Abstract
We investigated the safety and neuroregenerative potential of an adeno-associated virus (AAV2) containing human glial cell line-derived neurotrophic factor (GDNF) in an MPTP primate model of Parkinson’s disease. Dopaminergic function was evaluated by positron emission tomography with 6-[18F]fluoro-l-m-tyrosine (FMT) before and after AAV2-GDNF or phosphate-buffered saline infusion bilaterally into the putamen. FMT uptake was significantly increased bilaterally in the putamen of AAV2-GDNF but not phosphate-buffered saline-treated animals 6 months after infusion, indicating increased dopaminergic activity in the nigrostriatal pathways. AAV2-GDNF-treated animals also showed clinical improvement without adverse effects. These findings are consistent with our previous report in aged nonhuman primates that showed evidence of enhanced use of striatal dopamine and dopaminergic nigrostriatal innervation. Clinical improvement and evidence of functional recovery in the nigrostriatal pathway, and the absence of adverse effects, support the safety of this approach for the delivery of GDNF over a 6-month period.
Introduction
A number of studies have demonstrated the effectiveness of glial cell line-derived neurotrophic factor (GDNF) for the support of neuronal function in animal models of Parkinson’s disease (PD) (Bohn, 1999; Hurelbrink and Barker, 2004). Both rodent and nonhuman primate studies with striatal, nigral, and ventricular GDNF administration have shown beneficial effects, although these methods are limited in terms of protein distribution and the ability to deliver the protein chronically, both of which have safety implications for clinical use. In fact, the first randomized clinical trial of GDNF, in which monthly infusions of the protein were delivered via an implanted cerebroventricular catheter, failed to show a therapeutic effect but resulted in a number of adverse events (Nutt et al. 2003). The lack of therapeutic efficacy may have been due to the failure of GDNF to reach target tissues. This was supported by postmortem evidence in one patient showing a lack of regeneration or of GDNF diffusion into targeted brain regions (Kordower et al. 1999).
Another clinical trial that delivered GDNF by putaminal infusion also failed to show therapeutic effects beyond that shown in a control group (Lang et al., 2006). However, significant increases in positron emission tomography (PET) measures of striatal fluorine-labeled dihydroxyphenylalanine (F-dopa) uptake were observed, suggesting a subtherapeutic functional effect of GDNF. In addition to the lack of therapeutic effects, several patients developed neutralizing anti-GDNF antibodies, raising safety concerns perhaps related to the delivery method. Further concerns were raised after the observation of multifocal cerebellar Purkinje cell loss in monkeys after continuous putaminal infusion of GDNF over a 6-month period (Hovland et al., 2007). Although some PD patients experienced benefit after putaminal infusions of GDNF (Gill et al., 2003; Patel et al., 2005; Slevin et al., 2005, 2006), the overall failure of randomized trials and the safety concerns have prompted the exploration of alternative methods for administering viral vectors in nonhuman primates.
A lentiviral GDNF vector system evinced protective effects of GDNF in both aged nonhuman primates (NHPs) and in young 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned NHPs (Kordower et al., 2000). This study showed protection of dopaminergic innervation of the striatum, improved motor performance, and gene expression for 8 months. Although these findings were promising, lentiviral vectors have not been as well characterized as adeno-associated viral vectors for CNS applications. A study with an adeno-associated serotype 2 viral (AAV2) GDNF vector in marmosets found a protective effect against striatal delivery of 6-hydroxydopamine (6-OHDA) (Eslamboli, 2005). It must be emphasized that such studies did not explore the efficacy of GDNF in the context of a fully lesioned nigra, but only under conditions in which toxin was administered nearly contemporaneously with GDNF, raising the possibility that efficacy was due to protection of existing nigral neurons rather than to regeneration of dopaminergic function (Eberling et al., 1997). This is an important issue, particularly in the context of the failure of a phase 2 clinical trial of neurturin (NTN) delivered by striatal injections of an AAV2 encoding human NTN. The preclinical rationale for this study was based primarily on the neuroprotective effect of AAV2-NTN against 6-OHDA in rats (Gasmi et al., 2007) and MPTP in macaques (Kordower et al., 2006). However, Parkinsonian subjects recruited for the phase 1 and 2 clinical trials of AAV2-NTN (CERE-120) were enrolled at a stage of the disease (stage 3 on the Hoehn and Yahr scale) in which substantial loss of nigral innervation of the striatum had been present for at least 5 years (Marks et al., 2008). On this basis, we regard the failure of this phase 2 study as evidence that any neurotrophic therapy for PD must demonstrate the ability of the agent to stimulate regeneration in a stably lesioned animal. Accordingly, we investigated the safety and neuroregenerative potential of AAV2-GDNF in a stable MPTP primate model of PD. The present report represents an initial analysis of in vivo responses to AAV2-GDNF treatment of MPTP-lesioned NHP over the first 12 months of a 2-year study. Because our PD model is characterized by an extensive nigral lesion in the ipsilateral hemisphere and mild lesioning in the contralateral hemisphere, we were able to model early and advanced PD in the same animal. Our findings suggest that GDNF has the capacity to induce dopaminergic regeneration regardless of the degree of nigral injury, and thus augurs well for broad clinical application of AAV2-GDNF in the treatment of PD.
Materials and Methods
Experimental subjects
The protocol was approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco (San Francisco, CA) and the Animal Welfare and Research Committee at the Lawrence Berkeley National Laboratory. Eleven male rhesus monkeys (8–14 kg) and 1 female rhesus monkey (7 kg) were lesioned with 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) as previously described (Bankiewicz et al., 1986, 2000; Eberling et al., 1998). Briefly, MPTP lesioning consisted of one or two right intracarotid artery infusions of 2.0–4.0mg of MPTP-HCl followed by additional intravenous administrations of 0.2- to 0.5-mg/kg doses of MPTP-HCl. This method of lesioning produces nearly a complete dopaminergic lesion on the side of carotid artery infusion (ipsilateral side) and a partial lesion on the other side of the brain (contralateral side). Intravenous dosing with MPTP continued until the animal showed bilateral parkinsonian signs and a clinical rating scale (CRS) score between 21 and 26, as described below. Animals were not scheduled for surgery until they had achieved a stable behavioral disability rating in more than 10 assessments over about a 4-month period.
