et al., 2004; Seghier et al., 2004; Shivde and Thompson-Schill, 2004) and phonological vs. acoustic demands (Dehaene-Lambertz et al., 2005; LoCasto et al., 2004), so that the contribution of this region appears to be independent from earlier and later acoustic and semantic components of our task model.
These same studies that have identified activation in inferior frontal regions associated with phonological rehearsal also typically identify left lateralized activation in parietal cortex (e.g. Dehaene-Lambertz et al., 2005; Gold and Buckner, 2002; Jacquemot et al., 2003; Logie et al., 2003; Ravizza et al., 2004). These are often attributed to the workings of the phonological loop within the working memory system (Baddeley, 1986), although it has recently been suggested that this area might be more effectively attributed to increased attentional focus on phonological information (Chein et al., 2003). We note that the area of activation described in these studies tends to be somewhat lower than the left parietal activation found in Figure 4c with our sample of children. It is more consistent with the location of such activations in Figure 4e. Note that there is spatial overlap between the parietal activation (as well as frontal activation) in Figure 4c,e, which would prevent description of these as separate components without the use of ICA. The idea that these overlapping regions indeed make separate contributions to the task is supported by the fact that they are differentially correlated with the task demands. Whereas the activation seen in Figure 4c is negatively correlated with task performance and positively with age, the activation in Figure 4e displays no age or performance-related effects.
Verbal Semantic Processes
The most left-lateralized independent component, in Figure 4e, is likely associated with generation of semantic representations of the words that are heard. This would correspond to access to the semantic store specifically from the phonological input stream, rather than activation of an amodal semantic store. The activation pattern strongly resembles that found in verbal semantic processing and fluency tasks (e.g. Binder et al., 1995; Gaillard et al., 2003; Holland et al., 2001) and utilizes the left-hemispheric classical Wernicke-Geschwind network of Broca's area and Wernicke's area, including the inferior parietal lobule recently shown to be connected to these areas (Catani et al., 2005). The network of Broca's area and the inferior parietal lobule has previously shown to be involved in semantic decision tasks (Bullmore et al., 2000; Seghier et al., 2004; Shivde and Thompson-Schill, 2004). This network may also include an element of working memory. The frontal component and inferior parietal lobule may recruit the phonological loop as described earlier. Their presence in this ICA component may involve the storing of speech-based information (Awh et al., 1996; Schumacher et al., 1996; Smith et al., 1996a) using a covert articulatory mechanism (Baddeley, 1992) during the comparison of verbal and picture stimuli.
As previously mentioned, the left-lateralized component of Figure 4e (classical language areas) did not display a significant correlation either with age or with task performance. This may be a consequence of using stimuli selected because they are familiar to young children. However, previous pediatric fMRI studies (e.g. Gaillard et al., 2003; Holland et al., 2001) have found that this network is largely established by early childhood even for tasks where children can be expected to improve with age. In contrast, additional studies have also shown that the functional activation maps may become more left-lateralized with age for some aspects of language (e.g. Holland et al., 2001; Plante et al., 2006). However, these findings of change with age may reflect the contributions of other components that show age-related effects (e.g. the inferior frontal areas shown in Fig. 4c,f) but overlap with those shown in Figure 4e.
In addition to the lack of a significant age correlation for activation seen in Figure 4e (classical language areas), there was also no significant association with performance. This may be explained by how children of different ages processed the stimuli. In adult subjects, the ability to name pictures while “bypassing” Wernicke's and Broca's areas has been shown (Etard et al., 2000), and it was hypothesized that a direct transcoding from visual object to phonologic representations (“automatic labeling”) may occur in this case. Our older subjects may have employed such a strategy, given the vocabulary items should have been highly familiar to these individuals. In contrast, our younger subjects may have been employing a strategy which relied more heavily on the traditional language regions seen in Figure 4e. Such a difference in how the task was performed could have cancelled any simple age-related activation effect.
