Abstract
Objective
Literature data on the effects of CYP17 MspA1 polymorphism on age at menarche (AAM) are inconsistent. To reexamine this controversy, we performed a meta-analysis.
Study design
In total 16 studies containing more than 11000 individuals of various ethnicities were selected for the analyses. For 11 case-control studies, odds ratio (OR) was employed to evaluate the risk of late AAM for each study, using homozygote at the wild-type allele as a control group. For the 5 studies with continuous outcomes, the effect size was estimated using the Hedges' adjusted g, which is calculated based on the standardized mean difference between groups of subjects with early and late AAM.
Results
We did not find evidence for association of the MspA1 polymorphism with AAM in the combined case-control sample with mixed ethnic background (OR = 1.03, 95% CI: 0.90-1.18, P = 0.66), in the monoethnic case-control sample of Caucasian females (OR = 1.09, 95% CI: 0.99-1.20, P = 0.08) and in the combined sample with continuous traits (Hedges' g = 0.33 and -0.041, 95% CI: -0.14–0.80 and -0.18-0.10, P values 0.17 and 0.56 for the pooled population sample and monoethnic sample of Caucasian females, respectively).
Conclusion
Our study showed that CYP17 MspA1 polymorphism was not a significant independent risk factor of AAM. Further studies are needed to clarify the effects of the interaction between this gene and other genetic and/or environment factors on AAM.
Keywords: Age at Menarche, CYP17, Meta-Analysis, Publication Bias
1. Introduction
Age at menarche (AAM) is an important trait related to women's health. An early onset of menarche is associated with elevated risks of breast cancer [1] and endometrial cancer [2]. On the other hand, late menarche increases the risk of Alzheimer's disease [3] and osteoporosis [4], but decreases the incidence of coronary heart disease [3]. Therefore, from a clinical point of view, understanding the potential factors responsible for AAM may shed light on the pathophysiology of these diseases. Height, weight, body mass index (BMI), increased fat uptake, and maternal early menarche were reported as positive predictive factors of early menarche [5, 6], while sports activity seems to delay menarche [6]. Twin and family studies have suggested that about 53-74% variation of AAM can be attributed to genetic factors [7-9]. Several genes have been reported to be associated with AAM, such as estrogen receptor α (ER-α) [10, 11], sex hormone-binding globulin (SHBG) [12], androgen receptor (AR) [13], CYP19 [14], CYP3A4 and cytochrome P450c17α (CYP17) [15, 16].
AAM depends on the maturation of the female reproductive system and other endocrine organs. Estrogen plays an important role in the maturation and function of the reproductive system [17]. Menarche is initiated by the increased amplitude of estrogen exposure during puberty [18].
The human CYP17 gene, located on chromosome 10 (10q24.3) [19], is a key gene controlling biosynthesis of estrogen in the lipid precursor cells. A product of the gene, a steroidogenic enzyme P450c17α, displays both steroid 17α-hydroxylase and 17, 20-lyase activities in the estrogen biosynthesis pathway [20]. Supposedly, altering activity of the enzyme cytochrome P450c17α may influence estrogen biosynthesis. Hence, there is a possibility that the effect of different hormonal risk factors depends on different CYP17 genotypes. Some studies have suggested that the CYP17 gene is associated with hormonal risk factors, and thus the association between these factors (e.g., AAM, age at menopause and hormonal replacement therapy, etc.) and breast cancer depends on CYP17 genotypes [16, 21-23].
Three polymorphisms in the human CYP17 gene have been commonly used for association studies. One of these polymorphisms, MspA1 (a T→C nucleotide substitution 34 base pairs upstream of the translation initiation site in the 5′ promoter region), has been of particular interest as a candidate gene for the breast cancer risk [15, 21, 24-31]. A subset of the literature refers to the wild-type T allele as A1, and the variant C allele as A2 [7, 24]. In studies of association between the MspA1 polymorphism and breast or ovarian cancer risks this polymorphism was also analyzed for its effect on AAM. However, the results were contradictory and reported either the significant association [15, 16] or no association [21, 24-36].
The aim of this study is to investigate putative association between the CYP17 MspA1 polymorphism and AAM using a meta-analysis [37]. This method examines whether the aggregate data across several studies provides evidence of statistical significance. A meta-analysis has been commonly used to resolve ambiguities about association/non-association between various polymorphisms and complex traits [38-40]. The present study utilizes this approach to investigate putative association between the CYP17 MspA1 polymorphism and AAM using the data from the available association studies of breast cancer risk published during 1997-2007.
2. Materials and methods
2.1. Identification and eligibility of relevant studies
The sample data were obtained by conducting a search of literatures using PubMed and MEDLINE over the period from 1997 to 2007 to identify studies with information on the CYP17 MspA1 polymorphism and AAM. The search strategy was based on the various combinations of terms “breast cancer”, “ovarian cancer”, “menarche” and “CYP17”. In addition, the citations in the identified articles were screened to find additional publications on the topic. Any human population-based association study, regardless of sample size, was included.
