Abstract
Peroxisome proliferator-activated receptor gamma (PPARγ) plays a role in regulating a myriad of biological processes in virtually all brain cell types, including neurons. We and others have reported recently that drugs which activate PPARγ are effective in reducing damage to brain in distinct models of brain disease, including ischemia. However, the cell type responsible for PPARγ-mediated protection has not been established. In response to ischemia, PPARγ gene is robustly upregulated in neurons, suggesting that neuronal PPARγ may be a primary target for PPARγ-agonist mediated neuroprotection. To understand the contribution of neuronal PPARγ to ischemic injury, we generated conditional neuron-specific PPARγ knockout mice (N-PPARγ-KO). These mice are viable and appeared to be normal with respect to their gross behavior and brain anatomy. However, neuronal PPARγ deficiency caused these mice to experience significantly more brain damage and oxidative stress in response to middle cerebral artery occlusion. The primary cortical neurons harvested from N-PPARγ-KO mice, but not astroglia, exposed to ischemia in vitro demonstrated more damage and a reduced expression of numerous key gene products that could explain increased vulnerability, including: SOD1, catalase, glutathione S-transferase (GST), uncoupling protein-1 or transcription factor liver X receptor-alpha (LXRα). Also, PPARγ agonist based neuroprotective effect was lost in neurons from N-PPARγ neurons. Therefore, we conclude that PPARγ in neurons play an essential protective function, and that PPARγ agonists may have utility in neuronal self-defense, in addition to their well-established anti-inflammatory effect.
Keywords: cerebral ischemia, PPAR-gamma, conditional knockout, oxidative stress, neuroprotection, gene expression
INTRODUCTION
The peroxisome proliferator-activated receptors (PPARs), including α, γ, and δ/β encoded by separate genes, are members of the nuclear receptor superfamily of ligand-activated transcription factors (Kliewer et al., 1994; Mangelsdorf et al., 1995; Chinetti et al., 2000; Berger and Moller, 2002). Normally, PPARγ regulates gene transcription by binding to conserved DNA sequences termed peroxisome-proliferator-response-elements, as heterodimers with retinoic-X-receptor. Agonists of PPARγ include fatty acids, non-steroidal anti-inflammatory drugs (Lehmann et al., 1995) naturally occurring 15d-Δ12,14-PGJ2 (Forman et al., 1995; Kliewer et al., 1995; Ricote et al., 1998; Rohn et al., 2001), and a class of synthetic compounds, thiazolidinediones, (Stumvoll and Haring, 2002). PPARγ was originally characterized in adipose tissue as a regulator of lipid and glucose metabolism; however, new evidence indicates that PPARγ is present in most cell types where it mediates multimodal function (Giannini et al., 2004). Recently, PPARγ was identified also in neurons, but its function here is less clear. Activators of PPARγ, including 15d-PGJ2 or thiazolidinediones administered to animals prior to or after the onset of ischemia reduce ischemic damage (Shimazu et al., 2005; Sundararajan et al., 2005; Ou et al., 2006; Victor et al., 2006; Tureyen et al., 2007). The reduction of pro-inflammatory responses, anti-atherosclerotic activities and anti-oxidant effect was proposed as mechanisms underlying the beneficial effect of PPARγ activators (Jiang et al., 1998; Chinetti et al., 2000; Giannini et al., 2004; Pereira et al., 2005; Sundararajan et al., 2005; Zhao et al., 2005). We and others demonstrated that PPARγ activators protect neurons in culture from NMDA-induced cytotoxicity (Uryu et al., 2002; Zhao et al., 2006a), suggesting that PPARγ protects brain from ischemia via mechanisms that directly target neurons and are independent from inflammation or other extrinsic factors. In non-neuronal tissues, PPARγ promotes expression of numerous gene products whose profile of activity could be linked to oxidative stress suppression, proper mitochondrial function, or lipid and glucose metabolism and transport (Yoo et al., 1999; Akiyama et al., 2002; Girnun et al., 2002; Dello Russo et al., 2003; Romera et al., 2007). Assuming neural tissue could utilize PPARγ in a similar fashion to that of non-neuronal cells, a functional PPARγ in neurons could act to protect neurons from damage.
Here, to assess the role of neuronal-PPARγ in susceptibility to ischemic injury, we generated neuron-specific PPARγ-null mice by employing the Cre-loxP technology and taking advantage of alpha-Ca2+/calmodulin-dependent-protein kinase II (αCaM-KII) promoter to achieve neuronal specificity (Kelly et al., 1987; Tsien et al., 1996). These mutant mice were subjected to ischemic injury using unilateral middle cerebral/common carotid artery occlusion. We have also generated neuronal cultures from these mice to capture changes in gene expression in neurons as a consequence of PPARγ deletion. Our study revealed that neuronal PPARγ deficiency is associated with augmented ischemic damage, as judged by both morphological and functional assays. In addition, neurons from PPARγ mutant mice demonstrated impaired expression of many pro-survival gene products and suffered more damage including oxidative stress when subjected to ischemia in vitro.
MATERIAL AND METHODS
Animals
All experiments were performed in 3–6 months old male and female mice. All animals were congenic with the C57BL/6 strain. Mice were fed standard mouse diet, and housed in standard mouse cages on a 12-hour inverted light–dark cycle. Behavioral analyses were carried out between 10:00 AM to 4:00 PM. For measurement of mouse body weight, food intake, and cerebral blood vasculature, data were collected and compared within littermates. Blood glucose was determined on whole venous blood by using an automatic glucometer (One Touch Basic, Lifescan, and Mountain View, CA). All experimental procedures were approved by the Institutional Animal Care Committee of University of Texas Health Science Center at Houston.
Generation of neuronal-specific PPARγ mutant mice (N-PPARγ-KO)
Floxed (fl) PPARγ (PPARγfl/fl) mice (B6.129-Ppargtm2Rev, Jackson Laboratory, stock # 004584) were crossed withαCaM-KII-Cre mice (Cui et al., 2005) to generate neuronal-specific PPARγ knockout mice, αCaM-KII-Cre/PPARf/f (N-PPARγ-KO). Specifically, we utilized the Tg-62-Cre line containing a 6.2Kb of αCaM-KII promoter region (Cui et al., 2004). The αCaM-KII gene is known to confer neuron-specific expression with tissue-selectivity predominantly in the adult forebrain, including hippocampus, cortex and caudate-putamen (Kelly et al., 1987; Olson et al., 1995; Tsien et al., 1996). Therefore, by placing Cre cDNA under the control of αCaM-KII promoter, neuronal-specific Cre expression could be achieved (Tsien et al., 1996). The PPARγfl/fl mice have two loxP sites introduced on either side of the exons 1 and 2 of PPARγ. Cre-mediated deletion of exon 1 and 2 result in loss of PPARγ (He et al., 2003). Specifically, to generate N-PPARγ-KO, αCaM-KII-Cre mice were first crossed with PPARγfl/fl mice to generate αCaM-KII-Cre/PPARf/+ mice (approximately half of the progenies produced from these crosses). Second, αCaM-KII-Cre/PPARfl/+ mice were again mated with PPARγfl/fl mice to generate N-PPARγ-KO mice. This second crossing served to delete both copies of the PPARγ alleles in αCaM-KII-Cre expressing neurons. Both PPARγfl/fl andαCaM-KII-Cre mice were maintained on a C57BL/6 genetic background for at least 6 generations. PPARγfl/fl mice were used as the control. Three to four month old animals were used in the in vivo experiments and 1 day old post-natal pups (p1) were used in the tissue culture experiments.