Semantic Integration and Selection
In this experiment, the semantic information from the pictures and from the aurally presented noun is evaluated and matched in order to determine the final interpretation of which button to press. The component in Figure 4a involves the most posterior aspect of the superior temporal gyrus (BA 22) bilaterally and we propose that it is used for the semantic integration (Friederici et al., 2003), necessary for the comparison of different types of information in order to achieve a final interpretation. This component (with bilateral activation in posterior BA 22) was also found during ICA analysis of narrative comprehension in children (Schmithorst et al., 2006) with a delay in rise of the time course relative to the beginning of the narration; this was interpreted as BA 22 being involved with the higher-order process of integrating the sentences into the larger context of the narrative. This component is likely not involved to the same extent during the control task, since the control task only involves matching of a visual form, rather than comparing different types of semantic inputs.
Our task further required children both to make a choice between the pictured items based on semantic similarities between one of the pictures and the verbal label. Therefore, we might expect components related to the semantic selection aspect of the task. Note that the control task also required response selection, but the task demands in this condition had little or no semantic load. First, the child always selected the same item as the target, which appeared randomly on either the right or left side, therefore the job of classifying the correct image as the target was the same every trial. There was no coding of new object information across control trials. Second, the control items were not real-world objects and therefore did not convey general semantic or linguistic associations. Therefore, the critical component present in the semantic task was classifying of either right or left picture as semantic match to the word presented.
We propose that this element of semantically-driven selection is represented by the component seen in Figure 4f. Although this activation clearly overlaps spatially with those seen in Figure 4c,e, the time course differences among these components suggest this area is engaged in multiple functions (cf. Price and Friston (2005)). For component 4f, we propose that the dorsal inferior frontal gyrus is involved in selection among competing alternatives (e.g. the two pictures) from semantic memory, with semantic information maintained in the left temporal lobe, previously shown from lesion studies to be involved in modality-independent semantic representation (Saffran and Schwartz, 1994). This is consistent with the findings of Binder et al. (2004) who showed that activation in the superior temporal gyrus predicted accuracy of identification (was the sound detected), whereas activation in inferior frontal gyrus predicted response times in an auditory identification task (how fast was the decision made about detection). We would also expect that the cognitive substrates for decision-making would show age-related change, as does component 4f. The component in Figure 4f also showed a significant negative correlation with task performance, suggesting that less resources were needed when decisions were relatively easy for the subject to make.
Future Research and Limitations
A limitation of the current study was the utilization of a block-design as opposed to an event-related design, which would allow the distinguishing of correct from error trials, and provide more information on the relevance of each cortical region or network than was possible in the present study. Moreover, while performance was covaried for when analyzing age effects, the presence of error trials in the data still represents a confound. Future fMRI studies utilizing an event-related design may investigate how the use of the various cortical areas and their development with age differ when the stimulus consists of two pictures with disparate as opposed to similar semantic content; and when the pictures consist of easily recognizable stimuli (e.g. “fish”) for which the hypothesized direct object-to-phonological transcoding path is possible, as opposed to less easily recognizable stimuli for which a multitude of interpretations are possible (e.g. a group of circles which could represent “peas”, “pebbles”, or “balls”) and for which greater reliance on semantic decisions would be expected.
Newer, more sophisticated fMRI analysis techniques such as structural equation modeling (SEM) (Solodkin et al., 2004), path analysis (Bullmore et al., 2000), or dynamic causal modeling (DCM) (Mechelli et al., 2003) may also help to further refine current models of information flow in the brain. The ICA results can be used to generate a theoretical (predictive) model, the validity of which may then be tested with these procedures. In addition, future large-scale diffusion tensor imaging (DTI) studies will provide additional insight regarding the development of the relevant neuronal connections and their relevance to language and visual function; previous DTI studies have found increased anisotropy with age in white matter association areas (Schmithorst et al., 2002) and a correlation between frontal and posterior white matter anisotropy and IQ (Schmithorst et al., 2005).
We note that the control condition, designed to cancel out cortical regions related to primary auditory and visual processing, is not strictly necessary for an ICA analysis, as the algorithm will separate out the cortical regions related to different cognitive components. However, failure to include the control period limits the interpretability of results obtained using hypothesis-driven techniques such as GLM analyses (Worsley and Friston, 1995), which may be desirable as a complementary analysis strategy to data-driven approaches such as ICA.
CONCLUSION
A large-scale fMRI study was conducted on a group of over 280 children aged 5−18 performing a word–picture matching task. Using group ICA analysis, separate task-related regions were detected, including the posterior superior temporal gyrus bilaterally; the fusiform, inferior temporal, and middle occipital gyri bilaterally; the inferior frontal gyrus (BA 44) bilaterally and left precuneus; the right medial fusiform gyrus; a left-lateralized component including Broca's area, Wernicke's area, and the angular gyrus; and the ventral/anterior aspect of the inferior frontal gyrus (BA 47) bilaterally.
