Abstract
BACKGROUND AND PURPOSE:
Pentobarbital is known to affect cerebral metabolism; pentobarbital sedation is, however, frequently used for magnetic resonance (MR) imaging and MR spectroscopy, especially in children. Accurate assessment of the brain metabolite levels is important, particularly in neonates with suspected brain injury. We investigated whether pentobarbital sedation has any effect on the ratios of spectral metabolites lactate, N-acetylaspartate or choline in a group of premature neonates.
METHODS:
MR spectroscopy was performed in 43 premature neonates, all with normal concurrent MR imaging, and normal neuro-developmental outcome at 12 months of age. Of those neonates, 14(33%) required pentobarbital (Nembutal 1mg/Kg) sedation during MR spectroscopy, the remaining 29 neonates did not receive any sedation. Ratios of lactate, choline and N-acetylaspartate were calculated in the basal ganglia, thalami, and corticospinal tracts, and compared between those neonates with and without sedation.
RESULTS:
Small amounts of brain lactate were detected in all of the premature neonates. The basal ganglia lactate/choline and lactate / N-acetylaspartate ratios were significantly lower, by 17% and 25% respectively, in the neonates with pentobarbital sedation compared to the age-matched neonates without sedation (P < 0.05). Sedation did not affect the lactate level in the thalami or the corticospinal tracts. The N-acetylaspartate / choline ratios were unaffected by pentobarbital sedation.
CONCLUSION:
Pentobarbital sedation is associated with lower lactate/choline and lactate/ N-acetylaspartate ratios in the basal ganglia of premature neonates, as determined by proton MR spectroscopy. Investigators should be aware of this phenomenon for accurate interpretation of their MR spectroscopy results.
Introduction
Previous studies have shown that MR spectroscopy can provide clinically important assessment of neonatal brain metabolism 1-12. Elevated brain metabolite ratios, lactate to N-acetylaspartate (NAA) and lactate to choline, have been associated with poor neurodevelopmental status in neonates with hypoxic-ischemic encephalopathy 1, 10, 12, 13. The increase in brain lactate level is thought to be a result of anaerobic rather than oxidative energy metabolism during cellular hypoxia 14.
Frequently, neonates require sedation during MR examination in order to reduce motion and improve scan quality. It is known that the metabolism of the brain can be affected by barbiturate sedation 15, 16. In particular, barbiturates have been noted to decrease cerebral lactate concentration 17, 18. Therefore, we investigated whether pentobarbital sedation has any effect on the ratios of the central nervous system spectral metabolites lactate, choline, or N-acetylaspartate (NAA) in a group of premature neonates with normal neonatal MR examinations and normal neuro-developmental outcomes at 12 months of age.
Methods
Study Participants
As part of an ongoing study of the utility of neonatal brain MR imaging for the assessment of brain injury in premature neonates, 110 consecutive premature neonates (gestation age at birth < 34 weeks) were studied by head MRI between January 2001 and May 2004. The protocol was approved by our institutional Committee on Human Research. Participation in the study was voluntary; the infants were studied after informed consent from their parents was obtained.
Of those 110 patients, 43 neonates had normal developmental and neurological status at 12 months of age as evidenced by normal neuromotor scores and normal scores on the Mental Development Index of the Bayley Scales of Infant Development II. In addition, none of these 43 neonates had evidence of brain parenchymal injury on concurrent MR imaging studies that were reviewed by two pediatric neuroradiologists with experience in neonatal brain imaging. These 43 neonates comprised the group reported in this study. When possible, the infants were studied without sedation; but if necessary, for patient movement or in mechanically ventilated patients, pharmacological sedation with pentobarbital (Nembutal, 1 mg/Kg) was administered by a neonatologist according to our institution's sedation guidelines. Among the 43 neonates, 14 (33%) required sedation during the MR examination, the remaining 29 neonates did not receive sedation. The gestational age at the time of the MR examination was compared between the sedation and nonsedation groups. As the use of sedation was at the discretion of the transport team, we also compared other clinical variables reflecting overall illness severity between the sedation and nonsedation groups: gestational age at birth, 5-minute Apgar score, number of days of mechanical ventilation prior to the MR examination, and the presence / absence of systemic infection at the time of the MR examination.
