Abstract
Gliomas have a dismal prognosis, with the median survival of patients with the most common histology, glioblastoma multiforme, being only 12–15 months. Development of novel therapeutic agents is urgently needed. We have previously demonstrated that oncolytic measles virus strains derived from the Edmonston vaccine lineage have significant antitumor activity against gliomas [Phuong, L.K., Allen, C., Peng, K.W., Giannini, C., Greiner, S., Teneyck, C.J., Mishra, P.K., Macura, S.I., Russell, S.J., Galanis, E.C. (2003). Cancer. Res. 63, 2462–2469]. MV-CEA is an Edmonston vaccine lineage measles virus strain engineered to express the marker peptide carcinoembryonic antigen (CEA): CEA levels can serve as a correlate of viral gene expression. In support of a phase I clinical trial of intratumoral and resection cavity administration of MV-CEA to patients with recurrent gliomas, we assessed the neurotoxicity of MV-CEA in adult immune male rhesus macaques (Macaca mulatta). The animals' immune status and administration schedule mimicked the trial population and proposed administration schema. Macaca mulatta represents the prototype animal species for assessment of measles neurotoxicity. The animals were stereotactically administered either vehicle (n = 1) or MV-CEA at 2 × 105 or 2 × 106 TCID50 (each, n = 2) in the right frontal lobe in two injections on days 1 and 5. Macaques were closely monitored clinically for neurotoxicity. Body weight, temperature, complete blood count, CEA, clinical chemistries, coagulation, complement levels, immunoglobulin, measles antibody titers, viremia, and shedding (buccal swabs) were tested at multiple time points. Furthermore, cisterna magna spinal taps were performed on day 9 and 1 year after the first viral dose administration, and samples were analyzed for protein, glucose, cell differential, and presence of MV-CEA. Magnetic resonance imaging (MRI) was performed between 4 and 5 months after article administration to assess for subclinical neurotoxicity. To date, 36+ months from study initiation there has been no clinical or biochemical evidence of toxicity, including lack of neurological symptoms, fever, or other systemic symptoms and lack of immunosuppression. Quantitative RT-PCR analysis of blood, buccal swabs, and cerebrospinal fluid (CSF) was negative for MV-CEA at all time points, with the exception of viral genome deletion in the blood of one asymptomatic animal at the 2 × 106 TCID50 dose level on day 85. Vero cell overlays of CSF cells and supernatant were negative for viral recovery. There was no detection of CEA in serum or CSF at any time point. MRI scans were negative for imaging abnormalities and showed no evidence of encephalitis. Our results support the safety of CNS administration of MV-CEA in glioma patients. A clinical trial of intratumoral and resection cavity administration of MV-CEA in patients with recurrent glioblastoma multiforme is currently ongoing.
Introduction
Glioblastoma multiforme is the most frequent primary brain tumor in adults and accounts for the majority of 13,500 primary brain tumor patients diagnosed each year in the United States (Jemal et al., 2007). It is one of the most lethal malignancies, with a median survival of 12–15 months despite multimodality treatment including surgery, chemotherapy, and radiation therapy (Stupp et al., 2005). Gliomas represent a promising target for gene transfer approaches given their limited ability to metastasize, but despite encouraging preclinical data, significant clinical benefit has not materialized to date.
Our group is developing oncolytic measles virus strains as antitumor agents. Preclinical activity against a variety of tumor types has been demonstrated (Peng et al., 2002a; Phuong et al., 2003; Blechacz et al., 2006; McDonald et al., 2006). To address one of the major challenges in clinical gene transfer trials, that is, the ability to monitor viral propagation after administration to the patient, trackable measles virus strains were created by introducing soluble marker peptides in the viral H protein (Peng et al., 2002b). MV-CEA was constructed by introducing the human carcinoembryonic antigen (CEA) gene in position 1 of the viral H protein. CEA is a glycoprotein of molecular mass 180 kDa, a circulating half-life of 36 hr in humans, and no documented biological activity (Collatz et al., 1971; Ura et al., 1985). The normal serum concentration of CEA is less than 3 ng/ml. Circulating levels are frequently elevated in certain malignancies, such as colon cancer, in which CEA provides an important nonimmunogenic tumor marker. Because CEA is not expressed in gliomas (Dabbs et al., 2002), elevated levels of CEA in the serum of glioma patients after viral treatment could be attributed only to viral gene expression. Furthermore, as demonstrated in the case of colorectal cancer patients with CNS-only metastases, CEA produced in the CNS reaches the systemic circulation and can be detected in the serum (Ishikura et al., 1987).
Virotherapy with MV-CEA has resulted in significant antitumor activity in multiple established and primary glioma lines and glioma subcutaneous and orthotopic xenografts (Phuong et al., 2003; Allen et al., 2006). Of importance, CEA levels in the serum of treated animals both in the subcutaneous and orthotopic models increased before tumor regression, indicating viral gene expression, and returned to normal after tumor regression (Phuong et al., 2003).
