Abstract
PURPOSE
To evaluate the effects of human immunodeficiency virus (HIV) infection on proton metabolites in brain regions carrying the heaviest HIV load.
METHODS
We used two-dimensional proton MR spectroscopy with a preselected volume at the level of the third ventricle to measure N-acetyl-aspartate (NAA) and metabolites containing choline (Cho), and creatine (Cr) in the basal ganglia of eight cognitively impaired subjects who were seropositive for HIV and eight control subjects who were seronegative for HIV. Results are expressed as metabolite ratios.
RESULTS
In the thalamus and lenticular nuclei, NAA/Cr was not different between the two groups. NAA/Cho was decreased in both the thalamus and lenticular nuclei of the HIV-positive group compared with the HIV-negative group. Cho/Cr tended to be increased in both the thalamus and lenticular nuclei of the HIV-positive group.
CONCLUSIONS
The findings suggest no NAA differences between groups, consistent with negligible neuron loss in the region of the brain that carries the heaviest HIV load. The trends toward increased Cho/Cr are consistent with histopathologic findings of infiltration of subcortical gray matter structures with foamy macrophages, microglia, and lymphocytes, or possibly with gliosis.
Keywords: Acquired immunodeficiency syndrome (AIDS); Brain, magnetic resonance; Magnetic resonance, spectroscopy
The human immunodeficiency virus (HIV) has been found primarily in white matter and in deep gray matter structures of the brains of patients with acquired immune deficiency syndrome (AIDS). The basal ganglia are affected early in the course of the disease and carry the heaviest HIV load of all brain structures (1–4). The virus has been detected in multinucleated giant cells, macrophages, and microglia, but not in neurons (2). Nevertheless, pathologic studies show loss of neurons, dendrites, and synapses in neocortical regions of the brain in patients with AIDS (5–7), suggesting indirect effects of HIV on neurons.
Proton magnetic resonance (MR) spectroscopy allows noninvasive assessment of neuronal density by measuring N-acetyl-aspartate (NAA), an amino acid that is almost exclusively located in neurons and their processes but not in glial cells (8, 9). Proton MR spectroscopic studies of the brains of patients with HIV and AIDS, including our own work (10, 11), have focused on cerebral white matter and cortical gray matter (10–15), regions that show low to vanishing levels of viral infection (3). All proton MR spectroscopic studies found NAA loss in association with neurologic and/or cognitive impairment, consistent with neuronal damage and/or axonal degeneration. To evaluate the effects of HIV infection on proton metabolites in brain regions carrying the heaviest HIV load, we performed proton MR spectroscopy at the level of the third ventricle in eight cognitively impaired patients who were seropositive for HIV and in eight seronegative control subjects. Because proton MR spectroscopy is conceptionally a combination of MR imaging and MR spectroscopy, it allows imaging of the distribution of proton metabolites, facilitating the detection of metabolite variations throughout large regions of the brain.
Subjects and Methods
The eight patients who were seropositive for HIV were all men with a mean age of 38 ± 7 years. Their HIV infection was at stage B (symptomatic) or C (AIDS-indicator conditions) on the scale established by the Centers for Disease Control, and they had mild or moderate (n = 2) to severe (n = 6) cognitive impairment as determined by a wide range of neuropsychological tests (10, 11). Although all HIV-positive subjects were clinically symptomatic, none had developed opportunistic infections at the time of the study. Subjects were recruited from the HIV clinic and via fliers distributed with hot meals to impaired HIV-positive patients in the community. Four of the six HIV-positive subjects with severe cognitive impairment had participated previously in a study of cognitively impaired HIV-positive subjects that showed significantly decreased NAA signal in the supraventricular region of the brain (11). In that study, all the HIV-positive subjects participating here had NAA signal intensities more than 1 SD below the control mean.
The eight control subjects in this study who were sero-negative for HIV were all cognitively normal men with a mean age of 43 ± 16 years. They were recruited from the community. Candidates for the study were screened to exclude persons with current or past medical, neurologic, or psychiatric disorders or with alcohol or substance abuse, except for current medical and neuropsychiatric problems clearly related to the HIV infection. All subjects gave informed consent (approved by the local university's Committee on Human Research).