MR Imaging
All studies were performed on a 1.5-Tesla Signa EchoSpeed system (General Electric Medical Systems, Milwaukee, WI) using a MR-compatible incubator with a high sensitivity neonatal head coil 19. MR imaging of the brain was performed before MR spectroscopy and included 4-mm (1-mm “gap”) sagittal spin-echo (TR = 500, TE = 11, excitation = 2) images, 4-mm (2-mm “gap”) axial spin-echo (TR = 3000, TE = 60 and 120, excitation = 1) images, and high-resolution coronal 3-dimensional spoiled gradient echo images (partition size = 1.5 mm, TR = 36, TE = min, flip angle = 35, excitation = 2, FOV = 18cm). Using the point-resolved spectroscopy technique, a region of approximately 100 cm3 was selected which encompassed the majority of the newborn brain while excluding the calvarium and scalp tissues such as subcutaneous fat. The 3D MR spectroscopy array was 8 × 8 × 8 with 1 cm on-a-side. MR spectroscopy prescan included automatic 3D shimming and frequency shifting. Phase encoding in the point resolved spectroscopy-chemical shift imaging sequence was used to obtain 3D spectral arrays with a nominal spatial resolution of 1 cm3 20. After a 2- to 3-minute pre-scan procedure, the lactate-edited 3D MR spectroscopic imaging data were acquired in 17.5 minute with a TR of 1 and a TE of 144. The lactate editing method has been previously described by Star-Lack et al 21, and allows filtering of undesired signal contributions such as those from lipids which may be inadvertently included in the region of the brain selected for the 3D MR spectroscopic analysis. Both a summed and a difference spectra are generated using the lactate editing methods. The relative levels of choline, creatine, NAA, and lipid can be estimated from the summed spectra; where as the lactate level can be estimated from the difference spectra 21, 22. Any lipids would appear in the summed spectra if lipid contamination is present 21, 22. The edited lactate peak has the same phase (upright peak) as the other metabolites. The MR images and the raw MR spectroscopic imaging data were transferred off-line to a Sun UltraSparc workstation (Sun Microsystem, Moutain View, CA) for analysis using software developed at our institution for 3D MR spectroscopy processing. To assess the MR spectra in various anatomic locations in the brain, spectral voxels were retrospectively centered in the following regions bilaterally: basal ganglion (including the caudate head and anterior putamen), thalamus, and corticospinal tract (within the centrum semiovale) as defined by an experienced neuroradiologist (Fig 1). The MR spectroscopy data were Fourier-transformed and baseline-fitted; the peak intensities were determined for the choline, NAA, creatine and lactate resonances. Peak-height ratios of lactate/choline, lactate/NAA, and NAA/choline were calculated for each voxel. Because the creatine peak is typically small with lower signal to noise ratio in prematurely born infants, calculated ratios of lactate to creatine are less accurate and therefore not included in this analysis.
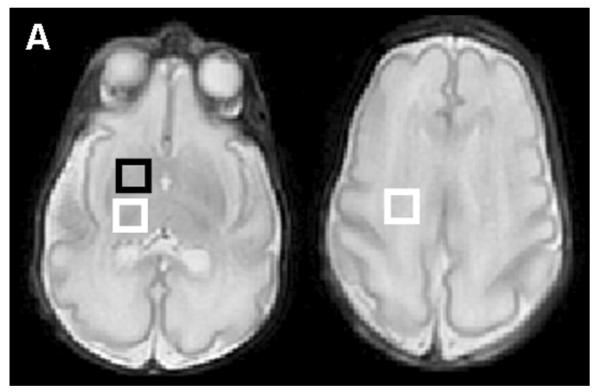
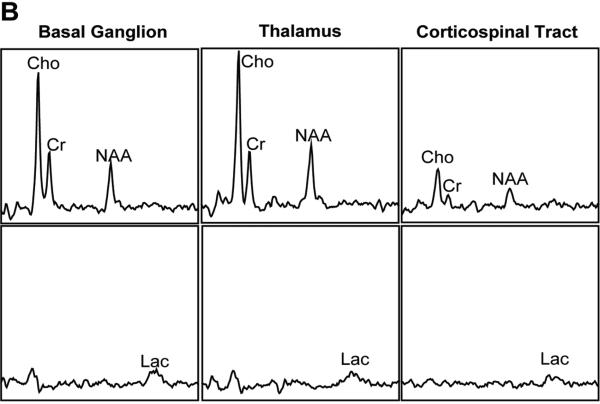
Fig. 1.