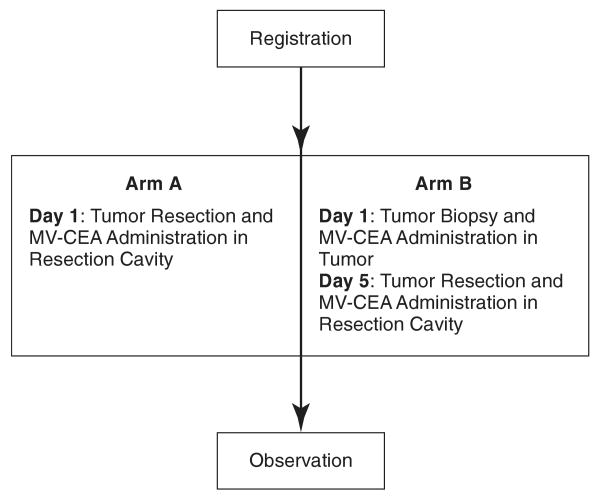
Recurrent glioblastoma multiforme remains confined in the CNS in the majority of patients. This allows us to explore intratumoral and resection cavity administration of MV-CEA, both as a means of targeting and in order to circumvent neutralization of the virus by anti-measles virus antibodies, which are present in more than 90% of the Western population as a result of immunization or natural infection (Cutts and Markowitz, 1994). The phase I trial of MV-CEA in recurrent glioblastoma patients represents the first human trial of a measles-based therapeutic approach in brain tumor patients. Patients with recurrent glioblastoma multiforme and preexisting immunity to measles, who are candidates for gross total or subtotal tumor resection, are eligible. The trial includes two arms and its schema is described in Fig. 1. Patients in group A will receive direct MV-CEA injections in the resection cavity. After dose escalation in arm A is completed up to a viral dose of 107 TCID50 (median tissue culture infective dose), accrual in arm B will be initiated. Arm B patients will have MV-CEA administered into their recurrent tumor. Five days later, and at the time that maximal viral replication is expected (Phuong et al., 2003), tumor resection will be performed, and a second viral dose will be administered at the resection cavity. The resected tumor will be examined for viral distribution by in situ hybridization and immunohistochemistry, in relationship to distance from the injection site. This two-step approach has been demonstrated to be informative in glioma trials employing other vector systems (Harsh et al., 2000; Lang et al., 2003). Primate toxicology studies in support of this phase I trial of intratumoral and resection cavity administration of MV-CEA in recurrent glioma patients are reported here.
FIG. 1.
Phase I trial of MV-CEA in recurrent glioblastoma multiforme patients: schema.
Materials and Methods
Animals
Five male adult measles-immune rhesus macaques (Macaca mulatta) were obtained from Alpha Genesis (Yemassee, SC). Macaques were examined by the attending veterinarians during the 6-week institutional quarantine, and were found to be healthy and seronegative for herpes B. All animals had protective antibody titers before study entry in order to mimic the immune status of the trial population.
Virus
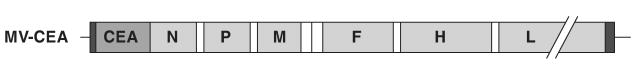
MV-CEA was constructed as previously described (Peng et al., 2002; and see Fig. 2). MV-CEA batch 04-MV-CP-01 was provided by the Mayo Clinic Viral Vector Production Laboratory (Rochester, MN). The titer of the preparation used was 1.9 × 107 TCID50/ml. The virus was stored at −70°C. Immediately before administration, the virus was thawed and diluted with sterile normal saline. Test and control article mixtures were kept on wet ice throughout. Syringes were loaded with a single dose of article just before injection. Excess active viral dilutions were titrated after dosing for confirmation of the dose administered.
FIG. 2.
Schematic representation of MV-CEA. The cDNA encoding the human CEA was inserted upstream of the nucleocapsid (N) gene. P, phosphoprotein gene; M, matrix protein gene; F, fusion protein gene; H, hemagglutinin gene; L, large protein gene. Adapted with permission from Peng, K.W., Facteau, S., Wegman, T., O'Kane, D., and Russell, S.J. (2002b). Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat. Med. 8, 527–531.
Administration schedule of the virus and vehicle control
Test and control article were administered on days 1 and 5 to mimic the proposed administration schedule for the high-dose arm of the human trial. Macaques were fasted for 12 hr and sedated with ketamine (15 mg/kg) and 0.5–xylazine (8.0 mg/kg) given intramuscularly. Cephazolan and atropine were administered intravenously. Macaques were intubated, catheterized, placed on a heat pad, and maintained on 1–3% isoflurane. Heart rate, respiration, pulse, and arterial oxygen saturation as determined by pulse oximetry (SpO2) were monitored. On day 1, one 4.0-mm hole was drilled into the skull according to the injection coordinates (see below). One hundred microliters of article was injected into the deep white matter of the right frontal lobe, 10 mm below the cortex of the brain using the following coordinates: 20–31.35 mm anterior to the intraaural line, 5 mm in front of the bregma, and 8 mm external to the midline. Two animals received 105 TCID50, two received 106 TCID50, and one animal received vehicle control on days 1 and 5. Test or control article was injected over an 8-min period with a Hamilton syringe that was fitted to the stereotactic frame and connected to a 26-gauge needle. The needle was slowly withdrawn over another 2- to 3-min period. The incision was sutured and coated with triple antibiotic ointment. On day 5, a second injection, using the same viral dose and stereotactic coordinates, was administered.
Clinical observations
Macaques were monitored daily by facility veterinary technicians and animal caretakers. In addition, trained study personnel assessed macaques daily for the first 3 months, and three times weekly thereafter for activity level, posture, facial symmetry, gait, behavior, motor function, level of consciousness, and clinical signs of coryza, conjunctivitis, and rash. Table 1 summarizes the data collection schedule in this toxicology study.
Table 1. Data Collection Schedule.
| Study day | Test article administration | Body weight/temperature | Blood sample collectiona | Viremia testing | Immune parametersb | Shedding | CSF examinationc |
|---|---|---|---|---|---|---|---|
| −2 | X | ||||||
| 1 | X | X | X | X | X | X | |
| 5 | X | X | X | X | X | ||
| 9 | X | X | X | X | X | ||
| 15 | X | X | X | ||||
| 22 | X | X | X | ||||
| 29 | X | X | X | X | |||
| 57 | X | X | X | X | |||
| 85 | X | X | X | X | |||
| 1 year | X | X | X | X | X |
Abbreviations: CEA, carcinoembryonic antigen; CSF, cerebrospinal fluid; MV, measles virus; QRT-PCR, quantitative real-time polymerase chain reaction.
