Abstract
Cell therapy has tremendous potential for the damaged heart, which has limited self-renewing capability. Bone marrow (BM) cells are attractive for cell therapy, as they contain diverse stem and progenitor cell populations that can give rise to various cell types, including cardiomyocytes, endothelial cells, and smooth muscle cells. Studies have shown BM cells to be safe and efficacious in the treatment of myocardial infarction. Possible therapeutic mechanisms mediated by both host and transplanted cells include cardiomyogenesis, neovascularization, and attenuation of adverse remodeling. In this review, different stem and progenitor cells in the bone marrow and their application in cell therapy are reviewed, and evidence for their therapeutic mechanisms is discussed
Introduction
Heart failure is a global health problem (109). In the United States, 5.3 million people have heart failure, and the prevalence of heart failure in people older than 65 reaches 1% (84). One of the major causes of heart failure is myocardial infarction (MI), which affects 920 thousand Americans every year and is a dominant cause of death in the United States. About 22% of male and 46% of female patients with MI will be disabled with heart failure within 6 years of MI. As the heart has limited regeneration capacity, lost cardiac muscle by MI is replaced by scar tissue, leading to progressive ventricular remodeling and congestive heart failure (76). Despite many breakthroughs in cardiovascular medicine, current therapies for heart failure are of limited benefit in regaining lost cardiac function.
Recently, cell therapy has emerged as an attractive therapeutic modality to repair damaged myocardium. Initial studies using committed myogenic cells, such as skeletal myoblasts (30, 50, 63) or fetal/neonatal cardiomyocytes (51, 52, 90), after MI have shown benefit in restoring lost heart function. The recent identification of various bone marrow (BM)-derived adult stem or progenitor cells capable of contributing to tissue regeneration has opened the possibility that these cells can be used to repair damaged heart tissue. Although BM transplantation has long been used in the treatment of hematologic diseases, BM also has stem/progenitor cells that can give rise to nonhematopoietic cells, including neural cells (12, 57), skeletal myoblasts (18, 23, 45), cardiomyocytes (29, 69), endothelium (4, 29, 69), and epithelium of lung, gut, and skin (44). The nonhematologic plasticity of BM cells in humans has further been supported by observations that BM cells can be detected in the brain (104) and heart (11, 46, 58, 79) as fully differentiated brain and cardiac cells after BM or heart transplantation.
Various BM cells have been examined for their ability to repair myocardial damage, and initial animal and limited human studies to date have shown therapeutic benefit. Controversy exists about the fate of transplanted cells and the therapeutic mechanism of BM cell therapy in the heart. In this review, we cover the various types of BM cells used to treat myocardial damage and their therapeutic effect in animal experiments and human clinical studies. We also review the mechanisms underlying the beneficial effect of BM cells on ischemic heart diseases.
Stem and Progenitor Cells in Bone Marrow
Before discussion of stem and progenitor cells in BM, we outline the difference between a stem cell and a progenitor cell. A stem cell is a cell that can self-renew and also can give rise to differentiated progeny, whereas a progenitor cell is a generic term for a dividing cell that can differentiate without evidence of self-renewing capability (95).
Endothelial progenitor cells
The development of the vascular system consists of two processes: vasculogenesis and angiogenesis. Vasculogenesis refers to the de novo formation of blood vessels from endothelial progenitor cells (EPCs) or angioblasts that differentiate into endothelial cells (ECs) (83), whereas angiogenesis is extension of preexisting vasculature by the sprouting of new capillaries through migration and proliferation of ECs (14). Until recently, vasculogenesis was thought to be restricted to embryonic development, whereas angiogenesis was considered to be responsible for neovascularization in embryos and adults. Now, postnatal vasculogenesis has been reported with the discovery of BM-derived EPCs, which circulate in adult peripheral blood (5), home to ischemic tissue, and incorporate into foci of neovascularization (6), leading to de novo blood vessel formation to augment oxygen and nutrient delivery to the ischemic tissue.
Diversity of EPC phenotypes
The definition of EPCs is complicated by the scarcity of specific or unique markers for EPCs. It is now apparent that different subsets of peripheral or BM-derived cells, including HSCs, monocytes, and circulating ECs, can differentiate into endothelium-like cells. BM-derived circulating EPCs (CEPCs) in the adult peripheral blood express a subset of HSC markers (43, 92). Human CEPCs can be identified as cells expressing CD34, CD133, and vascular endothelial growth factor receptor-2 (VEGFR-2) markers (20, 73, 92). CD133 (AC 133) is expressed in the immature HSCs, which can differentiate into endothelium-like cells involved in neovascularization but is absent in the mature ECs (20, 73). Furthermore, CD34+/VEGFR-2+ cells, expressing CXCR4 chemokine receptor, likely reflect immature progenitor cells (106). Ex vivo expanded human peripheral blood-derived EPCs expressed various EC markers, such as CD31, CD34, KDR, and VE-cadherin, bind lectins, and uptake Dil-acetylated low-density lipoprotein. The transplantation of these cells into ischemic tissue augments neovascularization with the incorporation of transplanted cells into new vessels as ECs (35). The uncertainty of definition of EPCs has been emphasized by the endothelial differentiation capacity of BM or peripheral blood-derived CD14+ cells or monocytes (80, 88, 103). Several studies suggested the overlap between endothelial phenotypes and monocytes, indicating that traditional endothelium-specific markers such as CD31, CD105, CD144, CD34, Tie-2, VEGFR-2, acetylated low-density lipoprotein uptake, and Ulex europaeus agglutinin-1 binding cannot specifically distinguish between EPCs and monocytes (80, 88, 114).
The origin of EPCs is further obscured by the two distinctive types of EPCs arising from different culture methods (53). “Early EPCs” are derived mainly from mononuclear cells or monocytes and do not proliferate after a few weeks (22, 27, 35, 37, 61, 80). Conversely, “Late EPCs” form colonies after >2 weeks in culture, have a cobblestone morphology, and rapidly proliferate (27, 115). The distinctive characteristics of these two types of EPCs are reinforced by the different expression of cell-surface markers. Early EPCs express panleukocyte and monocytic/macrophage markers such as CD45, CD11b, and CD14, whereas late EPCs do not. Early EPCs are also therapeutically effective in vivo, whereas evidence for therapeutic efficacy of late EPCs is limited (27, 115).
Recently, human adult peripheral blood (PB)- and umbilical cord blood (CB)-derived endothelial colony-forming cells (ECFCs) with high proliferative potential, which have almost identical characteristics to outgrowth endothelial cells (OECs) or late EPCs, were reported. The isolation method for ECFCs is similar to those reported for OECs. For culturing ECFCs, PB MNCs or CB MNCs are harvested and seeded on collagen type I-coated dishes, cultured for 1 day, nonadherent cells removed, and only adherent cells cultured (28, 114). Colonies of ECFCs emerge between 5 and 22 days after plating as monolayers of cobblestone-appearing ECs. ECFCs can form de novo vessels in vitro and have a phenotype indistinguishable from that of cultured ECs. However, their direct differentiation into ECs in vivo was demonstrated only by epifluorescent microscopy but not confirmed by rigorous imaging technologies with confocal imaging or in situ hybridization. Because of their similarities to fully differentiated ECs, their participation into new vessel formation in vivo is still questionable.
Controversies on the transdifferentiation potential of EPCs in vivo
Transdifferentiation of EPCs and whether this transdifferentiation plays a main role in the therapeutic benefit of EPCs in recovering cardiovascular function is controversial. Several recent studies demonstrated transdifferentiation of human peripheral blood CD34+ cells into endothelial lineage cells with incorporation into blood vessels (75, 112). However, other investigators claim that BM-derived cells do not transdifferentiate or incorporate into vessel walls (66, 118). These discrepancies may be due to the difference in cell types, the use of different animal models used to look for transdifferentiation, or the application of less or more rigorous criteria to define transdifferentiation. Considering these controversies, more-sophisticated methods with rigorous criteria, such as z-stacked confocal imaging and long-term in vivo tracking, are needed to elucidate the role of transdifferentiation of EPCs or more fundamentally to address the nature and identity of EPCs. Therapeutic mechanisms of EPCs in cell therapy in vivo also remain to be better characterized.