The experimental protocol was similar to ones described previously (10, 11). The subject's head was immobilized in a head coil by means of a pad molded around the head that, when a vacuum is applied, stays firm throughout the examination, thereby restricting head movement. We obtained axial MR images at 2500/30, 80 (repetition time/echo time) with contiguous 5.62-mm-thick sections angulated along the canthomeatal line. Immediately afterward, we performed localized shimming followed by water-suppressed two-dimensional proton MR spectroscopy (2000/272) with a preselected volume of interest at the level of the third ventricle. The volume of interest, which was selected by use of a point-resolved spectroscopic localization sequence (16), was 17 mm thick and had (depending on head size) typical left-right and anterior-posterior dimensions of 70 mm × 60 mm, respectively. The section thickness of the spectroscopic image corresponded to three MR imaging sections (3 mm × 5.72 mm = 17.16 mm), and its caudocranial offset was identical to the caudocranial offset of the middle MR imaging section. We applied 24 × 24 phase-encoding steps over a 210 mm2 field of view, yielding an in-plane resolution of less than 9 mm and a nominal voxel size of 1.3 mL. Two scans per phase-encoding step were obtained, for a total acquisition time of 38 minutes.
We processed the proton MR spectroscopic data sets using home-written spectroscopic imaging display software (17). Spectral processing included three-dimensional Fourier transform, zero filling to 1 K data points and exponential line broadening by 2 Hz. Spatial processing consisted of zero filling to 64 points in both dimensions and apodization with a mild gaussian filter, which resulted in an effective voxel size of 1.7 mL, small enough to obtain signal selectively from nuclei of the basal ganglia and thalami. We extracted single-voxel spectra from left and right central thalami, left and right lenticular nuclei (globus pallidus and putamen), and left and right caudate nuclei. The position of the voxels was chosen on the mathematical sum of the three spatially correlated MR images. Resonances for metabolites containing N-acetyl (primarily NAA), creatine (Cr), and choline (Cho) were curve-fitted and integrated by means of commercially available software (New Methods Research Inc, Syracuse, NY).
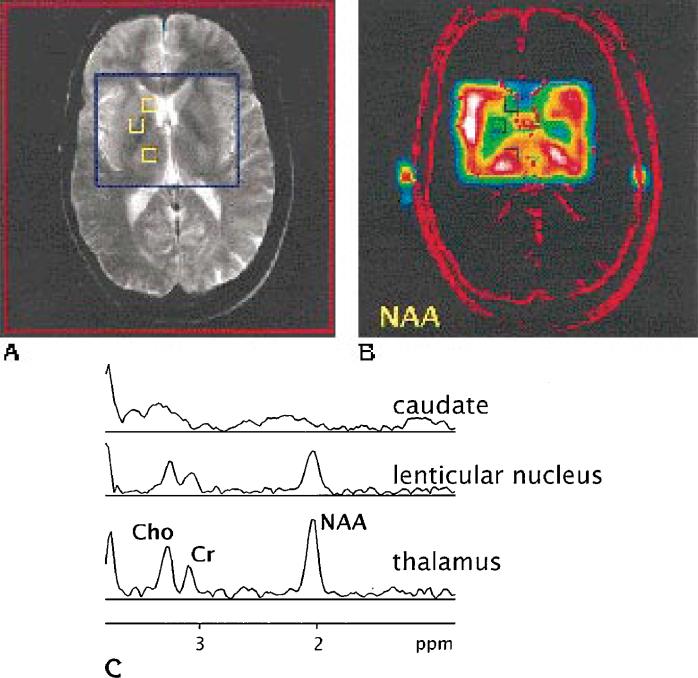
Figure 1 shows a typical MR data set obtained with the above methods from an HIV-positive subject: The sum of three T2-weighted axial MR images at the level of the third ventricle (Fig 1A) is superimposed with a blue rectangle, indicating the preselected volume of interest for proton MR spectroscopy, and with yellow squares, indicating location and size of the voxels extracted from the spectroscopic image. Figure 1B was reconstructed from the NAA signal in the volume of interest and is superimposed with a high-pass filtered MR image for better visual correlation. The spectroscopic image shows appreciable signal variations over the examined volume of interest, corresponding to the different subcortical brain nuclei. The regional signal variation is also reflected in the spectroscopic imaging spectra shown in Figure 1C.
