Abstract
The murine primordial follicle pool develops largely within 3 days after birth through germline nest breakdown and enclosure of oocytes within pre-granulosa cells. The mechanisms that trigger primordial follicle formation are likely influenced by a transition from the maternal to fetal hormonal milieu at the time of birth. High levels of maternal estrogen maintain intact germline nest in fetal ovary and decrease of estrogen after birth is permissive to follicle formation. In the present study, we measured an increase in neonatal serum follicle-stimulating hormone (FSH), which corresponded to falling estradiol (E2) levels during the critical window of primordial follicle formation (postnatal days 1-3). To determine whether fetal hormones contribute in an active manner to primordial follicle formation, 17.5 days post coitus (dpc) mouse fetal ovaries were cultured in vitro in two concentrations of E2 (levels meant to reflect maternal and fetal levels of E2) and FSH for six days. High levels of E2 (10-6 M) inhibited germline nest breakdown and this effect was significantly reduced when fetal ovaries were cultured in low E2 concentration (10-10 M). FSH facilitated germline nest breakdown and primordial follicle formation under both high and low E2 culture conditions. Low E2 was identified as being more permissive for the effects of FSH on primordial follicle formation by stimulating the up-regulation of Fshr and activin betaA subunit expression, pre-granulosa cell proliferation, and oocyte survival. The decrease of E2 plus FSH after birth are critical for primordial follicle formation and the expression of oocyte-specific transcription factors (Figla and Nobox), in that inappropriate expose to the FSH or E2 during follicle formation resulted in premature or delayed primordial folliculogenesis. In conclusion, with the drop of E2 level after birth, FSH promotes primordial follicle formation in mice by stimulating local activin signaling pathways and the expression of oocyte-specific transcription factors.
Keywords: Follicle-stimulating hormone, Activin, Ovary, Primordial follicle
Introduction
In females, the primordial follicle pool represents the total population of germ cells available during reproductive life [1]. In the fetal mouse ovary, primordial germ cell numbers increase by mitotic division between 10 and 12.5 days post coitus (dpc), and because of incomplete cytokinesis, the oogonia remain within a germline syncitium or “nest” until the initiation of primordial follicle formation [2]. Although the intrusion of pre-granulosa cell in the nest can be observed at 17.5 dpc [3], the majority of primordial follicle formation occurs within the first 3 days after birth. The pre-granulosa cells initiate terminal cytokinesis and establish connections to the oocyte, forming a primordial follicle. [2]. Most of the oocytes undergo apoptosis during primordial follicle formation, with one third becoming surrounded by pre-granulosa cells [1]. The resulting primordial follicle pool serves as the entire oocyte reserve, also known as the ovarian reserve, and must be restricted into growing and non-growing populations that serve the cyclical needs of the adult animal.
The hormonal and cellular mechanisms that control primordial follicle formation involve steroid and peptide hormones working both as paracrine and endocrine factors together with cell-cell interactions. Previous studies have shown that estrogen and progesterone inhibit germline nest breakdown during primordial follicle formation in vitro and in vivo [4; 5]. In the rodent, levels of estrogen and progesterone in the fetus and neonate are extraordinarily high, but drop precipitously after birth. Primordial follicles are assembled during this same time period [6-8]. Based on these observations, the current model suggests that maternal estrogen and progesterone exert an inhibitory effect on fetal germline nest breakdown and oocyte apoptosis [4]. The drop in estradiol levels after birth relieves the inhibitory action of this steroid on the ovary, and germline nest breakdown proceeds with coordinated oocyte apoptosis and primordial follicle formation.
In mice, follicle-stimulating hormone (FSH) is critical for pre-antral to later stage follicle development [9; 10], while early stage follicle development (primordial to primary stages) is thought to be independent of FSH. This is almost certainly the case in the adult animal, where primordial follicles are held in a relatively dormant state until activated by unknown factors. This physiological observation is supported by the relative paucity of FSHR on small follicles and their lack of blood supply. Less is known about the role of FSH in prepubertal follicle development, and specifically, the role of FSH in the first wave of primordial follicles that activate out of the germline nest. The presence of an appreciable amount of FSH in the serum of rodents and the FSH receptor (FSHR) in the ovary during primordial folliculogenesis has raised occasional questions regarding the role of FSH in this earliest stage of follicle formation [11-13]. This notion remains controversial because primordial follicles are present and can develop to late pre-antral stage in both FSH-deficient and Fshr mutation mouse ovaries, although the size of the primordial follicle pool has not been specifically addressed in these models. On the other hand, increased primordial follicle reserves are found in hypogonadal mice expressing transgenic human FSH [14-17]. These studies suggest that FSH may be involved in mouse primordial follicle formation. Indeed, FSH is important to primordial follicle formation in the hamster. Hamsters that are born to mothers treated with anti-FSH-antibody during gestation have significantly reduced numbers of primordial follicles [11]. Given the significance of the quantity and quality of the initial follicle pool to adult fertility, understanding the role FSH might play in the context of other regulators of ovarian function is an open and intriguing question. Moreover, the formation of follicles in vitro is an emerging area of interest and improving follicle quantity and quality is an important objective. Therefore, optimizing the media (including the addition of FSH) to support folliculogenesis is an underlying objective.
In addition to the hormones, several transcription factors are known to be involved in primordial follicle development; namely, Fig1a and Nobox. Figla knockout mice begin to lose oocytes at birth, which results in the failure of primordial follicle formation, whereas disruption of Nobox causes oocyte loss and a delay in germline nest breakdown in neonates [18, 19]. Moreover, activin A (a homodimer of two beta A subunits) is a peptide hormone secreted by granulosa cells and administration of activin A to neonatal mice increases the primordial follicle pool due to proliferation of oocytes and pre-granulosa cells [8]. Activin is a well-known regulator of follicle maturation and has idiosyncratic effects on the somatic cells that depend on the developmental status of the follicle. During follicle development, activin A is made by selective oogonia within the germ cell nest. Activin represses the expression of membrane-bound kit ligand in the pre-granulosa cells sitting outside the nest. Activin A levels decreases in concert with, and perhaps in anticipation of, nest breakdown which is permissive to membrane bound kit ligand expression in pre-granulosa cells, the ensuing rise of cKit expression in the oocyte, and assembly of a primordial follicle [20]. These factors may be critical downstream targets of hormone action in the neonatal ovary and the timing of hormone addition is critical to normal follicle formation and persistence.
Figure 1. The level of maternal and neonatal serum estradiol (E2) and Follicle-Stimulating Hormone (FSH), and the expression of FSH receptor (Fshr) in the fetal ovary during the time of primordial folliculogenesis.
(A). After birth, neonatal levels of E2 dropped rapidly within 3 days and then increased starting on day 4. In contrast, FSH levels increased dramatically from the day of birth to 3 days after birth. Both E2 and FSH levels increased by 7 days after birth. (B) The expression of FSH receptor mRNA was detectable in the neonatal ovary and increased significantly within the first 3 days after birth. DOB: day of birth; NB: newborn. Letters indicate statistically significant changes between each time point (P<0.05).