Blood parameters: CEA, hematology, coagulation, and clinical chemistry.
Immune parameters: complement, immunoglobulins, and MV antibody titers.
CSF examination (spinal tap): cell count and differential, morphology review, CEA, total protein, glucose, RNA isolation for QRT-PCR analysis, Vero cell overlay, and anti-measles antibody levels.
Blood draws
All blood draws and sample collection were performed under sedation, using ketamine (15 mg/kg) and xylazine (0.5–8.0 mg/kg), administered intramuscularly.
Hematology, coagulation, and chemistry profiles
For hematology studies, 100-μl samples of blood were collected into EDTA BD Vacutainer or BD Microtainer tubes (Becton Dickinson, Franklin Lakes, NJ) and mixed immediately to prevent clotting. Samples were kept at room temperature up to a maximum of 2 hr before assay. For coagulation assays, approximately 100-μl samples of blood were collected into sodium citrate tubes (final concentration of 10% sodium citrate in sample) and mixed immediately to prevent clotting. Samples were kept at room temperature up to a maximum of 2 hr before assay. Forty microliters was analyzed on citrate PT (prothrombin) cartridges and 40 μl was analyzed on citrate APTT (activated partial thromboplastin time) cartridges with the SCA2000 veterinary coagulation analyzer (Synbiotics, San Diego, CA). For other chemistry studies, serum was spun down from blood samples containing no anticoagulant. One hundred microliters of sample was analyzed on the VetScan chemistry system with comprehensive diagnostic profile (Abaxis, Union City, CA).
CD3/CD4/CD8 flow cytometric analysis
Blood was collected in EDTA tubes, split into six 100-μl aliquots, and stained with antibodies for 30 min at room temperature in the dark. All antibodies and reagents were purchased from BD Biosciences (San Jose, CA). One aliquot was stained with the three isotype controls: Alexa Fluor 488-conjugated mouse IgG1(κ) monoclonal isotype control (cat. no. 557782; BD Biosciences), phycoerythrin (R-PE)-conjugated mouse IgG1(κ) monoclonal isotype control (cat. no. 556650; BD Biosciences), and phycoerythrin–cyanine dye 7 (PE–Cy7)-conjugated mouse IgG1(κ) isotype control (cat. no. 557872; BD Biosciences). The second aliquot was stained with Alexa Fluor 488-conjugated mouse anti-human CD3 (cat. no. 557705; BD Biosciences), the third with PE-conjugated mouse anti-human CD4 (cat. no. 550630; BD Biosciences), and the fourth with PE–Cy7-conjugated anti-human CD8 (cat. no. 557872; BD Biosciences). The last two aliquots were double stained with anti-CD3 and anti-CD4 and, last, with anti-CD3 and anti-CD8. Red blood cells were lysed with FACS lysing solution (cat. no. 349202; BD Biosciences) twice for at least 20 min at room temperature in the dark. Samples were run on a BD FACScan (BD Biosciences), and analyzed with the provided CellQuest Pro software.
Immunological assays
Serum immunoglobulins (IgA, IgM, and IgG) were determined by nephelometry (as per the Dade Behring BN II nephelometer operations instruction manual; Dade Behring, Newark, DE) and serum total complement was determined by an automated liposome lysis assay through the Mayo Clinic Rochester Protein Immunology Laboratory. Assays for detection of anti-measles antibodies in the serum or cerebrospinal fluid (CSF) samples were performed at BioReliance Simian Serology Laboratory (Rockville, MD) (measles virus ELISA, cat. no. 80-421).
Serum CEA
Serum CEA was determined with a Beckman Coulter UniCel DxI 800 immunoassay system (Beckman Coulter Clinical Diagnostics Division, Brea, CA) at the Mayo Clinic Rochester Central Clinical Laboratory.
RNA isolation from buccal swabs and from peripheral blood mononuclear cell and CSF samples
Approximately 2.5 ml of whole blood was collected into a PAXgene collection tube and processed according to the manufacturer's instructions, using a PAXgene blood RNA kit (cat. nos. 762165 and 762164; Qiagen, Valencia, CA) with the optional on-column DNase treatment. Buccal swabs were obtained by swabbing each macaque's buccal mucosa, using 10 firm back-and-forth motions, with a sterile OmniSwab (cat. no. WB100035; Whatman Biosciences/GE Healthcare). The swabs were ejected into 400 μl of buffer RLT (with 2-mercaptoethanol added in accordance with the RNeasy micro kit (cat. no. 74004; Qiagen) and immediately placed on ice for up to 90 min. Samples were vortexed for 1 min, and lysates were transferred to a QIAshredder mini spin column and centrifuged for 5 min at maximal speed in a tabletop microcentrifuge. Supernatants were collected and frozen at less than −70°C until all samples were ready to be processed. Samples were then thawed and 1 volume of 70% ethanol was added and mixed with each lysate. Samples were applied to an RNeasy MinElute spin column and were centrifuged at 8000 × g for 15 sec; the flow-through was discarded, and 350 μl of buffer RW1 (cat. no. 74004; Qiagen) was added to each column, and then centrifuged at 8000 × g for 15 sec. The flow-through was discarded, and the columns were transferred to fresh 2-ml collection tubes. buffer RPE (500 μl) (cat. no. 74004; Qiagen) was added to each column, and the columns were centrifuged at 8000 × g for 15 sec. The flow-through was discarded, and 500 μl of 80% ethanol was added to each column. The columns were centrifuged at 8000 × g for 2 min. Flow-through and collection tubes were discarded, and the columns were transferred to fresh 2-ml collection tubes. The columns were centrifuged at full speed for 5 min. The columns wee transferred to new 1.5-ml collection tubes, and 20 μl of RNase-free water was added to each column, which were then allowed to sit for 1 min at room temperature. The columns were centrifuged at maximal speed for 1 min to elute the RNA. RNA was quantified by ultraviolet spectroscopy, and frozen at ≤ −70°C until quantitative realtime polymerase chain reaction (QRT-PCR) analysis.