Clinical rating scale
All ratings were performed by a single investigator blinded to the experimental conditions. The modified Parkinson’s CRS was developed by the Bankiewicz laboratory, and closely approximates those reported in the literature (Imbert et al., 2000). The scale evaluates 14 parkinsonian features, each of which receives a score from 0 to 3 in order of increasing severity (0, normal; 1, mild; 2, moderate; 3, severe). Individual scores are summed to arrive at a final score. Features evaluated include tremor (right and left sides), locomotion, “freezing,” fine motor skills (right and left hand), bradykinesia (right and left sides), hypokinesia, balance, posture, startle response, and gross motor skills (right and left hands). Normal animals score in the range of 0 to 4, and severely parkinsonian monkeys score over 20. CRS assessments were performed after MPTP lesioning to determine a stable baseline before treatment. Animals were assessed after AAV treatment both with and without l-dihydroxyphenylalanine (l-dopa) administration. Individual CRS ratings over 5–10 separate days were averaged for each time point to provide unbiased CRS scores at baseline and at 3-month intervals after treatment. Animals were treated with an intramuscular injection of l-dopa 30 min before CRS rating at a time when CRS (without l-dopa) was stable.
Magnetic resonance imaging
Baseline magnetic resonance imaging (MRI) was performed to enable accurate placement of the infusion cannula in the target structures. During the MRI procedure, NHPs were sedated with ketamine (Ketaset, 7mg/kg, intramuscular) and xylazine (Rompun, 3mg/kg, intramuscular). Each animal was positioned in the MRI-compatible stereotactic frame. Ear- and eye-bar positions were recorded, and an intravenous line was installed. Coronal images (1mm) were collated on a GE Signa 1.5-T system. Images were T1-weighted and taken in three planes with a repetition time of 700msec, an echo time of 20msec, and a flip angle setting of 30°. A scanning time of approximately 20min was employed, with a 15-cm field of view, a 192matrix, and a 2NEX (number of averages per signal unit). Each scan lasted approximately 20min. The coronal MRI images were used to determine the three-dimensional structure of the caudate nucleus and the putamen, and surgical coordinates were generated from magnified coronal images (×1.5).
AAV2-GDNF vector construction
The human GDNF cDNA was cloned into an AAV2 shuttle plasmid, and a recombinant AAV2 carrying GDNF under the control of the cytomegalovirus promoter was generated by a triple-transfection technique with subsequent purification by CsCl gradient centrifugation (Matsushita et al., 1998; Wright et al., 2003). AAV2-GDNF stock was concentrated to 1.1 × 1013 vector genomes per ml (vg/ml) as determined by quantitative PCR, and then diluted immediately before use to 3.3 × 1012 vector genomes (VG)/ml in phosphate-buffered saline (PBS)–0.001% (v/v) Pluronic F-68.
Stereotactic surgery and AAV2 infusion
Monkeys received bilateral infusions of AAV2-GDNF (n = 6) or PBS (n = 6) into the putamen at anterior and posterior levels by convection-enhanced delivery (CED). Infusions were performed with a step-design fused silica cannula (Krauze et al., 2005) with an outside diameter (at the tip) of 0.3mm by a sequential, ascending infusion rate method (0.2, 0.5, 0.8, 1, 1.5, and 2 µl/min for 10 min and 2.5 µl/min for 6 min) in which 75 µl/site was delivered into two sites in each putamen. After infusion, the cannula was left in place for 5 min before it was retracted, and the surgical site was then closed within anatomical layers. This method of delivery has been extensively used and refined by our group over more than a decade (Bankiewicz et al., 2000, 2006b; Forsayeth et al., 2006; Hadaczek et al., 2009).
Positron emission tomography methods and analysis
We have published extensively on the use of 6-[18F]fluoro-l-m-tyrosine (FMT)-PET to measure dopaminergic function in the human and primate brains (Jordan et al., 1997; Eberling et al., 1998, 2000, 2004, 2007, 2008; Oiwa et al., 2003). In our experience, the measurement of aromatic-l-amino-acid decarboxylase (AADC) activity, expressed either naturally in nigral neurons (Eberling et al., 2007) or via a neurorestorative gene therapy approach (Bankiewicz et al., 2000, 2006b; Forsayeth et al., 2006; Eberling et al., 2008), offers good signal-to-background and strong correlations between AADC activity and l-dopa responsiveness in primates (Forsayeth et al., 2006; Eberling et al., 2008). PET was performed 3 months before (baseline) and 6months after vector infusion. PET studies were performed on a Siemens ECAT EXACT HR PET scanner (Siemens, Malvern, PA) in three-dimensional acquisition mode. FMT was synthesized by a semiremote chemical synthesis apparatus (O’Neil and VanBrocklin, 1995) according to a modification of a previously described procedure (Namavari et al., 1993). Monkeys were anesthetized with an intramuscular injection of ketamine (15mg/kg), intubated, and placed on isoflurane anesthesia. All animals were pretreated with an intravenous injection of benserazide (2mg/kg), a peripheral decarboxylase inhibitor, 30 min before imaging. The animals were placed in a stereotactic frame and positioned in the PET scanner. Images were obtained in the coronal plane. Before the emission scan a 10-min transmission scan was obtained for attenuation correction. Subsequently, approximately 3–5 mCi of FMT was injected as a bolus in an antecubital vein and a 90-min dynamic acquisition sequence was acquired.
