Abstract
Activation of δ-opioid receptors (DOR) attenuates anoxic K+ leakage and protects cortical neurons from anoxic insults by inhibiting Na+ influx. It is unknown, however, which pathway(s) that mediates the Na+ influx is the target of DOR signal. In the present work, we found that in the cortex, 1) DOR protection was largely dependent on the inhibition of anoxic Na+ influxes mediated by voltage-gated Na+ channels; 2) DOR activation inhibited Na+ influx mediated by ionotropic glutamate NMDA receptors, but not that by non-NMDA receptors though both played a role in anoxic K+ derangement; and 3) DOR activation had little effect on Na+/Ca2+ exchanger-based response to anoxia. We conclude that, 1) DOR activation attenuates anoxic K+ derangement by restricting Na+ influx mediated by Na+ channels and NMDA receptors, and 2) non-NMDA receptors and Na+/Ca2+ exchangers, though involved in anoxic K+ derangement in certain degrees, are less likely the targets of DOR signal.
Keywords: anoxia, cortex, δ-opioid receptor, K+ homeostasis, Na+ channels, ionotropic glutamate receptor channels
Introduction
Hypoxia and ischemia induce neuronal injury in the brain, which is a leading cause of neurological disability and death. However, the treatment and prevention of hypoxic/ischemic brain injury remains a major medical challenge. The vast majority of the current research directed to finding treatments for hypoxic/ischemic brain injuries focused on the use of Ca2+ channel blockers, glutamate antagonists, antioxidants/free radical scavengers, and some agents that regulate cytokines and other intracellular molecules. However, many proving effective in animal models of stroke demonstrated minor or no efficacy in patients in clinical trials [1, 2]. Therefore, seeking novel approaches to protect neurons from hypoxic/ischemic injury still attracts much attention from both clinicians and scientists. Recent work in our laboratory [3–6] as well as others [7–12] have shown that activation of δ-opioid receptor (DOR) is neuroprotective against hypoxic/ischemic and excitotoxic stress. An increase in extracellular K+ due to K+ efflux is a typical response of the brain to hypoxic/ischemic stress and such derangement of K+ homeostasis is believed to be a crucial factor leading to neuronal injury or death under pathophysiological conditions, such as anoxic/ischemic stress [13–16]. DOR neuroprotection is, at least partially, related to DOR action against the derangement of K+ homeostasis during anoxia/ischemia [17, 18]. Furthermore, we found that DOR-mediated inhibition on Na+ influx constitutes a major mechanism underlying the DOR-protection against anoxic K+ derangement in the cortex because DOR protection against anoxic derangement of K+ homeostasis is largely abolished by low Na+ perfusion [19].
During anoxia/ischemia, massive Na+ enters into neurons [20–24], and the resultant intracellular Na+ accumulation is a major event that severely affects anoxic K+ derangement [19, 25, 26] and excitotoxicity-elicited K+ efflux [27] in neurons. In previous studies, potential routes for Na+ influx during anoxia/ischemia have been proposed, which include at least influx through voltage-gated Na+ channels [22, 28–31], ligand-gated Na+ channels [28, 30, 32] and entry through transporters/exchanges such as the Na+/Ca2+ exchanger [24, 33], albeit debate still exists on this issue. There is, however, no information available at all as to which pathway(s) mediating the Na+ influx is the target of DOR signals.
Several lines of evidence in our past studies have suggested a potential interaction between DOR and Na+ channels. For example, we observed that DOR down-regulation [34] is associated with an up-regulation of voltage-gated Na+ channels [35], and DOR activation attenuates hypoxic dysregulation of Na+ channels [36, 37]. These observations suggest an interactive regulation between DOR signals and Na+ homeostasis. It is very likely that voltage-gated Na+ channels are the major target of DOR signals in the DOR-mediated inhibition of hypoxic Na+ influx and the Na+-based K+ derangement.
Besides voltage-gated Na+ channels, other routes for Na+ influx may also be targeted by DOR. It has been well known that hypoxia/ischemia induces massive release of glutamate into the synaptic cleft [38]. A common feature of ion channel-linked glutamate receptors (including NMDA and non-NMDA receptors) is Na+ permeability [39]. Therefore, glutamate may increase intracellular Na+ concentration ([Na+]i) by activation of ionotropic receptor channels [28, 30, 32, 39]. DOR activation has been shown to prevent the release of glutamate from presynaptic vesicles, depress the amplitudes of stimulus-evoked excitatory postsynaptic potentials/currents of neocortical neurons [40, 41] as well as NMDA receptor activities in trigeminothalamic neurons [42], while inhibition of DOR potentiates deleterious effects mediated by NMDA receptors during anoxic insults in the turtle cortex [12]. Therefore, it is possible that DOR attenuates anoxic/ischemic Na+ influx by inhibiting ionotropic receptor channels.
Na+/Ca2+ exchangers are ubiquitously contained in neuronal plasma membrane and can operate either in the forward mode to extrude one Ca2+ ion by coupling a three Na+ ion influx or in the reverse mode to extrude three Na+ ions by a single Ca2+ ion entry; they play a fundamental role in controlling Na+ and Ca2+ homeostasis. Though reverse operation of Na+/Ca2+ exchangers has been demonstrated to be responsible for intracellular Ca2+ ([Ca2+]i) rise in the late phase of ischemia [23], both forward and reverse Na+/Ca2+ exchange can take place concurrently in the same cell [43]. Since [Ca2+]i has been shown to increase during O2 deprivation, the forward operation mode of Na+/Ca2+ exchange can be activated, thus extruding excessive Ca2+ loading with an increase in Na+ influx [33]. It is unknown, however, whether DOR inhibits this portion of anoxic Na+ influx.
To clarify these important issues and better understand the mechanism underlying the DOR protection against hypoxic ionic derangement in the cortex, we undertook this study in order to 1) examine the roles of voltage-gated tetrodotoxin (TTX)-sensitive Na+ channels, ionotropic glutamate receptor channels, and Na+/Ca2+ exchangers in anoxic K+ derangement in the cortex, and 2) investigate whether DOR targets these pathways, thereby attenuating Na+ influx-mediated K+ derangement during hypoxia.
Materials and Methods
Slice preparation
Experiments were performed in accordance with the guidelines of the Animal Care and Use Committee of Yale University School of Medicine, which is accredited by the American Association for Accreditation for Laboratory Animal Care. Slices of the frontoparietal cortex (400 μm) from 24–32 day old male C57BL/6 mice were prepared as described in our previous studies [17–19], and incubated in ACSF vigorously aerated with carbogen at least 90 min at ~35°C, then used for recording. ACSF consisted of (in mM) NaCl 125, KCl 3.1, NaHCO3 26, CaCl2 2.4, MgSO4 1.3, NaH2PO4 1.25, and dextrose 10 at pH 7.4.
Induction of anoxia in cortical slices
A slice was completely submerged ~0.5 mm below the ACSF surface (~3 ml/min flow rate) in the recording chamber and kept under normoxic conditions at 35.5 ± 0.5°C for at least 15 min before experimental measurements were taken. Anoxia was induced by switching from the control superfusate (95% O2, 5% CO2) to one continuously bubbled with 95% N2 and 5% CO2. Each slice was subjected to a single period of anoxia that continued for about 1.5 min after the onset of anoxic depolarization (as assessed by a rapid increase in extracellular [K+] that usually occurs within 10 min after the onset of anoxia), or for a period of 20 min if anoxic depolarization did not occur.