QRT-PCR for measles virus nucleoprotein mRNA
The assay has been optimized for primers, probe, and magnesium concentration, using the Stratagene (La Jolla, CA) Brilliant single-step quantitative RT-PCR core reagent kit and the Mx4000 QPCR system. The final 50-μl reaction contained 300 nM forward primer (5′-S-GAGAAGCCAGGGAGAGCTACAG-3′), 200 nM 5′-/S-56-FAM/AAACCGGGCCCAGCAGAGCA/3BHQ_1/-3′-TAMRA dual-labeled probe (FAM, 6-carboxy-fluorescein; TAMRA, 6-carboxy-tetramethyl rhodamine), 300 nM reverse primer (5′-S-GGGCAGCTCTCGCATCAC-3′), 4 mM Mg2+, and 1 μg of total RNA isolate to amplify 63 bases of measles virus nucleoprotein mRNA. 6-Carboxy-X-rhodamine (ROX) served as the reference dye. One cycle of RT reaction (30 min at 45°C) was applied followed by a denaturation step (10 min at 95°C) and 40 cycles of amplification (30 sec at 95°C and 1 min at 55°C), with fluorescence measured during the extension. A linear standard curve of 10-fold dilutions was run from 108 to 103 copies (limit of detection). The standard curve was generated on the basis of triplicate samples from a purified 61-bp RNA oligonucleotide (5′-GAAGCCAGGGAGAGCUACAGAGAAACCGGGCCCAGCAGAGCAAGUGAUGCGAGAGCUGCCC-3′; Dharmacon/Fisher Scientific, Lafayette, CO) specific to measles virus nucleoprotein mRNA.
CSF sampling
Cisterna magna taps were performed with a 25-gauge, 1-in. needle under sedation with ketamine, xylazine, and atropine, administered intramuscularly. Each CSF sample was gently mixed and immediately aliquoted; 0.4 ml was used for RNA isolation; 0.5 ml was used for determination of cell count, differential, and morphological review; and the remainder was centrifuged for 10 min at 2500 × g rpm at 4°C to obtain the CSF supernatant. The supernatant was aliquoted as follows: 0.1 ml for anti-measles antibody titer; 0.55 ml for glucose, total protein, and CEA assays; 0.5 ml for Vero cell overlays; and the remainder was archived at −70°C or less. The CSF pellet (with approximately 0.1 ml of supernatant) was also processed for Vero cell overlays.
CSF assays
CSF samples were kept on ice until assayed. Glucose was determined in a photometric glucose oxidase/peroxidase dry slide assay (VITROS analyzer; Ortho-Clinical Diagnostics, Raritan, NJ) and total protein was determined by reflectance spectrophotometry by the Mayo Clinic Rochester Central Clinical Laboratory. CSF CEA was determined on a Beckman Coulter UniCel DxI 800 (Beckman Coulter Clinical Diagnostics Division) through the Mayo Clinic Central Clinical Laboratory. CSF cell count, differential, and morphological review were performed at the Mayo Clinic Rochester General Hematopathology Laboratory.
Vero cell overlay of CSF samples
Twenty-four hours before assay, 1 × 105 Vero African green monkey kidney cells (American Type Culture Collection [ATCC], Manassas, VA) were plated on a 6-well plate and incubated at 37°C plus 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum (FBS). Wells were washed, and CSF supernatant or CSF cells were layered onto the cells with sufficient Opti-MEM (Invitrogen, Carlsbad, CA) to bring the total volume to 1.0 ml. Cells were incubated for 4 hr at 37°C plus 5% CO2, and then 3 ml of DMEM-5% FBS was added to the wells. Positive controls (SKOV3. ip1 cells infected with MV-CEA [1 × 103 TCID50], and CSF spiked with 1 × 105 viral TCID50) and negative controls were run in parallel with the primary cells/fluid to ensure assay quality. Cells were examined at 24, 48, 72, and 96 hr for development of syncytia (the characteristic measles virus-induced cytopathic effect).
Magnetic resonance imaging
At 4–5 months after study initiation, magnetic resonance imaging (MRI) of the head was performed in all subjects. Macaques were sedated with ketamine (15 mg/kg) and xylazine (0.5–8.0 mg/kg), administered intramuscularly. Atropine (0.5 mg/kg) was administered intravenously, and the animals were intubated, catheterized, and placed on a heat pad. Macaques were maintained on 1–3% isoflurane anesthesia during the procedure. Heart rate, respiration, pulse, and SpO2 were monitored. A 1.5T Signa MRI whole-body MRI scanner (GE Healthcare, Waukesha, Wisconsin) with an extremity coil was used for image acquisition. T1-weighted images without and with intravenous gadolinium chelate (0.1 mmol/kg), fast spin echo T2-weighted images, T2 fluid attenuation inversion recovery (FLAIR) images, and diffusion-weighted images of each subject's whole brain were obtained, using standard clinical pulse sequences slightly modified to the macaque brain. Sagittal images were performed with a slice thickness of 4 mm, and axial images were performed with slice thickness of 3 mm. All subject images were analyzed digitally and on film by a neuroradiologist (T.K.), who was blinded to prior intracerebral MV-CEA or vehicle-only administration. All studies were evaluated for the presence or absence of any brain parenchymal or meningeal signal abnormality or abnormal contrast enhancement.