Genotyping
The genotyping for determiningαCaM-KII-Cre transgenic, PPARγ-LoxP and N-PPARγ–KO mice was performed by PCR method. The primer sequence and PCR condition is listed in Table 1. The expression of Cre and recombinant PPARγ in N-PPARγ-KO mice or in the cultured neurons was tested with RT-PCR. The primer sequence and PCR condition is listed in Table 2. In testing the recombinant PPARγ in the N-PPARγ-KO mice, the primer pair listed in Table 2 amplifies a full PPARγ band at 700 bp and two recombinant PPARγ bands at 400 bp and 300 bp. The 300bp band has the exact sequence predicted from the deletion of the loxP-flanked region. The 400bp product is identical to the 300-bp product, except that an additional 100 bp of intronic sequence from the intron 3 of PPARγ was spliced into the transcript (Hevener et al., 2003).
Table 1.
Primer Sequence for Genotyping
| Gene Name | Primer Sequence | Usage | Product Size | PCR Condition | |
|---|---|---|---|---|---|
| CaM-KII-Cre | F | 5′ - GGGAGGTAGGAAGAGCGATG | genotyping CaM-KII-Cre mice | 1200~1500 bp | 94°C (2 min), 65°C (50s), and 72°C (1.5 min), 30 cycles. |
| B | 5′ - CCATGAGTGAACGAACCTGG | ||||
| PPAR fl/fl | F | 5′ - TGTAATGGAAGGGCAAAAGG | genotyping PPAR flfl mice | 214 bp (WT) and 250 bp (Lox-P) | 94°C (1 min), 55°C (30s), and 72°C (30s), 30 cycles. |
| B | 5′ - TGGCTTCCAGTGCATAAGTT | ||||
| recombinant PPAR | F | 5′ - GTCACGTTCTGACAGGACTGTGTGAC | genotyping N- PPAR -KO | 700 bp (full) and 400 and 300 bp (recombinant) | 94°C (1 min), 55°C (30s), and 72°C (50s), 35 cycles. |
| B | 5′ - TATCACTGGAGATCTCCGCCAACAGC |
Table 2.
Primer Sequence for RT-PCR
| Gene Name | Primer Sequence | Seqence ID | Cycle # | Product Size (bp) | |
|---|---|---|---|---|---|
| Cre | F | GCATTACCGGTCGATGCAACGAGTG | AF234173.1 | 30 | 374 |
| B | GAACGCTAGAGCCTGTTTTGCACGTTC | ||||
| PPAR | F | TTCTCAGACTCGAAGCAGCA | NM_013672 | 35 | 346 |
| B | CACAACATACTGCCCACCAG | ||||
| SOD1 | F | AGGCTGTACCAGTGCAGGAC | X 06683 | 28 | 300 |
| B | GTTTACTGCGCAATCCCAAT | ||||
| CAT | F | CCTCGTTCAGGATGTGGTTT | X 52108 | 32 | 325 |
| B | GGCATCCCTGATGAAGAAAA | ||||
| GST | F | AAGACTGCCTTGGCAAAAGA | NM_029555 | 26 | 345 |
| B | GCCAGTATCTGTGGCTCCAT | ||||
| UCP | F | TCTCAGCCGGCTTAATGACT | NM_009463 | 32 | 445 |
| B | GTCGGTCCTTCCTTGGTGTA | ||||
| LXR | F | GGATAGGGTTGGAGTCAGCA | NM_013839 | 26 | 347 |
| B | GCAGGACTTGAGGAGGTGAG | ||||
| LPL | F | GCTGGCCTTGAACTCTCAAC | NM_008509 | 28 | 379 |
| B | CGGCAACAATCCTGGTACTT | ||||
| GAPDH | F | TGTTCCTACCCCCAATGTGT | NM_001001303 | 22 | 396 |
| B | TGTGAGGGAGATGCTCAGTG | ||||
| SYNP | F | AGACAGGCAGGTGAAGAGGA | NM_009305 | 20 | 483 |
| B | GGGCCACAGTCAGACAAAAT | ||||
| EAAT2 | F | TCTGAGGAGGCCAATACCAC | NMMU11763 | 30 | 312 |
| B | GTCCTTGATGGCGATGATCT |
LacZ staining to detect the Cre expression pattern
Mice carrying Tg-Cre were crossed with β-actin promoter-loxP-stoploxP-LacZ transgenic mice as we described (Cui et al., 2005). The double transgenic mice (carrying Tg-Cre and Tg-LacZ) were examined at 25 days old. The staining procedure using X-gal solution was performed exactly as we described recently (Cui et al., 2005).
Ischemia model in mice
In this experiment, we utilized 5–6 months old animals. Focal ischemia was induced by left middle cerebral artery (MCA) and left common carotid artery (CCA) occlusion, as we described previously (Aronowski et al., 2000). Briefly, all animals were fasted overnight with free access to water and then anesthetized with 0.35 g/kg i.p. injection of chloral hydrate. A single injection of chloral hydrate provided 2h of continuous anesthesia. The rectal temperature was monitored and maintained at 36.5+/−0.5°C during ischemia and the first hour of reperfusion using a feed-forward temperature controller (YSI Model 72, Yellow Springs, OH) utilizing a heating lamp and warming blanket. An incision was made through the left temporalis muscle perpendicular to a line drawn between the external auditory canal and the lateral canthus of the left eye. Under direct visualization with a surgical microscope, a burr hole was drilled at 1.5 mm rostral to the fusion of the zygomatic arch with the squamosal bone to expose the left MCA rostral to the rhinal fissure. A 0.005 inch diameter stainless steel wire (Small Parts Inc, Miami, FL) was placed underneath the left middle cerebral artery (MCA) rostral to the rhinal fissure, proximal to the major bifurcation of the MCA, and distal to the lenticulostriate arteries. The artery was then lifted, and the wire rotated clockwise. The CCA was occluded using atraumatic Heifetz aneurysm clips. Reperfusion after 60 min of occlusion is established by first removing the aneurysm clip from the CCA, and then rotating the wire counterclockwise and removing it from beneath the MCA. Interruption of flow through the MCA was inspected under the microscope and verified by cerebral perfusion (CP) measurement using a laser Doppler flowmeter (LDF, model BPM2, Vasamedics Inc, St Paul, MN). We used LDF to monitor CP at baseline (prior to occlusion), during MCA/CCA occlusion (at 5 and 55 min form the onset of occlusion), as well as during reperfusion (5 min after reversal of occlusion). The CP was collected by placing LDF probe on the surface of the skull at the location (3.5 mm caudal and 1.5 mm laterally to the bregma) that representing the ischemic penumbra.
Infarction volume measurement
The infarction volume was measured as we described (Aronowski et al., 1997). Briefly, the mice were deeply anesthetized by intraperitoneal injection of 0.6 g/kg of chloral hydrate, intracardiac perfusion with 40 ml of ice-cold PBS was performed before animals were decapitated. The brain was removed and sliced into 2-mm coronal sections using a plastic mold. The sections were stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC) for 30 min at room temperature and fixed in 10% formalin. The image of each section was digitized and the infarction volumes were determined morphometrically using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Infarcts produced by our protocol are restricted to cortical tissue. The infarct volume (mm3) was calculated from the difference between the volume of contralateral cortex and the volume of the TTC stained (non-ischemic) portion of ipsilateral cortex of each rat. This indirect measure of infarct volume, based on the assumption that the volume of the ipsi- and contralateral cortex are the same prior to ischemia, corrects the total infarct volume for the edema component (Swanson et al., 1990).