The ICA procedure was able to identify spatially-overlapping regions that had different time courses and frequently displayed different associations with performance and age. Thus, ICA provides an advantage relative to more traditional analyses that mask such differences. When compared with an a priori model of the cognitive components of the task, the ICA resulted in components that were plausible fits to the model components. The results demonstrate the utility of this statistical approach for extracting cognitively-relevant information from subjects, such as the children studied here, who may not be able to tolerate the number of scan conditions that would be otherwise needed to dissociate the hypothesized cognitive processes.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of Dr. Anna Byars, Ph.D., in the administration of the Wechsler Full-Scale IQ and OWLS tests; and of Drs. Richard Strawsburg, M.D., and Mark Schapiro, M.D., for performing the neurological examinations.
Contract grant sponsor: U.S. National Institute of Child Health and Human Development; Contract grant numbers: R01-HD38578.
REFERENCES
- Abecassis M, Sera MD, Yonas A, Schwade J. What's in a shape? Children represent shape variability differently than adults when naming objects. J Exp Child Psychol. 2001;78:213–239. doi: 10.1006/jecp.2000.2573. [DOI] [PubMed] [Google Scholar]
- Adi-Japha E, Levin I, Solomon S. Emergence of representation in drawing: The relation between kinematic and referential aspects. Cogn Dev. 1998;12:25–51. [Google Scholar]
- Apperly IA, Williams E, Williams J. Three- to four-year-olds’ recognition that symbols have a stable meaning: Pictures are understood before written words. Child Dev. 2004;75:1510–1522. doi: 10.1111/j.1467-8624.2004.00754.x. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of storage and rehearsal in verbal working memory: Evidence from positron emission tomography. Psychol Sci. 1996;7:25–31. [Google Scholar]
- Baddeley AD. Working Memory. Oxford University Press; Oxford: 1986. [Google Scholar]
- Baddeley AD. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The chronoarchitecture of the human brain–natural viewing conditions reveal a time-based anatomy of the brain. Neuroimage. 2004;22:419–433. doi: 10.1016/j.neuroimage.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Beeson PM, Hillis AE. Comprehension and production of written words. In: Chapey R, editor. Language Intervention Strategies in Adult Aphasia. 4th ed Lippincott Williams & Wilkins; Baltimore: 2001. pp. 572–595. [Google Scholar]
- Beeson PM, Rapcsak SZ, Plante E, Chargualaf J, Chung A, Johnson S, Trouard T. The neural substrates of writing: A functional magnetic resonance imaging study. Aphasiology. 2003;17:647–665. [Google Scholar]
- Belin P, Zatorre RJ, Ahad P. Human temporal-lobe response to vocal sounds. Brain Res Cogn Brain Res. 2002;13:17–26. doi: 10.1016/s0926-6410(01)00084-2. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Loftus GR, Meltzoff AN. Object identification in preschool children and adults. Dev Sci. 2005;8:151–161. doi: 10.1111/j.1467-7687.2005.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok E. Symbolic representation across domains in preschool children. J Exp Child Psychol. 2000;86:223–243. doi: 10.1006/jecp.1999.2548. [DOI] [PubMed] [Google Scholar]
- Binder JR, Liebenthal E, Possing ET, Medler DA, Ward BD. Neural correlates of sensory and decision processes in auditory object identification. Nat Neurosci. 2004;7:295–301. doi: 10.1038/nn1198. [DOI] [PubMed] [Google Scholar]
- Binder JR, Rao SM, Hammeke TA, Frost JA, Bandettini PA, Jesmanowicz A, Hyde JS. Lateralized human brain language systems demonstrated by task subtraction functional magnetic resonance imaging. Arch Neurol. 1995;52:593–601. doi: 10.1001/archneur.1995.00540300067015. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Kessen W, Weiskopf S. The categories of hue in infancy. Science. 1976;191:201–202. doi: 10.1126/science.1246610. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Horwitz B, Honey G, Brammer M, Williams S, Sharma T. How good is good enough in path analysis of fMRI data? Neuroimage. 2000;11:289–301. doi: 10.1006/nimg.2000.0544. [DOI] [PubMed] [Google Scholar]