Measurements of extracellular potassium
Extracellular K+ concentrations ([K+]e) were measured using K+ sensitive microelectrodes as described previously [17–19]. Calibrations were carried out in triplicate by detecting the voltage responses generated in KCl solutions (1, 3.1, 5, 10, 20, 40, 80, 100, 160 mM), which were added with various concentrations of NaCl to keep constant ionic strength similar to that in interstitial fluid. For each concentration, the average of voltage changes in three separate tests was used as the final voltage change. Over this range electrode response was near ideal, showing a logarithmic relationship to [K+], with an average slope of 55.1 ± 0.2 mV per log10 unit increase in [K+] at 25°C (n = 81), which is stable between 25°C and 35°C with very minor fluctuation [26].
Electrical signals were recorded by a DC amplifier (IE-210, Warner Instrument Co., Hamden, CT) and digitized at a sampling rate of 100Hz. The following parameters were derived to assess K+ homeostasis: 1) the latency of anoxia-induced [K+]e increase (Latency), which was defined as the time from the beginning of anoxia to the time point when anoxia induced a K+ electrode voltage change greater than 1 mV; 2) maximal [K+]e ([K+]max), which was the peak change in [K+]e induced by anoxia; 3) the undershooting of [K+]e (undershoot), which referred to the minimal value of [K+]e during reoxygenation. The former two parameters, especially [K+]max, may reflect the degree of anoxia-induced disruption of K+ homeostasis. The [K+]e undershooting may be related to compensatory over-transportation of K+ from outside into inside of the membrane by Na+-K+ pumps [17], and may reflect the post-anoxic changes in K+ homeostasis. The changes in extracellular K+ activity are distinctly characterized by a two-phase response to anoxia [17, 26]. Since phase 2 induces a massive K+ efflux and marked [K+]e increase, which is believed to be a crucial factor leading to neuronal injury and death [13–16], we specifically focused on the changes in [K+]e in this phase (i.e. [K+]max) in this work and determined if DOR is protective from such massive K+ leakage. After recording of a stable baseline for at least 5 minutes, the slices were subject to experimental treatments. The electrophysiological recordings were continuously performed at least 75 min.
Drug administration
Drugs were applied to cortical slices by switching from control superfusate to one containing drugs, which was controlled by a perfusion system. All drugs were perfused for 20 min before induction of anoxia, and continued to the end of anoxic induction.
Chemicals
TTX, 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX), (+)MK 801 maleate, and KB-R7943 mesylate were purchased from Tocris Cookson Inc. (Ellisville, MI); Veratridine was purchased from Sigma Chemicals Co. (St. Louis, MO). UFP 512, a specific and potent DOR agonist [44], was synthesized by our research team.
Statistics
Data are expressed as mean ± SEM and the number of experiments (n) refers to the number of slices investigated. To ensure the independence of data, no more than 3 slices from the same mouse were used in the same experiments. One way ANOVA followed by Newman Keuls test was used for multiple pairwise tests. Changes were identified as significant if p <0.05.
Results
DOR protection against anoxic K+ derangement
First, we extend our previous studies to further validate our findings regarding the effect of DOR activation on anoxic K+ derangement. One μM UFP 512, a specific and potent DOR agonist [44], significantly attenuated anoxic increase in [K+]max from 35.35 ± 1.25 mM in control (n=19) to 25.20 ± 2.09 mM (n=27) (p<0.01), with a significantly prolonged latency (1.5 times of controls, p <0.001) (n=27). Increasing the concentration of UFP 512 up to 10 μM could not further or significantly attenuate anoxia-induced increase in [K+]max, but on the other hand enhanced the latency of the response (n=15). The undershoot was not affected by UFP 512 at 1–10 μM (p>0.05). These results are highly consistent with our previous observations [18].
Effect of Na+ channel blockade on the DOR protection
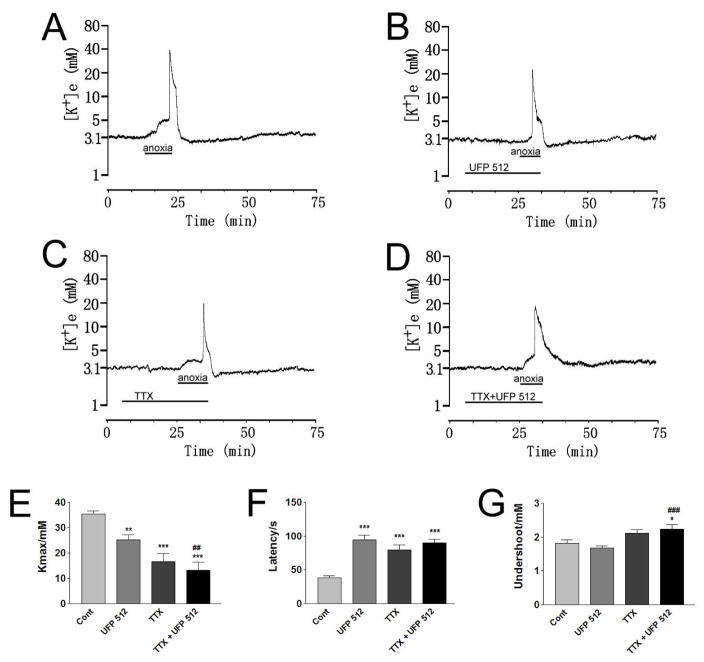
To explore whether DOR activation targets the voltage-gated Na+ channels in the attenuation of anoxic K+ derangement, we applied TTX, a potent and specific voltage-gated Na+ channel blocker to the cortical slices and tested its effect on DOR protection from anoxic K+ derangement. Firstly we tested the effect of TTX alone on anoxic K+ derangement. As shown in Fig. 1, 1 μM of TTX alone significantly decreased anoxia-induced [K+]max (p <0.001) and increased the response latency (p<0.001) (n=13). The occurrence of peak K+ increase was greatly delayed (from 7.9 ± 0.8 min in control to 13.8±1.5 min) (p<0.01) with the application of TTX to the cortical slices (n=13). During reoxygenation, the undershoot of [K+]e tended to decrease in TTX-perfused cortical slices though no statistical significance was found (n=13). These data suggest that inhibition of voltage-gated Na+ channels reduces anoxic K+ derangement in the cortex.
Fig. 1. Effect of TTX on DOR protection from anoxic K+ derangement.
Trace recordings of A: Control (Cont), B: UFP 512 (1 μM), C: TTX (1 μM) and D: TTX+UFP 512. E–G are statistical results of each recording parameter. *p <0.05, **p <0.01, ***p <0.001 as compared with the controls; ##p <0.01, ###p <0.001 vs. UFP 512. Note that TTX (1 μM) greatly attenuated anoxic K+ derangement (n=13). UFP 512 (1 μM) could not further attenuate anoxia-induced K+ derangement in the cortical slices with co-perfusion of TTX (n=14).