Neurological dysfunction (NDS) analysis
All behavioral tests in mouse were conducted in a quiet and low-lit room by an experimenter blinded with respect to the treatment groups. Pre-tests were conducted to exclude abnormally behaving animals. A combination score from a battery of behavioral tests (footfault, forelimb placing, postural reflex and cylinder tests) to measure the neurological functional deficits was used as we reported before (Zhao et al., 2007a).
Primary cortical neuron cultures
The neuronal culture was generated from cortices of littermates (within 24h after birth) of N-PPARγ-KO and the control (LoxP) mice. First, the cortices were dissected and dissociated by trituration as we described (Zhao et al., 2006a). The dissociated cells were plated on poly-L-lysine coated culture plates in Neurobasal medium with B27 at a cell density of 300 ~ 700/mm2. The cells were maintained in a CO2 incubator (5% CO2) at 37.0±0.5 °C. After 24h in culture, the cells were treated for 48h with 0.1 μM cytosine arabinoside (Ara-C) to inhibit glial growth. After 15 days in culture, the cells form extensive axonal and dendritic networks and are ready for the experiments. The expression of Cre and PPARγ in those neuronal cultures was determined at 5, 9 and 15d after being in culture in vitro.
Primary glial cultures
The primary glial cultures were prepared using the same methods as we described in the neuronal cultures except that the cells were cultured in DMEM+10% fetal bovine serum (FBS) without Ara-C. After 15 days in culture, the cells were subjected to a shaking procedure at 220 rpm for 2 hours to remove the neurons. The closely attached glial cells were used as the glia-enriched cells for measurement of the cell specificity to the OGD injury.
Determination of lactate dehydrogenase activity
The release of lactate dehydrogenase (LDH) into the culture medium was measured using a spectrophotometer and an LDH assay kit (Promega, Medison, WI) as we described (Zhao et al., 2006a).
Immunohistochemistry and neuronal counting
The cultured neurons grown on German Glass were either fixed in 4% paraformaldehyde at room temperature for 10 min (DAB staining) or were treated with 95% methanol containing 5% acetic acid at −15°C for 10 min. Immunostaining of microtubule associated protein (MAP2), Cre, NeuN or glial fibrillary acidic protein (GFAP) was performed by incubating cells with (1) rabbit polyclonal antibody for MAP2 (1:1000, Sigma-Aldrich for DAB), (2) chicken polyclonal antibody for MAP2 (1:1000, Abcam; immunofluorescence) (3) rabbit polyclonal antibody for Cre (Novagen; 1:1000), (4) goat polyclonal antibody for GFAP (1:1000) and (5) mouse monoclonal for NeuN (Chemicon, 1:1000) at 4°C for overnight. The MAP-2 signal was visualized with a goat anti-rabbit ABC Elite kit (Vector) followed by DAB (Vector). The goat anti-rabbit IgG-Alexa Fluor 488, rabbit anti-goat IgG-Alexa Fluor 546, goat anti-mouse IgG-Alexa Fluor 488 or goat anti-chicken IgG-Alexa Fluor 546 (all at 1:100, Invitrogen) were used to visualize the remaining antigens using a protocol we described recently (Zhao et al., 2006a). The Hoechst 33258 (1μg/ml, Invitrogen) was applied to stain the nuclei. The staining was captured using a Zeiss Axioscop 2 photomicroscope equipped with a motorized stage and CoolSnap ES (Photometrics) camera driven by MetaMorph software.
We used 10 μm thin cryosections of brains to stain for NeuN as we described previously (Felberg et al., 2002). The immunofluorescence images in 3 pre-selected areas of the cerebral cortex and dentate gyrus of hippocampus (same anatomical location was used for each animal) were recorded. The neurons (NeuN positive cells) were counted using a Metamorph program set for recognition of fluorescent pixels (immunofluorescent cells). Each area subjected to neuronal counting was set as 300 μm × 300μm. Six areas were counted per each animal. Three animals from each group were used for the analyses.
Oxygen-glucose-deprivation (OGD) injury model
Thirteen –15 days old primary cortical neurons or glia in culture were subjected to OGD exactly as we reported previously (Kim et al., 2004). Briefly, the culture media were replaced with the neurobasal medium without glucose and the cultures were placed in a gas-tight humidified chamber filled with 5% CO2/95% N2 for 1h for neurons and 2h for glia. At the end of OGD, the cultures were returned to their original culture condition and maintained for 24h. In the experiment using rosiglitazone (Rosig; 0.5μM final concentration), the compound was added to culture media 30min before induction of OGD and then maintained in the media during the entire recovery period.
Oxidative stress measurement
Oxidative stress in the cultured neurons was measured by assessing the oxidative damage to protein and lipid, as we reported (Zhao et al., 2007b). Briefly, samples containing 2 μg proteins of cell lysates were spotted on a nitrocellulose membrane using the Bio-Rad Dot Blot system. After air-drying, the membranes were probed with anti-nitrotyrosine (3′-NT, Upstate Biotechnology, 1 μg/ml) and anti-4-hydroxynone (4-HNE, Chemicon, AB5605) antibodies to measure the oxidative damage to proteins and lipids, respectively. The blots were incubated in goat anti-rabbit-HRP (1:5000) for 45 minutes and the antibodies were visualized with enhanced chemiluminescence (ECL; Pierce, Rockford, IL). Semi-quantification of the signal on X-ray film was achieved by analyses of optical density using a computer-assisted Kodak Analysis (EDAS 290)
Neurofilament (NF) degradation
Twenty μg of cell lysates from 15 day-old control and N-PPARγ-KO cultured neurons (generated from the cerebral cortices) at 24h after 1h of oxygen-glucose deprivation (OGD) were subjected to SDS-PAGE and followed by Western blotting. Rabbit anti-NF-M and NF-L antibodies (Abcam) were used as we described before (Aronowski et al., 1999). Immonopositive bands representing NF-M and NF-L were semi-quantified using densitometrical analysis, EDAS 290 system.
RT-PCR
The mRNA levels were semi-quantified by RT-PCR as we reported (Zhao et al., 2007a). We used mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene as an internal control. Each set of PCR reactions included the control samples run withoutRNA or the RT step was omitted to ensure that PCR productsresulted from amplification of the purified mRNA rather than of genomic DNA. The cycle number corresponding to the exponential amplification phase was determined individually for each gene and each preparation by the cycle-product curve. The sequences of primers and cycle number accepted for quantification are listed in Table 2. The PCR products were run on 1–2% agarose gels and stained with ethidium bromide. The image was digitized and the optical density of each band was quantified using a computer-assisted Kodak Analysis (EDAS) 290 system. The data (OD) were first normalized by internal control (GAPDH) and then expressed as percent change over the vehicle control.
Statistical Analysis
All data were expressed as mean±SEM. For in vitro experiments, we pooled the samples from three culture wells and repeated the experiments 3-times, unless otherwise stated in the figure legends. We performed statistical analyses with GraphPad and InStat programs. We used one-way analyses of variance (ANOVA) followed by the Newman-Keuls post-test for multiple comparison. Non-paired t-test was used when two groups were compared. Differences are considered significant if p ≥ 0.05.