Data were reconstructed via an ordered subset expectation maximization (OSEM) algorithm with weighted attenuation and scatter correction. PET data were quantified by means of a multiple-time graphical analysis (“Patlak plot”) with the time–activity curve for a region, the cerebellum, in which the tracer is nonspecifically bound as the input function. The Patlak model was fit with dynamic data from each region between 24 and 90 min. Parametric images of FMT influx (Ki) from both time points were generated and coregistered in order to identify functional changes after treatment. FMT influx in identical regions within the putamen was thereby measured before and after treatment for each monkey. Comparisons were made between baseline and posttreatment Ki values by paired t tests. Pearson product–moment correlation coefficients were used to assess relationships between Ki values and CRS scores.
Results
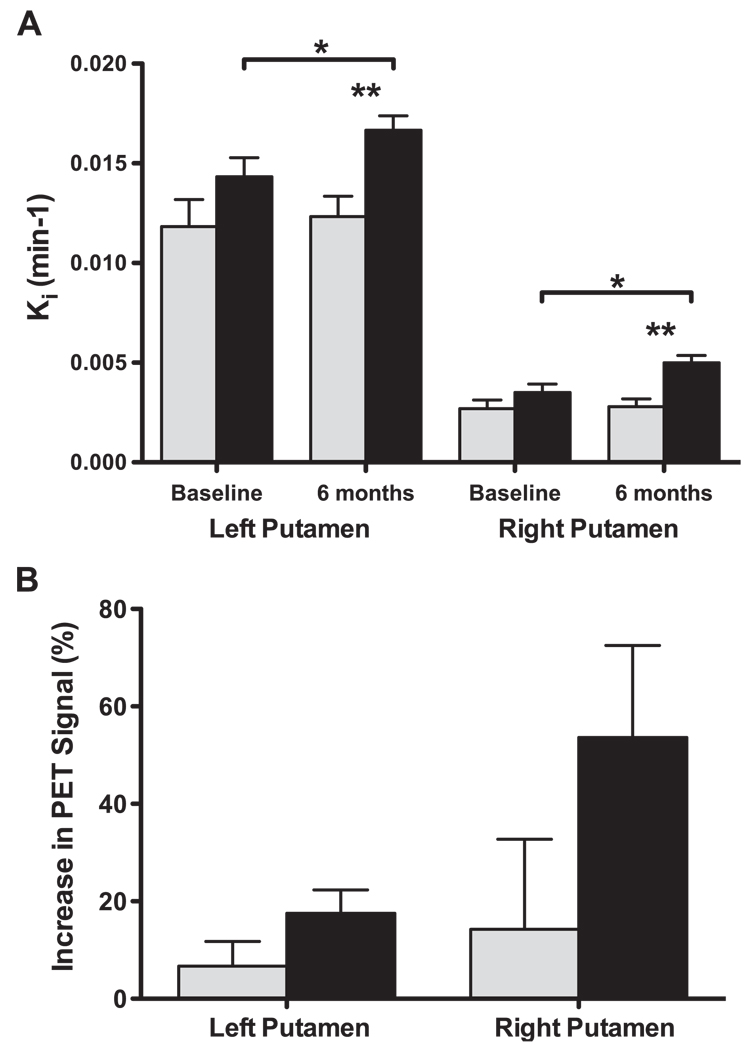
PET scans performed both before and after treatment displayed a considerable imbalance in FMT signal between the right and left putamen resulting, respectively, from the full and partial lesioning with MPTP (Fig. 1). AAV2-GDNF-treated monkeys generally displayed a clear bilateral increase in the intensity and distribution of FMT signal 6 months after treatment when compared with the baseline scans, whereas no apparent change in FMT uptake was evident in PBS-treated control monkeys (Fig. 1). Quantification of the PET signals showed a ∼ 4-fold difference in Ki values between the right (0.013 min−1) and left (0.003 min−1) putamen at baseline (Fig. 2A). In comparison, a similar sized cohort of unlesioned animals (n = 4) had a mean Ki of 0.023 ± 0.001 min−1. Six months after treatment the FMT uptake in AAV2-GDNF-infused monkeys was significantly (p < 0.05) increased bilaterally over baseline levels, with both hemispheres showing a similar increase in Ki of ∼0.002 min−1. However, given the large baseline PET difference between hemispheres, the percentage increase in FMT uptake after AAV2-GDNF treatment was considerably greater in the fully lesioned right putamen (54 ± 19%) than in the partially lesioned left putamen (18 ± 5%; Fig. 2B). Control animals did not show a significant change in FMT uptake for either right or left putamen. One control animal was notable in its sharp behavioral improvement after surgery despite the absence of an increase in PET signal.
FIG. 1.
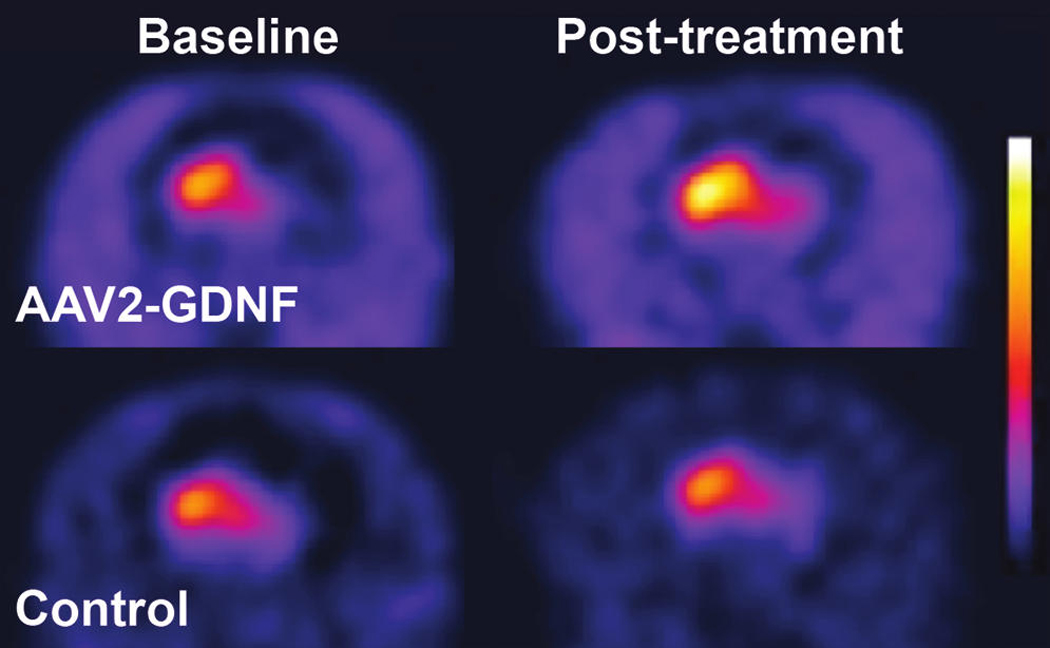
FMT-PET images before and after treatment. Representative parametric PET images show increased FMT uptake bilaterally for a GDNF-treated monkey but minimal change in FMT uptake for a control monkey.