- Burkhalter A. Development of forward and feedback connections between areas V1 and V2 of human visual cortex. Cereb Cortex. 1993;3:476–487. doi: 10.1093/cercor/3.5.476. [DOI] [PubMed] [Google Scholar]
- Burkhalter A, Bernardo KL, Charles V. Development of local circuits in human visual cortex. J Neurosci. 1993;13:1916–1931. doi: 10.1523/JNEUROSCI.13-05-01916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byars AW, Holland SK, Strawsburg RH, Bommer W, Dunn RS, Schmithorst VJ, Plante E. Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. J Child Neurol. 2002;17:885–890. doi: 10.1177/08830738020170122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Pekar JJ, Pearlson GD. Alcohol intoxication effects on simulated driving: Exploring alcohol-dose effects on brain activation using functional MRI. Neuropsychopharmacology. 2004;29:2097–2117. doi: 10.1038/sj.npp.1300543. [DOI] [PubMed] [Google Scholar]
- Carrow-Woolfolk E. Oral and Written Language Scales. American Guidance Service; Circle Pines, MN: 1996. [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chee MW, Soon CS, Lee HL, Pallier C. Left insula activation: A marker for language attainment in bilinguals. Proc Natl Acad Sci USA. 2004;101:15265–15270. doi: 10.1073/pnas.0403703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein JM, Ravizza SM, Fiez JA. Using neuroimaging to evaluate models of working memory and their implications for language processing. J Neurolinguistics. 2003;16:315–339. [Google Scholar]
- Clark EV. What's in a word? On the child's acquisition of semantics in his first language. In: Moore TE, editor. Cognitive Development and the Acquisition of Language. Academic Press; New York: 1973. pp. 65–110. [Google Scholar]
- Dehaene-Lambertz G, Pallier C, Serniclaes W, Sprenger-Charolles L, Jobert A, Dehaene S. Neural correlates of switching from auditory to speech perception. Neuroimage. 2005;24:21–33. doi: 10.1016/j.neuroimage.2004.09.039. [DOI] [PubMed] [Google Scholar]
- Dewhurst SA, Robinson CA. False memories in children. Evidence for a shift from phonological to semantic associations. Psychol Sci. 2004;15:782–786. doi: 10.1111/j.0956-7976.2004.00756.x. [DOI] [PubMed] [Google Scholar]
- Dobson V, Teller DY. Visual acuity in human infants: A review and comparison of behavioral and electrophysiological studies. Vision Res. 1978;18:1469–1483. doi: 10.1016/0042-6989(78)90001-9. [DOI] [PubMed] [Google Scholar]
- Duann JR, Jung TP, Kuo WJ, Yeh TC, Makeig S, Hsieh JC, Sejnowski TJ. Single-trial variability in event-related BOLD signals. Neuroimage. 2002;15:823–835. doi: 10.1006/nimg.2001.1049. [DOI] [PubMed] [Google Scholar]
- Etard O, Mellet E, Papathanassiou D, Benali K, Houde O, Mazoyer B, Tzourio-Mazoyer N. Picture naming without Broca's and Wernicke's area. Neuroreport. 2000;11:617–622. doi: 10.1097/00001756-200002280-00036. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Ruschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: Localizing syntactic and semantic processes. Cereb Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, Grandin CB. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. 2003;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathers AD, Bhatt R, Corbly CR, Farley AB, Joseph JE. Developmental shifts in cortical loci for face and object recognition. Neuroreport. 2004;15:1549–1553. doi: 10.1097/01.wnr.0000133299.84901.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand JR, Bookheimer SY. Dissociating neural mechanisms of temporal sequencing and processing phonemes. Neuron. 2003;38:831–842. doi: 10.1016/s0896-6273(03)00285-x. [DOI] [PubMed] [Google Scholar]
- Gerlach C, Law I, Gade A, Paulson OB. Perceptual differentiation and category effects in normal object recognition: A PET study. Brain. 1999;122(Part 11):2159–2170. doi: 10.1093/brain/122.11.2159. [DOI] [PubMed] [Google Scholar]
- Gershk-offStowe L, Smith LB. A curvilinear trend in naming errors as a function of early vocabulary growth. Cogn Psychol. 1997;34:37–71. doi: 10.1006/cogp.1997.0664. [DOI] [PubMed] [Google Scholar]