RESULTS
Neuron-specific PPARγ-deficient mice (N-PPARγ-KO)
To study PPARγ in neurons, we generated neuronal-specific PPARγ knockout mice (N-PPARγ-KO) using Cre-loxP recombination system. To achieve neuronal selectivity in PPAR deletion, we crossed PPARγfl/fl mice with mice expressing Cre under a well-characterized αCaM-KII promoter to drive Cre expression (Olson et al., 1995; Tsien et al., 1996; Cui et al., 2005). Here, we specifically utilized a Tg-Cre-62 line of mice which allows Cre-loxP - mediated recombination to be completed by the 3rd postnatal week and to be confined to the cortex, striatum and hippocampus in the forebrain (Fig. 1A; illustrates the Cre-loxP recombination pattern with lacZ staining)(Tsien et al., 1996; Cui et al., 2005). As expected, we determined a strong presence of Cre expression and 11 an abundance of Cre-mediated recombination of PPARγ-flox allele in tissue samples from the cerebral cortex of N-PPARγ mice, as determined at 3 months of age (Fig. 1B).
Figure 1.
(A), Photograph of LacZ staining in the Tg-62-Cre mouse showing the Cre LoxP recombination pattern. A sagittal section shows that the Cre loxP recombination (indicated by blue X-gal signals) is restricted to the forebrain regions and primarily the cerebral cortex, striatum and hippocampus. Eosin was used for counterstain. (B), Detection of mutant PPARγ mRNA by RT-PCR analysis. Messenger RNAs for the Cre and PPARγ were isolated from cerebral cortex of N-PPARγ-KO and LoxP (Control) mice at 3 months of age. Arrowheads indicate presence of Cre products (upper panel) and recombined 300bp and 400bp PPARγ PCR products (lower panel) (see methods section for more details). Note that expression of Cre corresponds with the appearance of unique RT-PCR bands specific to the recombined fragments of PPARγ. (C), Both the control (bottom of the picture) and N-PPARγ-KO mice have normal appearance. (D), The NeuN immunofluorescence (green) demonstrates neuronal density and distribution in N-PPARγ-KO (D, E, F panels) and the control (G, H, I panels) mice. Coronal section through a whole brain (D, G); hilus of dentate gyrus (E, I), and cerebral cortex (F, H) of representative mice at 3 months of age are shown. (J), Summary bar graph illustrating neuronal (NeuN-positive cells) count in the cerebral cortex and in denature gyrus of hippocampus of Lox-P (control) and N-PPARγ-KO (KO) mice. Each count covered a 300μm × 300μm area. Data are expressed as mean ± SEM (n=6 fields counted in 3 animals). (K), Expression of astroglia specific PPARγ gene target, EAAT2, is not affected in cortex of N-PPARγ-KO, as compared to control mice, confirming neuronal specificity of the knockout.
The N-PPARγ-KO mice have normal appearance (Fig. 1C) and seem to be normal with respect to their gross brain structures, as determined by visually inspecting neurons in both the hippocampus and cerebral cortex (Fig. 1D and G). The hippocampal (Fig. 1E) and cortical (Fig. 1F) neurons in N-PPARγ-KO mice seem morphologically intact and demonstrate similar neural architecture/anatomy (Fig. 1H and I) and density (Fig 1J) to the control mice, indicating that PPARγ deficiency alone is not sufficient to cause neuronal loss or defective brain development. This notion is further supported by a similar low abundance of TUNEL positive cells or apoptosis-like morphology in the Hoechst stained nuclei in cerebral cortex or hippocampus of N-PPARγ-KO mice, as determined at 1, 3 and 6 months of age (data not included). It is likely that N-PPARγ mutation did not affect PPARγ activity in astroglia, as the expression of excitatory amino acid transporter 2 (EAAT2) that is known to be under the control of PPARγ and expressed mainly in astrocytes (Romera et al., 2007) was not affected in the forebrain of N-PPARγ-KO (Fig 1K).
The neuronal PPARγ deficiency did not impact animal growth and body weight (suggestive of intact metabolism), body temperature, daily behavioral activity, brain vascular anatomy and reproducibility, as determined by examining the mice for up to six months after birth. There were no differences in blood glucose, blood pressure and plasma ions (Na+, K+, Ca2+, Cl−, NaHCO−,) compositions between the neuron-PPARγ-KO and the control mice (data not shown). At 5 months of age, body weight of N-PPARγ-KO and control mice were similar and were 24.7 ± 2.4 grams (n=8) vs. 24.1 ± 3.9 grams (n=8), respectively.
N-PPARγ-KO mice are more susceptible to focal cerebral ischemia
At approximately 3 months of age, randomly selected male and female N-PPARγ-KO mice and age and sex-matched littermate controls were subjected to a transient 60 min MCA/CCAo followed by three days of reperfusion. The damage produced with this ischemia method is restricted to the cortical tissue; overlapping with the distribution of Cre expression. In response to ischemia, N-PPARγ-KO mice developed 40% larger infarction, as compared to control mice (28.9 +/− 4.5 mm3 vs. 17.4 +/− 2.3 mm3 infarct volume) (Fig. 2A and C). The increased vulnerability to ischemic damage was most likely not dependent on gender, as both male and female N-PPARγ-KO mice demonstrated either strong trend (males) or statistical difference (females) toward increased vulnerability to ischemic damage (Fig. 2B). The larger volume of infarction in N-PPARγ-KO mice coincided with increased neurological dysfunction (Fig. 2D), indicating a close association between the volume of histological damage and neurological deficit.
Figure 2.
Neuronal PPARγ deficiency aggravates cerebral ischemic damage. N-PPARγ-KO (KO) and control (Lox-P) mice were subjected to reversible 60 min MCA/CCA occlusion. (A), The infarct volumes representing composite of all the control (white filling) and KO (grey filling) mice (individual mice are indicated as squares or diamonds within each group, (B) segregated by gender (M - male and F – female; number below each bar indicates the sample size). *p<0.05., and (C) infarct areas at six rostro-caudal plains were determined from morphometric analyses of TTC stained brains sections at 3 days after the onset of ischemia. Representative photographs of TTC-stained brain sections demarcate infarcts in control (Lox-P) and N-PPARγ-KO (KO) mice (included in panel A). Infarct volume in all groups was established at 3 days after ischemia. (D), Bar graph illustrating the neurological deficit composite score (NDS) as measured at 3 days after ischemia using a battery of behavioral tests. Number below each bar indicates the sample size. *p<0.05. (E), Bar graph illustrating cerebral perfusion values in the peri-ischemic areas of control (n=10) and N-PPARγ-KO mice (n=11) at four time points representing: 5 min prior to ischemia (Pre-ischemia; baseline), 5 min and 55 min after induction of ischemia, and 10 min after reversal of the occlusion (reperfusion) measured using Laser Doppler flowmetry. The results are presented as mean ± SEM.
The increased vulnerability to ischemic damage was likely not due to changes in cerebral perfusion (CP) as the values of perfusion in N-PPARγ-KO mice at baseline (prior to occlusion), during ischemia and upon reperfusion were indistinguishable between N-PPARγ-KO and control mice (Fig. 2E).
Finally there was no difference in surgery/stroke-related mortality between N-PPARγ-KO and control mice. The mortality was 26% and 29% for the control and knockout group, respectively. All deaths occurred within the first 24h after the stroke.