FIG. 2.
FMT-PET uptake (Ki) in the putamen. (A) Mean FMT uptake in fully lesioned (right) and partially lesioned (left) putamen at baseline and post-treatment for GDNF-treated (solid columns) and PBS control monkeys (shaded columns). GDNF-treated monkeys showed significant bilateral increases in Ki values at 6 months compared with baseline (paired t test, *p < 0.05), whereas control monkeys did not show a significant change. FMT-PET uptake in both hemispheres was also significantly greater at 6 months in the AAV2-GDNF monkeys compared with the controls (unpaired t test, **p < 0.01). (B) Percentage increase in FMT-PET uptake 6 months after treatment compared with the baseline PET scans was greater in the partially lesioned right putamen. Error bars indicate the SEM.
The extent of motor deficits was assessed at various times throughout the study both with and without l-dopa administration. AAV2-GDNF-treated monkeys displayed considerable improvements in their CRS scores over a 9-month period after treatment, with improvements maintained at the 12-month point (two-way analysis of variance [ANOVA], AAV2-GDNF vs. PBS treatment, p < 0.001 [Fig. 3]; note that two monkeys from each group were killed at 6 months). With the exception of one clear outlier, the PBS-treated controls displayed only a slight reduction in CRS score. A small amount of functional recovery is typical for this model; however, we currently have no explanation for the rapid recovery of one control monkey after the sham PBS treatment and have therefore included this outlier in all statistical analyses. Rarely, MPTP-lesioned animals have shown improved CRS for no obvious reason (Bankiewicz et al., 2006b). At the time of PET imaging (6 months after treatment), the AAV2-GDNF-treated monkeys displayed a significant 37 ± 6% (7.6 ± 1.0 point) improvement in CRS score over baseline, compared with an 18 ± 11% (4.1 ± 2.3 points) reduction in CRS score for the control animals. By 12 months, CRS scores had improved by 60 ± 6% (11.8 ± 1.1 points) for AAV2-GDNF monkeys and only 28 ± 12% (6.1 ± 2.4 points) for controls. The improvements in CRS scores 6 months after surgery, relative to baseline CRS, were independently compared with FMT-PET signal changes in the putamen. A positive linear correlation was found between the percentage improvement in CRS and the percentage increase in FMT uptake for both the left (r = 0.85, p < 0.05) and right (r = 0.97, p < 0.01) putamen of AAV2-GDNF-treated monkeys (Fig. 4A and B). No significant correlations were found for the PBS-treated control monkeys (Fig. 4C and D).
FIG. 3.
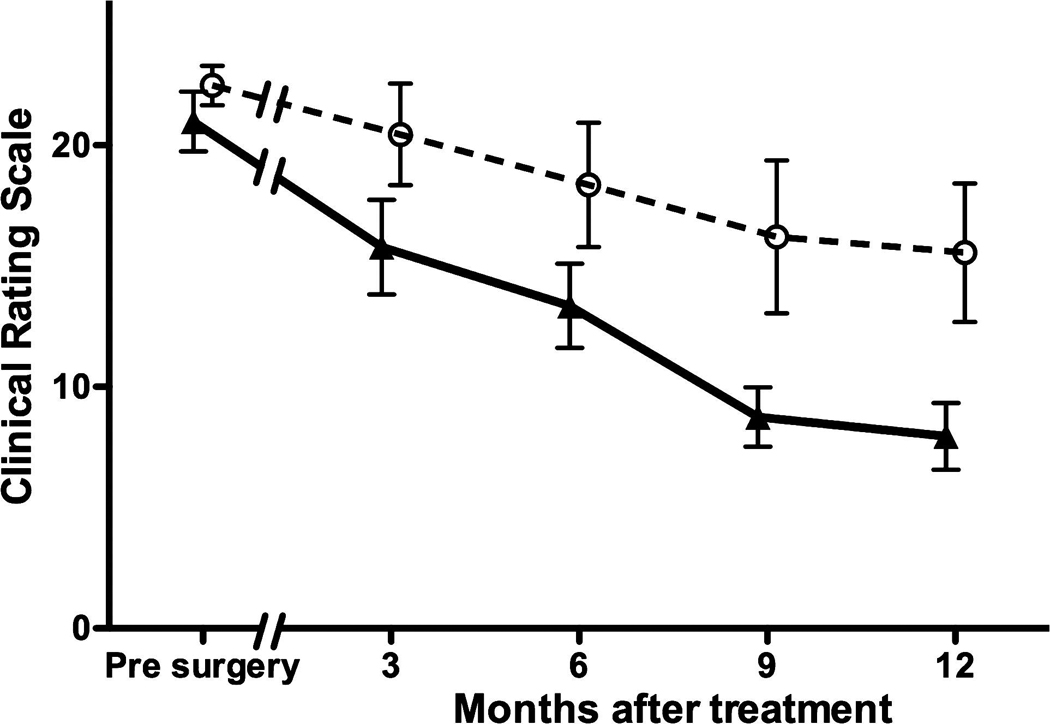
Clinical rating scale (CRS) scores. AAV2-GDNF-treated monkeys (triangles) showed rapid improvement in CRS score after treatment relative to controls (circles). A significant reduction in CRS score was observed to occur during the 9 months after treatment and was maintained at the longest time point of 12 months (two-way ANOVA, AAV2-GDNF vs. controls, p < 0.001). Error bars indicate the SEM.