Hematopoietic stem cells
A hematopoietic stem cell (HSC) is a cell that can self-renew and give rise to all blood cells. The transplantation of a single HSC can constitute all hematopoietic cells in an organism, and a HSC fulfills the requirements for being a stem cell (i.e., self-renewal capacity and potential to generate differentiated progeny). The use of HSCs to repair an infarcted myocardium in a murine model has been investigated (69). Recent studies have suggested the possibility that specific stem cell populations derived from murine BM could regenerate most of the key elements of myocardium and ameliorate ischemic cardiac dysfunction. The more-versatile potential of BM-derived HSCs has been documented with the use of side-population cells (cells characterized by their ability to expel Hoechst dye) (21) and with lineage-negative, c-kit-positive cells (cells that express a specific protein known as a tyrosine kinase, which adds phosphate groups to the amino acid tyrosine in other proteins; Lin-c-kit+) (68); both of which differentiated into ECs, vascular smooth muscle cells (SMCs), and cardiomyocytes in a murine MI model (29, 69). However, other studies suggested that HSCs do not readily acquire a cardiac phenotype in the injured heart, despite the improvement in ventricular function in the HSC-transplanted group (62). These studies were all performed with murine HSCs, and currently no studies have been performed with the human counterpart of these stem cells.
In terms of clinical application, the lack of these HSCs in BM and the unavailability of culture-expansion techniques may limit the therapeutic application of HSCs in ischemic heart diseases.
Mesenchymal stem cells
Nonhematopoietic mesenchymal stem cells (MSCs) are a common source of adult stem cells. They can be isolated from various tissues, have high proliferation capability, and are easy to collect. BM-derived MSCs have shown multilineage differentiation capacity (78). Both in vivo and in vitro studies have demonstrated that MSCs can undergo cardiomyogenic differentiation (91, 100, 107). This cardiomyogenic differentiation capacity was demonstrated with both human and animal MSCs. However, differentiation of MSCs into ECs has not yet been demonstrated clearly. Paracrine effects of MSCs have also been demonstrated. Media from MSC cultures exert therapeutic effects similar to those of direct MSC therapy, suggesting that paracrine effects via released cytokines may play a major therapeutic role in MSC therapy. Also, another group demonstrated that, in a model of murine hindlimb ischemia, MSC transplantation enhanced tissue repair via secretion of multiple cytokines, including vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), and placental growth factor (PlGF), rather than incorporation of MSCs into new or remodeling tissues (39-41).
Others reported that MSC viability can be enhanced with gene transfection [heme oxygenase-1 plasmid and adenoviral Akt (71)] with greater therapeutic effect on the injured myocardium. Recently, it was reported that direct MSC injection by using catheter is safe, reduces fibrosis, and augments myocardial regeneration (3). Another group demonstrated that MSC treatment decreases myocardial scar and improves cardiovasculogenesis (32).
The advantage of MSCs for potential clinical application lies in the ease of acquisition and isolation, high expansion capability in culture, and low immunogenicity. The low immunogenicity can render MSCs more-attractive candidate cells for treatment of MI because of their capability for modulating inflammatory reactions in acute MI and the utility of the allogeneic approach (reviewed in ref. 59). This extensive capacity for clinical scale expansion in vitro has facilitated the development of preclinical models as well as clinical studies designed to assess the safety, feasibility, and efficacy of MSC transplantation in a variety of diseases. However, the use of animal serum for culture expansion restricts the growth of pilot clinical trials with MSCs in humans.
Other multipotent bone marrow-derived stem and progenitor cells
A novel adult stem cell population, BM-derived multipotent stem cells (BMSCs), was clonally isolated from human and rat, beginning at the single-cell level from mixed total BM cell culture (117). Clonally isolated BMSCs exhibited unlimited self-renewal in culture for >140 population doublings and differentiated into three germ layers (endoderm, mesoderm, neuroectoderm). None of the characteristic marker panels (e.g., CD34, CD44, CD90, CD105, and CD117) defining HSCs, MSCs, and multipotent adult progenitor cells (MAPCs) match the profile of human BMSCs. The minimal expression of surface molecules appears to be a prerequisite for the plasticity of human BMSCs, as shown in other adult multipotent stem cells. The characteristics of BMSCs that distinguishes them from HSCs include absence of HSC marker expression, ex vivo expansion with an adherent culture system, and differentiation potential to give rise to three germlayer cells. BMSCs are also distinct from MSCs because of a different expression pattern of surface markers, ex vivo culture expansion on fibronectin-coated dishes, and the potential to differentiate into endodermal and ectodermal cells. Direct transplantation of human BMSCs into acutely infarcted myocardium attenuated cardiac dysfunction both by de novo differentiation into heart tissue, such as cardiomyocytes, ECs, and SMCs, and by paracrine (noncellular differentiation mechanisms) effects of transplanted cells. Furthermore, engrafted human BMSCs have been demonstrated to provoke proliferation of host myocardial cells and prevent apoptosis of host myocardial cells after ischemic injury, suggesting that endogenous angio/arteriogenesis and cardiomyogenesis could occur after stem cell transplantation (49, 117). It seems that augmenting cell-to-cell contact and multiple paracrine effects could lead to a higher degree of endogenous cardiomyogenesis and angiogenesis, as well as the differentiation of local stem cells.
The potential advantages of human BMSCs with respect to their potential for treating cardiovascular diseases include their culture expandability, the higher engraftment rate in ischemic and infarcted myocardium, and the demonstrated multifunctional capacity for cardiovascular tissue regeneration. Conversely, the difficulty of culturing techniques and the use of large quantities of animal serum are some of the hurdles that must still be overcome before entering the clinic.
A subpopulation of BM cells referred to as MAPCs has been isolated and shown to possess the most versatile potential of adult stem cells to date. MAPCs can be clonally expanded, proliferate indefinitely without obvious genetic instability, and differentiate into cells of all three embryonic germ layers in vitro (33, 81, 82, 89). When they are injected into blastocysts, chimerism can be detected in most if not all somatic tissues. On transplantation into a non-irradiated or minimally irradiated host, MAPCs engraft and differentiate into the hematopoietic lineage, as well as other lineages. MAPCs are distinguished from MSCs and BMSCs by the expression of embryonic stem cell markers such as Oct4, the use of multiple cytokines for cultivation, and the requirement of leukemia inhibitory factor for ex vivo expansion of murine MAPCs. However, MAPCs showed minimal engraftment into the myocardium after being injected into the embryonic blastocysts. The therapeutic, engraftment, and differentiation potentials of MAPCs are controversial in the study of cardiovascular disease models (74). Furthermore, the requirement of multiple expensive cytokines to culture MAPCs and the difficulty of culture techniques renders them less attractive for clinical use.
Therapeutic Effect of BM Cells: Animal and Human Studies
Our group and others investigated the effects of EPCs in a rat model of MI. EPCs expanded ex vivo and systemically administered after MI incorporate into foci of myocardial neovascularization and have a favorable impact on preservation of left ventricular function. Direct intramyocardial injection of human CD34+ cells has also been shown to induce new blood-vessel formation in the infarct vascular bed and to promote proliferation of preexisting vasculature after experimental MI of rats (37, 38, 43).
Other simplified approaches using unselected (19) or selected BM or peripheral blood mononuclear cells fractions (36, 93), based on the premise that BM or peripheral blood cells would include multiple cytokines and stem/progenitor cell populations, which could augment neovascularization in ischemic tissue, were reported to be successful in improving cardiac function after various models of myocardial ischemia. Our group, however, found potential adverse effects after adult BM cells transplantation (116). We observed unexpected severe intramyocardial calcification after direct transplantation of syngeneic unselected BM cells into the infarct myocardium (116). One of the possible contributing factors for the calcification may be the harsh local milieu in the infarcted myocardium that favors the deposition of calcium. Furthermore, uncontrolled (trans)differentiation of multipotent stem and progenitor cells and proliferation of osteoblasts in the injected cells can be another possible mechanism. However, the exact mechanism of this calcification remains to be elucidated.