Location of the voxels and normal neonatal proton spectra obtained at gestation age of 32 weeks from a prematurely born infant. The infant received pentobarbital sedation. (A) The basal ganglion voxel includes the head of caudate and the anterior putamen (black box, left image). The thalamic voxel is indicated by the white box in the left image. The corticospinal tract voxel includes the corticospinal tract within the centrum semiovale (white box in the right image). (B) Lactate-edited spectra. Top row shows the summed spectra in each voxel for Cho, Cr, NAA. The bottom row shows the difference spectra for each voxel; the difference spectra show only the Lac peaks. Cho, choline; Cr, creatine; NAA, N-acetylaspartate; Lac, lactate.
Statistical Analysis
Statistical analysis was performed using statistical software package SAS 9.0 (SAS Institute, Cary, NC). The clinical variables (mean adjusted gestational age at the time of MR spectroscopy, mean gestation age at birth, 5-minute Apgar score, number of days of mechanical ventilation prior to MR imaging, and the presence / absence of systemic infection at the time of the MR examination.) were compared between the sedation and non-sedation groups using either the paired t-test or the Fisher-exact test. Generalized estimating equation was used to evaluate the effect of sedation on the ratios of the spectral metabolites while controlling for the effect of having multiple regions of interest from the right and left sides of the brain in each patient, as well as adjusting for the above-mentioned clinical variables. For all tests, p-values less than 0.05 were considered statistically significant. Our p-values were not adjusted for multiple tests.
Results
Patient Clinical Data
Among the 43 neonates, 14 (33%) required sedation during the MR examination (adjusted gestational age range at the time of MR examination, 29.1 to 38.7 weeks; mean, 32.0 weeks), the remaining 29 neonates (adjusted gestational age range at the time of MR examination, 27.6 to 35.9 weeks; mean, 32.4 weeks) did not receive sedation. There was no significant difference in the adjusted gestational age at the time of MR examination between the neonates requiring sedation and those imaged without sedation (P = 0.21). There was no significant difference between the sedation and nonsedation groups in the mean 5-minute Apgar score (mean score of sedation group, 6.9; mean score of nonsedation group, 7.3; P = 0.53), or in the presence / absence of infection at the time of the MR examination (sedation group, 3 of 11 neonates with infection; nonsedation group, 6 of 23 neonates with infection; P = 1.0). There was, however, significant difference between the sedation and nonsedation groups in the gestational age at birth (mean age of sedation group, 27.1 weeks; mean age of nonsedation group, 29.1 weeks; P = 0.01), and the number of days of mechanical ventilation prior to MR imaging (mean duration of sedation group, 22 days; mean duration of nonsedation group, 7 days; P = 0.01). Therefore our multivariate statistical models were adjusted for gestational age at birth and the number of days of mechanical ventilation prior to the MR examination. As a result, any potential differences in metabolite ratios between the sedation and nonsedation groups will be independent of the effect of the gestational age at birth or the number of days of mechanical ventilation prior to MR examination.
MR Studies
The major metabolite ratios for the sedation and non-sedation groups are summarized in Table 1. Small amounts of lactate were seen in the basal ganglia, thalami and corticospinal tracts in all of the premature neonates in this study.
TABLE 1.