FIG. 4.
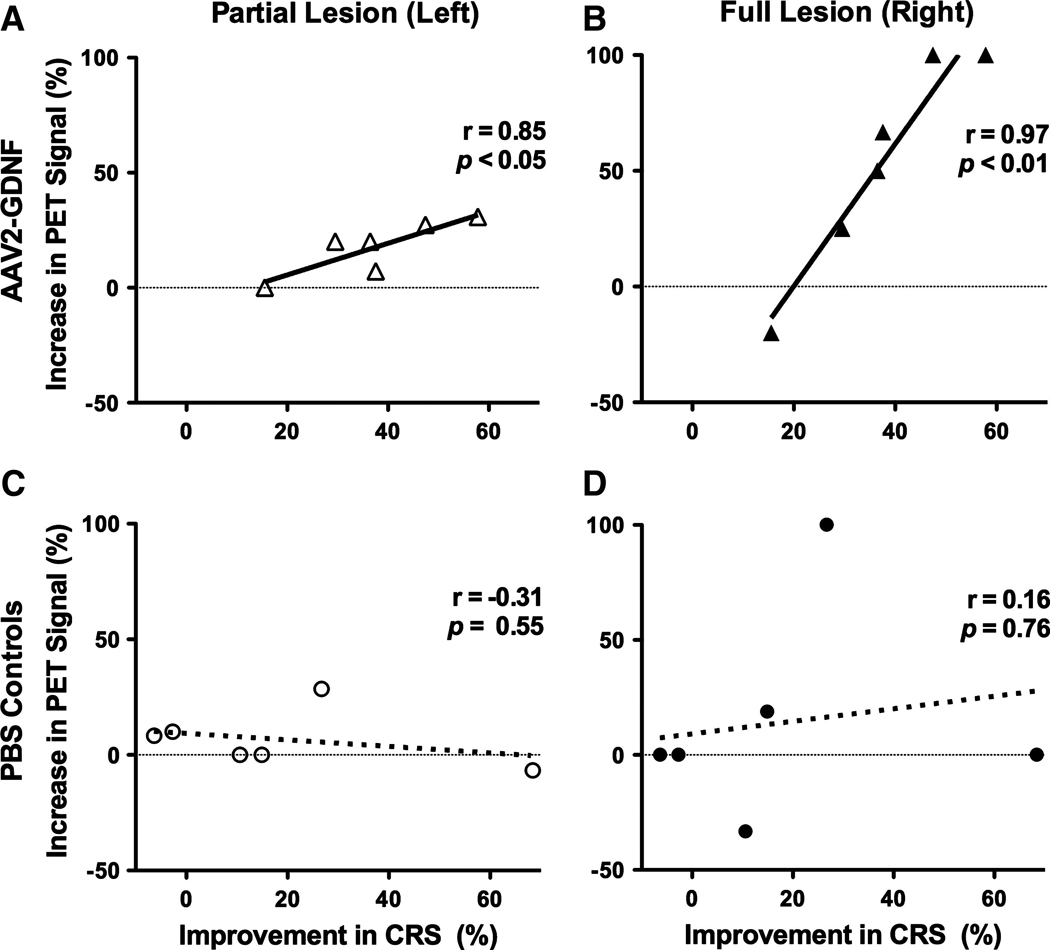
Bilateral increases in FMT uptake correlate with reductions in clinical rating scale (CRS) scores. Shown are independent correlations between the percentage change in CRS score 6 months after treatment and the percentage increase in FMT-PET signal for the left and right putamen of AAV2-GDNF (A and B) and control (C and D) monkeys. AAV2-GDNF monkeys displayed a significant linear correlation (solid lines) between changes in CRS score and PET signal for both the partially lesioned left putamen (A) and fully lesioned right putamen (B). Correlations for the control monkeys (dotted lines) were not statistically significant for either the partial (C) or the fully (D) lesioned hemisphere.
Comprehensive biochemical and histological analyses of the brains will be undertaken when the 24-month end-point monkeys are killed and the results will be published separately. Preliminary data at the 6- and 12-month end points showed bilateral expression of GDNF protein within the AAV2-GDNF-infused putaminal regions and an enhancement of tyrosine hydroxylase (TH) expression in both the partially lesioned (left) and fully lesioned (right) caudate–putamen when compared with the control PBS-infused monkeys (Fig. 5). No evidence of MPTP lesioning could be detected by TH immunostaining on the partially lesioned hemisphere of AAV2-GDNF-treated monkeys. Enhanced TH-positive staining in the fully lesioned hemisphere was most evident in the medial–ventral region of the putamen with the MPTP-induced lesioning of the nigrostriatal projection still clearly evident over most of the caudate–putamen. Further analysis of these brains, including histological assessment of the substantia nigra, may provide further insight into the basis of the enhancement and/or regeneration of TH-expressing neurons.
FIG. 5.