The successful results from initial experimental reports promoted the initiation of clinical studies. Several randomized control studies with different cell types (unselected BM cells, BM mononuclear cells, cultured EPCs), disease states (acute MI, chronic heart failure), and route of delivery (intracoronary delivery, injection during bypass surgery, intramyocardial injection) showed mixed results [reviewed in (85)], probably owing to these differences in study parameters. A recent meta-analysis based on the data from randomized control trials of BM cell treatment for acute MI revealed mild, but statistically significant improvement of heart function over control groups and potential safety of the treatment (55) and proposed the necessity for further study to determine the optimal cell type, dose, and long-term outcome. Taken together, these results suggest the possibility of BM cell therapy as an adjunct to reperfusion therapy for acute MI.
However, in contrast to the favorable outcome of BM cell therapy, a recent multicenter, randomized, placebo-controlled, double-blind study showed that transfer of autologous skeletal myoblasts (non-BM cells) to the patient with left ventricular dysfunction did not improve heart function, but increased arrhythmic events, warranting further investigation with myoblasts (56).
Mechanism of the Therapeutic Effect
In this section, we address the mechanisms of beneficial effects of BM-derived cells from two points of view: the role of BM cells or host tissue or both, and the pathophysiology of myocardial damage and regeneration. First, we describe the function of transplanted BM cells and host tissue cells. The role of transplanted cells will include transdifferentiation into or fusion with cardiac cells and their paracrine effect. Then, we address the pathophysiologic mechanisms underlying the myocardial damage and regeneration by BM cells.
The role of BM cells
Transdifferentiation versus fusion
Transdifferentiation
The loss of cardiac cells with resultant functional decline of the heart is a key pathogenic component of MI. Thus, the regeneration of lost cardiac cells by differentiation of BM cells into cardiac cells has been a major goal of cell therapy for ischemic heart disease. Initially, two groups reported the transdifferentiation of hematopoietic cells from BM into cardiomyocytes, ECs, and SMCs in an experimental model of MI (29, 69, 70). However, these results were called into question by other groups who reported that HSCs adopt mature hematopoietic fates in ischemic myocardium without transdifferentiation into cardiac cells (9, 62). The discrepancy in the results may arise partly from the different methods used to determine the fate of transplanted cells, as Murry et al. (62) suggested: whereas Orlic et al. (68) used fluorochrome-conjugated antibodies that detect proteins specific to certain types of cardiac cells, Murry et al. created intrinsic genetic markers, such as a cardiomyocyte-specific promoter linked to β-galactosidase reporter, which can be recognized without antibody staining. However, the results of Balsam et al. (9) are hard to reconcile with those of Orlic et al. because the Balsam group was not able to reproduce the experiments of the Orlic group by using similar methods. Differences in anesthesia or surgical techniques might have contributed to the discrepancies. In summary, transdifferentiation of HSCs into cardiomyocytes in vivo is currently controversial.
BM-derived MSCs have also been reported to differentiate into cardiomyocytes in vitro and in vivo. For example, bromodeoxyuridine (BrdU)-labeled rat MSCs (101), DiI-labeled swine MSCs (91), and β-galactosidase (LacZ)-expressing adenovirus-infected human MSCs (100) differentiate into cardiomyocytes, as suggested by the expression of cardiomyocyte-specific proteins in vivo. Furthermore, cardiomyocytes can be generated from BM-derived MSCs in vitro (24, 54). However, as in HSCs, the differentiation of MSCs into cardiomyocytes has recently been challenged (16). BMSCs (117) and MAPCs (33) are BM-derived stem cells that have also been studied in cardiac regeneration. BMSCs differentiate into cardiac cells, including cardiomyocytes, ECs, and SMCs in vitro and in vivo (117). MAPCs also differentiate into cardiomyocytes in vitro. However, in vivo differentiation of MAPCs in the setting of myocardial injury has not been reported because of the inability to detect it 1 week after transplantation (74). Peripheral blood-derived human adult EPCs differentiate into functional cardiomyocytes in vitro when cocultured with paraformaldehyde-fixed cardiomyocytes (8). However, in vivo cardiac transdifferentiation of EPCs has not been demonstrated.
Fusion
Most or all of these reports that BM cells can undergo differentiation into cardiac cells are based on the following approach: donor cells are prelabeled with genetic markers [green fluorescent protein (GFP) or LacZ] or chemical dyes (DiI or BrdU), transplanted into a recipient host, and tracked by these markers (Table 1). Differentiation is defined as co-expression of these tracking markers with concurrent expression of specific cell markers.
Table 1.
The Studies Addressing the Transdifferentiation of Bone Marrow Cells into Cardiac Cells
|
Differentiation into cardiac cells |
||||||||
|---|---|---|---|---|---|---|---|---|
| Type of bone marrow cells | Injection method | Identification of donor cells | Methods to identify cardiac cells | Cardiomyocytes | Endothelial cells | Smooth muscle cells | Functional improvement | References |
| Side population cells (mouse) | BMTa | Rosa26_β-galactosidase | IHCb stain | 0.02% | 3.30% | - | Yes | (29) |
| Lin-c-kit+ cells (mouse) | Intramyocardial injection | EGFPc and Y chromosome | 53±9% | 44±6% | 49±7% | Yes | (69) | |
| Mobilization of bone marrow cells | None | - | - | - | - | Yes | (70) | |
| Lin-c-kit+ or LSKd cells (mouse) | Intramyocardial injection | Cardiac-specific α-myosin heavy chain promoter_EGFPc | Expression of cardiomyocyte-specific promoter, IHCb staining | 0 | - | - | - | (62) |
| Cardiac-specific α-myosin heavy chain promoter_β-galactosidase | ||||||||
| β-actin-EGFPc | ||||||||
| LSKd cells, Thy1.1low LSKd cells (mouse) | Intramyocardial injection, parabiotic mice | β-Actin-EGFPc (C57BL=Ka-Thy-1.1) | IHCb staining | 0 | 0 | 0 | Yes | (9) |
| Whole bone marrow cells, LSKd CD45+ cells | Intramyocardial injection, BMT* | β-Actin-EGFPc | IHCb stainingI | Intramyocardial injection: 0 | - | - | - | (64) |
| α-Actin-EGFPc | Cytokine-induced bone marrow cell mobilization: Yes but rare (0.75% of all EGFP+ cells and 0.0065% of all cardiomyocytes) | |||||||
| Rat bone marrow cells cultured in the presence of 5-azacytidine | Intramyocardial injection | BrdU labeling during culture (autologous) | IHCb staining | Yes, but not quantified | Yes, but not quantified | - | Yes | (101) |
| Human MSCse | Intraventricular injection | Adenovirus carrying β-galactosidase transfection | IHCb staining | Yes, but not quantified | - | - | - | (100) |
| Autologous swine MSCse | Intramyocardial injection | DiI labeling | IHCb staining | Yes, but not quantified | - | - | Yes | (91) |
| Autologous rat MSCse | Intramyocardial injection | DAPI labeling | IHCb staining | No | Yes, but not quantified | Yes, but not quantified | Yes | (16) |
| Human BMSCsf | Intramyocardial injection | DiI labeling | IHCb staining | Yes, but not quantified | Yes, but not quantified | Yes, but not quantified | Yes | (117) |
| Murine MAPCsg | Intramyocardial injection | ROSA26_β-galactosidase | IHCb staining | No | No | No | Yes | (74) |
BMT, bone marrow transplantation