Results
Study treatment
The administration schedule and timing mimicked the high-dose schedule in arm B of the clinical trial. The highest total dose in the human clinical study is 2 × 107 TCID50 with 107 TCID50 administered stereotactically in the recurrent tumor on day 1 and another 107 TCID50 administered in the resection cavity after tumor resection on day 5. Dose equivalence between humans and macaques is as shown in Table 2.
Table 2. Dose Equivalence Between Humans and Macaques.
| Species | Brain weight (kg) | Dose (TCID50) | Equivalent human dose (TCID50) |
|---|---|---|---|
| Human, adult | 1.4 | 2 × 107 | 2 × 107 |
| Rhesus macaque (Macaca mulatta) | 0.090 | 2 × 106 | 3.1 × 107 |
Abbreviation: TCID50, median tissue culture infective dose.
Two animals received 105 TCID50 on days 1 and 5 (total dose, 2 × 105 TCID50), two animals received 106 TCID50 on days 1 and 5 (total dose, 2 × 106 TCID50), and one animal received vehicle control. On the basis of brain weight equivalence the high-dose macaques received 1.5 times the highest proposed dose in the clinical trial and three times the highest dose that normal human brain can be exposed to in the clinical trial.
General health status
Macaques remained normal in their activity level, posture, facial symmetry, gait, behavior, motor function, and level of consciousness throughout the study; no coryza, conjunctivitis, or rash was observed. Control and experimental macaques maintained and gained weight throughout the study. Overall there was no negative impact of the study treatment on the health of the macaques.
Hematology, coagulation, and chemistry profiles
The following parameters remained normal both for MV-CEA- and control-treated animals for the duration of the study. White blood cell count with three-part differential, red blood cells, platelets, hemoglobin, hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC), coagulation assays (PT and APTT), clinical chemistry profile to include albumin, alkaline phosphatase, alanine aminotransferase, amylase, total bilirubin, blood urea nitrogen, calcium, phosphorus, creatinine, glucose, sodium, potassium, and total protein. In summary, there was no evidence of hematologic, hepatic, or renal toxicity or of coagulation abnormalities as a result of MV-CEA treatment.
Immunologic assays
There was no evidence of immunosuppression after MV-CEA administration into the CNS. CD4+/CD8+ cell counts, complement, and specific immunoglobulin levels (IgA, IgM, and IgG) remained normal throughout the study. Macaques retained positive measles antibody titers throughout the study.
Serum CEA
All serum samples were negative (<0.5 ng/ml) for the presence of CEA at all time points. Because CEA represents a correlate of MV-CEA gene expression, no detection indicates lack of significant viral replication in the CNS.
QRT-PCR for measles virus nucleoprotein RNA in buccal swabs and blood
To determine whether the viral inoculum had been able to replicate and disseminate systemically, QRT-PCR analyses were performed on buccal swabs and blood samples. Buccal swabs were negative (limit of detection, 1000 copies/μg RNA) for the presence of measles virus nucleoprotein mRNA at all time points. With the exception of 5M01, the macaques were negative for the presence of measles virus nucleoprotein mRNA in the blood (peripheral blood mononuclear cells) at all time points tested. Macaque 5M01, one of the two animals that received a total dose of 2 × 106 TCID50, had one positive blood sample with 4844 genome copies/μg RNA at 3 months (day 85). However, all prior samples and subsequent samples at 6 months and 1 year were below the limit of detection. At no time did 5M01 exhibit clinical symptoms suggestive of infection, or laboratory abnormalities.
CSF assays
To investigate the possibility of subclinical MV-CEA-induced encephalitis, we sampled and analyzed CSF on day 9 and at 1 year from treatment initiation. Glucose and total protein levels were normal for all macaques at both the day 9 and 1-year time points. CSF was negative for the presence of CEA, anti-measles antibodies, replicating virus (Vero cell overlay; Fig. 3), and measles virus nucleoprotein mRNA (QRT-PCR). Total nucleated cell counts, red blood cell counts, and differentials were normal for all macaques, both at 9 days and 1 year (Table 3). Collectively these results indicate a lack of subclinical CNS infection.
FIG. 3.
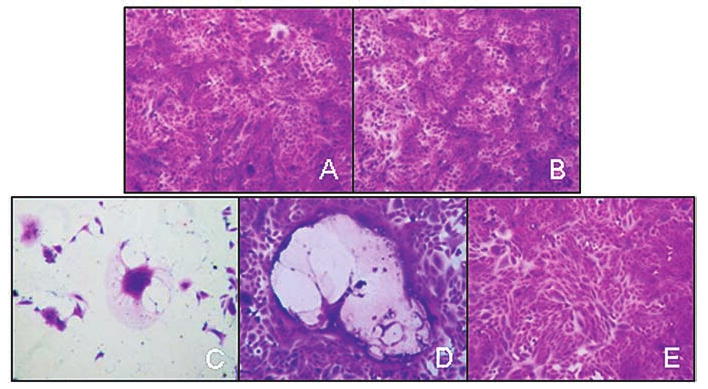
Vero cell overlay of CSF obtained on day 9 from a macaque treated with a high dose of virus. (A) Overlay of CSF supernatant; (B) overlay of cell pellet; (C and D) positive controls; (E) negative control.