As the A2 allele was initially suggested to increase expression of the gene [25], most studies divided the total sample into subsets of wild homozygous (A1A1) and combined variants (A1A2+A2A2). So, in this study we did alike. A total of 16 studies were identified to have the data on AAM and the CYP17 MspA1 polymorphism [15, 16, 21, 24-36]. Five of them reported mean AAM according to the genotype of CYP17 [16, 33-36] and the other 11 divided the subjects into groups early or late AAM [15, 21, 24-32]. Among these 11 articles, 10 partitioned the data with the threshold of 13 years [15, 21, 24-28, 30-32] and the other one partitioned with threshold of 14 [29]. Women with breast cancer or ovarian cancer were not excluded from the analysis because menarche affects disease states, but not vise versa.
Each study with binary outcomes provided a two-by-two table classifying subjects by AAM (early or late) and CYP17 MspA1 A2 allele (present or not). Each study with continuous outcomes provided sample sizes of the subgroups (CYP17 MspA1 A2 allele presents or not) and mean, standard deviation (sd) of AAM in each subgroup.
2.2. Data extraction
Following the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) statement for reporting meta-analysis of observational studies [41], we used a standardized reporting form to independently abstract data from each included study. The following information was sought from each report: authors, year of publication, country of origin, mean age of the sample, selection and ethnic background of the study population (Caucasian, Asian, African or Mixed), number of eligible and genotyped cases and controls. Relevant information is shown in details in Tables 1 and 2.
Table 1.
A summary of eligible case-control studies used for the meta-analysis of association between the CYP17 MspA1 polymorphism and AAM
| Author | Place of study | Ethnicity | Mean age | threshold | Number of samples | Power1 | |
|---|---|---|---|---|---|---|---|
| A1A1 | A1A2+A2A2 | (%) | |||||
| Feigelson et al. (1997) | USA | Mixed | 62 | 13 | 145 | 314 | 52.62 |
| Helzlsouer et al. (1998) | USA | Caucasian | 60.3 | 13 | 68 | 123 | 26.96 |
| Haiman et al. (1999) | USA | Caucasian | 58.3 | 13 | 394 | 678 | 89.09 |
| Mitrunen et al. (2000) | Finland | Caucasian | 56.2 | 13 | 391 | 549 | 73.54 |
| Goodman et al. (2001) | USA | Mixed | - | 13 | 96 | 173 | 35.39 |
| Ambrosone et al. (2003) | USA | Caucasian | 55.6 | 13 | 205 | 189 | 51.56 |
| Wu et al. (2003) | Singapore | Asian | >45 | 13 | 146 | 713 | 48.63 |
| Shin et al. (2005) | Korea | Asian | - | 14 | 70 | 264 | 24.33 |
| Verla-Tebit et al. (2005) | Germany | Caucasian | 41.7 | 13 | 502 | 925 | 94.91 |
| Chang et al. (2005) | Australia | Caucasian | 41.1 | 13 | 776 | 1181 | 99.19 |
| Einarsdottir et al. (2005) | Sweden | Caucasian | 63.2 | 13 | 933 | 1652 | 99.05 |
| Pooled | 3726 | 6761 | 100 | ||||
| Caucasian sample | 3269 | 5297 | 100 | ||||
The power was calculated based on the assumption that the true odds ratio was 1.5 at the significant level 0.05.
Table 2.
A summary of eligible studies with continuous outcomes used for the meta-analysis of association between the CYP17 MspA1 polymorphism and AAM
| Author | Place of study | Ethnicity | Mean age | Number of samples | |
|---|---|---|---|---|---|
| A1A1 | A1A2+A2A2 | ||||
| McCann et al. (2002) | USA | Caucasian | 55.64 | 205 | 190 |
| Gorai et al. (2003) | Japan | Asian | 59.9 | 101 | 149 |
| Onland-Moret et al. (2005) | Holland | Caucasian | 57.55 | 166 | 207 |
| Small et al. (2005) | USA | Mixed | 32.08 | 61 | 103 |
| Jasienska et al. (2006) | Poland | Caucasian | 29.52 | 21 | 37 |
| Pooled | 554 | 686 | |||
| Caucasian sample | 392 | 434 | |||
2.3. Statistical analysis
The effect sizes and pooled estimates of the effect across the studies were calculated with the Comprehensive Meta-Analysis software package [42]. For the studies with binary outcomes, odds ratio (OR) was used to evaluate the risk of late AAM for each study using homozygotes of the wild-type allele as a control group. The power of the study was estimated as the probability of finding a significant association between CYP17 MspA1 and AAM at the 0.05 significance level, assuming that the OR is 1.5. For the studies with continuous outcomes, the measure of the effect size was Hedges' adjusted g [43]. This is a commonly used estimator of the effect size that is calculated based on the standardized mean difference between two groups being studied. Heterogeneity across the studies was examined using the Q statistics and was considered significant at P < 0.10 [44]. Depending on whether heterogeneity was present or not, the meta-analysis was conducted using random effects or fixed effects model, respectively.