IHC, immunohistochemical
EGFP, enhanced green fluorescent protein
LSK, Lin-Sca-1+c-Kit+
MSCs, mesenchymal stem cells
BMSCs, bone marrow-derived multipotent stem cells
MAPCs, multipotent adult progenitor cells
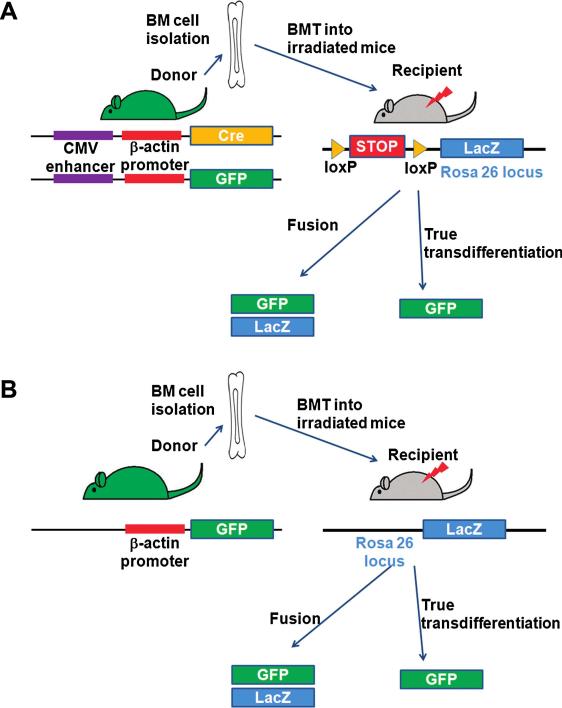
However, this approach cannot rule out the fusion of the BM-labeled cells with host cells (Fig. 1). The phenomenon of fusion as a mechanism for apparent differentiation was first demonstrated by two groups when somatic cells such as BM mononuclear cells (99) and brain cells (113) spontaneously fused with embryonic stem cells in vitro with subsequent epigenetic reprogramming of the somatic cells into embryonic stem cells. These results were reproduced in vivo by using state-of-the-art genetic tools, which demonstrated fusion, not true transdifferentiation, as the main mechanism of cardiomyocyte generation from BM cells (2) (Fig. 2A). Nigren et al. (64) also tested the transdifferentiation of BM cells into cardiomyocytes by using various approaches including direct injection of hematopoietic cells and cytokine-induced mobilization of BM cells with cardiac-restricted (α-actin promoter) and constitutively active (β-actin promoter) enhanced GFP (EGFP)/Cre recombinase-expressing donors and floxed LacZ-expressing recipients (64) (Fig. 2B). They found that cytokine mobilization, but not direct intramyocardial injection of BM cells generated cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. In contrast to BM cells, however, stem and progenitor cells derived from the heart can undergo true differentiation into cardiomyocytes in vivo without cell fusion (10, 67, 77). The lack of evidence for true transdifferentiation of BM cells into cardiomyocytes without fusion is also compatible with the results of two studies showing that cell fusion is the principal source of BM-derived hepatocytes (105, 108). Recent studies demonstrated the fusion of BM cells with hepatocytes with resultant restoration of liver function in a mouse model of liver disease (47, 105). The smaller magnitude of heterotypic fusion between BM cells and cardiomyocytes suggests that fusion may not be a main mechanism of therapeutic effects for BM-derived cells for the heart. It has not been demonstrated that BM cells can make cardiomyocytes without fusion, even though the plasticity of BM cells for other types of cells, such as hepatocytes and epithelial cells in the lung, liver, and skin, has been reported without cell fusion (25, 31).
FIG. 1. A method to distinguish differentiation from fusion in coculture system.
DiI-labeled bone marrow-derived multipotent stem cells (BMSCs) and carboxyfluorescein diacetate succinimidyl ester (CFDA-SE)-labeled neonatal rat cardiomyocytes (CFDA-SE-NRCM) are cocultured under direct contact and stained with cardiac troponin I (cTnI) to identify cardiomyocytes. Cells double-positive for DiI and cTnI are regarded to differentiate into cardiomyocytes without fusion, and those triple-positive for DiI, cTnI, and CFDA-SE are fused BMSCs with cardiomyocytes. D, differentiation; F, fusion. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 2. Methods to determine cell fusion or transdifferentiation from BM cells.
(A) Bone marrow (BM) cells are isolated from mice with constitutive Cre recombinase (Cre) and green fluorescent protein (GFP) protein expression and transplanted into lethally irradiated mice in which a stop cassette is floxed before the β-galactosidase (LacZ) reporter gene. Fused cells express LacZ via Cre-mediated recombination of floxed stop cassette and also express GFP, whereas transdifferentiated cells express only GFP. Donor cells express only GFP, whereas recipient cells express neither GFP nor LacZ. (B) BM cells are isolated from mice with constitutive GFP expression and transplanted into lethally irradiated mice with constitutive LacZ expression. Fused cells express both GFP and LacZ, and true transdifferentiation cells express only GFP. Donor cells express only GFP, and recipient cells express only LacZ. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Recent studies show that injuries or inflammation of the tissue induces the BM cells to form heterotypic-fusion hybrids with cardiomyocytes, skeletal muscle, hepatocytes, and Purkinje neurons (34, 65). However, the functional implication of the fusion of BM cells with cardiomyocytes has not been elucidated.
Lack of evidence for direct contribution of BM cells to fully functional cardiomyogenesis
As functional cardiomyocytes contract in an electromechanically orchestrated manner, the expression of cardiomyocyte-specific markers and fusion with recipient cardiomyocytes by bone marrow cells are not enough to prove the direct contribution of BM cells to the generation of functional cardiomyocytes. The evidence of electromechanical integration of the transplanted cells into the host myocardium is required to prove that the transplanted cells undergo phenotypic change into functional cardiomyocytes.
After transplantation, fetal or neonatal cardiomyocytes have been demonstrated to be electromechanically integrated into the normal heart, as evidenced by intravital imaging studies (71, 86, 119). Rubart et al. (86) demonstrated synchronous calcium transients in adjacent engrafted fetal and adult host cardiomyocytes by using multiphoton microscopy, and Zimmermann et al. (119) showed the electromagnetic integration of the transplanted neonatal cardiomyocytes in the infracted heart. Furthermore, Ott et al. (71) made beating hearts by using neonatal cardiac cells and decellularized matrix.
In contrast to fetal and neonatal cardiomyocytes, however, no convincing evidence for electrical or mechanical activation and integration of transdifferentiated or fused transplanted BM-derived cells has been reported, suggesting that the direct contribution by BM cells to fully functional cardiomyocytes remains unproven.
Paracrine effect
Therapeutic effects of BM-derived cell therapy on ischemic heart correlate poorly with generation of new cardiomyocytes, either by fusion or by transdifferentiation. Accordingly, paracrine effects have been proposed as a dominant mechanism of beneficial effect of BM cells. All BM cells that have been shown to be effective in the treatment of myocardial damage also secrete various angiogenic factors. Fuchs et al. (19) reported that BM cells released factors such as VEGF and monocyte chemotactic protein-1 (MCP-1), which increased the proliferation of host ECs in a dose-dependent manner. Kamihata et al. (36) showed that BM-derived MNCs express FGF-2 ≫ VEGF > angiopoietin-1 and that intramyocardiac injection of BM-derived MNCs increased cardiac levels of these angiogenic factors. Kinnaird et al. (39) showed that MSCs expressed wide array of arteriogenic cytokines, with hypoxic stress as one stimulus. MSCs in culture secreted VEGF, FGF-2, PlGF, and MCP-1 into the medium, and this conditioned medium enhanced proliferation of ECs and SMCs in a dose-dependent manner in vitro. The injection of the MSC-conditioned media also augmented the recovery of ischemic hindlimb in vivo. Cultured EPCs secrete VEGF, hepatocyte growth factor (HGF), granulocyte-colony-stimulating factor, and granulocyte-monocytes colony-stimulating factor in vitro (80), and intramyocardiac EPC transplantation induced prolonged expression of these factors in the ischemic heart. Overall, paracrine effects of BM-derived cells promote angiogenesis (19, 36), prevent the apoptosis of cardiac cells (43, 102), and activate resident cardiac stem/progenitor cells (15).They may also be the dominant therapeutic mechanism of BM-derived cell therapy.
