Abstract
The Mrgprd receptor is selectively expressed in nonpeptidergic nociceptors that innervate the outer layers of mammalian skin. The function of Mrgprd in nociceptive neurons and the physiologically-relevant somatosensory stimuli that activate Mrgprd-expressing (Mrgprd+) neurons are currently unknown. To address these issues, we studied three Mrgprd knockin mouse lines using an ex vivo somatosensory preparation to examine the role of the Mrgprd receptor and Mrgprd+ afferents in cutaneous somatosensation. In mouse hairy skin, Mrgprd, as marked by expression of GFP reporters, was expressed predominantly in the population of non-peptidergic, TRPV1-negative, C-polymodal nociceptors. In mice lacking Mrgprd, this population of nociceptors exhibited decreased sensitivity to cold, heat and mechanical stimuli. Additionally, in vitro patch clamp studies were performed on cultured DRG neurons from Mrgprd−/− and Mrgprd+/− mice. These studies revealed a higher rheobase in neurons from Mrgprd−/− mice than from Mrgprd+/− mice. Furthermore, in vitro the application of the Mrgprd ligand β-alanine significantly reduced the rheobase and increased the firing rate in neurons from Mrgprd+/− mice, but was without effect in neurons from Mrgprd−/− mice. Our results demonstrate that Mrgprd influences the excitability of polymodal nonpeptidergic nociceptors to mechanical and thermal stimuli.
Keywords: Sensory neuron, Dorsal Root Ganglion, Pain, Knockin mice, M-current, Skin
INTRODUCTION
Numerous studies have explored the rich physiological diversity of sensory neurons that innervate cutaneous tissues. Of particular interest, the nociceptive fibers that detect pain have multiple specializations, responding to either noxious chemical, thermal or mechanical stimuli, or combinations of these stimuli (Perl, 1984; Darian-Smith, 1984a, 1984b; Koerber et al., 1988; Price, 1988; Perl, 1992; Meyer et al., 1994; Koltzenburg et al., 1997; Lawson, 2002). These nociceptive fibers are also neurochemically diverse, with widely overlapping expression patterns of histochemical markers, including ion channels, growth factor receptors, proinflammatory neuropeptides, and other signaling molecules.
One family of signaling molecules, the Mas-related G protein-coupled receptors (Mrgprs; otherwise known as sensory neuron-specific receptors, SNSR), are predominantly expressed in small-diameter sensory neurons of the trigeminal ganglia and dorsal root ganglia (DRG; Dong et al., 2001; Lembo et al., 2002; Zylka et al., 2003). In the DRG, one member of this large family of receptors, Mrgprd (also known as MrgD, Gm499 and TGR7; Zhang et al., 2005), is activated by β–alanine (Shinohara et al., 2004) and has been found to inhibit KCNQ/M-type potassium channels and increase excitability of sensory neurons (Crozier et al., 2007). Mrgprd is expressed in unmyelinated sensory afferents that bind isolectin-B4 (IB4), and express the ectonucleotidase Prostatic Acid Phosphatase (previously known as Fluoride Resistant Acid Phosphatase) as well as the ATP-gated ion channel P2X3. Mrgprd is not extensively coexpressed with substance P (SP), calcitonin gene-related peptide (CGRP) or the capsaicin-, heat- and proton-activated ion channel, transient receptor potential vanilloid receptor-1 (TRPV1; Dong et al., 2001; Zylka et al., 2003; Zylka et al., 2005, 2008). These Mrgprd-expressing fibers have recently been shown to specifically innervate the stratum granulosum of the epidermis (Zylka et al., 2005), where they may indirectly transduce sensation via the release of ATP from keratinocytes (Dussor et al., 2008). Currently, it is not known what physiologically-relevant somatosensory stimuli activate Mrgprd+ neurons. Moreover, the role that Mrgprd receptor activation plays in the overall function of these afferents is unknown.
In this study, we identified and characterized the population of sensory afferents that express Mrgprd using sharp electrode electrophysiology and an ex vivo skin/nerve/DRG/spinal cord preparation. We found that Mrgprd+ neurons were polymodal in function, responding to mechanical and heat stimuli, as well as to cold stimuli in some cases. Deletion of Mrgprd resulted in a significantly reduced excitability in these sensory afferents, which consisted of a decreased sensitivity to thermal and mechanical stimuli.
To further characterize the biophysical properties of Mrgprd-containing cells, we also used dissociated patch clamp electrophysiology to record from Mrgprd heterozygous (+/−) and homozygous (−/−) mouse DRG neurons in culture. We found that Mrgprd acts as a modulator of cell excitability, in which the threshold for firing a single action potential (AP) is lower in heterozygous cells than in homozygous cells. Furthermore, we show that Mrgprd is directly activated by β-alanine in DRG neurons, as β-alanine reduces the AP threshold and increases the AP firing rate in neurons from heterozygous, but not homozygous mice.
METHODS
All procedures used in these experiments were reviewed and approved by the Institutional Care and Use Committees at the University of Pittsburgh, University of North Carolina at Chapel Hill and Caltech, and followed the guidelines of the International Association for the Study of Pain.
Knockin Mouse Lines
Mrgprd+/+ gene-conserving mice contain the intact Mrgprd gene followed by IRES-EGFPf (Zylka et al., 2005; Figure 1A). Two different Mrgprd−/− gene-deleting mice were also examined. In one knockin line, the open reading frame of Mrgprd was deleted and replaced with an in-frame fusion of EGFPf (also called Mrgprd-EGFPf−/−, MrgprdΔEGFPf; previously described in Zylka et al., 2005; Figure 1A). In a second knockin line, we subcloned the EGFP-CRE-frt-PGK-Neo-frt construct (Shin and Anderson, 2005) out-of-frame with the Mrgprd start codon, using previously described Mrgprd 129/SvJ targeting arms, to make the gene-deleting Mrgprd-Cre−/− mice (Figure 1A). In this second line, amino acids # 20 to 315 are deleted from the 321 amino acid coding region of MRGPRD (GenBank accession # AAK91800). Homologous recombination was performed in mouse CJ7 embryonic stem (ES) cells following standard procedures. Correctly targeted ES cell clones were identified by Southern blot hybridization using probes that flanked the 5′ and 3′ arms of the targeting constructs, as well as an internal neomycin probe. Chimeric mice were produced by blastocyst injection and were mated to human β-actin FLPe deleter mice (Jackson Laboratories; Rodriguez et al., 2000) to remove the frt-flanked selection cassette and then were mated to C57BL/6 mice to establish the line. All knock-in lines used for this study were backcrossed to C57BL/6 mice for five or more generations. For all three lines, GFP expression faithfully marked the neurons that typically express Mrgprd.