Fig 1.
A, T2-weighted axial MR image (2500/80) of an HIV-positive subject at the level of the third ventricle. The superimposed red square indicates the field of view used for metabolite proton MR spectroscopy, the blue rectangle indicates the position of the preselected point-resolved spectroscopic volume of interest encompassing the basal ganglia and both thalami.
B, Spatially corresponding spectroscopic image reconstructed from the NAA resonance of a water-suppressed two-dimensional proton MR spectroscopic data set (2000/272) with a preselected volume of interest. Superimposed in red is the high-pass filtered MR image for better spatial correlation. White denotes the highest, black the lowest signal intensity. The squares on both A and B represent the location and size of voxels from which spectra were extracted.
C, In spectra from the lenticular nucleus and thalamus, resonances from N-acetyl-containing metabolites (primarily NAA), from creatine-containing metabolites (Cr), and from choline-containing metabolites (Cho) can be clearly identified. The caudate nucleus spectrum is not recognizable.
Results of the spectral analysis for the different brain regions are expressed as ratios of metabolite signal integrals. Absolute signal intensities corresponding to individual metabolites are more informative but could not be evaluated because intersubject variability in coil loading, which affects absolute signal strengths, could not be accounted for reliably in these experiments. Since there were no left-right differences in a given brain nucleus (as determined by a paired t test) left and right voxels were averaged to obtain a better representation of metabolite levels. Within-group comparisons of regional differences were made using two-tailed paired t tests. Between-group comparisons were done with one-tailed t tests with equal variances (after verifying equal variance). A P value of less than .05 was considered statistically significant.
Results
MR imaging findings were normal for all subjects except for three HIV-positive patients (two with severe and one with moderate neuropsychological impairment) who had mild sulcal widening. No subcortical abnormalities were observed on the MR images.
NAA was unchanged and Cho was increased in the thalami and lenticular nuclei of HIV-positive subjects relative to the HIV-negative subjects. Proton MR spectroscopy showed metabolic differences in the deep gray matter structures between the HIV-positive and HIV-negative subjects (see Table): thalamic NAA/Cho was 13% lower in the HIV-positive group than in the HIV-negative group (P = .02), whereas Cho/Cr showed a concomitant 13% increase (P = .06). Thalamic NAA/Cr was unchanged between the groups. In the lenticular nucleus, the findings were qualitatively and quantitatively similar: NAA/Cho was 15% lower in the HIV-positive group than in the HIV-negative subjects (P = .05), Cho/Cr showed a concomitant but nonsignificant increase of 14% in HIV-positive subjects, and NAA/Cr was unchanged between the groups.
Metabolic differences in the thalamus and lenticular nucleus as shown by proton MR spectroscopy in cognitively impaired HIV-positive subjects and in cognitively normal HIV-negative control subjects (n = 8 in each group, mean ± SD)
| HIV Positive | P Value | HIV Negative | Percentage of Change, % | |
|---|---|---|---|---|
| Thalamus | ||||
| NAA/Cho | 1.9 ± 0.3 | .02 | 2.2 ± 0.2 | −13 |
| NAA/Cr | 3.4 ± 0.6* | . . . | 3.4 ± 0.7† | −2 |
| Cho/Cr | 1.8 ± 0.3 | .06 | 1.6 ± 0.2 | +13 |
| Lenticular nucleus | ||||
| NAA/Cho | 1.6 ± 0.3 | .05 | 1.9 ± 0.3 | −15 |
| NAA/Cr | 2.6 ± 0.6 | . . . | 2.7 ± 0.7 | −2 |
| Cho/Cr | 1.7 ± 0.5 | . . . | 1.5 ± 0.4 | +14 |
Note.—NAA indicates metabolites containing N-acetyl, primarily N-acetyl-aspartate; Cho, metabolites containing choline; and Cr, metabolites containing creatine.