The role of host tissue cells
Paracrine effect
The role of host tissue on the beneficial effect by BM cells was reported in an ischemic hindlimb model. Tateno et al. (97) treated 29 patients with ischemic lower-limb disease with implantation of peripheral mononuclear cells. Twenty-one of 29 patients’ conditions were ameliorated, whereas the remaining eight patients did not show a response to the treatment. The comparison of the responders with nonresponders disclosed that the plasma levels of C-reactive protein, interleukin-1β (IL-1β), interleukin-6 (IL-6), and VEGF were higher in the responders. C-reactive protein is an inflammatory biomarker (111), and IL-1β, IL-6, and VEGF are angiogenic and inflammatory cytokines.
To determine the role of IL-1β, they transplanted wild-type mononuclear cells into IL-1β-deficient mice, and IL-1β-deficient mononuclear cells, into wild-type mice. The implantation of IL-1β-deficient mononuclear cells improved the limb ischemia as efficiently as that of wild-type cells by increasing IL-1β in the ischemic tissue. However, transplantation of wild-type mononuclear cells into IL-1β-deficient mice failed to induce neovascularization, suggesting that IL-1β from host tissue plays a role in cell therapy-induced neovascularization.
Recently, our group clearly addressed the critical role of host tissue for sustained humoral effect of cell therapy in an MI model (15). We transplanted human EPCs into the ischemic myocardium of athymic mice and observed that most of transplanted cells disappeared from the heart within a week. However, EPC transplantation upregulated the levels of multiple cytokines associated with angiogenesis, antiapoptosis, cell proliferation, and BM cell mobilization for at least 2 weeks in the heart tissue.
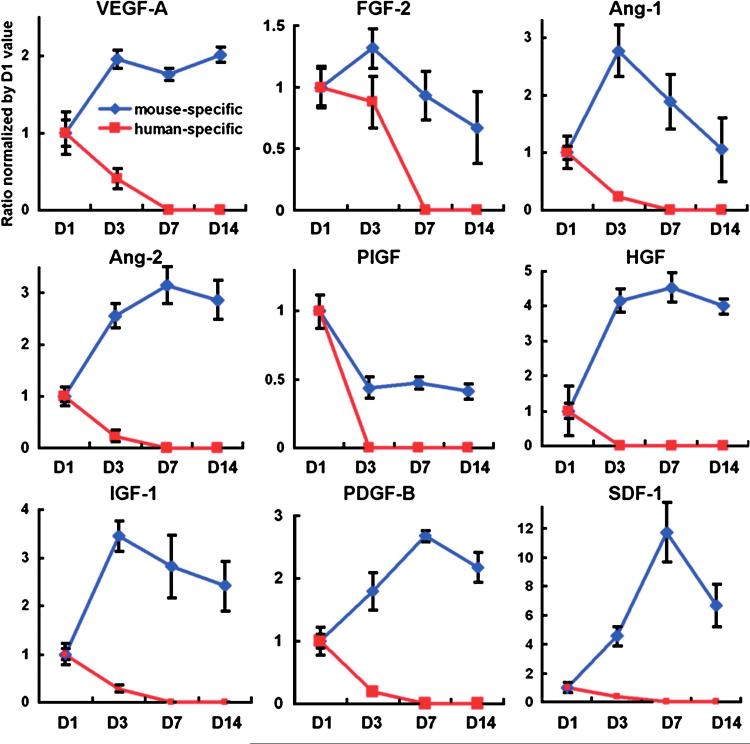
To explore the origin of the beneficial cytokine production, human and mouse specific primers and probes for each cytokine were designed. Real-time reverse transcription-polymerase chain reaction with these primers and probes showed that the expression levels of cytokines from the transplanted human cells were rapidly decreased to undetectable range within a week, whereas those from host (mouse) cells were increased 1 day after the transplantation and maintained over a 2-week period (Fig. 3), suggesting that sustained upregulation of beneficial cytokines was derived from host cells, but not from the transplanted cells (Fig. 4). This study further suggests that patients with severely damaged hearts or peripheral muscles may not properly respond to BM cell therapy.
FIG. 3. The sustained upregulation of the humoral factors in ischemic myocardium after cell transplantation is attributable to host cells.
To determine whether the upregulated cytokines after cell transplantation into infarcted myocardium were derived from injected donor cells or recipient host cells, human EPCs were transplanted into immunocompromised nude mice, and the levels of cytokines were measured by both human- and mouse-specific primers and probes for each cytokine, such as VEGF or FGF-2. The expression levels of cytokines from human EPCs (donor cells) were at their highest levels at day 1 and decreased to an undetectable range within 7 days. Most of the mouse (host)-specific cytokine levels continued to increase after day 1 and were maintained at higher than the baseline levels over a 14-day period (n=5 per each time point). Individual values were normalized to GAPDH and shown as fold difference from the values at day 1. VEGF-A, vascular endothelial growth factor-A; FGF-2, fibroblast growth factor-2; Ang-1, angiopoietin-1; Ang-2, angiopoietin-2; PlGF; placental growth factor; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor-1; PDGF-B, platelet-derived growth factor, B polypeptide; SDF-1, stromal cell-derived factor-1. Originally published in Journal of Experimental Medicine (DOI: 10.1084/JEM20070166; ref. 15). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
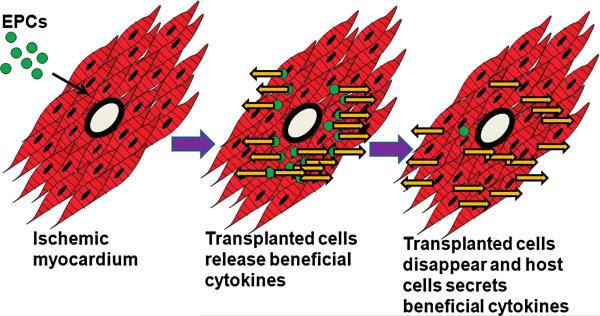
FIG. 4. The role of host cells for sustained paracrine or humoral effects in ischemic myocardium after EPC transplantation.
When endothelial progenitor cells (EPCs) are transplanted into ischemic myocardium, the transplanted cells release beneficial biologic factors in the beginning. However, over the 2-week period, the transplanted cells gradually disappeared, and thus the expression level of the factors decreased. In the later phase, the host cells that have been stimulated by the transplanted cells, secrete beneficial cytokines, and maintain the humoral effects >2 weeks. Yellow arrows, the secretion of beneficial cytokines. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Activation of endogenous cardiomyocytes
In addition to the secretion of beneficial cytokines, BM-derived cell therapy may activate endogenous cardiac progenitors to repair damaged heart tissue. The presence of endogenous cardiac stem and progenitor cells has been reported by several groups (10, 26, 48, 67, 77). Hierlihy et al. (26) first reported the presence of stem cell-like populations of cells from the heart by using their ability to efflux fluorescent dye, Hoechst 33342. Stem cells capable of excluding Hoechst were first identified by the Goodell group (21) as HSCs from BM, and they were termed side population (SP) cells. Since then, the Hoechst-exclusion method has been used to identify tissue-specific stem progenitor cells in other tissues, including skeletal muscle, lung, liver, heart, testis, kidney, skin, brain, and mammary gland, as well as heart [reviewed in (21)].
Coculture of cardiac SP cells with cardiomyocytes induced differentiation of SP cells into cardiomyocytes with the biochemical and electromechanical phenotype of cardiomyocytes in vitro (77). When transplanted into injured heart, the cardiac SP cells underwent differentiation into cardiac cells, including cardiomyocytes, ECs, and SMCs (72). Yamahara et al. (110) recently reported that cardiac SP cells are heterogeneous and include ECs, SMCs, mesenchymal stem/progenitor cells, and potentially cardiomyogenic cells. Pfister et al. (77) added CD31-Sca 1+ as another selection marker to obtain more enriched cardiomyogenic cells out of SP cells. For another type of cardiac stem cells, Beltrami et al. (10) isolated c-kit+ cells from the adult rat hearts and showed that these cells can be clonally expanded in vitro and undergo differentiation into cardiac cells, including cardiomyocytes, ECs, and SMCs both in vitro and in vivo. Two additional cardiac progenitor cells were identified by using other stem cell markers such as Sca-1 (67) and LIM-homeodomain transcription factor islet-1 (isl1) (48). As all these cardiac progenitor cells appear to be distinct from each other, the heart may have more than one endogenous progenitor population.