Table 3. Results of Cerebrospinal Fluid Analysis.
| Monkey ID | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5M02 | 5M04 | 5M05 | 5M01 | 5M03 | ||||||
| Dose MV-CEA (TCID50) | ||||||||||
| 0 | 2 ×105 | 2 × 105 | 2 × 106 | 2 × 106 | ||||||
| CSF parameter | Day 9 | One year | Day 9 | One year | Day 9 | One year | Day 9 | One year | Day 9 | One year |
| Glucose (mg/dl) | 41 | 47 | 39 | 44 | 37 | 45 | 44 | 50 | 49 | 47 |
| Total protein (mg/dl) | 16 | 16 | 18 | 20 | 24 | 32 | 19 | 18 | 26 | 39 |
| CEAa (ng/ml) | <0.5 | <0.1 | <0.5 | <0.1 | <0.5 | <0.1 | <0.5 | <0.1 | <0.5 | <0.1 |
| Anti-MVAb ELISAb (OD) | 0.00 | 0.00 | 0.00 | 0 | 0.01 | 0.02 | 0.07 | 0.00 | 0.10 | 0.04 |
| Total nucleated cell count (no./μl) | 4 | 2 | ND | 7 | ND | <1.0 | 59 | 3 | ND | 3 |
| Erythrocytes (no./μl) | 6 | 792 | ND | 0 | ND | 131 | 94 | 122 | ND | 32 |
| CSF differential | ||||||||||
| Neutrophils (%) | 6 | 7 | 4 | 11 | 7 | 2 | ||||
| Lymphocytes (%) | 93 | 78 | 59 | 47 | 80 | 69 | 66 | 57 | 84 | 54 |
| Monos/macros (%) | 5 | 9 | 33 | 51 | 14 | 15 | 23 | 29 | 16 | 42 |
| Eosinophils (%) | 2 | 3 | 1 | 2 | 2 | 5 | 11 | 7 | 2 | |
| Other cells (%) | 4 | |||||||||
| Comment (%) | c | c | c | c | c | c | c | c | c | c |
|
| ||||||||||
| Vero cell overlay (Pos/Neg) | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| QRT-PCR for MV-N gene (copies) | BLD | BLD | BLD | BLD | BLD | |||||
Abbreviations: BLD, below limit of detection for assay (<1000 copies/μg RNA); CEA, carcinoembryonic antigen; CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; ID, identification; monos/macros, monocytes/macrophages; MV-CEA, measles virus derivative producing carcinoembryonic antigen; ND, not data (laboratory did not obtain counts before Cytospin of sample); OD, opical density; QRT-PCR, quantitative real-time polymerase chain reaction; TCID50, median tissue culture infective dose.
Assay limit of detection improved from 0.5 to 0.1 ng/ml between sampling dates.
OD > 0.25 is positive.
No blasts or malignant cells seen.
MRI
To further address the possibility of subclinical neurotoxicity, MRI imaging was performed 4–5 months after MV-CEA administration. Images of all five subjects were without abnormalities. Specifically, the frontal brain parenchyma, including the regions corresponding to the MV-CEA injection sites, were normal on all MRI sequences including T1-weighted, T2-weighted, T2 FLAIR, and diffusion-weighted images; and there was no abnormal enhancement on T1-weighted gadolinium-enhanced images, including lack of meningeal inflammation (Fig. 4).
FIG. 4.
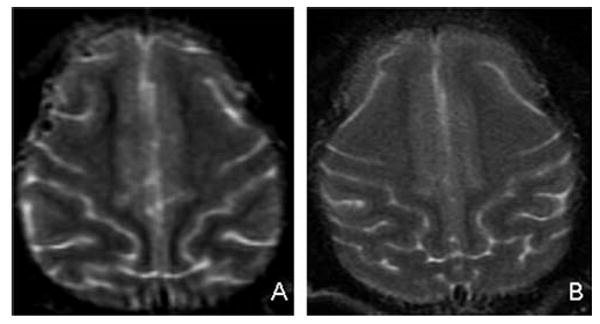
T2-weighted MRI images of the frontal injection site areas in a control macaque (A) and a high-dose macaque (B). There is no evidence of encephalitis or other abnormality in either animal.
Discussion
Oncolytic measles virus strains have emerged as novel antitumor agents with significant efficacy against glioma (Phuong et al., 2003; Allen et al., 2006; Paraskevakou et al., 2007). The measles virus derivative MV-CEA (a strain deriving from the Edmonston NSe strain and engineered to express the human carcinoembryonic antigen) was chosen as an optimal candidate for clinical translation. MV-CEA has significant antitumor activity against glioma (Phuong et al., 2003). In addition, because gliomas do not produce CEA, CEA production as the virus replicates and its detection in the serum could serve as a noninvasive monitoring strategy of viral gene expression, thus allowing treatment optimization. Development of noninvasive monitoring strategies is particularly desirable in glioma treatment, where repeat biopsies after viral administration into the tumor are not always safe or ethically justified.
The toxicology studies described in this paper were conducted in support of the clinical trial of MV-CEA in recurrent glioma patients. This phase I study includes two treatment arms. In the first arm (arm A), following resection of their recurrent glioblastoma multiforme, patients receive one dose of the virus, divided among 10 different injection sites in the resection cavity. In the second arm of the study (arm B), which is to be initiated after dose escalation in arm A has been completed, patients receive two doses of MV-CEA. The first dose is administered into the recurrent tumor under stereotactic guidance. Five days later, at a time corresponding to maximal viral replication (Phuong et al., 2003), the tumor is resected and the second dose is administered into the resection cavity. This trial represents the first human study of CNS administration of a measles-based oncolytic therapeutic agent.
Wild-type measles virus has been associated with subacute sclerosing panencephalitis (SSPE) several years after infection. There have been no cases of encephalitis or SSPE associated with a vaccine strain of measles virus, however (Billeter et al., 1994). In consultation with the U.S. Food and Drug Administration (FDA), it was decided that assessment of neurotoxicity of MV-CEA in nonhuman primates (rhesus macaques) was imperative before our trial was activated in order to maximize patient safety. Rhesus macaques (Macaca mulatta) are naturally susceptible to measles virus infection and have been extensively used to study measles virus pathogenesis. CNS inoculation of measles virus vaccine strains is the standard method by which the measles virus vaccine lots are tested for neurotoxicity.