P = .07 compared with lenticular NAA/Cr.
P = .06 compared with lenticular NAA/Cr.
NAA tends to be higher in thalami than in lenticular nuclei. The metabolic images and spectra in Figure 1 show signal variations over the examined volume of interest corresponding to the different brain nuclei. The underlying metabolic differences between thalamic and lenticular nuclei approach significance for NAA/Cr in both the HIV-positive and HIV-negative groups: NAA/Cr tended to be higher by more than 20% in the thalamus (3.4 in both groups) than in the lenticular nucleus (2.6 ± 0.6 in the HIV-positive group, P = .07; and 2.7 ± 0.7 in the HIV-negative subjects, P = .06), suggesting higher neuronal density in the thalami of both groups.
Susceptibility changes occur in the anterior basal ganglia. Although thalamic and lenticular spectra were of consistently good quality, spectra from the head of the caudate nucleus could not be obtained reliably in every examination. This is attributed to susceptibility changes in this area of the brain. Iron deposits in the caudate nucleus are not thought to be responsible for these susceptibility changes, since iron deposits are higher in the lenticular nucleus (eg, 18), which exhibits good-quality spectra with narrow resonances. To depict susceptibility changes in the region of the caudate nucleus, we obtained a water MR spectroscopy data set from a control subject at the level of the third ventricle. Experimental parameters were identical to those for metabolite MR spectroscopy, except that the water-suppression pulse was set +3 kHz away from all resonances. Figure 2 shows this water MR spectroscopic image and a stacked plot of water spectra obtained from a column of spectra at a location indicated on the spectroscopic image. Near the head of the caudate nucleus, a large frequency shift and a broadening of the water resonance are obvious. Apparently, localized shimming over the selected volume of interest does not compensate for the susceptibility changes in this brain region—changes that are most likely caused by susceptibility differences between air in the nasal cavity and sinuses and brain tissue (19). Therefore, water suppression and regional B0 field homogeneity were typically unsatisfactory for reliable metabolite measurements by proton MR spectroscopy in the region of the caudate nucleus.
Fig 2.
Proton MR spectroscopic image of water (H2O) at the level of the third ventricle obtained from a healthy control subject by means of the two-dimensional MR spectroscopic method described in Figure 1 without water suppression (A). The color scale denotes white as the highest and black as the lowest signal intensity. The superimposed pair of lines delineate the column of spectroscopic imaging voxels from which water spectra were obtained; these are displayed in a stacked plot in B. Clearly visible is a frequency shift and extensive broadening of the water resonance in the region of the head of the caudate nucleus, probably explaining the generally bad quality of caudate spectra (see Fig 1). The red line superimposed on the stacked plot of spectra corresponds to the frequency used for water suppression in metabolite proton MR spectroscopy.
Discussion
In cognitively impaired HIV-positive subjects, the NAA/Cr ratio in the thalamus and the lenticular nucleus was not significantly different from that in HIV-negative control subjects. This is in spite of the fact that four of the eight HIV-positive subjects had NAA signal intensities more than 1 SD below normal in the supraventricular area of the brain, a region that appears to be more vulnerable to neuronal damage by HIV (11). Since the NAA signal represents almost exclusively the putative neuronal marker NAA, our basal ganglia findings suggest negligible neuron loss in a region of the brain that carries the heaviest viral load. These in vivo findings are consistent with histopathologic findings of neuronal preservation in the basal ganglia (1, 2, 4).
The proton metabolite measurements suggest a trend toward increased Cho in both the thalami and lenticular nuclei of this small cohort of cognitively impaired HIV-positive subjects compared with HIV-negative control subjects. This in vivo finding is consistent with histopathologic findings of infiltration of the basal ganglia with foamy macrophages, microglia, and lymphocytes (1–3) or possibly with gliosis. It has been suggested that this infiltration has a role in AIDS dementia, and it warrants further study (3). Increased Cho was not detected in the supraventricular region of the brain in cognitively impaired HIV-positive subjects with normal MR imaging findings (10, 11), consistent with immunohistochemical findings of low levels of HIV infection in these brain regions (3). Increased Cho, however, has been reported in immunosuppressed HIV-positive subjects in regions of abnormal white matter signal hyperintensities on MR images (13), but it is not specific to HIV infection and has also been seen in elderly HIV-negative subjects with white matter signal hyperintensities on MR images (20). Our results of preserved NAA and increased Cho levels in subcortical gray matter structures have been corroborated recently in a proton MR spectroscopic study of patients with AIDS dementia complex (21).