In the present study, we examined the effects of hormonal factors, specifically E2 and FSH, on primordial follicle formation. We conclude that FSH can contribute to primordial folliculogenesis in mice, likely in combination with other local factors that have yet to be identified. Moreover, in vitro addition of FSH may facilitate early follicle formation and germ cell quality.
Materials and methods
Animals
CD-1 mice (Harlan, Indianapolis, IN) were maintained in accordance with the policies of Northwestern University's Animal Care and Use Committee. Mice were housed and bred at a constant temperature, humidity and photoperiod (12L, 12D) with food and water available ad libitum. Breeders (8-10 weeks old) were fed soy-free mouse chow (Harlan 7926) to limit exogenous phytoestrogen intake through food. Noon on the day a vaginal plug was found was defined as 0.5 dpc. At the time of delivery, eight pups were kept with each female to minimize differences in pup development caused by nutrient availability.
Hormone Measurements
Blood was collected from pregnant mice on 17.5 dpc and 19.5 dpc, from neonatal mice on the day of birth, and from newborn mice on days 1-7 after birth. The blood samples from neonatal mice were pooled from ten pups. Serum E2 and FSH levels were measured by radioimmunoassay (RIA, Ligand Assay and Analysis Core Laboratory, University of Virginia, Charlottesville, VA).
In vitro Ovary Organ Culture
Fetal ovaries were collected at 17.5 dpc and cultured in drops of media on a 0.4 μm membrane insert (Millicell-CM; Millipore Corp., Bedford, MA) placed in 24-well culture plates with 0.5 ml media in each well. Basal media used for culture was: DMEM-Ham's F-12 media (with L-glutamine, GIBCO BRL, Grand Island, NY) supplemented with 5× ITS (Sigma), 3 mg/ml BSA (MP Biomedicals, Solon, OH), and 0.25 mg/ml bovine fetuin (Sigma) [21]. Fetal ovaries were cultured in a 37°C chamber containing 5% CO2. 17β-estradiol (E2, Sigma-Aldrich, St. Louis, MO) was dissolved in absolute ethanol (Sigma) at a concentration of 0.1 M and then added to the basal culture media to achieve the desired final concentrations. Ethanol was added to media at the same concentration (≤0.1%) to serve as a vehicle control. Recombinant human FSH (Organon, Roseland, NJ) was dissolved in Phosphate Buffered Saline (PBS) at 10 mIU/μl as a stock solution and added to the culture media to achieve a final concentration of 10 mIU/ml. Ovaries were cultured under varying E2 concentrations in the presence or absence of FSH as shown in Table 1, with n=4 ovaries per treatment group.
Table 1. In vitro culture of 17.5 dpc mouse fetal ovaries under various E2 concentrations in the presence or absence of FSH (n=4 ovaries for each group).
| Group | 1st three days culture | 2nd three days culture |
|---|---|---|
| 1 | BM | BM |
| 2 | BM +10-6 M E2 | BM +10-6 M E2 |
| 3 | BM +10-10 M E2 | BM +10-10 M E2 |
| 4 | BM +10 mIU/ml FSH | BM +10 mIU/ml FSH |
| 5 | BM +10-6 M E2+10 mIU/ml FSH | BM +10-6 M E2+10 mIU/ml FSH |
| 6 | BM +10-10 M E2+10 mIU/ml FSH | BM +10-10 M E2+10 mIU/ml FSH |
| 7 | BM +10-6 M E2 | BM +10-6 M E2+10 mIU/ml FSH |
| 8 | BM +10-6 M E2 | BM +10-10 M E2 |
| 9 | BM +10-6 M E2 | BM +10-10 M E2+10 mIU/ml FSH |
| 10 | BM +10-6 M E2 | N/A |
| 11 | BM +10-6 M E2+ 10 mIU/ml FSH | N/A |
| 12 | BM +10-10 M E2 | N/A |
| 13 | BM +10-10 M E2+10 mIU/ml FSH | N/A |
BM: basal medium.
Follicle Counting
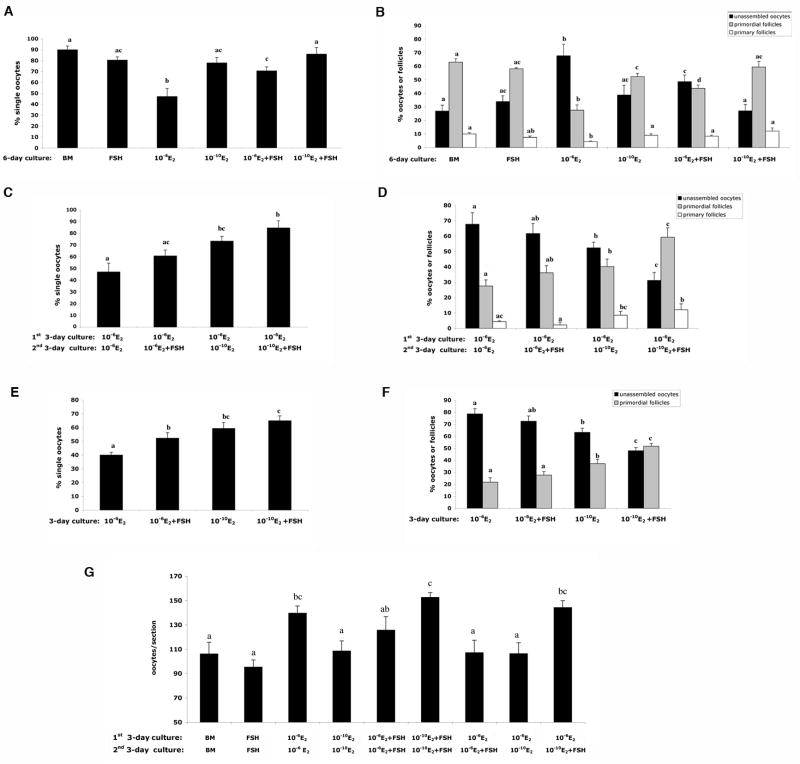
After 6 days in culture, four ovaries from each treatment were fixed in 4% paraformaldehyde for 4 hours then embedded in paraffin. Serial sections were cut at 5 μm and stained with hematoxylin. Two serial sections from the largest cross-section through the center of the each ovary were chosen for follicle/ oocyte counts, and the average was used as the follicle/ oocyte number of one ovary. Follicle counts were repeated in the ovaries from three independent experiments (n=12 ovaries per treatment group). To assess the progression of follicle formation, we counted three different categories of follicles/oocytes in ovarian sections according to the method in pervious study [5]. As shown in Figure 2, the most immature category is the ‘unassembled oocytes ’ that did not surround by pre-granulosa cells completely or oocytes were associated. The second category is “primordial follicles” where one oocyte was surrounded by several flatten pre-granulosa cells. “Primary follicles” were classified by an expanded egg cytoplasm with a complete layer of cuboidal granulose cells. Type B/C primordial follicles (the follicles have single layer of mixed flattened and cuboidal granulosa cells) were counted as primordial follicles. The percentages of three follicle/oocyte categories in each ovary (n=12) were undergone statistical analysis to access the primordial folliculogenesis under each treatment.