Primary neurons from N-PPARγ-KO mice show reduced expression of PPARγ and PPARγ-dependent genes
To better understand the profile and consequences of PPARγ deletion in neurons, we employed primary neuron-glia co-culture or neuron-enriched culture system and N-PPARγ-KO mice as a source of PPARγ null neurons. Earlier published studies demonstrated that the activity of αCaM-KII promoter is barely detectable at day 4 postnatal, but is increased as much as 10-fold by day 16 (Scholz et al., 1988; Burgin et al., 1990). In agreement with this study, the αCaM-KII promoter activity in neurons, as measured by Cre mRNA expression was negligible for up to nine days (Fig. 3A), followed by a robust increase at day 15. Additionally, Cre protein expression was confined to the nuclei, as determined using immunoreactivity for Cre protein in the neuron/glia co-culture system (Fig. 3B). Further studies using double immunostaining for Cre and cell-specific marker demonstrated that Cre co-localizes to nuclei of neurons (NeuN positive cells) (Fig 3C), but not astroglia (GFAP positive cells) (Fig 3D). The Cre expression in cultured neurons coincided with significant loss of PPARγ protein (Fig. 3E), demonstrating that it is possible to induce PPARγ gene knockdown in neurons using αCaM-KII promoter-driven Cre expression.
Figure 3.
(A), Photograph of RT-PCR gel demonstrating the Cre recombinase in neurons in culture that were produced from cortices of N-PPARγ-KO and control (Lox-P) mice, and (B), Immunohistochemistry for Cre protein in neuron-glial co-culture to show that the Cre is localized primarily to nuclei of cells with neuronal morphology (red arrows), but not in smaller neuroglia cells (blue arrowheads). (C and D) Immunofluorescence demonstrating that Cre immunopositive cells in cultured neurons co-localize to MAP-2 immunopositive (C), but not to GFAP immunopositive (D) cells in neuron-glia co-culture. Cells were stained for Cre (green), MAP-2 (neurons; red, C), GFAP (astroglia, red; D) and nuclei (Hoechst 33258; blue). Arrows in panel C identify cells positive for both Cre and MAP2, and arrows in panel D identify nuclei of GFAP positive cells. Note that immunofluorescence for Cre does not overlap with the location of nuclei of GFAP positive cells. (E) Bar graph (n=3) and a representative Western blot demonstrating PPARγ and β-actin protein level (determined via densitometrical analyses) in the neurons in culture that was generated from N-PPARγ-KO and the control (Lox-P) mice, at 15 days in culture. (F and G), Quantification of mRNA expression in neurons in culture. (F), Photographs of representative agarose gels of RT-PCR products, and (G), Bar graph showing densitometric quantification of RT-PCR products of the selected PPARγ-regulated genes in neuronal cultures generated from N-PPAR-KO (KO) and control (Lox-P) mice, as measured after 15 days in culture. Synaptophysin (SYNAPT) and GAPDH were used as reference controls. The data is expressed as mean +/− SEM (N=5–6). In E and G, *p≤0.05 from control.
In agreement with the consequences of deficient PPARγ expression in neurons, we observed significant reduction in PPARγ-dependent gene expression (Fig. 3F and G), as studied in 15-day-old cultured cortical neurons. These included genes encoding for enzymes involved in neutralization of reactive oxygen radicals (ROS), such as Cu, Zn-Superoxide dismutase (SOD1), catalase (CAT) and glutathione S-transferase (GST), enzyme involved in proper functioning of mitochondria, uncoupling protein-1 (UCP-1), enzymes involved in lipid metabolism, lipoprotein lipase (LPL), and transcription factor liver X receptor-alpha (LXRα). While we detected reduction in the expression of canonical PPARγ gene products, there was no change in expression in GAPDH or synaptophysin genes. This suggests that changes in gene expression is specific to PPARγ targets and that deficit of PPARγ in neurons is not sufficient to compromise neuronal integrity including the presence of synapse associated protein, synaptophysin that is often considered a surrogate marker for synapse density changes.
PPARγ-deficient in neurons renders neurons more susceptible to OGD-induced damage
To establish the relationship between PPARγ and neuronal susceptibility to ischemic injury, we first subjected the cultured neurons harvested from N-PPARγ-KO and control mice to oxygen-glucose-deprivation (OGD) injury. The damage level was assessed by quantifying LDH and the oxidative stress to proteins (3-NT) and lipids (4-HNE). Also, we analyzed the neurite damage based on neurofilament loss (using western blot) and gross morphological features of MAP2 immunohistochemical study. All these assays were conducted at 24h after OGD.
As predicted from the in vivo experiments, PPARγ-deficient neurons in response to OGD demonstrated more severe damage than the control neurons, as determined with assays for LDH release (Fig. 4A), morphological damage (Fig. 4B), neurofilament proteolysis (Fig. 4C) and oxidative stress (Fig. 4F). In agreement with the protective function of PPARγ, PPARγ-deficient neurons upon exposure to OGD injury did not benefit from the treatment with PPARγ activating agent, rosiglitazone, as compared to the control neurons (Fig. 4A). Since OGD-mediated damage is at least in part associated with oxidative stress, we tested the hypothesis that PPARγ deficiency reduces neuron’s ability to handle oxidative insult. Again, we used cultured neurons from N-PPARγ-KO mice and subjected them to a toxic level of hydrogen peroxide to model oxidative stress. In agreement with our postulate, PPARγ deficient neurons were significantly more vulnerable to hydrogen peroxide-induced injury (Fig. 4D).
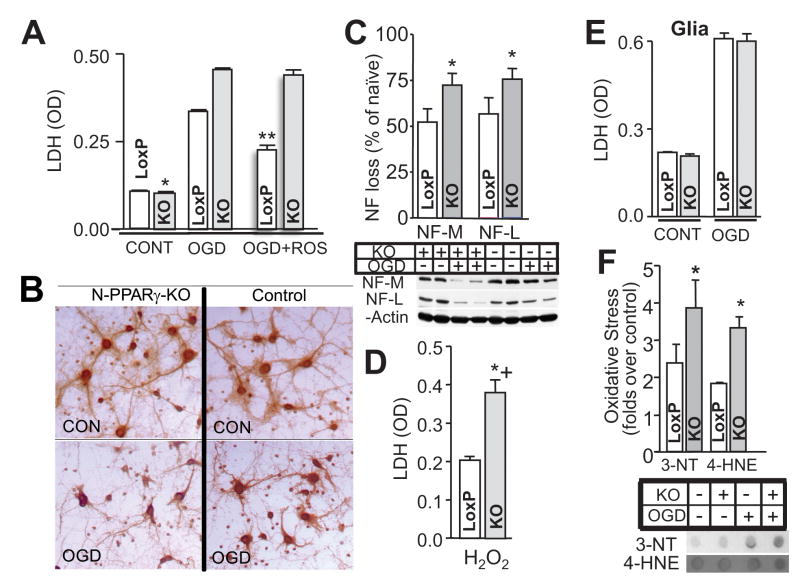
Figure 4.
PPARγ deficiency renders neurons more vulnerable to damage. Neuron-enriched cultures (A, B, C, D, F) and glia-enriched cultures (E), generated from the control (LoxP) and N-PPARγ-KO (KO) mice were exposed to oxygen-glucose deprivation injury (OGD) and the level of injury at 24 h was determined using: LDH assay (A and E), loss of MAP-2 integrity (B), oxidative stress (F; immuno-dot-blot for 3-nitrotyrosine, 3′-NT, and 4-hydroxynone, 4-HNE), and neurofilament hydrolysis (C; immunoblot for parent NF-L and NF-M protein). The effect of PPARγ agonist, rosiglitazone (ROSIG) on the OGD-induced injury in control and N-PPARγ-KO (KO) is also illustrated (A; last two bars). (D), Bar graph illustrating LDH release from neurons in culture generated from LoxP and N-PPARγ-KO mice in response to H2O2-induced injury. In A – F, n=3 independent experiments performed in triplicates (i.e. 3 culture well per experiment). *p≤0.05 from control; **p<0.05 from KO and LoxP without ROSIG.