Finally, for the primary outcomes, we performed a cumulative meta-analysis and a recursive cumulative meta-analysis to evaluate whether the summary OR changed as more data are accumulated. The cumulative meta-analysis was conducted stepwise after adding a single new study at a time. The recursive cumulative analysis estimates the relative change in each step and provides a measure of how much the effect size changes as evidence accumulates. For the case-control studies, the relative change was defined as the pooled OR at the next step divided by the pooled OR at the current step. For the studies with continuous outcomes, the relative change was defined as the pooled Hedges' g at the next step minus the g at the current step [45-47]. We also estimated publication bias by using both funnel plot and the method proposed by Egger et al. (1997) [48]. Funnel plot is a scatterplot of studies' effect sizes against standard errors. In the absence of bias the plot will resemble a symmetrical inverted funnel. Conversely, if there is bias, funnel plots will often be skewed and asymmetrical. The Egger's method is based on the funnel plot, where the standardized effect estimate is regressed on a measure the precision (1/Standard Error). The resulting publication bias statistics is an intercept of the regression, which will be significantly greater than zero in the presence of publication bias.
3. Results
3.1. Meta-analysis database
The eligible studies with binary outcomes included 10487 subjects in total (Table 1); all of them had genotype data. The sample size varied substantially (ranging from 83 to 2585 individuals), and so did the statistical power (24.33-99.19%). The power of the pooled sample was 100%. Among these 11 case-control studies, seven studies employed subjects of Caucasian descent; two studies used Asians and the other two used subjects of mixed ethnicities. After excluding the latter four, the total number of the subjects in the pooled sample became 8566 and the statistical power still reached 100% (Table 1).
The studies with continuous outcomes totaled 1240 subjects with a sample size varying from 58 to 395 (Table 2). Three studies employed subjects of Caucasian descent and the other two employed subjects of Asians and mixed ethnical background, respectively. After excluding the latter two, the total number of the subjects in the pooled sample became 826.
3.2. Meta-analysis
The tests for heterogeneity across the studies were performed before the studies were pooled for the meta-analysis. The statistically significant heterogeneity among the individual studies was observed for both studies with binary outcomes and continuous outcomes (Q = 19.48, P = 0.04 and Q = 63.33, P < 0.01, respectively). Therefore we used the random effects model in the subsequent analyses. There was no statistically association in overall groups (P = 0.66 for the studies with binary outcomes and P = 0.17 for the studies with continuous outcomes).
The results of the pooled analysis and the individual studies for association of the CYP17 MspA1 polymorphism and AAM are presented in Tables 3 and 4. The ORs of association between CYP17 MspA1 and AAM varied slightly (between 0.66 and 1.52) for the data from the studies with binary outcomes. One study showed a significant association (P < 0.01) between CYP17 and AAM and an increased risk of later AAM in women carrying the A2 allele ([15] Table 3). For the studies with continuous outcomes, the standard difference of means ranged from -0.15 to 1.44. Two studies [16, 36] showed significant difference between the genotypes (P < 0.01, Table 4).
Table 3.
The summary of ORs for the CYP17 MspA1 polymorphism and AAM of studies with binary outcomes.
| AAM | |||||||
|---|---|---|---|---|---|---|---|
| Study | A1A1 | A1A2+A2A2 | Weight | OR (95% CI) A1A2+A2A2 vs A1A1 |
P1 | ||
| Early AAM | Late AAM | Early AAM | Late AAM | ||||
| Feigelson et al. (1997) | 63 | 82 | 161 | 153 | 24.50 | 0.73 (0.49-1.08) | 0.12 |
| Helzlsouer et al. (1998) | 36 | 32 | 57 | 66 | 10.90 | 1.30 (0.72-2.36) | 0.38 |
| Haiman et al. (1999) | 203 | 191 | 348 | 330 | 62.24 | 1.01 (0.79-1.29) | 0.95 |
| Mitrunen et al. (2000) | 88 | 303 | 111 | 438 | 38.53 | 1.15(0.87-1.57) | 0.40 |
| Goodman et al. (2001) | 56 | 40 | 83 | 90 | 15.15 | 1.52 (0.92-2.51) | 0.10 |
| Ambrosone et al. (2003) | 88 | 117 | 91 | 98 | 24.33 | 0.81 (0.54-1.20) | 0.30 |
| Wu et al. (2003) | 17 | 129 | 118 | 595 | 13.03 | 0.66 (0.39-1.14) | 0.14 |
| Shin et al. (2005) | 8 | 62 | 37 | 227 | 5.80 | 0.79 (0.35-1.78) | 0.57 |
| Verla-Tebit et al. (2005) | 191 | 311 | 318 | 607 | 75.51 | 1.17 (0.94-1.47) | 0.17 |
| Chang et al. (2005) | 350 | 426 | 456 | 725 | 113.94 | 1.31 (1.09-1.57) | 0.00 |
| Einarsdottir et al. (2005) | 198 | 735 | 378 | 1274 | 101.61 | 0.91 (0.75-1.10) | 0.33 |
| Pooled (heterogeneity test: Q = 19.48, P = 0.04) | |||||||
| Random effect model | 1298 | 2428 | 2158 | 4603 | 485.54 | 1.03(0.90-1.18) | 0.66 |
| Pooled after excluding Asians and mixed populations: (heterogeneity test: Q = 10.50, P = 0.11) | |||||||
| Fixed effect model | 1154 | 2115 | 1759 | 3538 | 427.06 | 1.09(0.99-1.20) | 0.08 |
all P values are two-sided.
Table 4.