Measured Brain Metabolite Ratios
| Metabolite Ratios |
||||
|---|---|---|---|---|
| Anatomic Region | Group | Lactate/Choline | Lactate/NAA | NAA/Choline |
| Basal Ganglia | Non-sedation | 0.12±0.05* | 0.32±0.15 † | 0.40±0.10 |
| Sedation | 0.10±0.04* | 0.24±0.10 † | 0.42±0.06 | |
| Thalamus | Non-sedation | 0.08±0.05 | 0.20±0.18 | 0.44±0.10 |
| Sedation | 0.07±0.03 | 0.17±0.06 | 0.44±0.08 | |
| Corticospinal Tract | Non-sedation | 0.12±0.05 | 0.25±0.11 | 0.51±0.19 |
| Sedation | 0.13±0.06 | 0.26±0.14 | 0.52±0.10 | |
Note--- Data expressed as mean values with standard deviation.
denote a significant difference between the non-sedation and sedation groups (P = 0.03 and 0.01 respectively).
denote a significant difference between the non-sedation and sedation groups (P = 0.03 and 0.01 respectively).
NAA, N-acetylaspartate.
The basal ganglia lactate/choline and lactate/NAA ratios were significantly lower, by 17% and 25% respectively, in the neonates with pentobarbital sedation compared to those without sedation (P= 0.03 and 0.01 respectively). The lactate level did not change significantly in the thalami or the corticospinal tracts with sedation. There was no significant difference in the NAA/choline ratios between the sedation and the non-sedation groups.
Discussion
We found small amounts of lactate in the basal ganglia, thalami and corticospinal tracts in all of the premature neonates in this study. Although brain lactate level is known to increase in term infants who suffer hypoxic-ischemic injury or other parenchymal injuries, small amounts of lactate can be seen in the brains of premature neonates who are otherwise normal, and does not in itself indicate brain injury 2, 10. Indeed, none of the neonates in our study group was found to have brain parenchymal abnormality on concurrent MR imaging, and all had normal developmental and neurological outcome at 12 months of age.
Our data show that the basal ganglia lactate level was significantly lower in the group of neonates with pentobarbital sedation. Lactate is produced via anaerobic glycolysis. The relatively high level of lactate in the immature brain may be explained by the relatively low quantity of mitochondria and the consequent greater dependence of the immature brain on glycolytic rather than oxidative energy generation 2. Indeed, there is evidence that lactate is used as a metabolic fuel in the immature central nervous system 23. Anaerobic glycolysis may, therefore, be an important mechanism to satisfy the energy requirements of the immature brain. The impact of barbiturates on brain metabolic rate and glucose metabolism has been studied extensively. Cerebral blood flow and cerebral metabolic rate of oxygen utilization are reduced by about 30% with the onset of barbiturate sedation 24. Pentobarbital may also transiently reduce the cerebral metabolic rate for glucose to a greater degree than the metabolic rate of oxygen utilization 24. Barbiturates have been shown to alter the glucose metabolism of the brain such that the cellular glucose concentration is increased and the lactate concentration is reduced 17, 18, 25. The decreased basal ganglia lactate level in the sedation group may, therefore, be explained by a combination of the relative importance of glycolysis in the immature brain and the reduced glycolysis produced by pentobarbital. It is important to note that this observation in no way suggests that there is any danger to the use of pentobarbital for sedation of these infants. Pentobarbital has been used for pediatric sedation for many decades with no evidence of deleterious effects on the affected children. It is important, however, to recognize that metabolite values and metabolite ratios from proton MR spectroscopy may be affected by the use of pentobarbital.
We did not find any significant changes in the lactate level of the thalamus with sedation. Previous studies have shown that the thalami are the most mature region of the supratentorial brain in neonates 26, 27. It is conceivable that a more mature region such as the thalamus is differentially affected by sedation compared to the less mature basal ganglion. Or, perhaps, the more mature thalamus, with more mature energy pathways, may have a higher rate of oxidative metabolism than the basal ganglion. In either event, reduced glycolysis produced by pentobarbital might be less likely to result in a change in lactate production in the thalamus. Pentobarbital sedation did not appear to have any effect on the lactate level in the corticospinal tracts either, possibly because the corticospinal tracts are also more mature than the basal ganglia at this stage of development, with a greater dependence on oxidative metabolism. Indeed, the increased maturity of the thalami and corticospinal tracts with consequent higher oxidative metabolism is given as an explanation for why they are more affected than the basal ganglia in term neonatal hypoxic ischemic injury 28.