After the blockade of Na+ channels, DOR activation could not further reduce the anoxia-induced increase in [K+]max. As shown in Figure 1, UFP 512 (1 μM) in the presence of TTX (1 μM) did not significantly reduce the anoxia-induced increase in [K+]max as compared to that of TTX (1 μM) alone, There was only a slight increase in the latency of the response to anoxia with the occurrence of peak [K+]e in 14.4±1.6 min of anoxia (p>0.05) (n=14). These data suggest that DOR activation could not show additional protection from anoxic K+ derangement in the presence of voltage-gated Na+ channel blockade.
Effect of a Na+ channel opener on DOR protection
In order to further ascertain the role of DOR in the regulation of Na+ entry during anoxic stress, we asked if enhanced Na+ permeability through Na+ channels results in a more severe anoxic K+ derangement and if DOR activation attenuates such ionic disruption. Therefore, we examined the effect of DOR activation on anoxic K+ derangement in the condition of enhanced Na+ entry through Na+ channels. First, we determined whether an increase in Na+ entry through Na+ channels by veratridine, an alkaloid that increases Na+ channels permeability and prevents their inactivation, had any effect on anoxic K+ derangement. Perfusion of 1 μM veratridine, which increases Na+ channel permeability and prevents their inactivation, could not produce any obvious changes in [K+]e in most of the slices investigated (13/15) during 20 minutes of normoxia, but greatly enhanced anoxia-induced K+ derangement (Fig. 2). Under perfusion with 1 μM veratridine, a short period of anoxia (3.3±0.46 min vs. 7.9±0.8 min of anoxia in control, p<0.01) was sufficient to induce a major increase in [K+]e (Fig. 2). The anoxia-induced increase in maximal [K+]e was enhanced more than 50% from that of the control (p<0.001, n=14) by perfusion of 1 μM veratridine (Fig. 2) though no changes in the latency of response to anoxia and undershoot were observed (Fig. 2).
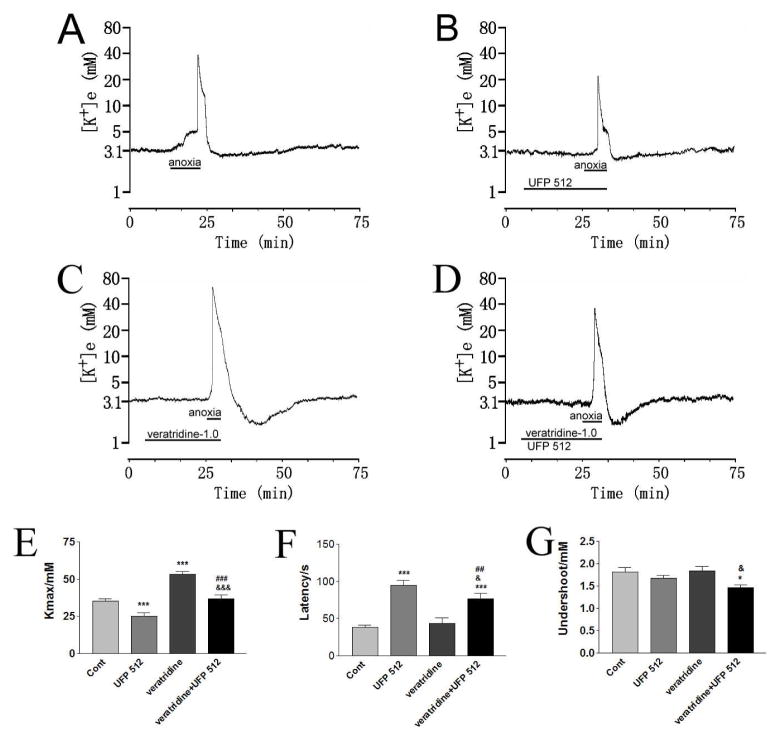
Fig. 2. Effect of DOR activation on veratridine-enhanced anoxic K+ derangement.
Trace recordings of A: Control (Cont), B: UFP 512 (1 μM), C: veratridine (1 μM) and D: veratridine+UFP 512. E–G are statistical results of each recording parameter. *p<0.05, **p <0.01, ***p<0.001 compared with the controls; ##p <0.01, ###p <0.001 compared to UFP 512-1.0; &p<0.05, &&&p<0.001 compared to veratridine. Note that perfusion of 1 μM veratridine itself for 20 minutes could not produce any obvious changes in [K+]e concentration in most of the slices investigated in normoxia, but greatly enhanced anoxia-induced K+ derangement, which could be greatly attenuated by DOR activation with 1 μM UFP 512 (n = 15).
Increasing the concentration of veratridine to 10 and 50 μM led to a significant increase in [K+]e within 3 min of perfusion even in normoxia. For example, perfusion of 10 and 50 μM veratridine induced the increase in [K+]max from basal level (around 3 mM) to 48.34±4.89 mM (n = 6) and 57.21±3.85 mM (n=5), respectively, and the peak [K+]e was observed in 2.2±0.2 min and 1.2±0.1 min of perfusion, respectively. Extended perfusion of veratridine (10 and 50 μM) seriously damaged the slices in normoxia. For instance, even a 20 min period of oxygen-glucose deprivation, which repeatedly induced an abrupt increase in [K+]e in the control slices (also see ref. 45), did not induce any abrupt increase in [K+]e after the slices recovered for >1 hour from veratridine perfusion in normal ACSF. Therefore, it was extremely difficult, if not impossible, to determine the effects of 10 and 50 μM veratridine on K+ derangement during anoxia with the drug treatment protocol used in our study.
Since 1 μM of veratridine greatly enhanced anoxia-induced K+ derangement, we tested the effect of DOR activation on veratridine-enhanced anoxic K+ derangement in cortical slices. As shown in Fig. 2, perfusion of 1 μM UFP 512 (n=15) greatly attenuated veratridine-enhanced anoxic K+ derangement. The anoxia-induced increase in [K+]max nearly returned to the control level (36.78±2.75 mM vs. 53.07±2.10 mM in veratridine, p <0.001; vs. 35.35 ± 1.25 mM in control, p>0.05), and the latency of the response to anoxia was prolonged (77± 7s vs. 43±7s in veratridine, p<0.001; vs. 38±3s in control, p<0.001). There was a definite trend toward delayed occurrence of peak increase in [K+]e to anoxia although no statistical significance was found (4.8±0.7 min vs. 3.3 ± 0.4 min in veratridine, p=0.08) (n=15).
Effect of ionotropic glutamate receptor blockers on DOR protection
Since voltage-gated Na+ channels are not the only pathway for Na+ entry, we asked whether other pathways for Na+ entry are targeted by DOR. A common feature of ionotropic glutamate receptor channels is Na+ permeability [39]. Previous studies have shown that elevation of [Na+]i glutamate receptor channels during hypoxia/ischemia is at least partially mediated by ionotropic [28, 30, 32]. We then applied (+)MK 801, a NMDA receptor blocker, and CNQX, a non-NMDA receptor blocker, respectively, to the cortical slices and then re-examined the effect of DOR activation on anoxic K+ derangement.