Figure 2. The histology of the primordial folliculogenesis in in vitro cultured 17.5 dpc mouse fetal ovaries.
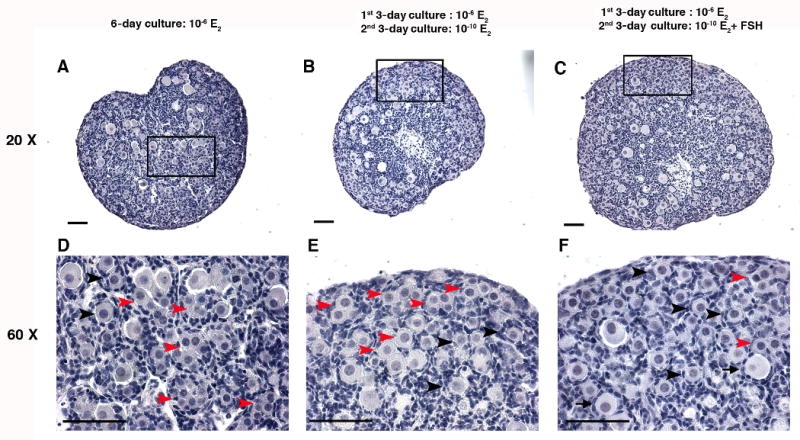
Histology of 17.5 dpc mouse fetal ovaries cultured in vitro in (A) 10−6 M E2 for 6 days; (B) 10-6 M E2 for 1st 3 days then 10-10 M E2 for 2nd 3 days; or (C) 10-6 M E2 for 1st 3 days and 10-10 M E2 plus 10 mIU/ml FSH for 2nd 3 days. (D-F) Higher magnification shows the structure of the unassembled oocytes, primordial follicles and primary follicles and in the ovaries cultured under the three conditions listed in A-C. Red arrowheads indicate unassembled oocytes: oocytes that did not surround by pre-granulosa cells completely or oocytes were associated; black arrowheads indicate primordial follicles: one oocyte was surrounded by several flatten pre-granulosa cells; black arrows indicate primary follicles: one oocyte with expanded cytoplasm surrounded by a complete layer of cuboidal granulosa cells. Bar=50 μm.
Assessment of Proliferating and Apoptotic Cells
After 5 days in culture, four ovaries of the treatment groups 2, 3, 5, 6, 8 and 9 (Table 1) were collected, fixed, embedded, and sectioned as described for follicle counting. Two slides with the largest cross-sections were selected for proliferating cell nuclear antigen (PCNA) staining using a PCNA staining kit (Zymed Laboratories, South San Francisco, CA) and TUNEL staining using the TACS-XL In Situ Apoptosis Detection Kit (R&D, Minneapolis, MN) following the manufacturer's protocols respectively. Sections were then counter stained with hematoxylin. Flattened PCNA positive pre-granulosa cells located around oocytes and the total number of oocytes within the same section was counted to assess the relevant proliferation rate of pre-granulosa cells. TUNEL positive oocytes and total oocytes within the same section were also counted to assess the ratio of apoptotic oocytes (n=12 ovaries per treatment group).
cAMP Enzyme Immunoassay (EIA)
After 5 days of culture, for the ovaries cultured in treatment groups 2, 3, 5, 6, 8 and 9 (Table 1), methylisobutylxanthine (Sigma) was added to the media at a final concentration of 0.5 mM. After incubation for 24 hours, media from different culture groups were collected for measurement of cAMP levels using the Amersham cAMP Biotrak Enzymeimmunoassay System (GE Healthcare, Buckinghamshire, UK). The data are reported as pmol cAMP per ml medium per 24 hours.
RNA Isolation and Real-Time PCR
For treatment groups 2, 3, 5, 6, 8 and 9 (Table 1), after 6 days in culture, total RNA was isolated from ovaries (n=8, ovaries from two experiments) using a Qiagen mini prep RNA isolation kit (Qiagen, Valencia, CA). On-column DNase digestion was performed using an RNase-free DNase kit (Qiagen) to eliminate DNA contamination. 1 μg of total RNA from each of the samples was reverse transcribed with MMV reverse transcriptase (Promega, Madison, WI). Real-time PCR was performed using primers specific for the Fshr, Inhba, Figla, Nobox Msy2 and Gapdh and the Taqman gene expression assay according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). The RNA isolation and PCR were repeated three times independently. The data of the treatment groups (2, 3, 5, 6, 8 and 9) were normalized to data of group 1, in which ovaries were cultured in basal medium.
Statistical Analysis
Data are presented as the mean ± SEM. One-way ANOVA was used to assess the hormone levels in serum, the follicle assembly, total oocyte number, cAMP levels, percentage of PCNA-positive cells, percentage of TUNEL-positive cells and gene expression under different treatments. All statistical calculations were carried out using the PRISM software (GraphPad Software, Inc.). A level of P< 0.05 was considered statistically significant.
Results
Coincident rise of FSH and drop in E2 during primordial follicle formation in neonatal mice
To explore the roles of FSH and E2 in primordial folliculogenesis, hormone levels were measured in pregnant mice at 17.5 dpc, 19.5 dpc and in neonatal mice at birth and up to 7 days after birth. As shown in Figure 1A, maternal E2 levels as well as the E2 levels in neonatal mice at birth were relatively higher and decreased within the first 3 days of birth in the neonates, which coincides with the known timeframe of primordial follicle formation in the mouse ovary. In contrast, neonatal serum FSH increased from the day of birth to neonatal day 7, with a particularly rapid increase from the day of birth to neonatal day 1 that peaked 3 days after birth. By day 7, both E2 and FSH levels were increasing, coincident with gonadotrophin-sensitive steroidogenesis in the mouse ovary [22]. Expression of FSH receptor (Fshr) mRNA was detected in the neonatal ovary over the first 7 days after birth (Figure 1B).
E2-inhibits and FSH reverses E2-dependent germline nest breakdown in vitro
To examine the relative effects of E2 and FSH on germline nest breakdown, ovaries from 17.5 dpc fetal mice were cultured under various concentrations of E2 in the presence or absence of FSH as listed in Table 1. The percentage of the single oocytes relative to total number of oocytes per section were examined to assess germline nest breakdown. Firstly, the inhibitory effect of high concentrations of E2 (10-6 M) on germline nest breakdown was verified in our culture system (Figure 2A, D; Figure 3A). As quantitated in Figure 3A, a significant difference on the percentage of single oocytes was measured between the fetal ovaries cultured in 10−6 M and 10-10 M E2: less single oocytes (47%) were found in the high dose group compared to 78% single oocytes in the low dose groups after 6 days of culture. A statistical difference was already detected after three days of hormone treatment with 40% and 59% of the single oocytes in the high and low dose E2 conditions, respectively (Figure 3E). When ovaries were cultured with high levels of E2 for first three days, then moved to low E2 levels (mimicking the transition from the maternal to fetal estrogen milieu) 73% oocytes were single oocytes indicating the low E2 is more permissive for germline nest breakdown (Figure 3C). Approximately 90% oocytes were single oocytes in the complete absence of E2. So estradiol limits the progression of oocytes out of nests in the perinatal mouse ovary, confirming observations by previous study and us [5; 23].