Summary of studies with continuous outcomes.
| AAM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | A1A1 | A1A2+A2A2 | P1 | |||||||
| Mean | Std | N | Mean | Std | N | Weight | Hedges' g (95% CI) | |||
| McCann et al. (2002) | 12.82 | 1.52 | 205 | 12.72 | 1.76 | 190 | 98.56 | 0.06 (-0.14-0.26) | 0.54 | |
| Gorai et al. (2003) | 14.10 | 1.30 | 101 | 13.66 | 1.22 | 249 | 70.94 | 0.35 (0.12-0.59) | 0.00 | |
| Onland-Miret et al. (2005) | 13.40 | 1.50 | 166 | 13.63 | 1.58 | 207 | 91.87 | -0.15 (-0.35-0.05) | 0.15 | |
| Small et al. (2005) | 12.46 | 0.22 | 61 | 12.16 | 0.20 | 103 | 30.80 | 1.44 (1.09-1.80) | 0.00 | |
| Jasienska et al. (2006) | 13.10 | 3.15 | 21 | 13.2 | 1.03 | 37 | 13.39 | -0.05 (-0.58-0.49) | 0.86 | |
| Pooled (heterogeneity test: Q = 63.33, P<0.01) | ||||||||||
| Random effect model | 554 | 786 | 305.57 | 0.33 (-0.14-0.80) | 0.17 | |||||
| Pooled after excluding Asians and mixed populations: (heterogeneity test: Q = 2.10, P = 0.35) | ||||||||||
| Fixed effects model | 392 | 434 | 203.83 | -0.041 (-0.18-0.10) | 0.56 | |||||
all P values are two-sided.
The above results are based on the pooled data from different ethnic groups and may thus carry a bias due to the ethnic heterogeneity. For example, the frequency of the A1A1 homozygote in Asians was significantly different from that in Caucasians (Tables 1, 2). Therefore, we also performed the meta-analyses for the pooled samples composed only of Caucasians.
The pooled Caucasian samples showed no heterogeneity for the studies with both types of outcomes (Q = 10.50, P = 0.11, Table 3 and Q = 12.10, P = 0.35, Table 4, respectively). The analysis under the fixed effects model showed no association between the CYP17 MspA1 polymorphism and AAM in both cases (P = 0.08 and P = 0.56, respectively).
3.3. Bias diagnostics
The results of the cumulative and recursive cumulative meta-analysis for case-control studies are shown in Figure 1. As more data were accumulated, the 95% CI became narrower, but there was no evidence that the magnitude of the cumulative effect estimates changed in the same direction.
Figure 1.
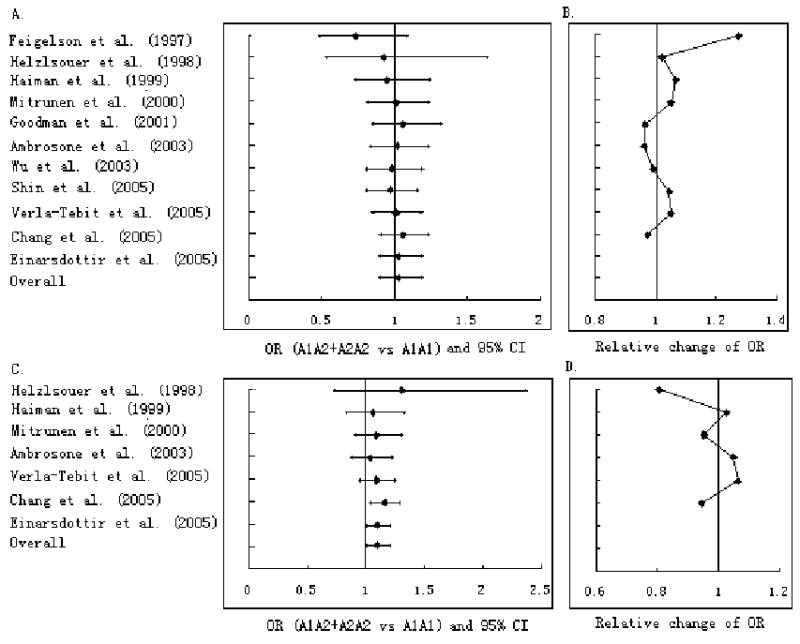
Cumulative meta-analysis (A and C) and recursive cumulative meta-analysis (B and D) of association between the CYP17 MspA1 polymorphism and AAM based on the studies with binary outcomes. Each line represents the OR and 95% CI of that study combined with all previous studies. A and B: all ethnicities; C and D: Caucasians only.
Figure 2 shows the result of the cumulative and recursive cumulative meta-analysis for the studies with continuous outcomes. While no change in the magnitude of the cumulative effect estimates was determined for the pooled sample of mixed ethnicity, the Hedges' g decreased from 0.06 to -0.41 in the aggregated Caucasian sample.
Figure 2.