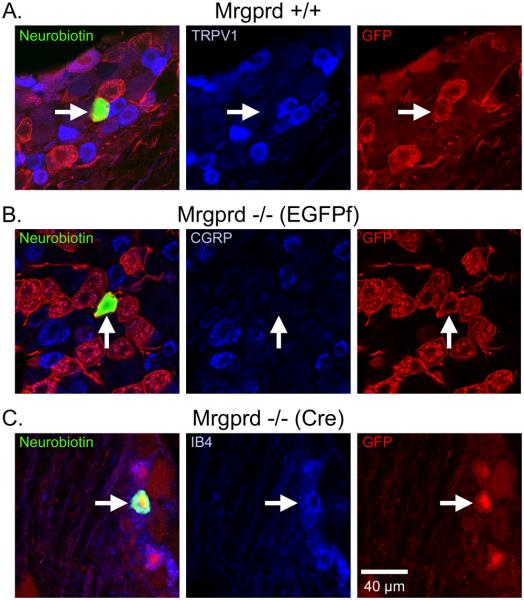
Figure 1. CPMs in Mrgprd knockin and WT mice bind IB4, but do not express either TRPV1 or CGRP.
Sample immunohistochemistry of recorded CPM cells in (A) Mrgpr+/+, (B) Mrgprd−/− (EGFPf ) and (C) Mrgprd−/− (Cre) as indicated by biotin-labeling (left panels, green). Labeling of TRPV1, CGRP and IB4 are shown for Mrgprd-IRES-EGFPf+/+, Mrgprd−/− (EGFPf) and Mrgprd−/− (Cre), respectively (middle panels, blue). GFP labeling is shown in the right panels (red). Arrows indicate recorded cell. Scale bar represents 40 μm (C, right panel).
Ex Vivo Preparation
The ex vivo somatosensory system preparation has been previously described in detail (Woodbury et al., 2001). Briefly, adult mice [C57BL/6 (Jackson Laboratory, Bar Harbor, ME) and Mrgprd knockin mice (Mrgprd+/+, Mrgprd-EGFPf−/−, and Mrgprd-Cre−/−)] were anesthetized via an intramuscular injection of ketamine and xylazine (90 and 10 mg/kg, respectively) and perfused transcardially with chilled (10°C), oxygenated (95% O2-5% CO2) artificial CSF (aCSF; in mmol/L: 1.9 KCl, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2, 26.0 NaHCO3, and 10.0 D-glucose), with 253.9 mmol/L sucrose. Spinal cord, L1-L4 DRGs, saphenous nerve, and innervated skin were dissected free in continuity. Following dissection, the preparation was transferred to a separate recording chamber containing chilled oxygenated aCSF in which the sucrose was replaced with 127.0 mmol/L NaCl. The skin was pinned out on a stainless steel grid located at the bath/air interface, such that the dermal surface remained perfused with the aCSF while the epidermis was exposed to the air. The platform provided stability during application of thermal and mechanical stimuli. The bath was then slowly warmed to 31°C prior to recording.
Recording and Stimulation
Dorsal root ganglion (DRG) cells were impaled with quartz filament microelectrodes (impedance >100 MΩ) containing 5% Neurobiotin (Vector Laboratories, Burlingame, CA) in 1 mol/L potassium acetate. Electrical search stimuli were delivered through a suction electrode on the saphenous nerve to locate sensory neuron somata with a peripheral axon innervating the skin. Peripheral receptive fields (RF) were localized with a fine paint brush, blunt glass probe and von Frey hairs. When cells were driven by the nerve but had no mechanical RF, a thermal search was performed by applying hot (∼52°C) and/or cold (∼0°C) physiological saline to the skin using a 10-mL syringe with a 20-gauge needle. If a thermal RF was located, the absence of mechanical sensitivity was confirmed by searching the identified RF using a glass probe. The response characteristics of the DRG cell were determined by applying computer controlled mechanical and thermal stimuli. The mechanical stimulator consisted of a constant force controller (Aurora Scientific Aurora, Ontario, Canada) attached to a 1-mm-diameter plastic disc. Computer controlled 5-second square waves of 5, 10, 25, 50, and 100 mN were applied to the cell's RF. Mechanical threshold was determined to be the lowest stimulus intensity of this series to elicit at least 1 AP within the first second of stimulus application. After mechanical stimulation, thermal stimuli were applied using a 3-mm2 contact area Peltier element (Yale University Machine Shop). The cooling stimulus was rapidly applied by the Peltier element through the thermal conduction of circulating ice-chilled water, which resulted in a drop in temperature from 31 to approximately 4°C. The temperature was then brought back up to 31°C, and after a 5-second pause the heating stimulus was applied, consisting of a 12-second heat ramp from 31 to 52°C followed by a 5-second plateau at 52°. The stimulus then ramped back down to 31°C in 12 seconds. The cooling and heating thermal thresholds were determined to be the temperatures at which the first AP was evoked. All responses were recorded digitally for off-line analysis (Spike2 software; Cambridge Electronic Design, Cambridge, UK). After physiological characterization, the cell was labeled by iontophoretically injecting Neurobiotin (2–3 cells per DRG). Peripheral conduction velocity was calculated from spike latency and the distance between the stimulating and recording electrodes.
Tissue Processing and Analysis of Recorded Cells
Once a sensory neuron was characterized and filled with Neurobiotin, the DRG containing the injected cell was removed and immersion fixed (4% paraformaldehyde in 0.1 M phosphate buffer for 30 minutes at 4°C). Ganglia were then blocked, embedded in 10% gelatin, postfixed overnight, and cryoprotected in 20% sucrose. Frozen sections (60 μm) were collected in phosphate buffer and reacted with primary antisera for GFP (chicken anti-GFP; Aves Labs. Inc.), and either TRPV1 (rabbit anti-TRPV1; Calbiochem, San Diego, CA) or CGRP (rabbit anti-CGRP; Chemicon, Temecula, CA). In many cases, the binding of isolectin B4 from Griffonia simplicifolia was also examined (IB4-647; Molecular Probes, Eugene, OR). After incubation in primary antiserum, tissue was washed and incubated in donkey anti-rabbit secondary antiserum (conjugated to Cy2, Cy3, or Cy5; Jackson Immunoresearch, West Grove, PA), and reacted with fluorescently-tagged avidin to label Neurobiotin-filled cells (Vector Laboratories). Distribution of fluorescent staining was determined using an Olympus confocal microscope and software (Fluoview; Olympus, La Jolla, CA). Sequential scanning was done to prevent bleed-through of the different fluorophores.