Figure 3. Primordial follicle formation in mouse fetal ovaries cultured in vitro under various concentrations of E2 in the presence or absence of FSH.
(A, C, E) Percentage of single oocytes in the ovaries of each culture group. (B, D, F) Percentage of unassembled oocytes, primordial follicles and primary follicles in each culture group. (G) Total oocytes in the 17.5 dpc mouse fetal ovary after 6 days of culture in vitro. Letters indicate statistically significant differences between treatment groups (P<0.05).
The addition of FSH was able to partially reverse the inhibitory effects of the high dose E2 (10-6 M E2) on germline nest breakdown. The percentage of single oocytes increased significantly from 47% in high E2 to 73% in high E2 plus FSH after 6 days of culture (Figure 3A), and from 40% to 53% after 3 days culture in the same treatments (10-6 M E2 v.s. 10 −6 M E2 plus FSH, Figure 3E). Although the percentage of single oocytes increased with FSH supplement in low E2 (10-10 M E2) after both 3 days and 6 days of culture, there were no significant differences (Figure 3A, E). Similarly, the addition of FSH with the drop of E2 on the forth day of culture promoted germline nest breakdown with 85% single oocytes compared that (73%) of the ovaries cultured under the decreased E2 on fourth day alone (Figure 3C). Notably, without the decrease of E2, FSH did not reverse the inhibitory effect of high E2 on germline nest breakdown significantly when FSH was supplemented on the fourth day of culture (Figure 3C).
FSH stimulates primordial follicle formation inhibited by E2 in vitro
The percentages of the follicles/oocytes were used to assess the primordial follicle formation in the ovaries cultured in varies E2 and FSH combination. As shown in Figure 3B, After 6 day culture, for the ovaries cultured in high E2, the percentage of primordial follicles increased from 28% (10-6 M E2) to 46% (10-6 M E2 plus FSH), but the addition of FSH on the forth day of culture did not lead to significant increase on primordial follicles compared with that of the ovaries cultured in high E2 for 6 days (Figure 3D). The switch of the E2 level from 10-6 M to 10-10 M on the fourth day of culture promoted the follicle formation significantly with 40% primordial follicles v.s. 28% in the ovaries cultured persistently in 10-6 E2 for 6 days. Most importantly, with the decrease of E2 on the forth day of culture, the addition of FSH at the same time could promote follicle formation significantly with 59% primordial follicles compared with that (36%) of the ovaries cultured under persistent high E2 and the supplement of FSH on fourth day of culture (Figure 3D). During the first 3 days culture, compared with the ovaries cultured in high E2, low E2 resulted in more primordial follicles, and FSH promoted follicle formation further in low E2 culture condition (Figure 3F). Thus, FSH rescued E2 inhibited primordial follicle formation and low E2 facilitated this effect of FSH.
FSH promotes oocyte survival during germline nest breakdown
To determine the oocyte survival rates during primordial folliculogenesis in various E2 and FSH culture conditions, the total number of oocytes per histologic section were counted in ovaries cultured in vitro for 6 days (Table 1, groups 1-9; Figure 3G). High levels of E2 inhibited oocyte loss (total oocytes/section: 140 ± 6). Ovaries cultured in high levels of E2 plus FSH, had a slightly lower total oocyte number (total oocytes/section: 126 ± 11), and total oocyte numbers were even lower in ovaries cultured in low levels of E2 (total oocytes/section: 109 ± 8). However, the addition of FSH appeared to reverse the effects of low E2 on oocyte loss (total oocytes/section: 153 ± 4). The positive effect of FSH on oocyte number was also measured in ovaries cultured in high E2 for 3 days then switched to low E2 and FSH on day 4 (total oocytes/section: 144 ± 6). These data suggest that FSH promotes oocyte survival during primordial follicle formation in in vitro.
FSH regulates pre-granulosa cell proliferation and oocyte apoptosis during in vitro primordial folliculogenesis
Since the ovaries cultured under different E2 and FSH conditions showed obvious changes in total oocyte number and in proportions of primordial follicles, the proliferation of pre-granulosa cells and oocyte apoptosis were examined by PCNA and TUNEL staining in ovaries cultured in vitro under the paradigm established above during the process of primordial follicle formation (Figure 4). More PCNA positive pre-granulosa cells were found in ovaries cultured in 10-10 M E2 compared with 10-6 M E2 (Figure 4E), and the addition of FSH further increased pre-granulosa cell proliferation (Figure 4A, B and E). Notably, although FSH stimulated pre-granulosa cell proliferation regardless of the level of E2 present in the culture media or whether the ovaries had been cultured in high levels of E2 and then switched to FSH-containing media on day 4, low level of E2 with FSH addition induced a higher level of relative pre-granulosa cell proliferation (Figure 4E). Thus, the drop in E2 is permissive to autonomous granulosa cell proliferation. Oocyte apoptosis was higher in ovaries cultured in low levels of E2 than in those cultured in high levels of E2 (Figure 4F), further suggesting that high E2 inhibits germline nest breakdown. Addition of FSH reduced the number of oocytes undergoing apoptosis in ovaries cultured in low E2, but this treatment increased apoptosis in ovaries cultured in high E2 (Figure 4F). Compared with the ovaries culture with the switch form high E2 to low E2 on the forth day of culture, the addition of FSH at the same of time of E2 decrease led to less apoptotic oocytes.
Figure 4. The proliferation of pre-granulosa cells and oocyte apoptosis in 17.5 dpc mouse fetal ovaries after 5 day-culture.