First, we determined whether (+)MK 801 or CNQX alone had any effect on anoxic K+ derangement. As shown in Figure 3, (+)MK 801 (10 μM) significantly decreased anoxia-induced [K+]max (p<0.001) with prolonged latency (p<0.01). The undershoot of [K+]e during reoxygenation had no significant changes (p>0.05) (n=13). In contrast, perfusion of CNQX (10 μM) only slightly decreased the anoxic increase in peak [K+]e (p>0.05) (n=12), and the undershoot of [K+]e during reoxygenation significantly attenuated (p<0.01) (n=12) (Fig. 3). Similar to that of (+)MK 801 (10 μM), the response latency to anoxia is significantly prolonged with CNQX (10 μM) perfusion (p<0.001) (n=12). These results suggest ionotropic glutamate receptors play a role in anoxic K+ derangement in the cortex.
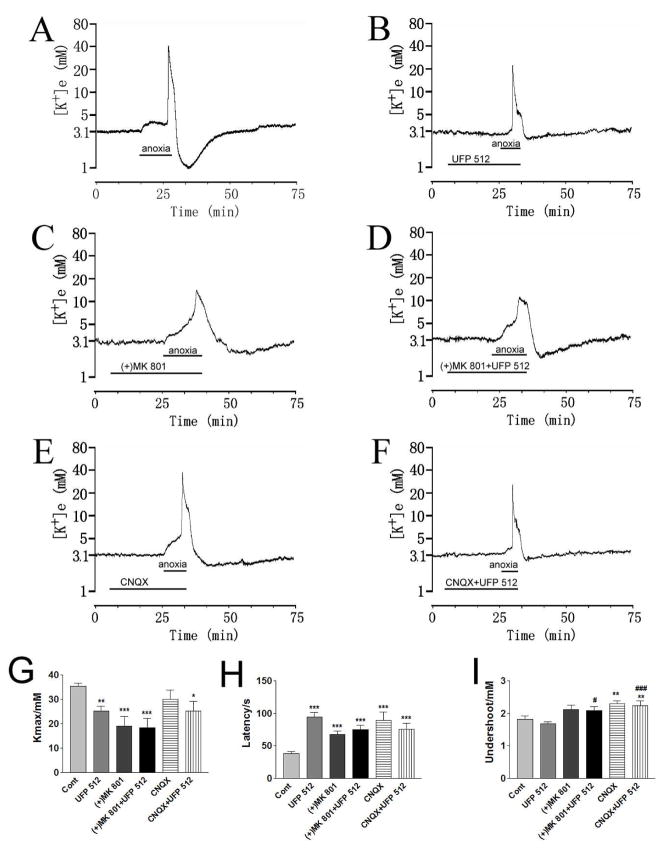
Fig. 3. Effect of ionotropic glutamate receptor blockers on the DOR protection from anoxic K+ derangement.
Trace recordings of A: Control (Cont), B: UFP 512 (1 μM), C: (+)MK 801 (10 μM), D: (+)MK 801+UFP 512, E: CNQX (10 μM), F: CNQX+UFP 512. G–I are statistical results of each recording parameter. *p<0.05, **p<0.01, ***p<0.001 compared to controls; #p <0.05, ###p <0.001 compared to UFP 512-1.0. Note that (+)MK 801 (10 μM) significantly decreased anoxia-induced [K+]max (n=13), whereas CNQX (10 μM) only slightly decreased the anoxia-induced increase in peak [K+]e (n=12). Blockade of NMDA receptor channels with (+)MK 801 (10 μM) reduced DOR-induced protection from anoxic K+ derangement (n=13); In the presence of CNQX (10 μM), UFP 512 (1 μM) further attenuated the anoxia-induced increase in [K+]max (n=14).
Co-perfusion of (+)MK 801 (10 μM) with UFP 512 (1 μM) did not produced any significant changes in anoxia-induced K+ derangement in cortical slices as compared to the groups of UFP 512 (1 μM) (n=27) or (+)MK 801 (10 μM) (n=13) alone (n=13) (Fig. 3). These data suggest that blockade of NMDA receptor channels reduced DOR-induced protection from anoxic K+ derangement.
Co-perfusion of CNQX (10 μM) with UFP 512 (1 μM) attenuated anoxia-induced increase in [K+]max to 25.29±3.97 mM (n = 14), a level same as that of UFP 512 (1 μM) alone (25.20±2.09 mM) (n=27) (Fig. 3). The undershoot of [K+]e was close to that of perfusion of CNQX (10 μM) alone (n=12), and the response latency to anoxia was similar to that of perfusion by either UFP 512 (1 μM) or CNQX (10 μM) alone (Fig. 3). Despite the presence of these drugs, the co-perfusion of UFP 512 (1 μM) and CNQX (10 μM) significantly attenuated anoxia-induced increase in [K+]max (p<0.05) with a prolonged latency of response to anoxia (p<0.01) and an attenuated undershoot during reoxygenation (p<0.01) in comparison to control (n=14) (Fig. 3).
Effect of a Na+/Ca2+ exchanger blocker on DOR protection
Anoxia may activate forward-operation of Na+/Ca2+ exchangers, and thereby contribute to Na+ influx and [Na+]i accumulation [33]. To determine the role of Na+/Ca2+ exchangers in the attenuation of anoxic K+ derangement by DOR activation, we applied KB-R7943, a potent Na+/Ca2+ exchangers blocker, to the cortical slices. Because KB-R7943 inhibits the reverse-operation of Na+/Ca2+ exchangers at the concentration of 1 μM, while inhibiting both the reverse- and forward-operation of Na+/Ca2+ exchangers at a concentration of 10 μM [24, 46], we initially tested the effects of KB-R7943 alone at 1 and 10 μM on the anoxic K+ derangement in our cortical model. As shown in Fig. 4, perfusion of 1 μM KB-R7943 significantly attenuated anoxia-induced increase in [K+]max (p <0.05) and the undershoot of [K+]e during reoxygenation (p<0.05) (n=13), while 10 μM KB-R7943 had no any effect on anoxia-induced increase in [K+]max and the undershoot (p>0.05) (n= 10). The response latency to anoxia was prolonged by KB-R7943 at both concentrations (p <0.001 and p<0.01, respectively). These results suggest that the reverse-operation of Na+/Ca2+ exchangers favors anoxic K+ derangement in the cortex.
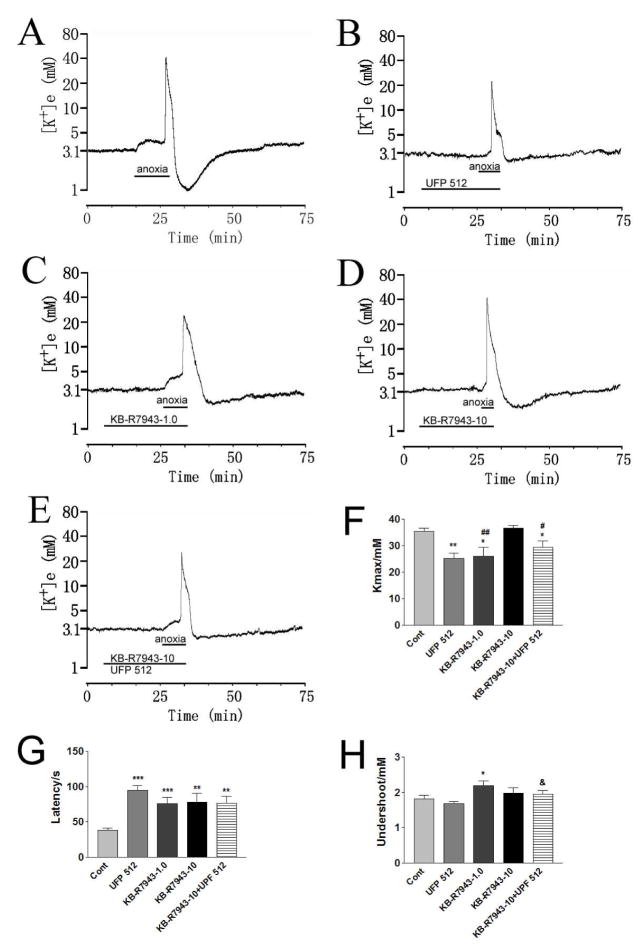
Fig. 4. Effect of Na+/Ca2+ exchanger blocker on DOR protection from anoxic K+ derangement.