DRG Culture
DRG from all spinal levels were dissected from adult Mrgprd+/− and Mrgprd−/− mice, digested with 1 mg/mL collagenase (Worthington, CSL1) and 5 mg/mL dispase (Invitrogen, 17105-041) for 30 minutes then triturated. The resulting cell suspension was filtered through a 70 μm cell strainer to remove debris. Cells were plated onto glass coverslips coated with 0.1 mg/mL poly-D-lysine and 5 μg/mL laminin. Cells were allowed to settle for three hours in DH10 medium (1:1 DMEM:Hams-F12, 10% horse serum, 100 U/mL penicillin and 100 μg/mL streptomycin) at 37°C, 5% CO2. Medium was replaced with fresh DH10 containing 25 ng/mL GDNF and 50 ng/mL NGF and cells were cultured for one-to-two days at 37°C, 5% CO2.
Patch Clamp Electrophysiology
GFP+ neurons were visualized using epifluorescence microscopy (Nikon Eclipse FN1, NIR Apo 40x/0.80W water immersion objective) and recorded using whole-cell patch clamp electrophysiology as previously described (Campagnola et al., 2008). Briefly, patch clamp equipment (Multiclamp 700B and Digidata 1440A) and software (pClamp10) were from Molecular Devices, Sunnyvale, CA. DRG neurons were perfused during recording in oxygenated (95% O2, 5% CO2) bath solution consisting of (in mM): 10 HEPES, 140 NaCl, 4 KCl, 2 MgCl2, 2 CaCl2, 5 glucose, pH 7.5, 300 mOsM. Pipette solution for DRG neurons contained (in mM): 10 HEPES, 135 KCl, 3 Mg-ATP, 0.5 Na2-ATP, 2 EGTA, 1.1 CaCl2, 5 glucose, pH 7.5, 300 mOsM. Patch pipettes were made of borosilicate glass and had a resistance of 3-6 MΩ when filled with pipette solution. Experiments were performed at room temperature (21–24°C). Threshold current was defined as the amount of current (1 s duration) required to generate one AP before drug addition. β-alanine (1 mM; Sigma, St. Louis, MO) was bath applied for 5 min, and then threshold current was injected for 1 s to measure changes in firing rate.
Data Analysis
Since there were no significant differences observed in the biophysical, mechanical, thermal or immunocytochemical characteristics between the cell types recorded from the two gene-deleting lines of Mrgprd−/− mice (Mrgprd-EGFPf−/− and Mrgprd-Cre−/−), data from these two lines were combined for analysis. Additionally, the analysis of knockin mouse data (Mrgprd+/+, Mrgprd+/−, and Mrgprd−/−), was limited to those CPM cells that were GFP+, thereby indicating a cell with the capacity to express Mrgprd (note that these cells are referred to as Mrgprd+ neurons throughout the text, and that Mrgprd+ neurons from the Mrgprd−/− mice do not contain functional copies of Mrgprd); whereas analysis of WT data consisted of all CPM cells recorded. Consequently the latter group undoubtedly includes some Mrgprd– cells. Data are expressed as means ± SE. Student's t-tests, ANOVA, and post hoc tests were used to analyze different aspects of the ex vivo responses of neurons to electrical, mechanical and thermal stimuli, and the in vitro responses to electrical stimuli and drug application. Heat data was normalized by multiplying the average AP spikes per degree by the percentage of cells responding at that temperature.
RESULTS
Classification and Distribution of Cutaneous Sensory Neurons
In these studies, neurons were sorted into subgroups depending upon their conduction velocities (CV) and responses to mechanical and thermal stimuli. Neurons with a conduction velocity of <1.2 m/s were classified as C-fibers, and all others were classified as A-fibers (presumably myelinated; Kress et al., 1992; Koltzenburg et al., 1997).
Although we recorded from both A-fibers and C-fibers, strong evidence suggests that Mrgprd-containing afferents are C-fibers (Zylka et al., 2005). Therefore it should be noted that we only examined enough A-fibers to verify that there were no differences in their response properties (data not shown). We also recorded from a number of cells that were driven by the peripheral stimulating electrode but were found to be both mechanically and thermally unresponsive; however, only cells that had a receptive field were included for analysis.
C-fibers were divided into six subclasses (Table 1): (1) fibers which responded to mechanical and heat (CMH), (2) fibers which responded to mechanical, heat and cool/cold stimuli (CMHC), (3) C-mechanical fibers that responded to mechanical, but not to thermal stimuli (CM), (4) C-mechanical cool/cold fibers that responded to mechanical and cooling but not heating (CMC), (5) C-heat fibers which responded to heat stimuli (CH), but were mechanically insensitive, and (6) C-cooling/cold fibers, which responded to decreasing skin temperature, but not to heat or mechanical stimuli (CC).
Table 1. Representative cell types recorded in Mrgprd+ and Mrgprd− mice.
Number of cells (n) and percent of population (percent) are listed for WT (column 1), Mrgprd+/+ (column 2), and Mrgprd−/− (column 3). Cell types include C-fiber groups: cold only (CC), heat only CH), mechano only (CM), mechanocold (CMC), mechanoheat (CMH) and mechanocold/heat (CMCH). Total numbers of cells are listed at the bottom for each strain.
| WT | Mrgprd+/+ | Mrgprd−/− | ||||||
|---|---|---|---|---|---|---|---|---|
| n | percent | n | percent | GFP+ | n | percent | GFP+ | |
| CC | 1 | 0.8 | 2 | 4.4 | − | 3 | 2.5 | 0/1 |
| CH | 15 | 12.7 | 6 | 13.3 | 0/2 | 11 | 9.1 | 0/1 |
| CM | 9 | 7.6 | 5 | 11.1 | 1/1 | 9 | 7.4 | 0/1 |
| CMC | 14 | 11.9 | 3 | 6.7 | 0/1 | 11 | 9.1 | 0/1 |
| CMH | 49 | 41.5 | 22 | 48.9 | 11/15 | 60 | 49.6 | 34/42 |
| CMCH | 30 | 25.4 | 7 | 15.6 | 5/6 | 27 | 22.3 | 21/23 |
| C fibers | 118 | 45 | 121 | |||||
A total of 284 C-fibers innervating hindlimb hairy skin via the saphenous nerve were intracellularly recorded and physiologically characterized from 19 WT (n = 118 cells), 9 Mrgprd+/+ (n = 45 cells), and 28 Mrgprd−/− (n = 121 cells) male mice. The distribution of these fibers across all six classes of C-fibers is shown in Table 1. Similar percentages of fibers were encountered in WT, Mrgprd+/+, and Mrgprd−/− mice.