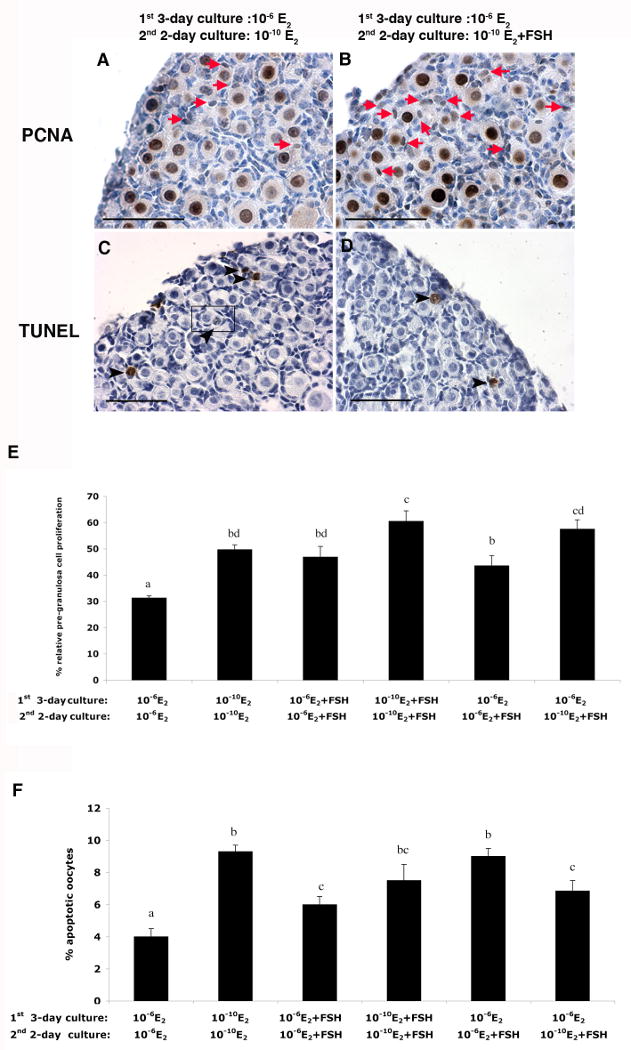
(A) PCNA and (C) TUNEL staining of ovaries cultured in 10-6 M E2 for 3 days and then 10-10 M E2 for 2 days. (B) PCNA and (D) TUNEL staining of ovaries cultured in 10-6 M E2 for 3 days and then 10-10 M E2 plus 10 mIU/ml FSH for 2 days. Red arrows indicate PCNA-positive proliferating pre-granulosa cells; black arrowheads indicate TUNEL-positive apoptotic oocytes; framed areas indicate nuclear pyknosis in germline nests. Bar=50 μm. (E) Percentage of relative pre-granulosa cell proliferation: PCNA positive pre-granulosa cells/total oocytes in 1 section of each ovary cultured under the indicated conditions (n=12). (F) Proportion of apoptotic oocytes: TUNEL positive oocytes/total oocytes in 1 section of each ovary cultured under the indicated conditions (n=12). Letters indicate statistically significant differences between treatment groups (P<0.05).
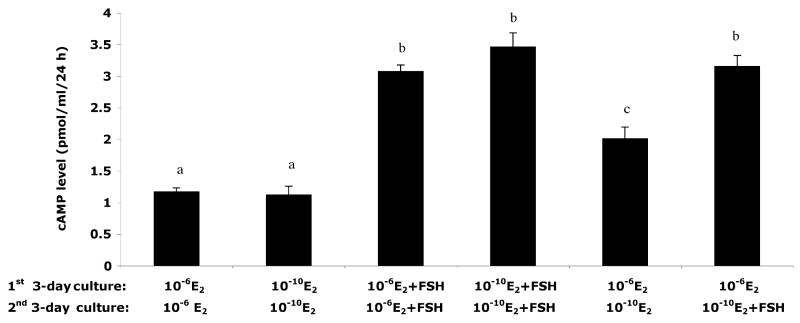
FSH increases cAMP production by in vitro cultured fetal ovaries
Based on the appreciable level of Fshr expression in the ovary after birth and to determine whether FSH-stimulated primordial folliculogenesis during in vitro fetal ovary culture involved the activation of the FSH receptor, the level of cAMP in the media was examined for each treatment group after 6 days of culture (Figure 5). Addition of FSH led to a significant increase in cAMP, regardless of the level of E2 present. A similar effect of FSH on cAMP levels was measured in media taken from ovaries that were switched from high E2 to low E2 on day 4 of culture. Taken together, the addition of FSH to in vitro cultured mouse fetal ovary led to elevated cAMP production, suggesting the FSH receptor is functional.
Figure 5. The stimulatory effect of FSH on the level of cyclic AMP in the media of different culture conditions.
Fetal ovaries were incubated with 0.5 mM methylisobutylxanthine for 24 h in terminal cultures. Compared with control (without FSH), the presence of FSH in culture led to a significant increase in cAMP production. Columns with different letters shows statistical differences (P < 0.05).
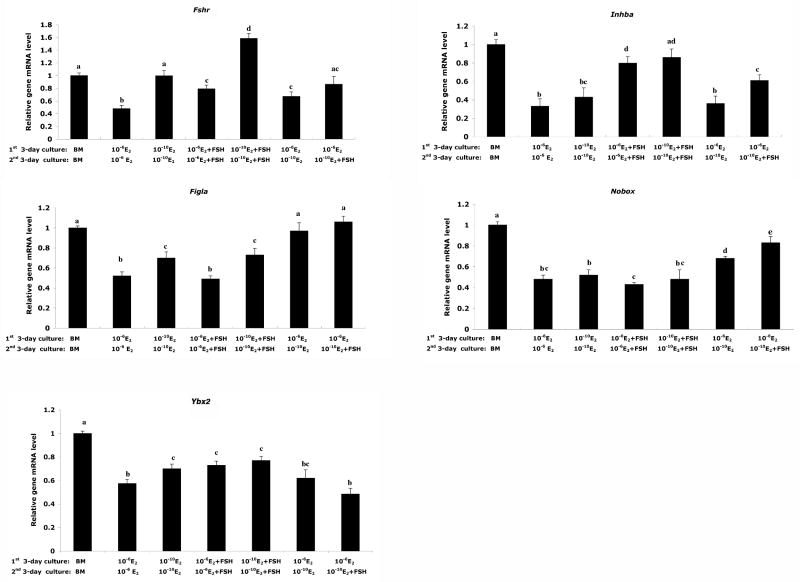
FSH regulates the expression of genes associated with primordial folliculogenesis
To elucidate the signaling factors that may mediate estradiol-mediated FSH-regulated primordial folliculogenesis in vitro, the expression level of five genes known to be involved in primordial folliculogenesis were examined in ovaries cultured under the paradigm described above (Figure 6). Changes in gene expression are shown relative to the levels seen in ovaries cultured in basal medium for 6 days. The expression of Fshr was up-regulated when the ovaries were cultured in low E2 or with FSH addition, but a greater increase was observed in ovaries cultured in low E2 (10-10 M E2) compared with that of ovaries cultured in high E2 plus FSH (10-6 M E2 + FSH), indicating low E2 facilitated the up-regulation of Fshr. The level of Fshr rose about three fold when ovaries were cultured under 10-10 M E2 with FSH simultaneously (Figure 6). The level of activin βA subunit gene (Inhba) increased significantly with FSH supplementation in both 10-6 M and 10-10 M E2 culture conditions, and the lower E2 level slightly enhanced the effect of FSH on the up-regulation of Inhba level (Figure 6). Ovarian expression of two oocyte -specific transcription factors, Figla and Nobox, showed similar patterns. Figla expression was elevated in lower E2 culture conditions and FSH did not promote Figla expression obviously compared to the parallel E2 culture condition (Figure 6). Although Nobox had higher expression level in lower E2 culture conditions, no significant differences were found. Notably, for both Figla and Nobox, the drop of E2 levels on the fourth day of culture (corresponding to day of birth) up-regulated gene expression significantly, and accompanying the drop of E2, the addition of FSH on the fourth day of culture increased the levels of both Figla and Nobox. As the cytoplasmic marker for diplotene oocytes, Msy2 (germ cell-specific Y box protein) had higher expression level in the ovaries cultured in low estradiol, and the presence of FSH promoted Msy2 expression significantly when the ovaries were cultured under high estradiol [19].