- Gershkoff-Stowe L, Smith LB. Shape and the first hundred nouns. Child Dev. 2004;74:1098–1114. doi: 10.1111/j.1467-8624.2004.00728.x. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Hoenig K, Scheef L. Mediotemporal contributions to semantic processing: fMRI evidence from ambiguity processing during semantic context verification. Hippocampus. 2005;15:597–609. doi: 10.1002/hipo.20080. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Hughes D, Woodcock J, Funnell E. Conceptions of objects across categories: Childhood patterns resemble those of adults. Br J Psychol. 2005;96:1–19. doi: 10.1348/000712604X15446. [DOI] [PubMed] [Google Scholar]
- Jacquemot C, Pallier C, LeBihan D, Dehaene S, Dupoux E. Phonological grammar shapes the auditory cortex: A functional magnetic resonance imaging study. J Neurosci. 2003;23:9541–9546. doi: 10.1523/JNEUROSCI.23-29-09541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancke L, Wustenberg T, Scheich H, Heinze HJ. Phonetic perception and the temporal cortex. Neuroimage. 2002;15:733–746. doi: 10.1006/nimg.2001.1027. [DOI] [PubMed] [Google Scholar]
- Jones SS, Smith LB. Object name learning and object perception: A deficit in late talkers. J Child Lang. 2005;32:223–240. doi: 10.1017/s0305000904006646. [DOI] [PubMed] [Google Scholar]
- Kellenbach ML, Hovius M, Patterson K. A pet study of visual and semantic knowledge about objects. Cortex. 2005;41:121–132. doi: 10.1016/s0010-9452(08)70887-6. [DOI] [PubMed] [Google Scholar]
- Kellman PJ, Spelke ES. Perception of partly occluded objects in infancy. Cogn Psychol. 1983;15:483–524. doi: 10.1016/0010-0285(83)90017-8. [DOI] [PubMed] [Google Scholar]
- Kovacs I. Human development of perceptual organization. Vision Res. 2000;40:1301–1310. doi: 10.1016/s0042-6989(00)00055-9. [DOI] [PubMed] [Google Scholar]
- Kovacs I, Kozma P, Feher A, Benedek G. Late maturation of visual spatial integration in humans. Proc Natl Acad Sci USA. 1999;96:12204–12209. doi: 10.1073/pnas.96.21.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau B, Leyton M. Perception, object kind, and object naming. Spat Cogn Comput. 1999;1:1–29. [Google Scholar]
- LoCasto PC, Krebs-Noble D, Gullapalli RP, Burton MW. An fMRI investigation of speech and tone segmentation. J Cogn Neurosci. 2004;16:1612–1624. doi: 10.1162/0898929042568433. [DOI] [PubMed] [Google Scholar]
- Logie RH, Venneri A, Della Sala S, Redpath TW, Marshall I. Brain activation and the phonological loop: The impact of rehearsal. Brain Cogn. 2003;53:293–296. doi: 10.1016/s0278-2626(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- McRae K, Cree GS. Factors underlying category-specific semantic deficits. In: Forde EME, Humphreys G, editors. Category-Specificity in Mind and Brain. Psychology Press; Hove, UK: 2002. pp. 211–250. [Google Scholar]
- Mechelli A, Price CJ, Noppeney U, Friston KJ. A dynamic causal modeling study on category effects: Bottom-up or top-down mediation? J Cogn Neurosci. 2003;15:925–934. doi: 10.1162/089892903770007317. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Juhasz C, Shen C, Chugani HT. Statistical parametric mapping: Assessment of application in children. Neuroimage. 2000;12:538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Horowitz FD. Visual motion perception in infancy: A review and synthesis. In: Salapatek P, Cohen L, editors. Handbook of Infant Perception. Academic Press.; New York: 1987. pp. 123–153. [Google Scholar]
- Nixon P, Lazarova J, Hodinott-Hill I, Gough P, Passingham R. The inferior frontal gyrus and phonological processing: An investigation using rTMS. J Cogn Neurosci. 2004;16:289–300. doi: 10.1162/089892904322984571. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–263. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Plante E, Schmithorst VJ, Holland SK, Byars AW. Sex differences in the activation of language cortex during childhood. Neuropsychologia. 2006;44:1210–1221. doi: 10.1016/j.neuropsychologia.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Functional ontologies for cognition: The systematic definition of structure and function. Cogn Neuropsychol. 2005;22:262–275. doi: 10.1080/02643290442000095. [DOI] [PubMed] [Google Scholar]