Apart from the cold response in CMHC cells, there was no statistical difference between the CMH and CMCH groups for any of the mouse lines, and therefore these populations were pooled together as CPM cells. As for the other C-fiber groups, no notable differences in AP features, and mechanical and / or thermal response characteristics were observed (data not shown).
Immunocytochemical analysis of CPMs
We identified Mrgprd+ neurons after ex vivo recordings by labeling DRG sections with antibodies against Neurobiotin and GFP. It should be noted that not all of the cells recorded in this study were stained with Neurobiotin for technical reasons. A total of 21 and 60 CPM cells from Mrgprd+/+ and Mrgprd−/− knockin mice, respectively, were recorded and labeled with Neurobiotin. Of those cells, the subsequent description of results applies to only 16 Mrgprd+/+ cells and 55 Mrgprd−/− cells that were confirmed to be GFP+ (Figure 1). A total of 79 WT cells were also recorded, and 28 of these were labeled with Neurobiotin. While all 79 WT cells were used for the biophysical, mechanical and thermal analysis, it should be noted that not all of these CPM cells will have contained Mrgprd, and therefore cells from Mrgprd+/+ mice are a more appropriate means for comparison.
In addition to verifying the presence of Neurobiotin and, in the case of the knockin mice, GFP, immunocytochemical analysis indicates that the CPMs of Mrgprd−/− mice express similar patterns of immunocytochemical markers as those contained in WT and Mrgprd+/+ mice (Figure 1). We found that most Neurobiotin-filled WT CPMs bound IB4 (18/23; cells positive / total cells examined), but did not express either TRPV1 (0/22) or CGRP (1/6). Most GFP+ and Neurobiotin-labeled CPM cells bound IB4 (Mrgprd+/+: untested; Mrgprd−/−: 32/32), but did not express TRPV1 (Mrgprd+/+: 0/16; Mrgprd−/−: 0/3) or CGRP (Mrgprd+/+: untested; Mrgprd−/−: 0/17; Figure 1), consistent with previous studies (e.g. Zylka et al., 2005).
Biophysical characteristics of CPM cells
Prior to immunocytochemistry, electrophysiological recordings were made from the CPM cells. With the recording electrode penetrating a DRG cell, search stimuli were pulsed from a suction electrode on the distal portion of the intact saphenous nerve just before it enters the skin. Action potentials were consequently evoked in cells whose axonal connection to the soma was intact. This provided multiple biophysical measurements for each of these cells, including membrane potential, half-amplitude width and conduction velocity (CV; calculated as the distance between the stimulating and recording electrodes, divided by the spike latency between the stimulus pulse and the triggered AP). No significant differences were observed in the characteristics for any of the mouse lines (data not shown).
CPMs in Mrgprd−/− mice exhibit decreased sensitivity to mechanical stimuli
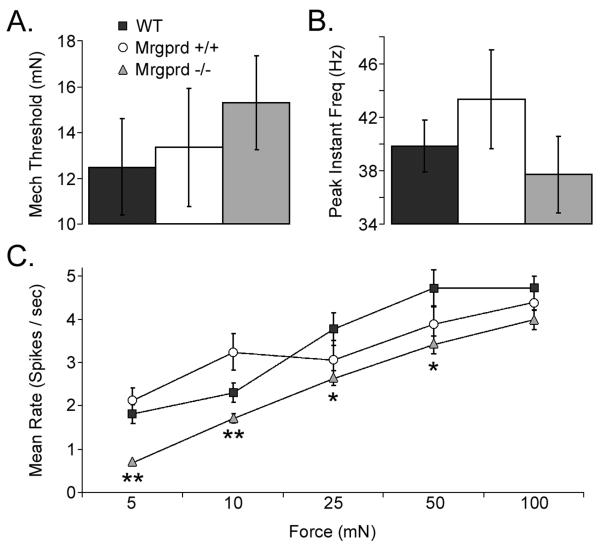
After it was determined that a cell could be driven peripherally, the search stimulus was turned off, and the peripheral receptive field (RF) of the CPM was isolated by brush, blunt glass probe, and von Frey hairs. CPM cells with identified RFs were then tested for their response to a computer-controlled mechanical device that applied 5, 10, 25, 50 and 100 mN stimuli. We observed that the average mechanical threshold was not significantly different between WT (12.49±2.10 mN), Mrgprd+/+ (13.33±2.57 mN), and Mrgprd−/− (15.28±2.04 mN; Figure 2B). Similarly, the peak instantaneous frequency remained unchanged (WT: 39.82±1.91 Hz; Mrgprd+/+: 43.32±3.71 Hz and Mrgprd−/− 37.69±2.87 Hz; Figure 2C). However, the mean firing rates for each of the stimuli was reduced in Mrgprd −/− mice versus WT and Mrgprd+/+ mice (Student's t-test: p<0.05; verified with ANOVA and Holm-Sidak post hoc tests; Figure 2D). There were no significant differences observed between WT and Mrgprd+/+ mice.
Figure 2. CPMs in Mrgprd−/− mice exhibit decreased mechanical sensitivity.
Sharp electrode recordings were made from WT (black), Mrgprd+/+ (white), and Mrgprd−/− (grey) CPM cells that were tested for their response to mechanical stimuli. No significant difference was observed between the mice in either the average mechanical threshold (A; mN) or in the mean peak instantaneous frequency (B; Hz); however, the mean rate for multiple force stimuli (C; spikes / sec; 5, 10, 25, 50 and 100 mN) were decreased in the Mrgprd−/− mice. Significance (p<0.05) is indicated below Mrgprd−/− in relation to either just WT (*), or to both WT and Mrgprd+/+ (**).