The neuron-selective PPARγ deletion did not appear to influence vulnerability of other brain cells to ischemia. Glia-enriched cultures from N-PPARγ-KO mice subjected to OGD demonstrated undistinguishable level of damage as compared to glia from the control mice (Fig. 4E).
DISCUSSION
In this study, in order to gain more insight into the role of neuronal PPARγ, we generated neuronal-specific PPARγ knockout mouse using LoxP-Cre-system. These mutant mice are fully viable, have intact brain anatomy and appear to develop normally. Because, we used αCaM-KII promoter to achieve selective expression of Cre in neurons, we were likely able to retain undisturbed prenatal developmental process, since the induction of αCaM-KII promoter (employed here to drive Cre expression; therefore mutation) does not occur until the early postnatal days (Burgin et al., 1990). In this study, we utilized a Tg-Cre-62 line of mice (Cui et al., 2004) which confines Cre recombinase expression to the forebrain, thus overlapping the middle cerebral artery territory (ischemia-affected aspect of the brain). Our results show that these PPARγ deficient mice suffer more extensive damage in response to MCA/CCA occlusion, as revealed by increased volume of infarction and more pronounced functional deficit. This aggravated damage is likely not due to altered cerebral perfusion, since laser Doppler data indicate that both the control and N-PPARγ-KO mice demonstrated similar level of blood perfusion reduction upon MCA occlusion, as well as similar level of perfusion during the early post-ischemic phase. The lack of PPARγ involvement in cerebral perfusion in this study is consistent with our earlier and others’ reports, showing that PPARγ-activating agents reduced ischemic damage without affecting intraischemic and early post-ischemic cerebral perfusion (Zhao et al., 2005; Victor et al., 2006; Tureyen et al., 2007). While PPARγ is expressed by blood vessel cells where it plays diverse regulatory roles (Beyer et al., 2008), its function in context of acute stroke, at least with respect to blood flow regulation appears to be limited. This suggests that some processes mediated via PPARγ within neurons protect neurons from ischemic damage. To test this hypothesis, we cultured cortical neurons from N-PPARγ-KO mice and probed their vulnerability by subjecting them to ischemia-like or oxidative stress injuries in vitro. In agreement with this hypothesis, PPARγ deficient neurons showed increased damage in response to ischemic or oxidative insult. Furthermore, in agreement with the pro-survival role of PPARγ, neurons deficient in PPARγ (in contrast to control neurons) did not benefit from the protective effect of PPARγ agonist, rosiglitazone. This experiment also indicates that rosiglitazone in fact requires PPARγ to provide neuroprotective effect. It is important to note that PPARγ deficiency does not affect the baseline neuronal health (including presence of synaptic vesicle glycoprotein, synaptophysin), suggesting that the role of PPARγ in neurons is in maintaining cytoprotective/defense capacity of these cells. These results are consistent with our earlier study demonstrating that PPARγ antagonists (GW9662 and T0070907) at the concentration sufficient to reverse neuroprotective effect of PPARγ agonists did not cause neuronal injury (Zhao et al., 2006a).
Based on earlier studies with agents activating PPARγ from our and other laboratories, it was demonstrated that PPARγ mitigates inflammation following ischemic and hemorrhagic stroke (Sundararajan and Landreth, 2004; Sundararajan et al., 2005; Zhao et al., 2005; Luo et al., 2006; Zhao et al., 2006b; Tureyen et al., 2007). Because inflammation is frequently recognized as a process contributing to the secondary brain damage (Garcia et al., 1994; del Zoppo et al., 2000; Aronowski and Hall, 2005), the anti-inflammatory effect of PPARγ agonists was proposed as a key mechanism underlying the beneficial role of PPARγ. However, in addition to its anti-inflammatory effect, PPARγ is known to regulate the expression of some important anti-oxidative enzymes such as catalase, SOD1 and GST (Yoo et al., 1999; Girnun et al., 2002; Park et al., 2004) that could effectively ameliorate oxidative stress, a recognized factor contributing to ischemic damage (Chan, 2001). Concurring with this notion, animals treated with PPARγ agonists demonstrated upregulated catalase and SOD1 in the injured brain (Park et al., 2004; Shimazu et al., 2005; Tureyen et al., 2007; Zhao et al., 2007a), suggesting that anti-oxidative effect of PPARγ could indeed participate in protecting ischemic brain. Specifically, our study demonstrated that microglia in culture treated with PPARγ agonist increased expression of catalase, reduced generation of H2O2 and ultimately became less damaging toward neurons, as tested in neurons-microglia co-cultures (Zhao et al., 2007a). Since both pro-inflammatory mediators and oxidative stress are the hallmarks of neurotoxicity via activated microglia (Gao et al., 2002; Qin et al., 2005), we initially proposed that inhibition of these microglia-mediated deleterious processes represents a key component of the beneficial effects of PPARγ.
Though, the role of PPARγ activation in ameliorating the adverse effect of microglia is likely, our present study with PPARγ deficient neurons strongly suggests that in addition to microglia, PPARγ is important in neuronal self-defense against ischemic and oxidative injuries. The analysis of gene expression in neurons cultured from N-PPARγ-KO mice showed that the disruption of PPARγ impairs expression of numerous genes that could improve neuronal resistance to ischemic and oxidative injury. First, PPARγ deficiency resulted in reduced expression of anti-oxidative catalase, SOD and GST, thus providing a likely explanation for increased oxidative damage to proteins and lipids, as well as increased susceptibility of these neurons to oxygen-glucose deprivation or oxidative (H2O2) injuries. Besides the gene products with anti-oxidative function, we also identified several other PPARγ-dependent genes with potential capacity to mediate anti-ischemic effects that were downregulated in neurons as a consequence of PPARγ deficiency. The first gene is the pleiotropic transcription factor, liver X-receptor-α (LXRα). Though, we have not tested this possibility here, the reduced LXRα expression may have a direct impact on the increased ischemic vulnerability. The existing data suggest that LXRα possess pro-survival functions (Joseph et al., 2004), and that LXR agonist reduces damage after focal cerebral ischemia injury (Sironi et al., 2008). Another PPARγ regulated gene reduced in PPARγ deficient neurons is lipoprotein lipase (LPL) an enzyme that functions to hydrolyze lipids and lipoproteins. Although the pro-survival function of LPL has not been studied, LPL is abundant in neurons and is upregulated in response to focal ischemia (Paradis et al., 2004). Further functional studies on the role of LPL in ischemia are warranted. Finally, we have identified a downregulation of uncoupling protein-1 (UCP1) in PPARγ deficient neurons, confirming the regulatory role of PPARγ on uncoupling proteins (Kelly et al., 1998; Villarroya et al., 2007). Uncoupling proteins are the inner mitochondrial membrane proteins that dissipate the proton gradient capable of affecting free radical generation and cell viability in the context of ischemia and reperfusion including in heart and brain (Hoerter et al., 2004; Chen et al., 2006). We have also determined that neuronal deficiency in PPARγ did not impact nNOS, eNOS and iNOS expression in cultured neurons (data not included).