- Ramus F. Outstanding questions about phonological processing in dyslexia. Dyslexia. 2001;7:197–216. doi: 10.1002/dys.205. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22:562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Richert RA, Lillard AS. Children's understanding of the knowledge prerequisites of drawing and pretending. Dev Psychol. 2002;38:1004–1015. doi: 10.1037//0012-1649.38.6.1004. [DOI] [PubMed] [Google Scholar]
- Saffran EM, Schwartz MF. Impairment of sentence comprehension. Philos Trans R Soc Lond B Biol Sci. 1994;346:47–53. doi: 10.1098/rstb.1994.0127. [DOI] [PubMed] [Google Scholar]
- Samuelson LK, Smith LB. Early noun vocabularies: Do ontology, category organization and syntax correspond? Cognition. 1999;73:1–33. doi: 10.1016/s0010-0277(99)00034-7. [DOI] [PubMed] [Google Scholar]
- Samuelson LK, Smith LB. They call it like they see it: Spontaneous naming and attention to shape. Dev Sci. 2005;8:182–198. doi: 10.1111/j.1467-7687.2005.00405.x. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Brown RD. Empirical validation of the triple-code model of numerical processing for complex math operations using functional MRI and group independent component analysis of the mental addition and subtraction of fractions. Neuroimage. 2004;22:1414–1420. doi: 10.1016/j.neuroimage.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20:535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Comparison of three methods for generating group statistical inferences from independent component analysis of fMRI data. J Magn Reson Imaging. 2004;19:365–368. doi: 10.1002/jmri.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Plante E. Cognitive modules utilized for narrative comprehension in children: A functional magnetic resonance imaging study. Neuroimage. 2006;29:254–266. doi: 10.1016/j.neuroimage.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy changes with age during childhood: A cross-sectional diffusion tensor imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor MR imaging study. Hum Brain Mapp. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Lauber E, Awh E, Jonides J, Smith EE, Koeppe RA. PET evidence for an amodal verbal working memory system. Neuroimage. 1996;3:79–88. doi: 10.1006/nimg.1996.0009. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, Pegna AJ, Annoni JM, Zimine I, Mayer E, Michel CM, Khateb A. Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum Brain Mapp. 2004;23:140–155. doi: 10.1002/hbm.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Hu X, Yacoub E, Ugurbil K. Neural correlates of visual form and visual spatial processing. Hum Brain Mapp. 1999;8:60–71. doi: 10.1002/(SICI)1097-0193(1999)8:1<60::AID-HBM5>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivde G, Thompson-Schill SL. Dissociating semantic and phonological maintenance using fMRI. Cogn Affect Behav Neurosci. 2004;4:10–19. doi: 10.3758/cabn.4.1.10. [DOI] [PubMed] [Google Scholar]
- Simons JS, Koutstaal W, Prince S, Wagner AD, Schacter DL. Neural mechanisms of visual object priming: Evidence for perceptual and semantic distinctions in fusiform cortex. Neuroimage. 2003;19:613–626. doi: 10.1016/s1053-8119(03)00096-x. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cereb Cortex. 1996a;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Smith LB, Jones SS, Landau B. Naming in young children: A dumb attentional mechanism? Cognition. 1996b;60:143–171. doi: 10.1016/0010-0277(96)00709-3. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Chen E, Small SL. Fine modulation in network activation during motor execution and motor imagery. Cereb Cortex. 2004;14:1246–1255. doi: 10.1093/cercor/bhh086. [DOI] [PubMed] [Google Scholar]
- Specht K, Reul J. Functional segregation of the temporal lobes into highly differentiated subsystems for auditory perception: An auditory rapid event-related fMRI-task. Neuroimage. 2003;20:1944–1954. doi: 10.1016/j.neuroimage.2003.07.034. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. In: Rayport M, editor. Thieme Medical Publishers; New York: 1988. p. 122. [Google Scholar]
- Thevenaz P, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum Brain Mapp. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited–again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]