Figure 6. The expression of FSH receptor (Fshr), activin beta A subunit (Inhba), Figla, Nobox and Msy2 in in vitro cultured 17.5 dpc mouse fetal ovaries.
Expression levels of Fshr, Inhba, Figla, Nobox and Msy2 in ovaries cultured in vitro under the indicated conditions were measured by real-time PCR. Expression levels are reported relative to those in ovaries cultured in basal medium for 6 days.
Discussion
Primordial follicle formation is a process that involves not only physical interactions between oocytes and somatic cells within the ovary, but also the actions and interactions of various hormones and local growth factors. The in vitro culture system used in the present study permits the evaluation of specific hormones and growth factors on primordial follicle formation. The observed 2-3 fold increase in FSH and drop in E2 after birth that coincides with the known period of primordial folliculogenesis in the mouse motivated us to explore the role of FSH in primordial follicle formation. Based on the evident decrease of E2 level during primordial folliculogenesis in our study, and the dose-dependent effects of E2 on germline cyst breakdown and primordial follicle formation in previous in vitro study [5], 17.5 dpc fetal ovaries were cultured in high level of E2 (10-6 M) and low level of E2 (10-10 M) with or without FSH. Through analyzing primordial follicle formation in the ovaries cultured in various hormone conditions, a role for FSH and E2 in the in vitro formation of primordial follicles was identified.
FSH is an essential stimulus for late-stage follicle development, though early-stage follicle development is thought to be independent of FSH. However, several researchers have documented a role for FSH in fetal and neonatal ovarian development and function in in vitro cultures [24; 25]. Moreover, the notion that primordial follicles are FSH-independent results from work in the adult animal, not at the time of follicle formation. FSH cannot be the only regulator of this process since, as was described earlier, deletion of the ligand or receptor results in primordial follicle formation. Clearly, primordial follicles can be formed without FSH, however, FSH may serve critical roles in timing the exit of oocytes from the nest and stimulating commensurate somatic cell expansion to ensure a correct number of follicles. Moreover, timely gene expression may increase the robustness of the ovarian reserve, which is entirely formed over an exceeding short 3-day window of time. Finally, FSH may serve as a peptide regulator where endocrine disruptors could be more damaging if the process depended solely on steroid action. These ideas must be tested further for their validity in vivo. In vitro, FSH addition contributes to better folliculogenesis, which is an important factor in media formulation for assembly of the primordial follicle pool.
Based on a previous study [26] and our own measurements of maternal and fetal E2 and FSH, we hypothesized that the shift to lower E2 and higher FSH levels that occurs after birth is critical for primordial folliculogenesis. When fetal ovaries were shifted from high to low E2 levels in in vitro culture, germline nest breakdown was evident, but there was a relatively high proportion of unassembled oocytes versus intact primordial follicles. In contrast, when the ovaries were shifted from high to low E2 levels in the presence of FSH during the culture, primordial follicle formation was significantly higher, suggesting an important role for FSH at the time of birth in supporting primordial folliculogenesis. Furthermore, we demonstrated that FSH treatment supported pre-granulosa cell proliferation, which is necessary for primordial follicle assembly and support of the enclosed oocytes.
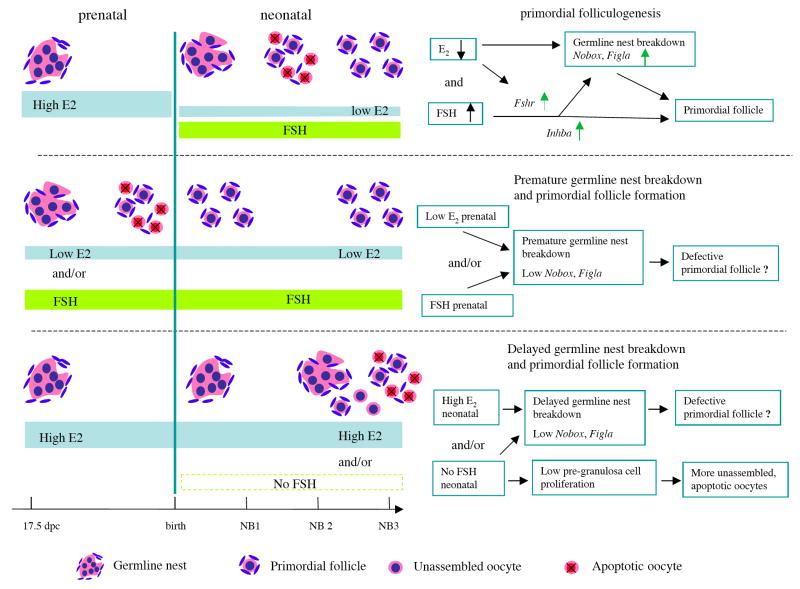
It is clear that not only the presence of FSH but the timing and coordination of changes in FSH and E2 around the time of birth are essential for supporting the normal process of primordial folliculogenesis (Figure 7). Prenatally, high E2 is important for preventing premature germline nest breakdown. In vitro, exposure of 17.5 dpc ovaries to low E2 and/or FSH for 6 days led to germline nest breakdown and primordial folliculogenesis. During the first 3 days of culture in these conditions, the oocytes that assemble into primordial follicles exhibited altered expression of germ cell-specific factors. Figla and Nobox are two important germ cell-specific transcription factors that are not only involved in primordial follicle formation, but are also important for regulating the expression of other oocyte -specific genes such as Zps [27], Gdf9 and Bmp15 [19]. These genes play critical roles in regulating later oocyte development and folliculogenesis. Figla expression was identical in ovaries cultured for 6 days in either low or high E2, although FSH was found promote its expression in both E2 conditions. And Nobox expression did not vary significantly in the ovaries cultured in persistent hormonal condition for 6 days. However, ovaries that were first cultured in high E2 and then switched to low E2 or low E2 plus FSH on day 4 produced the highest proportion of primordial follicles and had the highest expression levels of Figla and Nobox. Thus, the timing of the changes in ovarian exposure to FSH and E2 appears essential for regulating the process of primordial follicle formation, ensuring proper oocyte gene expression and support by pre-granulosa cells. Conversely, persistently high levels of E2 inhibited germline nest breakdown and Figla and Nobox expression, and delayed primordial folliculogenesis. The interrupted expression of oocyte-specific genes in both premature and delay formed primordial follicles suggesting that these follicles might contain defective oocytes. Interestingly, in the mice with promoted primordial follicle formation induced by neontal activin A administration, poor egg quality with defective spindle organization were found in pubertal ovaries. But those defective follicles were depleted rapidly from ovaries. There was no significant difference on the number of total follicles in adult ovaries between treatment and control mice [8]. Similar deletion of the follicles was also showed in the mice with delayed follicle formation by E2 injection after birth [23].