CPMs in Mrgprd−/− mice have decreased cold and heat sensitivity
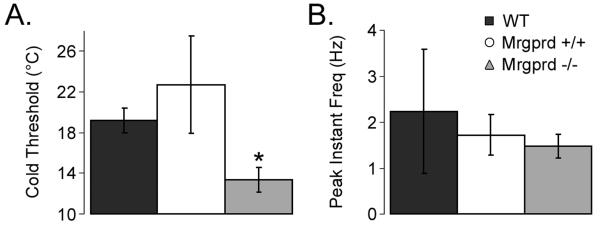
After examining the mechanical response of CPM cells, a small Peltier device was used to test for thermal response characteristics. In CPM cells that responded to cooling stimuli, the average cold threshold in Mrgprd+/+ mice was higher than those found in Mrgprd−/− mice (Figure 3A). From a baseline body temperature that was maintained at 31.0 °C, a rapid decrease in temperature to approximately 4°C resulted in action potentials that were evoked at relatively higher temperatures in WT (19.2±1.2 °C) and Mrgprd+/+ (22.7±4.8 °C) than those in Mrgprd−/− (13.4±1.3 °C; Student's t-test: p<0.05; verified with ANOVA and Holm-Sidak post hoc tests; Figure 3A). Maximum instantaneous frequency during cooling, however, was not significantly different between the mouse lines (WT: 2.23±1.35 Hz; Mrgprd+/+: 1.72±0.45 Hz; and Mrgprd−/−: 1.48±0.26 Hz; Figure 3B). For both hot and cold stimuli, no significant differences were observed between WT and Mrgprd+/+ mice.
Figure 3. CPMs in Mrgprd−/− mice exhibit decreased cold sensitivity.
Sharp electrode recordings were made from WT (black), Mrgprd+/+ (white), and Mrgprd−/− (grey) CPM cells that were tested for their response to cold stimuli. Mrgprd−/− CPM cells had lower cold thresholds (A; °C), but maintained a similar peak instantaneous frequency (B; Hz) compared to Mrgprd+/+ mice. Significance is indicated as p<0.05 (*).
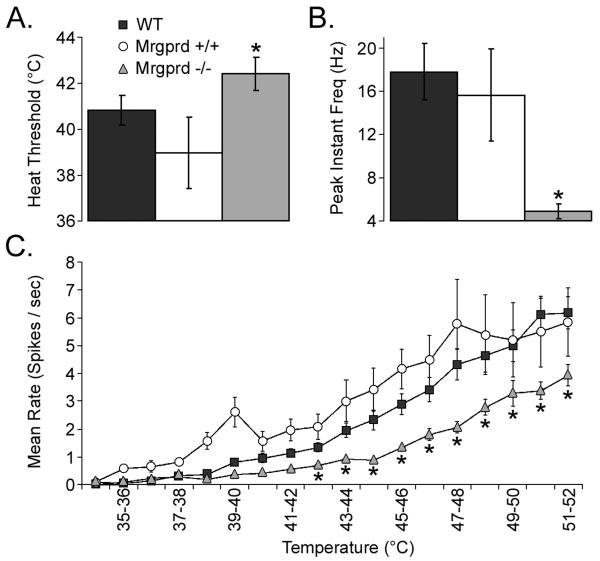
During heating ramps from 31.0 °C to 52.0 °C, CPMs in WT (40.8±0.6 °C) and Mrgprd+/+ (39.0±1.6 °C) exhibited lower heat thresholds versus Mrgprd−/− (42.4±0.7 °C; Student's t-test: p<0.05; verified with ANOVA; Figure 4A). Similarly, maximum instantaneous frequency during heating was significantly lower in Mrgprd−/− (4.88±0.67 Hz), versus WT (17.78±2.62 Hz) and Mrgprd+/+ (15.66±4.28 Hz; Student's t-test: p<0.05; verified with ANOVA and Dunn's Method post hoc tests; Figure 4B). The maximal firing rate per degree was also notably higher in WT (5.2±0.4 spikes/deg) and Mrgprd+/+ (5.2±0.8 spikes/deg) versus those in Mrgprd−/− mice (2.8±0.7 spikes/deg; Student's t-test: p<0.05; verified with ANOVA and Holm-Sidak post hoc tests; data not shown). In addition, mean firing rates per degree (44-52 °C, Student's t-test: p<0.05, verified with ANOVA and Holm-Sidak post hoc tests) were significantly higher in WT and Mrgprd+/+ than in Mrgprd−/− mice (Figure 4C).
Figure 4. CPMs in Mrgprd−/− mice exhibit decreased heat sensitivity.
Sharp electrode recordings were made from WT (black), Mrgprd+/+ (white), and Mrgprd−/− (grey) CPM cells that were tested for their response to heat stimuli. In comparison to WT and Mrgprd+/+ strains, the average thermal threshold of CPMs in Mrgprd−/− mice showed decreased excitability in terms of a higher heat threshold (A; °C) and a lower peak instantaneous frequency (B; Hz). A heat ramp (C) from 31 to 52°C showed a significant reduction in the average spikes / second in Mrgprd−/− mice between the temperatures of 43-52°C. Significance (p<0.05) is indicated below Mrgprd−/− in relation to both WT and Mrgprd+/+ (*).
It should be noted that in a related study (Cavanaugh et al., 2009), constitutive Mrgprd−/− mice did not exhibit any mechanical or thermal behavioral phenotype. This suggests that the decrease in neuronal excitability seen here is compensated for during development by other populations of neurons, including both sensory neurons as well as spinal circuits.
CPMs in Mrgprd−/− mice have decreased excitability in vitro
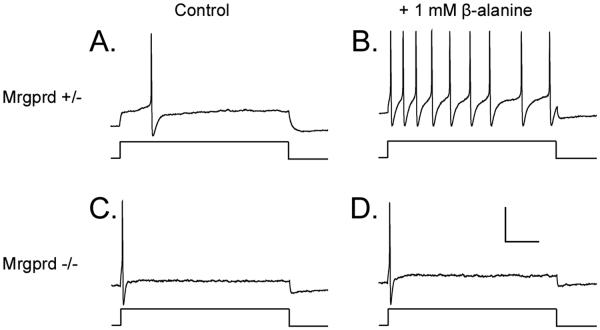
In addition to examining the knockin mice using the ex vivo electrophysiological technique, we also examined the excitability of GFP+ cells using an in vitro electrophysiological method. Patch-clamp studies revealed a higher rheobase in Mrgprd−/− mice (183.5 pA; n = 20) than in Mrgprd+/− mice (103.5 pA; n = 20; Student's t-test: p<0.05). When using threshold current injections, the application of β-alanine reduced the rheobase in Mrgprd+/− mice to 70.0 pA, and increased the firing rate by 3.03 fold (Student's t-test: p<0.05). In contrast, β-alanine had no effects in Mrgprd−/− mice (Figure 5; Table 2). There were no other significant differences between genotypes (e.g. Cm, Rin, MP, cell diameter).