Our analysis of mouse brain after cerebral ischemia showed that selective neuronal PPARγ deficiency causes significant increase in the infract volume, indicating that the tissue damage is embracing not only neurons, but also other non-neuronal cells. The reason why change in neuronal genotype/phenotype affects susceptibility of other than neurons cells to ischemic damage is not clear, though well-documented in the existing literature. For instance, excitotoxic insult (e.g. intracerebral injection of NMDA) preferentially damages neurons though the lesion produced around the area of excitotoxin injection consists of mixed cell loss. Additionally, studies with other cell specific mutant mice tested for ischemic vulnerability showed that neuronal-selective inhibition of inhibitor κB kinase-β (IKKβ) (Herrmann et al., 2004) reduced overall infarct size, while neuronal-selective HIF-1α deficiency leads to increase infarct volume (Baranova et al., 2007). One possible explanation for such inclusive cell loss is that the dying neurons adversely impact surrounding cells by the release/spillage of the cytotoxic content following their injury/lysis. Thus, in N-PPARγ-KO mice, ischemia-induced death of more vulnerable neurons in the transitional ischemic zone (distal penumbra) due to PPARγ deficiency may injure adjacent cells, causing infarct volume to enlarge. Alternatively, loss of non-neuronal cells may suggest the instrumental role of neurons in maintaining integrity of all the cells comprising the neurovascular unit. Taken as a whole, it is important to stress that our present study provides additional evidence that selective change in neuronal resistance to ischemia adversely affects survival of all brain cells in ischemia endangered zone.
Since estrogen receptors are linked to neuroprotection and anti-ischemic effect (McCullough et al., 2001), and PPARγ directly modulates transactivation of estrogen receptor-α (Houston et al., 2003), we were interested in establishing whether PPARγ deficiency may have different effect on ischemic vulnerability in male vs. female mice. Our data demonstrate that PPARγ deficiency increases ischemic brain damage in both genders, suggesting that changes in estrogen receptor expression or other gender-related cellular processes do not likely contribute to the overall increased vulnerability of N-PPARγ-KO mice.
In conclusion, our data unveil a previously unclear neuroprotective role for PPARγ in neuronal self-protection from insults such as ischemia and oxidative stress. The present data indicate that PPARγ in neurons is not essential for normal neuronal well-being, but it plays an important role in protecting neurons from damage by ischemic and oxidative injury. This cytoprotective role of PPARγ is likely due to induction of multiple pro-survival gene products and their multimodal effects. Finally, our data suggest that the neuroprotective function of PPARγ reinforces the growing body of evidence recognizing PPARγ as a logical target for neurological diseases with pathogenesis including ischemia and/or oxidative stress.
Acknowledgments
This project was supported by R01NS052791 and 1R21NS057284 grants from NIH/NINDS.
References
- Akiyama TE, Sakai S, Lambert G, Nicol CJ, Matsusue K, Pimprale S, Lee YH, Ricote M, Glass CK, Brewer HB, Jr, Gonzalez FJ. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol Cell Biol. 2002;22:2607–2619. doi: 10.1128/MCB.22.8.2607-2619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–279. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Aronowski J, Cho KH, Strong R, Grotta JC. Neurofilament proteolysis after focal ischemia; when do cells die after experimental stroke? J Cereb Blood Flow Metab. 1999;19:652–660. doi: 10.1097/00004647-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Aronowski J, Grotta JC, Strong R, Waxham MN. Interplay between the gamma isoform of PKC and calcineurin in regulation of vulnerability to focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:343–349. doi: 10.1097/00004647-200002000-00016. [DOI] [PubMed] [Google Scholar]
- Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27:6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Beyer AM, Baumbach GL, Halabi CM, Modrick ML, Lynch CM, Gerhold TD, Ghoneim SM, de Lange WJ, Keen HL, Tsai YS, Maeda N, Sigmund CD, Faraci FM. Interference with PPARgamma signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension. 2008;51:867–871. doi: 10.1161/HYPERTENSIONAHA.107.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC, Kelly PT. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990;10:1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chen SD, Wu HY, Yang DI, Lee SY, Shaw FZ, Lin TK, Liou CW, Chuang YC. Effects of rosiglitazone on global ischemia-induced hippocampal injury and expression of mitochondrial uncoupling protein 2. Biochem Biophys Res Commun. 2006;351:198–203. doi: 10.1016/j.bbrc.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- Cui Z, Lindl KA, Mei B, Zhang S, Tsien JZ. Requirement of NMDA receptor reactivation for consolidation and storage of nondeclarative taste memory revealed by inducible NR1 knockout. Eur J Neurosci. 2005;22:755–763. doi: 10.1111/j.1460-9568.2005.04257.x. [DOI] [PubMed] [Google Scholar]
- Cui Z, Wang H, Tan Y, Zaia KA, Zhang S, Tsien JZ. Inducible and reversible NR1 knockout reveals crucial role of the NMDA receptor in preserving remote memories in the brain. Neuron. 2004;41:781–793. doi: 10.1016/s0896-6273(04)00072-8. [DOI] [PubMed] [Google Scholar]
- del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Russo C, Gavrilyuk V, Weinberg G, Almeida A, Bolanos JP, Palmer J, Pelligrino D, Galea E, Feinstein DL. Peroxisome proliferator-activated receptor gamma thiazolidinedione agonists increase glucose metabolism in astrocytes. J Biol Chem. 2003;278:5828–5836. doi: 10.1074/jbc.M208132200. [DOI] [PubMed] [Google Scholar]
- Felberg RA, Grotta JC, Shirzadi AL, Strong R, Narayana P, Hill-Felberg SJ, Aronowski J. Cell death in experimental intracerebral hemorrhage: The “black hole” model of hemorrhagic damage. Ann Neurol. 2002;51:517–524. doi: 10.1002/ana.10160. [DOI] [PubMed] [Google Scholar]
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Yoshida Y, Lian J, Chen S, del Zoppo GJ. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat) Am J Pathol. 1994;144:188–199. [PMC free article] [PubMed] [Google Scholar]
- Giannini S, Serio M, Galli A. Pleiotropic effects of thiazolidinediones: taking a look beyond antidiabetic activity. J Endocrinol Invest. 2004;27:982–991. doi: 10.1007/BF03347546. [DOI] [PubMed] [Google Scholar]
- Girnun GD, Domann FE, Moore SA, Robbins ME. Identification of a functional peroxisome proliferator-activated receptor response element in the rat catalase promoter. Mol Endocrinol. 2002;16:2793–2801. doi: 10.1210/me.2002-0020. [DOI] [PubMed] [Google Scholar]
- He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann O, Baumann B, Schwaninger M. Inhibition of IKK-2 reduces infarct size in a model of cerebral ischemia. 10 th International Symposium on Pharmacology of Cerebral Ischemia Abstract; 2004. p. 69. [Google Scholar]
- Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J. Muscle-specific Pparg deletion causes insulin resistance. Nat Med. 2003;9:1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- Hoerter J, Gonzalez-Barroso MD, Couplan E, Mateo P, Gelly C, Cassard-Doulcier AM, Diolez P, Bouillaud F. Mitochondrial uncoupling protein 1 expressed in the heart of transgenic mice protects against ischemic-reperfusion damage. Circulation. 2004;110:528–533. doi: 10.1161/01.CIR.0000137824.30476.0E. [DOI] [PubMed] [Google Scholar]