Figure 7. The role of FSH and E2 levels and timing in primordial folliculogenesis in in vitro cultured mouse ovaries.
(a) During normal primordial follicle formation, represented in vitro by culturing 17.5 dpc mouse fetal ovaries for 3 days in high E2 and then 3 days in low E2 and FSH, a drop in E2 levels and presence of FSH permitted germline nest breakdown and up-regulation of oocyte expression of Figla and Nobox, which are critical for primordial folliculogenesis. At the same time, stimulation of FSH receptor (Fshr) gene expression and the activin (Inhba) signaling pathway conferred FSH responsiveness and promoted proliferation of pre-granulosa cells. (b) In the presence of persistently low E2 levels and/or with FSH addition, cultured fetal mouse ovaries exhibited premature germline nest breakdown and primordial follicle formation. Loss of the acute drop in E2 after birth (day 4 of in vitro culture) leads to low levels of Figla and Nobox in oocytes, which might lead to the assembly of primordial follicles with defective oocytes. (c) Exposure of cultured fetal ovaries to persistently high E2 levels inhibited germline nest breakdown, delayed primordial folliculogenesis, and repressed Figla and Nobox expression in oocytes. Loss of neonatal FSH exposure promoted oocyte apoptosis and decreased pre-granulosa cell proliferation, which in turn led to incomplete primordial follicle formation.
Although the stimulatory effect of FSH on primordial folliculogenesis was noted whether ovaries were cultured in either high or low E2, lower E2 levels appeared to be more permissive for primordial folliculogenesis. In ovaries cultured in lower E2, pre-granulosa cell expression of Fshr expression was upregulated, leading to higher FSH responsiveness and cAMP production. The presence of FSH in the culture media also promoted the gene expression of the activin βA subunit (Inhba), pre-granulosa cell proliferation and oocyte survival; Activin A has been shown previously to promote primordial folliculogenesis through the stimulation of oocyte and pre-granulosa cell proliferation [8].
Many members of TGF-beta family participate in regulating early follicle development [9]. Our previous work described an important role for activin A in early oocyte differentiation. Transgenic mouse models of disrupted activin signaling exhibited abnormal ovarian development and the formation of multiple-oocyte follicles (MOFs) [29; 30]. Furthermore, neonatal mice exposed to E2 had reduced activin βA subunit mRNA and protein expression [23]. Conversely, mouse ovaries injected with activin A on the day of birth had elevated germ and pre-granulosa cell proliferation and a large primordial follicle pool. In our in vitro cultured ovaries, the expression of the activin βA subunit (Inhba) was up-regulated primarily by FSH and to a lesser extent by low E2 levels. Previous studies in rats revealed that activin may be important for acquisition of FSH responsiveness in the neonatal ovary [31]. We hypothesize that the stimulatory effect of FSH on pre-granulosa cell proliferation during primordial folliculogenesis is modulated in part by activin A.
In the human ovary, primordial follicle formation starts at 18 weeks of gestation (second trimester) when relatively high steroid levels are present in the circulation [32; 33]. By the end of the first trimester, the human fetal pituitary starts to secrete gonadotropins (FSH and LH), and FSH levels peak around 20 weeks gestation, coincident with the time of germline nest breakdown and primordial follicle formation. At this time, FSH levels in the female fetus are several times higher than in the male fetus [34]. Furthermore, recent research on the human fetal ovary found that SMAD2/3 expression was found to be up-regulated during primordial follicle formation, which supports our proposal that the FSH regulated activin signal pathway might play an important role in human primordial folliculogenesis [35].
In summary, using an in vitro mouse fetal ovary culture model, we demonstrate the importance of FSH in regulating germline nest breakdown and primordial folliculogenesis. After birth, the presence of FSH combined with an acute drop in E2 concentration confers FSH responsiveness via up-regulation of activin βA subunit (Inhba) and Fshr and enables the expression of two germ cell-specific transcription factors (Figla and Nobox). Exposure of the ovaries to inappropriate levels of FSH or E2 or inappropriate timing of hormone exposure resulted in premature or delayed primordial folliculogenesis and altered expression of key genes in oocytes and pre-granulosa cells. These data enrich our understanding of the mechanisms of primordial follicle formation and may contribute to the elucidation of the mechanisms underlying low follicle reserve-related diseases in humans.
Acknowledgments
The authors gratefully acknowledge the assistance of Sarah Kiesewetter and Jen Jozefik in animal care concerns, and the PO1-funded Northwestern University Histology Core (Tyler Wellington, director) for all tissue processing. We thank Dr. Monica Antenos and Candace Tingen for discussion and critical reading of the manuscripts.
Grant Support: The work is supported by NIH grant P01HD21921 and 5 R01HD044464.
Footnotes
Childs, A. J. and R. A. Anderson “Activin A selectively represses expression of the membrane-bound isoform of Kit ligand in human fetal ovary.” Fertility and Sterility In Press, Corrected Proof.