Figure 5. β-alanine increases the firing rate of Mrgprd+/− but not Mrgprd−/− DRG neurons.
Whole-cell patch clamp recordings were made from cultured GFP+ DRG neurons from (A, B) Mrgprd+/− and (C, D) Mrgprd−/− mice. (A, C) The threshold current (1 s pulse duration, lower trace) required to evoke one AP (upper trace) was identified for each neuron. (B, D) β-alanine (1 mM) was then bath applied for 5 min. and the same amount of threshold current was injected into each neuron. (B) Neurons expressing one functional copy of Mrgprd increased their firing rate whereas (D) firing rate was not increased in Mrgprd−/− neurons. (A-D) In these cells, the threshold current was 100 pA. Scale bar (D) represents 40 mV (vertical) and 200 ms (horizontal).
Table 2. Deletion of Mrgprd reduces DRG neuron excitability and abolishes sensitivity to 1 mM β-alanine.
Ith = threshold current to generate one AP, determined using whole-cell patch clamp electrophysiology; n=20 GFP+ neurons per genotype. Data are presented as means ± s.e.m. P-values from paired t-tests are shown; N.S. = not significant (P>0.05).
| Ith (PA) | APs at Ith | |||||
|---|---|---|---|---|---|---|
| Genotype | Control | b-alanine | P-value | Control | b-alanine | P-value |
| +/− | 103.5 ± 17.4 | 70 ± 10.0 | 0.006 | 1.1 ± 0.1 | 3.2 ± 0.5 | 0.0002 |
| −/− | 183.5 ± 27.9 | 196 ± 29.3 | N.S. | 1.1 ± 0.1 | 0.9 ± 0.2 | N.S. |
| P-value | 0.02 | 0.0002 | ||||
DISCUSSION
This study examined the function of Mrgprd-containing sensory afferents and the role of Mrgprd as a modulator of cell excitability. Using an ex vivo preparation, we assessed the functional role of Mrgprd in cutaneous sensory neurons by examining three knockin mouse lines and comparing them to WT (C57BL/6) mice. Based on GFP and Neurobiotin co-labeling of recorded cells, we found that the Mrgprd receptor was almost exclusively localized in a large subset of C polymodal nociceptors (CPMs) that respond to mechanical stimuli, as well as to heat and sometimes cold stimuli. In the transgenic animals that we examined in this study, 76% and 92% of the CPM cells recorded from Mrgprd+/+ and Mrgprd−/− strains, respectively, exhibit expression of GFP driven by the Mrgprd promoter. The disparity in percentages could reflect the fact that in Mrgprd+/+ cells, EGFP (the surrogate marker for Mrgprd expression) is expressed after an IRES, while in Mrgprd−/− cells it is inserted as part of the coding sequence. Based on unpublished observations (M.J. Zylka and D. J. Anderson), the level of EGFP expression in the former line is about 10-fold lower than in the latter line. So the value of 76% could be an under-estimate, due to the lower expression of EGFP.
Deletion of Mrgprd significantly decreased sensitivity to mechanical and thermal stimuli. Specifically, a decreased firing rate was observed in cells lacking Mrgprd in response to lower mechanical forces and noxious heat. Additionally, thresholds of activation were remarkably lower in response to cold, and higher in response to hot stimuli. The decreased firing rates in response to mechanical and thermal stimuli, as well as the greater deviation from normal body temperatures that was required for CPMs to start firing suggests that Mrgprd may be necessary to maintain normal excitability. In support of this, in vitro patch clamp studies on cultured DRG neurons from mice lacking Mrgprd had a higher rheobase than heterozygous mice. The application of a known Mrgprd ligand, β-alanine, significantly reduced the rheobase and increased the firing rate in the neurons of heterozygous mice, but not in Mrgprd−/− mice.
Sensory neurons that express the Mrgprd receptor share multiple characteristics of unmyelinated nociceptors. Immunohistochemically these afferents bind IB4, and express P2X3, but not SP, CGRP or TRPV1 (Dong et al., 2001; Zylka et al., 2003; Zylka et al., 2005). Similarly, in this study as well as in previous studies in our laboratory using C57BL/6, Swiss Webster and C3H/BL6 WT mice, the vast majority of CPM fibers innervating mouse hairy skin characteristically bind IB4 and express P2X3, rarely express CGRP, but always lack TRPV1 (Albers et al., 2006; Lawson et al., 2008). Additionally, patch clamp of dissociated mouse DRG by Dussor and colleagues (2008), indicated that Mrgprd+ cells have somas with small diameters, long-duration APs, TTX-resistant Na+ currents, and Ca2+ currents inhibited by opioids. Finally, immunocytochemical analysis in this study confirmed that the majority of Neurobiotin-labeled CPM cells were in fact GFP+ (and hence normally Mrgprd+).
All of the CPM cells that contained Mrgprd in this study responded to cold and/or heat. It is well established that some of the key proteins involved in thermal transduction belong to the TRP family, including TRPV1 (Caterina et al., 1997), TRPV2 (Caterina et al., 1999), TRPA1 (Story et al., 2003), TRPM8 (Peier et al., 2002), and others. Previous work from our laboratory indicates that CPM cells are the largest population of heat-responding sensory afferents found in mouse hairy skin, but they lack the heat, capsaicin, and proton-sensitive TRPV1 channel (Zwick et al., 2002; Woodbury et al., 2004; Lawson et al., 2008). Instead, this protein appears to be isolated to a small population of mechanically-insensitive C-fibers that respond to heat (Lawson et al., 2008). In agreement with this data, Dussor and colleagues (2008) showed that dissociated cells containing Mrgprd fail to respond to either capsaicin or protons. Similarly, heat threshold and immunohistochemical evidence argue against the presence of heat-activated TRPV2 in the CPMs of WT animals (Woodbury et al., 2004; Lawson et al., 2008). As for the ionic currents evoked by cold stimuli in some Mrgprd+ CPMs, a previous report suggests that there is a lack of functional TRPM8 (≤25°C) and TRPA1 (≤17°C) as well, judging by the lack of a response to either the TRPM8 agonist menthol or the TRPA1 agonist cinnamaldehyde (Dussor et al., 2008). Thus how these sensory afferents transduce thermal signals remains to be determined.