Trace recordings of A: Control (Cont); B: UFP 512 (1 μM); C: KB-R7943 (1.0 μM) (KB-R7943-1.0); D: KB-R7943 (10 μM) (KB-R7943-10); E: KB-R7943-10+UFP 512. F–H are statistical results of each recording parameter. *p<0.05, **p<0.01, ***p <0.001 compared to controls; #p<0.05, ##p<0.01 compared to KB-R7943-10; &p <0.05 compared to UFP 512. Note that blockade of reverse operation of Na+/Ca2+ exchangers with 1 μM KB-R7943 significantly attenuated anoxia-induced K+ derangement (n=13), while blocking both reverse and forward operation mode with 10 μM KB-R7943 induced no change in anoxic increase in [K+]max and the undershoot with the response latency was prolonged (n=10). DOR activation still induced significant reduction of the anoxia-induced increase in [K+]max when Na+/Ca2+ exchangers was blocked by 10 μM of KB-R7943.
In the presence of 10 μM KB-R7943, perfusion of UFP 512 (1 μM) could still attenuated the anoxia-induced increase in [K+]max: a significant decrease occurred in comparison to KB-R7943 (10 μM) alone (p<0.05) (n=12) (Fig. 4) although there was no major change in the response latency and the undershoot. These results suggest that DOR may have little, in any, effect on Na+/Ca2+ exchangers-mediated Na+-K+ reaction to anoxia.
Discussion
In this study, we further confirmed that DOR activation is protective against anoxic increase in extracellular K+ (a typical anoxic response) in the cortex. More importantly, we found that (1) the DOR-mediated protection is largely dependent on the inhibition of anoxic Na+ influx mediated by voltage-gated Na+ channels; (2) DOR activation inhibits anoxic Na+ influx mediated by ionotropic glutamate NMDA receptors, but not that by non-NMDA receptors though both of them play a role in anoxic K+ derangement; and (3) DOR activation has little effect on the Na+/Ca2+ exchangers-based ionic responses to anoxia.
DOR and voltage-gated Na+ channels
Voltage-gated Na+ channels constitute the major route for Na+ influx into neurons in normal neuronal activities. However, it is still debated as to whether voltage-gated Na+ channels play a role in Na+ entry into neurons during anoxia/ischemia despite the fact that their blockade has been shown to attenuate/postpone anoxic depolarization, and prevent hypoxia/ischemia-induced neuronal injury and death [22, 31, 45, 46]. For example, by using different measuring methods, many authors have shown that blockade of voltage-gated Na+ channels partially or completely prevent anoxia-induced extracellular Na+ drop or excessive intraneuronal Na+ accumulation in various regions of the central nervous system [22, 28–31]. These studies support the idea that voltage-gated Na+ channels are the major source of Na+ influx during anoxia/ischemia. On the other hand, several reports oppose to this concept [21, 24]. The conflicting results from previous investigations may be attributed to multiple factors, including differences in the brain regions studied, tissue/cell preparation (acutely isolated/cultured neurons vs. brain slices with relatively intact microenviroment and neuronal connection), duration and severity of insults.
Our data obtained from studies with Na+ channel blocker (TTX) and opener (veratridine) and those of Na+ substitution with the impermeable N-methyl-D-glucamine [19] strongly support that voltage-gated Na+ channels play a key role in anoxic Na+ influx into neurons in the cortex. Moreover, our work suggests that TTX-sensitive voltage-gated Na+ channels is a critical target of DOR signals in the attenuation of Na+ influx-based K+ derangement in the cortex in anoxia. This is based on the following facts: (1) lowering the Na+ concentration and substitution with impermeable N-methyl-D-glucamine caused a concentration-dependent attenuation of anoxic K+ derangement, and under such lower Na+ conditions, DOR had little effect on anoxic K+ derangement [19]; (2) blockade of voltage-gated Na+ channels with TTX significantly attenuated anoxic K+ derangement triggered by a massive Na+ influx, while DOR had no additive effect on anoxic K+ derangement in the cortex when Na+ channels were blocked by TTX; and (3) veratridine (1 μM), a Na+ channel opener, further enhanced the anoxia-induced K+ derangement, which could be attenuated by DOR activation. In addition, our previous observations showed that DOR down-regulation [34] is associated with Na+ channel up-regulation [35], and DOR activation attenuates hypoxic dysregulation of Na+ channels [36, 37], suggesting an interactive regulation between DOR signals and Na+ homeostasis. More recently, we found that activation of DOR inhibits Na+ currents in Xenopus oocytes co-transfected with Na+ channels and DOR [47]. All these results prompt us believe that TTX-sensitive voltage-gated Na+ channels are the major pathway of hypoxic Na+ influx that induces massive K+ efflux during anoxia, and this pathway can be inhibited by DOR activation.
DOR and ionotropic glutamate receptor channels
A common feature of ionotropic glutamate receptors is Na+ permeability [39]. Anoxia/ischemia-induced massive glutamate release [38] promotes over-stimulation of postsynaptic ionotropic glutamate receptors to increase [Na+]i [28, 30, 32], which may affect anoxic K+ efflux, and induce neuronal injury [32, 46]. Kiedrowski observed that when Na+ was replaced with N-methyl-D-glucamine, the glutamate-elicited K+ efflux occurred only in the presence of Ca2+ and at a much lower rate, which could be blocked by MK 801 [27]. Croning et al [48] and Lopachin et al [30] also found that blockade of either NMDA or non-NMDA receptors prevented Na+ accumulation, which partially preserved intraneuronal K+ concentration and attenuated the rise in [K+]e in hippocampus during hypoxia/ischemia. Nevertheless, Műller and Somjen [45] found that in the same hippocampal region blocking ionotropic glutamate receptors did not affect anoxia-induced peak increase in [K+]e. Our results clearly demonstrated that in the cortex, blockade of ionotropic glutamate receptors attenuated anoxic K+ derangement (prolonged response latency and/or decreased [K+]max). Therefore, we believe that ionotropic glutamate receptors, particularly NMDA subtypes, are one of Na+ entry pathways during anoxia [27, 28, 30, 32], which may play a role in anoxic K+ derangement. However, we cannot rule out other possibilities. For example, ionotropic glutamate receptor channels also have permeability to Ca2+ and K+ [39], and blockade of these channels may directly decrease K+ efflux [13] and/or decrease [Ca2+]i [12, 23] that consequently leads to a decrease in Ca2+-based K+ efflux [27], e.g. through BK channels [18]. In any case, our data suggest that Na+ influx mediated by ionotropic glutamate receptors play a role in anoxia-induced K+ derangement in the cortex.