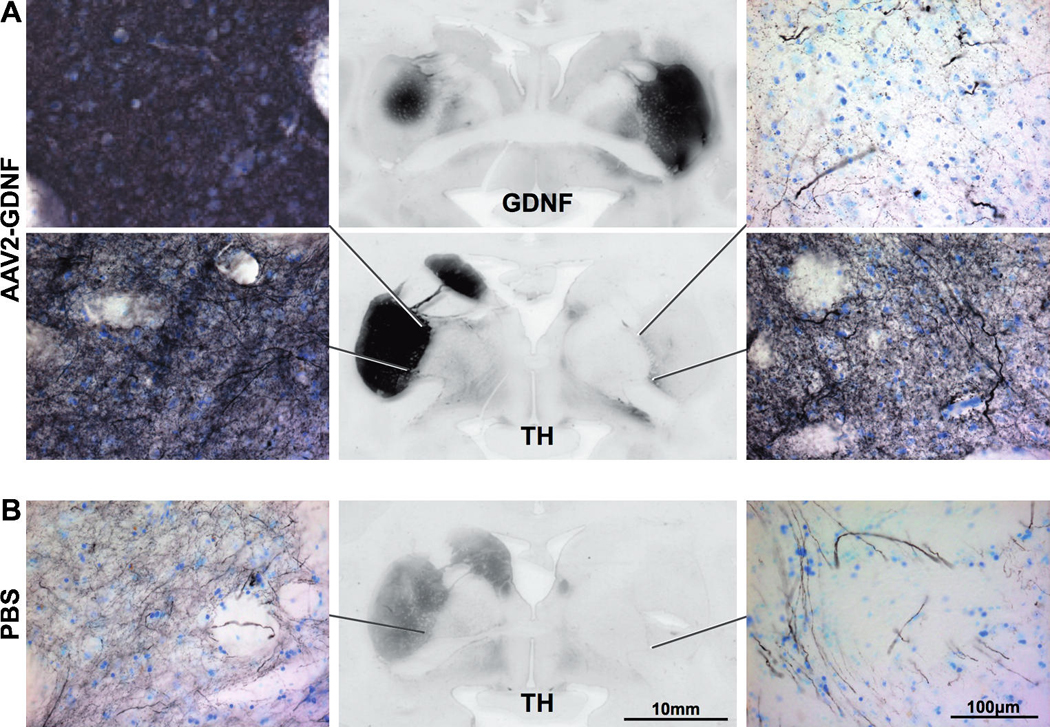
Enhanced expression of tyrosine hydroxylase (TH) after AAV2-GDNF delivery. Immunostaining for TH demonstrated almost complete destruction of TH-positive fibers in the fully MPTP lesioned (right) caudate–putamen compared with the partially lesioned (left) hemisphere. Twelve months after bilateral AAV2-GDNF delivery (A), and the subsequent upregulation of GDNF in the putamen (A, middle), TH expression in both hemispheres was enhanced relative to PBS-treated controls (B). Very intense TH staining was observed in the left putamen of AAV2-GDNF-treated monkeys (left) with no evident sign of MPTP-induced lesioning. Within the right putamen of AAV2-GDNF-treated monkeys, we observed upregulation of TH, particularly in the medial–ventral region (right). TH staining in the right putamen of PBS controls was restricted to a limited number of TH-positive fibers with none of the diffuse TH staining seen in either the left putamen or AAV2-GDNF-treated monkeys.
Discussion
We used an overlesioned hemiparkinsonian MPTP primate model to evaluate the safety and efficacy of AAV2-GDNF for PD. In this model, the nigrostriatal pathway is modestly lesioned in one hemisphere and severely lesioned in the other, resembling both early- and late-stage PD, respectively (Eberling et al., 1998). We chose this model to evaluate the effectiveness of GDNF at different stages of the degenerative process. FMT uptake was significantly increased by comparable magnitudes in the putamen in both hemispheres of AAV2-GDNF-treated monkeys, indicating increased dopaminergic activity in the nigrostriatal pathways. In addition, AAV2-GDNF-treated monkeys showed clinical improvement without the development of adverse effects, such as dyskinesia. The control monkeys did not show significant changes in FMT uptake with only slight recovery of clinical measures, as is often seen with the MPTP lesion model.
We reported the results of a parallel study in aged non-human primates treated with AAV2-GDNF and showed that this vector directed broadly distributed GDNF expression in the striatum, improved several clinically relevant measures of nigrostriatal function, and displayed no apparent adverse effects (Johnston et al., 2009). The use of aged and MPTP-treated NHP for the clinical development of novel therapeutics for PD addresses different critical issues for potential clinical application. The use of aged primates (>20 years) addresses issues related to the neuroregenerative potential of the aged brain. This is clearly significant because the mean age of onset for PD is about 60. Aged NHP undergo many of the changes seen in aged humans, including the loss of the dopaminergic phenotype in the substantia nigra, and improvements in dopaminergic function have been reported after treatment with growth factors (Kordower et al., 2000; Maswood et al., 2002; Grondin et al., 2003). The MPTP model is important because the clinical syndrome produced closely mimicks that seen in humans with PD, and therefore addresses questions regarding clinical efficacy (Fiandaca et al., 2008). In this respect, our studies resemble similar studies in aged and MPTP-treated, monkeys conducted before the phase 1 trial of CERE-120 (AAV2-NTN) (Kordower et al., 2006; Herzog et al., 2007; Marks et al., 2008; Palfi, 2008).
The present study differs from previous reports by demonstrating the neuroregenerative potential of AAV2-GDNF in a stable primate model of PD. We previously showed temporal changes in nigrostriatal degeneration by evaluating monkeys at various time points after MPTP administration (Eberling et al., 1997). These findings guided subsequent work aimed at neuroprotection by administering growth factors and neurotoxins closely in time in order to prevent the degeneration that occurs several days after a neurotoxic insult to the dopaminergic system (Kordower et al., 2000; Eslamboli et al., 2005). This neuroprotective strategy is problematic because it assumes that the cause of idiopathic PD is a neurotoxic insult. The use of the overlesioned primate model, in which the dopaminergic deficit was well established (>5 months before the administration of AAV2-GDNF), enabled the simultaneous evaluation of the neuroregenerative capacity of severely and mildly injured nigrostriatal pathways. The increased FMT uptake in both hemispheres suggests that AAV2-GDNF may be effective in addressing early and later stages of the disease process when applied to clinical populations. It is important to note in this context that the placebo-controlled phase 2 efficacy study of CERE-120 was reported to have failed to meet its end points (see http://www.ceregene.com/press_112608.asp), confirming early findings of modest clinical improvements that were not accompanied by changes in F-dopa uptake (Marks et al., 2008). The failure of this clinical trial to meet its primary objectives raises an important issue in addition to that raised previously. An often underappreciated factor is the key role of the vector infusion method used. In Kordower et al. (2006), distribution of neurturin immunoreactivity was restricted to a 3- to 4-mm radius, consistent with acute injection protocols. In contrast, the CED method produces complete coverage of the putamen by GDNF immunoreactivity (8- to 10-mm radius). This broad distribution of transgene within the putamen was also seen with AAV2-hAADC (Bankiewicz et al., 2006b; Cunningham et al., 2008), and correlated with efficacy in NHPs (Forsayeth et al., 2006) and in humans (Eberling et al., 2008). In contrast, highly focal delivery of AAV2-hAADC was associated with the appearance of l-dopa-induced dyskinesias in MPTP-lesioned NHPs (Bankiewicz et al., 2006a).