However, despite the presence of these resident progenitor cells, the function of these cells appears to be limited, as the majority of damaged cardiac tissue is replaced by noncontractile scar tissue. Several explanations for the restricted regenerative function of endogenous cardiac stem and progenitor cells are possible. The harsh microenvironment in the damaged heart may inhibit proliferation, migration, and differentiation of endogenous cardiac stem and progenitor cells or damage the immature cardiac cells differentiated from cardiac stem and progenitor cells. Additionally, this highly inflammatory milieu may enhance the proliferation of the scar-forming fibroblasts. However, the exact mechanism underlying the limited function of cardiac stem and progenitor cells remains to be explored.
It may be speculated that the favorable effects of BM cells might arise from either the augmentation of the regenerative capacity of these endogenous cardiac progenitor cells or the addition of new cardiac progenitor cells. In support of this hypothesis, our group showed that the transplantation of BMSCs increased BrdU- and Ki67-positive host ECs and cardiomyocytes, suggesting augmented angiogenesis and endogenous cardiomyogenesis (117). Mouquet et al. (77) also reported that mobilized BM cells contribute to cardiac SP cells after MI.
The pathophysiology of myocardial regeneration by BM cells
Muscular regeneration or repair?
As loss of contractile cardiac tissue plays a crucial role in aggravation of heart function after MI, the replacement of necrotic/scarred myocardium with viable new muscle is important in the treatment of ischemic heart diseases with large infarctions. Initial studies using cultured fetal or neonatal cardiomyocytes provided evidence for the possibility of muscular regeneration by showing that the transplanted cells were stably engrafted into the myocardium and formed cardiac tissue (51). The proof-of-concept of muscular regeneration by transplantation of cultured cardiomyocytes was further supported by recent studies demonstrating that transplanted cardiomyocytes can form contractile myocardial tissue with electromechanical integrity with adjacent host cardiac tissue (86, 119). However, it likely will not be feasible for BM cells, either through transdifferentiation or fusion, to generate a significant mass of cardiomyocytes, given the low rate of differentiation of BM cells into cardiomyocytes. From current evidence of low engraftment, it is possible but also unlikely that inducing cardiomyogenesis from endogenous cardiac stem/progenitor cells by BM cell therapy may yield a clinically meaningful cardiac mass.
Prevention of adverse remodeling
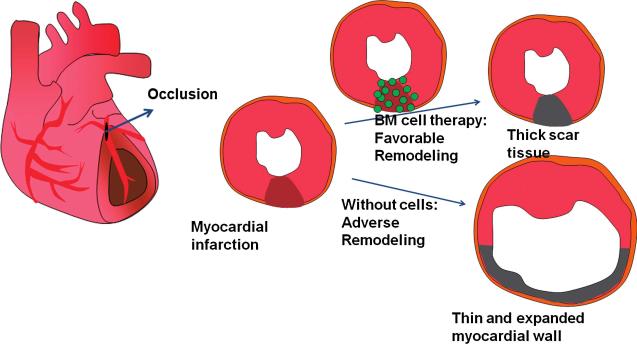
After MI, the injured wall becomes thin, and the ventricular cavity dilates over time, leading to a deterioration of heart function. This postinfarct remodeling is attenuated by β-blockers and angiotensin-converting enzyme inhibitors without muscular regeneration. The remodeling of the heart after myocardial damage has been suggested to be attenuated by cell therapy by using cardiomyocytes (17, 60, 90), skeletal myoblasts (7, 30, 98), and BM-derived cells (43, 91) (Fig. 5). Early neovascularization induced by transplantation of BM-derived cells prevented late myocardial remodeling by salvage of intrinsic cardiomyocytes (43). Furthermore, MSC-transplanted hearts had much thicker infarct scars and reduced ventricular dilation, suggesting that MSC therapy preserves heart function by prevention of remodeling (91).
FIG. 5. BM cell therapy attenuates the adverse remodeling of the heart after myocardial infarction.
Myocardial infarction is caused by occlusion of coronary arteries that supply oxygen and nutrients to the myocardium. In myocardial infarction without bone marrow (BM) cell transplantation, the damaged myocardium undergoes adverse remodeling, in which myocardial walls undergo thinning and the cardiac size expands over time, leading to dilative cardiac failure. When BM cells are applied, the ischemic myocardium in the border zone is partly salvaged by multiple cardioprotective effects of BM cells, which mitigate the thinning of infarcted wall and the expansion of the infarcted segment. This eventually attenuates cardiac-chamber dilatation and cardiac dysfunction. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Vascular regeneration through angiogenesis
In MI, most cells in the infarct center undergo necrosis, whereas cells in the periinfarct area survive. However, periinfarct cells are exposed to prolonged ischemia and can undergo apoptosis over time, leading to additional loss of contractile tissue and progressive dysfunction of the heart (13). Therapeutic intervention that can enhance the blood flow to the chronically ischemic cardiac cells may rescue endangered cardiomyocytes (1) (Fig. 6), enhance the electrical stability, and prevent infarct remodeling (87). All types of BM cells used for cardiac cell therapy have been shown to increase the number of blood vessels in the myocardium and ischemic hindlimb, including BM-derived mononuclear cells (36, 42, 96), EPCs (38), and MSCs (94).
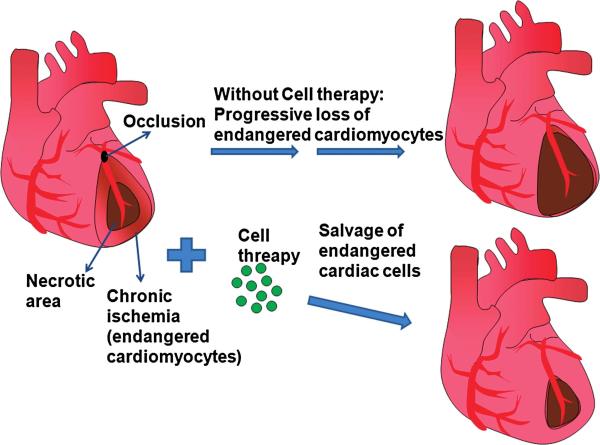
FIG. 6. Neovascularization induced by BM cells salvages the endangered cardiac cells in the ischemic area.
Myocardial infarction is caused by occlusion of coronary arteries, which supply oxygen and nutrients to the myocardium. The core of the affected area undergoes acute necrosis due to severe deprivation of oxygen and nutrients such as glucose, whereas the surrounding area of the necrotic core has relatively mild, but chronic ischemia. Without cell therapy, progressive loss of the endangered cardiomyocytes of the surrounding area occurs over time. However, when bone marrow (BM) cells are applied to the affected myocardium, the endangered cardiac cells are salvaged from further loss. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
In contrast to BM cells, fully differentiated mature ECs were not reported to improve neovascularization after ischemia (35, 43). At present, angiogenic effects of BM cells are more likely to explain the benefits of cell therapy rather than vasculogenic effects (differentiation into ECs).
Conclusion: Open Questions and Closing Perspectives
Progress in myocardial regeneration by using BM-derived cells has been rapid, with pilot clinical trials already showing the safety and efficacy of BM cell therapy in ischemic tissues. However, several key questions remain unanswered, and new techniques must be developed to advance this field. First, methods of cell delivery must be optimized. Despite >10 years of work in the field, many parameters have yet to be optimized, including the route of injection, the number of cells, the types of cells, and the timing of cell delivery. Second, mechanisms of the beneficial effects of cell therapy should be further elucidated (Fig. 7). Even though one plausible mechanism of BM cell therapy is the revascularization of the ischemic tissue, the involved molecular mechanisms have yet to be determined. Third, development of therapeutic modalities to induce both neovascularization and cardiomyogenesis are required, as both processes are intimately related in adult heart remodeling and regeneration. Neovascularization without cardiomyogenesis would lead mainly to revascularization of noncontractile scar tissue, even though some benefit may be derived from revascularization of periinfarct cardiomyocytes. Cardiomyogenesis without neovascularization would lead to apoptosis or necrosis of newly generated cardiomyocytes.