In the blockade of NMDA receptor channels by (+)MK 801, DOR activation could further attenuate anoxic K+ derangement. The reason was possibly that blockade of NMDA receptors decreased the anoxia-induced Na+ influx through the receptor-linked channels [28, 30, 32], which partially mimicked the effect of DOR activation. There is evidence showing that DOR activation prevents the release of glutamate from presynaptic vesicles and depresses the amplitudes of stimulus-evoked excitatory postsynaptic potentials/currents of neocortical neurons [40, 41] as well as NMDA receptor activities in trigeminothalamic neurons [42], whereas inhibition of DOR with naltrindole potentiates normaxic NMDA receptor currents [12]. Such depression may decrease K+ leakage because of the decrease in Na+-triggered activities (e.g., Na+-activated K+ channels, anoxic increase in excitability and action potential) [19, 25, 30, 31], though we could not rule out the involvement of Ca2+-activated K+ channels in this respect [18], since it has been suggested that DOR mediates NMDA receptor activity in a Gi-dependent manner and prevent deleterious NMDA receptor-mediated Ca2+ influx during anoxic insults in the turtle cortex [12].
In contrast to NMDA receptor blocker, non-NMDA receptor blockers had little effect on the DOR-induced attenuation of anoxic K+ derangement because DOR activation further attenuated anoxia-induced increase in [K+]max when non-NMDA receptor channels were blocked by CNQX. Though non-NMDA receptor channels also have Na+ permeability, they may not be the main pathway of Na+ influx during anoxia [23] because of their rapid desensitization after activation (decay half times ~30 ms vs. 250 ms for NMDA receptors in hippocampal cells) [49, 50]. Therefore, when they were blocked, DOR signals can still attenuate K+ efflux by decreasing Na+ influx mediated by pathways other than non-NMDA receptor channels.
DOR and Na+/Ca2+ exchangers
It is very interesting to note that a low concentration of KB-R7943 (1 μM) significantly attenuated anoxia-induced increase in [K+]max, whereas a higher concentration (10 μM) had no effect. KB-R7943 has been demonstrated to selectively inhibit reverse-operation of Na+/Ca2+ exchangers at a concentration of 1 μM, and both reverse- and forward-operations at a concentration of 10 μM [24, 46]. Both operations of Na+/Ca2+ exchange can take place concurrently in the same cell [43]. During anoxia, both [Na+]i and [Ca2+]i increase in neurons [21]. Therefore, this bidirectional exchange may operate in either mode depending on the changes in transmembrane potential as well as Na+ and Ca2+ gradients. It has been suggested that hypoxia-induced membrane depolarization [21, 31, 45] and a large increase in [Na+]i [20–24] favors reversing Na+/Ca2+ exchangers and Ca2+ entry/Na+ extrusion mode [32] though Ca2+ extrusion/Na+ entry mode may also concomitantly run during hypoxia [33]. Because either [Na+]i or [Ca2+]i causes K+ efflux [25, 27], both forward (leading to an increase in [Na+]i) and reversed (leading to an increase in [Ca2+]i) modes may contribute to anoxic K+ efflux in the cortex. However, it is likely that reserved mode-mediated Ca2+ entry contributes more to this effect since blocking this Ca2+ entry/Na+ extrusion operation with 1 μM of KB-R7943 [24, 46] resulted in a net decrease in anoxic K+ efflux. In other words, [Ca2+]i caused more K+ efflux than [Na+]i in the Na+/Ca2+ exchangers-related portion of anoxic K+ efflux. Interestingly, 10 μM KB-R7943, which blocks both the forward and reversed modes [24, 46], led to no change in [K+]max, suggesting a minor role in Na+/Ca2+ exchangers-based ionic events in anoxic K+ efflux overall compared to that of Na+ channels and glutamate receptors in the presence of these dominant Na+ entry pathways. There is a possibility that when Na+/Ca2+ exchangers was blocked, the Na+/Ca2+ exchangers-mediated Na+ influx and the subsequent ionic events were compensated by Na+ channels and/or glutamate receptors. In any case, our data suggest that Na+/Ca2+ exchangers is less important than Na+ channels and/or glutamate receptors in anoxic K+ efflux despite its involvement.
Our results show that in the presence of 10 μM KB-R7943, perfusion of UFP 512 (1 μM) still significantly attenuated the anoxia-induced increase in [K+]max when compared with 10 μM of KB-R7943 alone. Since both operation modes of Na+/Ca2+ exchangers were blocked at this concentration [24, 46], it was less likely that DOR signals targeted Na+/Ca2+ exchangers modulation.
This notion further confirms that the DOR effect on anoxic K+ derangement in the presence of Na+ channel or glutamate receptor blockers is specific, which renders us more confident on our conclusion that DOR protection against anoxic K+ derangement relies on inhibition of Na+ influx mediated by voltage-gated Na+ channels as well as NMDA receptors in the cortex.
The mechanisms of interaction between DOR and ion channels (Na+ channels and NMDA receptor channels) regarding DOR attenuation against anoxic K+ leakage are poorly understood at present. DOR belong to a family of G-protein-coupled metabotropic receptors. Their effect is mediated by G proteins and G-protein-dependent cytoplasmic second messengers involving protein kinases [12, 17, 51]. Voltage-gated Na+ channels and NMDA receptors are important targets modulated by metabotropic receptors via G protein/protein kinases [52, 53]. Our previous studies as well as those of others have suggested that DOR interacts with Na+ channels [19, 34–37, 47] and NMDA receptors and DOR activation reduces the activities of Na+ channels and NMDA receptors and vice versa [12, 40–42]. Therefore, it is possible that DOR targets Na+ channels and NMDA receptors via G-protein-protein kinases dependent pathways. In support of this notion is that DOR mediates NMDA receptor activity in a Gi-dependent manner and prevent deleterious NMDA receptor-mediated Ca2+ influx during anoxic insults in the turtle cortex [12], and DOR activation attenuates anoxic K+ leakage via protein kinase C-dependent pathway in the cortex [17].
In summary, our work shows that DOR activation attenuates anoxic K+ derangement by restricting Na+ entry through voltage-gated Na+ channels and NMDA receptor channels in the cortex. Non-NMDA receptor channels and Na+/Ca2+ exchangers, though involved in anoxic K+ derangement in certain degrees, are less likely the targets of DOR signals.
Acknowledgments
This work was supported in part by grants from National Institutes of Health (HD-34852) and American Heart Association (0755993T), and in part by the Division of Intramural Research of NIEHS and NIH.