While direct activation of known TRP channels by temperature seems unlikely in Mrgprd+ neurons, temperature could be sensed indirectly in Mrgprd+ afferents via the release of ATP from keratinocytes (Dussor et al., 2008). Mrgprd+ neurons express the ATP-gated receptor P2X3 (Zylka et al., 2005) and respond robustly to ATP with P2X3-like current kinetics (Dussor et al., 2008). In this way, changes in temperature (and perhaps other stimuli) are detected by skin cells, which then propagate signals to Mrgprd+ fibers in the stratum granulosum through the release of ATP. Such a role for keratinocytes in the sensation of the external environmental stimuli has been previously suggested (see reviews, Denda et al., 2007; Lumpkin and Caterina, 2007; and Peier et al., 2002). However, support for such a signaling mechanism is mixed. For example, P2X3−/− mice exhibit a reduced response of wide dynamic range neurons in the spinal cord dorsal horn to hindpaw heating (Souslova et al., 2000; Shimizu et al., 2005); however, behavioral testing indicated normal withdrawal from a hot plate and paradoxically indicated an enhanced avoidance of hot and cold temperatures in a thermal gradient (Shimizu et al., 2005). While these findings may reflect some form of compensatory mechanism exists, they also suggest that the mechanism of transduction is more complex than ATP release from kerotinocytes and activation of P2X3 receptors in this subset of nociceptive fibers. Although we tested for mechanical and thermal responsiveness in CPM fibers, we did not test these cells for responses to chemical stimuli using the ex vivo preparation. It is likely that these Mrgprd+ afferents are activated by molecules other than ATP, including the Mrgprd agonist, β-alanine (Shinohara et al., 2004). In this study, using a dissociated cell preparation, we demonstrated for the first time that Mrgprd+ neurons are directly sensitized by β-alanine. β-alanine, and the related dipeptide carnosine (β-alanyl-L-histidine), are present in high concentrations in vertebrate muscles and skin (Crush 1970; Nagai et al., 1986; Kohen et al., 1988) as well as rat sciatic nerve (Marks et al., 1970). In addition, β-alanine can be generated from carnosine by carnosinase enzymes. Taken together, this suggests β-alanine could be present and/or produced in skin and tonically activate Mrgprd on sensory afferents. Tonic activation of Mrgprd could in turn increase the excitability of Mrgprd+ neurons via inhibition of KCNQ/M-currents (Crozier, et al., 2007). The loss of this tonic activation could explain why Mrgprd+ neurons are less sensitive to thermal, mechanical, cold and electrical stimulation when the Mrgprd receptor is deleted. However, it should be noted that we have made repeated attempts, using the same the voltage step protocol used in rat sensory neurons (Crozier et al. 2005), but have been unable to reliably record M-currents in Mrgprd-GFP neurons. In addition, our colleague, Dr. Gregg Dussor, has also experienced the same difficulties in recording the M-current in these cells (G. Dussor, personal communication). Therefore, we were unable to directly test this hypothesis.
Numerous studies have shown that the C fiber population consists of multiple subsets of cells having a diverse pattern of histochemical markers, including neuropeptides, growth factor receptors, purinergic receptors, heat/cold-sensing receptors, proton-sensing receptors, IB4-binding, and others. The majority of these markers, however, are expressed in overlapping patterns in a wide range of cells types and/or tissue types. In this study, Mrgprd+ neurons accounted for 76-92% of the total CPM cells recorded from knockin animals. Considering work done by this laboratory and by others, the overwhelming localization of Mrgprd in CPM cells of the skin and its absence in other C fiber groups and tissue types, makes Mrgprd useful for targeting a major population of non-peptidergic C-polymodal nociceptors that innervate the skin.
The results presented in this study verify two key aspects of Mrgprd+ fibers. First, this receptor is localized in sensory afferents of the skin that specifically function as C-polymodal nociceptors. Secondly, it directly supports the idea that Mrgprd can influence cell excitability, at least in response to mechanical and thermal stimuli, as well as to its ligand β–alanine.
Acknowledgements
The authors wish to sincerely thank Collene Anderson and Weiwen Wang, for their excellent technical assistance, and Liching Lo for assistance in making the Mrgprd-CRE knockin mouse. This work was supported by grants to H.R.K from the National Institute of Health (R01 NS23275 and NS052848); grants to M.J.Z. from The Sloan Foundation, The Searle Scholars Program, The Klingenstein Foundation, The Whitehall Foundation, Rita Allen Foundation and NINDS (R01 NS060725); and a grant to D.J.A. from the National Institute of Health (P01 NS048499-05). M.J.Z. is a Rita Allen Foundation Milton E. Cassel Scholar.
Contributor Information
K.K. Rau, W1413 BST, Department of Neurobiology, University of Pittsburgh, Pittsburgh, PA 15261, 412-648-9086, krau@pitt.edu.
S.L. McIlwrath, W1402 BST, Department of Neurobiology, University of Pittsburgh, Pittsburgh, PA 15261, 412-648-8123, slm5@pitt.edu.
H. Wang, Campus Box 7545, Neuroscience Research Building, Cell and Molecular Physiology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, 919-966-2540, hongwa@email.unc.edu.
J.J. Lawson, Department of Neurobiology, University of Pittsburgh, Pittsburgh, PA 2664 Village Pier Lane, Hilliard, OH 43026, 724-244-1317, galvani_cajal@yahoo.com
M.P. Jankowski, W1413 BST, Department of Neurobiology, University of Pittsburgh, Pittsburgh, PA 15261, 412-648-9086, mpjst5@pitt.edu.
M.J. Zylka, Campus Box 7545, Neuroscience Research Building, Cell and Molecular Physiology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, 919-966-2540, zylka+mark@med.unc.edu.
D.J. Anderson, Division of Biology 216-76, Howard Hughes Medical Institute, California Institute of Technology, Pasadena, CA 91125, 626-395-8374, mancusog@caltech.edu.