In a cohort of comparable size, in which the supraventricular area of the brain of cognitively impaired HIV-positive subjects was studied with almost identical experimental design, spectral signal-to-noise ratio, and data processing methods (10, 11), we did not observe an increased Cho signal in regions of reduced NAA signal. In the present study, the power to detect differences between groups was similar to that of these earlier studies. The variance of the data was somewhat larger in the present study, possibly attesting to the greater metabolic heterogeneity among subjects of both groups in the subcortical gray matter nuclei than in the supraventricular regions of the brain.
In conclusion, in vivo proton MR spectroscopy has been used successfully for the metabolic evaluation of supraventricular (10, 11) and subcortical gray matter regions in HIV-infected patients and in uninfected control subjects. Susceptibility changes in the region of the anterior basal ganglia degrade the quality of spectra from these regions and may require localized shimming just in this small area of the brain. From a clinical point of view, previous (10–16, 21) and current proton MR spectroscopic findings are consistent with the understanding of a regional neuronal/axonal loss in advanced stages of HIV infection (22), and with neuronal preservation in the presence of microglia infiltration in deep gray matter structures of late-stage HIV-infected patients. These in vivo MR spectroscopic findings appear to reflect morphologic brain changes that have been well described in postmortem studies and that warrant further investigation of regional metabolic differences in a larger group of HIV-infected patients, preferably stratified by severity of clinical and/or neuropsychological impairment.
Acknowledgments
We are indebted to Dr Andrew Maudsley for his continuous efforts in developing and maintaining the spectroscopic imaging display software used for data analysis.
Supported by grant no. MHAZ 5 401 MH45680 from the National Institute of Mental Health (G.F.), grant no. RO1 AG10897 from the National Institutes of Health (M.W.W.), and by a grant from the DVA Medical Research Service (G.F., M.W.W.).
References
- 1.Pumarola-Sune T, Navia BA, Cordon-Cardo B, Cho ES, Price RW. HIV antigen in the brains of patients with AIDS dementia complex. Ann Neurol. 1987;21:490–496. doi: 10.1002/ana.410210513. [DOI] [PubMed] [Google Scholar]
- 2.Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- 3.Kure K, Weidenheim KM, Lyman WD, Dickson DW. Morphology and distribution of HIV-1 gp41-positive microglia in subacute AIDS encephalitis: pattern of involvement resembling a multisystem degeneration. Acta Neuropathol. 1990;80:393–400. doi: 10.1007/BF00307693. [DOI] [PubMed] [Google Scholar]
- 4.Masliah E, Achin CL, Ge N, et al. Spectrum of human immuno-deficiency virus: associated neocortical damage. Ann Neurol. 1992;32:231–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- 5.Gray F, Gherardi R, Scaravilli F. The neuropathology of the acquired immune deficiency syndrome (AIDS). Brain. 1988;111:245–266. doi: 10.1093/brain/111.2.245. [DOI] [PubMed] [Google Scholar]
- 6.Wiley CA, Masliah E, Morey M, et al. Neocortical damage during HIV infection. Ann Neurol. 1991;29:651–657. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- 7.Everall IP, Luthert PJ, Lantos PL. Neuronal loss in the frontal cortex in HIV infection. Lancet. 1991;337:1119–1121. doi: 10.1016/0140-6736(91)92786-2. [DOI] [PubMed] [Google Scholar]