Our results differed from those of Lundbom et al 29, who found no effect of barbiturate sedation on major brain metabolite levels including lactate. The differences can be attributed to different patient population. The group studied by Lundbom et al was comprised of healthy subjects between 20 to 32 years old, and had no detectable lactate peak at baseline (without barbiturate sedation). It has been shown that lactate level is lower in the adult brain than the term or preterm neonatal brain 2, presumably because mitochondria produce ATP almost entirely by aerobic metabolism in adults. A previous study has also demonstrated that the baseline lactate levels showed considerable inter-individual differences ranging from undetectable (less than 0.3mM) to barely detectable (about 1 mM) in healthy adults 30. It is not surprising, therefore, that no baseline lactate was detected in Lundbom's subjects; moreover, the absence of lactate likely would have precluded any potential metabolic changes resulting from barbiturate sedation from being detected. Our cohort, on the other hand, was comprised of prematurely born neonates who normally have a small, but easily detectable, amount of lactate in their brains. The presence of lactate at baseline made it possible to detect changes in lactate concentrations with pentobarbital sedation.
The 3D lactate-edited MR spectroscopy sequence used in our study has allowed the detection of small changes in lactate ratios between the neonates with sedation and those without. Although significant, these changes were small (17% and 25% lower in the sedation group for the lactate/choline and lactate/NAA ratios respectively) compared to the changes in lactate level associated with neonatal hypoxic-ischemic injury. In neonates with hypoxic-ischemic injury and poor developmental outcome, the lactate ratios may be two- to several folds higher compared to normal controls, depending on the timing of the MR study with respect to the time of injury 12, 31. Therefore, the use of pentobarbital sedation is unlikely to fully mask the relatively large lactate elevation in neonates with hypoxic brain injury. Nonetheless, it is important to note the potential effect of pentobarbital sedation on the lactate ratios at MR spectroscopy, especially in cases where the suspected metabolic changes may be subtle.
There are limitations to the current investigation, and therefore these preliminary results should be interpreted with caution. First, our sample size is small; therefore, a larger study is needed to validate these initial observations. Second, our results are limited to prematurely born neonates. It is known that significant differences are present in metabolite levels and physiology between premature and term neonates, and between neonates and older children. It is possible that barbiturate sedation can have different effect on a more mature human brain. Thus, additional studies are needed to investigate the effect of barbiturate sedation on the spectroscopy results in term neonates and infants.
Conclusion
In conclusion, our study suggests that pentobarbital sedation is associated with lower lactate level in the basal ganglion of premature neonates, as determined by proton MR spectroscopy. This may be explained by the decreased glucose metabolism as a result of pentobarbital sedation. Investigators should be aware of this phenomenon for accurate interpretation of their MR spectroscopy results.