There are limited prior data pertaining to administration of wild-type or attenuated measles virus strains in the CNS of primates. Invariably, these studies employed much lower doses as compared with the dose proposed to be used in the clinical trial, and in no study was a repeat administration schedule tested. In animal work performed in the early 1970s, Albrecht and coworkers found no clinical signs of encephalitis in rhesus monkeys that were inoculated in the right thalamus with low-passage wild type Edmonston or attenuated Schwartz strains of measles virus (Albrecht et al., 1972). Although measles virus was isolated from 4 of the 12 animals when sections of their brains were overlaid on Vero cells, there was no histological evidence of active measles infection. Administration of an attenuated Edmonston strain of measles virus (102.75 plaque-forming units [PFU]/0.1 ml) to grivet monkeys both intrathalamically (0.5 ml per thalamus) and intracisternally (0.2 ml) had no clinical toxicity and resulted in pathologic changes (gliosis with mixed cellular infiltrate) that were not different from those observed in animals injected with vehicle control (Buynak et al., 1962). Similarly, intracerebral inoculation of an attenuated strain of measles virus (101.8-103.5 PFU/ml) into the cerebral hemisphere (0.5 ml) and cisterna magna (0.25 ml) of cynomolgus monkeys produced no clinical toxicity. No virus was isolated from the CSF and no changes compatible with viral injury were observed at postmortem 21 days after viral inoculation (Enders et al., 1960). In contrast, when monkeys were infected intracerebrally with a variant of the measles virus vaccine strain (L-16) isolated after prolonged persistence in human cell culture NEr-2, pathologic changes developed in the CNS within 30 to 60 days postinoculation, including giant cell formation at the injection site (Sharova et al., 1987).
Our study employs 3- to 4-log higher doses as compared with previously reported experiments of CNS administration of measles virus strains in macaques. In addition, it uses a repeat administration schedule that mimicks the human trial. Animals in our study were immune to the virus, to mimic the study population and to address concerns regarding development of inflammation in the CNS of immune individuals after measles virus injection. The administration was performed in the frontal lobe, which is one of the most common sites of glioma recurrence. The highest total viral dose administered to the macaques in this study was 2 × 106 TCID50 By direct brain weight conversion, this is approximately equivalent to 3 × 107 TCID50 in an adult human or 1.5 times the total proposed maximal dose to be administered to human patients. In addition, because the maximal dose that the normal brain can receive in the human study is 107 TCID50, this dose is three times higher than the highest dose to which normal brain can be exposed in this trial. Our data therefore support the safety of the proposed approach in humans.
MV-CEA was well tolerated in this primate toxicology study. Macaques remained in good clinical condition, with no clinical evidence of toxicity, including lack of neurological symptoms, fever, rash, or other systemic symptoms during the study. There were no deaths or adverse clinical signs on the study. All hematology, clinical chemistry, and coagulation parameters remained normal. There was no clinical, laboratory, or imaging evidence of encephalitis, or of any other abnormality in MRIs obtained 4–5 months after the first article administration.
In addition, there is no convincing evidence that MV-CEA was able to disseminate away from the injection site. MV-CEA was not detected by QRT-PCR analysis of buccal swabs or CSF, Vero cell overlays of CSF cells, and supernatant were negative for measles-related pathology, and CEA was not detected in sera or CSF at any time point. One asymptomatic macaque had a positive 3-month blood sample for the presence of measles virus nucleoprotein by QRT-PCR analysis, but a repeat draw and all other blood samples were negative.
As per FDA recommendations, and because of concerns about the late development of encephalitis, the study was designed as a non-terminal study. The fact that the macaques remain clinically asymptomatic currently at 3+ years after study initiation supports the long-term safety of the proposed approach.
In addition to the macaque study, a second toxicology study was performed in 120 Ifnarko-CD46Ge transgenic mice. These mice express the measles virus CD46 receptor in a tissue distribution that replicates human CD46 expression and they lack the interferon-αβ receptor gene, which renders them susceptible to measles neurotoxicity (Mrkic et al., 1998). Although this is an artificial system because most human patients have intact interferon-αβ receptor pathways, in conjunction with the FDA, this model was employed to mimic the worst case scenario in our study population. Similar to the macaque studies, immune Ifnarko-CD46Ge mice were able to tolerate intracranial CNS administration of viral doses that were 35 times higher than the proposed dose in the human trial without neurotoxicity (Allen et al., 2008).
In summary, our toxicology study of CNS administration of MV-CEA in immune rhesus macaques proved the safety of the proposed approach. A phase I trial of intratumoral and resection cavity administration in measles-immune recurrent glioblastoma patients was activated in 2007. Five patients have been treated so far and doses up to 106 TCID50 in the resection cavity have been well tolerated, with no dose-limiting toxicity having been observed (E. Galanis, unpublished data). Accrual continues to the target dose of 2 × 107 TCID50.
Acknowledgments
The authors thank Dr. Stephen Russell for input and support that made this study possible. The authors also thank Dr. Kah-Whye Peng and the members of the Mayo Clinic Viral Vector Production Laboratory: Linda Gregory, Guy Griesmann, Kirsten Langfield, Julie Sauer, Sharon Stephan, Henry Walker, Troy Wegman, and Cindy Whitcomb. Finally, they thank Ms. Raquel Ostby for help with manuscript preparation. This work was supported by the Mayo Brain SPORE P50 108961, the Eisenberg Foundation, and the Siebens Foundation.
Footnotes
Author Disclosure Statement: No competing financial interests exist for all authors.