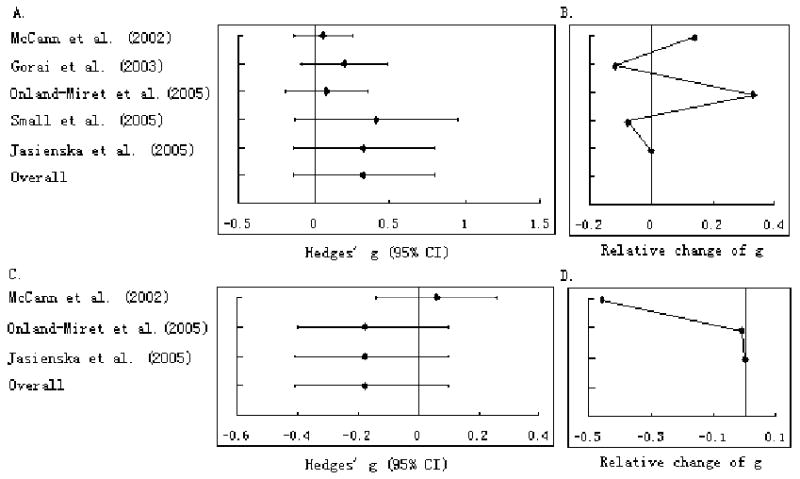
Cumulative meta-analysis (A and C) and recursive cumulative meta-analysis (B and D) of association between the CYP17 MspA1 polymorphism and AAM based on the studies with continuous outcomes. Each line represents Hedges' g and 95% CI of that study combined with all previous studies. A and B: all ethnicities; C and D: Caucasians only.
Figure 3 demonstrates the funnel plots for both case-control studies and studies with continuous outcomes. The plots are roughly symmetric, thus suggesting no publication bias. The publication bias statistics [49] were not significant for the studies with binary outcomes (intercept of the regression a = -0.98, t = 0.95, P = 0.36 for the total sample; intercept of the regression a = -0.52, t = 0.31, P = 0.77 for Caucasians), and so were for the studies with continuous outcomes (intercept a = 5.26, t = 0.90, P = 0.43 for the total sample; intercept a = -0.15, t = 0.06, P = 0.96 for Caucasians).
Figure 3.
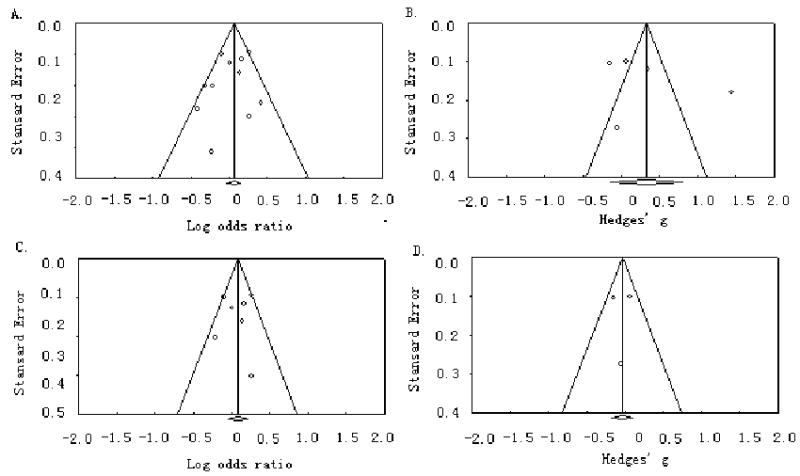
Funnel plots of the publication bias. A and B: all ethnicities; C and D: Caucasians only.
4. Comment
The presented meta-analysis summarizes the data of 16 observational studies (11 with binary outcomes and the others with continuous outcomes) about the effect of the CYP17 MspA1 polymorphism on AAM. Overall, the obtained results suggest no association between this polymorphism and AAM in both the pooled multiethnic samples and monoethnic Caucasian samples. However, the low P value (0.08) for the pooled Caucasian sample from studies with binary outcomes reserves some probability that the CYP17 MspA1 polymorphism may be a modifier, but likely is not a significant independent risk factor of AAM on a wide population basis.
The CYP17 MspA1 polymorphism has three genotypes: a homozygous wild type (A1A1), a heterozygous variant (A1A2), and the homozygous variant (A2A2). The T(A1)→C(A2) substitution was initially hypothesized to create an Sp-1 promoter site, which could lead to up-regulation of transcriptional activation of the variant allele and which in turn might affect the synthesis of estrogen [49]. Some studies reported higher estrogen levels in women carrying the A2 allele [22, 26]. Generally, there is limited evidence for the effect of the CYP17 genotype on estrogen mediated factors such as AAM. While some data suggested weak though not statistically significant association between the A2A2 genotype and earlier menarche [50], the majority of the studies, including those used in our meta-analysis, reported no such association [27, 29, 31, 51].
Indeed, among the 11 case-control studies included in the present analysis, only one [15] reported the positive result, namely, suggesting an increased risk for later AAM among women with the C (A2) allele (Table 3). There is a potential factor, which might produce this discrepancy. The subjects recruited in this study were on average younger than those in the other studies (Table 1). This might yield more accurate recall of AAM. As was recently showed, after 30 years, about 79 percent of women may recall their AAM with accuracy of within one year of original menarche [52]. The recall bias, albeit small, still exists [53]. In a case of the bias, it would most likely have been biased toward the null, leading to an underestimation of the true effect of the tested polymorphism.
Ideally, to avoid a bias in the results, the estimates of ORs should be adjusted for the factors known to contribute to AAM, such as age and ethnicity [51, 54]. However, since some of the studies used in the current meta-analysis did not contain respective data, the crude ORs were calculated using only tabular data from the published reports. This might affect the overall accuracy of the meta-analysis in either way.