Acknowledgments
This study was supported by the following grants: NIH R01 NS46432, RO1 NS40117, R21 NS40382, and the Neonatal Clinical Research Center (PCRC RR01271)
Reference
- 1.Barkovich AJ, Baranski K, Vigneron D, et al. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol. 1999;20(8):1399–405. [PMC free article] [PubMed] [Google Scholar]
- 2.Cady EB, Penrice J, Amess PN, et al. Lactate, N-acetylaspartate, choline and creatine concentrations, and spin-spin relaxation in thalamic and occipito-parietal regions of developing human brain. Magn Reson Med. 1996;36(6):878–86. doi: 10.1002/mrm.1910360610. [DOI] [PubMed] [Google Scholar]
- 3.Groenendaal F, Veenhoven RH, van der Grond J, Jansen GH, Witkamp TD, de Vries LS. Cerebral lactate and N-acetyl-aspartate/choline ratios in asphyxiated full-term neonates demonstrated in vivo using proton magnetic resonance spectroscopy. Pediatr Res. 1994;35(2):148–51. doi: 10.1203/00006450-199402000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Hanrahan JD, Sargentoni J, Azzopardi D, et al. Cerebral metabolism within 18 hours of birth asphyxia: a proton magnetic resonance spectroscopy study. Pediatr Res. 1996;39(4 Pt 1):584–90. doi: 10.1203/00006450-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Holshouser BA, Ashwal S, Luh GY, et al. Proton MR spectroscopy after acute central nervous system injury: outcome prediction in neonates, infants, and children. Radiology. 1997;202(2):487–96. doi: 10.1148/radiology.202.2.9015079. [DOI] [PubMed] [Google Scholar]
- 6.Huppi PS, Posse S, Lazeyras F, Burri R, Bossi E, Herschkowitz N. Magnetic resonance in preterm and term newborns: 1H-spectroscopy in developing human brain. Pediatr Res. 1991;30(6):574–8. doi: 10.1203/00006450-199112000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30(4):424–37. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- 8.Leth H, Toft PB, Peitersen B, Lou HC, Henriksen O. Use of brain lactate levels to predict outcome after perinatal asphyxia. Acta Paediatr. 1996;85(7):859–64. doi: 10.1111/j.1651-2227.1996.tb14168.x. [DOI] [PubMed] [Google Scholar]
- 9.Peden CJ, Rutherford MA, Sargentoni J, Cox IJ, Bryant DJ, Dubowitz LM. Proton spectroscopy of the neonatal brain following hypoxic-ischaemic injury. Dev Med Child Neurol. 1993;35(6):502–10. doi: 10.1111/j.1469-8749.1993.tb11680.x. [DOI] [PubMed] [Google Scholar]
- 10.Penrice J, Cady EB, Lorek A, et al. Proton magnetic resonance spectroscopy of the brain in normal preterm and term infants, and early changes after perinatal hypoxia-ischemia. Pediatr Res. 1996;40(1):6–14. doi: 10.1203/00006450-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Shu SK, Ashwal S, Holshouser BA, Nystrom G, Hinshaw DB., Jr. Prognostic value of 1H-MRS in perinatal CNS insults. Pediatr Neurol. 1997;17(4):309–18. doi: 10.1016/s0887-8994(97)00140-9. [DOI] [PubMed] [Google Scholar]
- 12.Zarifi MK, Astrakas LG, Poussaint TY, Plessis Ad A, Zurakowski D, Tzika AA. Prediction of adverse outcome with cerebral lactate level and apparent diffusion coefficient in infants with perinatal asphyxia. Radiology. 2002;225(3):859–70. doi: 10.1148/radiol.2253011797. [DOI] [PubMed] [Google Scholar]
- 13.Barkovich AJ, Westmark KD, Bedi HS, Partridge JC, Ferriero DM, Vigneron DB. Proton spectroscopy and diffusion imaging on the first day of life after perinatal asphyxia: preliminary report. AJNR Am J Neuroradiol. 2001;22(9):1786–94. [PMC free article] [PubMed] [Google Scholar]
- 14.Zaidan E, Sims NR. Reduced activity of the pyruvate dehydrogenase complex but not cytochrome c oxidase is associated with neuronal loss in the striatum following short-term forebrain ischemia. Brain Res. 1997;772(12):23–8. doi: 10.1016/s0006-8993(97)00833-0. [DOI] [PubMed] [Google Scholar]