In summary, interim in vivo findings in stably lesioned NHPs presented here demonstrate that AAV2-GDNF delivered by CED into the putamen exhibits significant neuroregenerative capacity in both mildly and severely lesioned nigrostriatal pathways. This model more closely recapitulates the situation encountered in PD patients in whom more than 60% of the nigra is lost before symptoms appear. This initial report will be followed by detailed postmortem analyses that, together with our study in aged NHPs (Johnston et al., 2009), will form the basis of an Investigational New Drug application for AAV2-GDNF.
Acknowledgments
The authors thank Avigen for preparing the AAV2-GDNF. This work was supported under a U54 Cooperative Translational Research Program from NIH-NINDS.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- Bankiewicz KS, Oldfield EH, Chiueh CC, Doppman JL, Jacobowitz DM, Kopin IJ. Hemiparkinsonism in monkeys after unilateral internal carotid artery infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Life Sci. 1986;39:7–16. doi: 10.1016/0024-3205(86)90431-5. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, Cunningham J, Budinger TF, Harvey-White J. Convection-enhanced delivery of AAV vector in parkinsonian monkeys: In vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp. Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Daadi M, Pivirotto P, Bringas J, Sanftner L, Cunningham J, Forsayeth JR, Eberling JL. Focal striatal dopamine may potentiate dyskinesias in parkinsonian monkeys. Exp. Neurol. 2006a;197:363–372. doi: 10.1016/j.expneurol.2005.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, Herscovitch P, Carson RE, Eckelman W, Reutter B, Cunningham J. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol. Ther. 2006b;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Bohn MC. A commentary on glial cell line-derived neurotrophic factor (GDNF): From a glial secreted molecule to gene therapy. Biochem. Pharmacol. 1999;57:135–142. doi: 10.1016/s0006-2952(98)00280-9. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Pivirotto P, Bringas J, Suzuki B, Vijay S, Sanftner L, Kitamura M, Chan C, Bankiewicz KS. Biodistribution of adeno-associated virus type-2 in nonhuman primates after convection-enhanced delivery to brain. Mol. Ther. 2008;16:1267–1275. doi: 10.1038/mt.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling JL, Bankiewicz KS, Jordan S, VanBrocklin HF, Jagust WJ. PET studies of functional compensation in a primate model of Parkinson’s disease. Neuroreport. 1997;8:2727–2733. doi: 10.1097/00001756-199708180-00017. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Jagust WJ, Taylor S, Bringas J, Pivirotto P, VanBrocklin HF, Bankiewicz KS. A novel MPTP primate model of Parkinson’s disease: Neurochemical and clinical changes. Brain Res. 1998;805:259–262. doi: 10.1016/s0006-8993(98)00710-0. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Pivirotto P, Bringas J, Bankiewicz KS. Tremor is associated with PET measures of nigrostriatal dopamine function in MPTP-lesioned monkeys. Exp. Neurol. 2000;165:342–346. doi: 10.1006/exnr.2000.7470. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Pivirotto P, Bringas J, Bankiewicz KS. Comparison of two methods for the analysis of [18F]6-fluoro-l-m-tyrosine PET data. Neuroimage. 2004;23:358–363. doi: 10.1016/j.neuroimage.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Bankiewicz KS, O’Neil JP, Jagust WJ. PET 6-[F]fluoro-l-m-tyrosine studies of dopaminergic function in human and nonhuman primates. Front. Hum. Neurosci. 2007;1:9. doi: 10.3389/neuro.09.009.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, Aminoff MJ. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70:1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- Eslamboli A. Assessment of GDNF in primate models of Parkinson’s disease: Comparison with human studies. Rev. Neurosci. 2005;16:303–310. doi: 10.1515/revneuro.2005.16.4.303. [DOI] [PubMed] [Google Scholar]
- Eslamboli A, Georgievska B, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Annett L, Kirik D. Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson’s disease. J. Neurosci. 2005;25:769–777. doi: 10.1523/JNEUROSCI.4421-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiandaca M, Forsayeth J, Bankiewicz K. Current status of gene therapy trials for Parkinson’s disease. Exp. Neurol. 2008;209:51–57. doi: 10.1016/j.expneurol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Forsayeth JR, Eberling JL, Sanftner LM, Zhen Z, Pivirotto P, Bringas J, Cunningham J, Bankiewicz KS. A dose-ranging study of AAV-hAADC therapy in parkinsonian monkeys. Mol. Ther. 2006;14:571–577. doi: 10.1016/j.ymthe.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK, Cunningham JJ, Printz MA, Kordower JH, Bartus RT. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: Long-term efficacy and tolerability of CERE-120 for Parkinson’s disease. Neurobiol. Dis. 2007;27:67–76. doi: 10.1016/j.nbd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Grondin R, Zhang Z, Ai Y, Gash DM, Gerhardt GA. Intracranial delivery of proteins and peptides as a therapy for neurodegenerative diseases. Prog. Drug Res. 2003;61:101–123. doi: 10.1007/978-3-0348-8049-7_4. [DOI] [PubMed] [Google Scholar]