Among the 5 studies with continuous outcomes, the two, which used non-Caucasian subjects [16, 36], suggested a decreased risk for later AAM among women with the C (A2) allele (Table 4). AAM has well-known ethnic background: e.g., African-American girls have earlier menarche than Asians and Caucasians [51, 54]. Homozygotes for the A2 variant of the CYP17 gene appear to be more common in Japanese (22%) and other East Asian (32%) populations than in Caucasians (14%) and African-Americans (13%) [51, 54]. The significant difference in the CYP17 allele frequencies between Caucasians and Asians (Table 2) suggests that ethnicity may indeed be a potential factor contributing to AAM and other traits associated with this gene. Similar differences in allele frequencies were reported for candidate genes of other complex traits with ethnic background, e.g., osteoporosis [55], cardiovascular disease [56], renal disease [57], and others.
There was some heterogeneity between the results of various studies. The heterogeneity may be caused by ethnic differences between study samples. Among the 11 case-control studies used in the present analysis, two employed samples of mixed ethnicities; two employed samples of Asians while the other seven used Caucasians (Table 1). Among the 5 studies with continuous outcomes, one employed a sample of mixed ethnicities; one used Asians and the other three used Caucasians (Table 2). After excluding the data of non-Caucasian subjects, no heterogeneity was determined among the remaining studies.
Several studies demonstrated that AAM shares substantial proportion of genetic variation with obesity and osteoporosis [7, 58, 59]. Along with the other results [14, 60-62], those are in support that AAM is a complex trait, and, therefore, potential contribution of many genes to it may be modest. From this point of view, the nearly significant association of the CYP17 MspA1 polymorphism does not completely rejects a probability that it may be a weak modifier of AAM in Caucasian females, especially when interacting with other genetic or environmental factors. For example, an interaction between the ER-α gene and the VDR gene had a significant effect on AAM, but neither is a significant independent risk factor [63]. Likewise, interaction between 5-HTTLPR and stressful life-style factors significantly influences depression symptoms, but the 5-HTTLPR is not a significant independent risk factor of depression [64]. Further studies of CYP17 are needed to determine whether there are the effects of the interaction between this gene and other genetic or environment factors on AAM.
Acknowledgments
This work was supported by grants from National Institute of Health, the State of Nebraska. The study also benefited from 211 State Key Research Fund by Xi'an Jiaotong University and the University of Hong Kong startup fund (to Volodymyr Dvornyk, Ph. D.).
References
- 1.Peeters PH, et al. Age at menarche and breast cancer risk in nulliparous women. Breast Cancer Res Treat. 1995;33:55–61. doi: 10.1007/BF00666071. [DOI] [PubMed] [Google Scholar]
- 2.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11:1531–43. [PubMed] [Google Scholar]
- 3.Rees M. The age of menarche. Orgyn. 1995:2–4. [PubMed] [Google Scholar]
- 4.Ito M, et al. Relation of early menarche to high bone mineral density. Calcif Tissue Int. 1995;57:11–4. doi: 10.1007/BF00298989. [DOI] [PubMed] [Google Scholar]
- 5.Chie WC, et al. Predictive factors for early menarche in Taiwan. J Formos Med Assoc. 1997;96:446–50. [PubMed] [Google Scholar]
- 6.Merzenich H, Boeing H, Wahrendorf J. Dietary fat and sports activity as determinants for age at menarche. Am J Epidemiol. 1993;138:217–24. doi: 10.1093/oxfordjournals.aje.a116850. [DOI] [PubMed] [Google Scholar]
- 7.Kaprio J, et al. Common genetic influences on BMI and age at menarche. Hum Biol. 1995;67:739–53. [PubMed] [Google Scholar]
- 8.Sharma K. Genetic basis of human female pelvic morphology: a twin study. Am J Phys Anthropol. 2002;117:327–33. doi: 10.1002/ajpa.10055. [DOI] [PubMed] [Google Scholar]
- 9.van den Akker OB, et al. Genetic and environmental variation in menstrual cycle: histories of two British twin samples. Acta Genet Med Gemellol (Roma) 1987;36:541–8. doi: 10.1017/s0001566000006929. [DOI] [PubMed] [Google Scholar]
- 10.Long JR, et al. The oestrogen receptor alpha gene is linked and/or associated with age of menarche in different ethnic groups. J Med Genet. 2005;42:796–800. doi: 10.1136/jmg.2004.028381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stavrou I, et al. Association of polymorphisms of the oestrogen receptor alpha gene with the age of menarche. Hum Reprod. 2002;17:1101–5. doi: 10.1093/humrep/17.4.1101. [DOI] [PubMed] [Google Scholar]
- 12.Xita N, et al. Association of SHBG gene polymorphism with menarche. Mol Hum Reprod. 2005;11:459–62. doi: 10.1093/molehr/gah178. [DOI] [PubMed] [Google Scholar]