References
- 1.Ginsberg MD. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green AR. Pharmacological approaches to acute ischaemic stroke: reperfusion certainly, neuroprotection possibly. Br J Pharmacol. 2008;153:S325–S338. doi: 10.1038/sj.bjp.0707594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang JH, Haddad GG, Xia Y. δ-, but not μ- and κ-, opioid receptor activation protects neocortical neurons from glutamate-induced excitotoxic injury. Brain Res. 2000;885:143–153. doi: 10.1016/s0006-8993(00)02906-1. [DOI] [PubMed] [Google Scholar]
- 4.Zhang JH, Gibney GT, Zhao P, Xia Y. Neuroprotective role of δ-opioid receptors in the cortical neurons. Am J Physiol. 2002;282:C1225–C1234. doi: 10.1152/ajpcell.00226.2001. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Qian H, Zhao P, Hong SS, Xia Y. Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through δ-opioid receptor. Stroke. 2006;37:1094–1099. doi: 10.1161/01.STR.0000206444.29930.18. [DOI] [PubMed] [Google Scholar]
- 6.Ma MC, Qian H, Ghassemi F, Zhao P, Xia Y. Oxygen sensitive δ-opioid receptor-regulated survival and death signals: novel insights into neuronal preconditioning and protection. J Biol Chem. 2005;280:16208–16218. doi: 10.1074/jbc.M408055200. [DOI] [PubMed] [Google Scholar]
- 7.Borlongan CV, Wang Y, Su TP. Delta opioid peptide (D-Ala 2, D-Leu 5) enkephalin: linking hibernation and neuroprotection. Front Biosci. 2004;9:3392–3398. doi: 10.2741/1490. [DOI] [PubMed] [Google Scholar]
- 8.Lim YJ, Zheng S, Zuo Z. Morphine preconditions purkinje cells against cell death under in vitro simulated ischemia-reperfusion conditions. Anesthesiology. 2004;100:562–568. doi: 10.1097/00000542-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Zhao P, Huang Y, Zuo Z. Opioid preconditioning induces opioid receptor-dependent delayed neuroprotection against ischemia in rats. J Neuropathol Exp Neurol. 2006;65:945–952. doi: 10.1097/01.jnen.0000235123.05677.4b. [DOI] [PubMed] [Google Scholar]
- 10.Su DS, Wang ZH, Zheng YJ, Zhao YH, Wang XR. Dose-dependent neuroprotection of delta opioid peptide [D-Ala2, D-Leu5]enkephalin in neuronal death and retarded behavior induced by forebrain ischemia in rats. Neurosci Lett. 2007;423:113–117. doi: 10.1016/j.neulet.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 11.Charron C, Messier C, Plamondon H. Neuroprotection and functional recovery conferred by administration of kappa- and delta-opioid agonists in a rat model of global ischemia. Physiol Behav. 2008;93:502–511. doi: 10.1016/j.physbeh.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Pamenter ME, Buck LT. δ-opioid receptor antagonism induces NMDA receptor-dependent excitotoxicity in anoxic turtle cortex. J Exp Biol. 2008;211:3512–3517. doi: 10.1242/jeb.021949. [DOI] [PubMed] [Google Scholar]
- 13.Yu SP, Yeh CH, Strasser U, Tian M, Choi DW. NMDA receptor-mediated K+ efflux and neuronal apoptosis. Science. 1999;284:336–339. doi: 10.1126/science.284.5412.336. [DOI] [PubMed] [Google Scholar]
- 14.Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science. 1997;278:114–117. doi: 10.1126/science.278.5335.114. [DOI] [PubMed] [Google Scholar]
- 15.Liu D, Slevin JR, Lu C, Chan SL, Hansson M, Elmér E, Mattson MP. Involvement of mitochondrial K+ release and cellular efflux in ischemic and apoptotic neuronal death. J Neurochem. 2003;86:966–979. doi: 10.1046/j.1471-4159.2003.01913.x. [DOI] [PubMed] [Google Scholar]
- 16.Wei L, Yu SP, Gottron F, Snider BJ, Zipfei GJ, Choi DW. Potassium channel blockers attenuate hypoxia- and ischemia-induced neuronal death in vitro and in vivo. Stroke. 2003;34:1281–1286. doi: 10.1161/01.STR.0000065828.18661.FE. [DOI] [PubMed] [Google Scholar]
- 17.Chao D, Donnelly DF, Feng Y, Bazzy-Asaad A, Xia Y. Cortical δ-opioid receptors potentiate K+ homeostasis during anoxia and oxygen-glucose deprivation. J Cereb Blood Flow Metab. 2007;27:356–368. doi: 10.1038/sj.jcbfm.9600352. [DOI] [PubMed] [Google Scholar]
- 18.Chao D, Bazzy-Asaad A, Balboni G, Xia Y. δ-, but not μ-, opioid receptor stabilizes K+ homeostasis by reducing Ca2+ influx in the cortex during acute hypoxia. J Cell Physiol. 2007;212:60–67. doi: 10.1002/jcp.21000. [DOI] [PubMed] [Google Scholar]
- 19.Chao D, Bazzy-Asaad A, Balboni G, Salvadori S, Xia Y. Activation of DOR attenuates anoxic K+ derangement via inhibition of Na+ entry in mouse cortex. Cereb Cortex. 2008;18:2217–2227. doi: 10.1093/cercor/bhm247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman JE, Haddad GG. Anoxia induces an increase in intracellular sodium in rat central neurons in vitro. Brain Res. 1994;663:329–334. doi: 10.1016/0006-8993(94)91281-5. [DOI] [PubMed] [Google Scholar]
- 21.Calabresi P, Marfia GA, Centonze D, Pisani A, Bernardi G. Sodium influx plays a major role in the membrane depolarization induced by oxygen and glucose deprivation in rat striatal spiny neurons. Stroke. 1999;30:171–179. doi: 10.1161/01.str.30.1.171. [DOI] [PubMed] [Google Scholar]
- 22.Banasiak KJ, Burenkova O, Haddad GG. Activation of voltage-sensitive sodium channels during oxygen deprivation leads to apoptotic neuronal death. Neuroscience. 2004;126:31–44. doi: 10.1016/S0306-4522(03)00425-1. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Sánchez M, Striggow F, Schrőder UH, Kahlert S, Reymann KG, Reiser G. Na+ and Ca2+ homeostasis pathways, cell death and protection after oxygen-glucose-deprivation in organotypic hippocampal slice cultures. Neuroscience. 2004;128:729–740. doi: 10.1016/j.neuroscience.2004.06.074. [DOI] [PubMed] [Google Scholar]
- 24.Sheldon C, Diarra A, Cheng YM, Church J. Sodium influx pathways during and after anoxia in rat hippocampal neurons. J Neurosci. 2004;24:11057–11069. doi: 10.1523/JNEUROSCI.2829-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guatteo E, Mercuri NB, Bernardi G, Knőpfel T. Intracellular sodium and calcium homeostasis during hypoxia in dopamine neurons of rat substantia nigra pars compacta. J Neurophysiol. 1998;80:2237–2243. doi: 10.1152/jn.1998.80.5.2237. [DOI] [PubMed] [Google Scholar]
- 26.Jiang C, Haddad GG. Effect of anoxia on intracellular and extracellular potassium activity in hypoglossal neurons in vitro. J Neurophysiol. 1991;66:103–111. doi: 10.1152/jn.1991.66.1.103. [DOI] [PubMed] [Google Scholar]