- Hadaczek P, Forsayeth J, Mirek H, Munson K, Bringas J, Pivirotto P, McBride JL, Davidson BL, Bankiewicz KS. Transduction of nonhuman primate brain with adeno-associated virus serotype 1: Vector trafficking and immune response. Hum. Gene Ther. 2009;20:225–237. doi: 10.1089/hum.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog CD, Dass B, Holden JE, Stansell J, III, Gasmi M, Tuszynski MH, Bartus RT, Kordower JH. Striatal delivery of CERE-120, an AAV2 vector encoding human neurturin, enhances activity of the dopaminergic nigrostriatal system in aged monkeys. Mov. Disord. 2007;22:1124–1132. doi: 10.1002/mds.21503. [DOI] [PubMed] [Google Scholar]
- Hovland DN, Jr, Boyd RB, Butt MT, Engelhardt JA, Moxness MS, Ma MH, Emery MG, Ernst NB, Reed RP, Zeller JR, Gash DM, Masterman DM, Potter BM, Cosenza ME, Lightfoot RM. Six-month continuous intraputamenal infusion toxicity study of recombinant methionyl human glial cell line-derived neurotrophic factor (r-metHuGDNF) in rhesus monkeys. Toxicol. Pathol. 2007;35:1013–1029. doi: 10.1177/01926230701481899. [DOI] [PubMed] [Google Scholar]
- Hurelbrink CB, Barker RA. The potential of GDNF as a treatment for Parkinson’s disease. Exp. Neurol. 2004;185:1–6. doi: 10.1016/j.expneurol.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Imbert C, Bezard E, Guitraud S, Boraud T, Gross CE. Comparison of eight clinical rating scales used for the assessment of MPTP-induced parkinsonism in the macaque monkey. J. Neurosci. Methods. 2000;96:71–76. doi: 10.1016/s0165-0270(99)00184-3. [DOI] [PubMed] [Google Scholar]
- Johnston LC, Eberling J, Pivirotto P, Hadaczek P, Federoff HJ, Forsayeth J, Bankiewicz K. Clinically relevant effects of AAV2-GDNF on the dopaminergic nigrostriatal pathway in aged rhesus monkeys. Hum. Gene Ther. 2009;20:XXX–XXX. doi: 10.1089/hum.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S, Eberling JL, Bankiewicz KS, Rosenberg D, Coxson PG, VanBrocklin HF, O’Neil JP, Emborg ME, Jagust WJ. 6-[18F]fluoro-l-m-tyrosine: Metabolism, positron emission tomography kinetics, and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine lesions in primates. Brain Res. 1997;750:264–276. doi: 10.1016/s0006-8993(96)01366-2. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Palfi S, Chen EY, Ma SY, Sendera T, Cochran EJ, Mufson EJ, Penn R, Goetz CG, Comella CD. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson’s disease. Ann. Neurol. 1999;46:419–424. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J, III, Gasmi M, Bartus RT. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann. Neurol. 2006;60:706–715. doi: 10.1002/ana.21032. [DOI] [PubMed] [Google Scholar]
- Krauze MT, Saito R, Noble C, Tamas M, Bringas J, Park JW, Berger MS, Bankiewicz K. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J. Neurosurg. 2005;103:923–929. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, Stoessl AJ, Olanow CW, Bartus RT. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: An open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- Maswood N, Grondin R, Zhang Z, Stanford JA, Surgener SP, Gash DM, Gerhardt GA. Effects of chronic intraputamenal infusion of glial cell line-derived neurotrophic factor (GDNF) in aged rhesus monkeys. Neurobiol. Aging. 2002;23:881–889. doi: 10.1016/s0197-4580(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ, Iwaki Y, Colosi P. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- Namavari M, Satyamurthy N, Phelps M, Barrio J. Synthesis of 6-[18F] and 4-[18F]fluoro-l-meta-tyrosines via regioselective radiofluorodestannylation. Appl. Radiat. Isot. 1993;44:527. doi: 10.1016/0969-8043(93)90165-7. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr, Lozano AM, Penn RD, Simpson RK, Jr, Stacy M, Wooten GF. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- Oiwa Y, Eberling JL, Nagy D, Pivirotto P, Emborg ME, Bankiewicz KS. Overlesioned hemiparkinsonian non human primate model: Correlation between clinical, neurochemical and histochemical changes. Front. Biosci. 2003;8:a155–a166. doi: 10.2741/1104. [DOI] [PubMed] [Google Scholar]
- O’Neil JP, VanBrocklin HF. Development of a semi-remote system for the routine production of [18F]6-fluoro-l-meta-tyrosine to study striatal lesions and therapeutic implants in primates. J. Lab. Comp. Radiopharm. 1995;37:655–657. [Google Scholar]
- Palfi S. Towards gene therapy for Parkinson’s disease. Lancet Neurol. 2008;7:375–376. doi: 10.1016/S1474-4422(08)70066-8. [DOI] [PubMed] [Google Scholar]
- Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: A two-year outcome study. Ann. Neurol. 2005;57:298–302. doi: 10.1002/ana.20374. [DOI] [PubMed] [Google Scholar]
- Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Research on Parkinson disease. J. Neurosurg. 2005;102:401. [PubMed] [Google Scholar]
- Slevin JT, Gash DM, Smith CD, Gerhardt GA, Kryscio R, Chebrolu H, Walton A, Wagner R, Young AB. Unilateral intraputaminal glial cell line-derived neurotrophic factor in patients with Parkinson disease: Response to 1 year each of treatment and withdrawal. Neurosurg. Focus. 2006;20:E1. doi: 10.3171/foc.2006.20.5.2. [DOI] [PubMed] [Google Scholar]
- Wright JF, Qu G, Tang C, Sommer JM. Recombinant adeno-associated virus: Formulation challenges and strategies for a gene therapy vector. Curr. Opin. Drug Discov. Dev. 2003;6:174–178. [PubMed] [Google Scholar]