H.R. Koerber, Department of Neurobiology, W1413 BST, Department of Neurobiology, University of Pittsburgh, Pittsburgh, PA 15261, 412-648-9086, rkoerber@pitt.edu.
REFERENCES
- Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. Glial cell-line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci. 2006;26:2981–2990. doi: 10.1523/JNEUROSCI.4863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnola L, Wang H, Zylka MJ. Fiber-coupled light-emitting diode for localized photostimulation of neurons expressing channelrhodopsin-2. J Neurosci Methods. 2008;169:27–33. doi: 10.1016/j.jneumeth.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. PNAS. 2009 doi: 10.1073/pnas.0901507106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier RA, Ajit SK, Kaftan EJ, Pausch MH. MrgD activation inhibits KCNQ/M-currents and contributes to enhanced neuronal excitability. J Neurosci. 2007;27:4492–4496. doi: 10.1523/JNEUROSCI.4932-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crush KG. Carnosine and related substances in animal tissues. Comp Biochem Physiol. 1970;34:3–30. doi: 10.1016/0010-406x(70)90049-6. [DOI] [PubMed] [Google Scholar]
- Darian-Smith I. Handbook of Physiology: The Nervous System - Sensory Processes. American Physiological Society; Washington, D.C.: 1984a. The sense of touch: performance and peripheral neural processes; pp. 739–788. [Google Scholar]
- Darian-Smith I. Handbook of Physiology: The Nervous System - Sensory Processes. American Physiological Society; Washington, D.C.: 1984b. Thermal sensibility; pp. 879–913. [Google Scholar]
- Denda M, Nakatani M, Ikeyama K, Tsutsumi M, Denda S. Epidermal keratinocytes as the forefront of the sensory system. Exp Dermatol. 2007;16:157–161. doi: 10.1111/j.1600-0625.2006.00529.x. [DOI] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Dussor G, Zylka MJ, Anderson DJ, McCleskey EW. Cutaneous sensory neurons expressing the Mrgprd receptor sense extracellular ATP and are putative nociceptors. J Neurophysiol. 2008;99:1581–1589. doi: 10.1152/jn.01396.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber HR, Druzinsky RE, Mendell LM. Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol. 1988;60:1584–1596. doi: 10.1152/jn.1988.60.5.1584. [DOI] [PubMed] [Google Scholar]
- Kohen R, Yamamoto Y, Cundy KC, Ames BN. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci U S A. 1988;85:3175–3179. doi: 10.1073/pnas.85.9.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J Neurophysiol. 1992;68:581–595. doi: 10.1152/jn.1992.68.2.581. [DOI] [PubMed] [Google Scholar]
- Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain. 2008;9:298–308. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp Physiol. 2002;87:239–244. doi: 10.1113/eph8702350. [DOI] [PubMed] [Google Scholar]
- Lembo PM, Grazzini E, Groblewski T, O'Donnell D, Roy MO, Zhang J, Hoffert C, Cao J, Schmidt R, Pelletier M, Labarre M, Gosselin M, Fortin Y, Banville D, Shen SH, Strom P, Payza K, Dray A, Walker P, Ahmad S. Proenkephalin A gene products activate a new family of sensory neuron--specific GPCRs. Nat Neurosci. 2002;5:201–209. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- Marks N, Datta RK, Lajtha A. Distribution of amino acids and of exo- and endopeptidases along vertebrate and invertebrate nerves. J Neurochem. 1970;17:53–63. doi: 10.1111/j.1471-4159.1970.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Campbell JN, Raja SN. Peripheral neural mechanisms of nociception. In: Wall PD, Melzack R, editors. Textbook of Pain. Churchill Livingstone; New York: 1994. pp. 13–44. [Google Scholar]
- Nagai K, Suda T, Kawasaki K, Mathuura S. Action of carnosine and beta-alanine on wound healing. Surgery. 1986;100:815–821. [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296(5575):2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Perl ER. Handbook of Physiology: The Nervous System - Senosry Processes. American Physiological Society; Washington, D.C.: 1984. Pain and nociception; pp. 915–975. [Google Scholar]
- Perl ER. Function of dorsal root ganglion neurons: an overview. In: Scott SA, editor. Sensory Neurons: Diversity, Development and Plasticity. Oxford University Press; New York: 1992. [Google Scholar]
- Price DD. Psychological and Neural Mechanisms of Pain. Raven Press; New York: 1988. [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Iida T, Guan Y, Zhao C, Raja SN, Jarvis MF, Cockayne DA, Caterina MJ. Enhanced thermal avoidance in mice lacking the ATP receptor P2X3. Pain. 2005;116:96–108. doi: 10.1016/j.pain.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Shin D, Anderson DJ. Isolation of arterial-specific genes by subtractive hybridization reveals molecular heterogeneity among arterial endothelial cells. Dev Dyn. 2005;233:1589–1604. doi: 10.1002/dvdy.20479. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Harada M, Ogi K, Maruyama M, Fujii R, Tanaka H, Fukusumi S, Komatsu H, Hosoya M, Noguchi Y, Watanabe T, Moriya T, Itoh Y, Hinuma S. Identification of a G protein-coupled receptor specifically responsive to beta-alanine. J Biol Chem. 2004;279:23559–23564. doi: 10.1074/jbc.M314240200. [DOI] [PubMed] [Google Scholar]
- Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, Nebenuis-Oosthuizen D, Smith AJ, Kidd EJ, Wood JN. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407(6807):1015–7. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Ritter AM, Koerber HR. Central anatomy of individual rapidly adapting low-threshold mechanoreceptors innervating the “hairy” skin of newborn mice: early maturation of hair follicle afferents. J Comp Neurol. 2001;436:304–323. [PubMed] [Google Scholar]
- Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Taylor N, Xie Y, Ford R, Johnson J, Paulsen JE, Bates B. Cloning and expression of MRG receptors in macaque, mouse, and human. Brain Res Mol Brain Res. 2005;133:187–197. doi: 10.1016/j.molbrainres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22:4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci U S A. 2003;100:10043–10048. doi: 10.1073/pnas.1732949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Sowa NA, Taylor-Blake B, Twomey MA, Herrala A, Voikar V, Vihko P. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron. 2008 Oct 9;60(1):111–22. doi: 10.1016/j.neuron.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]