- 27.Kiedrowski L. N-methyl-D-aspartate excitotoxicity: relationships among plasma membrane potential, Na+/Ca2+ exchange, mitochondrial Ca2+ overload, and cytoplasmic concentrations of Ca2+, H+, and K+ Mol Pharmacol. 1999;56:619–632. doi: 10.1124/mol.56.3.619. [DOI] [PubMed] [Google Scholar]
- 28.Lynch JJ, III, Yu SP, Canzoniero LMT, Sensi SL, Choi DW. Sodium channel blockers reduce oxygen-glucose deprivation-induced cortical neuronal injury when combined with glutamate receptor antagonists. J Pharmacol Exp Ther. 1995;273:554–560. [PubMed] [Google Scholar]
- 29.Fung ML, Croning MDR, Haddad GG. Sodium homeostasis in rat hippocampal slices during oxygen and glucose deprivation: role of voltage-sensitive sodium channels. Neurosci Lett. 1999;275:41–44. doi: 10.1016/s0304-3940(99)00728-4. [DOI] [PubMed] [Google Scholar]
- 30.Lopachin RM, Gaughan CL, Lehning EJ, Weber ML, Taylor CP. Effects of ion channel blockade on the distribution of Na, K, Ca, and other elements in oxygen-glucose deprived CA1 hippocampal neurons. Neuroscience. 2001;103:971–983. doi: 10.1016/s0306-4522(01)00035-5. [DOI] [PubMed] [Google Scholar]
- 31.Raley-Susman KM, Kass IS, Cottrell JE, Newman RB, Chambers G, Wang J. Sodium influx blockade and hypoxic damage to CA1 pyramidal neurons in rat hippocampal slices. J Neurophysiol. 2001;86:2715–2726. doi: 10.1152/jn.2001.86.6.2715. [DOI] [PubMed] [Google Scholar]
- 32.Kiedrowski L. Critical role of sodium in cytosolic [Ca2+] elevations in cultured hippocampal CA1 neurons during anoxic depolarization. J Neurochem. 2007;100:915–923. doi: 10.1111/j.1471-4159.2006.04308.x. [DOI] [PubMed] [Google Scholar]
- 33.Jeon D, Chu K, Jung K-H, Kim M, Yoon BW, Lee CJ, Oh U, Shin HS. Na+/Ca2+ exchanger 2 is neuroprotective by exporting Ca2+ during a transient focal cerebral ischemia in the mouse. Cell Calcium. 2008;43:482–491. doi: 10.1016/j.ceca.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Zhao P, Ma MC, Qian H, Xia Y. Down-regulation of delta-opioid receptors in Na+/H+ exchanger 1 null mutant mouse brain with epilepsy. Neurosci Res. 2005;53:442–446. doi: 10.1016/j.neures.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Xia Y, Zhao P, Xue J, Gu XQ, Sun X, Yao H, Haddad GG. Na+ channel expression and neuronal function in the Na+/H+ exchanger 1 null mutant mouse. J Neurophysiol. 2003;89:229–236. doi: 10.1152/jn.00488.2002. [DOI] [PubMed] [Google Scholar]
- 36.Xia Y, Cao H, Zhang JH, Chen NY, Siegel K, Agulnik M, Haddad GG. Effect of δ-opioid receptor activation on Na+ channel expression in cortical neurons subjected to prolonged hypoxia in culture. 2001 Program No. 740.6 SfN Abstract. Society for Neuroscience online. [Google Scholar]
- 37.Ma MC, Donnelly DF, Xia Y. Neuronal preconditioning inhibits hypoxia-induced sodium channel up-regulation via δ-opioid receptor. 2004 SfN Abstract. Society for Neuroscience online. [Google Scholar]
- 38.Camacho A, Massieu L. Role of glutamate transporters in the clearance and release of glutamate during ischemia and its relation to neuronal death. Arch Med Res. 2006;37:11–18. doi: 10.1016/j.arcmed.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Mayer ML, Westbrook GL. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28:197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka E, North RA. Opioid actions on rat anterior cingulated cortex neurons in vitro. J Neurosci. 1994;14:1106–1113. doi: 10.1523/JNEUROSCI.14-03-01106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostermeier AM, Schlosser B, Schwender D, Sutor B. Activation of μ- and δ-opioid receptors causes presynaptic inhibition of glutamatergic excitation in neocortical neurons. Anesthesiology. 2000;93:1053–1063. doi: 10.1097/00000542-200010000-00029. [DOI] [PubMed] [Google Scholar]
- 42.Wang XM, Mokha SS. Opioids modulate N-methyl-D-aspartic acid (NMDA)-evoked responses of trigeminothalamic neurons. J Neurophysiol. 1996;76:2093–2096. doi: 10.1152/jn.1996.76.3.2093. [DOI] [PubMed] [Google Scholar]
- 43.Yu SP, Choi DW. Na+-Ca2+ exchange currents in cortical neurons: concomitant forward and reverse operation and effect of glutamate. Eur J Neurosci. 1997;9:1273–1281. doi: 10.1111/j.1460-9568.1997.tb01482.x. [DOI] [PubMed] [Google Scholar]
- 44.Balboni G, Salvadori S, Guerrini R, Negri L, Giannini E, Jinsmaa Y, Bryant SD, Lazarus LH. Potent δ-opioid receptor agonists containing the Dmt-Tic pharmacophore. J Med Chem. 2002;45:5556–5563. doi: 10.1021/jm020336e. [DOI] [PubMed] [Google Scholar]
- 45.Műller M, Somjen GG. Na+ dependence and the role of glutamate receptors and Na+ channels in ion fluxes during hypoxia of rat hippocampal slices. J Neurophysiol. 2000;84:1869–1880. doi: 10.1152/jn.2000.84.4.1869. [DOI] [PubMed] [Google Scholar]
- 46.Breder J, Sabelhaus CF, Opitz T, Reymann KG, Schrőder UH. Inhibition of different pathways influencing Na+ homeostasis protects organotypic hippocampal slice cultures from hypoxic/hypoglycemic injury. Neuropharmacology. 2000;39:1779–1787. doi: 10.1016/s0028-3908(00)00027-7. [DOI] [PubMed] [Google Scholar]
- 47.Kang X, Gu Q, Ding G, Chao D, Wang Y, Balboni G, Lazarus LH, Xia Y. Delta-opioid receptor activation and sodium channel inhibition in Xenopus oocytes. Acta Physiol Sin. 2008;60(suppl):124. [Google Scholar]
- 48.Croning MDR, Grahame-Smith DG, Zetterstrőm TSC. Effect of (+)-MK-801 on the increase in extracellular [K+] seen in rat dorsal hippocampus, in vivo, during transient hypoxia as measured by ion-selective microelectrodes. Br J Pharmacol. 1993;100:179. [Google Scholar]
- 49.Vyklický L, Jr, Benveniste M, Mayer ML. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurons. J Physiol. 1990;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilding TJ, Huettner JE. Activation and desensitization of hippocampal kainate receptors. J Neurosci. 1997;17:2713–2721. doi: 10.1523/JNEUROSCI.17-08-02713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- 52.Szabo ST, Machado-Vieira R, Yuan P, Wang Y, Wei Y, Falke C, Cirelli C, Tononi G, Manji HK, Du J. Glutamate receptors as targets of protein kinase C in the pathophysiology and treatment of animal models of Mania. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, Yu FH, Surmeier DJ, Scheuer T, Catterall WA. Neuromodulation of Na+ channel slow inactivation via cAMP-dependent protein kinase and protein kinase C. Neuron. 2006;49:409–420. doi: 10.1016/j.neuron.2006.01.009. [DOI] [PubMed] [Google Scholar]