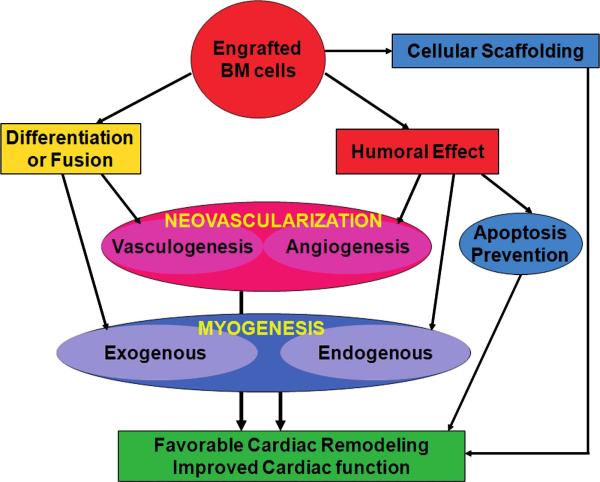
FIG. 7. Potential multimodal mechanisms underlying the therapeutic effect of BM cells in myocardial damage.
After transplantation, the engrafted bone marrow (BM)-derived stem or progenitor cells may undergo differentiation into or fuse with endothelial cells or cardiomyocytes, leading to vasculogenesis or exogenous myogenesis. Moreover, the BM cells can exert humoral effects by secreting various biologic factors, which induce angiogenesis (growth of preexisting vessels), endogenous myogenesis (proliferation of host cardiomyocytes), and prevention of apoptosis of endangered myocardial cells. Furthermore, the engrafted BM cells may serve as cellular scaffolds. These multimodal effects (i.e., neovascularization, myogenesis, prevention of apoptosis, and cellular scaffolding) work together and induce favorable cardiac remodeling and improvement of cardiac function. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Notwithstanding the remaining questions and other possible obstacles in the development of more effective and safe therapeutic strategies, BM-derived cell therapy holds enormous potential to ameliorate the health of many patients.
Acknowledgments
This work was supported in part by National Institutes of Health grants (HL079137, HL084471), Juvenile Diabetic Research Foundation Innovation Grant (5-2007-951), and a grant (SC4300) from Stem Cell Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, Republic of Korea.
Abbreviations
- acLDL
Acetylated low-density lipoprotein
- Ang-1
angiopoietin-1
- Ang-2
angiopoietin-2
- BM
bone marrow
- BMSCs
bone marrow-derived multipotent stem cells
- BrdU
bromodeoxyuridine
- CB
cord blood
- CEPCs
circulating endothelial progenitor cells
- CFDA-SE
carboxyfluorescein diacetate succinimidyl ester
- Cre
Cre recombinase
- cTnI
cardiac troponin I
- D
differentiation
- ECs
endothelial cells
- ECFCs
endothelial colony-forming cells
- EGFP
enhanced green fluorescent protein
- EPCs
endothelial progenitor cells
- F
fusion
- FGF-2
fibroblast growth factor-2
- GFP
green fluorescent protein
- HSCs
hematopoietic stem cells
- IGF-1
insulin-like growth factor-1
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- LacZ
β-galactosidase
- MCP-1
monocyte chemotactic protein-1
- MI
myocardial infarction
- MSCs
mesenchymal stem cells
- NRCMs
neonatal rat cardiomyocytes
- OECs
outgrowth endothelial cells
- PB
peripheral blood
- PDGF-B
platelet-derived growth factor, B polypeptide
- PlGF
placental growth factor
- SDF-1
stromal cell-derived factor-1
- SMCs
smooth muscle cells
- SP
side population
- UEA-1
Ulex europaeus agglutinin-1
- VEGF
vascular endothelial growth factor
- VEGFR-2
vascular endothelial growth factor receptor-2
References
- 1.Abbate A, Bussani R, Biondi-Zoccai GG, Rossiello R, Silvestri F, Baldi F, Biasucci LM, Baldi A. Persistent infarct-related artery occlusion is associated with an increased myocardial apoptosis at postmortem examination in humans late after an acute myocardial infarction. Circulation. 2002;106:1051–1054. doi: 10.1161/01.cir.0000030936.97158.c4. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 3.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkins BZ, Hueman MT, Meuchel J, Hutcheson KA, Glower DD, Taylor DA. Cellular cardiomyoplasty improves diastolic properties of injured heart. J Surg Res. 1999;85:234–242. doi: 10.1006/jsre.1999.5681. [DOI] [PubMed] [Google Scholar]
- 8.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 9.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 10.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 11.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 12.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 13.Bussani R, Abbate A, Biondi-Zoccai GG, Dobrina A, Leone AM, Camilot D, Di Marino MP, Baldi F, Silvestri F, Biasucci LM, Baldi A. Right ventricular dilatation after left ventricular acute myocardial infarction is predictive of extremely high peri-infarctual apoptosis at postmortem examination in humans. J Clin Pathol. 2003;56:672–676. doi: 10.1136/jcp.56.9.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 15.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Herve P, Etievent JP, Kantelip JP. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108(suppl 1):II253–II258. doi: 10.1161/01.cir.0000089186.09692.fa. [DOI] [PubMed] [Google Scholar]
- 17.Etzion S, Battler A, Barbash IM, Cagnano E, Zarin P, Granot Y, Kedes LH, Kloner RA, Leor J. Influence of embryonic cardiomyocyte transplantation on the progression of heart failure in a rat model of extensive myocardial infarction. J Mol Cell Cardiol. 2001;33:1321–1330. doi: 10.1006/jmcc.2000.1391. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, Weissman NJ, Leon MB, Epstein SE, Kornowski R. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37:1726–1732. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 20.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from ac133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 21.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulati R, Jevremovic D, Peterson TE, Witt TA, Kleppe LS, Mueske CS, Lerman A, Vile RG, Simari RD. Autologous culture-modified mononuclear cells confer vascular protection after arterial injury. Circulation. 2003;108:1520–1526. doi: 10.1161/01.CIR.0000089084.48655.49. [DOI] [PubMed] [Google Scholar]
- 23.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 24.Hakuno D, Fukuda K, Makino S, Konishi F, Tomita Y, Manabe T, Suzuki Y, Umezawa A, Ogawa S. Bone marrow-derived regenerated cardiomyocytes (cmg cells) express functional adrenergic and muscarinic receptors. Circulation. 2002;105:380–386. doi: 10.1161/hc0302.102593. [DOI] [PubMed] [Google Scholar]
- 25.Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004;305:90–93. doi: 10.1126/science.1098925. [DOI] [PubMed] [Google Scholar]
- 26.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–243. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 27.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 28.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 29.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain M, DerSimonian H, Brenner DA, Ngoy S, Teller P, Edge AS, Zawadzka A, Wetzel K, Sawyer DB, Colucci WS, Apstein CS, Liao R. Cell therapy attenuates deleterious ventricular remodeling and improves cardiac performance after myocardial infarction. Circulation. 2001;103:1920–1927. doi: 10.1161/01.cir.103.14.1920. [DOI] [PubMed] [Google Scholar]
- 31.Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- 32.Jaquet K, Krause KT, Denschel J, Faessler P, Nauerz M, Geidel S, Boczor S, Lange C, Stute N, Zander A, Kuck KH. Reduction of myocardial scar size after implantation of mesenchymal stem cells in rats: what is the mechanism? Stem Cells Dev. 2005;14:299–309. doi: 10.1089/scd.2005.14.299. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 34.Johansson CB, Youssef S, Koleckar K, Holbrook C, Doyonnas R, Corbel SY, Steinman L, Rossi FM, Blau HM. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol. 2008;10:575–583. doi: 10.1038/ncb1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 37.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 38.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, Hanley A, Silver M, Kearney M, Losordo DW, Isner JM, Asahara T. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 39.Kinnaird T, Stabile E, Burnett MS, Epstein SE. Bone-marrow-derived cells for enhancing collateral development: mechanisms, animal data, and initial clinical experiences. Circ Res. 2004;95:354–363. doi: 10.1161/01.RES.0000137878.26174.66. [DOI] [PubMed] [Google Scholar]
- 40.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 41.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi T, Hamano K, Li TS, Katoh T, Kobayashi S, Matsuzaki M, Esato K. Enhancement of angiogenesis by the implantation of self bone marrow cells in a rat ischemic heart model. J Surg Res. 2000;89:189–195. doi: 10.1006/jsre.2000.5828. [DOI] [PubMed] [Google Scholar]
- 43.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 44.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 45.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 46.Laflamme MA, Myerson D, Saffitz JE, Murry CE. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res. 2002;90:634–640. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- 47.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 48.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee N, Thorne T, Losordo DW, Yoon YS. Repair of ischemic heart disease with novel bone marrow-derived multipotent stem cells. Cell Cycle. 2005;4:861–864. doi: 10.4161/cc.4.7.1799. [DOI] [PubMed] [Google Scholar]