References
- 1.Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 2006;44:622–632. doi: 10.1002/dvg.20258. [DOI] [PubMed] [Google Scholar]
- 2.Pepling ME, Spradling AC. Mouse ovarian germ cell nests undergo programmed breakdown to form primordial follicles. Developmental Biology. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- 3.Mackay S, Smith RA. Mouse gonadal differentiation in vitro in the presence of fetal calf serum. Cell Differentiation and Development. 1989;27:19–28. doi: 10.1016/0922-3371(89)90041-5. [DOI] [PubMed] [Google Scholar]
- 4.Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biology of Reproduction. 2006;74:161–168. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- 6.Montano MM, Welshons WV, Vom Saal FS. Free estradiol in serum and brain uptake of estradiol during fetal and neonatal sexual differentiation in female rats. Biology of Reproduction. 1995;53:1198–1207. doi: 10.1095/biolreprod53.5.1198. [DOI] [PubMed] [Google Scholar]
- 7.Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- 8.Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK. Postnatal regulation of germ cells by activin: The establishment of the initial follicle pool. Developmental Biology. 2006;298:132–148. doi: 10.1016/j.ydbio.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 10.Woodruff TK, D'Agostino J, Schwartz NB, Mayo KE. Decreased inhibin gene expression in preovulatory follicles requires primary gonadotropin surges. Endocrinology. 1989;124:2193–2199. doi: 10.1210/endo-124-5-2193. [DOI] [PubMed] [Google Scholar]
- 11.Roy SK, Albee L. Requirement for follicle-stimulating hormone action in the formation of primordial follicles during perinatal ovarian development in the hamster. Endocrinology. 2000;141:4449–4456. doi: 10.1210/endo.141.12.7805. [DOI] [PubMed] [Google Scholar]
- 12.Dunkel L, Tilly JL, Shikone T, Nishimori K, Hsueh AJW. Follicle-stimulating hormone receptor expression in the rat ovary: increases during prepubertal development and regulation by the opposing actions of transforming growth factors β and α. Biology of Reproduction. 1994;50:940–948. doi: 10.1095/biolreprod50.4.940. [DOI] [PubMed] [Google Scholar]
- 13.O'Shaughnessy PJ, Marsh P, Dudley K. Follicle-stimulating hormone receptor mRNA in the mouse ovary during post-natal development in the normal mouse and in the adult hypogonadal (hpg) mouse: structure of alternate transcripts. Molecular and Cellular Endocrinology. 1994;101:197–201. doi: 10.1016/0303-7207(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 14.Kendall SK, Samuelson LC, Saunders TL, Wood RI, Camper SA. Targeted disruption of the pituitary glycoprotein hormone α-subunit produces hypogonadal and hypothyroid mice. Genes & Development. 1995;9:2007–2019. doi: 10.1101/gad.9.16.2007. [DOI] [PubMed] [Google Scholar]
- 15.Kumar TR, Wang Y, Lu NF, Matzuk MM. Follicle stimulating hormone is required for ovarian follicular maturation but not male fertility. Nature Genetics. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 16.Huhtaniemi IT, Aittomäki K. Mutations of follicle-stimulating hormone and its receptor effects on gonadal function. European Journal of Endocrinology. 1998;138:473–481. doi: 10.1530/eje.0.1380473. [DOI] [PubMed] [Google Scholar]
- 17.Allan CM, Wang Y, Jimenez M, Marshan B, Spaliviero J, Illingworth P, Handelsman DJ. Follicle-stimulating hormone increases primordial follicle reserve in mature female hypogonadal mice. Journal of Endocrinology. 2006;188:549–557. doi: 10.1677/joe.1.06614. [DOI] [PubMed] [Google Scholar]
- 18.Soyal SM, Amleh A, Dean J. FIGα, a germ cell-specific transcription factor required for ovarian follicle formation. Development. 2000;127:4645–4654. doi: 10.1242/dev.127.21.4645. [DOI] [PubMed] [Google Scholar]
- 19.Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupt early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- 20.Childs AJ, Anderson RA. Activin A selectively represses expression of the membrane-bound isoform of Kit ligand in human fetal ovary. Fertility and Sterility. doi: 10.1016/j.fertnstert.2009.03.095. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 21.Lei L, Zhang H, Jin S, Wang F, Fu M, Wang H, Xia G. Stage-specific somatic and germ cell interactions determine the primordial folliculogenesis. Journal of Cellular Physiology. 2006;208:640–647. doi: 10.1002/jcp.20702. [DOI] [PubMed] [Google Scholar]
- 22.Mannan MA, O'Shaughnessy PJ. Steroidogenesis during postnatal development in the mouse ovary. Journal of Endocrinology. 1991;130:101–106. doi: 10.1677/joe.0.1300101. [DOI] [PubMed] [Google Scholar]
- 23.Kipp JL, Kilen SM, Bristol-Gould S, Woodruff TK, Mayo KE. Neonatal exposure to estrogens suppresses activin expression and signaling in the mouse ovary. Endocrinology. 2007;148:1968–1976. doi: 10.1210/en.2006-1083. [DOI] [PubMed] [Google Scholar]
- 24.Terada N, Kuroda H, Namiki M, Kitamura Y, Matsumoto K. Augmentation of aromatase activity by FSH in ovaries of fetal and neonatal mice in organ culture. The Journal of Steroid Biochemistry and Molecular Biology. 1984;20:741–745. doi: 10.1016/0022-4731(84)90078-5. [DOI] [PubMed] [Google Scholar]
- 25.Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biology of Reproduction. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 26.Halpin DMG, Jones A, Fink G, Charlton HM. Postnatal ovarian follicle development in hypogonadal (hpg) and normal mice and associated changes in the hypothalamic pituitary axis. Journal of Reproduction and Fertility. 1986;77:287–296. doi: 10.1530/jrf.0.0770287. [DOI] [PubMed] [Google Scholar]
- 27.Liang L, Soyal SM, Dean J. FIGα, a germ cell specific transcription factor involved in the coordinate expression of the zone pellucida genes. Development. 1997;124:4939–4947. doi: 10.1242/dev.124.24.4939. [DOI] [PubMed] [Google Scholar]
- 28.Byskov AG. Differentiation of mammalian embryonic gonad. Physiological Reviews. 1986;66:71–117. doi: 10.1152/physrev.1986.66.1.71. [DOI] [PubMed] [Google Scholar]
- 29.Bristol-Gould SK, Hutten CG, Sturgis C, Kilen SM, Mayo KE, Woodruff TK. The development of a mouse model of ovarian endosalpingiosis. Endocrinology. 2005;146:5228–5236. doi: 10.1210/en.2005-0697. [DOI] [PubMed] [Google Scholar]
- 30.McMullen ML, Cho BN, Yates CJ, Mayo KE. Gonadal pathologies in transgenic mice expressing the rat inhibin α-subunit. Endocrinology. 2001;142:5005–5014. doi: 10.1210/endo.142.11.8472. [DOI] [PubMed] [Google Scholar]
- 31.Drummond AE, Dyson M, Mercer JE, Findly JK. Differential response of post-natal rat ovarian cells to FSH and activin. Molecular and Cellular Endocrinology. 1996;122:21–32. doi: 10.1016/0303-7207(96)03867-1. [DOI] [PubMed] [Google Scholar]
- 32.Mayo K, Jameson L, Woodruff TK. Eggs in the nest. Endocrinology. 2007;148:3577–3579. doi: 10.1210/en.2007-0590. [DOI] [PubMed] [Google Scholar]
- 33.Fulton N, Martins da Silva SJ, Bayne RA, Anderson RA. Germ cell proliferation and apoptosis in the developing human ovary. Journal of Clinical Endocrinology & Metabolism. 2005;90:4664–4670. doi: 10.1210/jc.2005-0219. [DOI] [PubMed] [Google Scholar]
- 34.Melmed S. The pituitary. Second. Blackwell publishing; 2002. p. 631. [Google Scholar]
- 35.Couttsa SM, Childsa AJ, Fultona N, Collinsa C, Baynea RAL, McNeillya AS, Andersonb RA. Activin signals via SMAD2/3 between germ and somatic cells in the human fetal ovary and regulates kit ligand expression. Developmental Biology. 2008;314:189–199. doi: 10.1016/j.ydbio.2007.11.026. [DOI] [PubMed] [Google Scholar]