- 15.Cheng MA, Theard MA, Tempelhoff R. Intravenous agents and intraoperative neuroprotection. Beyond barbiturates. Crit Care Clin. 1997;13(1):185–99. doi: 10.1016/s0749-0704(05)70301-8. [DOI] [PubMed] [Google Scholar]
- 16.Turner BK, Wakim JH, Secrest J, Zachary R. Neuroprotective effects of thiopental, propofol, and etomidate. Aana J. 2005;73(4):297–302. [PubMed] [Google Scholar]
- 17.Miller LP, Mayer S, Braun LD, Geiger P, Oldendorf WH. The effect of pretreatment with pentobarbital on the extent of [14C] incorporation from [U-14C]glucose into various rat brain glycolytic intermediates: relevance to regulation at hexokinase and phosphofructokinase. Neurochem Res. 1988;13(4):377–82. doi: 10.1007/BF00972488. [DOI] [PubMed] [Google Scholar]
- 18.Goodman JC, Valadka AB, Gopinath SP, Cormio M, Robertson CS. Lactate and excitatory amino acids measured by microdialysis are decreased by pentobarbital coma in head-injured patients. J Neurotrauma. 1996;13(10):549–56. doi: 10.1089/neu.1996.13.549. [DOI] [PubMed] [Google Scholar]
- 19.Dumoulin CL, Rohling KW, Piel JE, et al. Magnetic Resonance Imaging Compatible Neonate Incubator. Concepts in Magnetic Resonance. 2002;15:117–28. [Google Scholar]
- 20.Vigneron DB, Barkovich AJ, Noworolski SM, et al. Three-dimensional proton MR spectroscopic imaging of premature and term neonates. AJNR Am J Neuroradiol. 2001;22(7):1424–33. [PMC free article] [PubMed] [Google Scholar]
- 21.Star-Lack J, Spielman D, Adalsteinsson E, Kurhanewicz J, Terris DJ, Vigneron DB. In vivo lactate editing with simultaneous detection of choline, creatine, NAA, and lipid singlets at 1.5 T using PRESS excitation with applications to the study of brain and head and neck tumors. J Magn Reson. 1998;133(2):243–54. doi: 10.1006/jmre.1998.1458. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Vigneron DB, Cha S, et al. Relationship of MR-derived lactate, mobile lipids, and relative blood volume for gliomas in vivo. AJNR Am J Neuroradiol. 2005;26(4):760–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Leth H, Toft PB, Pryds O, Peitersen B, Lou HC, Henriksen O. Brain lactate in preterm and growth-retarded neonates. Acta Paediatr. 1995;84(5):495–9. doi: 10.1111/j.1651-2227.1995.tb13681.x. [DOI] [PubMed] [Google Scholar]
- 24.Drummond J, Shapiro H. Cerebral physiology. In: Miller RD, editor. Anesthesia. 4th ed. Churchill Livingston; New York: 1994. pp. 690–1. [Google Scholar]
- 25.Otsuka T, Wei L, Bereczki D, Acuff V, Patlak C, Fenstermacher J. Pentobarbital produces dissimilar changes in glucose influx and utilization in brain. Am J Physiol. 1991;261(2 Pt 2):R265–75. doi: 10.1152/ajpregu.1991.261.2.R265. [DOI] [PubMed] [Google Scholar]
- 26.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22(4):487–97. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 27.Tokumaru AM, Barkovich AJ, O'Uchi T, Matsuo T, Kusano S. The evolution of cerebral blood flow in the developing brain: evaluation with iodine-123 iodoamphetamine SPECT and correlation with MR imaging. AJNR Am J Neuroradiol. 1999;20(5):845–52. [PMC free article] [PubMed] [Google Scholar]
- 28.Barkovich AJ. Brain and spine injuries in infancy and childhood. In: Barkovich AJ, editor. Pediatric Neuroimaging. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 195–242. [Google Scholar]
- 29.Lundbom NM, Manner T, Komu M, Peltola O, Leino KA, Kirvela OA. Barbiturate anesthesia and brain proton spectroscopy. AJNR Am J Neuroradiol. 1999;20(8):1543–6. [PMC free article] [PubMed] [Google Scholar]
- 30.Merboldt KD, Bruhn H, Hanicke W, Michaelis T, Frahm J. Decrease of glucose in the human visual cortex during photic stimulation. Magn Reson Med. 1992;25(1):187–94. doi: 10.1002/mrm.1910250119. [DOI] [PubMed] [Google Scholar]
- 31.Barkovich AJ, Miller SP, Bartha A, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol. 2006;27(3):533–47. [PMC free article] [PubMed] [Google Scholar]