- 50.Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci U S A. 2003;100:7808–7811. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leor J, Patterson M, Quinones MJ, Kedes LH, Kloner RA. Transplantation of fetal myocardial tissue into the infarcted myocardium of rat: a potential method for repair of infarcted myocardium? Circulation. 1996;94:II332–II336. [PubMed] [Google Scholar]
- 52.Li RK, Jia ZQ, Weisel RD, Mickle DA, Zhang J, Mohabeer MK, Rao V, Ivanov J. Cardiomyocyte transplantation improves heart function. Ann Thorac Surg. 1996;62:654–660. doi: 10.1016/s0003-4975(96)00389-x. discussion 660-651. [DOI] [PubMed] [Google Scholar]
- 53.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 56.Menasche P, Alfieri O, Janssens S, McKenna W, Reich-enspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagege AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 57.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 58.Minami E, Laflamme MA, Saffitz JE, Murry CE. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;112:2951–2958. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- 59.Mishra PK. Bone marrow-derived mesenchymal stem cells for treatment of heart failure: is it all paracrine actions and immunomodulation? J Cardiovasc Med (Hagerstown) 2008;9:122–128. doi: 10.2459/JCM.0b013e32820588f0. [DOI] [PubMed] [Google Scholar]
- 60.Muller-Ehmsen J, Peterson KL, Kedes L, Whittaker P, Dow JS, Long TI, Laird PW, Kloner RA. Rebuilding a damaged heart: long-term survival of transplanted neonatal rat cardiomyocytes after myocardial infarction and effect on cardiac function. Circulation. 2002;105:1720–1726. doi: 10.1161/01.cir.0000013782.76324.92. [DOI] [PubMed] [Google Scholar]
- 61.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 63.Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996;98:2512–2523. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 65.Nygren JM, Liuba K, Breitbach M, Stott S, Thoren L, Roell W, Geisen C, Sasse P, Kirik D, Bjorklund A, Nerlov C, Fleischmann BK, Jovinge S, Jacobsen SE. Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nat Cell Biol. 2008;10:584–592. doi: 10.1038/ncb1721. [DOI] [PubMed] [Google Scholar]
- 66.O’Neill TJT, Wamhoff BR, Owens GK, Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res. 2005;97:1027–1035. doi: 10.1161/01.RES.0000189259.69645.25. [DOI] [PubMed] [Google Scholar]
- 67.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orlic D, Fischer R, Nishikawa S, Nienhuis AW, Bodine DM. Purification and characterization of heterogeneous pluripotent hematopoietic stem cell populations expressing high levels of c-kit receptor. Blood. 1993;82:762–770. [PubMed] [Google Scholar]
- 69.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 70.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 72.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, Yasuda N, Akazawa H, Uezumi A, Takeda S, Komuro I. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–341. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of vegfr-2 and ac133 by circulating human cd34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 74.Pelacho B, Nakamura Y, Zhang J, Ross J, Heremans Y, Nelson-Holte M, Lemke B, Hagenbrock J, Jiang Y, Prosper F, Luttun A, Verfaillie CM. Multipotent adult progenitor cell transplantation increases vascularity and improves left ventricular function after myocardial infarction. J Tissue Eng Regen Med. 2007;1:51–59. doi: 10.1002/term.7. [DOI] [PubMed] [Google Scholar]
- 75.Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein B, Kinzler KW, Lengauer C. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 76.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 77.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. Cd31- but not Cd31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 78.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 79.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 80.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 81.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 83.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 84.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart Disease and Stroke Statistics: 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 85.Rosenzweig A. Cardiac cell therapy: mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 86.Rubart M, Pasumarthi KB, Nakajima H, Soonpaa MH, Nakajima HO, Field LJ. Physiological coupling of donor and host cardiomyocytes after cellular transplantation. Circ Res. 2003;92:1217–1224. doi: 10.1161/01.RES.0000075089.39335.8C. [DOI] [PubMed] [Google Scholar]
- 87.Sadanandan S, Hochman JS. Early reperfusion, late reperfusion, and the open artery hypothesis: an overview. Prog Cardiovasc Dis. 2000;42:397–404. [PubMed] [Google Scholar]
- 88.Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J, Strasser RH, Daniel WG. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;49:671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scorsin M, Hagege A, Vilquin JT, Fiszman M, Marotte F, Samuel JL, Rappaport L, Schwartz K, Menasche P. Comparison of the effects of fetal cardiomyocyte and skeletal myoblast transplantation on postinfarction left ventricular function. J Thorac Cardiovasc Surg. 2000;119:1169–1175. doi: 10.1067/mtc.2000.104865. [DOI] [PubMed] [Google Scholar]
- 91.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. discussion 1926. [DOI] [PubMed] [Google Scholar]
- 92.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 93.Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001;103:897–903. doi: 10.1161/01.cir.103.6.897. [DOI] [PubMed] [Google Scholar]
- 94.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 95.Smith A. A glossary for stem-cell biology. Nature. 2006;441:1060. [Google Scholar]
- 96.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291:H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 97.Tateno K, Minamino T, Toko H, Akazawa H, Shimizu N, Takeda S, Kunieda T, Miyauchi H, Oyama T, Matsuura K, Nishi J, Kobayashi Y, Nagai T, Kuwabara Y, Iwakura Y, Nomura F, Saito Y, Komuro I. Critical roles of muscle-secreted angiogenic factors in therapeutic neovascularization. Circ Res. 2006;98:1194–1202. doi: 10.1161/01.RES.0000219901.13974.15. [DOI] [PubMed] [Google Scholar]
- 98.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 99.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 100.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 101.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–II256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 102.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 103.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 104.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 106.Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, Aicher A, Heeschen C, Fichtlscherer S, Zeiher AM, Dimmeler S. Impaired cxcr4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97:1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- 107.Wang JS, Shum-Tim D, Galipeau J, Chedrawy E, Eliopoulos N, Chiu RC. Marrow stromal cells for cellular cardiomyoplasty: feasibility and potential clinical advantages. J Thorac Cardiovasc Surg. 2000;120:999–1005. doi: 10.1067/mtc.2000.110250. [DOI] [PubMed] [Google Scholar]
- 108.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 109.World Health Organization . The World Health Report 2007. World Health Organization; Jun 6, 2008. online. http://www.who.int/whr/2007/en/index.html. [Google Scholar]
- 110.Yamahara K, Fukushima S, Coppen SR, Felkin LE, Varela-Carver A, Barton PJ, Yacoub MH, Suzuki K. Heterogeneic nature of adult cardiac side population cells. Biochem Biophys Res Commun. 2008;371:615–620. doi: 10.1016/j.bbrc.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 111.Yeh ET. Crp as a mediator of disease. Circulation. 2004;109:II11–II14. doi: 10.1161/01.CIR.0000129507.12719.80. [DOI] [PubMed] [Google Scholar]
- 112.Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood cd34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 113.Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 114.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 116.Yoon YS, Park JS, Tkebuchava T, Luedeman C, Losordo DW. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109:3154–3157. doi: 10.1161/01.CIR.0000134696.08436.65. [DOI] [PubMed] [Google Scholar]
- 117.Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, Hanley A, Scadova H, Qin G, Cha DH, Johnson KL, Aikawa R, Asahara T, Losordo DW. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 119.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]