- 13.Jorm AF, et al. Association of adverse childhood experiences, age of menarche, and adult reproductive behavior: does the androgen receptor gene play a role? Am J Med Genet B Neuropsychiatr Genet. 2004;125:105–11. doi: 10.1002/ajmg.b.20114. [DOI] [PubMed] [Google Scholar]
- 14.Guo Y, et al. Polymorphisms of estrogen-biosynthesis genes CYP17 and CYP19 may influence age at menarche: a genetic association study in Caucasian females. Hum Mol Genet. 2006;15:2401–8. doi: 10.1093/hmg/ddl155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang JH, et al. CYP17 genetic polymorphism, breast cancer, and breast cancer risk factors: Australian Breast Cancer Family Study. Breast Cancer Res. 2005;7:R513–21. doi: 10.1186/bcr1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorai I, et al. Estrogen-metabolizing gene polymorphisms, but not estrogen receptor-alpha gene polymorphisms, are associated with the onset of menarche in healthy postmenopausal Japanese women. J Clin Endocrinol Metab. 2003;88:799–803. doi: 10.1210/jc.2002-020353. [DOI] [PubMed] [Google Scholar]
- 17.Enmark E, Gustafsson JA. Oestrogen receptors - an overview. J Intern Med. 1999;246:133–8. doi: 10.1046/j.1365-2796.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- 18.Stoll BA. Western diet, early puberty, and breast cancer risk. Breast Cancer Res Treat. 1998;49:187–93. doi: 10.1023/a:1006003110909. [DOI] [PubMed] [Google Scholar]
- 19.Fan YS, et al. Localization of the human CYP17 gene (cytochrome P450(17 alpha)) to 10q24.3 by fluorescence in situ hybridization and simultaneous chromosome banding. Genomics. 1992;14:1110–1. doi: 10.1016/s0888-7543(05)80140-5. [DOI] [PubMed] [Google Scholar]
- 20.Dunning AM, et al. A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1999;8:843–54. [PubMed] [Google Scholar]
- 21.Ambrosone CB, et al. CYP17 genetic polymorphism, breast cancer, and breast cancer risk factors. Breast Cancer Res. 2003;5:R45–51. doi: 10.1186/bcr570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feigelson HS, et al. Cytochrome P450c17alpha gene (CYP17) polymorphism predicts use of hormone replacement therapy. Cancer Res. 1999;59:3908–10. [PubMed] [Google Scholar]
- 23.Hamajima N, et al. No association of the 5′ promoter region polymorphism of CYP17 with breast cancer risk in Japan. Jpn J Cancer Res. 2000;91:880–5. doi: 10.1111/j.1349-7006.2000.tb01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Einarsdottir K, et al. CYP17 gene polymorphism in relation to breast cancer risk: a case-control study. Breast Cancer Res. 2005;7:R890–6. doi: 10.1186/bcr1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feigelson HS, et al. A polymorphism in the CYP17 gene increases the risk of breast cancer. Cancer Res. 1997;57:1063–5. [PubMed] [Google Scholar]
- 26.Haiman CA, et al. The relationship between a polymorphism in CYP17 with plasma hormone levels and breast cancer. Cancer Res. 1999;59:1015–20. [PubMed] [Google Scholar]
- 27.Helzlsouer KJ, et al. Association between CYP17 polymorphisms and the development of breast cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:945–9. [PubMed] [Google Scholar]
- 28.Mitrunen K, et al. Steroid metabolism gene CYP17 polymorphism and the development of breast cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:1343–8. [PubMed] [Google Scholar]
- 29.Shin MH, et al. Genetic polymorphism of CYP17 and breast cancer risk in Korean women. Exp Mol Med. 2005;37:11–7. doi: 10.1038/emm.2005.2. [DOI] [PubMed] [Google Scholar]
- 30.Verla-Tebit E, Wang-Gohrke S, Chang-Claude J. CYP17 5′-UTR MspA1 polymorphism and the risk of premenopausal breast cancer in a German population-based case-control study. Breast Cancer Res. 2005;7:R455–64. doi: 10.1186/bcr1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu AH, et al. HSD17B1 and CYP17 polymorphisms and breast cancer risk among Chinese women in Singapore. Int J Cancer. 2003;104:450–7. doi: 10.1002/ijc.10957. [DOI] [PubMed] [Google Scholar]
- 32.Goodman MT, et al. CYP17 genotype and ovarian cancer: a null case-control study. Cancer Epidemiol Biomarkers Prev. 2001;10:563–4. [PubMed] [Google Scholar]
- 33.Jasienska G, et al. CYP17 genotypes differ in salivary 17-beta estradiol levels: a study based on hormonal profiles from entire menstrual cycles. Cancer Epidemiol Biomarkers Prev. 2006;15:2131–5. doi: 10.1158/1055-9965.EPI-06-0450. [DOI] [PubMed] [Google Scholar]
- 34.McCann SE, et al. The risk of breast cancer associated with dietary lignans differs by CYP17 genotype in women. J Nutr. 2002;132:3036–41. doi: 10.1093/jn/131.10.3036. [DOI] [PubMed] [Google Scholar]
- 35.Onland-Moret NC, et al. Cyp17, urinary sex steroid levels and breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:815–20. doi: 10.1158/1055-9965.EPI-04-0197. [DOI] [PubMed] [Google Scholar]