References
- Albrecht P, Shabo AL, Burns GR, Tauraso NM. Experimental measles encephalitis in normal and cyclophos-phamide-treated rhesus monkeys. J Infect Dis. 1972;126:154–161. doi: 10.1093/infdis/126.2.154. [DOI] [PubMed] [Google Scholar]
- Allen C, Vongpunsawad S, Nakamura T, James CD, Schroeder M, Cattaneo R, Giannini C, Krempski J, Peng KW, Goble JM, Uhm JH, Russell SJ, Galanis E. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006;66:11840–11850. doi: 10.1158/0008-5472.CAN-06-1200. [DOI] [PubMed] [Google Scholar]
- Allen C, Paraskevakou G, Liu C, Iankov ID, Msaouel P, Zollman P, Myers R, Peng KW, Russell SJ, Galanis E. Oncolytic measles virus strains in the treatment of gliomas. Exp Opin Biol Ther. 2008;8:213–220. doi: 10.1517/14712598.8.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter MA, Cattaneo R, Spielhofer P. Generation and properties of measles virus mutations typically associated with subacute sclerosing panencephalitis. In: Björnsson J, Carp RI, Love A, Wisniewski HM, editors. Slow Infections of the Central Nervous System: The Legacy of Dr Björn Sigurdsson. New York Academy of Sciences; New York: 1994. pp. 397–375. [DOI] [PubMed] [Google Scholar]
- Blechacz B, Splinter PL, Greiner S, Myers R, Peng KW, Federspiel MJ, Russell SJ, Larusso NF. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology. 2006;44:1465–1477. doi: 10.1002/hep.21437. [DOI] [PubMed] [Google Scholar]
- Buynak EB, Peck HM, Creamer V, Goldner H, Hilleman MR. Differentiation of virulent from avirulent measles strains. Am J Dis Child. 1962;103:406–473. [Google Scholar]
- Collatz E, von Kleist S, Burtin P. Further investigations of circulating antibodies in colon cancer patients: On the autoantigenicity of the carcinoembryonic antigen. Int J Cancer. 1971;8:298–303. doi: 10.1002/ijc.2910080215. [DOI] [PubMed] [Google Scholar]
- Cutts FT, Markowitz LE. Successes and failures in measles control. J Infect Dis. 1994;170(Suppl 1):S32–S41. doi: 10.1093/infdis/170.supplement_1.s32. [DOI] [PubMed] [Google Scholar]
- Dabbs DJ. Diagnostic Immunohistochemistry. Churchill Livingstone; Philadelphia, PA: 2002. [Google Scholar]
- Enders JF, Katz SL, Milovanovic VM, Holloway A. Studies of an attenuated measles-virus vaccine. Development and preparation of the vaccine: Technics for assay of effects of vaccination. N Engl J Med. 1960;263:153–159. doi: 10.1056/NEJM196007282630401. [DOI] [PubMed] [Google Scholar]
- Fine PEM. Herd immunity: History, theory, practice. Epidemiol Rev. 1993;15:265–302. doi: 10.1093/oxfordjournals.epirev.a036121. [DOI] [PubMed] [Google Scholar]
- Harsh GR, Deisboeck TS, Louis DN, Hilton J, Colvin M, Silver JS, Qureshi NH, Kracher J, Finkelstein D, Chiocca EA, Hochberg FH. Thymidine kinase activation of ganciclovir in recurrent malignant gliomas: A gene-marking and neuropathological study. J Neurosurg. 2000;92:804–811. doi: 10.3171/jns.2000.92.5.0804. [DOI] [PubMed] [Google Scholar]
- Ishikura A, Hunaki N, Watanabe K. Brain metastases of colorectal cancer: A case report. Gan No Rinsho. 1987;33:188–192. [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S, Berger MS, McDermott MW, Kunwar SM, Junck LR, Chandler W, Zwiebel JA, Kaplan RS, Yung WK. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: Biological and clinical results. J Clin Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- McDonald CJ, Erlichman C, Ingle JN, Rosales GA, Allen C, Greiner SM, Harvey ME, Zollman PJ, Russell SJ, Galanis E. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res Treat. 2006;99:177–184. doi: 10.1007/s10549-006-9200-5. [DOI] [PubMed] [Google Scholar]
- Mrkic B, Pavlovic J, Rulicke T, Volpe P, Buchholz CJ, Hourcade D, Atkinson JP, Aguzzi A, Cattaneo R. Measles virus spread and pathogenesis in genetically modified mice. J Virol. 1998;72:7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevakou G, Allen C, Nakamura T, Zollman P, James CD, Peng KW, Schroeder M, Russell SJ, Galanis E. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol Ther. 2007;15:677–686. doi: 10.1038/sj.mt.6300105. [DOI] [PubMed] [Google Scholar]
- Peng KW, Teneyck CJ, Galanis E, Kalli KR, Hartmann LC, Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002a;62:4656–4662. [PubMed] [Google Scholar]
- Peng KW, Facteau S, Wegman T, O'Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002b;8:527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- Phuong LK, Allen C, Peng KW, Giannini C, Greiner S, Teneyck CJ, Mishra PK, Macura SI, Russell SJ, Galanis EC. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–2469. [PubMed] [Google Scholar]
- Sharova OK, Bogomolva NN, Koptyaeva IB, Gordienko NM, Rozina EE. Pathomorphologic characterization of CNS damage in monkeys infected with persistent variant of measles virus vaccine strain L-16. Acta Virol. 1987;31:346–351. [PubMed] [Google Scholar]
- Stupp R, Mason W, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Ura Y, Ochi Y, Hamazu M, Ishida M, Nakajima K, Watanabe T. Studies on circulating antibody against carcinoembryonic antigen (CEA) and CEA-like antigen in cancer patients. Cancer Lett. 1985;24:283–295. doi: 10.1016/s0304-3835(15)30008-2. [DOI] [PubMed] [Google Scholar]