- 8.Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid. Neurosci Biobehav Rev. 1989;13:23–31. doi: 10.1016/s0149-7634(89)80048-x. [DOI] [PubMed] [Google Scholar]
- 9.Urenjak J, Williams SR, Gadian DG, Noble M. Specific expression of N-acetylaspartate in neurons, oligodendrociyte-type-2-astrocyte progenitors, and immature oligodendrocytes in-vitro. J Neurochem. 1992;59:55–61. doi: 10.1111/j.1471-4159.1992.tb08875.x. [DOI] [PubMed] [Google Scholar]
- 10.Meyerhoff DJ, MacKay S, Bachman L, et al. Reduced brain N-acetylaspartate suggests neuronal loss in cognitively impaired human immunodeficiency seropositive individuals: in vivo 1-H magnetic resonance spectroscopic imaging. Neurology. 1993;43:509–515. doi: 10.1212/wnl.43.3_part_1.509. [DOI] [PubMed] [Google Scholar]
- 11.Meyerhoff DJ, MacKay S, Poole N, Dillon WP, Weiner MW, Fein G. N-acetylaspartate reductions measured by 1H MRSI in cognitively impaired HIV-seropositive individuals. Magn Reson Imaging. 1994;12:653–659. doi: 10.1016/0730-725x(94)92460-0. [DOI] [PubMed] [Google Scholar]
- 12.Menon DK, Ainsworth JG, Cox IJ, et al. Proton MR spectroscopy of the brain in AIDS dementia complex. J Comput Assist Tomogr. 1992;16:538–542. doi: 10.1097/00004728-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Jarvik JG, Lenkinski RE, Grossman RI, Gomori JM, Schnall MD, Frank I. Proton MR spectroscopy of HIV-infected patients: characterization of abnormalities with imaging and clinical correlations. Radiology. 1993;186:739–744. doi: 10.1148/radiology.186.3.8430182. [DOI] [PubMed] [Google Scholar]
- 14.Chong WK, Sweeney B, Wilkinson ID, et al. Proton spectroscopy of the brain in HIV infection: correlation with clinical, immuno-logic, and MR imaging findings. Radiology. 1993;188:119–124. doi: 10.1148/radiology.188.1.8099750. [DOI] [PubMed] [Google Scholar]
- 15.Chong WK, Paley M, Wilkinson ID, et al. Localized cerebral proton MR spectroscopy in HIV infection and AIDS. AJNR Am J Neuroradiol. 1994;15:21–25. [PMC free article] [PubMed] [Google Scholar]
- 16.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 17.Maudsley AA, Lin E, Weiner MW. Spectroscopic imaging display and analysis. Magn Reson Imaging. 1991;10:471–485. doi: 10.1016/0730-725x(92)90520-a. [DOI] [PubMed] [Google Scholar]
- 18.Chen JC, Hardy PA, Clauberg M, et al. T2 values in the human brain: comparison with quantitative assays of iron and ferritin. Radiology. 1989;173:521–526. doi: 10.1148/radiology.173.2.2798884. [DOI] [PubMed] [Google Scholar]
- 19.Ericsson A, Weis J, Hemmingsson A, Wikstrom M, Sperber GO. Measurement of magnetic field variations in the human brain using a 3D-FT multiple gradient echo technique. Magn Reson Med. 1995;33:172–177. doi: 10.1002/mrm.1910330205. [DOI] [PubMed] [Google Scholar]
- 20.Constans JM, Meyerhoff DJ, Norman D, Fein G, Weiner MW. 1H and 31P magnetic resonance spectroscopic imaging of white matter signal hyperintensity areas in elderly subjects. Neuroradiology. 1995;37:615–623. doi: 10.1007/BF00593373. [DOI] [PubMed] [Google Scholar]
- 21.Barker P, Lee RR, McArthur JC. AIDS dementia complex: evaluation with proton MR spectroscopic imaging. Radiology. 1995;195:58–64. doi: 10.1148/radiology.195.1.7892496. [DOI] [PubMed] [Google Scholar]
- 22.Epstein LG, Gendelman HE. Human immunodeficiency virus type 1 infection of the central nervous system: pathogenic mechanisms. Ann Neurol. 1993;33:429–436. doi: 10.1002/ana.410330502. [DOI] [PubMed] [Google Scholar]