- 36.Small CM, et al. CYP17 genotype predicts serum hormone levels among pre-menopausal women. Hum Reprod. 2005;20:2162–7. doi: 10.1093/humrep/dei054. [DOI] [PubMed] [Google Scholar]
- 37.Rice JP. The role of meta-analysis in linkage studies of complex traits. Am J Med Genet. 1997;74:112–4. doi: 10.1002/(sici)1096-8628(19970221)74:1<112::aid-ajmg22>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 38.Langley K, et al. Association of the dopamine D4 receptor gene 7-repeat allele with neuropsychological test performance of children with ADHD. Am J Psychiatry. 2004;161:133–8. doi: 10.1176/appi.ajp.161.1.133. [DOI] [PubMed] [Google Scholar]
- 39.Thakkinstian A, et al. Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum Mol Genet. 2006;15:2784–90. doi: 10.1093/hmg/ddl220. [DOI] [PubMed] [Google Scholar]
- 40.Ye Z, Parry JM. The CYP17 MspA1 polymorphism and breast cancer risk: a meta-analysis. Mutagenesis. 2002;17:119–26. doi: 10.1093/mutage/17.2.119. [DOI] [PubMed] [Google Scholar]
- 41.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 42.Borenstein M, Borenstein H. Comprehensive Meta-Analysis. Englewood, NJ: Biostat Inc; 1999. [Google Scholar]
- 43.Hedges L, Olkin I. Statistical Methods for Meta-analysis. Academic Press; Orlando, Florida: 1985. [Google Scholar]
- 44.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 45.Ioannidis J, Lau J. Evolution of treatment effects over time: empirical insight from recursive cumulative metaanalyses. Proc Natl Acad Sci U S A. 2001;98:831–6. doi: 10.1073/pnas.021529998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ioannidis JP, et al. Replication validity of genetic association studies. Nat Genet. 2001;29:306–9. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- 47.Lau J, et al. Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med. 1992;327:248–54. doi: 10.1056/NEJM199207233270406. [DOI] [PubMed] [Google Scholar]
- 48.Egger M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carey AH, et al. Polycystic ovaries and premature male pattern baldness are associated with one allele of the steroid metabolism gene CYP17. Hum Mol Genet. 1994;3:1873–6. doi: 10.1093/hmg/3.10.1873. [DOI] [PubMed] [Google Scholar]
- 50.Weston A, et al. CYP17 genotype and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:941–4. [PubMed] [Google Scholar]
- 51.Lai J, et al. CYP gene polymorphisms and early menarche. Mol Genet Metab. 2001;74:449–57. doi: 10.1006/mgme.2001.3260. [DOI] [PubMed] [Google Scholar]
- 52.Must A, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. 2002;155:672–9. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]
- 53.Bean JA, et al. Variations in the reporting of menstrual histories. Am J Epidemiol. 1979;109:181–5. doi: 10.1093/oxfordjournals.aje.a112673. [DOI] [PubMed] [Google Scholar]
- 54.Sharp L, et al. CYP17 gene polymorphisms: prevalence and associations with hormone levels and related factors. a HuGE review. Am J Epidemiol. 2004;160:729–40. doi: 10.1093/aje/kwh287. [DOI] [PubMed] [Google Scholar]
- 55.Dvornyk V, Recker RR, Deng HW. Gene expression studies of osteoporosis: implications for microarray research. Osteoporos Int. 2003;14:451–61. doi: 10.1007/s00198-002-1373-0. [DOI] [PubMed] [Google Scholar]
- 56.Freedman BI, et al. The impact of ethnicity and sex on subclinical cardiovascular disease: the Diabetes Heart Study. Diabetologia. 2005;48:2511–8. doi: 10.1007/s00125-005-0017-2. [DOI] [PubMed] [Google Scholar]
- 57.Feehally J. Ethnicity and renal disease: questions and challenges. Clin Med. 2003;3:578–82. doi: 10.7861/clinmedicine.3-6-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo Y, et al. Genetic and environmental correlations between age at menarche and bone mineral density at different skeletal sites. Calcif Tissue Int. 2005;77:356–60. doi: 10.1007/s00223-005-0181-x. [DOI] [PubMed] [Google Scholar]
- 59.Wang W, et al. Genetic and environmental correlations between obesity phenotypes and age at menarche. Int J Obes (Lond) 2006;30:1595–600. doi: 10.1038/sj.ijo.0803322. [DOI] [PubMed] [Google Scholar]
- 60.Meyer F, et al. Dietary and physical determinants of menarche. Epidemiology. 1990;1(5):377–81. doi: 10.1097/00001648-199009000-00007. [DOI] [PubMed] [Google Scholar]
- 61.Sanchez-Andres A. Genetic and environmental factors affecting menarcheal age in Spanish women. Anthropol Anz. 2006;55:69–78. [PubMed] [Google Scholar]
- 62.Stavrou I, et al. Combined estrogen receptor alpha and estrogen receptor beta genotypes influence the age of menarche. Hum Reprod. 2006;21:554–7. doi: 10.1093/humrep/dei326. [DOI] [PubMed] [Google Scholar]
- 63.Xu H, et al. Interaction effects between estrogen receptor alpha and vitamin D receptor genes on age at menarche in Chinese women. Acta Pharmacol Sin. 2005;26(7):860–4. doi: 10.1111/j.1745-7254.2005.00122.x. [DOI] [PubMed] [Google Scholar]
- 64.Caspi A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]