- Houston KD, Copland JA, Broaddus RR, Gottardis MM, Fischer SM, Walker CL. Inhibition of proliferation and estrogen receptor signaling by peroxisome proliferator-activated receptor gamma ligands in uterine leiomyoma. Cancer Res. 2003;63:1221–1227. [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, Hogenesch J, O’Connell RM, Cheng G, Saez E, Miller JF, Tontonoz P. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Kelly LJ, Vicario PP, Thompson GM, Candelore MR, Doebber TW, Ventre J, Wu MS, Meurer R, Forrest MJ, Conner MW, Cascieri MA, Moller DE. Peroxisome proliferator-activated receptors gamma and alpha mediate in vivo regulation of uncoupling protein (UCP-1, UCP-2, UCP-3) gene expression. Endocrinology. 1998;139:4920–4927. doi: 10.1210/endo.139.12.6384. [DOI] [PubMed] [Google Scholar]
- Kelly PT, Shields S, Conway K, Yip R, Burgin K. Developmental changes in calmodulin-kinase II activity at brain synaptic junctions: alterations in holoenzyme composition. J Neurochem. 1987;49:1927–1940. doi: 10.1111/j.1471-4159.1987.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Kim DH, Zhao X, Tu CH, Casaccia-Bonnefil P, Chao MV. Prevention of apoptotic but not necrotic cell death following neuronal injury by neurotrophins signaling through the tyrosine kinase receptor. J Neurosurg. 2004;100:79–87. doi: 10.3171/jns.2004.100.1.0079. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J Neurochem. 2006;97:435–448. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke. 2001;32:796–802. doi: 10.1161/01.str.32.3.796. [DOI] [PubMed] [Google Scholar]
- Olson NJ, Masse T, Suzuki T, Chen J, Alam D, Kelly PT. Functional identification of the promoter for the gene encoding the alpha subunit of calcium/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1995;92:1659–1663. doi: 10.1073/pnas.92.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Z, Zhao X, Labiche LA, Strong R, Grotta JC, Herrmann O, Aronowski J. Neuronal expression of peroxisome proliferator-activated receptor-gamma (PPARgamma) and 15d-prostaglandin J(2)-Mediated protection of brain after experimental cerebral ischemia in rat. Brain Res. 2006;1096:196–203. doi: 10.1016/j.brainres.2006.04.062. [DOI] [PubMed] [Google Scholar]
- Paradis E, Clavel S, Julien P, Murthy MR, de Bilbao F, Arsenijevic D, Giannakopoulos P, Vallet P, Richard D. Lipoprotein lipase and endothelial lipase expression in mouse brain: regional distribution and selective induction following kainic acid-induced lesion and focal cerebral ischemia. Neurobiol Dis. 2004;15:312–325. doi: 10.1016/j.nbd.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Park EY, Cho IJ, Kim SG. Transactivation of the PPAR-responsive enhancer module in chemopreventive glutathione S-transferase gene by the peroxisome proliferator-activated receptor-gamma and retinoid X receptor heterodimer. Cancer Res. 2004;64:3701–3713. doi: 10.1158/0008-5472.CAN-03-3924. [DOI] [PubMed] [Google Scholar]
- Pereira MP, Hurtado O, Cardenas A, Alonso-Escolano D, Bosca L, Vivancos J, Nombela F, Leza JC, Lorenzo P, Lizasoain I, Moro MA. The nonthiazolidinedione PPARgamma agonist L-796,449 is neuroprotective in experimental stroke. J Neuropathol Exp Neurol. 2005;64:797–805. doi: 10.1097/01.jnen.0000178852.83680.3c. [DOI] [PubMed] [Google Scholar]
- Qin L, Liu Y, Qian X, Hong JS, Block ML. Microglial NADPH oxidase mediates leucine enkephalin dopaminergic neuroprotection. Ann N Y Acad Sci. 2005;1053:107–120. doi: 10.1196/annals.1344.009. [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Wong SM, Cotman CW, Cribbs DH. 15-deoxy-delta12,14-prostaglandin J2, a specific ligand for peroxisome proliferator-activated receptor-gamma, induces neuronal apoptosis. Neuroreport. 2001;12:839–843. doi: 10.1097/00001756-200103260-00043. [DOI] [PubMed] [Google Scholar]
- Romera C, Hurtado O, Mallolas J, Pereira MP, Morales JR, Romera A, Serena J, Vivancos J, Nombela F, Lorenzo P, Lizasoain I, Moro MA. Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPARgamma target gene involved in neuroprotection. Journal of Cerebral Blood Flow & Metabolism. 2007;27:1327–1338. doi: 10.1038/sj.jcbfm.9600438. [DOI] [PubMed] [Google Scholar]
- Scholz WK, Baitinger C, Schulman H, Kelly PT. Developmental changes in Ca2+/calmodulin-dependent protein kinase II in cultures of hippocampal pyramidal neurons and astrocytes. J Neurosci. 1988;8:1039–1051. doi: 10.1523/JNEUROSCI.08-03-01039.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T, Inoue I, Araki N, Asano Y, Sawada M, Furuya D, Nagoya H, Greenberg JH. A peroxisome proliferator-activated receptor-gamma agonist reduces infarct size in transient but not in permanent ischemia. Stroke. 2005;36:353–359. doi: 10.1161/01.STR.0000152271.21943.a2. [DOI] [PubMed] [Google Scholar]
- Sironi L, Mitro N, Cimino M, Gelosa P, Guerrini U, Tremoli E, Saez E. Treatment with LXR agonists after focal cerebral ischemia prevents brain damage. FEBS Lett. 2008;582:3396–3400. doi: 10.1016/j.febslet.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumvoll M, Haring HU. Glitazones: clinical effects and molecular mechanisms. Ann Med. 2002;34:217–224. [PubMed] [Google Scholar]
- Sundararajan S, Landreth GE. Antiinflammatory properties of PPARgamma agonists following ischemia. Drug News Perspect. 2004;17:229–236. doi: 10.1358/dnp.2004.17.4.829049. [DOI] [PubMed] [Google Scholar]
- Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume [see comments] J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, Vemuganti R. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- Uryu S, Harada J, Hisamoto M, Oda T. Troglitazone inhibits both post-glutamate neurotoxicity and low-potassium-induced apoptosis in cerebellar granule neurons. Brain Res. 2002;924:229–236. doi: 10.1016/s0006-8993(01)03242-5. [DOI] [PubMed] [Google Scholar]
- Victor NA, Wanderi EW, Gamboa J, Zhao X, Aronowski J, Deininger K, Lust WD, Landreth GE, Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur J Neurosci. 2006;24:1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- Villarroya F, Iglesias R, Giralt M. PPARs in the Control of Uncoupling Proteins Gene Expression. PPAR Res. 2007;2007:74364. doi: 10.1155/2007/74364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HY, Chang MS, Rho HM. Induction of the rat Cu/Zn superoxide dismutase gene through the peroxisome proliferator-responsive element by arachidonic acid. Gene. 1999;234:87–91. doi: 10.1016/s0378-1119(99)00176-6. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ou Z, Grotta JC, Waxham N, Aronowski J. Peroxisome-proliferator-activated receptor-gamma (PPARgamma) activation protects neurons from NMDA excitotoxicity. Brain Res. 2006a;1073–1074:460–469. doi: 10.1016/j.brainres.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang Y, Strong R, Grotta JC, Aronowski J. 15d-Prostaglandin J(2) activates peroxisome proliferator-activated receptor-gamma, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2006b;26:811–820. doi: 10.1038/sj.jcbfm.9600233. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sun G, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: Role for PPARg in microglia/macrophage. Ann Neurol. 2007a;61:352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, Grotta JC, Aronowski J. Transcription Factor Nrf2 Protects the Brain From Damage Produced by Intracerebral Hemorrhage. Stroke. 2007b;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J. The intracerebral application of the PPARgamma-ligand pioglitazone confers neuroprotection against focal ischaemia in the rat brain. Eur J Neurosci. 2005;22:278–282. doi: 10.1111/j.1460-9568.2005.04200.x. [DOI] [PubMed] [Google Scholar]